Abstract
Scedosporium apiospermum is a ubiquitous organism present in the environment and is rarely identified in rhinosinusitis. We report a case of invasive rhinosinusitis with Scedosporium apiospermum which made a definite diagnosis by metagenomic next-generation sequencing (mNGS) from a biopsy sample. The resection of the Scedosporium apiospermum pathological mass was performed with low-temperature plasma radiofrequency ablation. Six months of continuous oral voriconazole treatment was followed. The patient was asymptomatic with no signs of recurrence during the next 1-year follow-up.
Keywords: Scedosporium apiospermum, Invasive fungal rhinosinusitis, Metagenomic next-generation sequencing
Scedosporium apiospermum; Invasive fungal rhinosinusitis; Metagenomic next-generation sequencing.
1. Introduction
Invasive fungal rhinosinusitis (IFR) is a disease of the paranasal sinuses and nasal cavity that typically affects immunocompromised patients, such as diabetes mellitus. Aspergillus spp., Dematiaceae spp. and mucomycoses are the most commonly isolated pathogens. Infection caused by the Scedosporium apiospermum are relatively rare. It is a major challenge to make a quick and correct diagnosis in occasional cases due to the atypical physical findings, ambiguous symptoms and imaging manifestations. Currently, the gold standard for diagnosing IFR is evidence of pathogen identification in anatomic pathology specimens. Microbial culture or Gomori methenamine silver (GMS)-stained anatomic pathology specimens are helpful to confirm the presence of fungal organisms. However, because of the high negative rate, time-consuming and low detection rate to pathogenic bacteria of the existing technology, the misdiagnosis rate is often increased and the best time for treatment is missed. Here, we present a difficult-diagnosis case of IFR caused by Scedosporium apiospermum identified by metagenomic next-generation sequencing (mNGS) in 30 h from a biopsy tissue sample.
2. Case presentation
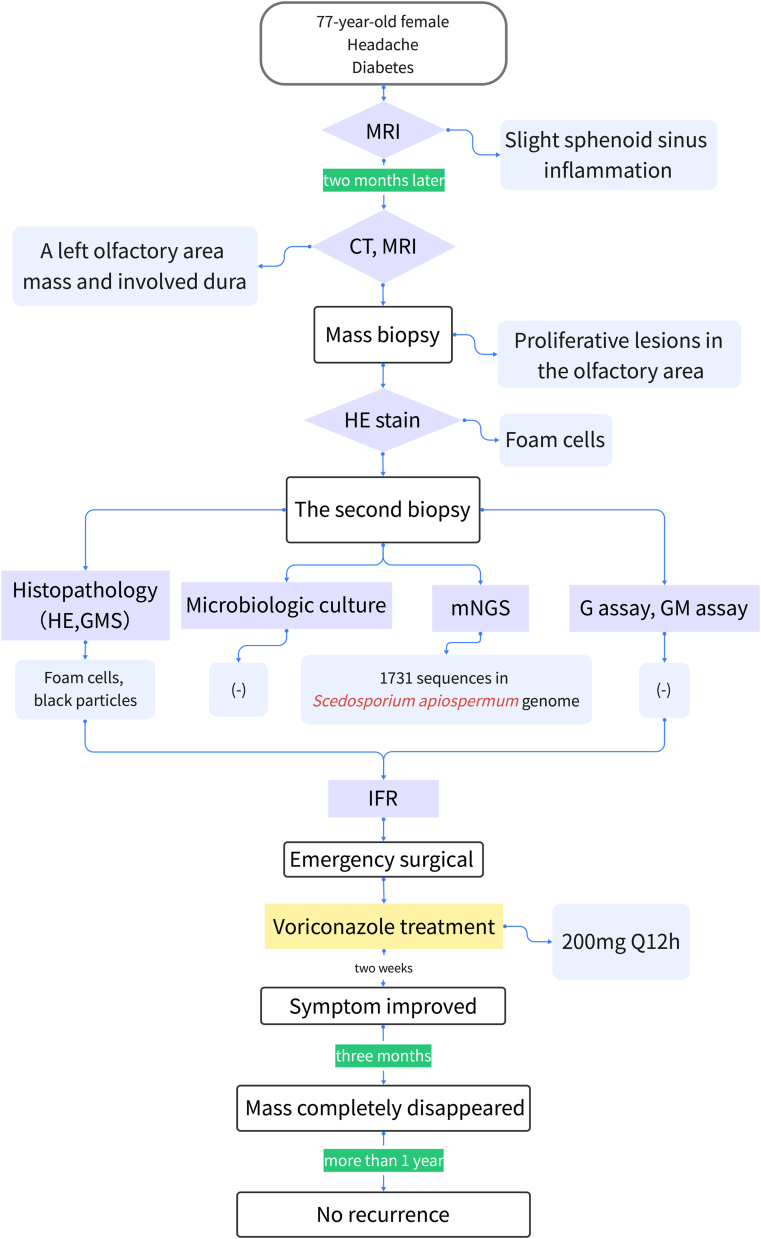
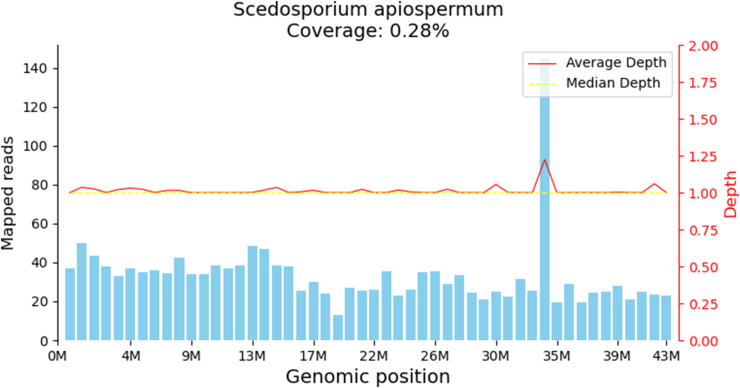
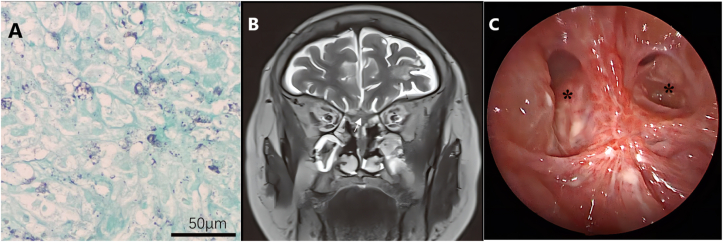
We report a 77-year-old female who suffered from persistent distending pain on the left side of the head and face with nasal obstruction and hyposmia. She had a history of diabetes, but her blood sugar was well-controlled. We attached the flowchart for diagnosis and treatment of this case to elucidate the process (Figure 1). The patient first received an examination in the neurology department, and a magnetic resonance imaging (MRI) scan showed slight sphenoid sinus inflammation. The patient responded poorly to analgesic treatment, computed tomography (CT) and MRI scans after two months later visualized a left olfactory area mass with dura invasion (Figure 2A, B). No abnormality was found on neurological examination. Transnasal endoscopic mass biopsy was performed by the otolaryngologist (Figure 2C). The biopsy specimen pathologic diagnosis was granulomatous lesions with a large number of foam cells. Subsequent immune-related tests were negative, except for erythrocyte sedimentation rate and C-reactive protein. Considering the high possibility of special infection, we subsequently performed microbiologic culture and GMS stain on pathology specimen obtained from the second biopsy. Meanwhile, we transported fresh biopsy tissue samples to Beijing Genomics Institute for mNGS. The detailed process of mNGS was added in Supplementary material. After 30 h, the results showed that an infrequent fungus, Scedosporium apiospermum, was highly replicated, with 1731 sequences (Figure 3). GMS-stained biopsy specimens showed black particles (Figure 4A). However, the tissue fungal culture got negative result. We also send patient blood sample for G assay and GM assay for aetiologic diagnosis but got negative result. According to the comprehensive judgment of medical history, clinical symptoms, laboratory examination, pathological examination and mNGS results, we speculated that the patient suffered IFR caused by Scedosporium apiospermum. Then we carried out emergency surgical exploration under general anaesthesia to clean out the resection of the Scedosporium apiospermum pathological mass through low-temperature plasma radiofrequency ablation. Both tissue GMS staining and microbiological culture after surgery yielded the same results as before. Voriconazole was administered after the operation, with a dose of intravenous (IV) 200 mg Q12 hours. The patient's headache gradually disappeared one week later. After 2 weeks, MRI showed that a small amount of the Scedosporium apiospermum mass still remained (Figure 4B). After 14 days of intravenous administration, the maintenance dose is oral 200 mg Q12 hours until 6 months after surgery. With more than 3 months of continuous oral voriconazole treatment, the mass completely disappeared (Figure 4C). During the next follow-up at 1 year without treatment, the patient was asymptomatic with no signs of recurrence on either clinical examination or MRI.
Figure 1.
The flowchart for diagnosis and treatment of this case.
Figure 2.
Preoperative CT scan showed that the skull base was involved by the olfactory area mass, and the bone of the skull base was damaged (A). MRI showed that the meninges were involved (B). A Scedosporium apiospermum mass was identified in the olfactory area during the surgical operation (C, black arrow indicates lesion location).
Figure 3.
The Scedosporium apiospermum genome sequences mapping by mNGS.
Figure 4.
GMS-stained results showed some black particles but could not find mycelia (A). MRI showed that a small mass remained 2 weeks after the surgery (B). The lesion completely disappeared after surgery and voriconazole treatment (C). ∗ indicates sphenoid sinus.
3. Discussion
IFR is a rare and fatal infection. Aspergillus, Candida, and Mucor species are the most common causative agents of IFR. Occasionally, other lesser known fungal species, such as Paecilomyces variotii, have been reported [1]. IFR caused by Scedosporium apiospermum, the aetiologic agent in our case, has been reported infrequently before. A retrospective review found isolated Scedosporium sp. in 5% of cases of acute IFR and in only one case of chronic IFR [2].
Based on the immune status of the patient and histopathological features, IFR has been divided into 3 categories: acute, chronic or granulomatous [3]. Acute IFR is often reported in immunosuppressed patients with rapidly progressive infections, and the mortality is as high as 50–80% [4]. Although granulomatous IFR is less progressive, an incorrectly diagnosed or missed case may also lead to central nervous system involvement and even to a life-threatening condition [5]. The diagnosis of IFR is challenging, especially for rare fungal-induced cases. In this case, the initially suspected diagnosis was olfactory neuroblastoma because a large mass could be visualized in the left olfactory area on MRI and CT scans. Surprisingly, pathological biopsy indicated that the specimens were rich in foam cells rather than tumour cells. We realized that this was a special infection, and made the second biopsy to detect pathogens. But both microbial culture and GMS stained anatomic pathology specimens failed to find the pathogen. G assay and GM assay was also negative. Finally, mNGS was employed, and the presence of rare Scedosporium apiospermum species was identified in 30 h.
Early diagnosis is a key factor affecting the prognosis of IFR patients, but the diagnosis of IFR is a major challenge because the identification of fungal pathogens is difficult. Singh et al. found that out of 76 IFR patient only 37 (48.68%) cases were found to be positive based on direct microscopy, culture, histopathology or polymerase chain reaction (PCR) [6]. Among these patients only 21 (27.6%) cases were found to be positive by culture and 14 (18.42%) by histopathology. Furthermore, several researches reported the positive rate of microbiology culture was less than 30% [7, 8, 9]. Mroeover, it is reported that the fungal pathogens were still difficult to detect after GMS-stained in false-negative frozen sections on H&E stain of IFR patients [10]. These evidences demonstrate that sometimes traditional identification methods, including time-consuming microbial culture and histopathology, hard to identify the pathogen quickly and precisely. Thus, a rapid and sensitive method for detection of pathogens in clinical samples is urgently needed to diagnose IFR early.
Recently, mNGS has emerged as a sensitive technology capable of detecting pathological organisms from human biopsy samples and body fluids [11] and provides an unbiased assay. Since mNGS ensures the detailed sequencing of the total DNA or RNA content of the microbiome [12]. In addition, the results can be obtained in a short period of time. Therefore, mNGS is a promising detection tool for the early diagnosis of IFR, especially for acute IFR and rare fungal species IFR.
For the treatment of IFR, surgical intervention removed infection tissues as soon as possible and combine with prolonged antifungal therapy should be processed. In the case, we performed emergency surgery to clean out the resection of the Scedosporium apiospermum mass and long-time antifungal agent voriconazole treatment. At the present, the duration and efficacy of antifungal therapy is not widely known due to a lack of large scales studies. A study reported that 57% of patients achieved response at a median of 103 days during the treatment of scedosporiosis with voriconazole [13]. For this patient, after we continue more than 3 months voriconazole treatment, MRI showed the remaining Scedosporium apiospermum mass completely disappeared. However, whether the treatment duration is proper should be further studied in the future IFR cases.
In conclusion, the application of mNGS in clinical diagnosis is considered to be very promising, not only because of its independence from culture techniques but also because of its short turnaround time and high sensitivity. However, mNGS is rarely used in clinical applications at present, and more cases are needed to evaluate its accuracy and practicability in the future.
Declarations
Author contribution statement
All authors listed have significantly contributed to the investigation, development and writing of this article.
Funding
This work was supported by the Key Clinical Discipline of Tianjin, National Natural Science Foundation of China (81971698), and Tianjin Natural Science Foundation (19JCYBJC27200).
Data availability statement
No data was used for the research described in the article.
Declaration of interest's statement
The authors declare no competing interests.
Additional information
Supplementary content related to this article has been published online at https://doi.org/10.1016/j.heliyon.2022.e12476.
Acknowledgements
Conception and design of this case report: wrote the manuscript: LYB, XX,CTZ; designed the manuscript: WW; revised the manuscript: GMZ. The authors thank the subjects who participated in this study.
Contributor Information
Wei Wang, Email: Wwei1106@hotmail.com.
Guimin Zhang, Email: zh_gm@163.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Swami T., Pannu S., Kumar M., Gupta G. Chronic invasive fungal rhinosinusitis by Paecilomyces variotii: a rare case report. Indian J. Med. Microbiol. 2016;34(1):103–106. doi: 10.4103/0255-0857.174126. [DOI] [PubMed] [Google Scholar]
- 2.Montone K.T., Livolsi V.A., Feldman M.D., Palmer J., Chiu A.G., Lanza D.C., et al. Fungal rhinosinusitis: a retrospective microbiologic and pathologic review of 400 patients at a single university medical center. Int. J. Otolaryngol. 2012;2012 doi: 10.1155/2012/684835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.deShazo R.D., O'Brien M., Chapin K., Soto-Aguilar M., Gardner L., Swain R. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch. Otolaryngol. Head Neck Surg. 1997;123(11):1181–1188. doi: 10.1001/archotol.1997.01900110031005. [DOI] [PubMed] [Google Scholar]
- 4.Fung M., Babik J., Humphreys I.M., Davis G.E. Diagnosis and treatment of acute invasive fungal sinusitis in cancer and transplant patients. Curr. Infect. Dis. Rep. 2019;21(12):53. doi: 10.1007/s11908-019-0707-4. [DOI] [PubMed] [Google Scholar]
- 5.Alarifi I., Alsaleh S., Alqaryan S., Assiri H., Alsukayt M., Alswayyed M., et al. Chronic granulomatous invasive fungal sinusitis: a case series and literature review. Ear Nose Throat J. 2021;100(5_suppl):720S–727S. doi: 10.1177/0145561320904620. [DOI] [PubMed] [Google Scholar]
- 6.Singh A.K., Gupta P., Verma N., Khare V., Ahamad A., Verma V., Agarwal S.P. Fungal rhinosinusitis: microbiological and histopathological perspective. J. Clin. Diagn. Res. 2017 Jul;11(7):DC10–DC12. doi: 10.7860/JCDR/2017/25842.10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaur R., Lavanya S., Khurana N., Gulati A., Dhakad M.S. Allergic fungal rhinosinusitis: a study in a tertiary care hospital in India. J. Allergy (Cairo) 2016;2016 doi: 10.1155/2016/7698173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prateek S., Banerjee G., Gupta P., Singh M., Goel M.M., Verma V. Fungal rhinosinusitis: a prospective study in a University hospital of Uttar Pradesh. Indian J. Med. Microbiol. 2013 Jul-Sep;31(3):266–269. doi: 10.4103/0255-0857.115634. [DOI] [PubMed] [Google Scholar]
- 9.Wang T., Zhang L., Hu C., Li Y., Wang C., Wang X., et al. Clinical features of chronic invasive fungal rhinosinusitis in 16 cases. Ear Nose Throat J. 2020;99(3):167–172. doi: 10.1177/0145561318823391. [DOI] [PubMed] [Google Scholar]
- 10.Crist H., Hennessy M., Hodos J., McGinn J., White B., Payne S., et al. Acute invasive fungal rhinosinusitis: frozen section histomorphology and diagnosis with PAS stain. Head Neck Pathol. 2019;13(3):318–326. doi: 10.1007/s12105-018-0965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo L.Y., Li Y.J., Liu L.L., Wu H.L., Zhou J.L., Zhang Y., et al. Detection of pediatric bacterial meningitis pathogens from cerebrospinal fluid by next-generation sequencing technology. J. Infect. 2019;78(4):323–337. doi: 10.1016/j.jinf.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg B., Sichtig H., Geyer C., Ledeboer N., Weinstock G.M. Making the leap from research laboratory to clinic: challenges and opportunities for next-generation sequencing in infectious disease diagnostics. mBio. 2015;6(6) doi: 10.1128/mBio.01888-15. e01888-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troke P., Aguirrebengoa K., Arteaga C., Ellis D., Heath C.H., Lutsar I. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob. Agents Chemother. 2008;52(5):1743–1750. doi: 10.1128/AAC.01388-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.