Abstract
The efficacy of sotorasib for patients with KRAS G12C-mutated lung cancer with poor performance status (PS) and active brain metastases remains unknown. Here, we present a case in which sotorasib was introduced as the third-line therapy for a patient whose PS worsened due to active multiple brain metastases and disseminated intravascular coagulation (DIC) caused by rapid tumor progression; a marked effect was observed. DIC and PS improved two weeks after the start of the administration, and multiple brain metastases disappeared. The effect lasted only approximately four months due to the development of a new liver metastasis, but sotorasib improved PS and the DIC status was reversed, allowing for further treatment. Sotorasib could be considered for introduction in patients with poor PS.
Keywords: KRAS G12C mutation, Sotorasib, Rapid response, Disseminated intravascular coagulation, Case report
Introduction
Sotorasib is the first small molecular inhibitor targeting oncogenic KRAS G12C protein to successfully enter clinical practice.1 A phase 2 trial evaluating the efficacy and safety of sotorasib as monotherapy in patients with NSCLC with KRAS G12C revealed an objective response rate of 37.1%, with a median progression-free survival (PFS) of 6.8 months.2 Although its efficacy is inferior to that of other molecularly targeted drugs for NSCLC, it is the first molecularly targeted drug for patients with KRAS G12C-mutated NSCLC,2 and there are great expectations for its clinical application. Although the effect of sotorasib on patients with poor performance status (PS) and active brain metastases remains unknown, we present here a case in which we observed a significant effect of sotorasib.
Case Presentation
A 62-year-old woman, a current 7.5 pack-year smoker, was introduced to our hospital for induction of the trial sotorasib treatment. She was diagnosed with having lung adenocarcinoma with clinical T2N2M1c stage IVB with programmed death-ligand 1 tumor proportion score of 10% and no targetable major oncogenic mutations at another hospital approximately one year ago. She was treated with a combination of CBDCA (area under the curve = 5), pemetrexed (500 mg/m2), and atezolizumab (1200 mg) as the first-line therapy. The PFS after the first-line treatment was approximately seven months. Docetaxel (75 mg/m2) was administered as the second-line therapy. The PFS after the second-line treatment was approximately two months. After these standard treatments, a comprehensive genomic profiling test using the FoundationOne panel was performed (Table 1).
Table 1.
Result of Gene Panel
 |
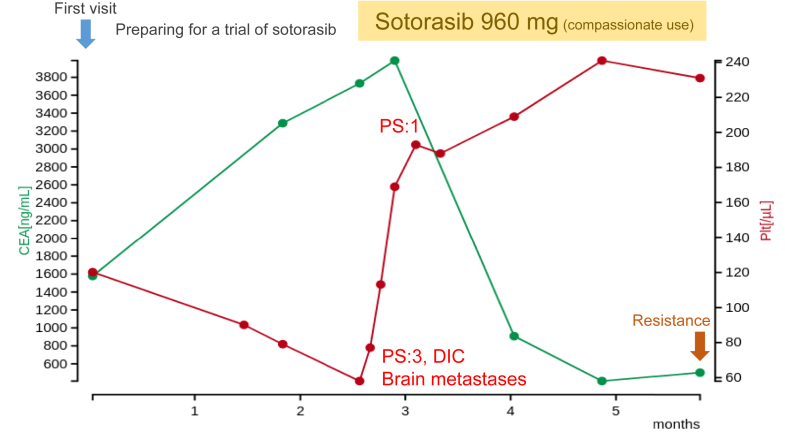
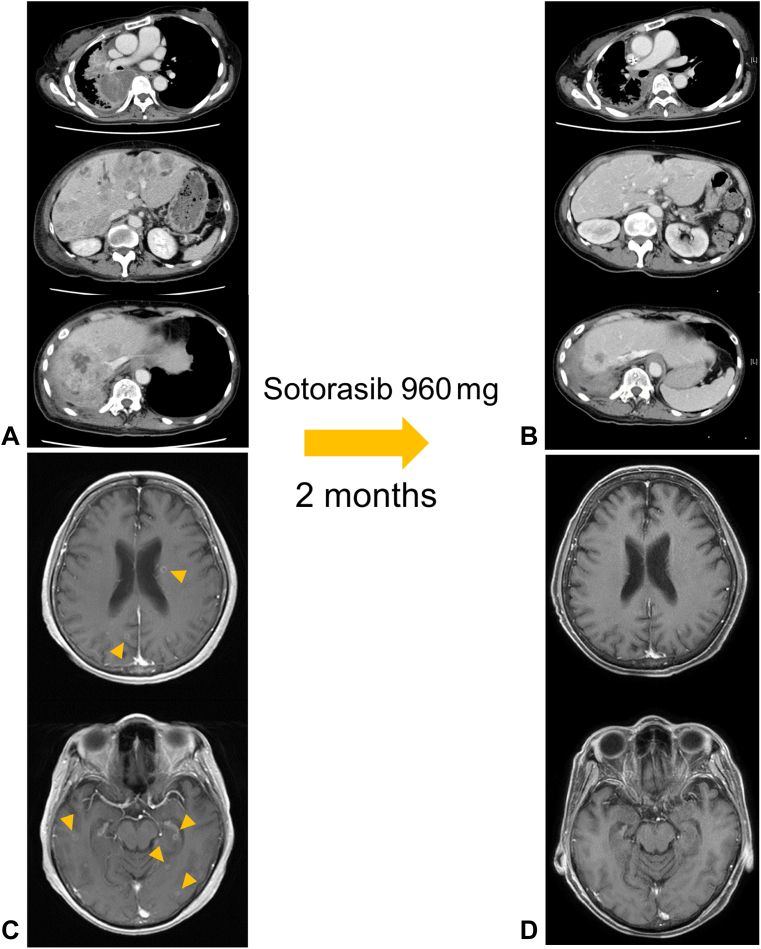
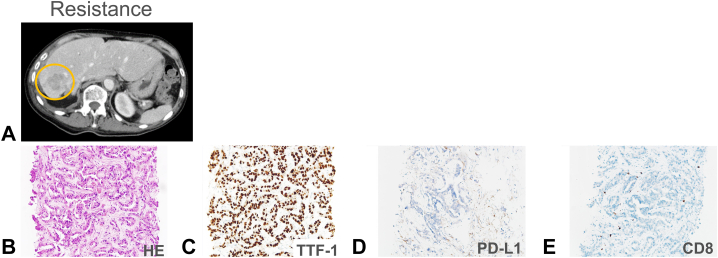
After referral to our hospital, her tumor progressed rapidly with the emergence of multiple brain metastases during her preparation for a sotorasib trial. Her platelet count rapidly decreased and her PS also reduced to 3 (Fig. 1). She was diagnosed with having disseminated intravascular coagulation (DIC) based on the International Society on Thrombosis and Haemostasis DIC score, as per the criteria, because her parameters were conclusive for DIC: platelet count: 58,000/μL; fibrinogen level: 87 mg/dL; prothrombin index: 45%; and D-dimer: 12.7 μg/mL. Enrollment in the trial became impossible because the eligibility criteria were not met, but the compassionate use program for sotorasib started in Japan at the same time. She successfully reached sotorasib on the program. Two weeks after beginning sotorasib (960 mg/body), her platelet counts rose to the reference range and her PS improved from 3 to 1 (Fig. 1). Two months after beginning sotorasib, the primary lung and multiple liver metastases had marked shrinkage (Fig. 2A and B) and the multiple brain metastases also disappeared (Fig. 2C and D). No toxicity due to sotorasib was observed during the treatment. Unfortunately, four months after sotorasib initiation, her carcinoembryonic antigen level had a tendency to rise again, and a new mass formation was observed in the liver (Fig. 3A and B). A biopsy of the same lesion revealed TTF-1–positive adenocarcinoma, which was diagnosed as metastatic recurrence of lung adenocarcinoma (Fig. 3C–E). When the next-generation sequence was performed on lung cancer-related genes, including KRAS and TP53, no mutation other than G12C appeared in KRAS, and the same TP53 G154V mutation was detected as before treatment (Table 1).
Figure 1.
Clinical course of the patient. CEA, carcinoembryonic antigen; DIC, disseminated intravascular coagulation; PS, performance status.
Figure 2.
Enhanced chest and abdominal CT images (A) before and (B) after the administration of sotorasib. Enhanced head MRI (C) before and (D) after the administration of sotorasib. Yellow arrowheads point to multiple brain metastases. CT, computed tomography; MRI, magnetic resonance imaging.
Figure 3.
(A) An abdominal contrast-enhanced CT image reveals a resistant lesion appearing in the liver with a yellow circle. Histopathologic analysis of biopsy specimen of the resistant lesion on the liver: (B) HE staining of the specimen, (C) strong positive TTF-1 nuclear staining, (D) with PD-L1 tumor proportion score of 0%, and (E) with scarce CD8-positive lymphocytes. CT, computed tomography; HE, hematoxylin and eosin; PD-L1, programmed death-ligand 1.
Discussion
Rapid and drastic response has been previously reported in NSCLC treatment with molecularly targeted agents.3 No previous reports of such response to sotorasib for KRAS G12C-mutated lung cancer exist; this is the first report. Although PFS after sotorasib treatment is shorter than that after other molecularly targeted agent treatments in NSCLC, PS may be improved with a rapid response, as in this case. Therefore, searching for KRAS G12C mutation is clinically valuable and the indication of sotorasib in patients with poor PS may be considered for the subsequent treatment.
The top mutations co-existing with KRAS G12C mutation in NSCLC are TP53 (47.07%), LRP1B (33.30%), STK11 (26.41%), KEAP1 (19.06%), and CDKN2A (15.96%).4 The efficacy of sotorasib can be affected by mutations in TP53, STK11, and KEAP1. The STK11 mutated and KEAP1 wild type category has the highest response rate, followed by the STK11 and KEAP1 wild type category.2 On the basis of the FoundationOne panel result of the present case, the STK11 and KEAP1 mutations were not detected (Table 1). A dramatic tumor shrinkage was observed, but a resistant lesion formed in the liver within a short period of time. Genetic analysis of the lesion detected no obvious resistant mutations.5 A mechanism of resistance to KRAS G12C inhibitors has also been reported to attenuate their effects by rapidly synthesizing KRAS G12C proteins after exposure to KRAS G12C inhibitors, rather than by acquiring new resistance mutations.6 It is possible that such mechanism other than acquired mutations led to the formation of resistant lesions within a short period.
The most significant adverse events with sotorasib are increases of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, with grade 3 increase reported in 6.3% and 5.6% of patients, respectively.2 The patient had massive multiple liver metastases, but no elevations in AST and ALT levels. Therefore, there was little concern about hepatic toxicity; there were no increases in AST or ALT level after introducing sotorasib. Platelet count decrease was reported at 3.2%, but no grade 3 or higher event was reported, and no case of sotorasib-associated DIC was reported. In the present case, sotorasib was introduced with DIC, but no adverse events such as bleeding or coagulopathy were observed.
Sotorasib was found to have durable anticancer activity for stable brain metastases previously treated with radiation or surgery.7 The phase 1 and 2 CodeBreaK 100 trial excluded patients with active untreated brain metastases. In the present case, sotorasib had a promising effect on such active brain metastases and can be expected to have a specific effect in controlling brain metastatic lesions. Another KRAS G12C inhibitor, adagrasib, was found to have good response to brain metastases (intracranial overall response rate: 32%; median intracranial duration of response: not reached)8 and is an equally promising agent.
Conclusions
Sotorasib resulted in remarkable tumor shrinkage and had adequate efficacy in a patient with KRAS G12C mutation with poor PS associated with rapid tumor growth and tumor-induced DIC. Although PFS after sotorasib treatment was only four months, the PS improvement was significant for the subsequent treatment. The indication for sotorasib can be considered for patients with KRAS G12C mutations with poor PS.
CRediT Authorship Contribution Statement
Kei Kunimasa: Conceptualization, Methodology, Investigation, Writing—original draft.
Motohiro Tamiya, Takako Inoue, and Takahisa Kawamura: Investigation.
Kazumi Nishino: Supervision.
Acknowledgments
Informed consent was obtained from the patient.
Footnotes
Disclosure: Dr. Kunimasa reports receiving honoraria for a lecture from AstraZeneca, Chugai Pharma, and Novartis. Dr. Tamiya reports receiving grants from Ono Pharmaceutical, Bristol-Myers Squibb, and Boehringer Ingelheim and honoraria for a lecture from Taiho Pharmaceutical, Eli Lilly, Asahi Kasei Pharmaceutical, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca, Chugai Pharmaceutical, Ono Pharmaceutical, and Bristol-Myers Squibb. Dr. Inoue reports receiving honoraria for a lecture from AstraZeneca. Dr. Nishino reports receiving a grant from Boehringer Ingelheim and honoraria for a lecture from Chugai Pharma, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Roche Diagnostics, Novartis, Pfizer, and Merck. Dr. Kawamura declares no conflict of interest.
Cite this article as: Kunimasa K, Tamiya M, Inoue T, Kawamura T, Nishino K. Rapid response to sotorasib of a patient with KRAS G12C-mutated lung cancer with cancer-associated disseminated intravascular coagulation: a case report. JTO Clin Res Rep. 2023;4:100442.
References
- 1.Canon J., Rex K., Saiki A.Y., et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 2.Skoulidis F., Li B.T., Dy G.K., et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ninomaru T., Okada H., Fujishima M., Irie K., Fukushima S., Hata A. Lazarus response to tepotinib for leptomeningeal metastases in a patient with MET exon 14 skipping mutation-positive lung adenocarcinoma: case report. JTO Clin Res Rep. 2021;2 doi: 10.1016/j.jtocrr.2021.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salem M.E., El-Refai S.M., Sha W., et al. Landscape of KRAS(G12C), associated genomic alterations, and interrelation with immuno-oncology biomarkers in KRAS-mutated cancers. JCO Precis Oncol. 2022;6 doi: 10.1200/PO.21.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awad M.M., Liu S., Rybkin I.I., et al. Acquired resistance to KRAS(G12C) inhibition in cancer. N Engl J Med. 2021;384:2382–2393. doi: 10.1056/NEJMoa2105281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue J.Y., Zhao Y., Aronowitz J., et al. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature. 2020;577:421–425. doi: 10.1038/s41586-019-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramalingam S., Skoulidis F., Govindan R., et al. P52.03 efficacy of sotorasib in KRAS p. G12C-mutated NSCLC with stable brain metastases: a post-hoc analysis of CodeBreaK 100. J Thorac Oncol. 2021;16(suppl):S1123. [Google Scholar]
- 8.Sabari J.K., Spira A.I., Heist R.S., et al. Activity of adagrasib (MRTX849) in patients with KRASG12C-mutated NSCLC and active, untreated CNS metastases in the KRYSTAL-1 trial. J Clin Oncol. 2022;40(suppl 17) LBA9009–LBA9009. [Google Scholar]