Abstract
Background
Breast cancer care has been affected by the COVID-19 pandemic. This systematic review aims to describe the observed pandemic-related changes in clinical and health services outcomes for breast screening and diagnosis.
Methods
Seven databases (January 2020–March 2021) were searched to identify studies of breast cancer screening or diagnosis that reported observed outcomes before and related to the pandemic. Findings were presented using a descriptive and narrative approach.
Results
Seventy-four studies were included in this systematic review; all compared periods before and after (or fluctuations during) the pandemic. None were assessed as being at low risk of bias. A reduction in screening volumes during the pandemic was found with over half of studies reporting reductions of ≥49%. A majority (66%) of studies reported reductions of ≥25% in the number of breast cancer diagnoses, and there was a higher proportion of symptomatic than screen-detected cancers. The distribution of cancer stage at diagnosis during the pandemic showed lower proportions of early-stage (stage 0–1/I-II, or Tis and T1) and higher proportions of relatively more advanced cases than that in the pre-pandemic period, however population rates were generally not reported.
Conclusions
Evidence of substantial reductions in screening volume and number of diagnosed breast cancers, and higher proportions of advanced stage cancer at diagnosis were found during the pandemic. However, these findings reflect short term outcomes, and higher-quality research examining the long-term impact of the pandemic is needed.
Keywords: Breast cancer, Screening, Diagnosis, Mammography, COVID-19, Pandemic
Highlights
-
•
Comprehensive review of COVID-19 impacts on breast cancer screening and diagnosis.
-
•
Consistent evidence of substantial reductions in screening volume globally.
-
•
Reductions in number of diagnosed breast cancers were found.
-
•
Higher stage cancers comprised a higher proportion of breast cancers.
-
•
Ongoing, high-quality research examining longer-term pandemic impacts is needed.
1. Introduction
On March 11, 2020, the World Health Organization declared coronavirus disease 2019 (COVID-19) a pandemic [1]. A shutdown or suspension of many non-essential medical services was imposed for COVID-19 management globally. Concerns about the impact of the pandemic on utilisation of health services have been raised since then [2,3]. Delays and disruptions to cancer screening and treatment have been reported in recently published systematic reviews [[3], [4], [5]]. However, there is no published systematic review specifically focused on breast cancer screening and diagnosis.
Breast cancer is the most commonly diagnosed neoplasm in women across the world [6]. A recent narrative review of modelling studies in seven countries by the COVID-19 and Cancer Global Modelling Consortium highlighted the potential for COVID-19-related disruptions to affect screening participation [7]. The possible consequences of screening and diagnostic delays include significant increases in breast cancer morbidity and mortality. To understand the actual impact of the pandemic on breast cancer, this systematic review aims to describe the change in clinical and health services outcomes related to breast screening and diagnosis using real-world data.
2. Materials and methods
2.1. Research question
How has the COVID-19 pandemic affected breast cancer screening and diagnosis?
2.2. Information sources and search strategy
We performed a literature search from January 1, 2020 to March 19, 2022 in seven electronic databases: Embase, Evidence-Based Medicine Reviews (EBMR), Global Health, Medline, Pre-Medline, CINAHL Complete and Scopus via three interfaces (Ovid, EBSCOHost and Scopus). The search strategy was reviewed by the search team prior to execution using the PRESS Checklist [8]. We selected medical subject heading terms and free-text keywords across four broad concepts (including breast cancer, screening, diagnosis, and COVID-19) using Boolean operators ‘and’ and ‘or’ to develop search strategy in each database, and iteratively tested it to ensure a sensitive search. Subject heading terms and keywords differed slightly across databases. The full search strategy is detailed in Appendix A.
2.3. Study eligibility criteria
Studies were eligible for inclusion if they: included asymptomatic women who attended breast cancer screening programs or services, or women with symptoms, suspicious lesions or newly-diagnosed breast cancer; investigated the COVID-19 pandemic as an exposure; reported comparisons to evaluate a potential change associated with the pandemic; reported outcomes of breast cancer screening (e.g. number of screens) or cancer diagnosis (e.g. stage at diagnosis); and reported observed outcomes. Only studies reported in English were considered. Studies were excluded if they did not report observed changes attributed to the pandemic on screening and/or diagnosis (e.g. hypothetical studies of impact of COVID-19, estimated or projected outcomes). Detailed inclusion and exclusion criteria are available in Appendix B.
2.4. Study selection process
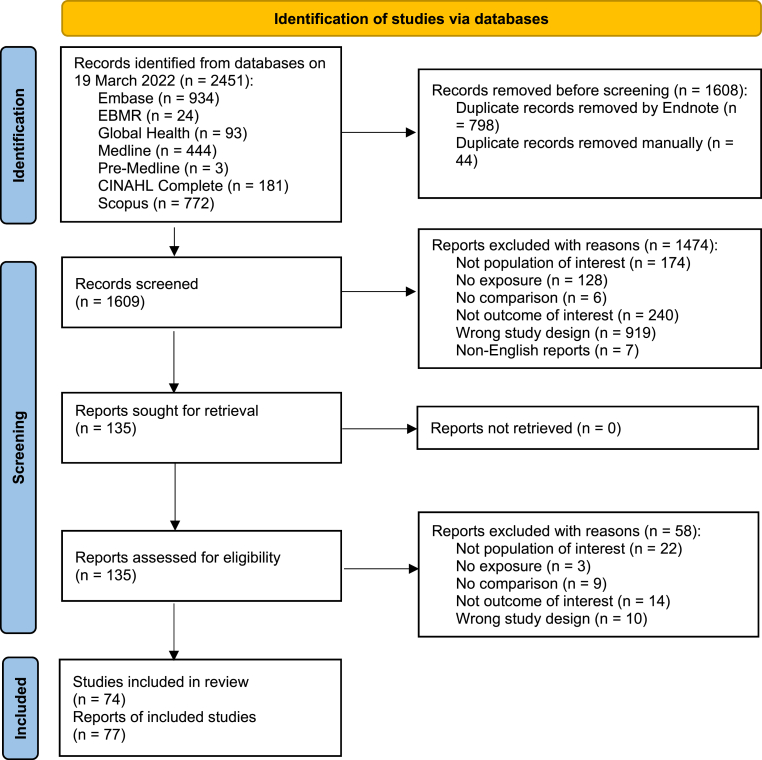
Titles and abstracts were screened by one author (TL) to determine whether studies met the eligibility criteria for full text assessment, and a sample of 12% was screened independently by another author (BN) to ensure consistent application of eligibility criteria. Any disagreement was resolved by discussion and consensus. Full text assessment was conducted by one author (TL) and verified by another author (BN). Discordant results were resolved by discussion/consensus or arbitration by a third reviewer (NH or MLM) if required. The PRISMA flowchart (Fig. 1) shows the study identification, screening and inclusion process.
Fig. 1.
PRISMA flow diagram.
2.5. Data extraction
Data extraction was performed by one author (TL), with another independent extraction by one of three other authors (PN, BN and KM). Disagreements were resolved by discussion and consensus or with arbitration by a third author (MLM) when needed.
The following data were extracted into an Excel spreadsheet using predefined cells: first author, publication year, country or region, publication type, study setting, study design, timeframe of COVID-19 pandemic and comparator periods, comparison type (pre-to-post, or fluctuation over multiple time points, or both), and outcomes related to three domains (i.e. screening, diagnosis and breast imaging). Details of data extraction are available in Appendix C.
2.6. Study appraisal and risk of bias assessment
Risk of bias and methodological quality assessment of eligible studies was performed by one author (TL), and a random sample of 12% was independently assessed by another author (MLM) with disagreements resolved by discussion and consensus. We used appraisal criteria adapted from both the NIH quality assessment tool [9] (items 1–6 and 9–11) and Cochrane EPOC risk of bias tool [10] (items 7–8 and 12) (Appendix D). Each question was answered as ‘yes’, ‘no’ or ‘not reported’, corresponding to ‘low’, ‘high’ or ‘unclear’ risk of bias. We rated the overall risk of bias for each individual study as high-risk if one or more of the questions were rated as high; and low if all questions were classified as low. Studies without a high-risk rating but one or more questions with unclear ratings were classified as unclear for overall risk of bias.
2.7. Data synthesis
Results were presented using a descriptive and narrative approach because the heterogeneity of study outcomes did not support pooling of results.
We created summary tables comprising methodological characteristics and reported outcomes within each domain for individual studies. We summarised study characteristics, including publication type, study region, study design, comparison type, duration of reported pandemic periods, and whether the pandemic period included 2021 as proportions of the total studies.
To synthesise the results for the domains of screening, and diagnosis and breast imaging, we created a table of outcomes that were reported by ≥ 20% of the total number of studies included in each domain.
2.8. Registration
This systematic review was prospectively registered in PROSPERO (International Prospective Register of Systematic Reviews) with registration number of CRD42021279436.
3. Results
3.1. Study selection
An initial 2451 citations were identified for title and abstract screening, of which 77 were eligible for inclusion (Fig. 1) [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87]]. For 3 studies with multiple publications reporting different or complementary outcomes, we selected the most recently published for inclusion [34,57,59], and extracted relevant data (where available) from the other superseded publications [[85], [86], [87]] but did not include them as separate papers. Therefore, a total of 74 studies were included in this review [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84]].
3.2. Study characteristics
AppxFigure E.1 (in Appendix E) summarises study characteristics of all included studies, and AppxTable F.1 (in Appendix F) displays study-specific characteristics. There were 38 [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]], 41 [14,15,20,21,29,30,[32], [33], [34],37,40,42,44,[49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76]] and 12 [20,21,42,65,[77], [78], [79], [80], [81], [82], [83], [84]] studies that reported breast screening, diagnosis, and breast imaging related outcomes, respectively. Any study could report outcomes in more than one domain, so 13 studies reported outcomes for both screening and diagnosis [14,15,20,21,29,30,[32], [33], [34],37,40,42,44], of which 3 reported outcomes in all three domains [20,21,42].
3.2.1. Publication type and study setting
The majority (74%) of the included studies were original research articles, and 26% (19/74) were other types of publications (e.g. letter or brief report) (AppxFigure E.1a). Of all 74 studies: 41% (30/74) were conducted in North America; 35% (26/74) were Europe-based; 15% (11/74) were Asia-based; 7% (5/74) were conducted in South America, and other studies (3%) came from Africa (1/74) and Oceania (1/74) (AppxFigure E.1b).
There was heterogeneity in the reported study design, and there were no prospective studies (AppxFigure E.1c). For study setting (AppxFigure E.1d), 24% of studies were based on breast cancer (or cancer) screening programs (13/74), and cancer or imaging registries (5/74); 34% (25/74) were a single institutional (or department-based) study; 42% (31/74) used a healthcare or community-based system or network, including 2 studies using health insurance claims.
3.2.2. Comparison types and timeline of before and after COVID-19 pandemic
All studies were natural experiments comparing periods before and after the pandemic [88]. Pre-to-post pandemic (86%, 64/74 studies) was the predominant comparison type, and comparison of fluctuations over multiple time points was reported in 7% (5/74) of studies; the remaining 5 studies (7%) had both comparison types (AppxFigure E.1e). Comparison of dichotomous pre-to-post time periods included pre vs during, pre vs peak/shutdown, and pre vs after-peak/reopening, and comparison of fluctuation included changes over multiple time points before and during the pandemic period, such as pre vs shutdown vs reopening.
AppxFigure E.2 displays the timeline of before and after pandemic periods for each individual study. A majority of the studies included the period from March to September 2020 as the main pandemic period, with a minimum duration of 1 month and a maximum of 18 months. Even though the starting timepoint of the reported pandemic period varied across all studies, over half (51%, 38/74) of studies reported the duration of pandemic of ≤6 months and 43% (32/74) of studies reported the duration period between 6 and 12 months; 4 studies (5%) reported a duration of over 1 year (AppxFigure E.1f). Some months in 2021 were included as part of the pandemic period in 8 (11%) studies (AppxFigure E.1g).
3.3. Risk of bias
AppxTable F.2 (in Appendix F) displays the risk of bias assessment results. The majority of studies were deemed to be at high risk of bias (92%, 68/74) with the remainder having an unclear risk of bias (8%, 6/74). No study was assessed as being at low risk of bias. The main reasons for high and unclear risk bias were study participants not being representative of population of interest (32%, 24/74); absence of formal tests of statistical significance for before-after changes (53%, 39/74); lack of multiple outcome measures (28%, 21/74), and limited consideration of whether the COVID-19 pandemic occurred independently of other changes (confounding factors) in the time-periods being compared (27%, 20/74 as high-risk; and 62%, 46/74 as unclear-risk).
3.4. Screening-related results
Screening-related outcomes were reported in 38 studies representing most world regions [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]]. Main outcomes from the individual studies are summarised narratively below, and detailed in Table 1 and AppxTable F.3-F.5 (Appendix F).
Table 1.
Summary of screening volume (number of screening mammograms/women or screening rate, n = 35).
| Study, Country/Region | Health service setting |
Pre-pandemic |
Pandemic |
Number of screening mammograms or number of women having screening |
||
|---|---|---|---|---|---|---|
| Time period | Time period | Services suspension/lockdown | Pre-pandemic vs Pandemic (data are frequency (N) unless specified) | Relative change in outcome (unless specified) | ||
| Al-Kuwari 2021, Qatar [11] | HCS | 01/01/2019 to 31/07/2019 | 01/01/2020 to 31/07/2020 | 11/03/2020–31/07/2020 | 4854 vs 2156 | ↓55.58% |
| Amran 2021, US [13] | HCS | 01/04/2019 to 31/12/2019 | 01/04/2020 to 31/12/2020 | NR | 55,678 vs 27,522 | ↓49% |
| Bakouny 2021, US [14] | HCS | Same months 2019: 02/03/2019 to 02/06/2019; Pre-peak: 01/12/2019 to 02/03/2020. |
Peak: 02/03/2020 to 02/06/2020; After-peak: 03/06/2020 to 03/09/2020. |
NR | Same months 2019 vs Peak: 24,660 vs 5305; Pre-peak vs Peak: 29,158 vs 5305; After-peak vs Peak: 24,788 vs 5305. |
Same months 2019 vs Peak: ↓77.67%; Pre-peak vs Peak: ↓81.49%; After-peak vs Peak: ↓78.06%. |
| Bentley 2021, Canada [15] | BCSP | 01/01/2019 to 31/12/2019 | 01/01/2020 to 31/12/2020 | 18/03/2020–30/05/2020 | 265,479 vs 185,154 | ↓30.2% |
| Bessa 2021, Brazil [16] | BCSP | 01/01/2019 to 31/12/2019 | 01/01/2020 to 31/12/2020 | 17/03/2020-NR | 1,948,471 vs 1,126,688 | ↓42.18% |
| Brugel 2021, France [17] | HCS | 01/01/2019 to 31/05/2019 | 01/01/2020 to 31/05/2020 | 17/03/2020–11/05/2020 | Jan: 2541 vs 2607; Feb: 2128 vs 2203; Mar: 2316 vs 965; Apr: 2288 vs 0; May: 2089 vs 636. |
Jan: ↑2%; Feb: ↑3%; Mar: ↓58%; Apr: ↓100%; May: ↓70%. |
| Chiarelli 2021a, Canada [19] | BCSP | 01/01/2019 to 29/02/2020 | Suspension: 01/03/2020 to 31/05/2020; Resumption: 01/06/2020 to 31/03/2021. |
23/03/2020–26/05/2020 | Pre vs Suspension vs Resumption: 822,862 vs 32,408 vs 394,559 |
Pre vs Suspension: ↓96.1%; Pre vs Resumption: ↓52.1%; Suspension vs Resumption: ↑1117.5%. |
| Chou 2020, Taiwan [20] | ASI | Week 1, 2019 to Week 22, 2019 | Week 1, 2020 to Week 22, 2020 | NR | NR | ↓51%, p < 0.001 |
| Collado-Mesa 2020, US [21] | HCS | 1/04/2018–2019 to 30/04/2018–2019 | 01/04/2020 to 30/04/2020 | 20/03/2020-Mid May to early June 2020 | 2722 vs 105 | ↓96% |
| Dabkeviciene 2021, Lithuania [22] | ASI | 01/02/2019 to 31/12/2019 | 01/02/2020 to 31/12/2020 | 18/03/2020–17/06/2020; 04/11/2020–31/12/2020. | 9704 vs 3653 | ↓62% |
| de Degani 2021, Argentina [23] | BCSP | 19/03/2019 to 19/09/2019 | 19/03/2020 to 19/09/2020 | 19/03/2020–19/09/2020 | 9918 vs 2098 | ↓78.85% (95% CI: 78.03–79.65%), p < 0.0001 |
| DeGroff 2021, US [24] | BCSP | 01/01/2015–2019 to 30/06/2015–2019 | 01/01/2020 to 30/06/2020 | NR | Total: 1,112,126 vs 71,704. Apr: 19,366 vs 2607; Jun: 17,385 vs 10,626. |
Total: ↓94%. Apr: ↓97%, p < 0.001; Jun: ↓39%, p < 0.001. |
| Fedewa 2021, Italy [25] | HCS | 01/07/2019 to 31/07/2019 | 01/07/2020 to 31/07/2020 | NR | 76,430 vs 74,340 | ↓2.7% |
| Gorin 2021, US [27] | ASI | 19/03/2019 to 09/05/2019 | 19/03/2020 to 09/05/2020 | 19/03/2020–09/05/2020 | 3339 vs 6 | ↓99.8% |
| Kang 2021b, Korea [29] | HCS | 01/02/2019 to 31/07/2019 | 01/02/2020 to 31/07/2020 | NR | Total: 20,923 vs 11,982. Peak (Feb–Apr): 8837 vs 3697; After-peak (May–Jul): 12,086 vs 8285. |
Total: ↓42.7%. Peak (Feb-Apr): ↓58.2%; After-peak (May-Jul): ↓31.4%. |
| Kidwai 2022, US [30] | ASI | 01/01/2019 to 31/12/2019 | 01/01/2020 to 31/12/2020 | NR | 435 vs 382 | ↓12% |
| Labaki 2021, US [33] | HCS | Pre-peak: 01/12/2019 to 02/03/2020 | 1st peak: 02/03/2020 to 02/06/2020; Period between two peaks: 03/06/2020 to 03/09/2020; 2nd peak: 04/09/2020 to 05/12/2020. |
NR | Pre-peak vs 1st peak: 29,305 vs 5379; Pre-peak vs Period between two peaks: 29,305 vs 24,876; Pre-peak vs 2nd peak: 29,305 vs 33,282. |
Pre-peak vs 1st peak: ↓82%; Pre-peak vs Period between two peaks: ↓15%; Pre-peak vs 2nd peak: ↑14%. |
| London 2022, US [34] | HCS | 01/01/2019 to 30/04/2020 | 01/01/2020 to 30/04/2021 | 01/03/2020–30/04/2020 | NR | Apr 1–15, 2020 (lowest): ↓89%; Jun 30- Jul 14, 2020 (recovery): ↑21%; Mar 2–16, 2021 (highest): ↑64%. |
| Losurdo 2022, Italy [35] | BCSP | 01/01/2019 to 31/12/2019 | 01/01/2020 to 31/12/2020 | 09/03/2020-NR | NR | ↓37.6% |
| Miller 2021, US [36] | ASI | Week 1, 2019 to Week 47, 2019 | Week 1, 2020 to Week 47, 2020 | Weeks 11–17 | 15,339 vs 13,841 | ↓9.8% |
| Norbash 2020, US [37] | HCS | Week 1, 2019 to Week 21, 2019 | Week 1, 2020 to Week 21, 2020 | NR | Nadir: in weeks 15–16: 12,027 vs 152 | Weekly change range: Weeks 1–10: ↑3%–16%; Weeks 11–13: ↓10%–96%; Weeks 14–20: ↓95%–99% (Nadir: ↓99% in weeks 15–16); Week 21: ↓73%. |
| Peng 2020, Taiwan [39] | BCSP | 01/01/2019 to 31/05/2019 | 01/01/2020 to 31/05/2020 | No suspension | Total: 496,207 vs 358,771 |
Total: ↓27.70%. Mar: ↓35% (p < 0.0001); Apr: ↓60% (p < 0.0001); May: ↓49% (p < 0.0001). |
| Ribeiro 2022, Brazil [40] | HCS | 01/01/2019 to 31/12/2019 | 01/01/2020 to 31/12/2020 | No lockdown | Total: 3,810,427 vs 218,371. Percentage of screening mammography performed in women aged 50–69 years: 64.8% vs 64.4%. |
Total: ↓42.62%. Percentage of screening mammography performed women aged 50–69 years: ↓0.4%. |
| Shen 2022, Taiwan [41] | BCSP | 01/01/2019 to 30/04/2019 | 01/01/2020 to 30/04/2020 | No suspension | Inreach (hospital): 150,903 vs 94,796; Outreach (mobile): 242,482 vs 211,730. |
Average monthly percentage change: Inreach (hospital): ↓41.43%; Outreach (mobile): ↓23.99%. |
| Sprague 2021, US [42] | CIR | 01/01/2019 to 31/07/2019 | 01/01/2020 to 31/07/2020 | NR | 190,454 vs 126,040 | ↓33.8% (95% CI: 27.4–39.7%) |
| Sutherland 2020, Australia [43] | BCSP | 01/03/2019 to 30/06/2019 | 01/03/2020 to 30/06/2020 | 24/03/2020–18/05/2020 | NR vs 58,478 | ↓51.5% |
| Tang 2022, US [44] | HCS | 17/03/2019 to 17/05/2019 | 17/03/2020 to 17/05/2020 | 17/03/2020–17/05/2020 | 180,724 vs 1681 | ↓99.1% |
| Tsai 2020, Taiwan [45] | BCSP | 01/01/2019 to 30/04/2019 | 01/01/2020 to 30/04/2020 | No suspension | 396,371 vs 308,463 | ↓22.2%, p < 0.001 |
| Velazquez 2021, US [46] | ASI | 01/01/2019 to 31/12/2019 | 01/01/2020 to 31/12/2020 | 16/03/2020–16/05/2020 | 5662 vs 3385 | ↓40% |
| Walker 2021a, Canada [47] | BCSP | 01/03/2019 to 31/12/2019 | 01/03/2020 to 31/12/2020 | Mid 03/2020-End 05/2020 | 605,889 vs 284,242 | ↓53.1% |
| Study, Country/Region | Health service setting | Pre-pandemic | Pandemic | Screening rate or use rate of mammograms | ||
| Time period | Time period | Services suspension/lockdown | Pre-pandemic vs Pandemic (data are in proportion (%) unless specified) | Relative change in outcome (unless specified) | ||
| Chen 2021, US [18] | HIC | 01/01/2019 to 31/07/2019 | 01/01/2020 to 31/07/2020 | NR | 4133 vs 2971 screens per 100,000 enrolees | ↓28.1% |
| Fedewa 2021, US [25] | HCS | 01/07/2019 to 31/07/2019 | 01/07/2020 to 31/07/2020 | NR | 53.9% vs 49.6% | Rate ratio: 0.92 (95% CI: 0.92–0.93) |
| Jidkova 2022, Belgium [28] | BCSP | 01/01/2019 to 31/12/2019 | 01/01/2020 to 31/12/2020 | 23/03/2020–28/06/2020 | NR | Absolute change: Entire year: ↓1.0% (95% CI: 0.8%–1.3%); Before shutdown (01/01–21/03): ↓1.4% (95% CI: 1.0%–1.9%); Reopen (05/07–31/12): ↓1.0% (95% CI: 1.3%–2.0%). |
| Kim 2022, US [31] | HCS | 01/01/2020 to 03/03/2020 | Stat-at-home: 04/03/2020 to 08/05/2020; Reopen: 09/05/2020 to 08/07/2020. |
04/03/2020–08/05/2020 | Odds Ratio (95% CI)d: Stay-at-home vs Pre: 0.34 (0.31–0.37); Reopen vs Pre: 0.49 (0.45–0.53); Reopen vs Stat-at-home: 1.44 (1.31–1.58). |
Stay-at-home vs Pre: ↓66%, p < 0.001; Reopen vs Pre: ↓51%, p < 0.001; Reopen vs Stat-at-home: ↑44%, p < 0.001. |
| Koczkodaj 2021, Poland [32] | BCSP | 01/01/2019 to 30/09/2019 | 01/01/2020 to 30/09/2020 | NR | 38.15% vs 35.92% | Absolute change: ↓2.2% |
| Whaley 2020, US [48] | HIC | 01/03/2019 to 30/04/2019 | 01/03/2020 to 30/04/2020. | NR | Mar: 358.4 per 10,000 women vs NR; Apr: 378.5 per 10,000 women vs NR. |
Mar: ↓41.6%c; Apr: ↓90.4%c. |
BCSP= Breast cancer (or cancer) screening program, HCS=Healthcare (or community-based) system/network/database, ASI = A single institution or department, CIR=Cancer or imaging registry, HIC=Health insurance claims, NR=Not reported, CI=Confidence interval.
Italics: computed data (see Appendix C).
Screening modality includes both mammography and magnetic resonance imaging.
Screening modality includes both mammography and ultrasound.
Controls for the age categories, state, year, and month.
Adjusted for age, race/ethnicity, enrolment in the patient portal (MyChart), COVID-19 risk score, and provider specialty.
3.4.1. Screening volume
As shown in Table 1, a relative reduction in screening volumes ranged from 2.7% to 100% during the pandemic period [17,25]; over half (54%, 19/35) of studies reported screening volume reductions of ≥49% [11,13,14,17,[19], [20], [21], [22], [23], [24],27,31,33,34,37,43,44,47,48]. Screening uptake reduced by 35%–100% during the pandemic peak in March–May 2020 [14,17,19,21,24,27,29,31,33,34,37,39,43,44,48]; many studies reported suspension of screening programs, or non-essential services shutdown or regional lockdown during this period [17,19,21,27,31,34,43,44]. However, screening volumes gradually recovered from May-September [14,17,19,24,28,29,31,33,34,37,39]. Due to this fluctuation, studies with relatively long study periods (10–12 months) reported smaller reductions (9.8%–62%) for cumulative screening [15,16,22,30,35,36,40,46,47] or even an increase (14%–64%) at mid-late stage of the pandemic, possibly indicating signs of recovery [33,34].
3.4.2. Positive screens
Studies based on organised screening programs generally reported a modest absolute increase (0.6%–2.3%) in the proportion of positive screens or recall rate [23,39,45,47], while other studies reported an absolute decrease (0.2%–2.2%) [14,20] (AppxTable F.3). In a similar pattern to screening volume, the number of abnormal (‘positive’) screening mammograms showed a relative decrease of 32%–49% during the pandemic peak [15,19].
3.4.3. Screening by age
There was heterogeneity in screening data by age and no consistent age-related patterns were found (AppxTable F.4). Compared to the pre-pandemic period, some studies found reductions in screening across all age groups during the pandemic [28,46]; some studies showed increased proportions of screening participants at younger age and decreased proportions in older age [20,36,45,47]; and one study reported the reverse [13].
3.4.4. Screening by ethnicity or race
All studies that reported screening by ethnicity or race were US-based, and most reported a reduction in screening volume or growth in cancellation rate across all ethnicity or race groups (AppxTable F.5). Compared to White or Non-Hispanic, non-White (including Black/African-American) or Hispanic groups experienced greater declines in screening participation [12,24,33,36,48], particularly at the pandemic peak [38], and slower recovery to the pre-pandemic level [46].
3.5. Diagnosis and breast imaging results
A subtotal of 41 [14,15,20,21,29,30,[32], [33], [34],37,40,42,44,[49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76]] and 12 [20,21,42,65,[77], [78], [79], [80], [81], [82], [83], [84]] studies reported diagnosis, and breast imaging outcomes, respectively. Key results from the individual studies are summarised narratively below, and presented in more details in Table 2 and AppxTable F.6-F.8 (Appendix F).
Table 2.
Summary of breast cancer diagnoses (number of breast cancer cases or percentage of women diagnosed with breast cancer, n = 32).
| Study, Country/Region | Health service setting |
Pre-pandemic |
Pandemic |
Number of breast cancer cases or number of women diagnosed with breast cancer |
||
|---|---|---|---|---|---|---|
| Time period | Time period | Services suspension/lockdown | Pre-pandemic vs Pandemic (data are frequency (N) unless specified) | Relative change in outcome (unless specified) | ||
| Bakouny 2021, US [14] | HCS | Same months 2019: 02/03/2019 to 02/06/2019; Pre-peak: 01/12/2019 to 02/03/2020. |
Peak: 02/03/2020 to 02/06/2020; After-peak: 03/06/2020 to 03/09/2020. |
NR | NR | Same months 2019 vs Peak: ↓61.29%; Pre-peak vs Peak: ↓62.44%; After-peak vs Peak: ↓52.87%. |
| Blay 2021, France [50] | HCS | 01/03/2019 to 31/07/2019 | 01/03/2020 to 31/07/2020 | 04/2020-NR | 10,525 vs 8428 | ↓20% |
| Bonadio 2021, Brazil [51] | ASI | 01/09/2019 to 31/01/2020 | 01/09/2020 to 31/01/2021 | NR | 457 vs 268 | ↓41.4% |
| Borsky 2022, UK [52] | ASI | 01/05/2019 to 31/10/2019 | 01/05/2020 to 31/10/2020 | 01/05/2020–31/07/2020 | 276 vs 163 | ↓40.9% |
| Chou 2021a, Taiwan [53] | ASI | 21/01/2019 to 31/07/2019 | 21/01/2020 to 31/07/2020 | NR | 128 vs 115 | ↓10%, p = 0.52 |
| Citgez 2021, Turkey [54] | ASI | Pre-peak: 01/12/2019 to 29/02/2020 | Peak: 01/03/2020 to 31/05/2020; After-peak: 01/06/2020 to 31/08/2020. |
NR | Pre-peak vs Peak: 72 vs 22 (daily average: 0.8 vs 0.24); Pre-peak vs After-peak: 72 vs 46 (daily average: 0.8 vs 0.51); Peak vs After-peak: 22 vs 46 (daily average: 0.24 vs 0.51). |
Pre-peak vs Peak: ↓69.4% (daily average: ↓70.0%); Pre-peak vs After-peak: ↓36.1% (daily average: ↓36.3%); Peak vs After-peak: ↑109.1% (daily average: ↑112.5%). |
| De Vincentiis 2021, Italy [55] | ASI | Week 11, 2018–2019 to Week 20, 2018–2019 | Week 11, 2020 to Week 20, 2020 | Weeks 11-20 | 47 vs 35 | ↓26% |
| Drescher 2022, US [56] | HCS | 04/03/2019 to 03/03/2020. | 04/03/2020 to 03/03/2021. | NR | 6135 vs 5257 | ↓14.3% |
| Eijkelboom 2021a, Netherlands [57] | CIR | Week 2, 2018–2019 to Week 35, 2018–2019 | Week 2, 2020 to Week 35, 2020 | Weeks 12–29 | Total: 7302 vs 5306. Average weekly incidence in each period of the pandemic year (per 100,000 women): 8.3 vs 7.6 vs 5.0 (screening suspension started) vs 2.1 (referrals ended) vs 3.7 vs 4.6 (screening restarted) vs 6.3 |
Total: ↓27% (weeks 9–35: ↓37%) |
| Ferrara 2021, Italy [58] | HCS | Week 11, 2018–2019 to Week 20, 2018–2019 | Week 11, 2020 to Week 20, 2020 | Weeks 11-20 | 620 vs 383 | ↓38.2% |
| Kaltofen 2021a, Germany [61] | ASI | 01/01/2019 to 30/06/2019 | 01/01/2020 to 30/06/2020 | 22/03/2020–05/05/2020 | Total: 170 vs 150. Lockdown (22/03–05/05): 30 vs 24. |
Total: ↓12%. Lockdown (22/03–05/05): ↓20%. |
| Kang 2021a, Korea [29] | HCS | 01/02/2019 to 31/07/2019 | 01/02/2020 to 31/07/2020 | NR | Total: 1669 vs 1369. Peak (Feb–Apr): 798 vs 638; After-peak (May–Jul): 871 vs 731. |
Total: ↓18.0%. Peak (Feb-Apr): ↓20.1%; After-peak (May-Jul): ↓16.1%. |
| Kempf 2021a, France [62] | HCS | Comparison 1: 01/03/2018–2019 to 31/05/2018–2019; Comparison 2: 01/06/2018–2019 to 30/09/2018–2019 |
Comparison 1 (lockdown): 01/03/2020 to 31/05/2020; Comparison 2 (after-lockdown): 01/06/2020 to 30/09/2020 |
17/03/2020–11/05/2020 | Comparison 1 (lockdown): 715 vs 507; Comparison 2 (after-lockdown): 870 vs 752. |
Comparison 1 (lockdown): ↓29%; Comparison 2 (after-lockdown): ↓14%. |
| Kidwai 2022, US [30] | ASI | 01/01/2019 to 31/12/2019 | 01/01/2020 to 31/12/2020 | NR | 4 vs 3 | ↓25% |
| Knoll 2021a, Austria [63] | ASI | Comparison 1: 16/03/2019 to 30/04/2019, and 03/11/2019 to 31/12/2019. Comparison 2: 01/05/2019 to 02/11/2019; |
Comparison 1 (two lockdowns): 16/03/2020 to 30/04/2020, and 03/11/2020 to 31/12/2020. Comparison 2 (periods between 2 lockdowns): 01/05/2020 to 02/11/2020. |
16/03/2020–30/04/2020, and 03/11/2020–31/12/2020. | Comparison 1 (two lockdowns): 115 vs 55; Comparison 2 (periods between two lockdowns): 148 vs 157. |
Comparison 1 (two lockdowns): ↓52%; Comparison 2 (periods between two lockdowns): ↑6%. |
| Koczkodaj 2021a, Poland [32] | BCSP | 01/01/2019 to 31/08/2019 | 01/01/2020 to 31/08/2020 | NR | 31,762 vs 31,414 | ↓1.1% |
| Labaki 2021a, US [33] | HCS | Pre-peak: 01/12/2019 to 02/03/2020 | 1st peak: 02/03/2020 to 02/06/2020; Period between two peaks: 03/06/2020 to 03/09/2020; 2nd peak: 04/09/2020 to 05/12/2020. |
NR | Pre-peak vs 1st peak: 587 vs 219; Pre-peak vs Period between two peaks: 587 vs 466; Pre-peak vs 2nd peak: 587 vs 593. |
Pre vs 1st peak: ↓63%; Pre vs Period between two peaks: ↓21%; Pre vs 2nd peak: ↑1%. |
| Linck 2022, France [64] | ASI | Reference: average 36 working days between 28/01/2019 and 03/07/2019. | Pre-lockdown: 27/01/2020 to 16/03/2020; Lockdown: 17/03/2020 to 05/05/2020; After-lockdown: 11/05/2020 to 01/07/2020. |
17/03/2020–11/05/2020 | Reference vs Lockdown: 40 vs 32; Reference vs After-lockdown: 40 vs 59; Pre-lockdown vs Lockdown: 43 vs 32; Pre-lockdown vs After-lockdown: 43 vs 59. |
Reference vs Lockdown: ↓20%; Reference vs After-lockdown: ↑48%; Pre-lockdown vs Lockdown: ↓26%; Pre-lockdown vs After-lockdown: ↑37%. |
| London 2022a, US [34] | HCS | 01/01/2019 to 31/12/2019 | 01/01/2020 to 31/12/2020 | 01/03/2020–30/04/2020 | NR | Jan: ↑26.2%; Feb: ↑8.5%; Mar: ↓13.7%; Apr: ↓50.5%. |
| Lowry 2021a, US [65] | CIR | 01/03/2019 to 30/09/2019 | 01/03/2020 to 30/09/2020 | NR | 2171 vs 1650 | ↓24% (95% CI: 17–31%), p < 0.001 |
| Morais 2022, Portugal [66] | ASI | 02/03/2019 to 01/07/2019 | 02/03/2020 to 01/07/2020 | 18/03/2020–02/05/2020 | 370 vs 227 | ↓38.6% (95%CI: 27.6%–48.0%) |
| O'Brien 2021, Ireland [67] | ASI | 01/02/2019 to 31/07/2019 | 01/02/2020 to 31/07/2020 | 17/03/2020–17/05/2020 | 197 vs 195 | ↓1% |
| Peacock 2021, Belgium [68] | HCS | 01/01/2019 to 31/12/2019 | 01/01/2020 to 31/12/2020 | 16/03/2020–30/06/2020 | NR | Total: ↓6% (Nadir in April: ↓50%) |
| Purushotham 2021, UK [69] | HCS | 01/01/2019 to 30/09/2019 | 01/01/2020 to 30/09/2020 | 20/03/2020-NR | 973 vs 686 | ↓29.5% |
| Ruiz-Medina 2021, Spain [70] | HCS | 13/03/2019 to 13/03/2020 | 13/03/2020 to 13/03/2021 | NR | 746 vs 551 | ↓26.1% |
| Skovlund 2021, Denmark [72] | CIR | 01/02/2015–2019 to 31/05/2015–2019 | 01/02/2020 to 31/05/2020 | 11/03/2020–05/2020 | NR | Mar–May: ↓30% (95% CI: 18%–40%). Feb: ↓17% (95% CI: 10%–23%); Mar: ↓21% (95% CI: −44% to 11%); Apr: ↓29% (95% CI: 18%–39%); May: ↓39% (95% CI: 32%–45%). |
| Tang 2022, US [44] | HCS | 17/03/2019 to 17/05/2019 | 17/03/2020 to 17/05/2020 | 17/03/2020–17/05/2020 | 703 vs 247 | ↓65% |
| Tsibulak 2020, Austria [74] | HCS | 16/03/2019 to 31/05/2019 | 16/03/2020 to 31/05/2020 | 16/03/2020–31/05/2020 | 351 vs 201 | ↓43% |
| van Wyk 2021, South Africa [75] | ASI | 01/04/2019 to 30/06/2019 | 01/04/2020 to 30/06/2020 | 26/03/2020-NR | By histopathology: 152 vs 102; By cytopathology: 95 vs 37. |
By histopathology: ↓32.9%; By cytopathology: ↓61.1%. |
| Vrdoljak 2021, Croatia [76] | CIR | 01/01/2019 to 31/12/2019 | 01/01/2020 to 31/12/2020 | 15/03/2020-Mid 05/2020; 26/10/2020-Mid 12/2020 |
2875 vs 2848 | Total: ↓1% (Apr–Jun: ↓24%) |
| Study, Country/Region | Health service setting | Pre-pandemic | Pandemic | Diagnosis rate (i.e. percentage of women diagnosed with breast cancer) | ||
| Time period | Time period | Services suspension/lockdown | Pre-pandemic vs Pandemic (data are in proportion (%) unless specified) | Absolute change in outcome (unless specified) | ||
| Bansal 2021, UK [49] | ASI | 01/04/2019 to 30/04/2019 | 01/04/2020 to 30/04/2020 | NR | 7% vs 5% | Absolute change: ↓2% |
| Drescher 2022, US [56] | HCS | 01/01/2019 to 03/03/2020 | Early: 04/03/2020 to 31/03/2020; Middle: 01/04/2020 to 09/06/2020; Late: 10/06/2020 to 31/05/2021. |
NR | NR | Incidence rate ratio (95% CI)b: Pre: 1.00; Early: 0.81 (0.73–0.89), p < 0.001; Middle: 0.57 (0.53–0.62), p < 0.001; Late: 0.95 (0.91–0.98), p = 0.002. |
| Tachibana 2021, Brazil [73] | ASI | 24/03/2019 to 31/12/2019 | 24/03/2020 to 31/12/2020 | 26/03/2020–21/06/2020 | Total: 8.5 (134/15,816) vs 12.4 (128/10,321) breast cancer per 1000 patients submitted to mammograms. During social isolation (24/03–21/06)): 6.4 (36/5661) vs 19.4 (18/927) breast cancer per 1000 patients submitted to mammograms; After social isolation (22/06–31/12): 9.7 (98/10,155) vs 11.7 (110/9394) per 1000 patients submitted to mammograms. |
Total: ↑3.9 breast cancer per 1000 patients submitted to mammograms, p = 0.002. During social isolation (24/03–21/06)): ↑13.0 breast cancer per 1000 patients submitted to mammograms, p < 0.001; After social isolation (22/06–31/12): ↑2.0 breast cancer per 1000 patients submitted to mammograms, p = 0.165. |
BCSP= Breast cancer (or cancer) screening program, HCS=Healthcare (or community-based) system/network/database, ASI = A single institution or department, CIR=Cancer or imaging registry, HIC=Health insurance claims, NR=Not reported, CI=Confidence interval.
Italics: computed data (see Appendix C).
Study sample also contains in situ tumour, and/or benign tumour cases.
Adjusted for the number of weeks for each study period.
3.5.1. Number of breast cancer diagnoses
There was a reduction in the number (or percentage) of women diagnosed with breast cancer (Table 2). Relative reductions in breast cancer diagnoses ranged from 1% [32,67,76] to 70% [54], with 66% (21/32) of studies reporting reductions of ≥25% [14,30,33,34,44,51,52,[54], [55], [56], [57], [58],[62], [63], [64],66,69,70,72,74,75]. The greatest decrease (20%–70%) was witnessed in the pandemic peak [14,29,33,34,54,56,[61], [62], [63],72], and a gradual recovery [14,29,33,54,56,62] or slight to moderate increases [33,63,64] were reported after the peak. Two studies (which used different measures) showed absolute reduction of 2% in percentage of women diagnosed with breast cancer [49], and increased breast cancer rates per 1000 patients who had mammograms [73].
3.5.2. Diagnosis by detection mode
During the pandemic, both the number of cancers diagnosed via screening and the number of symptomatic diagnoses were reduced, but the reduction in numbers was more evident for screen-detected compared to symptomatic cancers (AppxTable F.6) [44,52,54,57,60,65]. The general pattern across studies was a higher proportion of symptomatic diagnoses than the proportion diagnosed via screening [44,51,52,54].
3.5.3. Stage at diagnosis
Overall, the distribution of cancer stage at diagnosis in the pandemic period showed lower proportions of early-stage (including less stage 0–1, or I-II, or Tis and T1) and higher proportions of relatively more advanced cases than that in the pre-pandemic period [29,[51], [52], [53], [54],57,60,63,64,66,69,70], and this pattern was more obvious in the after-peak (versus the peak) period [29,54,64] (AppxTable F.7). Compared to the pre-pandemic, lower proportions of carcinoma in situ [44,57,63,74], and higher proportions of cancers with axillary node metastases or distant metastases [44,52,61,64] were found in the pandemic period (although noting small numbers and incomplete reporting for distant metastases at diagnosis). Two studies showed decreased proportions of T2-T4, because one study used all T-stages together with N+ and M1 stages as the denominator [61] and the other study reported an increased proportion of Tx stage [74].
3.5.4. Mammography volume
In medical imaging studies or studies with a mixed screening and symptomatic population (AppxTable F.8a), the number of mammography examinations during the pandemic [20,[77], [78], [79], [80], [81], [82], [83], [84]] generally showed a relative reduction of between 8.9% [84] and 92.7% [81], with 67% (6/9) of studies reporting cumulative (or average) reductions of 9%–37% [20,[77], [78], [79],82,83]. In an additional 6 studies of symptomatic populations (AppxTable F.8b) [20,21,29,37,42,73], a similar reduction was found for the number of diagnostic mammography (range: 4.7%–83.8%) with 50% (3/6) of studies observing reductions between 4% and 21% [20,29,42].
4. Discussion
This is the first systematic evidence review of the global impact attributed to the COVID-19 pandemic on breast cancer screening and diagnosis. Although we report heterogeneity in study outcomes, there were some key patterns in findings that are relevant to informing the recovery phase and identifying emerging priorities for breast cancer detection. Our study found consistent evidence of substantial reductions in screening volume, and similarly reductions in the number of diagnosed breast cancers and diagnostic mammography volume during the pandemic, particularly at the peak stage. These three observed patterns of changes attributed to the COVID-19 pandemic were generally consistent across countries (or regions) and across study settings, highlighting that screening participation, referral in symptomatic cancer diagnosis and other cancer diagnostic pathways have all been affected by the pandemic.
The above findings partially reflect the effect of the pandemic on populations as well as the global health system's response to the pandemic, such as suspension of screening programs, shutdown of non-urgent healthcare services, and enforced regional lockdowns, that accounted for some of the immediate reductions. Even though the above-noted response measures released health system capacity for better COVID-19 containment and mitigation, these measures to some extent compromised the delivery of health care services, including cancer prevention and control strategies, to general populations. Beyond the health system's response, other factors that may have contributed to the reductions were COVID-19 related anxiety and fear of COVID-19 infection [89], and decreased or difficult access to healthcare providers imposed by COVID-19 safety protocols [35].
In keeping with the reduction in screening volume, there was a decrease in the proportion of screen-detected cancers and a relative increase in cases diagnosed clinically through symptomatic presentations. This aligns with our review findings on stage at diagnosis of breast cancer. Lower proportions of early-stage and higher proportions of relatively more advanced cases were reported in the pandemic compared to the pre-pandemic period. Concomitantly, greater proportions of cancers with nodal and distant metastases were found. Even though the stage distributions might raise concerns about potentially worse long-term outcomes, these results should be interpreted with the caveat that these are the percentage of cases from the diagnosed breast cancers, and do not reflect population rates. Moreover, the association between diagnosis delay and prognosis has not been well-established [90]. While a delay of 3–6 months has been found to be associated with worse long-term prognosis and shorter survival [91], a shorter delay of 6–12 weeks may not affect the overall outcome [92]. It is also possible that diagnosis of the more advanced cancers was less affected by the pandemic as these are more likely to present with symptoms rather than through screening.
The reductions in breast screening volume and number of diagnosed breast cancers found in our study were also noted in other systematic reviews of the impact of COVID-19 pandemic related to cancer healthcare. One review identified significant declines in cancer screening or tests and cancer diagnosis rate, and found an increase in advanced cancers, but this study only included 17 publications [4]. One meta-analysis with relatively smaller number of papers (13 publications) reported incidence rate ratios of 0.10–0.63 for cancer screening services [5]. Another review also identified a remarkable frequency (up to 79%) of delays and disruptions in all cancer care attributed to the pandemic [3].
We not only found a large reduction in breast screening volumes and breast cancer diagnoses during the pandemic peak, but also found a slight to moderate rebound in these outcomes after the peak, which highlighted a possible recovery of cancer screening and diagnostic services. We further noted a slow persistent recovery or increase in both screening volume and cancer diagnosis where data were available (i.e. studies with longer time period after the onset of the pandemic) [33,34,63,64]. This reduction-rebound trend was also identified in a recent meta-analysis, which also reported decreases in the number of screening examinations of 45%–52% for cancer screening services [93]. Moreover, compared to studies which covered short duration of the pandemic, the reduction of screening volume was relatively less substantial in those that covered longer periods [15,16,22,30,35,36,40,46,47]. This might provide an insight that the impact of the pandemic on breast cancer screening and diagnosis may not be as sustained as expected. However, this cannot be determined without evidence of long-term outcome such as breast cancer mortality, survival and quality of life. It is therefore essential that ongoing research examines the longer-term impact of the pandemic.
This review also found that in studies from the US, non-White women were less likely to have breast cancer screening (than White women) during the pandemic in the context of reduction in screening volume more generally. This is consistent with other research that has shown a disproportionate effect of the pandemic on the health of minority groups [94,95]. These groups could be a focus for ‘catch-up screening’ during the pandemic recovery to manage any incremental effect from the pandemic on existing disparities.
This review has some limitations. Firstly, most of the included studies reported short-term impact and lacked data on long-term health outcomes. It is therefore unknown whether the pandemic will lead to worse longer-term outcomes, such as increased breast cancer mortality rates. Also, we did not extract (secular) trend from the included studies because the majority (94%) of studies reported the pandemic period of 1 year or less. Secondly, we used a narrative description without a meta-analysis due to considerable heterogeneity of reported outcomes. We provided the range of relative percentage change for screening volume and number of diagnoses, but we did not provide summary estimates (e.g. medians) due to the variability in reporting of these two outcomes in the included studies. Thirdly, as our review includes studies from all over the world, there is a possibility there may be studies with overlapping populations because many studies used aggregated data. However we ruled out superseded publications from same study at data synthesis to minimise the influence.
In addition, the studies in our review were generally at high risk of bias, reflecting both the observational nature of the comparisons and the urgency of reporting data to inform timely responses to a global pandemic. Although many studies accounted for seasonal variation by comparing the same calendar months across pandemic periods, other potential confounding of pre-versus-during pandemic comparisons was not commonly considered in the analyses or interpretation of results. Interrupted time series analysis, where temporal trends are assessed through multiple measurements over time, was used relatively infrequently. Although the impact of COVID-19 pandemic is likely to be dominant in the included studies, an interrupted time series approach would provide stronger evidence of the magnitude of changes in breast cancer screening and diagnostic outcomes [96].
5. Conclusions
This systematic review identified considerable reductions in breast screening volume and number of diagnosed breast cancers during the pandemic peak, and a relatively smaller reduction was noted after the peak. Changes in proportions of detection mode and stage at diagnosis were found, with higher proportions of cases diagnosed through symptomatic presentations and greater proportions of relatively more advanced stage at diagnosis during the pandemic. As our review includes studies reporting outcomes for early-mid-phase of the pandemic, high-quality studies examining the long-term impact are needed.
Funding
Funded by the National Breast Cancer Foundation, Chair in Breast Cancer Prevention (grant #EC-21-001 awarded to NH). BN is supported by a National Health and Medical Research Council Emerging Leader Research Fellowship (grant #1194108). MLM is funded by a National Breast Cancer Foundation Investigator Initiated Research Scheme grant (grant #IIRS-20-011). NH is supported by National Health and Medical Research Council Investigator grant (grant #1194410) and the National Breast Cancer Foundation (grant #EC-21-001).
Declaration of competing interest
The authors declared no conflict of interest.
Ethics
Ethical approval was not required.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2023.01.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Ghebreyesus T.A. World Health Organization; Feb: 2020. WHO Director-General's opening remarks at the mission briefing on COVID-19; p. 19. [Google Scholar]
- 2.Moynihan R., Sanders S., et al. Impact of COVID-19 pandemic on utilisation of healthcare services: a systematic review. BMJ Open. 2021;11(3):e045343. doi: 10.1136/bmjopen-2020-045343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riera R., Bagattini Â.M., et al. Delays and disruptions in cancer health care due to COVID-19 pandemic: systematic review. JCO Glob Oncol. 2021;7:311–323. doi: 10.1200/GO.20.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alkatout I., Biebl M., et al. Has COVID-19 affected cancer screening programs? A systematic review. Front Oncol. 2021:11. doi: 10.3389/fonc.2021.675038. [no pagination)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayo M., Potugari B., et al. Cancer screening during the COVID-19 pandemic: a systematic review and meta-analysis. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1109–1117. doi: 10.1016/j.mayocpiqo.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tao Z., Shi A., et al. Breast cancer: epidemiology and etiology. Cell Biochem Biophys. 2015;72(2):333–338. doi: 10.1007/s12013-014-0459-6. [DOI] [PubMed] [Google Scholar]
- 7.Figueroa J.D., Gray E., et al. The impact of the Covid-19 pandemic on breast cancer early detection and screening. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGowan J., Sampson M., et al. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 9.National Heart L., Institute B., et al. 2019. Study quality assessment tools. Availabe online.https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools accessed on 1206) [Google Scholar]
- 10.Cochrane Effective Practice and Organisation of Care (EPOC) 2017. Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors.https://epoc.cochrane.org/sites/epoc.cochrane.org/files/public/uploads/Resources-for-authors2017/suggested_risk_of_bias_criteria_for_epoc_reviews.pdf Available from: Accessed. [Google Scholar]
- 11.Al-Kuwari M.G., Abdulmalik M.A., et al. The impact of COVID-19 pandemic on the preventive services in Qatar. J Publ Health Res. 2021;10(1):1910. doi: 10.4081/jphr.2021.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amornsiripanitch N., Chikarmane S.A., et al. Patients characteristics related to screening mammography cancellation and rescheduling rates during the COVID-19 pandemic. Clin Imag. 2021;80:205–210. doi: 10.1016/j.clinimag.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amram O., Robison J., et al. Socioeconomic and racial inequities in breast cancer screening during the COVID-19 pandemic in Washington state. JAMA Netw Open. 2021;(5):e2110946. doi: 10.1001/jamanetworkopen.2021.10946. no pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakouny Z., Paciotti M., et al. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):458–460. doi: 10.1001/jamaoncol.2020.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bentley H., Woods R., et al. Can Assoc Radiol J; 2021. Hindsight is 2020: understanding the impact of the COVID-19 pandemic on a provincial population-based breast screening program. [DOI] [PubMed] [Google Scholar]
- 16.Bessa J.D.F. Breast Imaging Hindered During Covid-19 Pandemic, in Brazil. Rev. Saude Publica. 2021;55:1–8. doi: 10.11606/s1518-8787.2021055003375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brugel M., Carlier C., et al. Dramatic changes in oncology care pathways during the COVID-19 pandemic: the French ONCOCARE-COV study. Oncol. 2021;26(2):e338–e341. doi: 10.1002/onco.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen R.C., Haynes K., et al. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. 2021;7(6):878–884. doi: 10.1001/jamaoncol.2021.0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiarelli A.M., Walker M.J., et al. Adherence to guidance for prioritizing higher risk groups for breast cancer screening during the COVID-19 pandemic in the Ontario Breast Screening Program: a descriptive study. CMAJ Open. 2021;9(4):E1205–E1212. doi: 10.9778/cmajo.20200285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou C.P., Pan H.B., et al. Impact of the COVID-19 pandemic on the volume of mammography examinations in Southern Taiwan. Breast J. 2020;27(1):89–91. doi: 10.1111/tbj.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collado-Mesa F., Kaplan S.S., et al. Impact of COVID-19 on breast imaging case volumes in South Florida: a multicenter study. Breast J. 2020;26(11):2316–2319. doi: 10.1111/tbj.14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabkeviciene D., Vincerzevskiene I., et al. The impact of the COVID-19 pandemic on cancer patient's management-Lithuanian cancer center experience. Healthcare. 2021;9(11):9. doi: 10.3390/healthcare9111522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Degani Lopez G., Duarte L., et al. vol. 15. ecancermedicalscience; 2021. (The impact of the COVID-19 pandemic on cancer care in the public health subsector, province of Santa Fe, Argentina). [no pagination)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeGroff A., Miller J., et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January-June 2020, in the United States. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedewa S.A., Cotter M.M., et al. Cancer.; 2021. Changes in breast cancer screening rates among 32 community health centers during the COVID-19 pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher-Borne M., Isher-Witt J., et al. Understanding COVID-19 impact on cervical, breast, and colorectal cancer screening among federally qualified healthcare centers participating in "Back on track with screening" quality improvement projects. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorin S.N.S., Jimbo M., et al. The future of cancer screening after COVID-19 may be at home. Cancer. 2021;127(4):498–503. doi: 10.1002/cncr.33274. [DOI] [PubMed] [Google Scholar]
- 28.Jidkova S., Hoeck S., et al. Flemish population-based cancer screening programs: impact of COVID-19 related shutdown on short-term key performance indicators. BMC Cancer. 2022;22(1):183. doi: 10.1186/s12885-022-09292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang Y.J., Baek J.M., et al. Impact of the COVID-19 pandemic on the diagnosis and surgery of breast cancer: a multi-institutional study. J Breast Cancer. 2021;24(6):491–503. doi: 10.4048/jbc.2021.24.e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kidwai N. Routine cancer screening delays due to pandemic at veteran affairs. J Natl Med Assoc. 2022;114(1):12–15. doi: 10.1016/j.jnma.2021.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim E., Kojima N., et al. Impact of COVID-19 on primary care quality measures in an academic integrated health system. J Gen Intern Med. 2022;26:26. doi: 10.1007/s11606-021-07193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koczkodaj P., Sulkowska U., et al. SARS-CoV-2 as a new possible long-lasting determining factor impacting cancer death numbers. Based on the example of breast, colorectal and cervical cancer in Poland. Nowotwory. 2021;71(1):42–46. [Google Scholar]
- 33.Labaki C., Bakouny Z., et al. Recovery of cancer screening tests and possible associated disparities after the first peak of the COVID-19 pandemic. Cancer Cell. 2021;39(8):1042–1044. doi: 10.1016/j.ccell.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.London J.W., Fazio-Eynullayeva E., et al. Evolving effect of the COVID-19 pandemic on cancer-related encounters. JCO Clin Cancer Inform. 2022;6:e2100200. doi: 10.1200/CCI.21.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losurdo P., Samardzic N., et al. The real-word impact of breast and colorectal cancer surgery during the SARS-CoV-2 pandemic. Updates Surg. 2022;3 doi: 10.1007/s13304-021-01212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller M.M., Meneveau M.O., et al. Impact of the COVID-19 pandemic on breast cancer screening volumes and patient screening behaviors. Breast Cancer Res Treat. 2021;189(1):237–246. doi: 10.1007/s10549-021-06252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norbash A.M., Moore A.V., Jr., et al. Early-stage radiology volume effects and considerations with the coronavirus disease 2019 (COVID-19) pandemic: adaptations, risks, and lessons learned. J Am Coll Radiol. 2020;17(9):1086–1095. doi: 10.1016/j.jacr.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patt D., Gordan L., et al. Considerations to increase rates of breast cancer screening across populations. Am J Manag Care. 2022;28(3 Spec):14. doi: 10.37765/ajmc.2022.88855. . No.) [DOI] [PubMed] [Google Scholar]
- 39.Peng S.M., Yang K.C., et al. Impact of the COVID-19 pandemic on a population-based breast cancer screening program. Cancer. 2020;126(24):5202–5205. doi: 10.1002/cncr.33180. [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro C.M., Correa F.M., et al. Short-term effects of the COVID-19 pandemic on cancer screening, diagnosis and treatment procedures in Brazil: a descriptive study, 2019-2020. Epidemiol Serv Saude. 2022;31(1):e2021405. doi: 10.1590/S1679-49742022000100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen C.T., Hsieh H.M., et al. Different impacts of cancer types on cancer screening during COVID-19 pandemic in Taiwan. J Formos Med Assoc. 2022;15:15. doi: 10.1016/j.jfma.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sprague B.L., Lowry K.P., et al. Changes in mammography use by women's characteristics during the first 5 Months of the COVID-19 pandemic. J Natl Cancer Inst. 2021;113(9):1161–1167. doi: 10.1093/jnci/djab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutherland K., Chessman J., et al. Impact of COVID-19 on healthcare activity in NSW, Australia. Public Health Res Pract. 2020;30(4) doi: 10.17061/phrp3042030. [DOI] [PubMed] [Google Scholar]
- 44.Tang A., Neeman E., et al. Care in the time of COVID-19: impact on the diagnosis and treatment of breast cancer in a large, integrated health care system. Breast Cancer Res Treat. 2022;191(3):665–675. doi: 10.1007/s10549-021-06468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai H.Y., Chang Y.L., et al. Effects of the COVID-19 pandemic on breast cancer screening in Taiwan. Breast. 2020;54:52–55. doi: 10.1016/j.breast.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Velazquez A.I., Hayward J.H., et al. Trends in breast cancer screening in a safety-net hospital during the COVID-19 pandemic. JAMA Netw Open. 2021;4(8):e2119929. doi: 10.1001/jamanetworkopen.2021.19929. (no pagination)(8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker M.J., Meggetto O., et al. Measuring the impact of the COVID-19 pandemic on organized cancer screening and diagnostic follow-up care in Ontario, Canada: a provincial, population-based study. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whaley C.M., Pera M.F., et al. Changes in health services use among commercially insured US populations during the COVID-19 pandemic. JAMA Netw Open. 2020;3(11) doi: 10.1001/jamanetworkopen.2020.24984. [no pagination)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bansal G.J., Saleem Z. The symptomatic breast services in a university hospital: pandemic peak compared to the pre-pandemic year and future implications. Ir J Med Sci. 2022;191(6):2475–2479. doi: 10.1007/s11845-021-02910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blay J.Y., Boucher S., et al. Delayed care for patients with newly diagnosed cancer due to COVID-19 and estimated impact on cancer mortality in France. ESMO Open. 2021;6(3) doi: 10.1016/j.esmoop.2021.100134. [no pagination)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonadio R.C., Messias A.P., et al. ecancermedicalscience; 2021. Impact of the COVID-19 pandemic on breast and cervical cancer stage at diagnosis in Brazil; p. 15. [no pagination)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borsky K., Shah K., et al. Pattern of breast cancer presentation during the COVID-19 pandemic: results from a cohort study in the UK. Future Oncol. 2022;18(4):437–443. doi: 10.2217/fon-2021-0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chou C.P., Lin H.S. Delayed breast cancer detection in an asian country (Taiwan) with low covid-19 incidence. Cancer Manag Res. 2021;13:5899–5906. doi: 10.2147/CMAR.S314282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Citgez B., Yigit B., et al. The change in incidence of breast cancer by stage: how is it changed after the COVID-19 pandemic? a single-center retrospective study. Ann Ital Chir. 2021;92(5):488–493. [PubMed] [Google Scholar]
- 55.De Vincentiis L., Carr R.A., et al. Cancer diagnostic rates during the 2020 'lockdown', due to COVID-19 pandemic, compared with the 2018-2019: an audit study from cellular pathology. J Clin Pathol. 2021;74(3):187–189. doi: 10.1136/jclinpath-2020-206833. [DOI] [PubMed] [Google Scholar]
- 56.Drescher C.W., Bograd A.J., et al. Cancer case trends following the onset of the COVID-19 pandemic: a community-based observational study with extended follow-up. Cancer. 2022;128(7):1475–1482. doi: 10.1002/cncr.34067. [DOI] [PubMed] [Google Scholar]
- 57.Eijkelboom A.H., de Munck L., et al. Impact of the suspension and restart of the Dutch breast cancer screening program on breast cancer incidence and stage during the COVID-19 pandemic. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrara G., De Vincentiis L., et al. Cancer diagnostic delay in northern and Central Italy during the 2020 lockdown due to the coronavirus disease 2019 pandemic. Am J Clin Pathol. 2021;155(1):64–68. doi: 10.1093/ajcp/aqaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gathani T., Reeves G., et al. Impact of the COVID-19 pandemic on breast cancer referrals and diagnoses in 2020 and 2021: a population-based study in England. Br J Surg. 2022;109(2):e29–e30. doi: 10.1093/bjs/znab426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guven D.C., Sahin T.K., et al. Newly diagnosed cancer and the COVID-19 pandemic: tumour stage migration and higher early mortality. BMJ Support Palliat Care. 2021;28 doi: 10.1136/bmjspcare-2021-003301. [DOI] [PubMed] [Google Scholar]
- 61.Kaltofen T., Hagemann F., et al. Archives of Gynecology and Obstetrics.; 2021. Changes in gynecologic and breast cancer diagnoses during the first wave of the COVID-19 pandemic: analysis from a tertiary academic gyneco-oncological center in Germany. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kempf E., Lame G., et al. New cancer cases at the time of SARS-Cov2 pandemic and related public health policies: a persistent and concerning decrease long after the end of the national lockdown. Eur J Cancer. 2021;150:260–267. doi: 10.1016/j.ejca.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knoll K., Reiser E., et al. Arch Gynecol Obstet; 2021. The impact of COVID-19 pandemic on the rate of newly diagnosed gynecological and breast cancers: a tertiary center perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Linck P.A., Garnier C., et al. Impact of the COVID-19 lockdown in France on the diagnosis and staging of breast cancers in a tertiary cancer centre. Eur Radiol. 2022;32(3):1644–1651. doi: 10.1007/s00330-021-08264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lowry K.P., Bissell M., et al. Radiology; 2021. Breast biopsy recommendations and breast cancers diagnosed during the COVID-19 pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morais S., Antunes L., et al. The impact of the coronavirus disease 2019 pandemic on the diagnosis and treatment of cancer in Northern Portugal. Eur J Cancer Prev. 2022;31(2):204–214. doi: 10.1097/CEJ.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Brien A.C., McCartan D., et al. The COVID-19 impact on symptomatic breast cancer referrals and diagnosis. Ir Med J. 2021;114(4) [no pagination)] [Google Scholar]
- 68.Peacock H.M., Tambuyzer T., et al. Decline and incomplete recovery in cancer diagnoses during the COVID-19 pandemic in Belgium: a year-long, population-level analysis. ESMO Open. 2021;6(4) doi: 10.1016/j.esmoop.2021.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Purushotham A., Roberts G., et al. ecancermedicalscience; 2021. The impact of national non-pharmaceutical interventions ('lockdowns') on the presentation of cancer patients; p. 15. [no pagination)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruiz-Medina S., Gil S., et al. Significant decrease in annual cancer diagnoses in Spain during the COVID-19 pandemic: a real-data study. Cancers. 2021;13(13) doi: 10.3390/cancers13133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salem C., Hajj M.-A., et al. Radiology management of a 'breast unit' during COVID-19 pandemic: a single institution experience. Future Oncol. 2020;16(35):2917–2922. doi: 10.2217/fon-2020-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skovlund C.W., Friis S., et al. Hidden morbidities: drop in cancer diagnoses during the COVID-19 pandemic in Denmark. Acta Oncol. 2021;60(1):20–23. doi: 10.1080/0284186X.2020.1858235. [DOI] [PubMed] [Google Scholar]
- 73.Tachibana B.M.T., Ribeiro R.L.M., et al. The delay of breast cancer diagnosis during the COVID-19 pandemic in Sao Paulo, Brazil. Einstein (Sao Paulo) 2021;19:eAO6721. doi: 10.31744/einstein_journal/2021AO6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsibulak I., Reiser E., et al. Decrease in gynecological cancer diagnoses during the COVID-19 pandemic: an Austrian perspective. Int J Gynecol Cancer. 2020;30(11):1667–1671. doi: 10.1136/ijgc-2020-001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Wyk A.C., de Jager L.J., et al. The initial impact of the COVID-19 pandemic on the diagnosis of new cancers at a large pathology laboratory in the public health sector, Western Cape Province, South Africa. S Afr Med J. 2021;111(6):570–574. [PubMed] [Google Scholar]
- 76.Vrdoljak E., Balja M.P., et al. COVID‐19 pandemic effects on breast cancer diagnosis in Croatia: a population‐ and registry‐based study. Oncol. 2021;26(7):e1156–e1160. doi: 10.1002/onco.13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alelyani M., Alghamdi A., et al. The impact of the COVID-19 pandemic on medical imaging case volumes in aseer region: a retrospective study. Medicines (Basel) 2021;8(11):12. doi: 10.3390/medicines8110070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crisan C., Cainap C., et al. Decrease of oncological patients' hospital visits during Covid-19 pandemic; the experience of a tertiary Romanian centre. J BUON. 2021;26(3):1121–1126. [PubMed] [Google Scholar]
- 79.Doshi A.H., Kihira S., et al. Impact of COVID-19 social distancing regulations on outpatient diagnostic imaging volumes and no-show rates. Clin Imag. 2021;76:65–69. doi: 10.1016/j.clinimag.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lacson R., Shi J., et al. Exacerbation of inequities in use of diagnostic radiology during the early stages of reopening after COVID-19. J Am Coll Radiol. 2021;18(5):696–703. doi: 10.1016/j.jacr.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lang M., Yeung T., et al. Imaging volume trends and recovery during the COVID-19 pandemic: a comparative analysis between a large urban academic hospital and its affiliated imaging centers. Acad Radiol. 2020;27(10):1353–1362. doi: 10.1016/j.acra.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Le Bihan Benjamin C., Simonnet J.A., et al. Monitoring the impact of COVID-19 in France on cancer care: a differentiated impact. Sci Rep. 2022;12(1):4207. doi: 10.1038/s41598-022-07984-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naidich J.J., Boltyenkov A., et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on imaging case volumes. J Am Coll Radiol. 2020;17(7):865–872. doi: 10.1016/j.jacr.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patt D., Gordan L., et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Infor. 2020;(4):1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eijkelboom A.H., de Munck L., et al. Impact of the COVID-19 pandemic on diagnosis, stage, and initial treatment of breast cancer in The Netherlands: a population-based study. J Hematol Oncol. 2021;14(1):64. doi: 10.1186/s13045-021-01073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gathani T., Clayton G., et al. The COVID-19 pandemic and impact on breast cancer diagnoses: what happened in England in the first half of 2020. Br J Cancer. 2021;124(4):710–712. doi: 10.1038/s41416-020-01182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.London J.W., Fazio-Eynullayeva E., et al. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform. 2020;4(4):657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Vocht F., Katikireddi S.V., et al. Conceptualising natural and quasi experiments in public health. BMC Med Res Methodol. 2021;21(1):32. doi: 10.1186/s12874-021-01224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vanni G., Materazzo M., et al. Vol. 34. 2020. Breast cancer and COVID-19: the effect of fear on patients' decision-making process; p. 1651. (Vivo). 3 suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caplan L. Delay in breast cancer: implications for stage at diagnosis and survival. Front Public Health. 2014;2:87. doi: 10.3389/fpubh.2014.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kothari A., Fentiman I.S. Diagnostic delays in breast cancer and impact on survival. Int J Clin Pract. 2003;57(3):200–203. 22. [PubMed] [Google Scholar]
- 92.Bleicher R.J. Timing and delays in breast cancer evaluation and treatment. Ann Surg Oncol. 2018;25(10):2829–2838. doi: 10.1245/s10434-018-6615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Teglia F., Angelini M., et al. Global association of COVID-19 pandemic measures with cancer screening: a systematic review and meta-analysis. JAMA Oncol. 2022;8(9):1287–1293. doi: 10.1001/jamaoncol.2022.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tai D.B.G., Shah A., et al. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2021;72(4):703–706. doi: 10.1093/cid/ciaa815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Katikireddi S.V., Lal S., et al. Unequal impact of the COVID-19 crisis on minority ethnic groups: a framework for understanding and addressing inequalities. J Epidemiol Community Health. 2021;75(10):970. doi: 10.1136/jech-2020-216061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kontopantelis E., Doran T., et al. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ Br Med J (Clin Res Ed) 2015;350:h2750. doi: 10.1136/bmj.h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.