Abstract
Spinal cord injuries (SCIs) are devastating. In SCIs, a powerful traumatic force impacting the spinal cord results in the permanent loss of nerve function below the injury level, leaving the patient paralyzed and wheelchair-bound for the remainder of his/her life. Unfortunately, clinical treatment that depends on surgical decompression appears to be unable to handle damaged nerves, and high-dose methylprednisolone-based therapy is also associated with problems, such as infection, gastrointestinal bleeding, femoral head necrosis, obesity, and hyperglycemia. Nanomaterials have opened new avenues for SCI treatment. Among them, performance-based nanomaterials derived from a variety of materials facilitate improvements in the microenvironment of traumatic injury and, in some cases, promote neuron regeneration. Nanoparticulate drug delivery systems enable the optimization of drug effects and drug bioavailability, thus contributing to the development of novel treatments. The improved efficiency and accuracy of gene delivery will also benefit the exploration of SCI mechanisms and the understanding of key genes and signaling pathways. Herein, we reviewed different types of nanomaterials applied to the treatment of SCI and summarized their functions and advantages to provide new perspectives for future clinical therapies.
Keywords: Spinal cord injury, Inorganic nanomaterial, Organic nanomaterial, Bioderived nanomaterial, Neuroprotection, Neuroregeneration
Graphical abstract
1. Introduction
The spinal cord is an important component of the central nervous system, acting as a link between the brain and peripheral nervous tissues by transmitting vital information and reflexes. Spinal cord injury (SCI) is temporary or permanent damage to spinal cord function under the action of an external force. An estimated 250,000 to 500,000 new cases of SCI are reported worldwide each year with young people accounting for the majority of the cases [1]. Approximately 40% of all SCI patients have traffic accident injuries. Other causes include falling, work injuries, sports, and violence.
An SCI may have serious consequences. Depending on the level, site, and force of the injury, SCI patients may present with different neurological disorders. The most severe SCI can result in the complete loss of sensory and motor function below the injured level, called complete paraplegia. However, some patients experience other consequences of SCI. Studies have shown a high prevalence of SCI respiratory complications, which were reported in 84% of patients with SCI above the fourth cervical (C4) vertebra level [2], and 34% of the patients require tracheal intubation for ventilation assistance [3]. The mortality rate of patients admitted with emergent SCI was estimated to range between 4% and 17% [4]. Some SCIs may also be accompanied by bowel or bladder incontinence and long-term complications, such as muscle atrophy, cramps, bedsores, and infections, which impact both patients and their families, causing physical and psychological distress and financial burdens [5].
The clinical treatment of SCI is typically high-dose methylprednisolone (MP) combined with appropriate surgical decompression. However, these approaches have some problems. Although surgical treatments reestablish spinal stability and relieve compression for nerve recovery, the complete repair of the central nerve remains elusive. The application of high-dose MP also has problems, such as low bioavailability, low aggregation efficiency, indirect damage to other organs, a short treatment time window, and other shortcomings [[6], [7], [8], [9]]. Therefore, it is necessary to seek more effective treatment methods to address these problems.
The applications of functional nanomaterials in biomedical research are well documented, especially in the field of cancer [[10], [11], [12]]. In recent years, therapeutic nanoparticles (NPs) and their delivery have also attracted widespread attention as an emerging engineering technology for SCI treatment [13], and nanotheranostics have shown advantages in multiple aspects. The nanoscale size of functional nanomaterials makes it possible for drugs to cross the blood‒spinal cord barrier (BSCB) and accumulate in the lesion area [14,15]. Efficient transport will greatly improve the bioavailability of drugs, which facilitates the optimization of MP drugs in clinical use [16] and the possibility of developing a series of new preparations, including nonhormonal drugs [17,18], proteases [19], and genes [17,20]. Furthermore, the properties of the material itself in combination with the drug may offer various new advantages, conferring NPs with specific therapeutic capabilities, such as excellent antioxidant and anti-inflammatory properties [17], immunomodulatory ability [21], and axonal growth promotion [18]. These applications will affect SCI pathology not only by inhibiting adverse changes in the microenvironment but may eventually reverse their development in a beneficial direction. Based on the present findings, we believe that NPs can play a pivotal role in future SCI treatment.
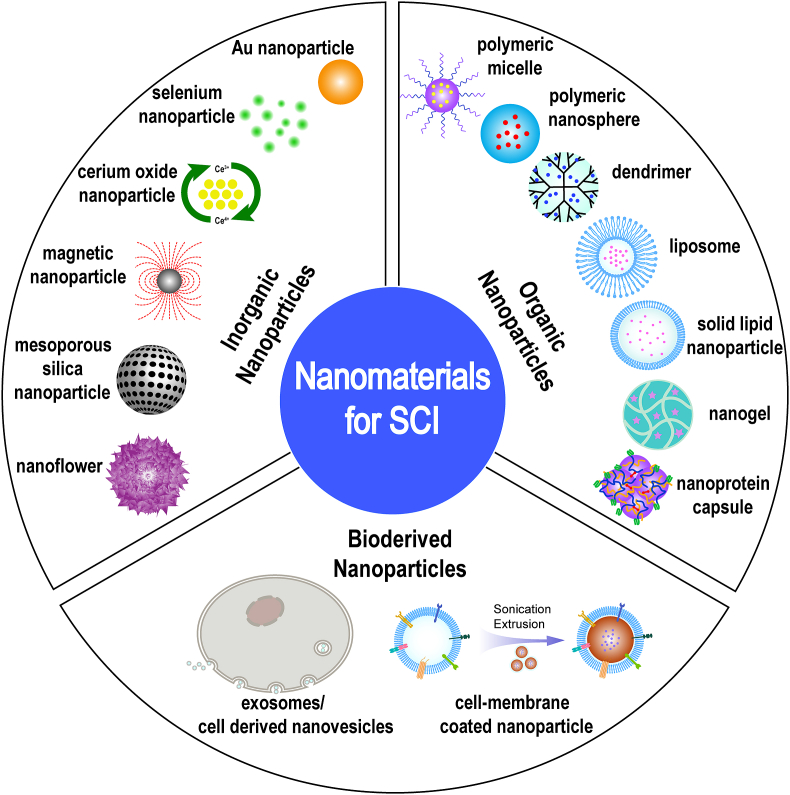
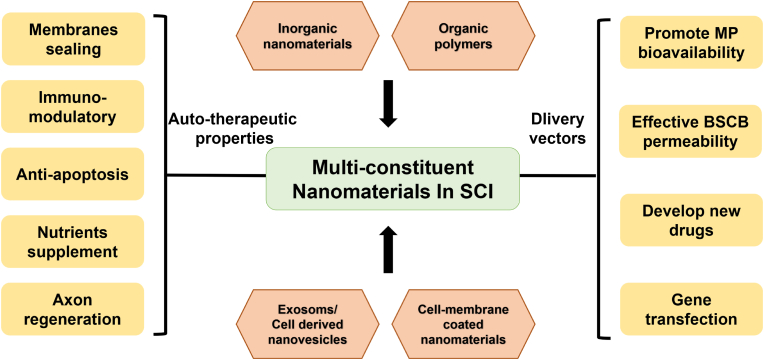
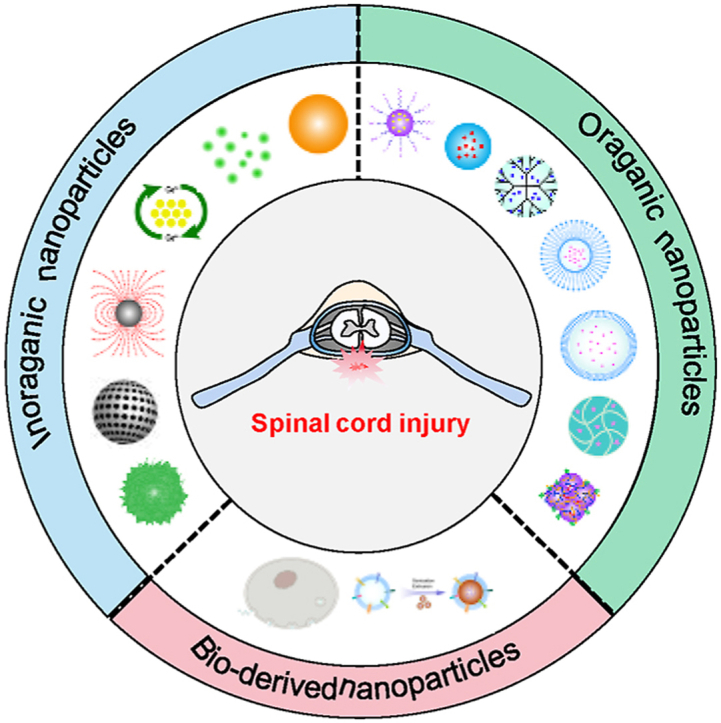
This review focuses on the current use of functional nanomaterials with particle sizes ranging from 1 to 1000 nm for the treatment of SCI and categorizes them into inorganic NPs, organic NPs, and bioderived NPs according to their source composition (Fig. 1). We also examined the design and structural composition of these NPs and their role in the treatment of SCI by describing the therapeutic properties of the components, their role in the pathological mechanism of secondary injury and their transport effects as nanovectors (Fig. 2). The aim of this review is to provide more comprehensive information for understanding the research status of NPs in the treatment of SCI and to advance new ideas for the further study of pathological mechanisms and clinical solutions.
Fig. 1.
Illustration of the classification and structure of nanomaterials for the treatment of SCI.
Fig. 2.
Schematic diagram of the role of nanomaterials in SCI treatment.
2. Role of nanomaterials in SCI treatment
2.1. The functional effects of nanomaterials in the SCI pathological mechanism
2.1.1. Pathological mechanisms of SCI and drug treatment strategy
SCI can be divided pathologically into primary injuries and secondary injuries [22]. In a primary injury, the death of neural cells occurs immediately at the site of the original injury, which is often accompanied by vertebral fracture, dislocation, blood vessel damage, hemorrhage, hematoma, ligament tear, and soft tissue damage. In contrast, secondary injury is initiated by the primary injury and then leads to a series of pathological changes, including edema, inflammation, excitotoxicity, oxidative stress, and ion imbalance. These changes can occur minutes or weeks after the primary injury.
At present, surgical treatment technology has entered a bottleneck stage. The irreparability and irreplaceability of the central nervous system cause the main trouble. Scholars have tried to reconstruct the spinal fibers by implanting different grafts such as 3D printed nerve guide conduits, polymer scaffolds and electrospun polymers [[23], [24], [25], [26]]. However, these attempts are still in the experimental phase. We also hope that in the future, fibered surgeries will be able to make a breakthrough to completely solve the central nerve fiber damage repair problem. The purpose of drug therapy is to restrain the undesirable aspects of pathological changes in secondary injury, including lipid peroxidation, reactive oxygen species production, inflammatory response burst, and apoptosis. By improving the microenvironment of SCI, uninjured nerve fibers are protected from further damage, whereas some injured nerve fibers of primary injury are also protected from further aggravation. The preservation of these neurons will be an important factor in long-term functional recovery. However, traditional approaches employ whole-body high-dose infusions to increase intramedullary drug concentrations, which are indistinguishable from the concept of “The more we eat, the more we absorb”. The design of functional nanomaterials will revolutionize all of this.
2.1.2. Therapeutic properties of functional nanomaterials affect the SCI pathological process
According to the characteristics described above, all judgments of the changes in the pathological process are important to better understand the situation of neurological outcomes. Nanomaterials affect these processes through neuroprotective or neuroregenerative therapeutic properties. Specifically, these approaches can be divided into the following three aspects. The first approach involves the direct protection and maintenance of nerve cells, including the sealing effect of the organic polymers poly(ethylene glycol) (PEG) and chitosan [[27], [28], [29], [30]], to protect neurons from outside toxins and inorganic graphene NPs to enhance electrical conductivity [31].
The second approach is to indirectly protect nerve cells by improving the damaged microenvironment during the process of secondary injury, which is similar to the current idea of clinical drug therapy for SCI. Most nanomaterials achieve this therapeutic purpose by assisting drug delivery, such as stable SiO2 [32] and high drug-loaded MoS2 [33] vectors in inorganic nanomaterials. Of course, a range of organic nanomaterials, including polymer micelles, liposomes, nanospheres, dendrimers, nanoproteins and nano prodrugs, are used. Through ingenious structural designs, these nanomaterials can effectively deliver different types of drugs to the lesion site to play a therapeutic role. It is worth noting that the targeted groups linking and the bioderived membrane-coated NPs inheriting the characteristics of cell activities can further enhance transportation efficiency and drug accumulation. In addition, some nanomaterials themselves have therapeutic effects similar to pharmaceuticals, such as inorganic iron NPs [34], CeO2 NPs [35] and Se NPs [36], which exhibit antioxidant functions. We should also pay attention to the systematic injection of the organic nanomaterial poly(lactic-co-glycolic acid) (PLGA), which can reduce the inflammatory injury microenvironment in SCI by modulating the invasion of innate immune cells [21].
The third approach is to facilitate neural regeneration for SCI treatment, and most nanomaterials stimulate axonal restoration for substantial functional recovery. For instance, inorganic Gold NPs modified by PEG have been shown to promote the remyelination of Schwann cells [37], which is beneficial for axon regrowth. Natural polymeric polysialic acid can also promote the remyelination of axons [18]. After polysialic acid treatment, significant axonal extension was observed in the spinal cord. The nanogel material designed by Papa is specifically phagocytized by A1 astrocytes, thus inhibiting astrocytosis [38]. After SCI, active astrocyte proliferation plays a key role in scar formation and limits the regeneration of axons and their functional activity [39]. Similarly, bone mesenchymal stem cell (BMSC) exosomes also effectively inhibit scar formation and promote axonal growth [40]. The promotion of nerve regeneration can also be achieved through gene delivery, and exosome material transfections have demonstrated success based on phosphatase and tensin homolog (PTEN) gene suppression [20]. PTEN siRNA delivery not only reduced glial scar tissue formation but also promoted the penetration and extension of BDA + regenerative fibers. Another approach is to directly stimulate neurogenesis. For example, bioderived human placental mesenchymal stem cell (HPC) exosomes promote the reactivation and recruitment of endogenous neural progenitor cells, thus promoting neural regeneration [41]. Similar therapeutic effects were also achieved based on the transportation of a plasmid for nerve growth factor (NGF) expresssaion using an organic nanobubble structure [42].
3. Nanomaterials act as efficient vectors for SCI delivery
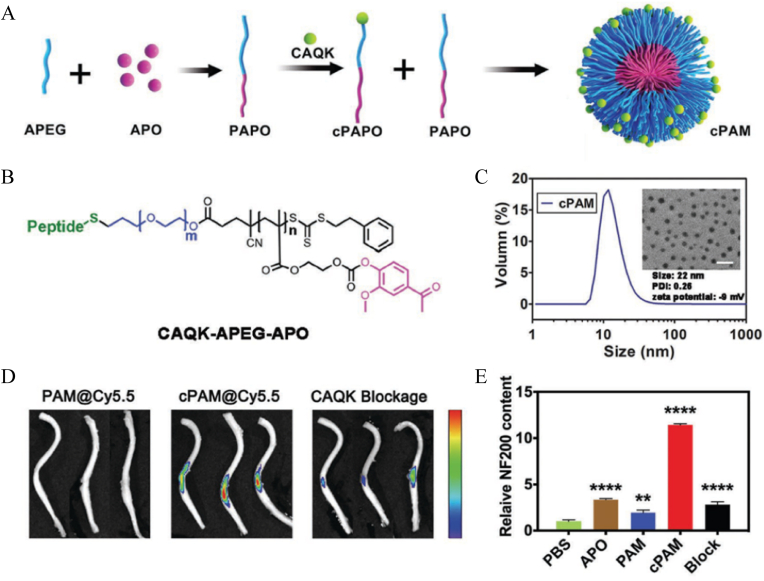
Drug administration for SCI treatment mainly includes local delivery and systemic delivery. Local administration ensures that the drug reaches the damaged spinal cord tissue accurately, but the injection process may also cause new damage. The process of open surgery is too cumbersome. Systemic administration, however, is a promising approach that could be administered as soon as possible after injury. The main problem with noninvasive administration is how to effectively reach the target position in the body. Nanomaterials can effectively reach the damaged area through a process similar to the enhanced permeability and retention effect. In addition, the disruption of the BSCB during the injury phase also allows nanomaterials to reach the damage site [43]. The study of Saxena et al. [44] also demonstrated that NPs of 200 nm could extravasate into the cord parenchyma at the lesion site within 96 h postinjury. Furthermore, active directional delivery can also be accomplished by linking targeting groups. This approach is common in the design and construction of organic NPs. For example, activated cell-penetrating peptide specifically recognizes matrix metalloproteinases and demonstrates an affinity for them [45], whereas sialic acid binds to the E-selectin receptor on endothelial cell membranes [17], effectively assisting NPs transmission through the BSCB. Tang et al. [46] made full use of the characteristics of chondroitin sulfate proteoglycan accumulation in the process of scar formation after SCI and used a CAQK peptide connection to enhance the targeting effect (Fig. 3A–C). The results showed that scar tissue-homing peptide modification greatly enhanced the accumulation and retention of NPs in the injured area (Fig. 3D), thereby ameliorating damaged axon symptoms by promoting neurofilament 200 (NF200) expression (Fig. 3E). In addition, small penetrating peptides, such as transactivating transduction protein and apamin [47,48], and microtubule-associated protein 2 specific recognition antibodies can also be applied [49]. Table 1 describes these molecular groups and their mechanism of action in detail. These nanomaterials will also be further discussed in subsequent subsections.
Fig. 3.
Illustration of the NPs for SCI targeting delivery. (A) Presentation of the synthesis process of the targeting polymeric NPs, characteristic as (B) chemical structure and (C) size distribution and TEM image. (D) Showing that CAQK targeting group effectively improved the accumulation of the NPs at the lesion site. (E) Showing that the targeting delivery significantly promoted the expression of NF200, thus alleviating axon damage. Copyright 2020, Small [46].
Table 1.
Potential groups facilitating polymeric NPs active targeting accumulation and their mechanisms.
| Active targeting group | Year | Mechanisms | Reference |
|---|---|---|---|
| Transactivating transduction protein (TAT) | 2010, 2017 | Crossing BSCB | [48,117] |
| Cystine-alanine-L-Glutamine-lysine (CAQK) | 2020, 2022 | Binding chondroitin sulfate proteoglycan | [46,105] |
| Activated cell-penetrating peptide | 2021 | Binding MMP; crossing BSCB | [45] |
| Bovine or human serum albumin (BSA or HSA) | 2017 | Binding albumin receptors at the lesion site | [16] |
| Microtubule-associated protein 2 antibody | 2018 | Targeting neural membrane | [49] |
| Apamin, an-18-amino acid peptide | 2014 | Binding apamin receptor | [47] |
| Sialic acid | 2019 | Binding E-selectin receptor on the endothelial cell membrane | [17] |
| 2-methacryloyloxyethyl phosphorylcholine (MPC) | 2019 | Nicotinic acetylcholine receptors (nAChRs) | [129] |
4. Functional nanomaterials for SCI treatment
4.1. Inorganic nanomaterials
Inorganic nanomaterials are relatively facile to construct, and a high yield can be obtained. The obtained nanomaterials typically exhibit uniform sizes, mostly tens of nanometers, with high dispersion. The intrinsic properties of these inorganic elements, such as the antioxidant effect of selenium, may be helpful for SCI therapy. Appropriate modifications can also improve their water solubility and bioavailability. However, it is well known that the safety of inorganic nanomaterials for in vivo applications requires further extensive studies.
4.1.1. Gold NPs (AuNPs)
AuNPs were first reported by Michael Faraday in 1857 [50]. In his research, he reduced gold salts to create colloidal gold, which was markedly different from bulk gold. However, the first application of AuNPs in the biomedical field was in reported the 1970s with the use of an immunogold labeling technique by Faulk and Taylor [51]. Different modifications of AuNPs have been used to develop applications for the treatment of SCI.
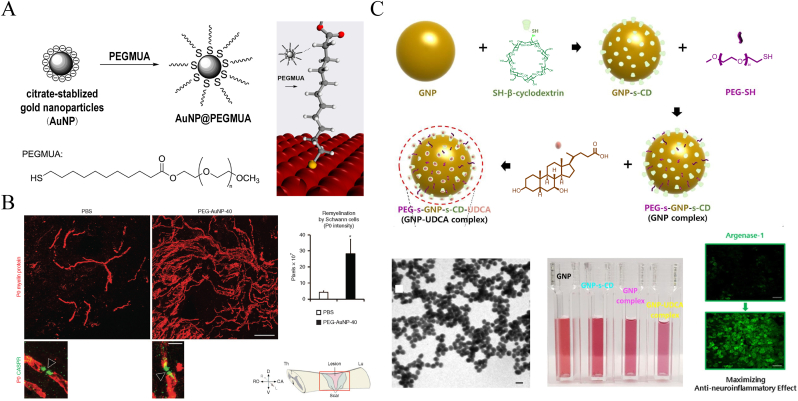
Florentia Papastefanaki et al. [37] referred to Horst Weller's method [52] of using monodentate thiolated PEG chemically linked to AuNPs to fabricate PEG-coated AuNPs with diameters of 14 and 40 nm (Fig. 4A). These colloidal gold NPs retained the sealing neuroprotective properties of PEG and exhibited higher bioavailability. The 40 nm NPs reduced microglia-related inflammation, protected motor neurons, and substantially promoted the myelin regeneration of Schwann cells (Fig. 4B). It was found that the expression of P0 myelin protein was significantly up regulated, and the remyelination myelin sheath reformation effectively contributed to the restoration of regrown axons. After intrathecal injection treatment, the Basso Mouse locomotor rating scale was significant improved after 8 weeks, compared with the PBS group. Similarly, R-dihydrolipoic acid enantiomer-modified Au nanoclusters (AuNCs) showed strong immune-suppressing responses for use in SCI therapy [53]. In addition to these therapeutic properties, AuNPs could also be used as drug carriers by binding to thiol end groups (-SH) [54,55]. Seil Sohn et al. [56] used AuNPs conjugated with mono-(6-mercapto-6-deoxy)-β-CD and further loaded them with ursodeoxycholic acid for SCI treatment (Fig. 4C). This AuNP complex that ranges from 20 to 40 nm in size significantly limited proinflammatory cytokine expression and increased antineuroinflammatory effects by promoting arginase-1 protein expression. Recently, Mei et al. [57] developed novel AuNCs conjugated to positively charged berberine through electrostatic interactions. One hundred 20-nm NPs stabilized by bovine serum albumin (BSA) showed effective anti-apoptotic ability and regulated M2 macrophage polarization.
Fig. 4.
Illustration of the fabrication of Au NPs and application in SCI treatment. (A) The preparation of AuNPs with monodentate thiolated PEG ligand. Copyright 2013 Langmuir [52]. (B) Remyelination promotion of Schwann cells in mice after the treatment by PEG modified AuNPs. Copyright 2015 Molecular Therapy [37]. (C) Showing that the synthesis process of the AuNP complex, their presentations in TEM and cuvettes, and the anti-neuroinflammatory effects. Copyright 2019 Chemical Engineering Journal [56].
4.1.2. Iron oxide NPs
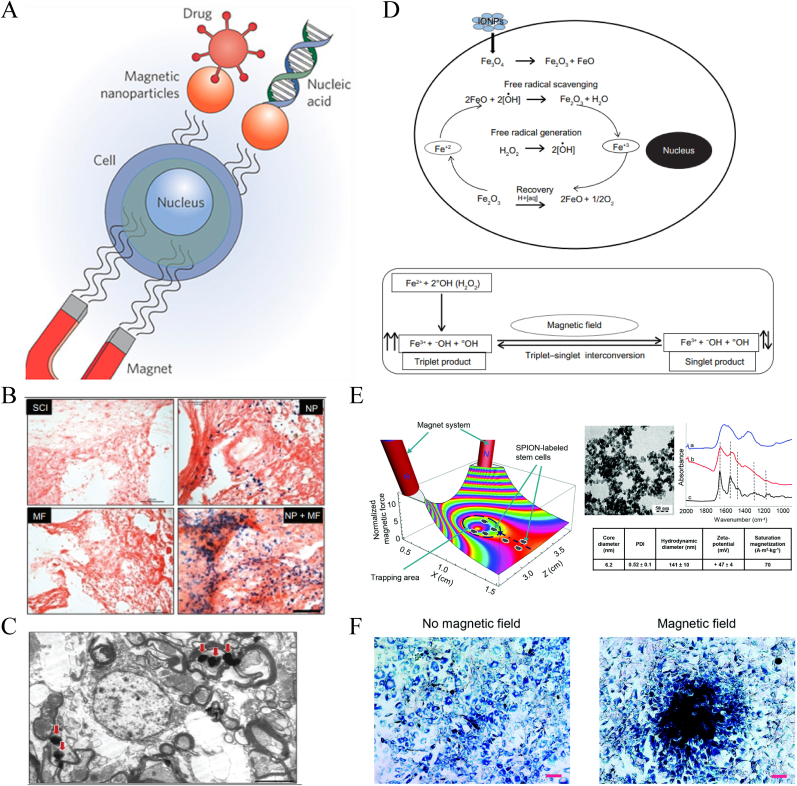
Iron oxide NPs (IONPs), such as Fe3O4 and Fe2O3, are also easy to produce and are widely used for imaging, drug delivery, and photothermal therapy [58,59]. When combined with external electromagnetic fields, IONPs play an effective role in transfection and delivery in the treatment of SCI (Fig. 5A) [60]. Ajay Pal et al. [34] confirmed that IONP exposure in a magnetic field effectively promoted the recovery of spinal function by attenuating free radical-induced damage. The study found that the magnetic field significantly increased the retention and accumulation of IONPs at the damage center (Fig. 5B and C) and that the enhanced antioxidant capacity was associated with alterations in the electron spin relaxation state caused by the magnetic field (Fig. 5D). Their subsequent work showed that exposure to a magnetic field may further stretch axonal terminals and cause sprouting, thus promoting regeneration.
Fig. 5.
Schematic illustration of IONP delivery combined with external magnetic field for SCI treatment. (A) Schematic showing that magnetic NPs targeting guided into cells under the action of an external magnetic field. Copyright 2009 nature nanotechnology [60]. (B, C) Prussian blue staining for iron and TEM imagese exhibiting the aggregations of IONPs in the lesion stie mediated by external magnetic field. (D) Mechanism of free radical scavenging by IONPs. (E) Showing 3D simulation of the distribution changes of superparamagnetic iron oxide-labelled stem cell under normalized magnetic gradient force (X–Z-plane), and the characteristics of superparamagnetic iron oxide NPs. (F) Distributions of superparamagnetic iron oxide-labelled cells with or without magnetic field. (B, C, D) Copyright 2013 International Journal of Nanomedicine [34]. (E–F) Copyright 2015 Nanoscale [61]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Taking advantage of the magnetic orientation, IONP vehicles can also realize directional transfection. Šárka Kubinová et al. [61] used superparamagnetic iron oxide NPs to label MSCs for intrathecal transplantation therapy and found significantly higher concentrations of superparamagnetic iron oxide-labelled stem cells in the vicinity of the damaged lesions under magnetic guidance, achieving effective stem cell delivery (Fig. 5E and F). Similar studies were conducted by Aleem Ahmed Khan et al. [62] and Jung-Keug Park et al. [63]. Stuart et al. [64] applied a static/oscillating magnetic field to promote in vivo transfection of oligodendrocyte precursor cell transplant cells. Magnetic NP-mediated transfection improved transfection efficiency and did not affect cell proliferation or differentiation potential. The protocol could also be conducted with plasmids for functional gene transfection, promoting neural regeneration by encoding the expression of therapeutic fibroblast growth factor 2.
Magnetic NPs could also be modified for drug delivery. Jing Wang et al. [65] used a thermal method to synthesize magnetic NPs containing PEG and then loaded tacrolimus using a coprecipitation method. The modified NPs with an average diameter of 90 nm significantly increased the immunosuppressive effect of tacrolimus and increased the neuronal length and the nerve regeneration rate.
4.1.3. Cerium oxide NPs
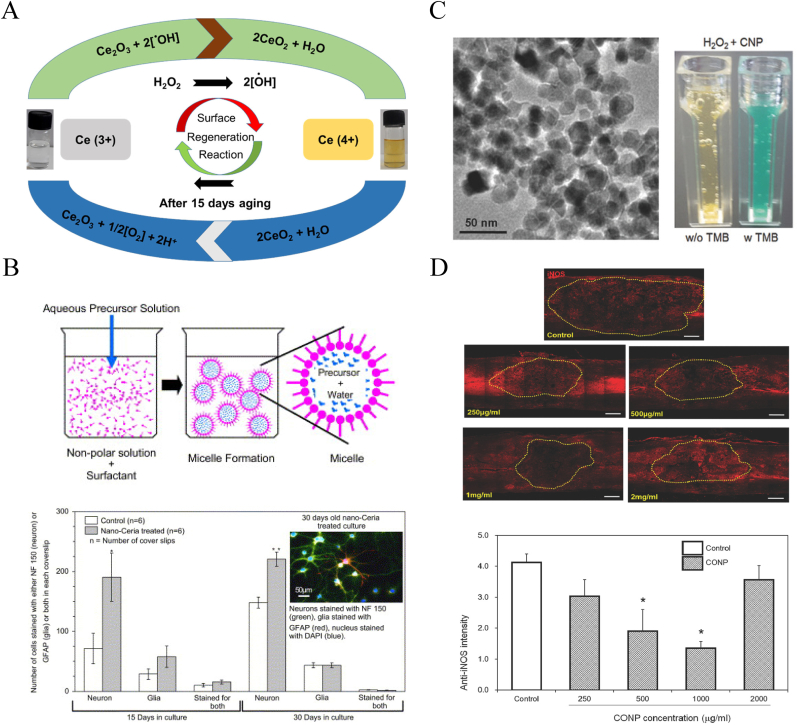
Cerium oxide NPs (CeNPs), also known as nanoceria, are receiving attention as potential antioxidant agents and free-radical scavengers. CeO2 at the nanoscale level coexists with Ce3+ and Ce4+ ions, which are involved in redox cycling reactions [66]. These reactions allow CeNPs to react catalytically with superoxide and hydrogen peroxide (H2O2) so that all noxious intracellular radical oxygen species (ROS) are abated via a self-regenerating mechanism (Fig. 6A).
Fig. 6.
Schematic illustration of CeNPs auto-catalytic behavior and free radical scavenging property in the treatment of SCI. (A) Surface regeneration reaction mechanism of CeNPs by Ce (3+) and Ce (4+) ionic cycle changes. Copyright 2021 Inorganic Chemistry [66]. (B) Microemulsion synthesis process of CeNPs and their protection ability for neural survival in vitro. Copyright 2007 Biomaterials [67]. (C) TEM presentation of CeNPs and their H2O2 monitoring ability presentation in cuvettes. (D) Showing CeNPs anti-oxidation therapeutic ability and the inhibition of ROS-induced inflammation in vivo after SCI. (C–D) Copyrght 2017 Advanced Science [35].
Mainak Das et al. [67] prepared CeNPs using a microemulsion method and found that their autocatalytic properties facilitated effectively treatment of animal models of spinal cord cell injury induced by H2O2 (Fig. 6B). Xiutong Fang et al. [68] further improved the synthetic steps in this in vitro therapeutic model using ionic liquid to enhance the solubility of CeNPs. Then, they loaded the inorganic NPs onto chitosan to form a CeO2/chitosan nanocomposite, thereby increasing its bioavailability and reducing its toxicity. Similarly, Xiujin Chen et al. [69] fabricated nanocerium oxide-loaded poly(ε-caprolactone) (PCL) with resveratrol to form CeNPs. This method improved the distribution of the CeNPs and enhanced their catalytic performance. This nanomaterial exhibited good solubility and continuous release in vitro. The H2O2 damage test also showed good spinal cord cell viability.
Jong-Wan Kim et al. [35] applied CeNPs to the in vivo treatment of SCI rats for the first time. These researchers produced a batch of CeNPs with an average particle size of 19.5 nm and injected different concentrations via local subdural injection into the spinal cord of T9 contusion rats. CeNPs exhibited good ROS scavenger and antioxidant capacities and significantly decreased inducible nitric oxide synthase in the lesion. CeNPs administration also showed excellent anti-inflammatory and anti-apoptotic therapeutic effects by regulating the expression levels of the relative molecules. The optimal therapeutic doses were 500 and 1000 μg/mL (Fig. 6C and D).
4.1.4. Selenium NPs
Selenium, as a component of selenoprotein, is a normal trace element in the human body and has a variety of structures and antioxidant functions [70]. Selenium was previously reported to play a vital role in the continuation of physiological activities in the nervous system, such as signal transmission and neuronal development [71]. Other studies confirmed that selenium had a certain protective effect on epilepsy and neuralgia [72,73]. The therapeutic effect of selenium in SCI requires further study.
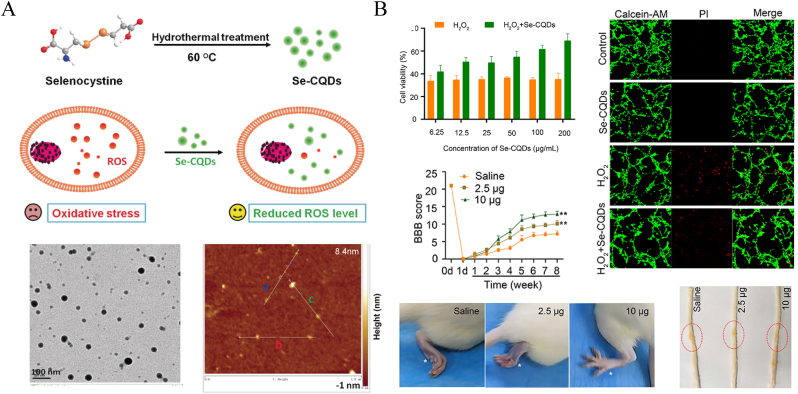
Tianfeng Chen et al. [36] prepared stable selenium NPs (SeNPs) by reacting sodium selenite and ascorbic acid. Polysaccharide-protein complex/PG-6 peptide (PLGLAG) was used to decorate the SeNPs to improve their stability and accumulation. The NPs loaded with monosialotetrahexosylganglioside/tetramethylpyrazine drugs showed great ROS scavenger and antioxidant therapeutic effects and effectively inhibited mitochondrial dysfunction, G2/M phase arrest, and apoptosis. The in situ injection therapy also represented an effective treatment. One study found that the attenuation of ROS overproduction by SeNPs was related to the inhibition of the p53 and MAPK pathways. Luo et al. [74] used a simpler method to fabricate selenium-doped carbon quantum dots (Se-CQDs) based on the hydrothermal treatment of L-selenocystine (Fig. 7A) [75]. The NPs exhibited a stable particle size of approximately 40 nm and good water solubility. Local Se-CQD injection in vivo produced good ROS-scavenging and anti-apoptotic effects, thereby protecting spinal nerves and alleviating secondary damage (Fig. 7B). At 8 weeks post operation, the SeNPs treated rats got obvious neurofunctional recovery, and the Basso, Beattie, Bresnahan (BBB) locomotor score was significantly higher than that of saline group.
Fig. 7.
Schematic illustration of the preparation of SeNPs and their theraupetic effects on SCI. (A) The synthesis process of SeNPs by the hydrothermal treatment of selenocystine and their characteristic presentations of TEM and Atomic force microscopy height profile analysis. Copyright 2017 Angewandte Chemie [75]. (B) Showing that SeNPs efficiently ameliorate SCI via eliminating ROS, manifestation in vitro and in vivo. Copyright 2020 International Journal of Nanomedicine [74].
Moosa Javdani et al. [76] orally administered SeNPs to rats with SCI and found that the NPs exhibited good anti-inflammatory effects. A significant reduction in white blood cells, including lymphocytes, neutrophils, and monocytes, in the blood was noted after NPs treatment compared to the control group. Histopathological image analysis also showed a significant decrease in inflammation and hemorrhage during the third and fourth weeks in the treatment group.
4.1.5. Silica NPs
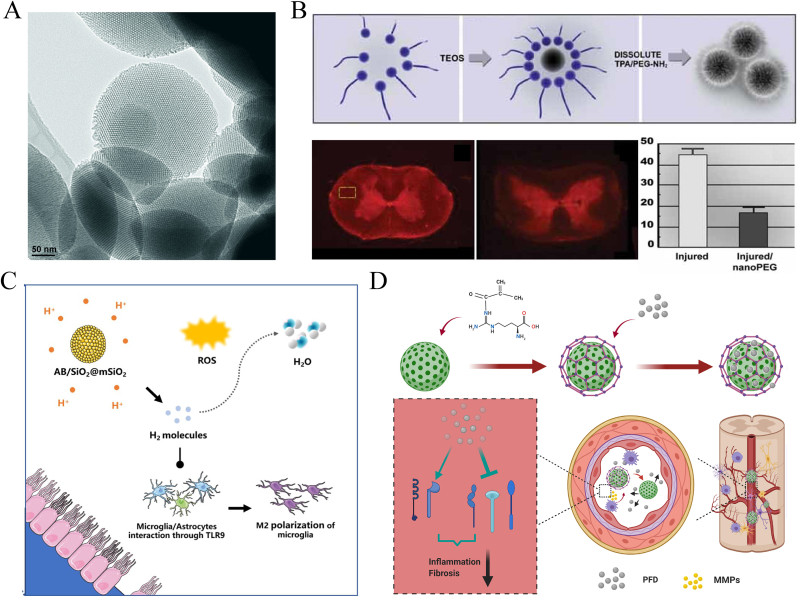
Silica, or silicon dioxide, NPs (SiNPs) are one of the most common and easily synthesized inorganic NPs. The physical parameters of SiNPs, such as size, shape, and porosity, can be easily tuned for special biological applications. The large surface of SiNPs can also be modified by attaching biocompatible, targeting, and imaging ligands [77,78]. Among them, the mesoporous form of silica is commonly used as a nanocarrier (Fig. 8A).
Fig. 8.
Schematic illustration of the applications of functionalized SiNPs in SCI. (A) Showing TEM image of MSN structure prepared by co-condensation method. Copyright 2007 Advanced Functional Materials [78]. (B) Silica colloid modified by PEG and its membrane sealing effect in SCI. Copyright 2008 Small [79]. (C, D) Showing the utilization of MSN for SCI deliver. (C) Shield transportation of ammonia borane for responsive H2 release. Copyright 2021 Regenerative Biomaterials [86]. (D) Macrophage-mediated degradable gelatin-coated MSN sturcture carrying pirfenidone for controlled release in SCI treatment. Copyright 2021 Nanomedicine: Nanotechnology, Biology, and Medicine [32].
Youngnam Cho et al. [79] attached PEG-NH2 covalently to the SiNP surface, prepared polymer-surfaced silica colloids (PSCs/PSiNPs), and applied it for the treatment of SCI in guinea pigs (Fig. 8B). Due to their PEG-sealing ability, PSCs can effectively protect the somatosensory-evoked potential of the spinal cord and prevent the intracellular accumulation of harmful substances, such as horseradish peroxidase. The NPs also exhibit good drug-carrying capacity. These researchers further doped tetramethyl rhodamine (TMR) dye as a mimicking drug into the PSiNPs to construct TMR-PSiNPs [80]. The NPs exhibited the therapeutic ability mentioned above, and the silica network effectively shielded the drug mimic. By incorporating a fluorescent TMR label onto PSiNPs, they showed the preferential targeting ability of these NPs to injured parenchyma. In addition, they used cetyltrimethylammonium bromide as a template and an acidic-extraction method to fabricate PEG-modified mesoporous silica NPs (MSNs) [81]. The NPs effectively protected neural cells from acrolein damage. Recently, Sara Daneshjou et al. [82] further demonstrated that MSNs exhibited good thermal stability at 37 °C, effectively protecting chondroitinase from degradation and inhibiting glial scar-associated chondroitin sulfate proteoglycan molecules from accumulating in the lesion [83,84]. Similar to the study by Wang et al. [85], the intrathecal injection of porous selenium@SiO2 nanocomposites reduced the toxicity of selenium, sustained its release, and exerted antioxidant effects. Liu et al. [86] constructed ammonia borane-loaded MSNs. The drug-loaded MSNs were sensitive to acidic pH in SCI, and then ammonia borane decomposed to release H2 gas, exerting a regulatory effect on oxidative and inflammatory imbalance (Fig. 8C). Zhang et al. [32] used MSNs to encapsulate pirfenidone, an anti-inflammatory and anti-fibrosis drug with a short half-life, to provide good gatekeeping properties to help avoid premature leakage. These researchers designed amino-MSNs based on the surface attachment of aminopropyltrimethoxysilane and then conjugated methacrylate gelatin so that the modified MSNs specifically decomposed under the action of matrix metalloproteinases to achieve the release of pirfenidone under both spatial and temporal control (Fig. 8D) [32].
4.1.6. Other inorganic NPs
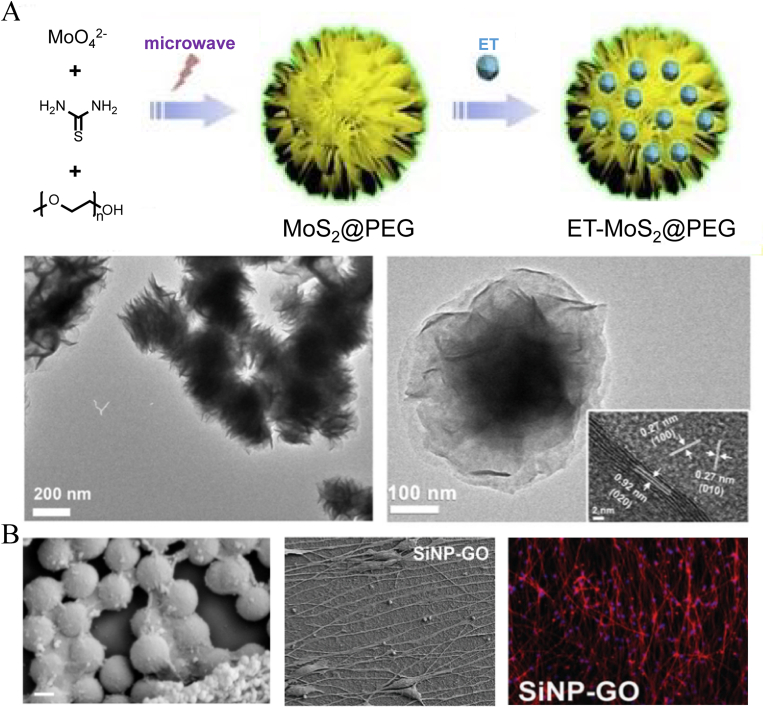
Sun et al. [33] designed a novel nanoflower structure constructed of molybdenum disulfide (MoS2) nanomaterials. The MoS2@PEG nanoflowers were synthesized via a microwave-assisted hydrothermal route and entrapped the anti-inflammatory drug etanercept (ET) between layers of MoS2 nanosheets. The construction method resulted in a high drug-loading capacity (∼60%) and achieved a continuous release rate of up to 144 h. In vivo treatment showed a long-lasting anti-inflammatory effect (Fig. 9A). This lamellar stacking form is more common in carbon-based NPs, especially graphene oxide (GO) NPs, which are promising candidates for SCI therapy given their good chemical and electrical conductivity [31]. Ki-Bum Lee et al. [87] used a GO nanosheet to cover the surface of 300-nm SiNPs and found that the acquired hybrid NPs could effectively promote the differentiation of human neural stem cells (NSCs). Compared to other substrates, only the addition of GO and GO-modified silica groups showed obvious axonal growth (Fig. 9B). Zhu et al. [88] studied GO NPs with different particle sizes and found that GO NPs at 417 nm and 663 nm were better able to maintain the self-renewal of mouse NSCs in the absence of epidermal growth factor (EGF) and basic fibroblast growth factor. Among them, 417-nm GO NPs promoted the migration of mouse NSCs by significantly upregulating vinculin expression. To avoid the cytotoxicity of graphene and the difficulty of physical absorption in vivo, Mehrdad Mahkam et al. [89] further grafted chitosan and PEG onto the surface of GO nanosheets. In situ injection of these modified hybrid NPs at the SCI site effectively promoted the functional recovery and regeneration of neurites. At 14 days postinjury, H&E staining showed lower cavity regions and hemorrhage in the treatment group. And the BBB scores of mice hind limbs exhibited remarkable improvement. GO and other carbon-based nanomaterials have also been developed and prepared as nanotubes, nanofibers, and nanoribbons [90,91]. However, due to the obvious difference between the configurations and particle shapes, these are not discussed further in this review, and relevant research can be found in other published literature.
Fig. 9.
Schematic illustration of nanoflower in SCI treatment. (A) The preparation process of ET loaded MoS2@PEG nanoflowers and their morphological features under the TEM. Copyright 2019, Journal of Colloid and Interface Science [33]. (B) Showing of the SiNPs modified by GO nanosheet and their promotion on human NSCs differentiation.Copyright 2013, Advanced Materials [87].
4.2. Organic nanomaterials
Organic NPs differ from inorganic NPs in that they are more biodegradable and biocompatible. They are the most widely studied and applied drug delivery vehicles to date. Organic NPs are mainly composed of polymers with various components and properties, which can be designed with different structures to increase cargo capacity, reduce side effects, or improve release efficiency or transfection efficiency according to the actual need. Organic NPs can also be endowed with active, targeted delivery capabilities by linking specific functional groups. These functional groups would be particularly important in the treatment of SCI, enabling polymeric NPs to effectively cross the BSCB barrier and accumulate in the injured area.
Some organic polymers themselves can also exert therapeutic effects on SCI. For example, PEG and chitosan can be used as membrane fusion agents [[27], [28], [29], [30]], and PLGA NPs with a diameter of 500 nm can effectively regulate the recruitment of immune cells [21]. The natural polymer polysialic acid has the ability to promote axon lengthening and nerve regeneration [18]. Making full use of these characteristics can achieve good synergistic effects in the delivery of therapy in SCI. Fig. 10A and B demonstrates the neuroprotective role of PEG-based NPs in the treatment of SCI. After treatment with the NPs, the membrane was fully protected, and the erosion caused by the influx of calcium ions was effectively avoided. Their physical composition will be further described in subsequent subsections [92]. Fig. 10C and D shows the PLGA chemical structure, transmission electron microscopy (TEM) characteristics of PLGA NPs, and in vivo therapeutic ability to reprogram immune cell distributions [21].
Fig. 10.
Schematic illustration of PEG and PLGA polymers exerting therapeutic effects on SCI. (A–B) Showing the structure of PEG-based block copolymer and its neuronal membrane protection for SCI treatment. The green fluorescence reflects the level of Ca2+ influx into axons. Compared with the untreated group (a), PEG-based NPs treated group (c) was significantly improved. (b) Stands for the healthy one. Copyright 2010 Nature Nanotechnology [92]. (C–D) Showing the PLGA chemical structure, TEM characteristics of PLGA NPs, and in vivo therapeutic ability to reprogram the distribution of immune cells. The NPs treated group exhibited less accumulation of immune cells in the lesion site, compared with that of the PBS group. Copyright 2019 PNAS [21]. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4.2.1. Polymeric micelles
Micelles are the most common structure of drug-loaded NPs of organic polymers, which are usually composed of amphiphilic polymers linked by organic polymerization. Due to the differences in hydrophilic and hydrophobic polarity, the polymer self-assembles in the aqueous phase, and the drug is encapsulated in the center of the physical structure [93]. PEG is the most commonly used polymer in micellar organic structures and is often used as the hydrophilic end of a micelle.
As described above for PEG membrane sealing, Cheng et al. [92] synthesized self-assembled monomethoxy PEG-poly(D,L-lactic acid) (mPEG-PLA) di-block copolymer micelles, which demonstrated a good compound action potential restorative effect in treating SCI (Fig. 10A and B). Guo Gang's group constructed similar PEG-related biodegradable copolymer structures, diblock mPEG-PCL [94] and triblock mPEG-poly(l-lactide)-poly(trimethylene carbonate) (mPEG-PLLA-PTMC) (Fig. 11A) [95], and used them to load the hydrophobic drugs dexamethasone acetate (DA) and zonisamide (dopaminergic recovery), respectively, thus synergizing the neuroprotective effect. Another example is that PEG–PLA diblock copolymer micelle-coated ibuprofen showed anti-inflammatory and antioxidative effects and promoted the recovery of motor function [96].
Fig. 11.
Schematic illustration of structural compositions and the characteristics of polymeric micelles in SCI treatment. (A) Chemical presentation of mPEG-PLLA-PTMC triblock copolymer and TEM image of the self-assembled nanomicelles. Copyright 2017 International Journal of Nanomedicine [95]. (B) Comb-like structure of PCL-based micelles and their treatment to revert activation process of microglia in vitro [97]. (C) Showing the structure of polysialic acid-based micelles, their morphological features and the ability to promote on myelin sheath regeneration. Copyright 2019 Nano Letters [18].
In addition to traditional block polymerization, PEG can also be designed as a comb-like structure. Using PEG chains as the hydrophilic end and poly-(2-hydroxyethyl methacrylate) as the backbone, Papa et al. [97] employed emulsion-free radical polymerization to finally synthesize unique comb-like structural PEG-PCL micelles by conjugating the hydrophobic PCL chains (Fig. 11B). The size of the NPs loaded with minocycline (MC) was approximately 100 nm, in which the PEG chain ensured stability, and the length of the PCL chain could be adjusted to alter the release rate of the MC. Due to the incorporation of PCL chains, the NPs were selectively taken up by the activated microglia and were completely degraded in approximately one week. The NPs could effectively inhibit the activated microglia and then alleviate the inflammatory response of secondary injury. In follow-up research, they further demonstrated that loading NPs with MC could effectively regulate the recruitment and distribution of M1 and M2 macrophages at the injury site [98], which was related to chemotaxis by the chemokine CCL2 [99].
PLGA is one of the most common synthetic copolymers in polymeric micellar constructions. PLGA is approved by the Food and Drug Administration (FDA) and is widely used for medical applications. The compound is produced by the ring-opening polymerization of two different monomers, glycolic acid and lactic acid, forming a random or block copolymer structure. PLGA exhibits good biocompatibility and degradability, so it is often used as part of a variety of polymer carriers [100]. Shen et al. [45] fabricated biocompatible polymeric PLGA-poly(ethylene imine) (PEI)-mPEG (PPP) micelles to entrap the tumor necrosis factor-α (TNF-α) blocker ET for SCI anti-inflammatory treatment. By attaching an activated cell-penetrating peptide to the shell, the ET@PPP NPs acquired a specific affinity for matrix metalloproteinases and accumulated in the targeted lesion site during systemic delivery (Fig. 12B). Similarly, Wang et al. [17] modified PEG-PLGA micelles with sialic acid and prepared MC drug-loaded NPs (SAPPM) for targeted delivery to the lesion site. As sialic acid monomers could specifically bind to E-selectin expressed on endothelial cells in SCI [101], SAPPM effectively improved treatment outcomes (Fig. 12A). The polysialic acid is also an important carbohydrate component on the surface of cell membranes and plays a vital role in the migration and differentiation of neural precursors, neuronal guidance, synapse formation, and axon path-finding [102,103]. Wang et al. [18] developed a novel functional polysialic acid-based nanomicelle for delivering MC (PSM) to treat SCI based on an acylation reaction with octadecylamine (Fig. 11C). The PSM NPs exhibited the sustained drug release of MC to the lesion site of rats with SCI and promoted the neural regeneration and extension of long axons throughout the glial scar. At 12 weeks postinjury, TEM images showed better myelination of myelin sheaths in PSM group compared with other groups (Fig. 11C). Meanwhile, the hindlimb motor functions of PSM group got better restored, according to the BBB scores [18].
Fig. 12.
Schematic diagram of polymeric micelles modified by targeting groups. (A) Showing sialic acid-driven PEG-based micelles for SCI targeting transportation by specifically combining with E-selection. Copyright 2019 Biomaterials [17]. (B) Showing SCI targeting delivery of PPP micelles induced by activated cell-penetrating peptide. Copyright 2021, Small [45].
Chitosan is one of the most extensively studied natural polymers with the potential to build micelle structures by modifying the reactive hydroxyl and amine groups it contains. Wei Wu et al. [104] used ferulic acid to substitute for the sugar residues of glycol chitosan, and the obtained conjugates could self-assemble in water to form core-structure nanoproducts. Here, the hydrophilic end of glycol chitosan was located on the outside, whereas the hydrophobic ferulic acid was located on the inside. The particle size also varied with different degrees of sugar residue substitution. NPs with a degree of substitution of 12.8 and a particle size of 236 nm were well distributed in both the gray and white matter. The NPs exhibited the combined therapeutic capability of ferulic acid and glycol chitosan. Jugular vein injection treatment effectively rescued axons and neuronal cells and alleviated the inflammatory response of astrocytes and microglial cells at the injured site. Zein is another type of natural polymer derived from corn protein with an amphiphilic molecular structure. Li et al. [105] applied its properties to packaging metformin and self-assembled it into NPs, the outer layer of which was modified by crosslinking CAQK with NHS-PEG5000-MAL. The CAQK peptides selectively bound to chondroitin sulfate proteoglycan to promote the site-specific accumulation of nanodrugs, whereas metformin exhibited good antioxidant, anti-inflammatory, antiapoptotic, and neuroprotective effects.
4.2.2. Polymeric nanospheres
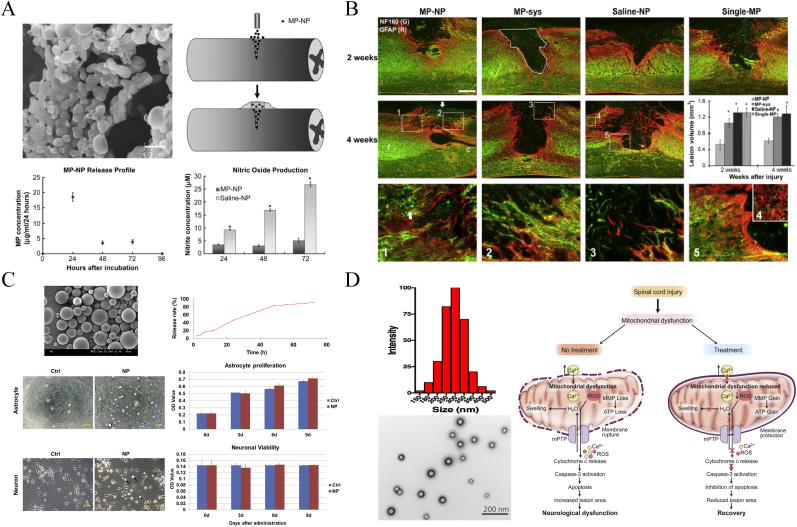
Polymeric nanospheres are spherical in shape and have a solid polymeric network core that allow drugs retained inside or adsorbed on the surface [106]. PLGA nanospheres are commonly used in SCI treatment to improve the efficacy of MP or to develop applications for other drugs. Young-tae Kim et al. [107,108] fabricated PLGA-based MP NPs based on a modification of the double-emulsion (water/oil/water phase) method and administered it through a single local injection at the injury site (Fig. 13A and B). The NPs were biodegradable and enabled the sustained release of MP to injured spinal cord tissue, and the novel method optimized the MP dose to approximately 1/20th of the conventional systemic MP administration of 30 mg/kg, minimizing the side effects associated with high-dose MP. In the fluorescence images, the treatment group presented significant reduction of lesion volume compared with other groups, especially at 4 weeks postinjury (Fig. 13 B). Some NF160+ staining axons could be observed at the treatment group while the control groups did not, and behavioral assessments by Grid walking and Beam walking also showed significant improvement. Similarly, Wu and Zhou [16] prepared novel composite NPs composed of PLGA, chitosan, and albumin for the delivery of MP and MC using the emulsion solvent evaporation method. Albumin-MP + MC-NPs controlled the codelivery of the 2 drugs, improving the therapeutic efficacy after reducing the dose by 10-fold. The NPs achieved site-specific delivery through BSA linked to chitosan on the surface, avoiding the shorter half-life of the drugs during intravenous administration. PLGA exhibits excellent prospects in the development of new drugs, such as encapsulating the antioxidant enzymes SOD and catalase to protect mitochondria from oxidative stress (Fig. 13D) [109] and the local delivery of flavopiridol (Fig. 13C) [110], a cyclin-dependent kinase inhibitor, to limit astrocyte proliferation, migration, and inflammatory factor synthesis. As another example, PLGA-mediated estrogen (E2) delivery effectively attenuated glial activation and inflammatory responses following SCI, thus protecting neuronal cells and improving myelination and motor function [111].
Fig. 13.
Schematic diagram of PLGA based nanospheres for drug delivery in SCI treatment. (A) The morphology of PLGA nanospheres loaded with MP under SEM, local delivery for dorsal over-hemisection lesioned spinal cord and their in vitro inhibition of inflammation. (B) Showing that SCI lesion range was significantly reduced after the NPs therapy. (A–B) Copyright 2009 Biomaterials [93]. (C) PLGA nanosphere loading flavopiridol to inhibit astrocytes proliferation in vitro. Copyright 2014 Biomaterials [110]. (D) PLGA NPs encapsulating antioxidant enzymes to keep neural cells from apoptosis by attenuating mitochondrial dysfunction. Copyright 2020 Journal of Controlled Release [111].
4.2.3. Dendrimers
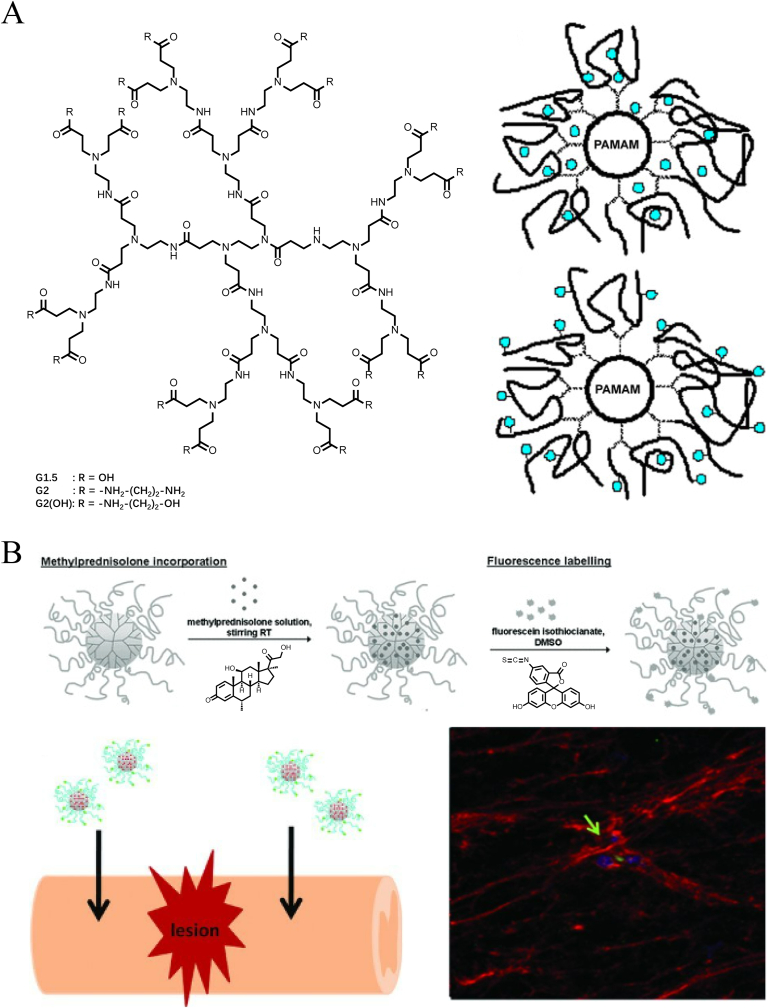
In contrast to the physical structure of micelles and nanospheres, dendrimers are highly ramified, spherical, and low-dispersity synthetic molecules (Fig. 14A) [112]. The structure of these macromolecules is extremely precise and controlled so they can be designed to achieve an adjustable molecular weight and excellent drug-loading capacity (Fig. 14A) [113]. Susana R. Cerqueira et al. [114] surface-engineered polyamidoamine (PAMAM) branched polymers with carboxymethylchitosan to fabricate a 100-nm dendrimer. The dendrimer NPs loaded with MP were successfully internalized by glial cells and exhibited sustained drug release inside for 14 days, thereby exerting anti-inflammatory effects (Fig. 14B). The sustained-release phase, in a sense, could attenuate the side effects on microglia by the direct acute action of MP. In the partially hemisected rat SCI model given a local injection, BBB function scores for locomotion were significantly improved in the treated animals compared to the control group.
Fig. 14.
Schematic illustration of dendrimers structure and their drug delivery for SCI treatment. (A) Chemical structure of PAMAM (G2) and its two drug-loaded forms (incorporation in the bulk and bonding to functional groups). Copyright 2008 Journal of Controlled Release [112], 2010 Progress in Polymer Science [113]. (B) The preparation of MP loaded CMCht/PAMAM dendrimer NPs and local injections for SCI treatment. Fluorescence image showed the internalization of the NPs at the lesion site. Copyright 2013, Small [114].
4.2.4. Liposomes and solid lipid NPs
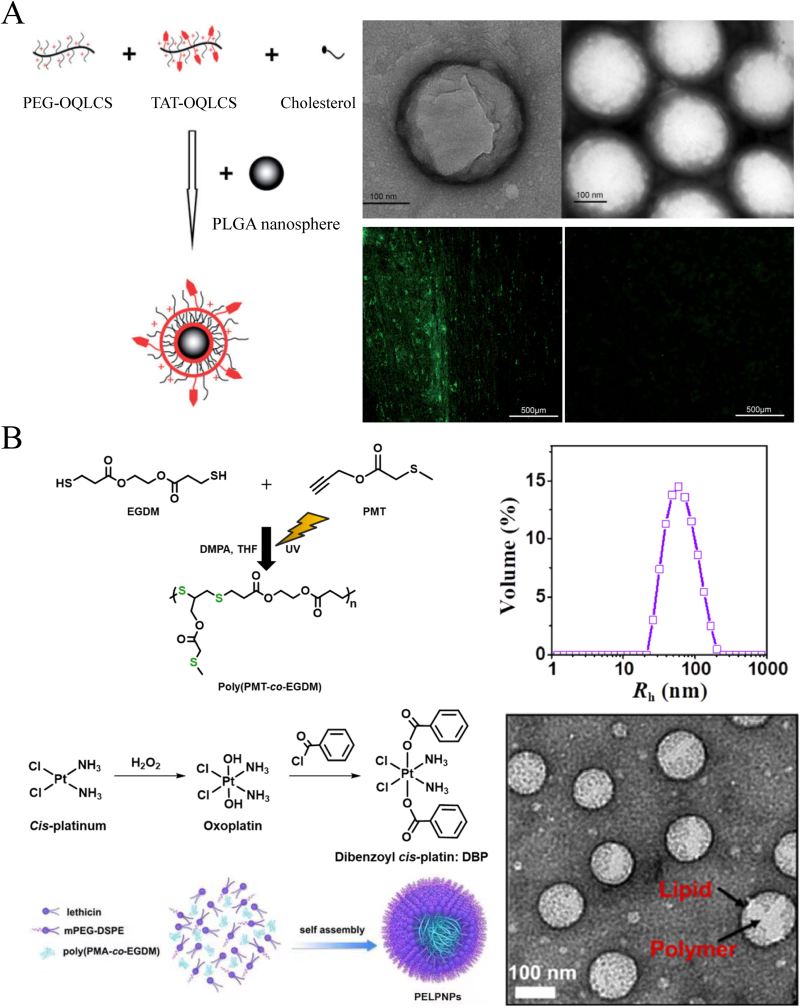
A liposome is an artificial spherical vesicle with at least one bilayer structure that is mostly comprised of phospholipids and similar amphipathic lipids, making the inner core and each layer independent regions [115]. As the composition of the peripheral structure is close to that of cell membranes, it is more conducive to fusion with cells and the transportation of contents. Steinmetz et al. [116] used phosphatidylcholine and cholesterol to synthesize phospholipid vesicles and encapsulated rolipram, a type 4 phosphodiesterase inhibitor protein that hydrolyzes cAMP, to treat T8 contusive SCI rats. Liposomal clodronate could effectively deplete peripheral macrophages and limit proinflammatory cytokine production, improving myelinated tissue sparing and enhancing locomotor recovery through immune-based therapy. Tarun Saxena et al. [44] mixed 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(poly(ethylene glycol))-2000] (mPEG2000-DSPE) in ethanol at 60 °C and extruded it repeatedly to prepare liposomal NPs. Then, NPs were encapsulated in Chicago sky blue, a macrophage migration inhibitory factor (MIF), for SCI treatment. With the addition of PEG, the stealth liposomes could avoid phagocytosis by the immune system and achieve long circulation times. The delivery of Chicago sky blue significantly improved white matter integrity and reduced BSCB leakage. Shi et al. [117] synthesized cationic PEGylated amphiphilic octadecyl-quaternizedlysine-modified chitosan (PEG-OQLCS) and cationic TAT-conjugated amphiphilic octadecyl-quaternizedlysine-modified chitosan (TAT-OQLCS) and then mixed the two amphipathic lipids with cholesterol and PLGA NPs to self-assemble polymeric liposomes (Fig. 15A). The core/shell NPs loaded with cyclosporin A could facilitate the transport of drugs across the BSCB by TAT-targeted binding and then promote their immunosuppressive and neuroprotective effects.
Fig. 15.
Schematic illustration of structural compositions of liposomes and SLNs and their application in SCI treatment. (A) The preparation of liposome-core/shell NPs by conjugation with TAT targeting group. The morphology of the NPs and their targeting delivery ability were presented by TEM and fluorescence images. Copyright 2010 Biomaterials [117]. (B) The fabrication process of ROS-scavenging SLNs which contain high density of thioether groups, incorporated with PEG-DSPE and lecthin self-assembly to form NPs, characterized as DLS an TEM. Copyright 2021 Applied Materials Today [121].
Unlike liposomes, solid lipid NPs (SLNs) or lipid NPs (LNPs) have only a single layer lipid external structure because the inner core is composed of a lipophilic substance, which is stabilized by a surfactant (emulsifier) [118,119]. Hari Prasad Joshi et al. [120] mixed carbon monoxide-releasing molecule-2 (CORM-2) palmityl alcohol, Tween 40, Span 40, and Myri S40 to prepare solid lipid NPs using a nanotemplate engineering technique. The intraperitoneal injection of CORM-2-SLNs for 8 consecutive days after SCI allowed the slow release of CO and prevented BSCB disruption and endothelial cell death. Zhang et al. [121] synthesized 69.8-nm lipid-polymer shell/core NPs composed of a poly[propargyl(methylthio)acetate-coethylene glycol di(β-mercaptopropionate)] (poly(PMT-co-EGDM)) core, which was prepared by simple thiol-yne click polymerization, and a protective lipid outer layer consisting of PEG-DSPE and lecithin (Fig. 15B). Because the structure contained a high density of thioether groups, the NPs could scavenge and eliminate ROS to exhibit antiapoptotic and anti-inflammatory roles in SCI therapy. At 28 days postinjury, the locomotor function was significantly improved in the NPs treated group, as assessed by the BBB scores.
Liposomes and SLNs are more advantageous for the long-cycle administration of different drugs and are also beneficial for gene transfection. This notion is exemplified by the transfection of the pras40 gene via the commercial cationic liposome N,N,N-Trimethyl-2-3-bis((1-oxo-9-octa-decenyl)oxy)-(Z,Z)-1-propanaminium methyl sulfate) (DOTAP) to protect motor neurons from death [122]. In addition, the vascular endothelial growth factor (VEGF) gene and plasmid pEGFP-GDNF cDNA was administered through dimethylaminoethane-carbamoyl-cholesterol liposomes to promote angiogenesis and axonal regeneration, respectively [123,124], and transcription factor interferon factor 5 (IRF5) was delivered by LNP to regulate the polarization rate of M1 or M2 macrophages [125]. All these findings provide new ideas for the future development of SCI treatments.
4.2.5. Polyelectrolyte complex NPs (PCNs) and nanoprotein capsules
The structure of PCNs is formed by electrostatic complexation between cationic and anionic polymers. Because the protein itself is negatively charged, it can combine with a cationic polymer to form NPs, thus effectively maintaining the activity of the core protein, providing a high protein load, and enhancing its functional properties. These NPs are called nanoprotein capsules. Polymers with polyelectronic properties are suitable for the design of these structures.
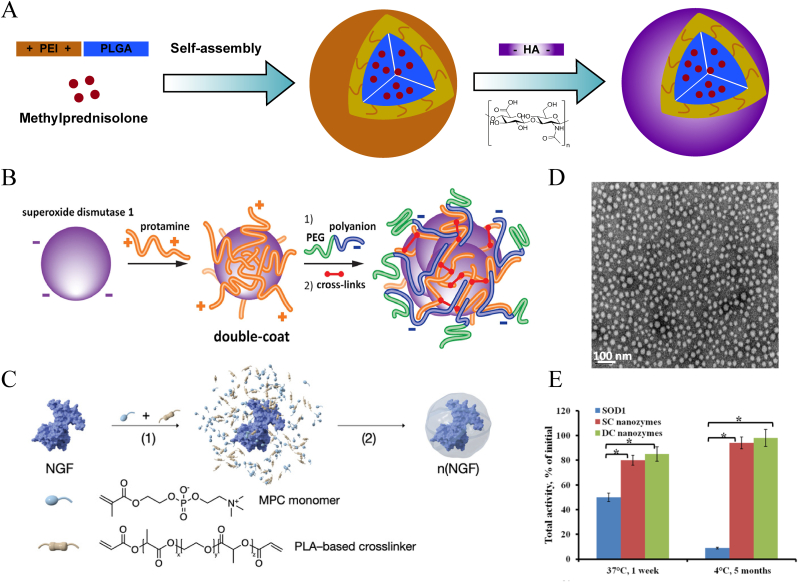
Youngnam Cho et al. [126] prepared chitosan NPs based on the interaction of cations and anions, which were formed at an equivalent mass ratio of chitosan to polyanionic dextran sulfate and sodium tripolyphosphate (TPP). These 100- to 200-nm diameter NPs effectively reduced reduce release of the intracellular lactate dehydrogenase after acrolein attack, restored the integrity of damaged neuronal membrane, and maintained Somatosensory Evoked Potential stability, improving the condition of adult guinea pig spinal cords after severe crushing/compression injuries. Similarly, So-Jung Gwak et al. [127] used the ionotropic gelation technique to mix dissolved chitosan, TPP and hyaluronic acid (HA) to form chitosan-TPP/HA NPs for DNA transfection. Compared to common polyethyleneimine NPs, the polymer carrier offered the advantages of lower cytotoxicity and higher transfection efficiency. Similar to PEI-PLGA polymers, these NPs have also been designed to encapsulate MP with HA coated on the outer layer by electrostatic interactions (Fig. 16A). Local administration improved the bioavailability of MP drugs, and HA was helpful in improving the internalization of NPs [128].
Fig. 16.
Schematic diagram of the structure of PCNs and nanoprotein capsules. (A) The synthesis process of the PCN by electrostatic interaction between cationic polymer PEI and anionic polymer HA. According to 2021 Journal of Nanomaterials [128]. (B) and (C) Showing that the negatively charged proteins of SOD1 or NGF entangled with polyelectrolyte polymers to form nanoprotein capsules. (D, E) The presentation of TEM of SOD1 nanozymes and their preservation for the protein activity. (B–E) Copright 2018 Journal of Controlled Release and 2019 Advanced Materials [19,129].
For nanoproteins, N.V. Nukolova et al. [19] made full use of the charge properties on the surface of enzyme protein. Specifically, cationic polymers (protamine or polyarginine cation) were used as a bridge to cross-link enzyme protein internally and mPEG113-block-poly(L-glutamic acid sodium salt)50 (PGlu-PEG) externally and finally formed a SOD1: polycation: polyanion complex (Fig. 16B). Due to electrostatic interactions, the multilayer polyionic structure was stable and effectively protected the core enzymes (Fig. 16D and E). The complex showed longer enzymatic activity in plasma of up to 1 week. Through the neutralization of ROS, the double-coated nanozymes significantly inhibited the inflammatory response and reduced the complications caused by postacute secondary injury processes. NGF and other neurotrophins are important for neural regeneration in SCI, but they are associated with unfavorable biodistribution and insufficient bioavailability upon systemic delivery. Lu et al. [129] completely utilized the electrostatic interactions by simply employing MPC, PLA-based crosslinker and NGF in situ polymerization to construct NPs, thus perfectly protecting the proteins (Fig. 16C). The ingenious doping of the MPC took full advantage of the basic characteristics of neural information transmission via nAChRs, thereby enhancing their penetration ability across the BSCB. This transportation method could provide sufficient NGF support for neural maturation and axonal network regeneration. At 21 days postinjury, the treatment group exhibited higher locomotor rating scale scores than that of the PBS group.
4.2.6. Other organic NPs
In addition to the relatively mainstream methods outlined above for constructing organic NPs, a number of rare and specific nanostructures are used to treat SCI, such as a nanogel structure composed of a space network that is synthesized by physical or chemical bonding, a nanoprodrug structure self-assembled from a polymer-pharmaceutical chemical connector, and ultrasound-mediated nanobubbles for fast drug release.
4.2.6.1. Nanogel
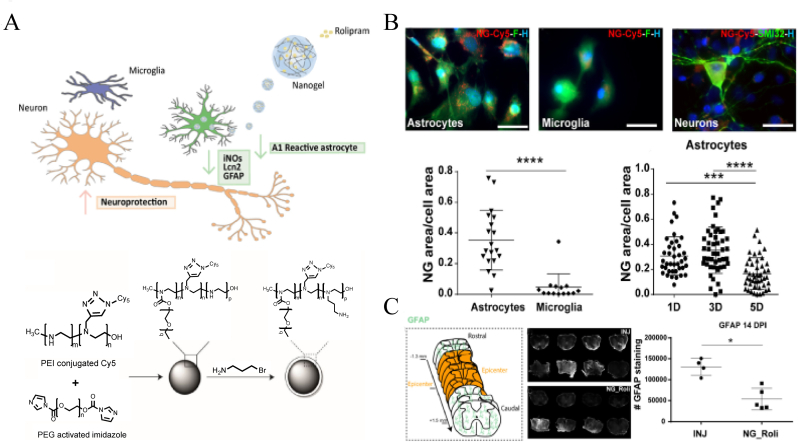
Papa et al. [130] utilized emulsification-evaporation to promote the cross-linking of PEG and PEI chains through interactions between imidazole and amine moieties, developing a novel nanogel structure (Fig. 17A). The nanovector inherited the 3D structure and swelling properties of the hydrogel with a hydrodynamic diameter ranging from 155 nm to 215 nm. They further [38] found that the nanogel loaded with Rolipram could be selectively taken up by activated astrocyte cells and effectively preserve neurons and reduce astrocytosis in vivo (Fig. 17B and C).
Fig. 17.
Schematic illustration of the structure of nanogel and its treatment in SCI. (A) Showing the preparation process of Rolipram loaded nanogel. (B) Showing that the nanogels were more likely to be uptaken by the astrocytes. (C) Spinal cord sections stained with anti-GFAP presented a significant reduction of hypertrophic astrocytes in the lesion site in the treated group. Copyright 2020 ACS Nano [130].
4.2.6.2. Nanoprodrug
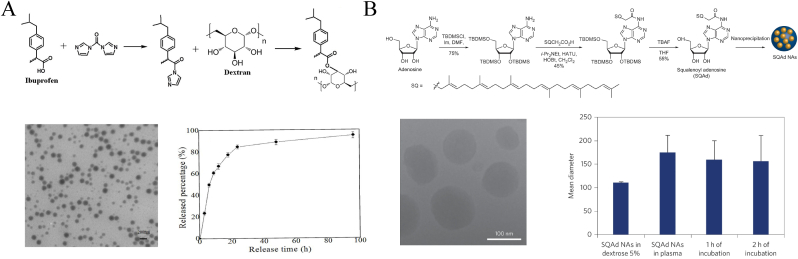
Nanoprodrugs are amphiphilic complexes formed by chemical bonding between polymers or other biological molecules and the drug, which are self-assembled to form micelle-like NPs. In a study by Yuan et al. [131], the polyphenolic compound quercetin was docked to HSA to fabricate a nanosphere complex of approximately 210.37 nm. The key amino acid residues of HSA, such as Ala-29, Leu-238, and His-242, exhibited a spontaneous binding ability to quercetin through hydrogen bonds and van der Waals interactions. This HSA-based nanocomplex demonstrated good dispersion and stable characteristics, suggesting a promising method for carrying bioactive molecules, such as phenolics, to exert antioxidant effects. Qi et al. [132] used ibuprofen to modify a dextran copolymer by a direct esterification reaction to synthesize an amphiphilic prodrug shell and further prepared MP-loaded NPs using the nanoprecipitation method. In this way, not only the controlled release of MP but also the synergetic therapeutic effects of the two drugs could be achieved (Fig. 18A). Alice Gaudin et al. [133] used “squalenoylation” technology to conjugate adenosine to lipid squalene and prepare SQAd NPs using a nanoprecipitation technique (Fig. 18B). The NPs effectively protected fragile adenosine from metabolization and extended its retention time in blood, thus significantly improving microcirculation at the lesion site after SCI and promoting the recovery of motor function in rats.
Fig. 18.
Schematic diagram of covalent attachment of polymers to clinical drugs to form nanoprodrug structures. (A) Illustration of the synthesis of buprofen modified dextran-based NPs and their drug-carrying capacity. Copyright 2017 Oncotarget [132]. (B) The synthesis process of SQAd NPs, their TEM characteristic image and long-term stability. Copyright 2014 Nature Nanotechnology [133].
4.2.6.3. Nanobubbles
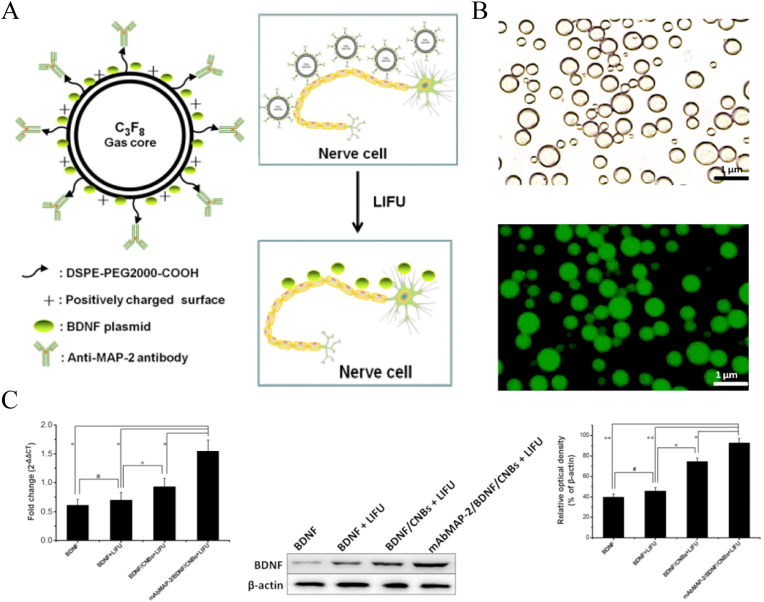
In recent years, the application of ultrasound-mediated microbubble destruction (UTMD) in gene transfection therapy has attracted more attention. Compared to viral vectors and other nonviral vectors, UTMD made the transfection system for gene therapy more stable and efficient [134,135]. Song et al. [49] prepared PLGA nanobubbles filled with perfluorocarbon gas to encapsulate an NGF plasmid using a double-emulsion process. After ultrasonic irradiation, NGF was significantly released from NGF/PLGA nanobubbles and highly expressed. In another study [42], researchers developed a cationic nanobubble based on DSPE-PEG2000-COOH and cholesterol with loading a brain-derived neurotrophic factor (BDNF) plasmid relying on the positively charged surfaces of the nanobubbles (Fig. 19A). Then, they conjugated microtubule-associated protein 2 antibody to the nanobubble to further increase its targeting ability to the membrane of neurons (Fig. 19B). After gene transfection, the mRNA level of BDNF expression in the treatment group was significantly higher than that in the other groups (Fig. 19C). And the treated rats also got better improvement of BBB scores.
Fig. 19.
Illustration of the structure of nanobubbles and the application in SCI treatment. (A) Showing targeted gene delivery nanobubble under LIFU irradiation. (B) The morphology of nanobubbles and their successful binding to microtubule-associated protein 2, assessed by CLSM. (C) Nanobubble delivery therapy via LIFU-mediation significantly increaseed the expression of BDNF in vivo. Copyright 2018 Biochemical and Biophysical Research Communications [42].
4.3. Bioderived nanomaterials
Organic polymer NPs have been used with biodegradable polymers to construct nanomaterials that can be adapted to the microenvironment of living organisms, thus improving the bioavailability of drugs in vivo. However, some issues in the application process, such as PEG immunogenicity problems after application for long periods, are noted. Therefore, it is necessary to develop nanomaterials that are more suitable for in vivo transition. Bioderived NPs (or biogenic NPs), such as exosomes and membrane-coated NPs, are derived from organic living cells themselves and have a high degree of nonimmunogenicity, making it easier for them to evade immune surveillance during systemic delivery. Bioderived NPs also carry natural proteins, RNA, and cell contents, which further promote information communication processes between cells, thus facilitating cell membrane fusion, improving delivery and transfection efficiency, and replenishing nutrients in the damaged area.
4.3.1. Exosomes and cell derived nanovesicles
Derived from biological cells, exosomes are small endosomal-originating membrane vesicles, 50–200 nm in diameter, secreted by most living cells [136]. Under physiological conditions, exosomes in the human body play a role in delivering biological information between cells [137,138]. Most exosomes are extracted from multipotent MSCs, such as those found in bone marrow, peripheral blood, umbilical cord blood, adipose tissue, and human skin. However, there are related research reports on the use of exosomes derived from macrophages, neuronal stem cells, pericytes, and other cells for SCI treatments. Previous studies have shown that extracellular vesicles, including exosomes play an important role in the development of secondary injury by transporting parent cell-specific signaling cargoes, such as signaling lipids, genetic information, cytokines, receptors, to change the function of receptor cells inside and outside the central nervous system [139]. As a cell-free therapeutic approach, extracellular vesicles inherit biocompatibility while reducing the uncertainty of stem cell differentiation compared to exosomes, which will be a potential for SCI therapeutic [140]. Table 2 lists the different cell-derived exosomes used for SCI treatment.
Table 2.
Unloaded exosomes for SCI treatment.
| Name | Year | Origin | Admin | Mechanisms | Reference |
|---|---|---|---|---|---|
| BMSC-Exons | 2021 | BMSC | IVa | Regulate TLR4/myd88/NF-κB signal pathway; reduce apoptosis and inflammation | [149] |
| MSC-exo | 2019 | BMSC | IV | Down-regulate the phosphorylated NFκBP65 subunit; I nhibit A1 astrocytes activation | [145] |
| MSCs-exosomes | 2017 | BMSC | IV | Anti-inflammatory, anti-apoptosis, promote angiogenesis | [144] |
| BMSC-Exons | 2019 | BMSC | IV | Inhibit inflammation, apoptosis, and scar formation promote axon | [40] |
| BMSC-Exons | 2020 | BMSC | IV | Promote autophagy; reduce neuronal apoptosis | [147] |
| BMSCs-Exo | 2019 | BMSC | IV | Inhibit the complement mRNA and the activation of NF-kb signaling pathway | [146] |
| BMSCs-Exos | 2019 | BMSC | IV | Activate Wnt/b-catenin signaling pathway Inhibit cell apoptosis and reduce tissue damage |
[148] |
| MSC-exosomes | 2021 | HPC | IV | Activate neural progenitor cells, promote regeneration | [41] |
| NSCs-Exons | 2020 | NSCs | IV | High expression of VEGF-A, improve spinal cord microvascular activity of endothelial cells (SCMEC) | [153] |
| HPC-exosomes | 2020 | HPC | IV | Promote angiogenesis | [151] |
| Exosomes | 2019 | Perictes | IV | Regulate endothelial cells, Protect bscb integrity, improve microcirculation |
[155] |
IV, intravenously injection.
Exosomes carry intracellular substances, including proteins, lipids, and nucleic acids, which enable them to play a unique role in the treatment of different diseases. However, exosomes are obtained at a low yield, and a significant number of cells is consumed. As cell generations increase, production is greatly reduced. Thus, researchers have tried to improve the technical methods to produce controllable cell derived nanovesicles [[141], [142], [143]]. For example, J. Park et al. [143] used appropriate centrifugation and filtration methods to generate large numbers of self-assembled spherical nanovesicles with a diameter of ∼100 nm. This generation process not only greatly increased the quantity but also improved the intracellular content concentrations in the vesicles compared to the conventional exosomes.
4.3.1.1. Therapeutic effects of various cell derived exosomes and nanovesicles
BMSCs are the most commonly applied stem cell lines used to produce exosomes as well as in the treatment of SCI. In 2017, Huang et al. [144] used BMSC exosomes to treat SCI in rats intravenously and found for the first time that the systemic administration of this type of exosome effectively attenuated inflammation and apoptosis and promoted angiogenesis after SCI. Liu et al. [40] also confirmed similar effects in their research. They also found that BMSC exosomes could inhibit scar formation and promote axon regeneration, and this process was related to the suppression of the activation of neurotoxic A1 astrocytes. Jia et al. [145] further studied the mechanism of the inhibition of A1 astrocyte activation by BMSC exosomes in SCI and found that the inhibitory effect was related to the downregulation of the phosphorylated NFκBP65 subunit. Zhao et al. [146] found that BMSC exosomes could effectively inhibit the activation of NF-κB signaling by binding to microglial cells and effectively reduce the synthesis and release of complement mRNA in SCI.
Scholars also studied how BMSC exosomes reduced cell apoptosis to improve SCI. Gu et al. [147] found that the inhibition of apoptosis was related to the activation of early autophagy by BMSC exosomes. However, Li et al. [148] and Fan et al. [149] confirmed that BMSC exosomes could effectively reduce neuronal cell apoptosis in SCI by regulating the Wnt/b-catenin and TLR4/MyD88/NF-κB signaling pathways, respectively. In addition, a study by Jia et al. [150] reported that the anti-apoptotic ability of BMSC-extracellular vesicles was related to the downregulation of the NF-κBp65 signaling pathway. After pathway downregulation, pericyte migration is effectively reduced, and the integrity of the BSCB is effectively maintained, thereby improving SCI.
In addition to common BMSC exosomes, exosomes derived from HPCs, human umbilical cord MSCs (HUCs), NSCs, and pericytes can be used to extract exosomes for SCI treatment. Zhang et al. [151] found that HPC exosomes applied to SCI in rats could promote angiogenesis at the injury site, thus improving neurologic function. Zhou et al. [41] also found that this type of exosome could activate the reactivation of endogenous neurogenesis and promote nerve regeneration. Pasquale Romaneli et al. [152] used HUC exosomes to conduct related studies and found that they exhibited anti-inflammatory and anti-scarring effects against SCI injury. Zhong et al. [153] found that NSC exosomes highly expressed VEGF-A, which could effectively mediate the angiogenic activity of spinal microvascular endothelial cells and facilitate spinal cord functional recovery. Rong et al. [154] confirmed that NSC derived small extracellular vesicles promoted the expression of the autophagy-related proteins LC3B and Beclin-1 when treating SCI in rats, which was beneficial for reducing the occurrence of nerve apoptosis. Pericytes play an important role in spinal cord microcirculation. Yuan et al. [155] used pericyte-derived exosomes to treat a rat SCI model and found that they could easily be taken up by endothelial cells. Experimental intravenous injections effectively improved microcirculation blood flow, protected the BSCB, and reduced apoptosis.
4.3.1.2. Delivery function of exosomes and cell derived nanovesicles
Exosomes and cell derived nanovesicles not only play a therapeutic role themselves but also make them good delivery vehicles given their natural biological advantages. As noted in Table 3, in the current research on SCI, exosomes and cell derived nanovesicles are primarily used for gene delivery. More recently, exosomes and cell derived nanovesicles have also been used as drug carriers. Li et al. [156] used BSMC exosomes to deliver the miR-544 repair gene and found that it reduced nerve cell apoptosis. Huang et al. [157] used the same method to deliver the miR-126 gene and found that it could effectively promote the regeneration of blood vessels and nerves after SCI, thereby inhibiting nerve cell apoptosis. Luo et al. [158] applied BMSC exosomes deliver high GIT1 gene expression levels to a rat model of SCI injury. The exosomes also had the ability to promote axonal regeneration and inhibit neuroinflammatory responses.
Table 3.
Exosomes and cell derived nanovesicles loaded with genes or drug for SCI treatment.
| Name |
Year |
Origin |
Administration |
Mechanism |
Reference |
|---|---|---|---|---|---|
| Gene-loaded | |||||
| ExoPTEN | 2019 | HBMSC | Intranasal | Delivery of sirna to inhibit PTEN expression; promote axon regeneration and angiogenesis | [20] |
| miR-126-loaded exosomes | 2019 | BMSC | IV | Promote angiogenesis and neuron regeneration, inhibit apoptosis | [157] |
| miR-21 and miR19b-loaded exosomes | 2019 | MSC | IV | Regulate PTEN, PDCD4 genes expression, inhibit neurons death | [159] |
| PTENP1-shRNA loaded exosomes | 2019 | PC12 | IV | Inhibit PTEN expression, promote mir-21 and mir-19 expression, and reduce neurons apoptosis | [161] |
| miR-544-loaded exosomes | 2020 | BMSC | IV | Deliver the therapeutic gene, reduce nerve cell apoptosis | [156] |
| miR-133b-loaded exosomes | 2018 | BMSC | IV | Activate ERK1/2, STAT3, and CREB signaling pathways Protect neurons, promote axon regeneration |
[165] |
| GIT1-loaded exosomes | 2021 | BMSC | IV | Promote axon regeneration, inhibit neuroinflammatory response, reduce apoptosis | [158] |
| ANGPTL3-loaded exosomes | 2021 | human urine stem cell (HUMSC) | Intrathecal | Regulate PI3K/AKT signaling pathway, promote angiogenesis | [164] |
| miR-181c loaded exosomes | 2021 | BMSC | IV | Inhibit PTEN and NF-kb pathways, reduce apoptosis | [176] |
| Exosomal miR-124-3p | 2020 | Neurons | IV | Regulate PI3K/AKT/NF-κB signaling cascade, Inhibit the activation of M1 microglial and A1 astrocytes |
[162] |
| miR-26a loaded exosomes | 2019 | BMSC | IV | Activate the PTEN/AKT/mtor signaling pathway, reduce glial scar formation, promote axon regeneration | [177] |
| miR-125a loaded exosomes | 2021 | BMSC | Intrathecal | Reduce the expression of IRF5; Enhance M2 polarization |
[167] |
| miR-199a-3p/145-5p loaded exosomes | 2021 | HUMSC | IV | Regulate NGF and trka signal pathways, promote nerve regeneration | [168] |
| miR-133b-modified exosomes | 2019 | Adipose-derived stem cells | IV | Promote the expression of mir-133b, NF, GAP-43, GFAP and MBP genes, promote axon regeneration | [166] |
| Drug-loaded | |||||
| Exos-Ber | 2021 | M2 macrophage | IV | Delivery of berberine, anti-inflammatory therapy, M2 macrophages derived targeting ability | [171] |
| Exo + Res | 2020 | Microglial | IV | Loaded with resveratrol, regulate P13K signaling pathway, activate autophagy, and inhibit cell apoptosis | [170] |
In many studies on exosomal gene delivery for SCI treatment, the PTEN gene is regarded as a key therapeutic target. Delivery therapy can directly or indirectly regulate the expression level of the PTEN gene or affect different PTEN gene signaling pathways, thereby exerting different therapeutic effects. Guo et al. [20] used human BMSC exosomes to encapsulate PTEN siRNA to construct ExoPTEN NPs, which were intranasally administered to treat SCI in rats (Fig. 20A). The results showed that the treatment effectively inhibited the expression of PTEN, promoted axonal regeneration and angiogenesis, and effectively improved the recovery of motor function in the rats. At 8 weeks postinjury, β–III–Tubulin level detection which stands for axonal regeneration was significantly increased in the NPs treated group compared to the untreated SCI rats. Meanwhile, the recovery of BBB locomotor scoring was in sharp contrast to that of the mean untreated transection rats. Xu et al. [159] fabricated extracellular vesicles derived from PC12 cells and human mesenchymal cells and loaded them with the miR-21/miR-19b therapeutic gene for SCI treatment. The combination of the delivery gene could guide neuronal cell differentiation and reduce neuronal cell apoptosis, exerting a therapeutic effect. PTENP1, a PTEN pseudogene, was reported to sponge both miR-21 and miR-19b, negatively regulating the effects of binding of these two genes with PTEN, thus inhibiting cell proliferation [160]. Wang et al. [161] used PC12 cell-derived exosomes to encapsulate PTENP1-shRNA for SCI in rats and found that it could remove the inhibitory effect of PTENP1, effectively enhance the expression of miR-21 and miR-19b, and subsequently promote nerve function repair.
Fig. 20.
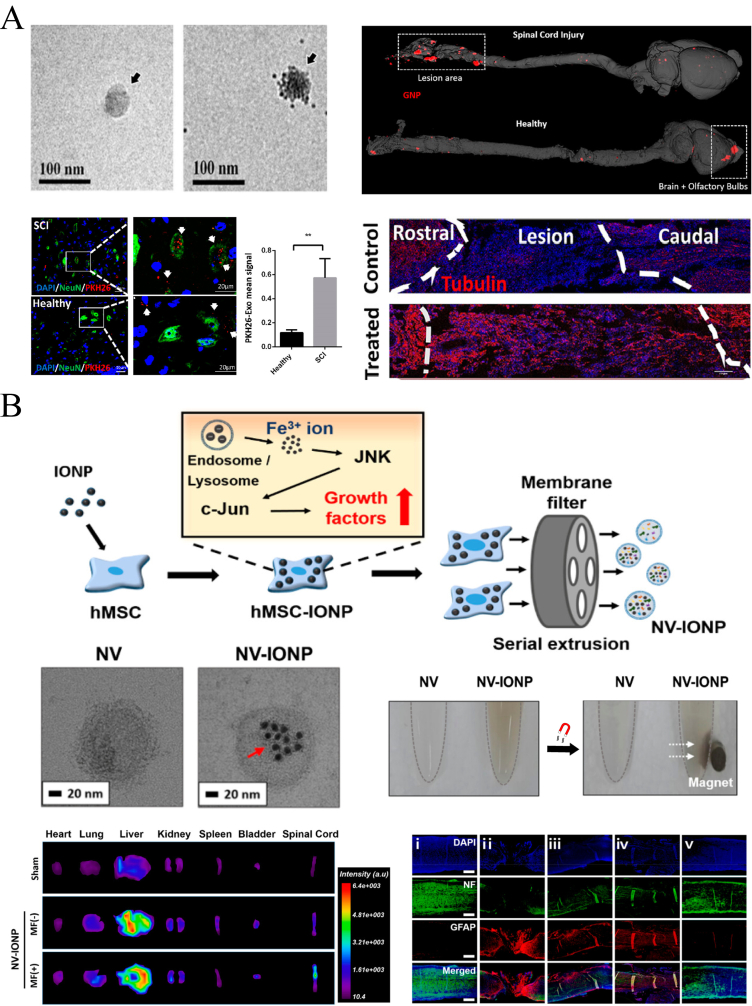
Schematic illustration of exosome and composite nanovesicle delivery for the treatment of SCI. (A) Showing TEM presentation of MSC exosomes and its targeting delivery for PTEN siRNA to the lesion site. Tubulin staining presented regenerative corticospinal tract fibers after exosomes therapy. Copyright 2019 ACS Nano [20]. (B) Showing the fabrication of composite nanovesicles with IONP and their targeting delivery ability by magnetic field. The therapeutic effects were reflected by NF and GFAP immunofluorescence stainings at 28 days after injury. Copyright 2018, Nano Letters [172].
In addition to the PTEN gene, the P13k gene and related pathways have also served as therapeutic targets. Jiang et al. [162] used neuron-derived exosomes to carry the miR-124-3p gene. The fabricated NPs inhibited the activation of M1 microglial cells and A1 astrocytes by regulating the PI3K/AKT/NF-κB signaling cascade, eventually promoting functional recovery following SCI in mice. The MSC exosomes modified by the related gene miR-124 have previously been shown to promote neurogenesis in traumatic brain injury [163]. Cao et al. [164] used human urine stem cells to construct ANGPTL3-loaded exosomes and applied them for SCI through local intrathecal injection. This delivery treatment effectively mediated the PI3K/AKT signaling pathways and promoted angiogenesis in the SCI model.
Other gene delivery systems and regulators of related signal transduction pathways should be mentioned. Li et al. [165] used BSMC exosomes to deliver the miR-133b gene and found that it could activate the ERK1/2, STAT3, and CREB signaling pathways, protecting neurons and promoting axonal regeneration. Ren et al. [166] found that adipose-derived stem cell-derived exosomes modified by miR-133b promoted the expression of NF, growth associated protein 43 (GAP-43), glial fibrillary acidic protein (GFAP), and myelin basic protein (MBP). Zhao et al. [167] recently found that BMSC-derived exosomal microRNA-125a downregulated IRF5 gene expression through gene delivery and promoted the activation of M2 macrophages, thereby reducing the inflammatory response in SCI. Wang et al. [168] reported that miR-199a-3p/145-5p highly expressed in exosomes derived from HUCs could mediate the NGF/TrkA signaling pathway in the treatment of SCI, promoting nerve differentiation and outgrowth. Another report suggested that the exosomes modified by miR-146a-5p could reduce the deleterious effects of neurotoxic astrocytes and protect neurological biofunctions after the injury [169].
Exosomes and cell derived nanovesicles also perform well in drug delivery. Fan et al. [170] used microglial cell-derived exosomes to encapsulate resveratrol, effectively increasing its solubility. The drug-loaded NPs inhibited apoptosis post-SCI by regulating the P13K signaling pathway to activate autophagy. Gao et al. [171] used M2 macrophage-derived exosomes to deliver berberine in SCI treatment and found that they exhibited good anti-inflammatory effects. In addition, exosomes derived from M2 macrophages increased the efficacy of targeted therapy.
Han Young Kim et al. [172] used human MSC derived nanovesicles with IONPs to construct composite nanovesicles, namely, NV-IONPs (Fig. 20B). Due to the introduction of IONPs, NPs with an average size of 150 nm exhibited magnet-guided targeting capability. In vivo delivery to treat SCI showed that external magnetic attraction could significantly increase the aggregation of NPs in the lesion. The experiment also found that after intervention with IONPs, the number of functional components, such as mRNA and proteins, carried in the nanovesicles was also significantly increased, indirectly improving the neuroprotective therapeutic and antiapoptotic effects. Thus, NV-IONPs represent a very promising treatment.
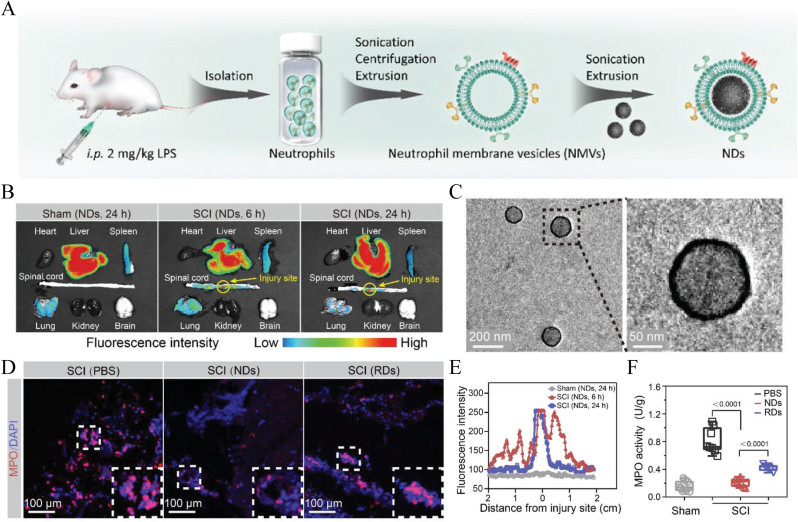
4.3.2. Membrane-coated NPs
Membrane-coated NPs are biomimetic NPs composed of biomembranes extracted from natural cells (red blood cells, white blood cells, cancer cells, stem cells, platelets, and bacterial cells) with encapsulated nanocores. This technique could be tailored as needed to design specific biohybrid nanosystems that incorporate the natural bioactivity of cells and the therapeutic properties of synthetic nanomaterials. The synthetic methods include extrusion and sonication. Presently, membrane-coated NPs have been used in a series of biomedical applications, such as in vivo drug delivery and tumor targeting, at a very early stage for SCI.
Bi et al. [173] were the first to take advantage of the property wherein neutrophils infiltrate inflamed areas and applied neutrophil membranes to encapsulate a polydopamine supporting core to fabricate biomimetic NPs using a sonication and extrusion method. Through ligand‒receptor binding, the NPs act as decoys to neutralize multiple types of chemokines, preventing neutrophil infiltration and inflammation progression in SCI. In addition, the protected polydopamine could also play a strong antioxidant and anti-inflammatory role (Fig. 21). By reprograming the microenvironment, demyelination and glial scar formation was effectively reduced,thus promoting neural regeneration. At 28 days postinjury, the neurological functional recovery of the treated group showed significant BBB improvement, compared with the PBS group. Similar to neutrophils, macrophages are also recruited during the inflammatory processes of secondary SCI. Tang et al. [174] found that macrophage membrane-camouflaged liposomes loaded with MC effectively accumulated at the trauma site after SCI. The targeting mechanism may be attributed to the interaction between integrin α4 and Mac-1 on macrophage membranes and vascular cell adhesion molecule-1 on impaired microvascular endothelium. In addition, this targeted aggregation ability was independent of the 3 different macrophage subtypes (M0, M1, and M2). Moreover, macrophage membranes can also be used to coat the exosome nucleus to fabricate macrophage membrane-fused exosome-mimetic nanovesicles (MF-NVs) [175]. Integrins α4 and β1 derived from the envelope membrane enhanced the binding ability and enabled the nanovesicles to effectively accumulate in the diseased site, representing another method to improve targeted delivery.
Fig. 21.
Schematic illustration depicts neutrophil membrane-coated NPs with anti-inflammatory and anti-oxidative properties as decoys to treat SCI. (A) Stimulating rats to extract neutrophils and the preparation process of neutrophil membrane-coated NPs, characterizated as TEM images (C). (B) Fluorescence images showed the targeting distribution of neutrophil membrane-coated NPs in SCI lesions. Intensity quantified as (E). And (D) exhibited a significant reduction of MPO + staining infiltration neutrophil cells, compared with other groups, quantitied as (F). Copyright 2021 Advanced Functional Materials [173].
5. Conclusions and prospects
SCI is currently one of the most complicated and intractable diseases in the world, causing great distress in people's lives. The current therapeutic strategies are fraught with complications and problems, which have increasingly aroused concern. Continued investigations of abundant synthetic NPs have shown great potential for clinical applications. Completely understanding the advantages and disadvantages of each nanomaterial will help us to better utilize and develop these nanomaterials for SCI treatment.
The preparation technology of inorganic NPs is more mature, and each batch production is more uniform and controllable. Although a number of studies have shown that inorganic NPs are biocompatible with neural cells in vitro, questions remain about their biosafety in vivo applications. Especially for metal NPs, such as Au, Fe and Ce, their properties can improve the negative pathological changes in SCI; however, the neurotoxicity caused by the deposition of these elements in the central nervous system remains a concern. Metal NPs are more suitable for the detection of disease, positioning, and imaging technology research. MSNs indeed serve as good drug carriers, providing stable protection for the core cargo. However, despite attempts by scholars to improve its availability through PEG modification, the use of MSNs in the clinic is still limited by how they can be efficiently degraded and metabolized in vivo. Go NPs and their derivatives are more suitable for constructing conductive materials, such as hydrogels, to repair SCI electrophysiological damage, and their size, surface charge, and layer numbers all have corresponding effects on biological systems. The toxicity in vivo remains to be further studied. In recent years, the discovery of selenium NPs has provided a new idea for the development of inorganic NPs in the future. As a trace element, selenium exhibits better biosafety and good antioxidant activity, making it suitable for the treatment of pathological changes in secondary injury. However, the dosage should be further standardized.
Organic NPs are a hot spot in the research of various types of nanomaterials, and they are also the nanomaterials with the greatest possibility for clinical translation in the short term. Some of these polymeric materials have even been approved for clinical use, such as PEG and PLGA. Organic NPs are synthesized artificially by chemical methods using degradable polymer materials. Compared with inorganic NPs, the biological safety is greatly improved, the raw materials are cheap, and the physicochemical properties are more controllable. Therefore, these nanomaterials are more likely to achieve biological engineering conversion in the short term. Micelles, liposomes and polymeric nanospheres account for the majority of studies, and a number of PEG- or PLGA-based nanomaterials have been approved by the FDA for marketed drugs. However, most of the current studies are repetitive and seek to improve the bioavailability of drugs through systematic passive targeting delivery or to develop new drugs as SCI therapies. We should focus on the design and optimization of active targeting in assembly design in future research to increase the accumulation of drugs at the lesion site. In addition, the relationship between biopolymers and different cell lines and molecules in the pathological process of SCI as well as the influence on immune regulation and treatment need to be further explored. Dendritic biomacromolecules can effectively package drugs into the structure and increase the loading rate, but the current delivery methods mostly rely on local administration. Their toxicity in systemic delivery needs to be further assessed. Nanoprodrugs are a useful method to protect delivered drugs by modifying them. However, compared to conventional delivery methods, how to release the drug effectively in the body and how to maintain the efficacy of the drug after covalent bonding are issues to be considered. Nanogel structures have not been widely used in the treatment of SCI. The question arises as to whether the sustained release properties of the gel itself are separate from the delivery requirements in the acute phase of SCI and whether the expansion process of the nanomaterials will cause further damage to the spinal cord. The nanobubble structure is also a novel structure, but the stability of this kind of bubble structure in the treatment of SCI in vivo remains to be further studied. Nanoprotein capsule studies are indeed very impressive, which may open up novel ideas for directions. We can make full use of key metabolic enzymes, cytokines, and neurotransmitters after SCI to assemble nanodrugs by simple electrostatic interactions and then perform in-depth research and treatment on the mechanisms and immunity. How to further improve the stability and targeting delivery capability of this nanomaterial in long-term drug delivery is a problem that needs to be solved.
Regarding bioderived NPs, one of the advantages is that the escape ability of MPS is further enhanced compared with the synthesized polymers. If the nanomaterials were derived from autologous cells, there would be no issue with immunogenicity. Such nanomaterials are more suitable for gene transfection studies due to their high loading and lack of safety risk of viral vectors. However, some problems are noted. As far as exosomes are concerned, their internal components are too complex, which will simultaneously bring nontherapeutic substances into the injured site, and the impact of these substances on SCI is uncertain. Furthermore, exosomes are mostly extracted from stem cells, and their differentiation in vivo is uncertain. The possibility of tumor cell transformation is noted, which is problematic. At present, research on biomembrane coating technology in SCI remains in the initial stage. The hybrid method of using a natural cell shell and artificial core will have great advantages in all nanomaterials. For example, making full use of the early infiltration of platelets and neutrophils into the lesion site, we may easily achieve active targeted delivery of drugs. We can also design different cell-membrane NPs to explore the role of different cells after injury by enhancing or reducing specific intercellular connections. This information will help us to further understand the pathological process of SCI and provide help for more effective treatment. However, autologous harvesting requires a complex process of cell extraction, a large amount of money, and specific storage technologies and equipment. The use of engineered cell extraction may cause immune rejection. The novel nanomaterial is currently far from clinical application.
Overall, although there are still many obstacles ahead, the future is bright. Utilizing a variety of ingredients, NPs made with advanced technological methods will be effective in the treatment of SCI, eventually providing a new treatment approach for paralyzed patients. A bold prediction is that the pathological process of SCI could potentially be reversed or even completely resolved through a simple intravenous nanodrug. It will be quite encouraging.
Credit author statement
Weiquan Gong: Conceptualization, Writing-original draft. Tianhui Zhang: Conceptualization, Funding acquisition. Mingxue Che: Writing-review & editing. Yongjie Wang: Conceptualization, Writing-review & editing. Chuanyu He: Writing-review & editing. Lidi Liu: Writing-review & editing. Zhenshan Lv: Writing-review & editing. Chunsheng Xiao: Conceptualization, Writing-review & editing, Supervision. Hao Wang: Conceptualization, Writing-review & editing, Supervision. Shaokun Zhang: Conceptualization, Funding acquisition, Writing review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We acknowledge the financial support from the National Natural Science Foundation of China (82272468 and 52103163). This study was supported by grant from the Department of Science and Technology of Jilin Province (grant number: 20190304028YY and 20200404103YY).
Contributor Information
Hao Wang, Email: wangh@ciac.ac.cn.
Shaokun Zhang, Email: shaokun@jlu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Quadri S.A., Farooqui M., Ikram A., Zafar A., Khan M.A., Suriya S.S., Claus C.F., Fiani B., Rahman M., Ramachandran A., Armstrong I.I.T., Taqi M.A., Mortazavi M.M. Recent update on basic mechanisms of spinal cord injury. Neurosurg. Rev. 2020;43(2):425–441. doi: 10.1007/s10143-018-1008-3. [DOI] [PubMed] [Google Scholar]
- 2.Berney S., Bragge P., Granger C., Opdam H., Denehy L. The acute respiratory management of cervical spinal cord injury in the first 6 weeks after injury: a systematic review. Spinal Cord. 2011;49(1):17–29. doi: 10.1038/sc.2010.39. [DOI] [PubMed] [Google Scholar]
- 3.Mu Z., Zhang Z. Risk factors for tracheostomy after traumatic cervical spinal cord injury. J. Orthop. Surg. 2019;27(3) doi: 10.1177/2309499019861809. [DOI] [PubMed] [Google Scholar]
- 4.Ahuja J.R.W. Christopher S., Satoshi Nori Kotter Mark R.N. Claudia druschel, armin curt & Michael G. Fehlings, traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.19. [DOI] [PubMed] [Google Scholar]
- 5.Bickenbach J. World report on disability. Lancet. 2011;377(9782) doi: 10.1016/s0140-6736(11)60844-1. 1977. [DOI] [PubMed] [Google Scholar]
- 6.Tyler J.Y., Xu X.M., Cheng J.X. Nanomedicine for treating spinal cord injury. Nanoscale. 2013;5(19):8821–8836. doi: 10.1039/c3nr00957b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin J.M., Bradke F. Therapeutic repair for spinal cord injury: combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020;12(3) doi: 10.15252/emmm.201911505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall E.D. Antioxidant therapies for acute spinal cord injury. Neurotherapeutics. 2011;8(2):152–167. doi: 10.1007/s13311-011-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahuja C.S., Wilson J.R., Nori S., Kotter M.R.N., Druschel C., Curt A., Fehlings M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P., Zhang Y., Ding X., Shen W., Li M., Wagner E., Xiao C., Chen X. A multistage cooperative nanoplatform enables intracellular Co-delivery of proteins and chemotherapeutics for cancer therapy. Adv. Mater. 2020;32(46) doi: 10.1002/adma.202000013. [DOI] [PubMed] [Google Scholar]
- 11.Sun S., Wang D., Yin R., Zhang P., Jiang R., Xiao C. A two-in-one nanoprodrug for photoacoustic imaging-guided enhanced sonodynamic therapy. Small. 2022;18(26) doi: 10.1002/smll.202202558. [DOI] [PubMed] [Google Scholar]
- 12.Shen W., Zhang Y., Wan P., An L., Zhang P., Xiao C., Chen X. Antineoplastic drug-free anticancer strategy enabled by host-defense-peptides-mimicking synthetic polypeptides. Adv. Mater. 2020;32(36) doi: 10.1002/adma.202001108. [DOI] [PubMed] [Google Scholar]
- 13.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., Habtemariam S., Shin H.S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd B.J., Galle A., Daglas M., Rosenfeld J.V., Medcalf R. Traumatic brain injury opens blood-brain barrier to stealth liposomes via an enhanced permeability and retention (EPR)-like effect. J. Drug Target. 2015;23(9):847–853. doi: 10.3109/1061186X.2015.1034280. [DOI] [PubMed] [Google Scholar]
- 15.Figley S.A., Khosravi R., Legasto J.M., Tseng Y.F., Fehlings M.G. Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J. Neurotrauma. 2014;31(6):541–552. doi: 10.1089/neu.2013.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bin S., Zhou N., Pan J., Pan F., Wu X.F., Zhou Z.H. Nano-carrier mediated co-delivery of methyl prednisolone and minocycline for improved post-traumatic spinal cord injury conditions in rats. Drug Dev. Ind. Pharm. 2017;43(6):1033–1041. doi: 10.1080/03639045.2017.1291669. [DOI] [PubMed] [Google Scholar]
- 17.Wang X.J., Shu G.F., Xu X.L., Peng C.H., Lu C.Y., Cheng X.Y., Luo X.C., Li J., Qi J., Kang X.Q., Jin F.Y., Chen M.J., Ying X.Y., You J., Du Y.Z., Ji J.S. Combinational protective therapy for spinal cord injury medicated by sialic acid-driven and polyethylene glycol based micelles. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119326. [DOI] [PubMed] [Google Scholar]
- 18.Wang X.J., Peng C.H., Zhang S., Xu X.L., Shu G.F., Qi J., Zhu Y.F., Xu D.M., Kang X.Q., Lu K.J., Jin F.Y., Yu R.S., Ying X.Y., You J., Du Y.Z., Ji J.S. Polysialic-acid-based micelles promote neural regeneration in spinal cord injury therapy. Nano Lett. 2019;19(2):829–838. doi: 10.1021/acs.nanolett.8b04020. [DOI] [PubMed] [Google Scholar]
- 19.Nukolova N.V., Aleksashkin A.D., Abakumova T.O., Morozova A.Y., Gubskiy I.L., Kirzhanova capital Ie C.A.C., Abakumov M.A., Chekhonin V.P., Klyachko N.L., Kabanov A.V. Multilayer polyion complex nanoformulations of superoxide dismutase 1 for acute spinal cord injury. J. Contr. Release. 2018;270:226–236. doi: 10.1016/j.jconrel.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 20.Guo S., Perets N., Betzer O., Ben-Shaul S., Sheinin A., Michaelevski I., Popovtzer R., Offen D., Levenberg S. Intranasal delivery of mesenchymal stem cell derived exosomes loaded with phosphatase and tensin homolog siRNA repairs complete spinal cord injury. ACS Nano. 2019;13(9):10015–10028. doi: 10.1021/acsnano.9b01892. [DOI] [PubMed] [Google Scholar]
- 21.Park J., Zhang Y., Saito E., Gurczynski S.J., Moore B.B., Cummings B.J., Anderson A.J., Shea L.D. Intravascular innate immune cells reprogrammed via intravenous nanoparticles to promote functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. U.S.A. 2019;116(30):14947–14954. doi: 10.1073/pnas.1820276116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tator C.H., Duncan E.G., Edmonds V.E., Lapczak L.I., Andrews D.F. Neurological recovery, mortality and length of stay after acute spinal cord injury associated with changes in management. Paraplegia. 1995;33(5):254–262. doi: 10.1038/sc.1995.58. [DOI] [PubMed] [Google Scholar]
- 23.Liu K., Yan L., Li R., Song Z., Ding J., Liu B., Chen X. 3D printed personalized nerve guide conduits for precision repair of peripheral nerve defects. Adv. Sci. 2022;9(12) doi: 10.1002/advs.202103875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Y., Xue F., Liu K., Li B., Fu C., Ding J. Physical and biological engineering of polymer scaffolds to potentiate repair of spinal cord injury. Mater. Des. 2021;201 doi: 10.1016/j.matdes.2021.109484. [DOI] [Google Scholar]
- 25.Zhang Q., Shi B., Ding J., Yan L., Thawani J.P., Fu C., Chen X. Polymer scaffolds facilitate spinal cord injury repair. Acta Biomater. 2019;88:57–77. doi: 10.1016/j.actbio.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 26.Ding J., Zhang J., Li J., Li D., Xiao C., Xiao H., Yang H., Zhuang X., Chen X. Electrospun polymer biomaterials. Prog. Polym. Sci. 2019;90:1–34. doi: 10.1016/j.progpolymsci.2019.01.002. [DOI] [Google Scholar]
- 27.He G., Ma L.L., Pan J., Venkatraman S. ABA and BAB type triblock copolymers of PEG and PLA: a comparative study of drug release properties and "stealth" particle characteristics. Int. J. Pharm. 2007;334(1–2):48–55. doi: 10.1016/j.ijpharm.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Allen T.M. Stealth liposomes: five years on. J. Liposome Res. 2008;2(3):289–305. doi: 10.3109/08982109209010210. [DOI] [Google Scholar]
- 29.Bazile D., Prud'homme C., Bassoullet M.T., Marlard M., Spenlehauer G., Veillard M., Stealth Me. PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J. Pharmacol. Sci. 1995;84(4):493–498. doi: 10.1002/jps.2600840420. [DOI] [PubMed] [Google Scholar]
- 30.Cho Y., Shi R., Borgens R.B. Chitosan produces potent neuroprotection and physiological recovery following traumatic spinal cord injury. J. Exp. Biol. 2010;213(Pt 9):1513–1520. doi: 10.1242/jeb.035162. [DOI] [PubMed] [Google Scholar]
- 31.Wang S.X., Lu Y.B., Wang X.X., Wang Y., Song Y.J., Wang X., Nyamgerelt M. Graphene and graphene-based materials in axonal repair of spinal cord injury. Neural Regen. Res. 2022;17(10):2117–2125. doi: 10.4103/1673-5374.335822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang B., Ding Z., Dong J., Lin F., Xue Z., Xu J. Macrophage-mediated degradable gelatin-coated mesoporous silica nanoparticles carrying pirfenidone for the treatment of rat spinal cord injury. Nanomedicine. 2021;37 doi: 10.1016/j.nano.2021.102420. [DOI] [PubMed] [Google Scholar]
- 33.Sun G., Yang S., Cai H., Shu Y., Han Q., Wang B., Li Z., Zhou L., Gao Q., Yin Z. Molybdenum disulfide nanoflowers mediated anti-inflammation macrophage modulation for spinal cord injury treatment. J. Colloid Interface Sci. 2019;549:50–62. doi: 10.1016/j.jcis.2019.04.047. [DOI] [PubMed] [Google Scholar]
- 34.Pal A., Singh A., Nag T.C., Chattopadhyay P., Mathur R., Jain S. Iron oxide nanoparticles and magnetic field exposure promote functional recovery by attenuating free radical-induced damage in rats with spinal cord transection. Int. J. Nanomed. 2013;8:2259–2272. doi: 10.2147/IJN.S44238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J.W., Mahapatra C., Hong J.Y., Kim M.S., Leong K.W., Kim H.W., Hyun J.K. Functional recovery of contused spinal cord in rat with the injection of optimal-dosed cerium oxide nanoparticles. Adv. Sci. 2017;4(10) doi: 10.1002/advs.201700034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao S., Lin Y., Du Y., He L., Huang G., Chen B., Chen T. Designing multifunctionalized selenium nanoparticles to reverse oxidative stress-induced spinal cord injury by attenuating ROS overproduction and mitochondria dysfunction. J. Mater. Chem. B. 2019;7(16):2648–2656. doi: 10.1039/c8tb02520g. [DOI] [PubMed] [Google Scholar]
- 37.Papastefanaki F., Jakovcevski I., Poulia N., Djogo N., Schulz F., Martinovic T., Ciric D., Loers G., Vossmeyer T., Weller H., Schachner M., Matsas R. Intraspinal delivery of polyethylene glycol-coated gold nanoparticles promotes functional recovery after spinal cord injury. Mol. Ther. 2015;23(6):993–1002. doi: 10.1038/mt.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papa S., Rossi F., Vismara I., Forloni G., Veglianese P. Nanovector-mediated drug delivery in spinal cord injury: a multitarget approach. ACS Chem. Neurosci. 2019;10(3):1173–1182. doi: 10.1021/acschemneuro.8b00700. [DOI] [PubMed] [Google Scholar]
- 39.Anderson M.A., Burda J.E., Ren Y., Ao Y., O'Shea T.M., Kawaguchi R., Coppola G., Khakh B.S., Deming T.J., Sofroniew M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532(7598):195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu W., Wang Y., Gong F., Rong Y., Luo Y., Tang P., Zhou Z., Zhou Z., Xu T., Jiang T., Yang S., Yin G., Chen J., Fan J., Cai W. Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J. Neurotrauma. 2019;36(3):469–484. doi: 10.1089/neu.2018.5835. [DOI] [PubMed] [Google Scholar]
- 41.Zhou W., Silva M., Feng C., Zhao S., Liu L., Li S., Zhong J., Zheng W. Exosomes derived from human placental mesenchymal stem cells enhanced the recovery of spinal cord injury by activating endogenous neurogenesis. Stem Cell Res. Ther. 2021;12(1):174. doi: 10.1186/s13287-021-02248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song Z., Ye Y., Zhang Z., Shen J., Hu Z., Wang Z., Zheng J. Noninvasive, targeted gene therapy for acute spinal cord injury using LIFU-mediated BDNF-loaded cationic nanobubble destruction. Biochem. Biophys. Res. Commun. 2018;496(3):911–920. doi: 10.1016/j.bbrc.2018.01.123. [DOI] [PubMed] [Google Scholar]
- 43.Maikos J.T., Shreiber D.I. Immediate damage to the blood-spinal cord barrier due to mechanical trauma. J. Neurotrauma. 2007;24(3):492–507. doi: 10.1089/neu.2006.0149. [DOI] [PubMed] [Google Scholar]
- 44.Saxena T., Loomis K.H., Pai S.B., Karumbaiah L., Gaupp E., Patil K., Patkar R., Bellamkonda R.V. Nanocarrier-mediated inhibition of macrophage migration inhibitory factor attenuates secondary injury after spinal cord injury. ACS Nano. 2015;9(2):1492–1505. doi: 10.1021/nn505980z. [DOI] [PubMed] [Google Scholar]
- 45.Shen K., Sun G., Chan L., He L., Li X., Yang S., Wang B., Zhang H., Huang J., Chang M., Li Z., Chen T. Anti-inflammatory nanotherapeutics by targeting matrix metalloproteinases for immunotherapy of spinal cord injury. Small. 2021;17(41) doi: 10.1002/smll.202102102. [DOI] [PubMed] [Google Scholar]
- 46.Wang J., Li D., Liang C., Wang C., Zhou X., Ying L., Tao Y., Xu H., Shu J., Huang X., Gong Z., Xia K., Li F., Chen Q., Tang J., Shen Y. Scar tissue-targeting polymer micelle for spinal cord injury treatment. Small. 2020;16(8) doi: 10.1002/smll.201906415. [DOI] [PubMed] [Google Scholar]
- 47.Wu J., Jiang H., Bi Q., Luo Q., Li J., Zhang Y., Chen Z., Li C. Apamin-mediated actively targeted drug delivery for treatment of spinal cord injury: more than just a concept. Mol. Pharm. 2014;11(9):3210–3222. doi: 10.1021/mp500393m. [DOI] [PubMed] [Google Scholar]
- 48.Gao S.J., Liu Y., Wang H.J., Ban D.X., Cheng S.Z., Ning G.Z., Wang L.L., Chang J., Feng S.Q. New approach to treating spinal cord injury using PEG-TAT-modified, cyclosporine-A-loaded PLGA/polymeric liposomes. J. Drug Target. 2017;25(1):75–82. doi: 10.1080/1061186x.2016.1191082. [DOI] [PubMed] [Google Scholar]
- 49.Song Z., Wang Z., Shen J., Xu S., Hu Z. Nerve growth factor delivery by ultrasound-mediated nanobubble destruction as a treatment for acute spinal cord injury in rats. Int. J. Nanomed. 2017;12:1717–1729. doi: 10.2147/ijn.S128848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faraday M., The Bakerian Lecture X. —experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. London, A. 1997;147:145–181. doi: 10.1098/rstl.1857.0011. [DOI] [Google Scholar]
- 51.Faulk W.P., Taylor G.M. An immunocolloid method for the electron microscope. Immunochemistry. 1971;8(11):1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- 52.Schulz F., Vossmeyer T., Bastús N.G., Weller H. Effect of the spacer structure on the stability of gold nanoparticles functionalized with monodentate thiolated poly(ethylene glycol) ligands. Langmuir. 2013;29(31):9897–9908. doi: 10.1021/la401956c. [DOI] [PubMed] [Google Scholar]
- 53.Lin S., Li D., Zhou Z., Xu C., Mei X., Tian H. Therapy of spinal cord injury by zinc modified gold nanoclusters via immune-suppressing strategies. J. Nanobiotechnol. 2021;19(1):281. doi: 10.1186/s12951-021-01035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heo D.N., Ko W.K., Moon H.J., Kim H.J., Lee S.J., Lee J.B., Bae M.S., Yi J.K., Hwang Y.S., Bang J.B., Kim E.C., Do S.H., Kwon I.K. Inhibition of osteoclast differentiation by gold nanoparticles functionalized with cyclodextrin curcumin complexes. ACS Nano. 2014;8(12):12049–12062. doi: 10.1021/nn504329u. [DOI] [PubMed] [Google Scholar]
- 55.Park C., Youn H., Kim H., Noh T., Kook Y.H., Oh E.T., Park H.J., Kim C. Cyclodextrin-covered gold nanoparticles for targeted delivery of an anti-cancer drug. J. Mater. Chem. 2009;19(16):2310–2315. doi: 10.1039/b816209c. [DOI] [Google Scholar]
- 56.Kim S.J., Ko W.-K., Heo D.N., Lee S.J., Lee D., Heo M., Han I.-B., Kwon I.K., Sohn S. Anti-neuroinflammatory gold nanocomplex loading ursodeoxycholic acid following spinal cord injury. Chem. Eng. J. 2019;375 doi: 10.1016/j.cej.2019.122088. [DOI] [Google Scholar]
- 57.Zhou Z., Li D., Fan X., Yuan Y., Wang H., Wang D., Mei X. Gold nanoclusters conjugated berberine reduce inflammation and alleviate neuronal apoptosis by mediating M2 polarization for spinal cord injury repair. Regen. Biomater. 2022;9 doi: 10.1093/rb/rbab072. rbab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharifi M., Rezayat S.M., Akhtari K., Hasan A., Falahati M. Fabrication and evaluation of anti-cancer efficacy of lactoferrin-coated maghemite and magnetite nanoparticles. J. Biomol. Struct. Dyn. 2020;38(10):2945–2954. doi: 10.1080/07391102.2019.1650114. [DOI] [PubMed] [Google Scholar]
- 59.Khan S., Sharifi M., Hasan A., Attar F., Edis Z., Bai Q., Derakhshankhah H., Falahati M. Magnetic nanocatalysts as multifunctional platforms in cancer therapy through the synthesis of anticancer drugs and facilitated Fenton reaction. J. Adv. Res. 2021;30:171–184. doi: 10.1016/j.jare.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plank C. Nanomedicine: silence the target. Nat. Nanotechnol. 2009;4(9):544–545. doi: 10.1038/nnano.2009.251. [DOI] [PubMed] [Google Scholar]
- 61.Tukmachev D., Lunov O., Zablotskii V., Dejneka A., Babic M., Sykova E., Kubinova S. An effective strategy of magnetic stem cell delivery for spinal cord injury therapy. Nanoscale. 2015;7(9):3954–3958. doi: 10.1039/c4nr05791k. [DOI] [PubMed] [Google Scholar]
- 62.Vishwakarma S.K., Bardia A., Paspala S.A., Khan A.A. Magnetic nanoparticle tagged stem cell transplantation in spinal cord injury: a promising approach for targeted homing of cells at the lesion site. Neurol. India. 2015;63(3):460–461. doi: 10.4103/0028-3886.158294. [DOI] [PubMed] [Google Scholar]
- 63.Cho H., Choi Y.K., Lee D.H., Park H.J., Seo Y.K., Jung H., Kim S.C., Kim S.M., Park J.K. Effects of magnetic nanoparticle-incorporated human bone marrow-derived mesenchymal stem cells exposed to pulsed electromagnetic fields on injured rat spinal cord. Biotechnol. Appl. Biochem. 2013;60(6):596–602. doi: 10.1002/bab.1109. [DOI] [PubMed] [Google Scholar]
- 64.Jenkins S.I., Pickard M.R., Granger N., Chari D.M. Magnetic nanoparticle-mediated gene transfer to oligodendrocyte precursor cell transplant populations is enhanced by magnetofection strategies. ACS Nano. 2011;5(8):6527–6538. doi: 10.1021/nn2018717. [DOI] [PubMed] [Google Scholar]
- 65.Wang J., Xie T., Long X., Gao R., Kang L., Wang Q., Jiang J., Ye L., Lyu J. The effect of tacrolimus-containing polyethylene glycol-modified maghemite nanospheres on reducing oxidative stress and accelerating the healing spinal cord injury of rats based on increasing M2 macrophages. Arab. J. Chem. 2022;15(1) doi: 10.1016/j.arabjc.2021.103534. [DOI] [Google Scholar]
- 66.Yadav N., Singh S. Polyoxometalate-mediated vacancy-engineered cerium oxide nanoparticles exhibiting controlled biological enzyme-mimicking activities. Inorg. Chem. 2021;60(10):7475–7489. doi: 10.1021/acs.inorgchem.1c00766. [DOI] [PubMed] [Google Scholar]
- 67.Das M., Patil S., Bhargava N., Kang J.F., Riedel L.M., Seal S., Hickman J.J. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28(10):1918–1925. doi: 10.1016/j.biomaterials.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang X., Song H. Synthesis of cerium oxide nanoparticles loaded on chitosan for enhanced auto-catalytic regenerative ability and biocompatibility for the spinal cord injury repair. J. Photochem. Photobiol., B. 2019;191:83–87. doi: 10.1016/j.jphotobiol.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 69.Dong L., Kang X., Ma Q., Xu Z., Sun H., Hao D., Chen X. Novel approach for efficient recovery for spinal cord injury repair via biofabricated nano-cerium oxide loaded PCL with resveratrol to improve in vitro biocompatibility and autorecovery abilities. Dose Response. 2020;18(3) doi: 10.1177/1559325820933518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uguz A.C., Naziroglu M. Effects of selenium on calcium signaling and apoptosis in rat dorsal root ganglion neurons induced by oxidative stress. Neurochem. Res. 2012;37(8):1631–1638. doi: 10.1007/s11064-012-0758-5. [DOI] [PubMed] [Google Scholar]
- 71.Wirth E.K., Conrad M., Winterer J., Wozny C., Carlson B.A., Roth S., Schmitz D., Bornkamm G.W., Coppola V., Tessarollo L., Schomburg L., Kohrle J., Hatfield D.L., Schweizer U. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. Faseb. J. 2010;24(3):844–852. doi: 10.1096/fj.09-143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naziroglu M. Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem. Res. 2009;34(12):2181–2191. doi: 10.1007/s11064-009-0015-8. [DOI] [PubMed] [Google Scholar]
- 73.Schweizer U., Brauer A.U., Kohrle J., Nitsch R., Savaskan N.E. Selenium and brain function: a poorly recognized liaison. Brain Res. Rev. 2004;45(3):164–178. doi: 10.1016/j.brainresrev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Luo W., Wang Y., Lin F., Liu Y., Gu R., Liu W., Xiao C. Selenium-doped carbon quantum dots efficiently ameliorate secondary spinal cord injury via scavenging reactive oxygen species. Int. J. Nanomed. 2020;15:10113–10125. doi: 10.2147/IJN.S282985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li F., Li T., Sun C., Xia J., Jiao Y., Xu H. Selenium-doped carbon quantum dots for free-radical scavenging. Angew. Chem., Int. Ed. Engl. 2017;56(33):9910–9914. doi: 10.1002/anie.201705989. [DOI] [PubMed] [Google Scholar]
- 76.Javdani M., Habibi A., Shirian S., Kojouri G.A., Hosseini F. Effect of selenium nanoparticle supplementation on tissue inflammation, blood cell count, and IGF-1 levels in spinal cord injury-induced rats. Biol. Trace Elem. Res. 2019;187(1):202–211. doi: 10.1007/s12011-018-1371-5. [DOI] [PubMed] [Google Scholar]
- 77.Qhobosheane M., Santra S., Zhang P., Tan W. Biochemically functionalized silica nanoparticles. Analyst. 2001;126(8):1274–1278. doi: 10.1039/b101489g. [DOI] [PubMed] [Google Scholar]
- 78.Slowing I.I., Trewyn B.G., Giri S., Lin V.S.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007;17(8):1225–1236. doi: 10.1002/adfm.200601191. [DOI] [Google Scholar]
- 79.Cho Y., Shi R., Borgens R., Ivanisevic A. Repairing the damaged spinal cord and brain with nanomedicine. Small. 2008;4(10):1676–1681. doi: 10.1002/smll.200800838. [DOI] [PubMed] [Google Scholar]
- 80.Cho Y., Shi R., Ivanisevic A., Borgens R.B. Functional silica nanoparticle-mediated neuronal membrane sealing following traumatic spinal cord injury. J. Neurosci. Res. 2010;88(7):1433–1444. doi: 10.1002/jnr.22309. [DOI] [PubMed] [Google Scholar]
- 81.Cho Y., Shi R., Borgens R.B., Ivanisevic A. Functionalized mesoporous silica nanoparticle-based drug delivery system to rescue acrolein-mediated cell death. Nanomedicine. 2008;3(4):507–519. doi: 10.2217/17435889.3.4.507. [DOI] [PubMed] [Google Scholar]
- 82.Rezaei S., Dabirmanesh B., Zare L., Golestani A., Javan M., Khajeh K. Enhancing myelin repair in experimental model of multiple sclerosis using immobilized chondroitinase ABC I on porous silicon nanoparticles. Int. J. Biol. Macromol. 2020;146:162–170. doi: 10.1016/j.ijbiomac.2019.12.258. [DOI] [PubMed] [Google Scholar]
- 83.Sato N., Shimada M., Nakajima H., Oda H., Kimura S. Cloning and expression in Escherichia coli of the gene encoding the Proteus vulgaris chondroitin ABC lyase. Appl. Microbiol. Biotechnol. 1994;41(1):39–46. doi: 10.1007/BF00166079. [DOI] [PubMed] [Google Scholar]
- 84.Prabhakar V., Capila I., Soundararajan V., Raman R., Sasisekharan R. Recombinant expression, purification, and biochemical characterization of chondroitinase ABC II from Proteus vulgaris. J. Biol. Chem. 2009;284(2):974–982. doi: 10.1074/jbc.M806630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang W., Huang X., Zhang Y., Deng G., Liu X., Fan C., Xi Y., Yu J., Ye X. Se@SiO2 nanocomposites suppress microglia-mediated reactive oxygen species during spinal cord injury in rats. RSC Adv. 2018;8(29):16126–16138. doi: 10.1039/c8ra01906a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y., Wang Y., Xiao B., Tang G., Yu J., Wang W., Xu G., Ye X. pH-responsive delivery of H2 through ammonia borane-loaded mesoporous silica nanoparticles improves recovery after spinal cord injury by moderating oxidative stress and regulating microglial polarization. Regener. Biomater. 2021;8(6) doi: 10.1093/rb/rbab058. rbab058. [DOI] [Google Scholar]
- 87.Solanki A., Chueng S.T., Yin P.T., Kappera R., Chhowalla M., Lee K.B. Axonal alignment and enhanced neuronal differentiation of neural stem cells on graphene-nanoparticle hybrid structures. Adv. Mater. 2013;25(38):5477–5482. doi: 10.1002/adma.201302219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin L., Zhuang X., Huang R., Song S., Wang Z., Wang S., Cheng L., Zhu R. Size-dependent effects of suspended graphene oxide nanoparticles on the cellular fate of mouse neural stem cells. Int. J. Nanomed. 2020;15:1421–1435. doi: 10.2147/ijn.S225722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yari-Ilkhchi A., Ebrahimi-Kalan A., Farhoudi M., Mahkam M. Design of graphenic nanocomposites containing chitosan and polyethylene glycol for spinal cord injury improvement. RSC Adv. 2021;11(33):19992–20002. doi: 10.1039/d1ra00861g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stout D.A. Recent advancements in carbon nanofiber and carbon nanotube applications in drug delivery and tissue engineering. Curr. Pharmaceut. Des. 2015;21(15):2037–2044. doi: 10.2174/1381612821666150302153406. [DOI] [PubMed] [Google Scholar]
- 91.Kim C.Y., Sikkema W.K.A., Kim J., Kim J.A., Walter J., Dieter R., Chung H.M., Mana A., Tour J.M., Canavero S. Effect of Graphene Nanoribbons (TexasPEG) on locomotor function recovery in a rat model of lumbar spinal cord transection. Neural Regen. Res. 2018;13(8):1440–1446. doi: 10.4103/1673-5374.235301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi Y., Kim S., Huff T.B., Borgens R.B., Park K., Shi R., Cheng J.X. Effective repair of traumatically injured spinal cord by nanoscale block copolymer micelles. Nat. Nanotechnol. 2010;5(1):80–87. doi: 10.1038/nnano.2009.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng C.-X., Zhao Y., Liu Y. Recent advances in self-assembled nano-therapeutics. Chin. J. Polym. Sci. 2017;36(3):322–346. doi: 10.1007/s10118-018-2078-y. [DOI] [Google Scholar]
- 94.Wang Y., Wu M., Gu L., Li X., He J., Zhou L., Tong A., Shi J., Zhu H., Xu J., Guo G. Effective improvement of the neuroprotective activity after spinal cord injury by synergistic effect of glucocorticoid with biodegradable amphipathic nanomicelles. Drug Deliv. 2017;24(1):391–401. doi: 10.1080/10717544.2016.1256003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li J., Deng J., Yuan J., Fu J., Li X., Tong A., Wang Y., Chen Y., Guo G. Zonisamide-loaded triblock copolymer nanomicelles as a novel drug delivery system for the treatment of acute spinal cord injury. Int. J. Nanomed. 2017;12:2443–2456. doi: 10.2147/IJN.S128705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang Y., Zhang L., Huang M., Sui R., Khan S. Reconstruction of the cervical spinal cord based on motor function restoration and mitigation of oxidative stress and inflammation through eNOS/Nrf2 signaling pathway using ibuprofen-loaded nanomicelles. Arab. J. Chem. 2021;14(8) doi: 10.1016/j.arabjc.2021.103289. [DOI] [Google Scholar]
- 97.Papa S., Rossi F., Ferrari R., Mariani A., De Paola M., Caron I., Fiordaliso F., Bisighini C., Sammali E., Colombo C., Gobbi M., Canovi M., Lucchetti J., Peviani M., Morbidelli M., Forloni G., Perale G., Moscatelli D., Veglianese P. Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury. ACS Nano. 2013;7(11):9881–9895. doi: 10.1021/nn4036014. [DOI] [PubMed] [Google Scholar]
- 98.Papa S., Caron I., Erba E., Panini N., De Paola M., Mariani A., Colombo C., Ferrari R., Pozzer D., Zanier E.R., Pischiutta F., Lucchetti J., Bassi A., Valentini G., Simonutti G., Rossi F., Moscatelli D., Forloni G., Veglianese P. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials. 2016;75:13–24. doi: 10.1016/j.biomaterials.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 99.Shechter R., Miller O., Yovel G., Rosenzweig N., London A., Ruckh J., Kim K.W., Klein E., Kalchenko V., Bendel P., Lira S.A., Jung S., Schwartz M. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38(3):555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Astete C.E., Sabliov C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006;17(3):247–289. doi: 10.1163/156856206775997322. [DOI] [PubMed] [Google Scholar]
- 101.Jubeli E., Moine L., Vergnaud-Gauduchon J., Barratt G. E-selectin as a target for drug delivery and molecular imaging. J. Contr. Release. 2012;158(2):194–206. doi: 10.1016/j.jconrel.2011.09.084. [DOI] [PubMed] [Google Scholar]
- 102.Ghosh M., Tuesta L.M., Puentes R., Patel S., Melendez K., El Maarouf A., Rutishauser U., Pearse D.D. Extensive cell migration, axon regeneration, and improved function with polysialic acid-modified Schwann cells after spinal cord injury. Glia. 2012;60(6):979–992. doi: 10.1002/glia.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.El Maarouf A., Petridis A.K., Rutishauser U. Use of polysialic acid in repair of the central nervous system. Proc. Natl. Acad. Sci. U. S. A. 2006;103(45):16989–16994. doi: 10.1073/pnas.0608036103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu W., Lee S.Y., Wu X., Tyler J.Y., Wang H., Ouyang Z., Park K., Xu X.M., Cheng J.X. Neuroprotective ferulic acid (FA)-glycol chitosan (GC) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials. 2014;35(7):2355–2364. doi: 10.1016/j.biomaterials.2013.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li T., Jing P., Yang L., Wan Y., Du X., Wei J., Zhou M., Liu Z., Lin Y., Zhong Z. CAQK modification enhances the targeted accumulation of metformin-loaded nanoparticles in rats with spinal cord injury. Nanomedicine. 2022;41 doi: 10.1016/j.nano.2022.102526. [DOI] [PubMed] [Google Scholar]
- 106.Zielińska A., Carreiró F., Oliveira A.M., Neves A., Pires B., Venkatesh D.N., Durazzo A., Lucarini M., Eder P., Silva A.M., Santini A., Souto E.B. Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules. 2020;25(16) doi: 10.3390/molecules25163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim Y.T., Caldwell J.M., Bellamkonda R.V. Nanoparticle-mediated local delivery of Methylprednisolone after spinal cord injury. Biomaterials. 2009;30(13):2582–2590. doi: 10.1016/j.biomaterials.2008.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chvatal S.A., Kim Y.T., Bratt-Leal A.M., Lee H., Bellamkonda R.V. Spatial distribution and acute anti-inflammatory effects of Methylprednisolone after sustained local delivery to the contused spinal cord. Biomaterials. 2008;29(12):1967–1975. doi: 10.1016/j.biomaterials.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andrabi S.S., Yang J., Gao Y., Kuang Y., Labhasetwar V. Nanoparticles with antioxidant enzymes protect injured spinal cord from neuronal cell apoptosis by attenuating mitochondrial dysfunction. J. Contr. Release. 2020;317:300–311. doi: 10.1016/j.jconrel.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haque A., Drasites K.P., Cox A., Capone M., Myatich A.I., Shams R., Matzelle D., Garner D.P., Bredikhin M., Shields D.C., Vertegel A., Banik N.L. Protective effects of estrogen via nanoparticle delivery to attenuate myelin loss and neuronal death after spinal cord injury. Neurochem. Res. 2021;46(11):2979–2990. doi: 10.1007/s11064-021-03401-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ren H., Han M., Zhou J., Zheng Z.F., Lu P., Wang J.J., Wang J.Q., Mao Q.J., Gao J.Q., Ouyang H.W. Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials. 2014;35(24):6585–6594. doi: 10.1016/j.biomaterials.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 112.Buhleier E., Wehner W., Vögtle F. Cascade"- and "Nonskid-Chain-like. Syntheses of Molecular Cavity Topologies, Synthesis. 1978;32:155–158. doi: 10.1055/s-1978-24702. 02. [DOI] [Google Scholar]
- 113.Oliveira J.M., Salgado A.J., Sousa N., Mano J.F., Reis R.L. Dendrimers and derivatives as a potential therapeutic tool in regenerative medicine strategies—a review. Prog. Polym. Sci. 2010;35(9):1163–1194. doi: 10.1016/j.progpolymsci.2010.04.006. [DOI] [Google Scholar]
- 114.Cerqueira S.R., Oliveira J.M., Silva N.A., Leite-Almeida H., Ribeiro-Samy S., Almeida A., Mano J.F., Sousa N., Salgado A.J., Reis R.L. Microglia response and in vivo therapeutic potential of methylprednisolone-loaded dendrimer nanoparticles in spinal cord injury. Small. 2013;9(5):738–749. doi: 10.1002/smll.201201888. [DOI] [PubMed] [Google Scholar]
- 115.Torchilin V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006;58(14):1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 116.Iannotti C.A., Clark M., Horn K.P., van Rooijen N., Silver J., Steinmetz M.P. A combination immunomodulatory treatment promotes neuroprotection and locomotor recovery after contusion SCI. Exp. Neurol. 2011;230(1):3–15. doi: 10.1016/j.expneurol.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 117.Wang H., Zhang S., Liao Z., Wang C., Liu Y., Feng S., Jiang X., Chang J. PEGlated magnetic polymeric liposome anchored with TAT for delivery of drugs across the blood-spinal cord barrier. Biomaterials. 2010;31(25):6589–6596. doi: 10.1016/j.biomaterials.2010.04.057. [DOI] [PubMed] [Google Scholar]
- 118.Mehnert W., Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Deliv. Rev. 2001;47(2–3):165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 119.Saupe A., Gordon K.C., Rades T. Structural investigations on nanoemulsions, solid lipid nanoparticles and nanostructured lipid carriers by cryo-field emission scanning electron microscopy and Raman spectroscopy. Int. J. Pharm. 2006;314(1):56–62. doi: 10.1016/j.ijpharm.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 120.Joshi H.P., Kumar H., Choi U.Y., Lim Y.C., Choi H., Kim J., Kyung J.W., Sohn S., Kim K.T., Kim J.K., Han I.B. CORM-2-Solid lipid nanoparticles maintain integrity of blood-spinal cord barrier after spinal cord injury in rats. Mol. Neurobiol. 2020;57(6):2671–2689. doi: 10.1007/s12035-020-01914-5. [DOI] [PubMed] [Google Scholar]
- 121.Zhang T., Lin F., Liu W., Liu Y., Guo Z., Xiao C., Zhuang X., Chen X. Reactive oxide species-scavenging lipid-polymer nanoparticles for neuroprotection after spinal cord injury. Appl. Mater. Today. 2021;24 doi: 10.1016/j.apmt.2021.101109. [DOI] [Google Scholar]
- 122.Yu F., Narasimhan P., Saito A., Liu J., Chan P.H. Increased expression of a proline-rich Akt substrate (PRAS40) in human copper/zinc-superoxide dismutase transgenic rats protects motor neurons from death after spinal cord injury. J. Cerebr. Blood Flow Metabol. 2008;28(1):44–52. doi: 10.1038/sj.jcbfm.9600501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu K.W., Chen Z.Y., Jin D.D., Hou T.S., Cao L., Fu Q. Cationic liposome-mediated GDNF gene transfer after spinal cord injury. J. Neurotrauma. 2002;19(9):1081–1090. doi: 10.1089/089771502760341983. [DOI] [PubMed] [Google Scholar]
- 124.Gwak S.J., Yun Y., Yoon D.H., Kim K.N., Ha Y. Therapeutic use of 3beta-[N-(N',N'-Dimethylaminoethane) carbamoyl] cholesterol-modified PLGA nanospheres as gene delivery vehicles for spinal cord injury. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li J., Liu Y., Xu H., Fu Q. Nanoparticle-delivered IRF5 siRNA facilitates M1 to M2 transition, reduces demyelination and neurofilament loss, and promotes functional recovery after spinal cord injury in mice. Inflammation. 2016;39(5):1704–1717. doi: 10.1007/s10753-016-0405-4. [DOI] [PubMed] [Google Scholar]
- 126.Chen B., Bohnert D., Borgens R.B., Cho Y. Pushing the science forward: chitosan nanoparticles and functional repair of CNS tissue after spinal cord injury. J. Biol. Eng. 2013;7:15. doi: 10.1186/1754-1611-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gwak S.J., Jung J.K., An S.S., Kim H.J., Oh J.S., Pennant W.A., Lee H.Y., Kong M.H., Kim K.N., Yoon D.H., Ha Y. Chitosan/TPP-hyaluronic acid nanoparticles: a new vehicle for gene delivery to the spinal cord. J. Biomater. Sci. Polym. Ed. 2012;23(11):1437–1450. doi: 10.1163/092050611X584090. [DOI] [PubMed] [Google Scholar]
- 128.Chen C., Sun X., Yang Q., Ma X., Vassallo A. Hyaluronic acid-coated nanoparticles for the localized delivery of methylprednisolone to the injured spinal cord. J. Nanomater. 2021;2021 doi: 10.1155/2021/5358046. [DOI] [Google Scholar]
- 129.Xu D., Wu D., Qin M., Nih L.R., Liu C., Cao Z., Ren J., Chen X., He Z., Yu W., Guan J., Duan S., Liu F., Liu X., Li J., Harley D., Xu B., Hou L., Chen I.S.Y., Wen J., Chen W., Pourtaheri S., Lu Y. Efficient delivery of nerve growth factors to the central nervous system for neural regeneration. Adv. Mater. 2019;31(33) doi: 10.1002/adma.201900727. [DOI] [PubMed] [Google Scholar]
- 130.Papa S., Veneruso V., Mauri E., Cremonesi G., Mingaj X., Mariani A., De Paola M., Rossetti A., Sacchetti A., Rossi F., Forloni G., Veglianese P. Functionalized nanogel for treating activated astrocytes in spinal cord injury. J. Contr. Release. 2021;330:218–228. doi: 10.1016/j.jconrel.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 131.Yuan F., Wang P., Yang Y., Shi P., Cheng L. Quercetin-albumin nano-complex as an antioxidant agent against hydrogen peroxide-induced death of spinal cord neurons as a model of preventive care study. Arab. J. Chem. 2020;13(11):8172–8182. doi: 10.1016/j.arabjc.2020.09.051. [DOI] [Google Scholar]
- 132.Qi L., Jiang H., Cui X., Liang G., Gao M., Huang Z., Xi Q. Synthesis of methylprednisolone loaded ibuprofen modified dextran based nanoparticles and their application for drug delivery in acute spinal cord injury. Oncotarget. 2017;8(59):99666–99680. doi: 10.18632/oncotarget.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gaudin A., Yemisci M., Eroglu H., Lepetre-Mouelhi S., Turkoglu O.F., Dönmez-Demir B., Caban S., Sargon M.F., Garcia-Argote S., Pieters G., Loreau O., Rousseau B., Tagit O., Hildebrandt N., Le Dantec Y., Mougin J., Valetti S., Chacun H., Nicolas V., Desmaële D., Andrieux K., Capan Y., Dalkara T., Couvreur P. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat. Nanotechnol. 2014;9(12):1054–1062. doi: 10.1038/nnano.2014.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhigang W., Zhiyu L., Haitao R., Hong R., Qunxia Z., Ailong H., Qi L., Chunjing Z., Hailin T., Lin G., Mingli P., Shiyu P. Ultrasound-mediated microbubble destruction enhances VEGF gene delivery to the infarcted myocardium in rats. Clin. Imag. 2004;28(6):395–398. doi: 10.1016/j.clinimag.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 135.Takahashi M., Kido K., Aoi A., Furukawa H., Ono M., Kodama T. Spinal gene transfer using ultrasound and microbubbles. J. Contr. Release. 2007;117(2):267–272. doi: 10.1016/j.jconrel.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 136.Thery C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 137.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 138.Ratajczak J., Miekus K., Kucia M., Zhang J., Reca R., Dvorak P., Ratajczak M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 139.Rufino-Ramos D., Albuquerque P.R., Carmona V., Perfeito R., Nobre R.J., Pereira de Almeida L. Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J. Contr. Release. 2017;262:247–258. doi: 10.1016/j.jconrel.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 140.Dutta D., Khan N., Wu J., Jay S.M. Extracellular vesicles as an emerging frontier in spinal cord injury pathobiology and therapy. Trends Neurosci. 2021;44(6):492–506. doi: 10.1016/j.tins.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jang S.C., Kim O.Y., Yoon C.M., Choi D.S., Roh T.Y., Park J., Nilsson J., Lötvall J., Kim Y.K., Gho Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 142.Jo W., Jeong D., Kim J., Cho S., Jang S.C., Han C., Kang J.Y., Gho Y.S., Park J. Microfluidic fabrication of cell-derived nanovesicles as endogenous RNA carriers. Lab Chip. 2014;14(7):1261–1269. doi: 10.1039/c3lc50993a. [DOI] [PubMed] [Google Scholar]
- 143.Jo W., Kim J., Yoon J., Jeong D., Cho S., Jeong H., Yoon Y.J., Kim S.C., Gho Y.S., Park J. Large-scale generation of cell-derived nanovesicles. Nanoscale. 2014;6(20):12056–12064. doi: 10.1039/c4nr02391a. [DOI] [PubMed] [Google Scholar]
- 144.Huang J.H., Yin X.M., Xu Y., Xu C.C., Lin X., Ye F.B., Cao Y., Lin F.Y. Systemic administration of exosomes released from mesenchymal stromal cells attenuates apoptosis, inflammation, and promotes angiogenesis after spinal cord injury in rats. J. Neurotrauma. 2017;34(24):3388–3396. doi: 10.1089/neu.2017.5063. [DOI] [PubMed] [Google Scholar]
- 145.Wang L., Pei S., Han L., Guo B., Li Y., Duan R., Yao Y., Xue B., Chen X., Jia Y. Mesenchymal stem cell-derived exosomes reduce A1 astrocytes via downregulation of phosphorylated NFkappaB P65 subunit in spinal cord injury. Cell. Physiol. Biochem. 2018;50(4):1535–1559. doi: 10.1159/000494652. [DOI] [PubMed] [Google Scholar]
- 146.Zhao C., Zhou X., Qiu J., Xin D., Li T., Chu X., Yuan H., Wang H., Wang Z., Wang D. Exosomes derived from bone marrow mesenchymal stem cells inhibit complement activation in rats with spinal cord injury. Drug Des. Dev. Ther. 2019;13:3693–3704. doi: 10.2147/dddt.S209636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gu J., Jin Z.S., Wang C.M., Yan X.F., Mao Y.Q., Chen S. Bone marrow mesenchymal stem cell-derived exosomes improves spinal cord function after injury in rats by activating autophagy. Drug Des. Dev. Ther. 2020;14:1621–1631. doi: 10.2147/dddt.S237502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li C., Jiao G., Wu W., Wang H., Ren S., Zhang L., Zhou H., Liu H., Chen Y. Exosomes from bone marrow mesenchymal stem cells inhibit neuronal apoptosis and promote motor function recovery via the wnt/β-catenin signaling pathway. Cell Transplant. 2019;28(11):1373–1383. doi: 10.1177/0963689719870999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fan L., Dong J., He X., Zhang C., Zhang T. Bone marrow mesenchymal stem cells-derived exosomes reduce apoptosis and inflammatory response during spinal cord injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway. Hum. Exp. Toxicol. 2021;40(10):1612–1623. doi: 10.1177/09603271211003311. [DOI] [PubMed] [Google Scholar]
- 150.Lu Y., Zhou Y., Zhang R., Wen L., Wu K., Li Y., Yao Y., Duan R., Jia Y. Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front. Neurosci. 2019;13:209. doi: 10.3389/fnins.2019.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang C., Zhang C., Xu Y., Li C., Cao Y., Li P. Exosomes derived from human placenta-derived mesenchymal stem cells improve neurologic function by promoting angiogenesis after spinal cord injury. Neurosci. Lett. 2020;739 doi: 10.1016/j.neulet.2020.135399. [DOI] [PubMed] [Google Scholar]
- 152.Romanelli P., Bieler L., Scharler C., Pachler K., Kreutzer C., Zaunmair P., Jakubecova D., Mrowetz H., Benedetti B., Rivera F.J., Aigner L., Rohde E., Gimona M., Strunk D., Couillard-Despres S. Extracellular vesicles can deliver anti-inflammatory and anti-scarring activities of mesenchymal stromal cells after spinal cord injury. Front. Neurol. 2019;10:1225. doi: 10.3389/fneur.2019.01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhong D., Cao Y., Li C.J., Li M., Rong Z.J., Jiang L., Guo Z., Lu H.B., Hu J.Z. Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp. Biol. Med. 2020;245(1):54–65. doi: 10.1177/1535370219895491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rong Y., Liu W., Wang J., Fan J., Luo Y., Li L., Kong F., Chen J., Tang P., Cai W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019;10(5):340. doi: 10.1038/s41419-019-1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yuan X., Wu Q., Wang P., Jing Y., Yao H., Tang Y., Li Z., Zhang H., Xiu R. Exosomes derived from pericytes improve microcirculation and protect blood-spinal cord barrier after spinal cord injury in mice. Front. Neurosci. 2019;13:319. doi: 10.3389/fnins.2019.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li C., Li X., Zhao B., Wang C. Exosomes derived from miR-544-modified mesenchymal stem cells promote recovery after spinal cord injury. Arch. Physiol. Biochem. 2020;126(4):369–375. doi: 10.1080/13813455.2019.1691601. [DOI] [PubMed] [Google Scholar]
- 157.Huang J.H., Xu Y., Yin X.M., Lin F.Y. Exosomes derived from miR-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience. 2020;424:133–145. doi: 10.1016/j.neuroscience.2019.10.043. [DOI] [PubMed] [Google Scholar]
- 158.Luo Y., Xu T., Liu W., Rong Y., Wang J., Fan J., Yin G., Cai W. Exosomes derived from GIT1-overexpressing bone marrow mesenchymal stem cells promote traumatic spinal cord injury recovery in a rat model. Int. J. Neurosci. 2021;131(2):170–182. doi: 10.1080/00207454.2020.1734598. [DOI] [PubMed] [Google Scholar]
- 159.Xu G., Ao R., Zhi Z., Jia J., Yu B. miR-21 and miR-19b delivered by hMSC-derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. J. Cell. Physiol. 2019;234(7):10205–10217. doi: 10.1002/jcp.27690. [DOI] [PubMed] [Google Scholar]
- 160.Poliseno L., Salmena L., Zhang J., Carver B., Haveman W.J., Pandolfi P.P. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465(7301):1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Z., Song Y., Han X., Qu P., Wang W. Long noncoding RNA PTENP1 affects the recovery of spinal cord injury by regulating the expression of miR-19b and miR-21. J. Cell. Physiol. 2020;235(4):3634–3645. doi: 10.1002/jcp.29253. [DOI] [PubMed] [Google Scholar]
- 162.Jiang D., Gong F., Ge X., Lv C., Huang C., Feng S., Zhou Z., Rong Y., Wang J., Ji C., Chen J., Zhao W., Fan J., Liu W., Cai W. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J. Nanobiotechnol. 2020;18(1):105. doi: 10.1186/s12951-020-00665-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yang Y., Ye Y., Kong C., Su X., Zhang X., Bai W., He X. MiR-124 enriched exosomes promoted the M2 polarization of microglia and enhanced Hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway. Neurochem. Res. 2019;44(4):811–828. doi: 10.1007/s11064-018-02714-z. [DOI] [PubMed] [Google Scholar]
- 164.Cao Y., Xu Y., Chen C., Xie H., Lu H., Hu J. Local delivery of USC-derived exosomes harboring ANGPTL3 enhances spinal cord functional recovery after injury by promoting angiogenesis. Stem Cell Res. Ther. 2021;12(1):20. doi: 10.1186/s13287-020-02078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li D., Zhang P., Yao X., Li H., Shen H., Li X., Wu J., Lu X. Exosomes derived from miR-133b-modified mesenchymal stem cells promote recovery after spinal cord injury. Front. Neurosci. 2018;12:845. doi: 10.3389/fnins.2018.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ren Z.W., Zhou J.G., Xiong Z.K., Zhu F.Z., Guo X.D. Effect of exosomes derived from MiR-133b-modified ADSCs on the recovery of neurological function after SCI. Eur. Rev. Med. Pharmacol. Sci. 2019;23(1):52–60. doi: 10.26355/eurrev_201901_16747. [DOI] [PubMed] [Google Scholar]
- 167.Chang Q., Hao Y., Wang Y., Zhou Y., Zhuo H., Zhao G. Bone marrow mesenchymal stem cell-derived exosomal microRNA-125a promotes M2 macrophage polarization in spinal cord injury by downregulating IRF5. Brain Res. Bull. 2021;170:199–210. doi: 10.1016/j.brainresbull.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 168.Wang Y., Lai X., Wu D., Liu B., Wang N., Rong L. Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5p-mediated NGF/TrkA signaling pathway in rats. Stem Cell Res. Ther. 2021;12(1):117. doi: 10.1186/s13287-021-02148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lai X., Wang Y., Wang X., Liu B., Rong L. miR-146a-5p-modified hUCMSC-derived exosomes facilitate spinal cord function recovery by targeting neurotoxic astrocytes. Stem Cell Res. Ther. 2022;13(1):487. doi: 10.1186/s13287-022-03116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fan Y., Li Y., Huang S., Xu H., Li H., Liu B. Resveratrol-primed exosomes strongly promote the recovery of motor function in SCI rats by activating autophagy and inhibiting apoptosis via the PI3K signaling pathway. Neurosci. Lett. 2020;736 doi: 10.1016/j.neulet.2020.135262. [DOI] [PubMed] [Google Scholar]
- 171.Gao Z.S., Zhang C.J., Xia N., Tian H., Li D.Y., Lin J.Q., Mei X.F., Wu C. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 2021;126:211–223. doi: 10.1016/j.actbio.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 172.Kim H.Y., Kumar H., Jo M.J., Kim J., Yoon J.K., Lee J.R., Kang M., Choo Y.W., Song S.Y., Kwon S.P., Hyeon T., Han I.B., Kim B.S. Therapeutic efficacy-potentiated and diseased organ-targeting nanovesicles derived from mesenchymal stem cells for spinal cord injury treatment. Nano Lett. 2018;18(8):4965–4975. doi: 10.1021/acs.nanolett.8b01816. [DOI] [PubMed] [Google Scholar]
- 173.Bi Y., Duan W., Chen J., You T., Li S., Jiang W., Li M., Wang G., Pan X., Wu J., Liu D., Li J., Wang Y. Neutrophil decoys with anti-inflammatory and anti-oxidative properties reduce secondary spinal cord injury and improve neurological functional recovery. Adv. Funct. Mater. 2021;31(34) doi: 10.1002/adfm.202102912. [DOI] [Google Scholar]
- 174.Tang W., Yang Y., Yang L., Tang M., Chen Y., Li C. Macrophage membrane-mediated targeted drug delivery for treatment of spinal cord injury regardless of the macrophage polarization states. Asian J. Pharm. Sci. 2021;16(4):459–470. doi: 10.1016/j.ajps.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee J.R., Kyung J.W., Kumar H., Kwon S.P., Song S.Y., Han I.B., Kim B.S. Targeted delivery of mesenchymal stem cell-derived nanovesicles for spinal cord injury treatment. Int. J. Mol. Sci. 2020;21(11) doi: 10.3390/ijms21114185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang M., Wang L., Huang S., He X. Exosomes with high level of miR-181c from bone marrow-derived mesenchymal stem cells inhibit inflammation and apoptosis to alleviate spinal cord injury. J. Mol. Histol. 2021;52(2):301–311. doi: 10.1007/s10735-020-09950-0. [DOI] [PubMed] [Google Scholar]
- 177.Chen Y., Tian Z., He L., Liu C., Wang N., Rong L., Liu B. Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury. Stem Cell Res. Ther. 2021;12(1):224. doi: 10.1186/s13287-021-02282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.