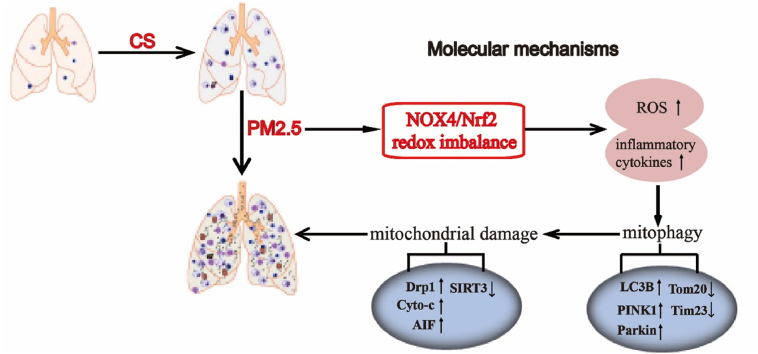
Abstract
The increasing abundance of fine particulate matter (PM2.5) in the environment has increased susceptibility to acute exacerbation of COPD (AECOPD). During PM2.5 exposure, excessive reactive oxygen species (ROS) production triggers a redox imbalance, which contributes to damage to organelles and disruption of homeostasis. At present, there are limited data on whether NOX4/Nrf2 redox imbalance increases susceptibility to acute exacerbation of COPD (AECOPD), and the underlying mechanism is unclear. Therefore, the current study was aimed to evaluate the role of NOX4/Nrf2 redox balance on AECOPD induced by PM2.5-CS-exposure. Here, we report that PM2.5 exacerbates cytotoxicity by enhancing NOX4/Nrf2 redox imbalance-mediated mitophagy. First, exposure to a low-dose of PM2.5 (200 μg/ml) significantly exacerbated oxidative stress and mitochondrial damage by increasing the ROS overproduction, enhancing the excessive NOX4/Nrf2 redox imbalance, decreasing the mitochondrial membrane potential (MMP), and enhancing the mitochondrial fragmentation that were caused by a low-dose of CSE (2.5%). Second, coexposure to PM2.5 and CSE (PM2.5-CSE) induced excessive mitophagy. Third, PM2.5 exacerbated CS-induced COPD, as shown by excessive inflammatory cell infiltration, inflammatory cytokine production and mucus hypersecretion, goblet cell hyperplasia, NOX4/Nrf2 redox imbalance, and mitophagy, these effects triggered excessive ROS production and mitochondrial damage in mice. Mechanistically, PM2.5-CS-induced excessive levels of mitophagy by triggering redox imbalance, leading to greater cytotoxicity and AECOPD; however, reestablishing the NOX4/Nrf2 redox balance via NOX4 blockade or mitochondria-specific ROS inhibitor treatment alleviated this cytotoxicity and ameliorated AECOPD. PM2.5 may exacerbate NOX4/Nrf2 redox imbalance and subsequently enhance mitophagy by increasing the ROS and mito-ROS levels, thereby increasing susceptibility to AECOPD.
Keywords: PM2.5, NOX4/Nrf2 redox imbalance, Mitophagy, Acute exacerbation of COPD
Graphical abstract
1. Introduction
Air pollution is a problem that affects human health worldwide. Increasing numbers of epidemiological and clinical studies have shown that long-term exposure to air pollution, in particular, exposure to airborne particulate matter 2.5 (PM2.5), increases the morbidity and mortality rates of respiratory diseases, such as asthma, acute exacerbation of chronic obstructive pulmonary disease (AECOPD), and lung cancer [1,2]. Thus, it is of great importance to explore the potential molecular mechanisms by which air pollutants trigger airway injury in order to identify new effective therapeutic strategies. Previous studies have reported multiple mechanisms by which PM2.5 causes pathology, including mechanisms related to inflammation, oxidative stress, aging and airway epithelial barrier impairment [[3], [4], [5]]. However, the detailed molecular mechanisms underlying the adverse effects of PM2.5 remain to be further investigated.
Mitochondria are sensitive targets of PM2.5, and PM2.5 exposure causes obvious mitochondrial dysfunction. Damaged mitochondria produce large amounts of reactive oxygen species (ROS), resulting in intracellular redox imbalance and eventually leading to extensive mitochondrial damage [6,7]. Dysfunctional mitochondria exhibit fragmentation and membrane depolarization, produce large amounts of ROS and release apoptotic proteins in response to stressors, ultimately activating the mitochondrial cell death pathway [8]. To disrupt the vicious cycle of mitochondrial damage and ROS production, cells protect themselves from mild damage via mitophagy, which is a cellular quality control mechanism. However, it has also been reported that the accumulation of substantial levels of mitochondrial ROS leads to abnormal or excessive mitophagy, which in turn causes cell death and disease [9]. It has been reported that PM2.5-induced ROS-mediated increases in mitophagy contribute to liver and vessel fibrosis [10,11]. Excessive autophagy induced by PM2.5 causes lung injury [12]. Mitophagy is usually considered to be a mechanism of cell survival, as it eliminates damaged mitochondria. However, it has also been reported that abnormal or excessive mitophagy contributes to cell death. Studies have shown that PM2.5 can cause different degrees of oxidative damage to the lung, heart, and liver tissues of mice and thereby exert obvious toxic effects, which can result in heart disease, stroke, respiratory infections, liver cancer and so on [13,14]. However, the role of ROS in initiating mitophagy and cell death remains largely unknown. We hypothesized that PM2.5 may induce AECOPD via a mechanism that involves the regulation of mitophagy by oxidative stress.
The antioxidant capacity of patients with COPD decreases in response to chronic exposure to toxicants, and oxidative stress is considered to be one of the main predisposing factors of COPD [15,16]. ROS affect many cellular functions, and the maintenance of a proper redox balance between ROS and antioxidant capacity is important for the maintenance of the normal physiological function of cells. Under pathological conditions, redox imbalance leads to increased ROS generation, which may lead to oxidative damage. Redox imbalance is associated with deficient activation of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), which is a master regulator of antioxidant genes, and sustained activation of NADPH oxidase 4 (NOX4), which is an enzyme that generates reactive oxygen species. NOX4 is a member of NOx family that produces ROS and is rapidly expressed when the body is stimulated by stressors [17,18]. On the other hand, antioxidative defenses also play a critical role in the development and progression of many diseases that are characterized by redox imbalance, including COPD. The antioxidant defense network is regulated by Nrf2, and inhibition of Nrf2 results in excessive ROS production, making the body vulnerable to damage by toxic substances [19,20]. It has been reported that many diseases can be alleviated by regulating the NOX4/Nrf2 pathway to inhibit ROS production. It has been reported that the NOX4 protein abundance in the airway smooth muscle cells of COPD patients increases with disease severity and is negatively associated with pulmonary function [21]. Other reports have confirmed the role of Nrf2 in protecting against lung inflammation via the activation of anti-protease and antioxidants in alveolar macrophages in an Nrf2-knockout mouse model [22]. Oxidative stress is one of the main mechanisms by which exposure to PM2.5 induces tissue damage [10]. Epidemiological studies have shown that sustained exposure to high levels of PM2.5, the concentration of which varies in different seasons, increases the risk of AECOPD [5]. A previously unknown mechanism underlying NOX4/Nrf2 redox balance may help to explain the more frequent development of AECOPD in response to PM2.5 exposure. However, how to reestablish the NOX4/Nrf2 redox balance and reduce ROS production in individuals with PM2.5-CS exposure-induced AECOPD is not well understood.
In the present study, we proposed whether NOX4/Nrf2 redox balance could regulating mitophagy alleviate lung injury and reduce the susceptibility to PM2.5-CS-induced AECOPD. Therefore, we demonstrated that reestablishing the NOX4/Nrf2 redox balance via NOX4 blockade or mitochondria-specific ROS inhibitor treatment reduced ROS production and mitophagy and decreased susceptibility to PM2.5-CS-induced AECOPD in vitro and in vivo.
2. Materials and methods
2.1. Materials
PM2.5 (SRM1649b) was obtained from NIST (MD, USA). Cigarettes were purchased from Hongta Tobacco (Group) Co., Ltd. (Yunnan, China). GKT137831 and mito-TEMPO were obtained from Med Chem Express (New Jersey, USA). Specific antibodies against Nrf2 (ab137550), p-mTOR (ab109268), SIRT3 (ab189860), VCAM (ab174279), ICAM (ab179707) and β-actin (ab8226) were purchased from Abcam (Cambridge, UK). Specific antibodies against caspase3 (9662S), Bax (5023S), Bcl2 (3498S), cleaved caspase-3 (9664S), Drp1 (8570S), AIF (4642), Cyto-c (4272), LC3B (2775S), P62 (5114S), ATG3 (3415), Tom20 (42406S), and Parkin (4211S) were purchased from Cell Signaling Technology (Danvers, USA). A specific antibody against PINK1 (P0076) was provided by Sigma–Aldrich (St. Louis, MO, USA). Specific antibody against NOX4 (Cat NO.380874) and MUC5ac (Cat NO.381811) were obtained from ZENBIO (Nanjing, China). Specific antibodies against Tim23 (Cat No.11123-1-AP) and p-creb (Cat No.28792-1-AP) were obtained from Proteintech (Chicago, USA). The BCA protein assay kit (lot # tk274307, USA) was obtained from Thermo Fisher Scientific. NOX4 siRNA was obtained from HanBio Technology (Shanghai, China). Cell Counting Kit-8 (CCK-8) and LDH kits were obtained from Beyotime (Shanghai, China).
2.2. Preparation of cigarette smoke extracts (CSEs) and PM2.5
A peristaltic pump was used to collect the smoke from cigarettes, and the smoke of one cigarette was bubbled through 10 ml of DMEM. The pH of the CSE solution was then adjusted to 7.2, and the solution was passed through a 0.22‐μm filter to remove bacteria and large particles. The solution was considered to have a concentration of 10% CSE, and the concentration was adjusted as indicated based on the experimental needs. PM2.5 was dissolved in DMSO to a concentration of 400 mg/ml and then shaken and ultrasonicated for 2 h. After centrifugation, the supernatant was collected and stored at −20 °C.
2.3. Cell culture
Beas-2b cells were maintained in DMEM supplemented with 10% FBS and antibiotics in an incubator in 5% CO2 at 37 °C.
2.4. Cell viability and LDH measurement
BEAS-2b cells were cultured in 96-well plates. One hour before treatment with or without PM2.5 (200 μg/ml) and CSE (2.5%), the cells were treated with GKT 137831 (25 μM) or mito-TEMPO (100 μM). The Cell Counting Kit-8 or LDH kit was used to assess cell viability.
2.5. Intracellular ROS and mitochondrial ROS assay
DCFH-DA (Beyotime Shanghai, China) and MitoSOX (Invitrogen, USA) were used to measure the production of intracellular ROS and mitochondrial ROS. After treatment, BEAS-2b cells were incubated at 37 °C for 40 min or 15 min with DCFH-DA or MitoSOX, respectively, and then observed by fluorescence microscopy.
2.6. TMRM staining
After treatment, BEAS-2b cells were stained with TMRM for 20 min. Then, the red fluorescence intensity was observed by fluorescence microscopy.
2.7. Immunofluorescence assay
After treatment, BEAS-2b cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.2% Triton X-100 for 5 min and blocked with 5% BSA for 1 h at room temperature. Tom20 and Tim23 monoclonal antibodies (1:200) were coincubated with the cells at 4 °C overnight. After washing with PBS, the cells were coincubated with a fluorescent secondary antibody for 1 h and then stained with DAPI. The images were captured under a fluorescence microscope.
2.8. RNA interference assay
Small interfering RNAs (siRNAs) targeting NOX4 (si 1, si 2 and si 3) were designed by HanBio Technology (Shanghai, China). BEAS-2b cells were transfected with these molecules using RNA-FIT (HanBio Technology, Shanghai, China).
2.9. Transmission electron microscope (TEM) assay
After treatment, BEAS-2b cells and lung tissues were fixed in 2.5% glutaraldehyde, and then, sections were generated with an ultrathin microtome. The samples were stained and imaged with a transmission electron microscope.
2.10. Autophagic flux assay
mRFP-GFP-LC3 adenoviral vectors were designed by HanBio Technology (Shanghai, China) and used to assess the autophagic flux. The autophagic flux was observed and analyzed with a confocal microscope.
2.11. Animal experiments
Male BALB/c mice (Beijing Vital River Laboratory Animal Technology Co., Ltd.; Certificate SYXK2019-0012) aged 6–8 weeks were randomly divided into 5 groups: 1) control group; 2) COPD group; 3) AECOPD group; 4) AECOPD+GKT (60 mg/kg, ip) group; 5) AECOPD+mito-TEMPO (20 mg/kg, ip) group. To establish COPD, LPS (50 μg/mouse) was administered by intranasal instillation on the 1st and 14th days, and the mice were then exposed to cigarette smoke (CS; 3 times/day, nine cigarettes in total) for up to 4 weeks via a PAB-S200 animal passive smoking exposure system (Biolab Technology, Beijing, China). The mice in the AECOPD group were intranasally instilled with PM2.5 (10 mg/kg) daily for 7 consecutive days beginning on the 21st day.
2.12. Giemsa and ROS staining
The mice were euthanized, and endotracheal intubation was performed. PBS (500 μl) was injected into the lungs via endotracheal intubation, and the alveoli were flushed twice to collect the BALF. The total cell numbers were counted, and the cells were stained using a Giemsa and ROS staining kit.
2.13. ELISA
The levels of the cytokines IL-1, IL-6, IL-8 and TNF-α in the BALF were measured using ELISA kits according to the manufacturer's instructions (BioLegend Inc., CA, USA).
2.14. Hematoxylin and eosin (HE), PAS and terminal transferase dUTP nick-end labeling (TUNEL) staining
Lung tissues were fixed in 4% paraformaldehyde, embedded, sliced, dehydrated, and then stained with H&E, PAS and TUNEL.
2.15. SOD, GSH, MPO and MDA assay
SOD, GSH, MPO and MDA assay kits were used to evaluate the level of oxidative stress in BEAS-2b cells and lung tissues according to the manufacturers’ instructions.
2.16. Western blotting assay
Cells or lung tissues were lysed at 4 °C with RIPA lysis buffer supplemented with protease and phosphatase inhibitors. The total protein concentration was measured by the BCA method. After separation in SDS-polyacrylamide gels, the proteins were transferred to PVDF membranes. The membranes were blocked with 5% nonfat milk and incubated overnight with specific primary antibodies. After washing, the membranes were incubated with secondary antibodies at room temperature for 1 h. The data were analyzed using ImageJ software.
2.17. Statistical analysis
All the experimental data were obtained from at least three independent experiments. All the data were analyzed by GraphPad Prism (GraphPad Software, San Diego, CA, USA). P values of less than 0.05 were considered to indicate statistically significant differences. All the values are presented as the mean ± SEM. One-way analysis of variance (ANOVA) was used to analyze multiple groups, and then a posttest was performed.
3. Results
3.1. Effect of PM2.5 on oxidative stress and mitochondrial damage in Beas-2b cells
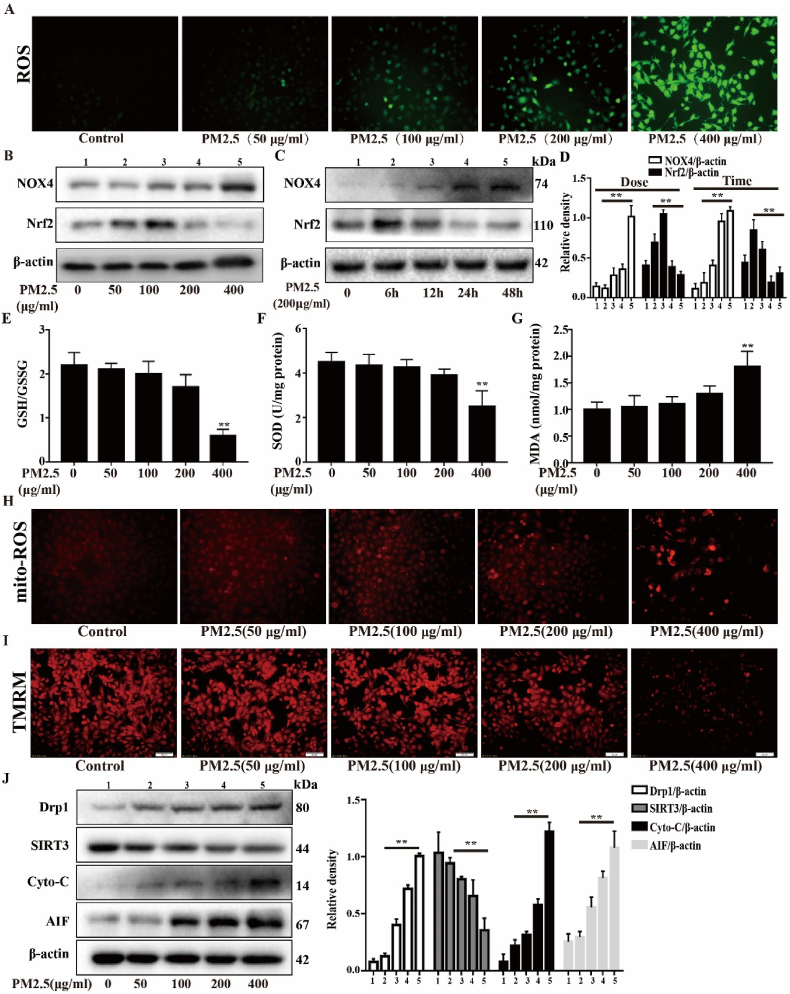
Oxidative stress plays a major role in PM2.5-mediated lung injury. To analyze the level of oxidative stress in Beas-2b cells treated with PM2.5, the level of ROS generation, the ratio of NOX4/Nrf2 expression, and the expression levels of GSH/GSSG, SOD and MDA were examined. As shown in Fig. 1A, PM2.5 significantly increased the levels of ROS in a dose-dependent manner, leading to redox imbalance. This imbalance was associated with deficient activation of Nrf2, which is a master regulator of antioxidant genes, and sustained activation of NOX4 in a dose- and time-dependent manner (Fig. 1B–D). Moreover, the levels of oxidant and antioxidant enzymes, including GSH/GSSG, SOD and MDA, were obviously changed in high-dose PM2.5 (400 μg/ml) group (Fig. 1E–G). These data indicate that PM2.5 triggers oxidative stress in Beas-2b cells via a NOX4/Nrf2 redox imbalance in a dose-dependent manner. To examine the mitochondrial damage induced by PM2.5, the mito-ROS levels, mitochondrial membrane potential and mitochondrial function-related protein levels were measured. Compared with the control and low-dose PM2.5 groups, the high-dose PM2.5 (400 μg/ml) group exhibited significant increases in mito-ROS generation and decreased in membrane potential (Fig. 1H and I). Then, we measured the protein expression of cyto-c, AIF and DRP1, which play key roles in mitochondrial fission. The results showed that the expression levels of DRP1, cyto-c and AIF were significantly increased in the PM2.5 (400 μg/ml) group compared with the control and low-dose PM2.5 groups. SIRT3, which is a protein that regulates mitochondrial metabolism, was decreased by PM2.5 in a dose-dependent manner (Fig. 1J). The cytotoxicity and apoptosis were significantly increased in the PM2.5 (400 μg/ml) group compared with the control and low-dose PM2.5 groups (Supplementary Fig. 1). These data suggest that PM2.5 triggers oxidative stress and mitochondrial damage in a dose-dependent manner in Beas-2b cells.
Fig. 1.
Effects of PM2.5 on oxidative stress and mitochondrial damage in vitro. (A) After different dose of PM2.5 exposed for 24 h in Beas-2b cells, total ROS were measured in Beas-2b cells. (B) Western blot analysis showed the expression levels of NOX4 and Nrf2 in dose-depend manner, (C) time-depend manner and the relative density. (E–G) After treated with different concentration of PM2.5 for 24 h in Beas-2b cells, the expression levels of oxidants (MDA) and antioxidants (GSH/GSSG and SOD) were determined. (H) The mitochondrial ROS in different concentration of PM2.5 were determined by MitoSOX staining. (I) Mitochondrial membrane potential were measured by TMRM staining in different concentration of PM2.5 for 24 h. (J) Western blot analysis showed the expression levels of Drp1, SIRT3, Cyto-c and AIF in different concentration of PM2.5 (50, 100, 200, 400 μg/mL) for 24 h. The values were showed as the mean ± SD. (**p < 0.01).
3.2. Effect of PM2.5 on mitophagy in Beas-2b cells
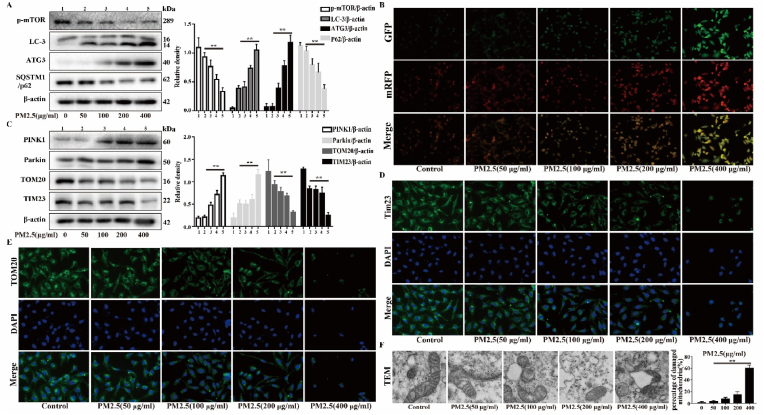
Mitophagy, which is a specific form of autophagy, controls mitochondrial quality and mitochondrial ROS production via the degradation of damaged mitochondria. However, excessive mitophagy activation can mediate cell death and cause disease. To evaluate the autophagy induced by PM2.5, the expression levels of proteins that regulate autophagy were measured. The results showed that the expression levels of LC3-II and ATG3 were increased in a dose-dependent manner, while the expression levels of p62 and mTOR were decreased by PM2.5 (Fig. 2A). The autophagic flux was analyzed with tandem fluorescent mCherry-GFP-LC3B in Beas-2b cells. The PM2.5 (400 μg/ml) group exhibited more autophagosomes and autolysosomes than the control and low-dose PM2.5 groups, indicating that the autophagic flux was enhanced (Fig. 2B). Subsequently, we assessed whether PM2.5 promotes mitophagy. The results showed that the expression levels of Parkin and PINK1 were increased in a dose-dependent manner, while the expression levels of TIM23 and TOM20 were decreased by PM2.5 (Fig. 2C). IF staining of TIM23 and TOM20 revealed that the fluorescence intensity of TIM23 and TOM20 gradually decreased with the increase of PM2.5 dose (Fig. 2D and E). Next, mitochondrial damage and mitophagy were assessed by TEM. As shown in Fig. 2F, the mitochondrial structure was disrupted, and mitochondrial vacuolation and mitophagy were observed. These results suggest that PM2.5 promotes mitophagy in a dose-dependent manner in Beas-2b cells.
Fig. 2.
Effects of PM2.5 on mitophagy in vitro. Beas-2b cells were treated with different concentration of PM2.5 (50, 100, 200, 400 μg/mL). (A) Western blot analysis showed the expression levels of p-mTOR, LC3-B, ATG3 and SQSTM1/p62. (B) Autophagic flux was detected by tandem fluorescent mCherry-GFP-LC3B. (C) Western blot analysis showed the expression levels of PINK1, Parkin, TOM20 and TIM23. (D and E) Immunofluorescence assay showed the expression levels of TIM23 and TOM20. (F) Transmission electron microscopy images showed the mitophagy and mitochondrial damage. The values were showed as the mean ± SD. (**p < 0.01). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. PM2.5-CSE causes oxidative stress and mitochondrial damage in Beas-2b cells
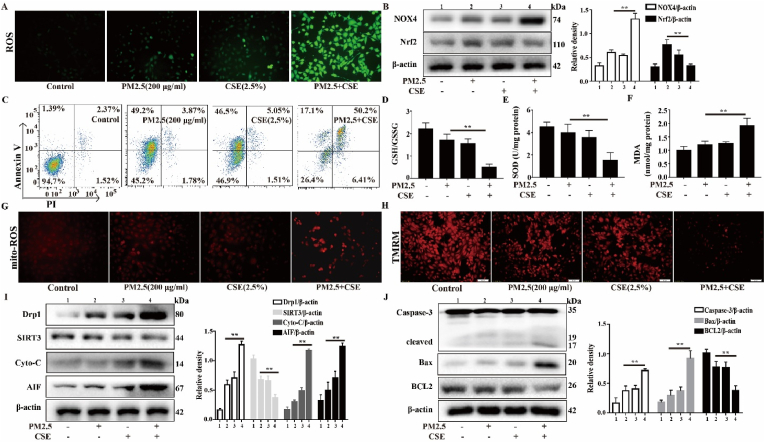
We have shown that higher doses of PM2.5 (400 μg/ml) or CSE (5%) are associated with significant cytotoxicity. According to our previous research results, exposure to a lower concentration of PM2.5 or CSE alone resulted in no obvious oxidative stress or mitochondrial damage in Beas-2b cells. Therefore, the appropriate working concentrations of PM2.5 (200 μg/ml) and CSE (2.5%) were selected for the subsequent experiments. Oxidative stress plays a major role in AECOPD. To study the oxidative stress that is induced by PM2.5-CSE in Beas-2b cells, the level of ROS production, the ratio of NOX4/Nrf2 expression, and the expression levels of GSH/GSSG, SOD and MDA were examined. As shown in Fig. 3A, PM2.5-CSE induced an obvious increase in the generation of ROS. Compared with the control and the PM2.5 or CSE alone groups, the PM2.5-CSE group showed a significant increase in NOX4/Nrf2 redox imbalance and apoptosis (Fig. 3B and C). In addition, PM2.5-CSE induced an increase in the MDA levels but a decrease in the GSH/GSSG and SOD levels, leading to redox imbalance (Fig. 3D–F). These results show that PM2.5 or CSE alone did not increase oxidative stress. However, PM2.5-CSE significantly increased oxidative stress via NOX4/Nrf2 redox imbalance in Beas-2b cells. To examine the mitochondrial damage that is induced by PM2.5-CSE, the mito-ROS levels, mitochondrial membrane potential and mitochondrial function-related protein levels were evaluated. Compared with the control, low-dose PM2.5 or CSE groups, a significant increase mito-ROS generation and decrease in membrane potential was observed in the PM2.5-CSE group (Fig. 3G and H). Then, we measured the protein levels of DRP1, which plays a key role in mitochondrial fission, as well as cyto-c and AIF. The results showed that the expression levels of DRP1, cyto-c and AIF were significantly increased in the PM2.5-CSE group compared with the control, low-dose PM2.5 and CSE groups. In contrast, the protein expression level of SIRT3, which is an important regulator of mitochondrial metabolism, was reduced (Fig. 3I). We also examined the levels of the apoptosis- and anti-apoptosis-related proteins cleaved-caspase-3, Bax and BCL2. The results showed that PM2.5-CSE increased the expression levels of cleaved-caspase-3 and Bax and decreased the expression levels of BCL2 compared with the control, low-dose PM2.5 or CSE (Fig. 3J). The cytotoxicity and apoptosis were significantly increased in PM2.5-CSE group compared with the control and low-dose PM2.5 or CSE groups (Supplementary Fig. 2). These data suggest that low concentrations of PM2.5 and CSE trigger oxidative stress and mitochondrial damage in Beas-2b cells.
Fig. 3.
PM2.5-CSE causes oxidative stress and mitochondrial damage in Beas-2b cells. Beas-2b cells were exposed to PM2.5 (200 μg/mL) and CS (2.5%) respectively, or PM2.5 (200 μg/mL) combined with CS (2.5%). (A) Total ROS were measured. (B) Western blot analysis showed the expression levels of NOX4 and Nrf2. (C) Annexin V-FITC/PI staining was used to detected apoptotic cells by flow cytomety. (D–F) The expression levels of oxidants (MDA) and antioxidants (GSH/GSSG and SOD) were determined. (G) The mitochondrial ROS were determined by MitoSOX staining. (H) Mitochondrial membrane potential were measured by TMRM staining. (I and J) Western blot analysis showed the expression levels of Drp1, SIRT3, Cyto-c, AIF, Caspase-3, Bax and BCL2. The values were showed as the mean ± SD. (**p < 0.01).
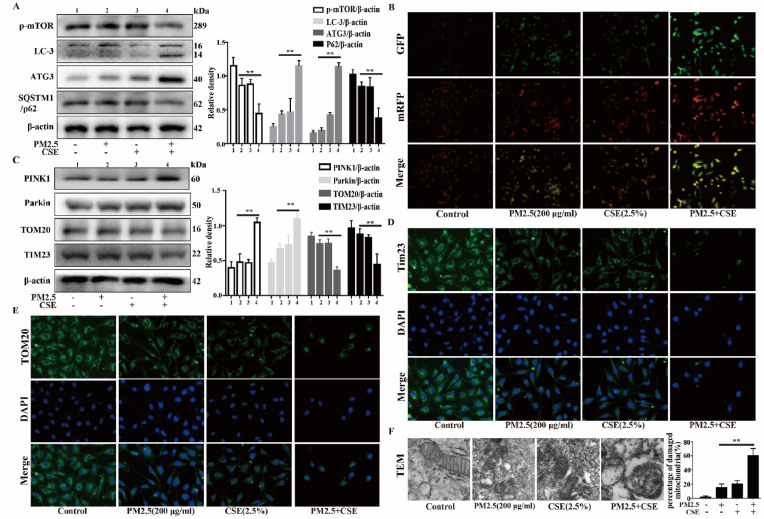
3.4. PM2.5-CSE triggers excessive mitophagy in Beas-2b cells
To evaluate the autophagy that is induced by PM2.5-CSE, the expression levels of proteins that regulate autophagy were measured. The results showed that the expression levels of LC3-II and ATG3 were increased, while the expression levels of p62 and mTOR were decreased in the PM2.5-CSE group (Fig. 4A). The autophagic flux was assessed with tandem fluorescent mCherry-GFP-LC3B in Beas-2b cells. The PM2.5-CSE group exhibited more autophagosomes and autolysosomes than the control and low-dose PM2.5 or CSE groups, indicating that the autophagic flux was enhanced (Fig. 4B). Mitophagy, which is a specific form of autophagy, controls mitochondrial quality and mitochondrial ROS via the degradation of damaged mitochondria. However, excessive activation of mitophagy can mediate cell death and cause disease. In this study, we found that PM2.5-CSE induces excessive mitophagy. The results showed that the expression levels of Parkin and PINK1 were significantly increased, while the expression levels of TOM20 and TIM23 were significantly decreased in the PM2.5-CSE group compared with the control and low-dose PM2.5 or CSE groups (Fig. 4C). IF staining of TIM23 and TOM20 revealed that the fluorescence intensity was obviously decreased in the PM2.5-CSE group (Fig. 4D and E). Next, mitochondrial damage and mitophagy were assessed by TEM. As shown in Fig. 4F, the mitochondrial structure was disrupted, and mitochondrial vacuolation and mitophagy were observed in the PM2.5-CSE group. These results suggest that low concentrations of PM2.5 and CSE promote mitophagy in Beas-2b cells.
Fig. 4.
PM2.5-CSE triggers excessive mitophagy in Beas-2b cells. Beas-2b cells were exposed to PM2.5 (200 μg/mL) and CS (2.5%) respectively, or PM2.5 (200 μg/mL) combined with CS (2.5%). (A) Western blot analysis showed the expression levels of p-mTOR, LC3-B, ATG3 and SQSTM1/p62. (B) Autophagic flux was detected by tandem fluorescent mCherry-GFP-LC3B. (C) Western blot analysis showed the expression levels of PINK1, Parkin, TOM20 and TIM23. (D and E) Immunofluorescence assay showed the expression levels of TIM23 and TOM20. (F) Transmission electron microscopy images showed the mitophagy and mitochondrial damage. The values were showed as the mean ± SD. (**p < 0.01). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
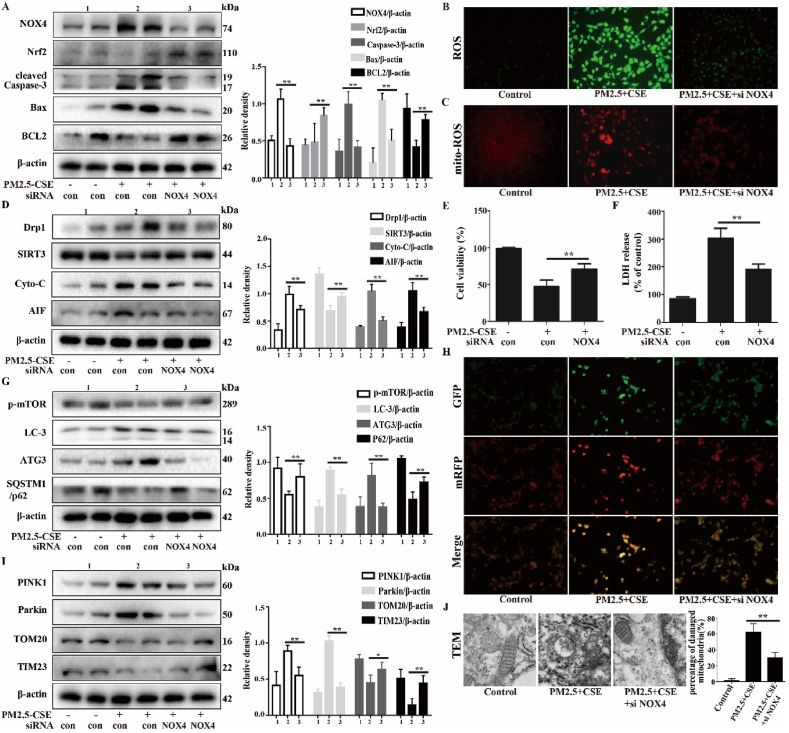
3.5. Effect of the NOX4/Nrf2 redox balance on PM2.5-CSE-induced oxidative stress, mitochondrial damage and mitophagy in Beas-2b cells
Studies have reported that ROS production is mainly dependent on NOX4/Nrf2 redox imbalance. Our previous study indicated that PM2.5-CSE causes NOX4/Nrf2 redox imbalance and triggers excessive ROS production, which leads to excessive mitophagy and mitochondrial damage. Therefore, NOX4 siRNA was transfected into Beas-2b cells, and PM2.5-CSE-induced oxidative stress, mitophagy and mitochondrial damage were evaluated. As shown in Fig. 5A–C, inhibiting NOX4 regulated the NOX4/Nrf2 redox balance and inhibited PM2.5-CSE-induced ROS generation. The PM2.5-CSE-induced cytotoxicity was decreased by NOX4 siRNA (Fig. 5E and F). In addition, the PM2.5-CSE-induced mitochondrial damage and mitophagy were also improved by suppressing NOX4 (Fig. 5D, G - J). NOX4 inhibitors, GKT137831, and mitochondria-targeted antioxidant, Mito-TEMPO, have the same effect (Supplementary Fig. 3). These results suggest that regulating the NOX4/Nrf2 redox balance can inhibit PM2.5-CSE-induced ROS production and improve mitochondrial damage and mitophagy.
Fig. 5.
Effects of the NOX4/Nrf2 redox balance on the oxidative stress, mitochondrial damage and mitophagy induced by PM2.5-CSE. Beas-2b cells were transfected with control siRNA or NOX4 siRNA, then exposed to PM2.5 (200 μg/mL) combined with CS (2.5%). (A) Western blot analysis showed the expression levels of NOX4, Nrf2, cleaved Caspase-3, Bax and BCL2. (B and C) Total ROS and mitochondrial ROS were measured by DCFH-DA and MitoSOX staining. (D) Western blot analysis showed the expression levels of Drp1, SIRT3, Cyto-c and AIF. (E) Cell viability was detected by Cell Counting Kit-8. (F) LDH release was detected by LDH kit. (G) Western blot analysis showed the expression levels of p-mTOR, LC3-B, ATG3 and SQSTM1/p62. (H) Autophagic flux was detected by tandem fluorescent mCherry-GFP-LC3B. (I) Western blot analysis showed the expression levels of PINK1, Parkin, TOM20 and TIM23. (J) Transmission electron microscopy images showed the mitophagy and mitochondrial damage. The values were showed as the mean ± SD. (*p < 0.05, **p < 0.01). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.6. PM2.5 exacerbated oxidative stress and the NOX4/Nrf2 redox imbalance in CS-exposed mice
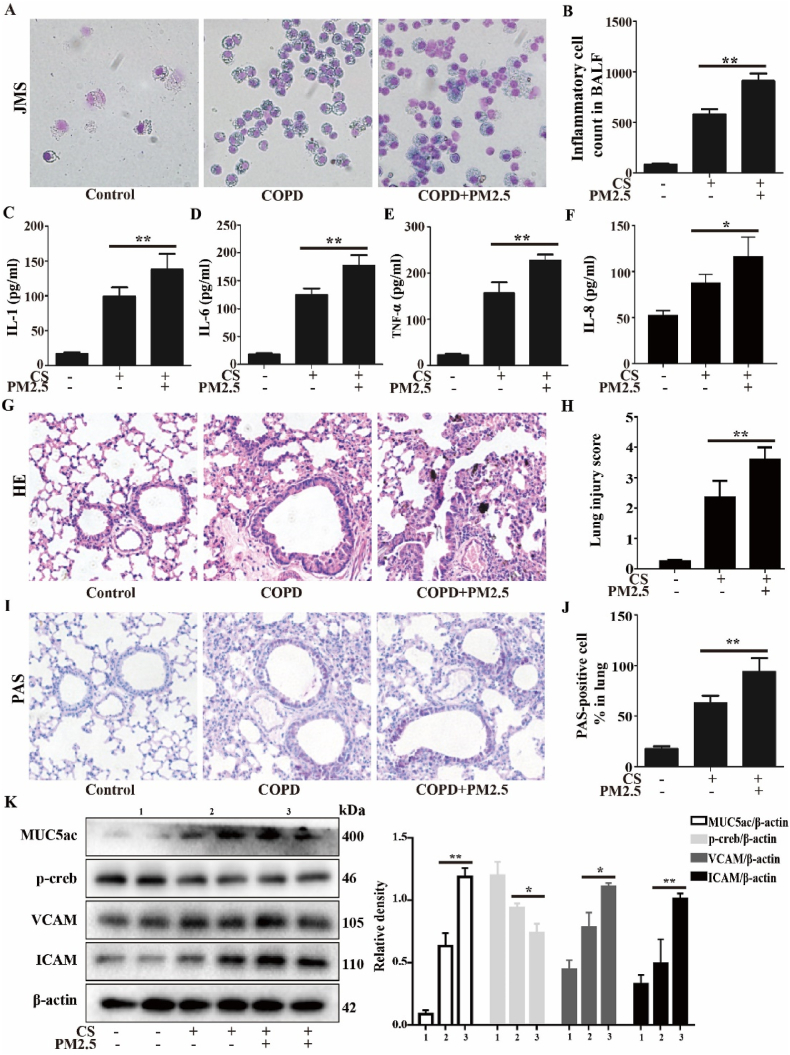
The CS-exposed mice exhibited airway inflammation, tissue damage and mucin hypersecretion compared with the control mice. Compared to the CS group, the CS + PM2.5 group exhibited more severe airway inflammation, tissue damage and mucin hypersecretion, and inflammatory cell infiltration and inflammatory cytokine production were significantly higher (Fig. 6A–F). The pathological changes in mouse lungs were assessed by H&E and PAS staining (Fig. 6G–J). The results showed that the alveolar walls of the CS + PM2.5 group were more severely thickened, with extensive neutrophil infiltration and numerous inflammatory cell foci around blood vessels, than those of the CS group. Compared to the CS group, goblet cell hyperplasia and mucus secretion were significantly increased in the CS + PM2.5 group. In addition, the expression levels of mucus secretion-related proteins were also significantly increased (Fig. 6K). These results suggest that PM2.5 exacerbated airway inflammation, tissue damage and mucin hypersecretion in CS-exposed mice.
Fig. 6.
PM2.5 exacerbated the airway inflammation, tissue damage and mucin hypersecretion in CS-exposed mice. A COPD mouse model was induced after 4 weeks of exposure to CS. The COPD mice were intranasally instilled with or without PM2.5 (10 mg/kg) daily for 7 consecutive days beginning on the 21 st day. (A) BALF was stained with Giemsa staining. (B) The inflammatory cell numbers in BALF. (C–F) The levels of IL-1, IL-6, TNF-α and IL-8 in BALF were determined by ELISA assay. (G and H) Lung tissue was stained with hematoxylin and eosin (H&E) and the lung injury score. (I and J) Lung tissue was stained with PAS and the percentage of PAS-positive cells. (K) Western blot analysis showed the expression levels of MUC5ac, p-creb, VCAM and ICAM. The values were showed as the mean ± SD. n = 3–5. (*p < 0.05, **p < 0.01).
3.7. PM2.5 exacerbated oxidative stress and NOX4/Nrf2 redox imbalance in CS-exposed mice
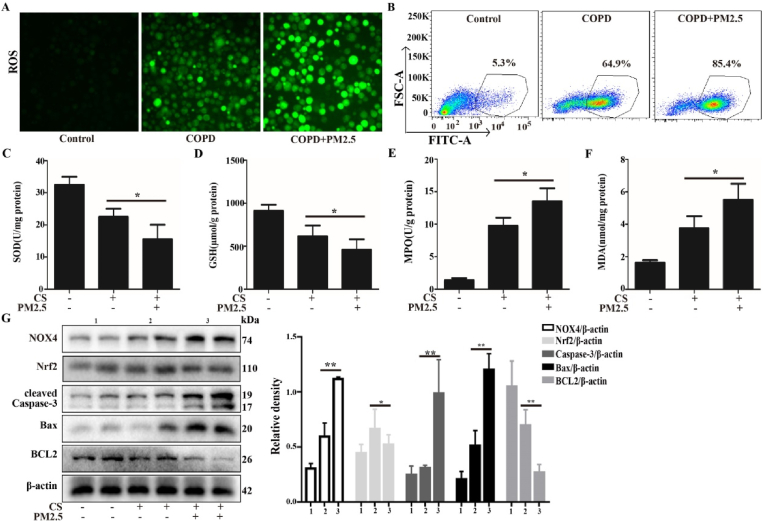
Our in vitro experiments demonstrated that PM2.5-CSE triggers a NOX4/Nrf2 redox imbalance, leading to ROS overproduction and resulting in mitochondrial damage and mitophagy. In vivo, we analyzed the effect of PM2.5 on oxidative stress, ROS generation and NOX4/Nrf2 redox imbalance in CS-exposed mice. As shown in Fig. 7A–F, the levels of ROS, MPO and MDA in the BALF were significantly increased, while the antioxidant enzymes SOD and GSH were obviously decreased compared with those in the CS group. Next, we analyzed the NOX4/Nrf2 redox imbalance state. Consistent with the in vitro results, the CS + PM2.5 group exhibited obviously increased NOX4/Nrf2 redox imbalance and apoptosis compared with the CS group (Fig. 7G). These results suggest that oxidative stress caused by the PM2.5-induced NOX4/Nrf2 redox imbalance exacerbates lung injury in CS-exposed mice.
Fig. 7.
PM2.5 exacerbated oxidative stress and NOX4/Nrf2 redox imbalance in CS-exposed mice. A COPD mouse model was induced after 4 weeks of exposure to CS. The COPD mice were intranasally instilled with or without PM2.5 (10 mg/kg) daily for 7 consecutive days beginning on the 21 st day. (A and B) Total ROS in BALF were measured respectively by DCFH-DA staining and flow cytometry. (C–F) The expression levels of antioxidants (SOD and GSH) and oxidants (MDA and MPO)were determined. (G) Western blot analysis showed the expression levels of NOX4, Nrf2, cleaved Caspase-3, Bax and BCL2. The values were showed as the mean ± SD. n = 3–5. (*p < 0.05, **p < 0.01).
3.8. PM2.5 exacerbated mitochondrial damage and mitophagy in CS-exposed mice
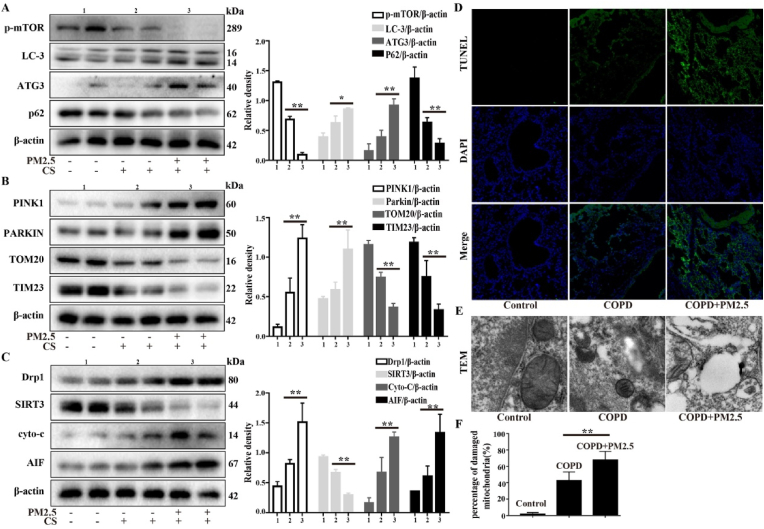
Studies have shown that mitochondrial homeostasis plays a critical role in COPD. Next, we explored whether PM2.5 could cause mitochondrial damage and mitophagy in CS-exposed mice. AECOPD model mice showed more significant activation of key mitochondrial damage and mitophagy-related proteins, including LC3-II, ATG3, PINK1, Parkin, Drp1, cyto-c and AIF, and inhibition of mTOR, p62, TOM20, TIM23 and SIRT3 than the CS group (Fig. 8A–C). In addition, TUNEL staining and TEM showed that the CS + PM2.5 group exhibited more severe mitochondrial damage and mitophagy than the COPD group, as revealed by a greater number of TUNEL-positive cells, disruption of the mitochondrial structure, and mitochondrial vacuolation and mitophagy (Fig. 8D–F). These results suggested that PM2.5 exacerbated mitochondrial damage and mitophagy in CS-exposed mice.
Fig. 8.
PM2.5 exacerbated mitochondrial damage and mitophagy in CS-exposed mice. A COPD mouse model was induced after 4 weeks of exposure to CS. The COPD mice were intranasally instilled with or without PM2.5 (10 mg/kg) daily for 7 consecutive days beginning on the 21 st day. (A–C) Western blot analysis showed the expression levels of p-mTOR, LC3-B, ATG3, SQSTM1/p62, PINK1, Parkin, TOM20, TIM23, Drp1, SIRT3, Cyto-c and AIF. (D) Lung tissue was stained with TUNEL. (E and F) Transmission electron microscopy images showed the mitophagy and mitochondrial damage in lung. The values were showed as the mean ± SD. n = 3–5. (*p < 0.05, **p < 0.01). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.9. The role of NOX4/Nrf2 redox balance in ameliorating the airway inflammation, tissue damage and ROS generation induced by PM2.5 in CS-exposed mice
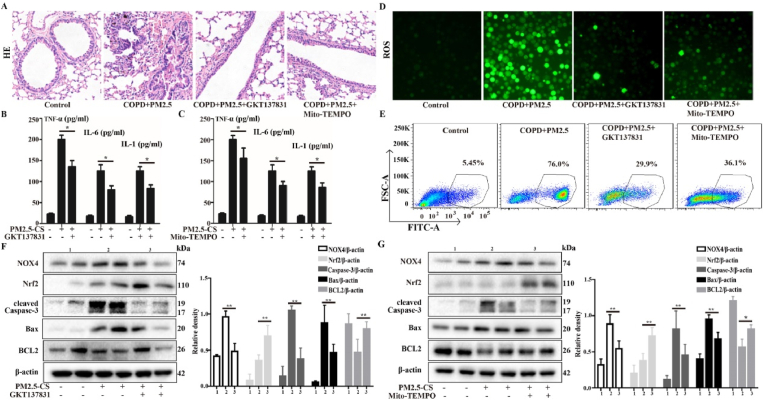
Our previous study indicated that CS + PM2.5 causes NOX4/Nrf2 redox imbalance and triggers excessive ROS production, which leads to mitochondrial damage and mitophagy, resulting in AECOPD. Therefore, ROS inhibitors were used to treat AECOPD model mice. Among these inhibitors, Mito-TEMPO is a mitochondria-targeted antioxidant, and GKT137831 is an inhibitor of NOX4; both of these agents can inhibit the production of ROS. The results showed that inhibition of ROS production improved pathology, airway inflammation and oxidative stress; the thickened alveolar walls with extensive neutrophil infiltration and the numerous inflammatory cell foci around blood vessels that were observed in the CS + PM2.5 group were significantly improved in the GKT137831 or Mito-TEMPO treatment groups (Fig. 9A). The levels of the inflammatory cytokines TNF-α, IL-1 and IL-6 and ROS in the BALF were obviously reduced in the GKT137831 and Mito-TEMPO treatment groups (Fig. 9B–E). Next, we analyzed the NOX4/Nrf2 redox imbalance state. Consistent with the in vitro results, GKT137831 or Mito-TEMPO treatment obviously improved the NOX4/Nrf2 redox imbalance compared with the CS + PM2.5 group (Fig. 9F and G). These results suggest that the inhibition of ROS production reestablishes the NOX4/Nrf2 redox balance and can ameliorate PM2.5-induced lung injury in CS-exposed mice.
Fig. 9.
The role of the NOX4/Nrf2 redox balance in ameliorating the airway inflammation, tissue damage and ROS generation induced by PM2.5 in CS-exposed mice. A COPD mouse model was induced after 4 weeks of exposure to CS. COPD mice were intranasally instilled with or without PM2.5 (10 mg/kg) daily for 7 consecutive days beginning on the 21 st day. In parallel, before cigarette smoke and PM2.5 administer, the mice were treat with or without GKT137831 or Mito-TEMPO (i. p.) to mice. (A) Lung tissue was stained with hematoxylin and eosin (H&E). (B and C) The levels of TNF-α, IL-6 and IL-1 in BALF were determined by ELISA assay. (D and E) Total ROS in BALF were measured respectively by DCFH-DA staining and flow cytometry. (F and G) Western blot analysis showed the expression levels of NOX4, Nrf2, cleaved Caspase-3, Bax and BCL2. The values were showed as the mean ± SD. n = 3–5. (*p < 0.05, **p < 0.01).
3.10. The role of NOX4/Nrf2 redox balance in improving mitochondrial damage and excessive mitophagy in CS-exposed mice
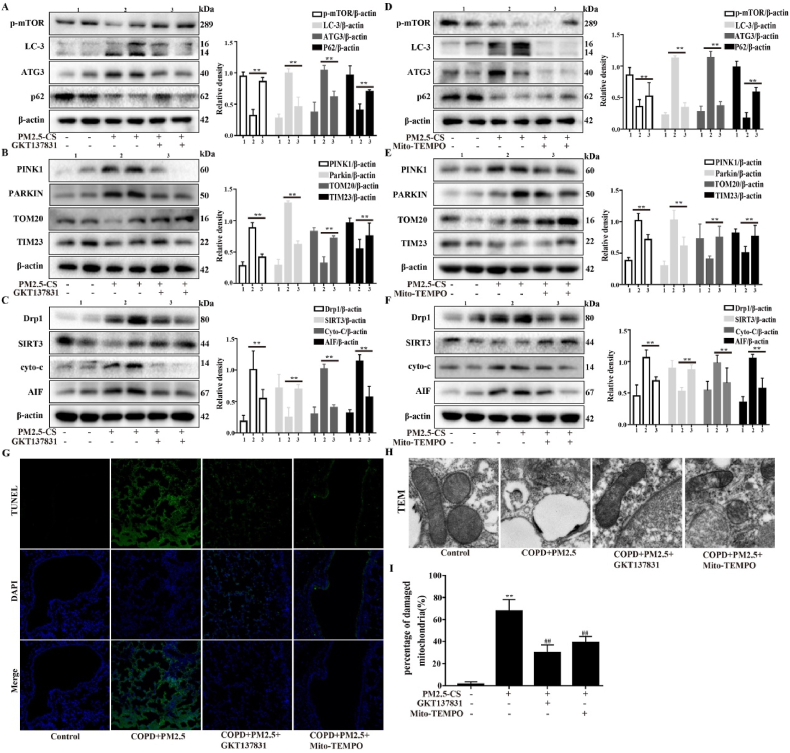
Subsequently, the therapeutic effects of GKT137831 and Mito-TEMPO on PM2.5-induced mitophagy and mitochondrial damage were investigated in CS-exposed mice. The mice in the treatment group showed more significant activation of the key mitophagy and mitochondrial-related proteins mTOR, p62, TOM20, TIM23 and SIRT3 and inhibition of LC3-II, ATG3, PINK1, Parkin, Drp1, cyto-c and AIF than the AECOPD model mice (Fig. 10A- F). Both of these agents can inhibit the production of ROS and autophagy. The results showed that inhibition of ROS production improved mitophagy and mitochondrial damage, as shown by a decreased number of TUNEL-positive cells (Fig. 10G), and the damage to the mitochondrial structure, mitochondrial vacuolation and mitophagy were decreased according to TEM (Fig. 10H and I). These results suggested that inhibiting ROS production to reestablish the NOX4/Nrf2 redox balance can ameliorate PM2.5-induced mitochondrial damage and mitophagy in CS-exposed mice.
Fig. 10.
The role of the NOX4/Nrf2 redox balance in improving mitochondrial damage and excessive mitophagy in CS-exposed mice. A COPD mouse model was induced after 4 weeks of exposure to CS. COPD mice were intranasally instilled with or without PM2.5 (10 mg/kg) daily for 7 consecutive days beginning on the 21 st day. In parallel, before cigarette smoke and PM2.5 administer, the mice were treat with or without GKT137831 or Mito-TEMPO (i.p.) to mice. (A–F) Western blot analysis showed the expression levels of p-mTOR, LC3-B, ATG3, SQSTM1/p62, PINK1, Parkin, TOM20, TIM23 Drp1, SIRT3, Cyto-c and AIF. (G) Lung tissue was stained with TUNEL. (H and I) Transmission electron microscopy images showed the mitophagy and mitochondrial damage in lung. The values were showed as the mean ± SD. n = 3–5. (**p < 0.01). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Acute exacerbation of COPD (AECOPD) reduces respiratory function and negatively affects disease progression and prognosis, leading to high healthcare costs [23,24]. Therefore, the management of AECOPD remains a clinical challenge. Recent studies have shown that PM2.5 is closely related to the morbidity and mortality of AECOPD [25]. PM2.5 induces AECOPD through several mechanisms, including mechanisms related to oxidative stress, airway inflammation, airway epithelial damage and airway immunity. These mechanisms all involve a central link, namely, mitochondrial dysfunction [26]. Studies have reported that oxidative stress plays an important role in inducing mitophagy and mitochondrial dysfunction, which characterized by redox balance disruption and subsequent tissue and cell damage. Moreover, ROS production is mainly dependent on NOX4/Nrf2 redox imbalance. In this study, we aimed to explore whether increased susceptibility to acute exacerbation of COPD-induced by PM2.5 was associated with redox imbalance and mitophagy. We first verified that the NOX4-Nrf2 redox imbalance triggers excessive mitophagy and mitochondrial dysfunction and then showed that regulating the NOX4-Nrf2 redox balance by inhibiting ROS production could reduce susceptibility to PM2.5-induced acute exacerbation of COPD.
During the development of AECOPD, ROS are overproduced, leading to severe redox imbalance and increasing sensitivity to exogenous stimuli, such as PM2.5. In our study, we found that PM2.5-CSE caused greater decreases in GSH/GSSG and SOD and increases in MDA than PM2.5 or CSE alone in Beas-2b cells. Consistent with the in vitro results, PM2.5 caused greater decreases in GSH and SOD and increases in MDA in CS-exposed mice than in COPD mice, suggesting the critical role of oxidative stress in amplifying the susceptibility of CS-exposed mice to PM2.5-induced AECOPD. The role of oxidative stress in the pathogenesis of COPD is well known. However, the mechanisms have not been clarified. In this study, we show that an aberrant upregulation of the ROS-generating enzyme Nox4 coupled with a deficiency in Nrf2 results in a sustained redox imbalance that promotes persistent ROS production, resulting in mitochondrial damage and excessive mitophagy. We have shown that targeting Nox4 at the time of injury ameliorates the development of PM2.5-induced lung injury in CS-exposed mice. These studies demonstrate that targeting Nox4 in PM2.5-induced AECOPD is sufficient to correct this redox imbalance and promote resolution.
Studies have proven that mitochondria are the main targets of PM2.5. Mitochondrial redox imbalance plays a vital role in COPD. In the present study, we found that exposure to PM2.5 (400 μg/ml) caused mitochondrial ROS overproduction and reduced the MMP. Moreover, increases in the DRP1, cyto-c and AIF levels, decreases in the SIRT3 levels, and mitochondrial fragmentation were also observed after PM2.5 exposure in this study. Interestingly, neither PM2.5 (200 μg/ml) nor CSE (2.5%) exposure alone induced significant mitochondrial damage, whereas the combined exposure (PM2.5-CSE) was sufficient to cause mitochondrial dysfunction. These results were coincided with the in vivo results of PM2.5 exposure in COPD model mice. These results suggest that low-dose PM2.5-CSE triggered excess ROS, contributing to mitochondrial dysfunction in vitro and in vivo.
Mitophagy plays a critical role in the maintenance of mitochondrial integrity by regulating the relationship between healthy mitochondria and damaged mitochondria to control mitochondrial quality. However, it has also been reported that abnormal or excessive mitophagy contributes to cell death via excessive increases in mitochondrial ROS [9]. It has been reported that increased ROS-mediated mitophagy induced by PM2.5 contributes to liver and vessel fibrosis [10,11]. Our previous research showed that PM2.5- induced excessive autophagy was responsible for lung injury [12]. The continuous accumulation of ROS induced by PM2.5 initiates excessive mitophagy in CS-exposed mice, and the underlying mechanism remains unclear. Our results indicated that PM2.5-CSE obviously activated mitophagy, as shown by measurement of the autophagic flux by tandem fluorescent mCherry-GFP-LC3B, electron microscopy analysis of mitochondria and IF staining of TOM20 and TIM23. In addition, PM2.5-CSE induced higher expression of LC3-II, ATG3, PINK1 and Parkin, and decreased expression of p-mTOR, p62, TOM20 and TIM23 compared with PM2.5 or CSE alone. Importantly, these results were further confirmed in mice. These data suggested that PM2.5 combined with cigarettes induces a large amount of ROS production and overactivates mitophagy to cause tissue damage, ultimately leading to the acute exacerbation of COPD.
In this study, we provide sufficient evidence that targeting redox imbalance and mitophagy overactivation contributes to increased susceptibility to PM2.5-induced acute exacerbation of COPD. Therapeutic strategies that regulate the redox balance are available in clinical practice, but it is unclear whether strategies to enhance antioxidant defenses would be equally effective. Downregulation of Nrf2 is relevant to increase in markers of oxidative stress, mitochondrial dysfunction and alterations in energy metabolism [27,28]. Next we will explore whether restoring Nrf2 expression can prevent these alterations. Combined with our findings of decreased Nrf2 expression during COPD, these findings suggest that strategies that target Nrf2 to further enhance antioxidant pathways may not be effective. Therefore, strategies that more directly target the sources of ROS production may be more specific and effective. In the present study, we first evaluated whether reestablishing the NOX4-Nrf2 redox balance to inhibit ROS production could reduce PM2.5-CSE-induced cytotoxicity. The results showed that the inhibition of ROS overproduction by NOX4 siRNA, GKT137831 and Mito-TEMPO could regulate the NOX4-Nrf2 redox balance and inhibit excessive mitophagy and mitochondrial dysfunction to protect against cell damage (Fig. 5 and Supplementary Fig. 3). Notably, it has been reported that GKT137831 and Mito-TEMPO can inhibit autophagy or mitophagy by scavenging ROS or mtROS. Subsequently, we evaluated whether treatment of mice with GKT137831 and Mito-TEMPO could alleviate PM2.5-induced AECOPD in CS-exposed mice by inhibiting ROS and mitophagy. The results indicated that regulating NOX4-Nrf2 redox balance using compounds such as GKT 137831 or Mito-TEMPO may reduce the effects of PM2.5 on AECOPD by inhibiting ROS and excessive mitophagy. Therefore, NOX4 inhibitors, such as GKT 137831, or mitochondria-targeted antioxidants, such as Mito-TEMPO, might have potential for use in the treatment of PM2.5-induced AECOPD.
5. Conclusion
In summary, we demonstrated that NOX4-Nrf2 redox imbalance triggers the ROS overproduction and mediates the excessive mitophagy that are induced by PM2.5, which might increase susceptibility to AECOPD. Targeting NOX4 inhibits ROS production, thereby inhibiting the overactivation of mitophagy and improving the acute exacerbation of COPD induced by PM2.5 combined with cigarette smoke in vitro and in vivo. Therapeutic strategies aimed at reestablishing the NOX4-Nrf2 redox balance may preserve the mitochondrial function of lung epithelial cells and protect against PM2.5-induced AECOPD.
Declaration of competing interest
The authors declare they have no conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81870030 and No. 82070043), Major National Science and Technology Projects (Grant No. 2017ZX10103004), Research on Prevention and Control of Major Chronic Non-communicable Diseases (2016YFC1304500), and the Natural Science Foundation of Jilin (Grant No. JLSCZD2019-019).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2022.102587.
Contributor Information
Xinxin Ci, Email: cixinxin@jlu.edu.cn.
Liping Peng, Email: penglp@jlu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gordon S.B., Bruce N.G., Grigg J., Hibberd P.L., Kurmi O.P., Lam K.B., Mortimer K., Asante K.P., Balakrishnan K., Balmes J., Bar-Zeev N., Bates M.N., Breysse P.N., Buist S., Chen Z., Havens D., Jack D., Jindal S., Kan H., Mehta S., Moschovis P., Naeher L., Patel A., Perez-Padilla R., Pope D., Rylance J., Semple S., Martin W.J., 2nd Respiratory risks from household air pollution in low and middle income countries. Lancet Respir. Med. 2014;2(10):823–860. doi: 10.1016/S2213-2600(14)70168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Zheng X.Y., Chung K.F., Zhong N.S. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016;388(10054):1939–1951. doi: 10.1016/S0140-6736(16)31597-5. [DOI] [PubMed] [Google Scholar]
- 3.Santibanez-Andrade M., Quezada-Maldonado E.M., Osornio-Vargas A., Sanchez-Perez Y., Garcia-Cuellar C.M. Air pollution and genomic instability: the role of particulate matter in lung carcinogenesis. Environ. Pollut. 2017;229:412–422. doi: 10.1016/j.envpol.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y.F., Li Z.Y., Dong L.L., Li W.J., Wu Y.P., Wang J., Chen H.P., Liu H.W., Li M., Jin C.L., Huang H.Q., Ying S.M., Li W., Shen H.H., Chen Z.H. Inactivation of MTOR promotes autophagy-mediated epithelial injury in particulate matter-induced airway inflammation. Autophagy. 2020;16(3):435–450. doi: 10.1080/15548627.2019.1628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun X.W., Lin Y.N., Ding Y.J., Li S.Q., Li H.P., Zhou J.P., Zhang L., Shen J.M., Li Q.Y. Surfaxin attenuates PM2.5-induced airway inflammation via restoring surfactant proteins in rats exposed to cigarette smoke. Environ. Res. 2022;203 doi: 10.1016/j.envres.2021.111864. [DOI] [PubMed] [Google Scholar]
- 6.Dan Dunn J., Alvarez L.A., Zhang X., Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annesley S.J., Fisher P.R. Mitochondria in health and disease. Cells. 2019;8(7) doi: 10.3390/cells8070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara H., Kuwano K., Araya J. Mitochondrial quality control in COPD and IPF. Cells. 2018;7(8) doi: 10.3390/cells7080086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu J., Wang L., Zhang L., Zheng F., Wang F., Leng J., Wang K., Heroux P., Shen H.M., Wu Y., Xia D. Mono-2-ethylhexyl phthalate drives progression of PINK1-parkin-mediated mitophagy via increasing mitochondrial ROS to exacerbate cytotoxicity. Redox Biol. 2021;38 doi: 10.1016/j.redox.2020.101776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu Y.N., Wang G.H., Zhou F., Hao J.J., Tian L., Guan L.F., Geng X.K., Ding Y.C., Wu H.W., Zhang K.Z. PM2.5 induces liver fibrosis via triggering ROS-mediated mitophagy. Ecotoxicol. Environ. Saf. 2019;167:178–187. doi: 10.1016/j.ecoenv.2018.08.050. [DOI] [PubMed] [Google Scholar]
- 11.Ning R., Li Y., Du Z., Li T., Sun Q., Lin L., Xu Q., Duan J., Sun Z. The mitochondria-targeted antioxidant MitoQ attenuated PM2.5-induced vascular fibrosis via regulating mitophagy. Redox Biol. 2021;46 doi: 10.1016/j.redox.2021.102113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Y., Fan X., Gu W., Ci X., Peng L. Hyperoside relieves particulate matter-induced lung injury by inhibiting AMPK/mTOR-mediated autophagy deregulation. Pharmacol. Res. 2021;167 doi: 10.1016/j.phrs.2021.105561. [DOI] [PubMed] [Google Scholar]
- 13.Xing Y.F., Xu Y.H., Shi M.H., Lian Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016;8(1):E69–E74. doi: 10.3978/j.issn.2072-1439.2016.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng H., Eckel S.P., Liu L., Lurmann F.W., Cockburn M.G., Gilliland F.D. Particulate matter air pollution and liver cancer survival. Int. J. Cancer. 2017;141(4):744–749. doi: 10.1002/ijc.30779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkham P.A., Barnes P.J. Oxidative stress in COPD. Chest. 2013;144(1):266–273. doi: 10.1378/chest.12-2664. [DOI] [PubMed] [Google Scholar]
- 16.Climent M., Viggiani G., Chen Y.W., Coulis G., Castaldi A. MicroRNA and ROS crosstalk in cardiac and pulmonary diseases. Int. J. Mol. Sci. 2020;21(12) doi: 10.3390/ijms21124370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quijano C., Trujillo M., Castro L., Trostchansky A. Interplay between oxidant species and energy metabolism. Redox Biol. 2016;8:28–42. doi: 10.1016/j.redox.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao X., Schottker B. Reduction-oxidation pathways involved in cancer development: a systematic review of literature reviews. Oncotarget. 2017;8(31):51888–51906. doi: 10.18632/oncotarget.17128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock M., Hafstad A.D., Nabeebaccus A.A., Catibog N., Logan A., Smyrnias I., Hansen S.S., Lanner J., Schroder K., Murphy M.P., Shah A.M., Zhang M. Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise. Elife. 2018;7 doi: 10.7554/eLife.41044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gogoi K., Manna P., Dey T., Kalita J., Unni B.G., Ozah D., Baruah P.K. Circulatory heavy metals (cadmium, lead, mercury, and chromium) inversely correlate with plasma GST activity and GSH level in COPD patients and impair NOX4/Nrf2/GCLC/GST signaling pathway in cultured monocytes. Toxicol. Vitro : Int. J. Publ. Assoc. BIBRA. 2019;54:269–279. doi: 10.1016/j.tiv.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Liu X., Hao B., Ma A., He J., Liu X., Chen J. The expression of NOX4 in smooth muscles of small airway correlates with the disease severity of COPD. BioMed Res. Int. 2016;2016 doi: 10.1155/2016/2891810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii Y., Itoh K., Morishima Y., Kimura T., Kiwamoto T., Iizuka T., Hegab A.E., Hosoya T., Nomura A., Sakamoto T., Yamamoto M., Sekizawa K. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J. Immunol. 2005;175(10):6968–6975. doi: 10.4049/jimmunol.175.10.6968. [DOI] [PubMed] [Google Scholar]
- 23.Vogelmeier C.F., Criner G.J., Martinez F.J., Anzueto A., Barnes P.J., Bourbeau J., Celli B.R., Chen R., Decramer M., Fabbri L.M., Frith P., Halpin D.M., Lopez Varela M.V., Nishimura M., Roche N., Rodriguez-Roisin R., Sin D.D., Singh D., Stockley R., Vestbo J., Wedzicha J.A., Agusti A. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 24.McGuire A., Irwin D.E., Fenn P., Gray A., Anderson P., Lovering A., MacGowan A. The excess cost of acute exacerbations of chronic bronchitis in patients aged 45 and older in England and Wales. Value Health : J. Int. Soc. Pharmacoecon. Outcomes Res. 2001;4(5):370–375. doi: 10.1046/j.1524-4733.2001.45049.x. [DOI] [PubMed] [Google Scholar]
- 25.Ni L., Chuang C.C., Zuo L. Fine particulate matter in acute exacerbation of COPD. Front. Physiol. 2015;6:294. doi: 10.3389/fphys.2015.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo L., Otenbaker N.P., Rose B.A., Salisbury K.S. Molecular mechanisms of reactive oxygen species-related pulmonary inflammation and asthma. Mol. Immunol. 2013;56(1–2):57–63. doi: 10.1016/j.molimm.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Barrera C., Valenzuela R., Rincon M.A., Espinosa A., Echeverria F., Romero N., Gonzalez-Manan D., Videla L.A. Molecular mechanisms related to the hepatoprotective effects of antioxidant-rich extra virgin olive oil supplementation in rats subjected to short-term iron administration. Free Radic. Biol. Med. 2018;126:313–321. doi: 10.1016/j.freeradbiomed.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Valenzuela R., Rincon-Cervera M.A., Echeverria F., Barrera C., Espinosa A., Hernandez-Rodas M.C., Ortiz M., Valenzuela A., Videla L.A. Iron-induced pro-oxidant and pro-lipogenic responses in relation to impaired synthesis and accretion of long-chain polyunsaturated fatty acids in rat hepatic and extrahepatic tissues. Nutrition. 2018;45:49–58. doi: 10.1016/j.nut.2017.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.