Abstract
Summary
A retrospective study of 121 patients who stopped denosumab (Dmab) then received no treatment (NT), risedronate (RIS), alendronate (ALN), or zoledronic acid (ZOL). Bone density (spine and hip) during and after Dmab discontinuation was measured. Treatment with ALN or ZOL, not NT and RIS, mitigated BMD loss after Dmab discontinuation.
Introduction
Denosumab (Dmab) discontinuation is associated with bone loss and multiple vertebral fractures. The purpose was to compare bone mineral density (BMD) change in patients following Dmab discontinuation with no subsequent treatment (NT) and three bisphosphonate (BP) treatments: risedronate (RIS), alendronate (ALN), and zoledronic acid (ZOL).
Methods
In a review of 121 patients aged 71.2 ± 8.1 years, discontinuing Dmab (mean 5.4 doses), 33 received NT and 88 received BP (22 RIS; 34 ALN; 32 ZOL). BMD change after 1 year was compared between groups at the lumbar spine (LS), femoral neck (FN), and total hip (TH). Risk factors for bone loss after Dmab discontinuation were compared between groups and incidence of vertebral fractures was determined.
Results
Following Dmab discontinuation, LS mean change (g/cm2; 95% CI) was for NT: − 0.041 (− 0.062 to − 0.021); RIS: − 0.035 (− 0.052 to − 0.017); ALN: − 0.005 (− 0.020 to 0.009); and ZOL: − 0.009 (− 0.025 to 0.008). Differences in LS were found between NT and ALN (p = 0.015), and NT and ZOL (p=0.037), but not between NT and RIS. The only significant difference in TH was found between NT and ZOL (p 0.034) with no group differences in FN. BMD gains during Dmab treatment were associated with BMD loss after Dmab discontinuation. In a subset, discontinuation after Dmab treatment (> 5 doses) followed by ALN (n = 22) and ZOL (n = 11) showed no difference in BMD. Five of 7 vertebral fractures occurred after Dmab discontinuation in NT.
Conclusion
Subsequent treatment with ALN or ZOL but not NT and RIS mitigates BMD loss after Dmab discontinuation.
Supplementary information
The online version contains supplementary material available at 10.1007/s00198-022-06648-9.
Keywords: Bisphosphonates, Bone mineral density, Denosumab, Discontinuation, Osteoporosis, Subsequent treatment
Introduction
Denosumab (Prolia), a human monoclonal antibody that targets RANKL (receptor activator of nuclear factor-kappa B ligand), has been reported to have antiresorptive activity. The pivotal FREEDOM [1] and its extension trial [2] showed continued increase in bone mineral density (BMD) at the lumbar spine and total hip without plateauing, plus significant reduction in vertebral, nonvertebral, and hip fractures while taking denosumab (Dmab). However, Dmab discontinuation has been associated with reduction of BMD gained during treatment [3, 4], rebound of bone turnover markers (BTMs) overriding pretreatment status [5, 6], and increased risk of multiple vertebral fractures [3, 7–10].
There have been studies that show treatment is needed after Dmab discontinuation [3, 11, 12]. However, some have combined agents in the analysis, combining all oral bisphosphonates (BP) [3, 13, 14] or combining BP and selective estrogen receptor modulator (SERM) [11]. Therefore, it is difficult to determine which BP to use to prevent this rebound phenomenon. There is a lack of data on the effect of different types of BP (oral versus IV BP) on BMD change, and which patient characteristics lead to risk for BMD loss after Dmab discontinuation. Therefore, we conducted a retrospective chart review of several Dmab discontinuation pathways, using data from prior patients.
Material and methods
Study design and participants
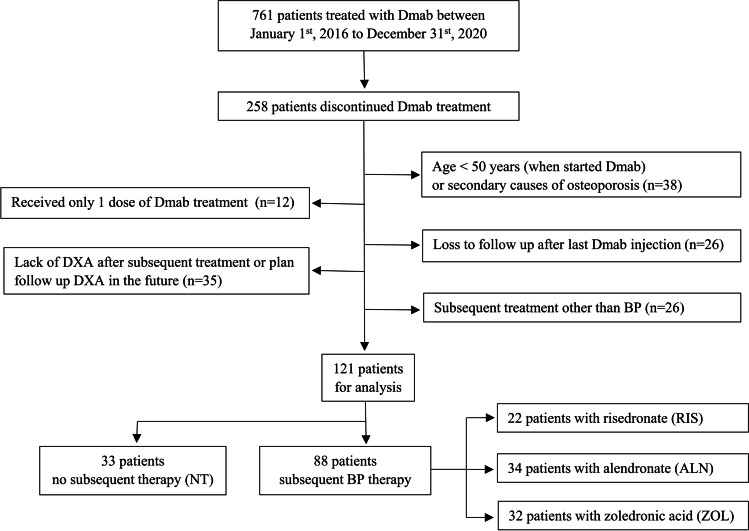
This study was a monocentric retrospective chart review performed at the Hospital for Special Surgery (HSS), New York. We used the data from patients with primary osteoporosis, at an outpatient Metabolic Bone Diseases clinic, who were treated with Dmab (60 mg sc injection every 6 months, at least two injections) between January 1, 2016, and December 31, 2020 (n = 761). We identified 258 patients who discontinued Dmab treatment. Among them, we excluded 137 patients for the following reasons (see Fig. 1): age or secondary osteoporosis (n = 38); only one Dmab dose (n = 12); loss to follow-up (n = 26); lack of BMD data (n = 35) or treatment after Dmab was not BP (n = 26).
Fig. 1.
The study flowchart. Dmab, denosumab; DXA, dual-energy X-ray absorptiometry; BP, bisphosphonates
The remaining 121 patients were used in this analysis. There were 33 that did not receive any treatment (no treatment group, NT), even though they were advised and warned about the risk of the rebound phenomenon and for multiple vertebral fractures. In the group that received subsequent therapy with BP (n = 88), the choice of treatment was based on physician’s decision in consultation with the patient; oral BP: risedronate (RIS) 35 mg/week (n = 22), alendronate (ALN) 70 mg/week (n = 34), and zoledronic acid (ZOL) one dose of 5 mg (n = 32). This subsequent treatment was received approximately 6 months after the last Dmab injection, per the European Calcified Tissue Society (ECTS) position statement [15]. All patients received both daily calcium supplementation (total calcium 1000–1200 mg/day) and vitamin D supplementation (oral cholecalciferol, 1000–2000 IU/day) if needed, and dose adjusted according to their 25(OH)D level.
This study protocol was reviewed and approved by the Institutional Review Board at HSS. The informed consent was waived due to the retrospective chart review and anonymous data.
Measurements
Areal BMD was measured in grams per centimeter at the lumbar spine (LS) using L1–L4, femoral neck (FN), and total hip (TH) by DXA. DXA scans were performed by Hologic (Horizon® DXA system) with a least significant change (LSC) of 0.024 g/cm2, 0.032 g/cm2, and 0.017 g/cm2 for the lumbar, femoral neck, and total hip, respectively. LS that was uninterpretable due to extensive degeneration, significant scoliosis, and instrumentation/artifact were excluded from the analysis. Likewise, instrumentation/artifact at proximal femur was excluded. We used the term DXA1 at the time of first Dmab initiation, DXA2 at the time of last Dmab injection, and DXA3 for the follow-up DXA approximately 12 months after receiving BP (in no treatment group median follow-up, DXA3 was 15 months after the last Dmab injection). We calculated absolute change (g/cm2) in LS, FN, and TH BMD to evaluate BMD gained during Dmab treatment (DXA2-DXA1), BMD change after Dmab discontinuation ± subsequent therapy (DXA3-DXA2), and BMD change between the end of follow-up and the Dmab initiation (DXA3-DXA1).
Thoracolumbar spine X-rays, MRIs, or vertebral fracture assessments (VFA) were reviewed at each DXA measurement from the radiologists’ report. The decision to include vertebral imaging was made by the treating physician and was completed in 81% of patients. Morphometric vertebral fractures were diagnosed as newly developed or progression of at least one grade, per the semiquantitative method described by Genant [16]. All cases with morphometric vertebral fractures were confirmed by the corresponding author.
Serum C-telopeptide (CTX) was measured in a subset of patients.
Treatment outcomes
The primary outcome was the absolute change in BMD between no subsequent treatment, risedronate, alendronate, and zoledronic acid at the spine and hip following Dmab discontinuation (DXA3-DXA2) at approximately 12 months after receiving BP (18 months after the last dose of Dmab). The secondary outcomes were the absolute change in BMD during Dmab treatment (DXA2-DXA1), and the absolute change in BMD at follow-up for subsequent treatment (including no treatment) from Dmab initiation (DXA3-DXA1) in each group. The patient-related factors for bone loss after Dmab discontinuation (DXA3-DXA2) were also assessed.
Statistical analysis
Continuous data were presented as mean ± standard deviation (SD) and categorical data were summarized as numbers (percent). Patients’ demographics, BMD measurements, and treatment characteristics at the time of Dmab discontinuation were compared among the four groups using analysis of variance (ANOVA) and chi-square tests for categorical data. For any significant difference, post hoc analysis with the Bonferroni method were conducted. Within each group and for each site (LS, FN, TH), paired t-tests were used to compare BMD gained during Dmab treatment (DXA2-DXA1) and net BMD change post-Dmab discontinuation compared to baseline (DXA3-DXA1), and between before and after Dmab discontinuation (DXA3-DXA2). To characterize the effect of post-Dmab treatment on BMD, absolute BMD change after Dmab discontinuation (DXA3-DXA2) was compared between no additional treatment as the reference group and the rest of the groups for the three sites (LS, FN, TH) using ANOVA and post hoc analysis with the Bonferroni method. Multivariate linear regression models were used to delineate the predictive values of variables of interest on BMD change after Dmab discontinuation. One model was constructed per site for LS, FN, and TH. Analysis excluded those cases with missing data for each variable of interest. Alpha was set to 0.05 for all analyses. Statistical analysis was performed with MATLAB 2022a (MathWorks, USA).
Results
One hundred and twenty-one patients were included in the analysis (Fig. 1). The mean age was 71.2 ± 8.1 years (mean ± SD) with 112 females (92.6%). In this study, the participant racial distribution was as follows: 89.3% White, 4.1% Asian, and 1.7% Other, and for 5% of participants race data was missing. For ethnicity, most of the participants (95.9%) were not Hispanic or Latino, 2.5% were Hispanic or Latino, and for 1.7% of participants the ethnicity data was missing. The mean BMD T-score when Dmab was initiated in each group is shown in Table 1. The mean T-score is in the low bone mass range although each patient had osteoporosis at some site before initiation, or they had low bone mass with a recent fragility fracture (45/121 of participants). Patient characteristics at the time of Dmab discontinuation are shown in Table 1. No statistically significant differences between the 4 groups were found for most variables (Table 1) including basic metabolic laboratory results (corrected Ca, 25[OH]D, PTH, Cr, and eGFR; data not shown) and bone turnover markers (data not shown). However, BP therapy was started later in zoledronic acid group (6.4 ± 1.0 months) when compared to both oral BP (5.8 ± 2.0 months in RIS group and 5.5 ± 1.1 months in ALN group, p 0.022). The total number of Dmab injections was also significantly different between the no treatment group (4.6 ± 1.6 doses) and alendronate group (6.5 ± 2.8 doses, p = 0.008). BMD at LS, FN, and TH (g/cm2 and T-score) at discontinuation of Dmab was significantly different between 4 groups (p for all < 0.05). In the post hoc analysis, the difference was significant only between NT and groups that received subsequent therapy (Table 1). The most common reasons for Dmab discontinuation were the coronavirus disease (COVID) pandemic and patient’s refusal for further treatment (NT), and BMD goal achievement (T-score better than − 2.5) in the oral and IV BP groups. Two people (6.1%) in the no treatment group received dental care and stopped Dmab without notifying their physicians, and osteonecrosis of the jaw (ONJ) was suspected in one patient. One patient each from the alendronate and zoledronic acid groups stopped because of suspected side effects from Dmab, although one ALN subject discontinued Dmab due to insurance coverage issues.
Table 1.
Baseline characteristics of patients at the time of Dmab discontinuation (unless otherwise noted)
| Characteristics | No treatment (n = 33) | Risedronate (n = 22) | Alendronate (n = 34) | Zoledronic acid (n = 32) | p value |
|---|---|---|---|---|---|
| Age (years) | 72.2 ± 9.0 | 68.7 ± 8.2 | 72.0 ± 7.5 | 71.0 ± 7.5 | 0.386 |
| Women (n, %) | 32 (97.0%) | 22 (100%) | 30 (88.2%) | 28 (87.5%) | 0.186 |
| Body mass index (kg/m2) | 22.3 ± 2.8 | 22.7 ± 2.9 | 24.3 ± 4.9 | 23.2 ± 3.0 | 0.131 |
| Charlson Comorbidity Index (range) | 1 (0–3) | 1 (0–3) | 1 (0–3) | 1 (0–4) | 0.353 |
| Oral/IV BP use before Dmab (n, %) | 12 (36.4%) | 11 (50.0%) | 15 (44.1%) | 8 (25.0%) | 0.239 |
| TPTD use before Dmab (n, %) | 9 (27.3%) | 4 (18.2%) | 6 (17.6%) | 10 (31.3%) | 0.517 |
| Prior vertebral fragility fracture (n, %) | 5 (15.2%) | 2 (9.1%) | 5 (14.7%) | 4 (12.5%) | 0.916 |
| Prior non-vertebral fragility fracture (n, %) | 17 (51.5%) | 8 (36.4%) | 17 (50.0%) | 19 (59.4%) | 0.426 |
| Prior any fracture (n, %) | 21 (63.6%) | 11 (50.0%) | 21 (61.8%) | 22 (68.8%) | 0.572 |
| BMD T-score before Dmab initiation | |||||
|
- Lumbar spine - Femoral neck - Total hip |
− 2.3 ± 1.0 − 2.4 ± 0.6 − 2.0 ± 0.6 |
− 2.4 ± 0.7 − 2.0 ± 0.5 − 1.8 ± 0.5 |
− 1.9 ± 0.8 − 2.1 ± 0.6 − 1.7 ± 0.5 |
− 1.9 ± 0.7 − 2.0 ± 0.5 − 1.6 ± 0.7 |
0.052 0.060 0.052 |
| Start treatment after last Dmab injection (months) | N/A | 5.8 ± 2.0 | 5.5 ± 1.1 | 6.4 ± 1.0a | 0.022 |
| Number of Dmab injection | 4.6 ± 1.6b | 5.2 ± 2.4 | 6.5 ± 2.8 | 5.3 ± 2.6 | 0.013 |
| BMD (g/cm2) | |||||
|
- Lumbar spine - Femoral neck - Total hip |
0.829 ± 0.122c 0.603 ± 0.081d 0.717 ± 0.079d |
0.856 ± 0.064 0.647 ± 0.051 0.762 ± 0.049 |
0.895 ± 0.117 0.646 ± 0.081 0.762 ± 0.076 |
0.895 ± 0.076 0.648 ± 0.055 0.762 ± 0.088 |
0.035 0.028 0.047 |
| BMD T-score | |||||
|
- Lumbar spine - Femoral neck - Total hip |
− 1.9 ± 1.1c − 2.2 ± 0.7d − 1.9 ± 0.6d |
− 1.7 ± 0.6 − 1.8 ± 0.5 − 1.5 ± 0.4 |
− 1.3 ± 1.0 − 1.8 ± 0.7 − 1.5 ± 0.6 |
− 1.3 ± 0.7 − 1.8 ± 0.5 − 1.5 ± 0.7 |
0.016 0.022 0.035 |
| Reasons for Dmab discontinuation | |||||
|
- Achieve BMD goal - Patient’s preference - COVID pandemic - Dental care - Side effects from Dmab - Other reasons |
4 (12.1%) 10 (30.3%) 16 (48.5%) 2 (6.1%) 0 (0%) 1 (3.0%) |
21 (95.5%) 0 (0%) 1 (4.5%) 0 (0%) 0 (0%) 0 (0%) |
24 (70.6%) 2 (5.9%) 6 (17.6%) 0 (0%) 1 (2.9%) 1 (2.9%) |
30 (93.8%) 1 (3.1%) 0 (0%) 0 (0%) 1 (3.1%) 0 (0%) |
< 0.001 < 0.001 < 0.001 0.143 0.637 0.650 |
Data are presented as mean ± standard deviation (SD) unless otherwise noted
Dmab, denosumab; BP, bisphosphonates; IV, intravenous; TPTD, teriparatide; N/A, not applicable; pts, patients; BMD, bone mineral density; COVID, coronavirus disease
Significant p values are shown in bold and were calculated from analysis of variance (ANOVA) for continuous data, chi-square tests for categorical data. Post hoc analysis with Bonferroni method resulted in: azoledronic acid difference from other groups, bdifference between no treatment and alendronate, cno treatment difference from alendronate and zoledronic acid, dno treatment difference from all other groups
Eighty-one patients had serum CTX measured post-Dmab discontinuation (mean follow-up time of 16.1 months after the last Dmab injection in the no treatment group and approximately 12 months in the BP groups). Average serum CTX was (mean ± SD) 633.4 ± 306.4 pg/mL, 358.3 ± 202.6 pg/mL, 380.5 ± 240.4 pg/mL, and 259.4 ± 126.9 pg/mL in the no treatment group, alendronate group, risedronate group, and zoledronic acid group, respectively. There was a significant difference in serum CTX during follow-up between the 4 groups (p < 0.001); in the subgroup analysis, the significance was between the no treatment group and each BP group (p = 0.002 vs ALN, p = 0.018 vs RIS, p < 0.001 vs ZOL).
Absolute change in BMD after Dmab discontinuation according to subsequent treatment (DXA3-DXA2)
Group comparison by ANOVA resulted in significant group differences at all skeletal sites (Table 2). From the last Dmab injection (DXA2) to the follow-up DXA (DXA3), the absolute LS BMD (g/cm2) significantly decreased by − 0.041 with a 95% confidence interval (CI) − 0.062 to − 0.021 in the no treatment group (used as reference). In patients who received subsequent treatment with risedronate, the follow-up DXA also significantly decreased by − 0.035 (95% CI − 0.052 to − 0.017). The absolute change of DXA3-DXA2 was not different between the no treatment group and the risedronate group. Patients who received either alendronate or zoledronic acid after Dmab discontinuation demonstrated a smaller absolute BMD change when compared to the reference NT group (p 0.015 and 0.037, respectively) (Table 2). Absolute FN and TH BMD were significantly decreased in the no treatment group, − 0.013 (95% CI − 0.023 to − 0.003) and − 0.016 (95% CI − 0.025 to − 0.007), respectively. The mean absolute change of DXA3-DXA2 was not different between the no treatment group and the risedronate group at both FN and TH but the no treatment group was different from zoledronic acid at TH (p = 0.034) (Table 2).
Table 2.
The associations between subsequent therapy (oral, IV BP) and no subsequent treatment with absolute change (g/cm2) in LS, FN, and TH BMD between last denosumab injection (DXA2) and follow-up (DXA3)
| Parameters | Treatment | Mean absolute change (95% confidence interval) |
p valuea |
|---|---|---|---|
| LS BMD Change (g/cm2) |
No treatment (n = 29) Risedronate (n = 20) Alendronate (n = 28) Zoledronic acid (n = 27) |
− 0.041 (− 0.062 to − 0.021) − 0.035 (− 0.052 to − 0.017) − 0.005 (− 0.020 to 0.009) − 0.009 (− 0.025 to 0.008) |
Reference > 0.999 0.015 0.037 |
| FN BMD change (g/cm2) |
No treatment (n = 31) Risedronate (n = 20) Alendronate (n = 34) Zoledronic acid (n = 31) |
− 0.013 (− 0.023 to − 0.003) − 0.011 (− 0.029 to 0.006) 0.004 (− 0.007 to 0.014) 0.004 (− 0.006 to 0.013) |
Reference > 0.999 0.160 0.181 |
| TH BMD change (g/cm2) |
No treatment (n = 31) Risedronate (n = 20) Alendronate (n = 34) Zoledronic acid (n = 31) |
− 0.016 (− 0.025 to − 0.007) − 0.017 (− 0.027 to − 0.007) − 0.006 (− 0.017 to 0.004) 0.001 (− 0.006 to 0.008) |
Reference > 0.999 0.673 0.034 |
IV, intravenous; BP, bisphosphonates; BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; LS, lumbar spine; FN, femoral neck; TH, total hip; ALN, alendronate; ZOL, zoledronic acid
Significant p values are shown in bold
aGroup comparison by analysis of variance (ANOVA): LS (p = 0.004); FN (p = 0.047); TH (p = 0.016). Adjusted p value was calculated from post hoc analysis with the Bonferroni method
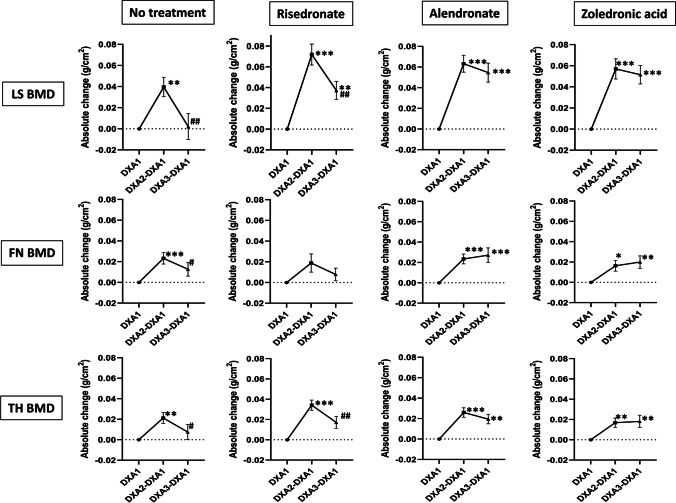
Absolute BMD change during Dmab treatment and after Dmab discontinuation
The graphs of absolute change in BMD (g/cm2) are found in Fig. 2. During Dmab treatment (DXA2-DXA1), absolute LS, FN, and TH BMD (g/cm2) increased significantly within each group when compared to Dmab initiation (DXA1) without significant differences between the 4 groups at all three skeletal sites. The loss of bone from Dmab discontinuation (DXA3-DXA2) was minimized by ALN or ZOL but not by RIS or NT. In fact, the bone loss with discontinuation for the LS BMD is equal to a change of − 0.49% for ALN and − 1.05% for ZOL vs − 4.00% for RIS and − 4.91% for NT. When absolute change in BMD after DMAB discontinuation was compared between BP groups (data not shown), there was no significant difference between zoledronic acid vs alendronate at any of three sites but alendronate was different from risedronate for LS BMD (p = 0.037) and zoledronic acid was better for TH BMD (p 0.032) than RIS. When follow-up LS BMD was compared to Dmab initiation (DXA3-DXA1), the mean LS DXA values returned to pre Dmab levels during the off-treatment interval in NT subjects, while for the risedronate group the LS BMD loss was about 50% smaller, with even more modest losses evident with ALN and ZOL.
Fig. 2.
Absolute change (mean ± SEM) in LS, FN, and TH BMD during denosumab treatment and after receiving no subsequent therapy or received subsequent treatment with risedronate, alendronate, or zoledronic acid. DXA1 (baseline), BMD at the time of Dmab initiation; DXA2-DXA1, BMD gained during Dmab treatment; DXA3-DXA1, BMD after Dmab discontinuation (± subsequent therapy) when compared to DXA1 (baseline); SEM, standard error of the mean; LS, lumbar spine; FN, femoral neck; TH, total hip; BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; Dmab, denosumab. Differences were compared using paired t-tests. *Significant change when compared to Dmab initiation (*p value < 0.05; **p value < 0.01; ***p value < 0.001). #Significant change when compared to Dmab discontinuation (#p value < 0.05; # #p value < 0.01)
Patient-related factors for predicting BMD change after Dmab discontinuation
Multivariate linear regression was analyzed in all patients. The results with coefficient (95% CI) and p value are illustrated in Table 3. Patients receiving alendronate or zoledronic acid therapy following denosumab experienced significantly less BMD loss in all 3 locations compared to those who received NT or RIS (LS BMD, p < 0.001; FN BMD, p = 0.014; TH BMD, p < 0.001). Patients with larger BMD gains during Dmab treatment experienced significantly greater loss of LS and FN BMD after Dmab discontinuation at each anatomic site (LS BMD, p = 0.001; FN BMD, p = 0.001). Higher BMI was associated with a greater decrease in TH BMD upon Dmab discontinuation (p = 0.006).
Table 3.
The relationship between percent change in LS, FN, and TH BMD (dependent variable) among the last denosumab injection (DXA2) and follow-up (DXA3) and patient-related factors (independent variables) by using multivariable regression models
| Patient-related factors | LS BMD change (%) | FN BMD change (%) | TH BMD change (%) | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | Coefficient (95% CI) | p value | |
| Age (per 1 year increase) | − 0.05 (− 0.18 to 0.08) | 0.461 | 0.03 (− 0.08 to 0.13) | 0.637 | − 0.01 (− 0.08 to 0.07) | 0.944 |
| BMI (per 1 kg/m2 increase) | − 0.02 (− 0.30 to 0.26) | 0.904 | − 0.03 (− 0.26 to 0.20) | 0.782 | − 0.25 (− 0.42 to − 0.07) | 0.006 |
| Prior vertebral Fx | − 0.87 (− 3.95 to 2.21) | 0.577 | − 0.84 (− 3.26 to 1.58) | 0.492 | − 0.18 (− 1.99 to 1.63) | 0.842 |
| Any fragility Fx during Dmab | − 1.66 (− 6.22 to 2.89) | 0.471 | − 1.49 (− 5.09 to 2.10) | 0.412 | − 1.17 (− 3.82 to 1.49) | 0.386 |
| BP before Dmab | 1.78 (− 0.25 to 3.81) | 0.085 | 1.13 (− 0.54 to 2.80) | 0.183 | 0.92 (− 0.32 to 2.16) | 0.145 |
| Total Dmab doses | 0.06 (− 0.35 to 0.48) | 0.764 | − 0.17 (− 0.51 to 0.17) | 0.323 | − 0.21 (− 0.46 to 0.04) | 0.095 |
| ALN or ZOL vs. RIS or NT after Dmab | 3.76 (1.76 to 5.81) | < 0.001 | 2.14 (0.44 to 3.83) | 0.014 | 2.45 (1.22 to 3.75) | < 0.001 |
| LS T-score (start Dmab) | 0.26 (− 1.01 to 1.52) | 0.687 | ||||
| FN T-score (start Dmab) | 0.85 (− 0.57 to 2.26) | 0.240 | ||||
| TH T-score (start Dmab) | − 0.67 (− 1.70 to 0.36) | 0.200 | ||||
| LS BMD change (during Dmab) | − 0.30 (− 0.47 to − 0.13) | 0.001 | ||||
| FN BMD change (during Dmab) | − 0.26 (− 0.41 to − 0.11) | 0.001 | ||||
| TH BMD change (during Dmab) | − 0.07 (− 0.24 to 0.09) | 0.366 | ||||
LS, lumbar spine; FN, femoral neck; TH, total hip; BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; BMI, body mass index; Fx, fractures; Dmab, denosumab; BP, bisphosphonates; UNL, upper normal limit; 95% CI, 95% confidence interval
Significant p values are shown in bold. All variables listed were included in each regression model
A sensitivity analysis was done by excluding the patients from the no treatment group (n = 33) and then using the same variables for the regression models (Table 3). After excluding the NT group, the results were the same, indicating that the associations noted in Table 3 were not primarily attributable to the NT group.
Efficacy of alendronate vs zoledronic acid to preserve BMD after prolonged (Dmab > 5 doses) or shorter Dmab treatment (Dmab ≤ 5 doses)
In patients who received more than 5 doses of Dmab, we compared the BMD changes between DXA3-DXA2 in patients who received subsequent therapy with either alendronate (n = 22) or zoledronic acid (n = 11). The baseline characteristics were comparable between two groups (as shown in Supplement 1). The mean ages were 72.2 years and 73.9 years, and the mean number of Dmab injections were 8.0 and 8.1 doses in the alendronate and zoledronic acid groups, respectively. The interval between last Dmab and the first BP dose was slightly less in the alendronate group (mean ± SD; 5.9 ± 0.7 months; 6.5 ± 0.8 months, p 0.060). BMD change at all 3 sites after Dmab discontinuation was not different between the ZOL and ALN groups following long-term Dmab (mean LS BMD change: 0% ALN vs − 3.9% ZOL, p 0.074; FN BMD: − 0.2% ALN vs − 0.7% ZOL, p 0.733; TH BMD: − 0.8% ALN vs − 0.7% ZOL, p 0.919). BMD change was also not different between alendronate and zoledronic acid after discontinuation following shorter Dmab treatment (all p > 0.05).
Vertebral fractures after Dmab discontinuation
A total of 7 female patients (5.8%) sustained vertebral fracture (n = 5 [15.2%], no treatment; n = 1 [2.9%], alendronate; n = 1 [3.1%] zoledronic acid) after Dmab discontinuation. Multiple vertebral fractures (more than 1 level) occurred in 4 out of 5 instances of vertebral fractures in the no treatment group; however, only single-level vertebral fracture occurred in the alendronate and zoledronic acid groups. Most patients with vertebral fractures (85.7%) had the clinical symptom of back pain, except one patient in no treatment group. The details for each patient are shown in Table 4. The small number of fractures precluded statistical analysis.
Table 4.
Characteristics of patients at the time of vertebral fragility fracture after Dmab discontinuation
| Patients | Sites of Fx | Treatment after Dmab discont | Age at Dmab discont (years) | Prior fragility Fx | Prior treatment | Total Dmab (doses) | Fragility Fx during Dmab treatment | Serum CTX (or urine NTX) / Serum BSAP | BMD T-Score post Dmab (LS, FN, TH) | % BMD change with Dmab treatment (LS, FN, TH) |
% BMD change after stop Dmab (± subsequent treatment) (LS, FN, TH) |
Time since last Dmab to vertebral Fx (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No.1 | T11 (Mild) (Asymp) | None | 83.5 | No | No | 5 | No | *823 (CTX) / 16.5 | LS − 1.8, FN − 2.7, TH − 1.8 | LS + 12.3%,FN + 15.2%, TH + 9.4% | LS − 12.7%, FN − 6.2%, TH − 4.4% | 27 |
| No.2 |
T6 (Mod), T10 (Mod), T7 (Mild) (Symp) |
None | 73.9 | T6 (mild) |
RIS (2 yrs) (stop 4yrs before Dmab) |
6 | No | 314 (CTX) / 11.5 | LS − 1.8, FN − 0.7, TH − 1.0 | LS + 7.4%, FN + 8.8%, TH + 6.6% | LS − 6.5%, FN − 0.4%, TH + 0.1% | 13 |
| No.3 |
T12 (Mod), L1 (Mod) (Symp) |
None | 82.1 |
L1 (mild) L2 (mild) |
ALN (5 yrs) (stop 2yrs before Dmab) |
3 | No | 413 (CTX) / 6.8 | LS − 4.3, FN − 3.1, TH − 2.7 | LS 0%, + FN 1.0%, TH + 4.6% | LS − 0.9%, FN + 1.8%, TH + 3.4% | 15 |
| No.4 |
T8 (Sev), L2 (Mild), L4 (Mild) (Symp) |
None | 58.8 | Proximal humerus, patella |
TPTD (18 mo) (then start Dmab) |
4 | No | 41 (NTX) / 15.1 | LS − 3.5, FN − 3.2, TH − 2.0 | LS + 4.5%, FN + 4.0%, TH + 0.7% |
N/Aa, N/Aa, N/Aa |
9 |
| No.5 |
L1 (Mod), L3 (Mild) (Symp) |
None | 79 | Distal radius |
TPTD (18 mo) (then start Dmab) |
2 | No | 67 (NTX) / 13.1 | LS − 3.7, FN − 3.6, TH − 2.8 | LS + 4.4%, FN + 2.9%, TH + 1.5% | LS − 5.6%, FN − 0.2%,TH + 0.5% | 10 |
| No.6 | T10 (Mod) (Symp) | ALN | 66.4 | No | No | 10 | No | 312 (CTX) / 17.2 | LS − 2.0, FN − 2.1, TH − 1.5 | LS + 11.9%, FN − 1.4%, TH + 4.2% | LS − 8.4%, FN + 1.1%, TH + 0.3% | 10 |
| No.7 | T5 (Mod) (Symp) | ZOL | 75.8 | Humerus shaft | No | 4 | No | *310 (CTX) / 18.4 | LS + 0.4, FN − 1.0, TH − 0.7 | LS + 16.1%, FN + 1.7%, TH + 8.1% | LS + 0.1%,FN + 11.2%, TH + 1.8% | 20 |
Fx, fracture; discont, discontinuation; Dmab, denosumab; BP, bisphosphonates; Mild, mild vertebral compression fracture; Mod, moderate vertebral compression fracture; Sev, severe vertebral compression fracture; Asymp, asymptomatic; Symp, symptomatic; ALN, alendronate; RIS, risedronate; ZOL, zoledronic acid; TPTD, teriparatide; mo, months; yrs, years; CTX, C-terminal telopeptide of type I collagen; NTX, urine cross-linked N-telopeptide of type I collagen; BSAP, bone-specific alkaline phosphatase; N/A, not available
aCannot be evaluated due to vertebral fracture and bilateral hip arthroplasty (treatment for femoral head insufficiency fracture and severe osteoarthritis of another hip)
*BTM was not collected at the time of fracture—for patient No.1 CTX was tested at 27 months after the last Dmab injection and for patient No. 7 CTX was collected 12 months after receiving ZOL
Discussion
In this retrospective study, we found that sequential treatment with alendronate and zoledronic acid, but not risedronate, could mitigate BMD loss after Dmab discontinuation when compared to no subsequent therapy. Approximately half of the patients in the no treatment group had lower BMD approximately 1 year after discontinuation as compared to their BMD when initiating Dmab treatment. Decreases in LS and FN BMDs after Dmab discontinuation were associated with BMD gained during Dmab treatment at each site. Vertebral fractures occurred in about 6% of patients and were uncommon in patients who received subsequent treatment with BP.
In patients who did not receive subsequent treatment after Dmab discontinuation, mean BMD loss in the present study is 4.9%, which is slightly lower than that of previous studies (5–11%) [3–5, 17]. Our patients may have less BMD loss because of shorter duration of Dmab treatment and less gain in BMD on Dmab, since there were 5 injections in our study versus 14–20 injections in others [3, 4, 17]. Another reason might be prior BP treatment (before Dmab) possibly causing less BMD gain during Dmab treatment compared to treatment-naïve [18]; nearly two-thirds of our patients (64%) in this group were pre-treated with BP or TPTD before Dmab. Younger age [11, 19] has been reported as a risk factor for bone loss, while prevalent fractures [20] have been shown to have no impact on BMD loss after Dmab discontinuation. Studies of the effects of prior bisphosphonate therapy on rates of post-Dmab bone loss have been inconsistent [20–24].
In the current study, age, prevalent fractures, and previous antiresorptive treatment were not associated with BMD loss after Dmab discontinuation. We found that higher BMI was associated with a greater decrease in TH BMD upon Dmab discontinuation, although this might be a chance finding.
The effect of alendronate after Dmab discontinuation was assessed by a post hoc analysis in a recent study [25] using the data from one group of patients in the original Denosumab Adherence Preference Satisfaction (DAPS) study [26]. Participants used Dmab therapy for 1 year (n = 115), then had 1 year of alendronate. Mean BMD at all 3 sites were maintained in most patients; however, it should be noted that the duration of Dmab treatment was relatively short, and might be unusual in clinical practice.
There are few studies regarding risedronate after Dmab discontinuation. A case series of 5 women after the FRAME extension study [27] (1 year of ROMO or placebo, then 2 years of Dmab), followed by 1 year of risedronate, showed a mean change of − 9.9% in LS BMD and − 3.9% in TH BMD. This result might be dictated by both the higher percentage of BMD gained and therefore the potential for greater BMD loss after receiving risedronate (LS BMD + 17.1% and TH BMD + 10.6% during ROMO). Moreover, a retrospective study by Laroche et al. (n = 18), used risedronate for only 3 months, then measured BMD at follow-up after 9 months free of BP. The mean bone loss was − 4.6% at LS and − 1.8% at TH BMD. The mean duration of Dmab treatment was not different from our study (38.7 months; our study 33.9 months in risedronate group, p 0.292). Our results confirmed that 1 year of risedronate might not be strong enough to prevent bone loss after Dmab discontinuation. This may be caused by a lower suppression effect in bone turnover markers and a lower gain in BMD shown in direct comparative studies between approved doses of risedronate and alendronate [28, 29].
In general, zoledronic acid has been shown to maintain [30–32] or minimize loss [33] of BMD gained after Dmab discontinuation in patients who received Dmab treatment for a short period (≤ 2.5 years). Until now, there are only two complete RCTs that study the effect of zoledronic acid after Dmab treatment. Firstly, Anastasilakis et al. [32] assigned 57 postmenopausal (treated with Dmab for mean of 2.4 years) to receive a single dose of zoledronic acid (n = 27) or two doses of Dmab (n = 30). A single dose of zoledronic acid could maintain BMD gained in LS and FN at 24 months, and still maintained up to 36 months in a recent report [34]. Secondly, Sølling et al. [19] assigned postmenopausal women and men who were previously treated with a longer Dmab duration (mean 4.6 years) to receive zoledronic acid at 6 months or 9 months, or when BTMs were increased (n = 20 per group). In this study, BMD at 12 months after the first ZOL injection was significantly decreased at LS, FN, and TH in all of three treatment groups. The longer Dmab treatment duration (mean ± SD, 4.6 ± 1.6 years vs 2.8 ± 1.3 years in ZOL group from our study) might be the main reason for the contradictory results. Recently, the 2-year outcomes were reported from 58 participants [35]. The LS, FN, and TH BMD were mostly maintained during 12 to 24 months after the initial ZOL injection without difference between the three groups similar to our results at 12 months.
For patients who received Dmab treatment for a longer duration (more than 2.5 years) and are no longer considered at high risk for fragility fractures, Dmab discontinuation was considered. The recent position statement by ECTS 2020 recommends switching to zoledronic acid at 6 months after the last Dmab injection [36]. In one study, a single dose of zoledronic acid could preserve 66% of BMD gained at LS and 49% at TH for 2 years in postmenopausal women (n = 120) treated with Dmab for 2–5 years (mean 3 years) [22]. A recent prospective study compared a single dose of zoledronic acid in patients who received > 6 doses of Dmab (n = 20) versus ≤ 6 doses (n = 27) [37]. LS BMD 1 year after zoledronic acid was maintained (+ 1.0%) in the ≤ 6 doses group, while it significantly decreased 7.0% in the > 6 doses group (p < 0.001). Our results support this; LS BMD changed + 0.7% in patients who received Dmab < 6 doses (n = 21), but significantly decreased 3.9% (p = 0.010) in the ≥ 6 doses group (n = 11) (data not shown). In contrast, the FN BMD was not different between two groups (p = 0.079) [37], consistent with our result (p = 0.220). This may be a result of the relationship between gain in BMD on Dmab and subsequent loss when discontinuing Dmab reported by us and others [10, 11, 25] or a bit more on the pool of dormant osteoclasts that are suddenly reactivated following discontinuation of long-term denosumab treatment [38].
Previously published articles described the results for alendronate and zoledronic acid as one group [13] in patients on long-term Dmab treatment (> 2.5 years), so it was impossible to determine the effect of alendronate alone in long-term Dmab patients. The present study shows that alendronate could maintain BMD gained as well as zoledronic acid not only after shorter Dmab treatment (≤ 5 doses) (supporting the recent recommendation by ECTS 2020 [36]), but also after prolonged Dmab treatment (Dmab > 5 doses). A recent retrospective study was also consistent with our result [11]. Thus, in spite of the differences in continuous oral and a single intravenous administration, both the alendronate and the zoledronic acid appear to be effective in preventing post-Dmab bone loss in the large majority of the patients. The proper dosing of BP therapy following Dmab therapy is still a subject of controversy. Some suggest repeat infusion of zoledronic acid 3 or 6 months after the first dose of zoledronic acid if BTMs are still persistently increased [36]. This may make a difference in retention of BMD gained when compare to once-yearly zoledronic acid.
Our results need to be confirmed in prospective trials. To the best of our knowledge, there is one ongoing randomized clinical trial that addresses this question: comparing 4 months of alendronate therapy with once yearly zoledronic acid in male or female populations aged more than 50 years after Dmab used for 2–3 years (NCT05091099) [39].
A large recent study found that delaying Dmab injection (≥ 9 months after the last Dmab injection) might increase the risk of fracture as compared to persistent Dmab users, not only for vertebral fractures (RR 4.7), but also major osteoporotic fractures (RR 3.2) and hip fractures (RR 5.3) [40]. However, a recent narrative review concludes that the increasing risk of non-vertebral fractures remains unclear [41]. The exact mechanism of the occurrence of vertebral fractures after Dmab discontinuation is unknown. These fractures are typically clinical [8, 9, 42], and uncommon in patients who received subsequent treatment with BP [14, 22, 32, 43]. Multiple vertebral fractures were mostly observed in groups with no subsequent treatment [3, 22, 32]. We obtained consistent results, observing vertebral fractures in about 15% of patients, with most not on treatment after Dmab discontinuation. Moreover, prior BP before Dmab may reduce but did not prevent vertebral fractures after Dmab discontinuation [8, 9, 44].
The most common reason for Dmab discontinuation in our study in patients who received subsequent therapy with BP, was the achievement of BMD goal (T-score better than − 2.5). This reason was the same as previous studies [45, 46]; however, some studies report the most common reason being invasive dental procedures [47] or other non-toxic reasons [14]. Since the COVID-19 outbreak was a global pandemic, it was the most common reason for discontinuation within the no treatment group in our study. Our results support that oral alendronate might be a good option if Dmab is not feasible within 7 months, as during the pandemic [48], or needs to be delayed for other reasons and to avoid an in-person visit for ZOL. A case series reported 20 cases of vertebral fractures following Dmab discontinuation during COVID pandemic [49]. Importantly, physicians should emphasize the increased risk of fractures after delaying/missing a Dmab dose to their patients. In our study, one case of osteonecrosis of the jaw (ONJ) was the suspected cause of Dmab discontinuation. In the long-term original trial of Dmab [2], the rate of ONJ was low (5.2 per 10,000 participant-years). Moreover, the final diagnosis in our patient did not meet the criteria of the American Association of Oral and Maxillofacial Surgeons (AAOMS)-2022 update [50], so the risk of uncommon events versus benefits of continuing Dmab treatment should be discussed with patients during each outpatient visit to improve medication adherence.
There are several limitations that should be considered. First, the lack of randomization due to retrospective design of the study left the choice of BP/follow-up regimen to the physician. Second, the number of participants in each group may be too small to draw definite conclusions. Third, the cases of vertebral fracture after Dmab discontinuation might be higher than our report because we used X-rays to evaluate vertebral deformities instead of the more sensitive MRI alternative [51]. Also, vertebral imaging was available in 81% of our patients so we might have missed morphometric vertebral fractures. The duration of follow-up DXA (DXA3) varies up to 2.5 years in the NT group; however, the percentage changes of both LS and TH BMD were not significantly different among the different time points in one study (12–23 months vs 24–30 months vs 31–42 months after the last Dmab injection) [22]. Lastly, the four groups were similar with the exception that the no treatment group had slightly lower BMD at the time of Dmab discontinuation. However, because of this difference, we analyzed the data as the absolute value rather than percent change during the discontinuation period.
Despite these limitations, our study population reflects the patients from routine clinical practice, with or without prior fractures or previous treatment with BP before Dmab which improves the external validity of our results (real-world data). We have the no treatment group as a reference, to compare and report changes in BMD as a RCT, providing no treatment, would be unethical. In addition, all three BP groups were well matched in baseline characteristics at the time of Dmab discontinuation. Advantages also include that the number of patients in the risedronate group is greater than that in previous studies and we were able to compare alendronate with zoledronic acid in the ability to attenuate BMD loss after long-term Dmab treatment.
Conclusion
Subsequent treatment with weekly alendronate or once-yearly zoledronic acid mitigates BMD loss at the LS, FN, and TH after Dmab discontinuation. There is no significant difference between the two treatments at the LS, FN, and TH, even following long-term Dmab treatment. Patients receiving no treatment or those receiving risedronate post Dmab, both demonstrate significant bone loss with no significant differences between these groups. Data from a prospective study is needed to identify risk factors for BMD loss after Dmab discontinuation, even while being treated with a BP.
Supplementary information
Below is the link to the electronic supplementary material.
Declarations
Ethics approval
This study protocol was reviewed and approved by the Institutional Review Board of Hospital for Special Surgery (HSS), New York (the study ID 2021–1960-AM1).
Consent to participate/publication
Not applicable.
Conflicts of interest
ZW, JEL, and JEY have nothing to declare. TT has received a speaker honorarium from Amgen. JWN has received study medication from Eli Lilly and Radius. JML was paid as a consultant from ON Foundation, Kuros, Lenoss, Radius Health, Merck, Terumo BCT, and Mesentech. JML has received a research support from Merck, Novartis, and Radius Health. JML is a board member/committee appointment of ON Foundation, UCB/Amgen, and Lenoss.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 2.Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–523. doi: 10.1016/s2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 3.McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM. Observations following discontinuation of long-term denosumab therapy. Osteoporos Int. 2017;28:1723–1732. doi: 10.1007/s00198-017-3919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanchetta MB, Boailchuk J, Massari F, Silveira F, Bogado C, Zanchetta JR. Significant bone loss after stopping long-term denosumab treatment: a post FREEDOM study. Osteoporos Int. 2018;29:41–47. doi: 10.1007/s00198-017-4242-6. [DOI] [PubMed] [Google Scholar]
- 5.Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, Liu Y, San Martin J. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–229. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972–980. doi: 10.1210/jc.2010-1502. [DOI] [PubMed] [Google Scholar]
- 7.Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D, Lamy O. Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int. 2016;27:1923–1925. doi: 10.1007/s00198-015-3380-y. [DOI] [PubMed] [Google Scholar]
- 8.Lamy O, Gonzalez-Rodriguez E, Stoll D, Hans D, Aubry-Rozier B. Severe rebound-associated vertebral fractures after denosumab discontinuation: 9 clinical cases report. J Clin Endocrinol Metab. 2017;102:354–358. doi: 10.1210/jc.2016-3170. [DOI] [PubMed] [Google Scholar]
- 9.Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. 2017;32:1291–1296. doi: 10.1002/jbmr.3110. [DOI] [PubMed] [Google Scholar]
- 10.Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM Trial and its extension. J Bone Miner Res. 2018;33:190–198. doi: 10.1002/jbmr.3337. [DOI] [PubMed] [Google Scholar]
- 11.Everts-Graber J, Reichenbach S, Gahl B, Ziswiler HR, Studer U, Lehmann T. Risk factors for vertebral fractures and bone loss after denosumab discontinuation: a real-world observational study. Bone. 2021;144:115830. doi: 10.1016/j.bone.2020.115830. [DOI] [PubMed] [Google Scholar]
- 12.Grassi G, Chiodini I, Palmieri S, Cairoli E, Arosio M, Eller-Vainicher C. Bisphosphonates after denosumab withdrawal reduce the vertebral fractures incidence. Eur J Endocrinol. 2021;185:387–396. doi: 10.1530/eje-21-0157. [DOI] [PubMed] [Google Scholar]
- 13.Leder BZ, Tsai JN, Jiang LA, Lee H. Importance of prompt antiresorptive therapy in postmenopausal women discontinuing teriparatide or denosumab: The Denosumab and Teriparatide Follow-up study (DATA-Follow-up) Bone. 2017;98:54–58. doi: 10.1016/j.bone.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Ebina K, Hashimoto J, Kashii M, et al. Effects of follow-on therapy after denosumab discontinuation in patients with postmenopausal osteoporosis. Mod Rheumatol. 2021;31:485–492. doi: 10.1080/14397595.2020.1769895. [DOI] [PubMed] [Google Scholar]
- 15.Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Zillikens MC. Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi: 10.1016/j.bone.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 17.Popp AW, Varathan N, Buffat H, Senn C, Perrelet R, Lippuner K. Bone mineral density changes after 1 year of denosumab discontinuation in postmenopausal women with long-term denosumab treatment for osteoporosis. Calcif Tissue Int. 2018;103:50–54. doi: 10.1007/s00223-018-0394-4. [DOI] [PubMed] [Google Scholar]
- 18.Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, San Martin J, Bone HG. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010;25:72–81. doi: 10.1359/jbmr.090716. [DOI] [PubMed] [Google Scholar]
- 19.Sølling AS, Harsløf T, Langdahl B. Treatment with zoledronate subsequent to denosumab in osteoporosis: a randomized trial. J Bone Miner Res. 2020;35:1858–1870. doi: 10.1002/jbmr.4098. [DOI] [PubMed] [Google Scholar]
- 20.Everts-Graber J, Reichenbach S, Ziswiler HR, Studer U, Lehmann T. A single infusion of zoledronate in postmenopausal women following denosumab discontinuation results in partial conservation of bone mass gains. J Bone Miner Res. 2020;35:1207–1215. doi: 10.1002/jbmr.3962. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann T, Aeberli D. Possible protective effect of switching from denosumab to zoledronic acid on vertebral fractures. Osteoporos Int. 2017;28:3067–3068. doi: 10.1007/s00198-017-4108-y. [DOI] [PubMed] [Google Scholar]
- 22.Japelj M, Vidmar G, Rajic AS, Pfeifer M, Kocjan T (2018) Bone mineral density decline following denosumab discontinuation might not be attenuated with previous bisphosphonate therapy. Endocrine Abstracts. Bioscientifica
- 23.Liebich G, Stoll D, Stoll D, Gonzalez-Rodriguez E, Gonzalez-Rodriguez E, Hans D, Lamy O, Aubry-Rozier B, Aubry-Rozier B (2019) Can we avoid the loss of bone mineral density one year after denosumab discontinuation? The reolaus bone project. World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases, Paris
- 24.Aubry-Rozier B, Liebich G, Stoll D, Gonzalez-Rodriguez E, Hans D, Lamy O (2019) OP0085 Can we avoid the loss of bone mineral density one year after denosumab discontinuation? The Reolaus Bone Project. BMJ Publishing Group Ltd
- 25.Kendler D, Chines A, Clark P, Ebeling PR, McClung M, Rhee Y, Huang S, Stad RK. Bone mineral density after transitioning from denosumab to alendronate. J Clin Endocrinol Metab. 2020;105:e255–264. doi: 10.1210/clinem/dgz095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, Borenstein J, Kendler DL. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int. 2012;23:317–326. doi: 10.1007/s00198-011-1780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewiecki EM, Dinavahi RV, Lazaretti-Castro M, Ebeling PR, Adachi JD, Miyauchi A, Gielen E, Milmont CE, Libanati C, Grauer A. One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: results of the FRAME Extension Study. J Bone Miner Res. 2019;34:419–428. doi: 10.1002/jbmr.3622. [DOI] [PubMed] [Google Scholar]
- 28.Rosen CJ, Hochberg MC, Bonnick SL, et al. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151. doi: 10.1359/jbmr.040920. [DOI] [PubMed] [Google Scholar]
- 29.Naylor KE, Bradburn M, Paggiosi MA, Gossiel F, Peel NFA, McCloskey EV, Walsh JS, Eastell R. Effects of discontinuing oral bisphosphonate treatments for postmenopausal osteoporosis on bone turnover markers and bone density. Osteoporos Int. 2018;29:1407–1417. doi: 10.1007/s00198-018-4460-6. [DOI] [PubMed] [Google Scholar]
- 30.Kondo H, Okimoto N, Yoshioka T, et al. Zoledronic acid sequential therapy could avoid disadvantages due to the discontinuation of less than 3-year denosumab treatment. J Bone Miner Metab. 2020;38:894–902. doi: 10.1007/s00774-020-01126-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramchand SK, David NL, Lee H, Eastell R, Tsai JN, Leder BZ. Efficacy of zoledronic acid in maintaining areal and volumetric bone density after combined denosumab and teriparatide administration: DATA-HD Study Extension. J Bone Miner Res. 2021;36:921–930. doi: 10.1002/jbmr.4259. [DOI] [PubMed] [Google Scholar]
- 32.Anastasilakis AD, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Makras P. Zoledronate for the prevention of bone loss in women discontinuing denosumab treatment. A prospective 2-year clinical trial. J Bone Miner Res. 2019;34:2220–2228. doi: 10.1002/jbmr.3853. [DOI] [PubMed] [Google Scholar]
- 33.Horne AM, Mihov B, Reid IR. Bone loss after romosozumab/denosumab: effects of bisphosphonates. Calcif Tissue Int. 2018;103:55–61. doi: 10.1007/s00223-018-0404-6. [DOI] [PubMed] [Google Scholar]
- 34.Makras P, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Anastasilakis AD. The three-year effect of a single zoledronate infusion on bone mineral density and bone turnover markers following denosumab discontinuation in women with postmenopausal osteoporosis. Bone. 2020;138:115478. doi: 10.1016/j.bone.2020.115478. [DOI] [PubMed] [Google Scholar]
- 35.Sølling AS, Harsløf T, Langdahl B. Treatment with zoledronate subsequent to denosumab in osteoporosis: a 2-year randomized study. J Bone Miner Res. 2021;36:1245–1254. doi: 10.1002/jbmr.4305. [DOI] [PubMed] [Google Scholar]
- 36.Tsourdi E, Zillikens MC, Meier C, et al. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. 2020 doi: 10.1210/clinem/dgaa756. [DOI] [PubMed] [Google Scholar]
- 37.Makras P, Appelman-Dijkstra NM, Papapoulos SE, van Wissen S, Winter EM, Polyzos SA, Yavropoulou MP, Anastasilakis AD. The duration of denosumab treatment and the efficacy of zoledronate to preserve bone mineral density after its discontinuation. J Clin Endocrinol Metab. 2021;106:e4155–e4162. doi: 10.1210/clinem/dgab321. [DOI] [PubMed] [Google Scholar]
- 38.Fassio A, Adami G, Benini C, et al. Changes in Dkk-1, sclerostin, and RANKL serum levels following discontinuation of long-term denosumab treatment in postmenopausal women. Bone. 2019;123:191–195. doi: 10.1016/j.bone.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 39.The optimal sequential therapy after long term denosumab treatment. https://clinicaltrials.gov/ct2/show/study/NCT05091099 Accessed May 13, 2022
- 40.Triptoshkolnik L, Fund N, Rouach V, Chodick G, Shalev V, Goldshtein I. Fracture incidence after denosumab discontinuation: Real-world data from a large healthcare provider. Bone. 2020;130:115150. doi: 10.1016/j.bone.2019.115150. [DOI] [PubMed] [Google Scholar]
- 41.Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A (2021) Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med 10(1):152. 10.3390/jcm10010152 [DOI] [PMC free article] [PubMed]
- 42.Polyzos SA, Terpos E. Clinical vertebral fractures following denosumab discontinuation. Endocrine. 2016;54:271–272. doi: 10.1007/s12020-016-1030-6. [DOI] [PubMed] [Google Scholar]
- 43.Laroche M, Couture G, Ruyssen-Witrand A, Constantin A, Degboé Y. Effect of risedronate on bone loss at discontinuation of denosumab. Bone Rep. 2020;13:100290. doi: 10.1016/j.bonr.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tripto-Shkolnik L, Rouach V, Marcus Y, Rotman-Pikielny P, Benbassat C, Vered I. Vertebral fractures following denosumab discontinuation in patients with prolonged exposure to bisphosphonates. Calcif Tissue Int. 2018;103:44–49. doi: 10.1007/s00223-018-0389-1. [DOI] [PubMed] [Google Scholar]
- 45.Lamy O, Stoll D, Aubry-Rozier B, Rodriguez EG. Stopping denosumab. Curr Osteoporos Rep. 2019;17:8–15. doi: 10.1007/s11914-019-00502-4. [DOI] [PubMed] [Google Scholar]
- 46.Burckhardt P, Faouzi M, Buclin T, Lamy O. Fractures after denosumab discontinuation: a retrospective study of 797 cases. J Bone Miner Res. 2021;36:1717–1728. doi: 10.1002/jbmr.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong N, Shin S, Lee S, Kim KJ, Rhee Y. Raloxifene use after denosumab discontinuation partially attenuates bone loss in the lumbar spine in postmenopausal osteoporosis. Calcif Tissue Int. 2022 doi: 10.1007/s00223-022-00962-4. [DOI] [PubMed] [Google Scholar]
- 48.Yu EW, Tsourdi E, Clarke BL, Bauer DC, Drake MT. Osteoporosis management in the era of COVID-19. J Bone Miner Res. 2020;35:1009–1013. doi: 10.1002/jbmr.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minisola S, Cipriani C, Vigna E, Sonato C, Colangelo L, Monti F, Pepe J. COVID pandemic and denosumab adherence. Osteoporos Int. 2022;33:943–944. doi: 10.1007/s00198-021-06274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D. American Association of Oral and Maxillofacial Surgeons' Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J Oral Maxillofac Surg. 2022;80:920–943. doi: 10.1016/j.joms.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Anastasilakis AD, Evangelatos G, Makras P, Iliopoulos A. Magnetic resonance imaging has an advantage over conventional spine X-rays in the evaluation of rebound-associated vertebral fractures following denosumab discontinuation. Endocrine. 2020;69:516–518. doi: 10.1007/s12020-020-02333-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.