Abstract
Background
Chronic anal fissure is a common benign anorectal disease with a high recurrence rate. Pelvic floor physical therapy has been proven effective in the short-term management in patients with chronic anal fissure and pelvic floor dysfunction (PAF-trial). The aim of this study was to determine the outcomes of the PAF-trial and fissure recurrence in patients who completed the 2 months of pelvic floor physical therapy at 1-year follow-up.
Methods
Electromyographic registration of the pelvic floor, digital rectal examination, visual analog scales, patient-related outcome measurements, and quality of life were assessed at baseline and at 1-year after inclusion. The primary outcome was muscle tone at rest during electromyographic registration of the pelvic floor at baseline and at 1-year follow-up. Secondary outcomes contained fissure recurrence, pain ratings, pelvic floor dysfunction, complaint reduction measured with a proctology specific patient-reported outcome measurement, and quality of life.
Results
The treatment protocol was followed by 133 patients. Ninety-seven patients (71%) completed the 1-year follow-up, 48 women (49.5%) and 49 men (50.5%) with a mean age of 44.4 ± 11.6 years (range 19–68). In the total group of patients, mean resting electromyographic values of the pelvic floor significantly improved from baseline to follow-up at 1 year (mean estimated difference 2.20 μV; 95% CI, 1.79 to 2.61; p < 0.001). After 1 year, the fissure recurred in 15 patients (15.5%). VAS-pain significantly decreased from baseline to follow-up (mean estimated difference 4.16; 95% CI, 3.75 to 4.58; p < 0.001). Dyssynergia was found in 72.9% at baseline and decreased to 14.4% at 1-year follow-up (p < 0.001). Complaint reduction measured with the Proctoprom significantly improved from baseline to 1-year follow-up (p < 0.001). Quality of life (RAND-36) significantly improved in eight of nine domains at 1-year follow-up. No significant improvement was found in the domain vitality.
Conclusions
In the PAF-trial, we demonstrated that pelvic floor physical therapy yields a significant and clinical benefit in the time course and therefore should be advocated as adjuvant conservative treatment in patients with chronic anal fissure.
Trial registration
The trial is registered at the Dutch Trial registry (NTR7581) https://trialsearch.who.int
Keywords: Chronic anal fissure, Pelvic floor physical therapy, Treatment, Recurrence, Long-term follow-up
Introduction
Background and objectives
Chronic anal fissure (CAF) is a frequent and disabling anorectal disorder. Optimal management of CAF is quite challenging, mainly because of its recurrent nature. Initial conservative therapy includes normalization of the defecation pattern by a fiber-enriched diet to ensure the regular passage of soft stools [1]. Treatment with ointments is aimed at reducing elevated internal sphincter tone for which nitro-glycerine as well as calcium channel blockers achieve good results [2]. When conservative treatment fails local botulinum toxin injections and/or fissurectomy and lateral internal sphincterotomy are possible treatment options. Botulinum toxin is often used for CAF, but has a recurrence rate of 41.7% [3]. In the Netherlands, the first step of surgical treatment is fissurectomy [4]. The long-term effect of fissurectomy has been proven successful with recurrence rates between 6 and 12% [5, 6], although the mean time for obtaining wound healing is about 10 weeks [6]. Lateral internal sphincterotomy remains the surgical treatment of choice for fissures that are refractory to medical treatment and is recommended in guidelines [7, 8]. The recurrence rate of lateral internal sphincterotomy is low (6.9%) [3]; however, there is a potential risk of incontinence [3, 9–11].
To fill the gap in treatment modalities between conservative management and surgery, we recently performed a randomized controlled trial to investigate the effect of pelvic floor physical therapy in the treatment of CAF (PAF-trial). This trial demonstrated that pelvic floor physical therapy was effective in patients with CAF and concomitant pelvic floor dysfunction. Patients had clinically relevant and significant improvements in all outcomes, clinical healing of the fissure, pain ratings, diminished pelvic floor dyssynergia, and complaint reduction [12].
The aim of this study was to determine the outcomes of the PAF-trial and fissure recurrence at 1-year follow-up.
Materials and methods
Study design
This was a study of the long-term results of PFPT, originally evaluated in a single-center randomized controlled trial (PAF-trial) [12]. The PAF-trial included 140 patients with CAF and pelvic floor dysfunction. Patients were randomized to 2 study groups, an intervention group starting immediately after inclusion with PFPT and a control group receiving postponed PFPT after 8 weeks after inclusion. The present study was a long-term follow-up, using the same outcomes as in the RCT [13].
Baseline and follow-up
Baseline and follow-up appointments at 1 year from baseline with the surgeon and principal investigator, an experienced pelvic floor physical therapist, consisted of a clinical examination provided through inspection to investigate the healing of the fissure. Resting anal sphincter pressure, pelvic floor muscle tone, and function were measured by a careful digital rectal examination and scored as decreased, normal, and increased [14, 15]. Pelvic floor dysfunction was defined by the presence of increased pelvic floor muscle tone and/or dyssynergia detected by digital rectal examination [14, 16]. Besides that, pelvic floor muscle tone was measured with EMG (μV) [14] with an intra-anal probe (MAPle, ®Novuqare Pelvic Health B.V. CE 0344, Rosmalen, the Netherlands).
Patients were requested to fill in 3 validated self-administered questionnaires at baseline and 1-year follow-up. To quantify the average intensity of pain during defecation, a visual analog scale (VAS) from 0 (no pain) to 10 (most intense pain) was used [17]. The Proctoprom, a patient-related outcome measurement, was used to assess the impact of proctologic complaints on different aspects of a patient’s life and to evaluate the effect of treatment [18]. To access the impact of global quality of life, the validated Dutch version of Short-Form RAND-36, Health Status Inventory, version 2 was used [19]. The RAND-36 comprises 36 items and entails nine subscales: physical functioning, bodily pain, role limitation due to physical health problems, vitality, general health perception, social functioning, role limitation due to emotional problems, mental health, and health change perception.
Participants
Men and women aged 18 years or older presenting with CAF and pelvic floor dysfunction were recruited at the Proctos Clinic in the Netherlands from December 2018 until July 2021. CAF was defined as a longitudinal ulcer in the squamous epithelium with one or more signs of chronicity including hypertrophied anal papilla and sentinel tag and exposed internal sphincter muscle with symptoms presenting longer than 6 weeks or recurrent fissures.
All patients had failed conservative treatment with fiber and/or laxatives and ointment (diltiazem or isosorbide dinitrate) used for at least 6 weeks and with accurate instructions about how to apply. All patients had sufficient understanding of the Dutch language (reading and writing) and were able to complete online questionnaires. We considered patients who were not able to undergo a digital rectal examination, not eligible for this study. Patients with an abscess or fistula, Crohn’s disease or ulcerative colitis, anorectal malignancy, prior rectal radiation, and pregnancy were excluded from the study.
Interventions
At baseline, patients in both groups received information about toilet behavior, the pelvic floor, and lifestyle advice. All patients continued their conservative measures including the use of ointment (diltiazem or isosorbide dinitrate) during the treatment period.
PFPT consisted of 5 face-to-face appointments of 45 min in a period of 8 consecutive weeks, using a treatment protocol. Details of this treatment protocol were prescribed earlier [13]. Data collection of the questionnaires was facilitated by a secure on-line system called Castor EDC [20]. Patients received the questionnaires by e-mail through the Castor system at baseline and 1-year follow-up.
Outcome measures
Primary outcome was muscle tone at rest during EMG-registration of the pelvic floor at baseline and 1-year follow-up.
Secondary outcomes contained fissure recurrence, average pain intensity during defecation on a VAS-scale, pelvic floor (dys)function, complaint reduction measured with the Proctoprom [18], and quality of life measured with the Short-Form RAND-36 [19].
All outcomes were measured at baseline and 1-year follow-up.
Statistical analysis
Data were analyzed using Statistical Packages for Social Sciences (SPSS, Chicago, II, USA, version 26.0). Descriptive methods were used to assess quality of data, homogeneity of treatment groups, and endpoints. Normality of the data were analyzed with histograms. Data are presented using mean (SD), median (min–max) for the numeric and non-normal variables and frequency (percentages) for categorical variables. A paired t-test and Wilcoxon signed rank was used to compare continuous variables within groups. McNemar was used to compare categorical variables within groups. Comparison between groups for continuous variables was made by repeated measure analysis of variance using a mixed model after transformation of the data to enhance normality, with treatment, time (categorical), and their interaction as fixed effects and with random patient effects. In addition, data at each time point were compared with independent samples t-tests, Mann–Whitney U test, and Chi-square test depending on the variables. All p values were two-tailed, and statistical significance was taken as a p value of less than 0.05. Multiple imputation for incomplete records was not needed because less than 5% of the data was missing. An interim analysis was not performed for this study.
Results
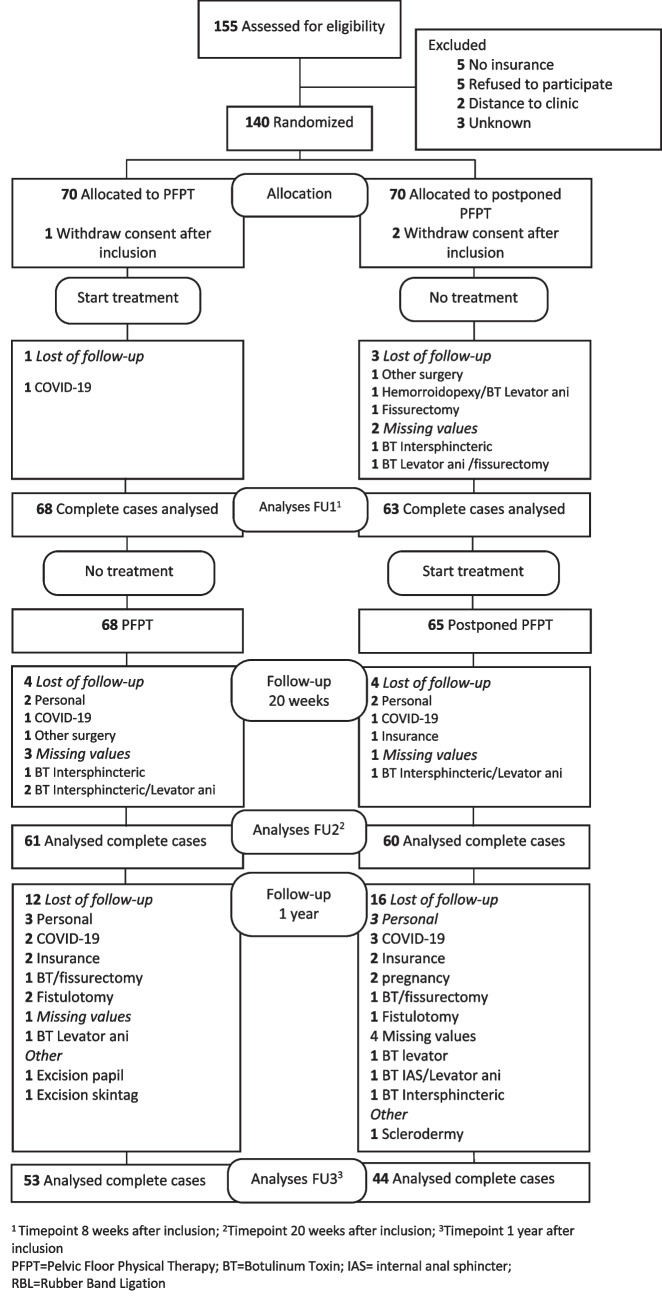
Between 10 December 2018 and 13 July 2021, 140 patients were randomized to PFPT (n = 70) and a control group (postponed PFPT) (n = 70). Baseline characteristics were similar between the 2 groups (Table 1). After randomization, 1 patient in the PFPT group and 2 patients in the postponed PFPT group withdrew after inclusion. Ninety-seven patients completed the 1-year follow-up, 48 women (49,5%), 49 men (50,5%) with a mean age of 44.4 ± 11.6 (range 19–68). In total, 40 patients were lost of follow-up from baseline to 1-year follow-up. Details of the loss of follow-up, missing values, and other surgery are shown in Fig. 1.
Table 1.
Demographics at baseline
| Variable | Total group (n = 140) | PFPT group (n = 70) | Postponed PFPT (n = 70) |
|---|---|---|---|
| Age, years mean (SD) (range) | 44.5 (11.1) (19–79) | 44.2 (10.7) (23–66) | 44.7 (11.6) (19–79) |
| Gender, women/men, n (%) | 72 (51.4)/68 (48.6) | 37 (52.9)/33 (47.1) | 35 (50)/35 (50.0) |
| Duration of complaints (%) | |||
| 0–2 months | 12.1 | 12.9 | 11.4 |
| 2–6 months | 22.9 | 18.6 | 27.1 |
| 6–12 months | 14.3 | 12.9 | 15.7 |
| 12–36 months | 22.1 | 24.3 | 20.0 |
| > 3 years | 28.6 | 31.4 | 25.7 |
| Location of fissure (%) | |||
| Anterior | 14.3 | 12.9 | 15.7 |
| Posterior | 77.9 | 78.6 | 77.1 |
| Other | 7.9 | 8.6 | 7.1 |
Fig. 1.
CONSORT diagram
There were no reported negative side effects or serious adverse events in both groups.
Primary outcome
Mean resting electromyographic values of the pelvic floor in the total group of patients significantly improved from baseline to 1-year follow-up (mean estimated difference 2.20 μV; 95% CI, 1.79 to 2.61; p < 0.001).
In the PFPT-group, the mean tone of the pelvic floor at rest measured with EMG decreased significantly from baseline to 1-year follow-up (mean estimated difference 2.39 μV; 95% CI, 1.79 to 2.99; p < 0.001). In the postponed PFPT-group, the mean tone of the pelvic floor at rest measured with EMG significantly decreased from baseline to follow-up at 1 year (mean estimated difference 1.97 μV; 95% CI, 1.42 to 2.52; p < 0.001) (Table 2).
Table 2.
Study measures at baseline and 1-year follow-up. Comparisons within and between treatment groups are repeated measurements
| Total group | PFPT group | Postponed PFPT group | MD between groups | Group vs. time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline n = 140 |
1 year n = 97 |
p value | Baseline n = 70 |
1 year n = 53 |
p value | Baseline n = 70 |
1 year n = 44 |
p value | p value | p value | |
| Rest EMG pelvic floor mean (sd) (μV) (n) | 6.7 (2.9) | 4.7 (1.9) (94) | < 0.001* | 6.9 (2.9) | 4.8 (1.9) (52) | < 0.001* | 6.5 (2.8) | 4.5 (1.9) (42) | < 0.001* | p = .303b | < 0.001f |
| VAS-pain mean (sd) (n) | 5.3 (1.6) | 1.0 (1.4) (94) | < 0.001a | 5.5 (1.6) | 1.1 (1.2) (53) | < 0.001a | 5.2 (1.6) | .93 (1.6) (41) | < 0.001a | p = .509b | < 0.001f |
| Proctoprom mean (sd) (n) | 5.1 (2.1) | 2.1 (1.9) (84) | < 0.001a | 5.2 (2.0) | 2.1 (1.9) (34) | < 0.001a | 5.0 (2.2) | 2.1 (1.9) (30) | < 0.001a | p = .662c | < 0.001f |
| Dyssynergia DRE, yes (%) (n) | 72.9 | 14.4 (96) | < 0.001d | 67.1 | 9.4 (53) | < 0.001d | 78.6 | 20.5 (43) | < 0.001d | p = .112e | NA |
| Tenderness traction puborectalis, yes (%) (n) | 75.0 | 9.3 (96) | < 0.001d | 70.0 | 7.5 (53) | < 0.001d | 80 | 11.4.(43) | < 0.001d | p = .495e | NA |
PFPT pelvic floor physical therapy, EMG electromyography, VAS visual analog scale, RT digital rectal examination, NA not applicable
*Paired t-test
aWilcoxon signed rank test
bUnpaired t-test
cMann-Whitney U test
dMcNemar
eChi-square test
fRepeated measurement analyses
The mean estimated difference between groups at 1-year follow-up was − 0.427 μV; 95% CI, − 1.25 to 0.391 (p = 0.303).
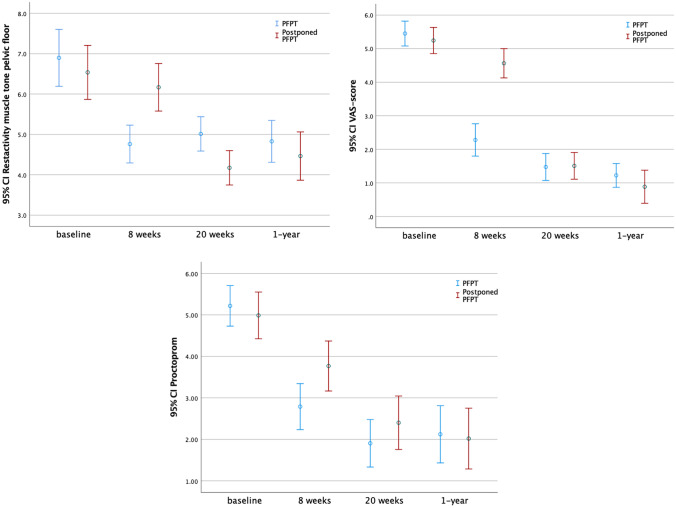
Regarding the analysis of repeated measures, pelvic floor muscle tone at 1 year from baseline, measured with EMG, was reduced in favor of the PFPT-group (p < 0.001) (Fig. 2; Table 2).
Fig. 2.
Repeated measurement analyses
Secondary outcomes
Fissure recurrence
In the total group of patients, after 1 year, the fissure recurred in 15 patients (15.5%), 60% women and 40% men. In the PFPT-group, in 11.3% vs. 20.5% in the postponed PFPT-group, no significant difference was found between groups at 1-year follow-up.
Pain
VAS-pain was significantly reduced in the total group of patients from baseline to follow-up at 1 year (mean estimated difference 4.23; 95% CI, 3.82 to 4.66; p < 0.001).
In the PFPT group and the postponed PFPT group, the pain score measured with VAS decreased significantly from baseline to 1-year follow-up (p < 0.001) (Table 2).
No significant differences were found between groups at 1-year follow-up (Table 2).
Regarding the analysis of repeated measures, the PFPT group was found to be more effective for reducing pain compared to the postponed PFPT group at 1 year from baseline (p < 0.001) (Fig. 2; Table 2).
Pelvic floor function
Increased pelvic floor muscle tone measured with digital rectal examination was found in 87.1% of the total group of patients at baseline and in 19 patients (19.6%) at 1-year follow-up (p < 0.001). There are 14 patients in the PFPT group vs. 5 patients in the postponed PFPT group, but there were no significant differences between groups.
Tenderness with traction on the puborectalis muscle, by digital rectal examination, was found in the total group of patients in 75% at baseline vs. 9.3% at 1-year follow-up (p < 0.001). At 1-year follow-up, tenderness with traction on the puborectalis muscle was painful in 7.5% in the PFPT group vs. 11.4% in postponed PFPT group. No significant differences were found between groups at 1-year follow-up (Table 2).
Dyssynergia diagnosed by digital rectal examination was found in the total group of patients in 72.9% at baseline vs. 14.4% at 1-year follow-up (p < 0.001). In the PFPT-group in 9.4% vs. 20.5% in the postponed PFPT-group at 1-year follow-up, no significant differences were found between groups at 1-year follow-up (Table 2).
Patient-related outcome measurement
The Proctoprom scores in the total group, the PFPT-group, and postponed PFPT group decreased significantly from baseline to follow-up at 1 year (p < 0.001). At 1 year, no significant difference in Proctoprom scores was found between groups (Table 2).
Regarding the analysis of repeated measures, the PFPT group experienced significantly more reduction of complaints than the control group at 1 year from baseline (p < 0.001) (Fig. 2; Table 2).
Quality of life
In the total patients’ group, the mean scores significantly improved in the domains of the RAND-36 from baseline to 1-year follow-up, bodily pain, health change (p < 0.001), physical functioning, physical role, general health, social functioning, emotional role, and mental health (p < 0.05) (Table 3, Fig. 3). No significant improvement was found in the domain vitality.
Table 3.
Study measures at baseline and 1-year follow-up. Comparison within and between treatment groups
| Quality of life scale SF-36 | Total group | PFPT group | Control group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline mean (SD) n = 100 |
1-year mean (SD) n = 61 |
p value | Baseline mean (SD) n = 52 |
1-year mean (SD) n = 31 |
p value | Baseline mean (SD) n = 48 |
1-year mean (SD) n = 30 |
p value | |
| Physical functioning | 82.9 (20.9) | 90.6 (14.0) | p = .006b | 84.9 (18.7) | 92.1 (8.3) | p = .110b | 80.7 (23.0) | 89.0 (18.1) | p = .031b |
| Bodily pain | 55.0 (26.5) | 75.8 (22.3) | < 0.001b | 54.8 (25.2) | 75.1 (19.7) | p = .008b | 55.3 (28.2) | 76.5 (25.0) | p = .003b |
| Physical role | 56.0 (44.1) | 75.8 (35.6) | p = .047b | 57.7 (43.3) | 78.2 (30.8) | p = .123b | 54.2 (45.4) | 73.3 (40.4) | p = .258b |
| Vitality | 55.5 (18.7) | 60.8 (16.5) | p = .112b | 55.5 (20.4) | 61.6 (18.4) | p = .200a | 55.5 (16.9) | 60.0 (14.5) | p = .125a |
| General health | 66.9 (18.8) | 70.2 (15.9) | p = .048a | 71.2 (17.7) | 73.7 (14.5) | p = .483b | 62.3 (19.0) | 66.7 (16.8) | p = .050b |
| Social functioning | 72.4 (24.2) | 84.4 (19.5) | p = .005b | 70.2 (23.6) | 86.3 (15.3) | p = .092b | 74.7 (24.8) | 82.5 (23.1) | p = .041b |
| Emotional role | 70.7 (41.4) | 86.3 (29.4) | p = .018b | 76.3 (36.4) | 84.9 (32.0) | p = .794b | 64.6 (45.8) | 87.8 (29.9) | p = .006b |
| Mental health | 66.2 (14.5) | 70.4 (14.7) | p = .037a | 65.9 (14.6) | 71.7 (13.1) | p = .302b | 66.5 (14.7) | 69.1 (16.3) | p = .025b |
| Health change | 46.5 (24.6) | 65.2 (22.9) | < 0.001a | 49.5 (26.4) | 66.9 (24.5) | p = .010a | 43.2 (22.3) | 63.3 (21.5) | < 0.001a |
Values in bold indicate a significance of p<0.001
aPaired t-test
bWilcoxon signed rank test
Fig. 3.
Quality of life, at baseline and 1-year follow-up
Discussion
Principal findings
This is the first study with a long-term follow-up demonstrating the efficacy of PFPT in patients with CAF and pelvic floor dysfunction. The results from this follow-up study show that PFPT resulted in significant and clinically relevant long-term improvement regarding mean resting tone of the pelvic floor, recurrence rate, changes in dyssynergia of the pelvic floor, pain, complaints, and quality of life. Furthermore, the improvement at the short-term follow-up at 20 weeks [12] was sustained at the long-term follow-up for all outcomes.
Pelvic floor muscle tone and function measured with EMG-biofeedback decreased from baseline to follow-up and demonstrated an effective and efficient treatment modality. Biofeedback is the mainstay in the treatment of anorectal dysfunctions [21, 22] and is commonly utilized in PFPT. In this trial, we established that EMG-biofeedback in CAF with pelvic floor dysfunction yields a high percentage of clinical benefit, in the short-, medium-, and long-term period.
The long-term efficacy of PFPT including biofeedback on dyssynergia has already been proven in randomized control trials in patients with constipation [23, 24], although no long-term studies were performed in patients with CAF.
Pelvic floor muscle tone, based on digital rectal examination significantly decreased from baseline to follow-up after 1 year. A comprehensive careful digital rectal examination is an important topic to obtain information on anorectal anatomy and function [15, 25]. Although digital rectal examination to investigate muscle tone and dyssynergia is recommended in clinical guidelines [8, 14], only 23% of the surgeons investigate the pelvic floor during digital rectal examination in patients with CAF [4]. In our study, we found that a large percentage of the patients had an increased pelvic floor muscle tone and this could be a contributing factor in the pain patients experience after defecation [26]. It is therefore important to investigate the pelvic floor muscles during digital rectal examination in patients with CAF.
In almost 75% of the patients, dyssynergia of the pelvic floor was found at baseline. Pelvic floor dyssynergia is thought to be a learned and acquired behavioral disorder of defecation, where an inability to coordinate the abdominal, recto-anal, and pelvic floor muscles during attempted defecation exists [27]. Although patients improved in their dyssynergic pattern, it is possible that this learned behavior does tend to lose the benefit over a period of time [28] which could influence fissure recurrence. It is important to encourage patients to continue practicing their exercises and learned techniques. A clinical follow-up could be beneficial to re-evaluate and to repeat the learned skills [28]. In our trial we scheduled at least 2 follow-up appointments after the treatment period in 1 year. This could have positively influenced the outcome of treatment. Besides that, a clinical follow-up could reinforce the adherence rate which are described in behavioral interventions such as PFPT. Important barriers to adherence are difficulties remembering to do the exercises and finding time to do them [29].
The recurrence rate in our study was 15.5%, which is low compared to other current treatments in CAF. When clinical factors related to recurrence were analyzed, gender, duration of complaints, location of the fissure, and prior treatment were not significantly related to the long-term recurrence. In half of our patients the recurrence was influenced by stool changes. Special attention should be paid to avoid constipation and remain a good lifestyle to avoid recurrence. The use of extra 20–25 g/day of fiber should be recommended to ensure avoidance and constipation [30, 31].
The first results from our study confirmed that both groups significantly improved at 20 weeks of follow-up on all outcomes, although the PFPT-group improved faster than the postponed group. At 1-year follow-up, no significant difference was found between groups, even though a higher recurrence rate (20.5%) was found in the postponed PFPT group. More patients from the postponed PFPT-group received botulinum toxin (3.8% vs. 9.1%). Thus, we would recommend starting with PFPT as soon as possible after at least 6 weeks of using ointment (diltiazem or isosorbide dinitrate) and good regulation of the defecation pattern.
Seven patients in our study developed a superficial fistula during the trial. Suppurative lesions are commonly found with CAF and mostly due to diseases of the anal glands, or the result of infection of the lymphoid tissues, which become chronically infected [32]. It is unknown which proportion of fistulas are due to a fissure and at what time lapse it becomes evident. In the Netherlands, only 57% of the gastrointestinal surgeons scheduled a physical follow-up after 6–8 weeks and 46% scheduled telephone call or according to the needs of the patients [4]. The development of other anorectal complaints could therefore be missed.
Conservative management of chronic anal fissure is associated with significant improvement in patient-related outcome scores. In our study, we used the Proctoprom to detect changes over time, the patient’s state of health measures, and the effect of treatment [18]. The study showed a significant effect of disease burden from the patient’s point of view at long-term follow-up. In a study by Wilson et al. [33] on bowel function reported outcome measures in 37 patients with CAF, an association was found with a statistically significant change in social impact, stool-related aspects, and the mean score of global functioning. The patients received counseling including fiber supplementation, toileting strategies, and the use of ointment but were not treated with PFPT. These baseline strategies were also effective in the patients from the postponed PFPT who did also improve on Proctoprom-scores [12].
In the PAF-trial, we found significant improvements in all nine domains of the RAND-36 at 20-week follow-up, and this result sustained in eight of nine domains at 1-year follow-up, except for the domain vitality. The domain vitality measures energy/fatigue. It is possible that this domain is less influenced by this anorectal disease. On the other hand, when patients improve with 5 points on the RAND-36, this could be interpreted as clinically relevant [34].
Strengths
This is the first study of the long-term results of using PFPT in the treatment of CAF. The main strengths of this study are the well-powered, prospective randomized control trial design and the design of the study in which all patients received the same treatment of PFPT with a long-term follow-up.
The willingness to participate and adherence of the patients to the trial procedures and the intervention were high, which can be seen by a relatively low rate of loss of follow-up (29%) even during the COVID pandemic.
Limitations
First, the pelvic floor physical therapist was also the principal investigator, and consequently, investigator’s bias could not be ruled out. Secondly, COVID-19 did have some influence on our study. During the pandemic, a small number of patients were lost to follow-up because they were diagnosed with COVID-19 at the follow-up appointment.
Some of our patients were lost of follow-up because they were treated with surgery or for personal circumstances. This may have caused non-response bias.
Clinical implications
Clinical guidelines of leading societies do not recommend PFPT as a treatment option for CAF. Our findings provide strong evidence that also in the long run, PFPT is effective in the treatment of CAF and pelvic floor dysfunction.
Conclusions
Pelvic floor physical therapy provided sustained improvement in pelvic floor muscle tone, pain ratings, patient’s satisfaction, and quality of life in patients with chronic anal fissure after 1-year follow-up.
Author contribution
Category 1—[a] Conception and design: D. A. van Reijn, I. J. M. Han-Geurts, and H. W. Elzevier; [b] Acquisition of data: Daniëlle A. van Reijn; [c] Analyses and interpretation of data: D. A. van Reijn, I. J. M. Han-Geurts, H. W. Elzevier, H. Putter, and R. C. M. Pelger. Category 2—[a] Drafting the article: D. A. van Reijn, I. J. M. Han-Geurts, and H. W. Elzevier; [b] Revising it for intellectual content: D. A. van Reijn, I. J. M. Han-Geurts, H. W. Elzevier, H. Putter, and R. C. M. Pelger. Category 3—[a] Final approval of the completed article: D. A. van Reijn, I. J. M. Han-Geurts, H. W. Elzevier, H. Putter, and R. C. M. Pelger.
Data availability
Data is available and applicable.
Declarations
Ethics approval
Ethics approval for the trial was granted by the Medical Ethics Review Committee of the Leiden University Medical Centre (P18.090).
Conflict of interest
The authors declare no competing interests.
Footnotes
The original online version of this article was revised due to the incorrect total of patients who completed the treatment protocol mentioned in the abstract section under results.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/18/2023
A Correction to this paper has been published: 10.1007/s00384-023-04316-w
References
- 1.Felt-Bersma RJ, Bartelsman JF. Haemorrhoids, rectal prolapse, anal fissure, peri-anal fistulae and sexually transmitted diseases. Best Pract Res Clin Gastroenterol. 2009;23(4):575–592. doi: 10.1016/j.bpg.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Steele SR, Madoff RD. Systematic review: the treatment of anal fissure. Aliment Pharmacol Ther. 2006;24(2):247–257. doi: 10.1111/j.1365-2036.2006.02990.x. [DOI] [PubMed] [Google Scholar]
- 3.Boland PA, Kelly ME, Donlon NE, Bolger JC, Larkin JO, Mehigan BJ, et al. Management options for chronic anal fissure: a systematic review of randomised controlled trials. Int J Colorectal Dis. 2020;35(10):1807–1815. doi: 10.1007/s00384-020-03699-4. [DOI] [PubMed] [Google Scholar]
- 4.van Reijn-Baggen DA-O, Dekker L, Elzevier HW, Pelger RCM, Han-Geurts IJM. Management of chronic anal fissure: results of a national survey among gastrointestinal surgeons in the Netherlands. Int J Colorectal Dis. 2022;37(1432–1262 (Electronic)):973–978. doi: 10.1007/s00384-022-04115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schornagel IL, Witvliet M, Engel AF. Five-year results of fissurectomy for chronic anal fissure: low recurrence rate and minimal effect on continence. Colorectal Dis. 2012;14(8):997–1000. doi: 10.1111/j.1463-1318.2011.02840.x. [DOI] [PubMed] [Google Scholar]
- 6.Zeitoun JD, Blanchard P, Fathallah N, Benfredj P, Lemarchand N, de Parades V. Long-term Outcome of a Fissurectomy (2018) A prospective single-arm study of 50 operations out of 349 initial patients. Ann Coloproctol 34(2):83–87. 10.3393/ac.2017.06.12 [DOI] [PMC free article] [PubMed]
- 7.Stewart DB, Sr, Gaertner W, Glasgow S, Migaly J, Feingold D, Steele SR. Clinical practice guideline for the management of anal fissures. Dis Colon Rectum. 2017;60(1):7–14. doi: 10.1097/DCR.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 8.Wald A, Bharucha AE, Limketkai B, Malcolm A, Remes-Troche JM, Whitehead WE, et al. ACG clinical guidelines: management of benign anorectal disorders. Am J Gastroenterol. 2021;116(10):1987–2008. doi: 10.14309/ajg.0000000000001507. [DOI] [PubMed] [Google Scholar]
- 9.Casillas S, Hull TL, Zutshi M, Trzcinski R, Bast JF, Xu M. Incontinence after a lateral internal sphincterotomy: are we underestimating it? Dis Colon Rectum. 2015;48:1193–1199. doi: 10.1007/s10350-004-0914-3. [DOI] [PubMed] [Google Scholar]
- 10.Garg P, Garg M, Menon GR. Long-term continence disturbance after lateral internal sphincterotomy for chronic anal fissure: a systematic review and meta-analysis. Colorectal Dis. 2013;15(3):e104–e117. doi: 10.1111/codi.12108. [DOI] [PubMed] [Google Scholar]
- 11.Ebinger SM, Hardt J, Warschkow R, Schmied BM, Herold A, Post S, et al. Operative and medical treatment of chronic anal fissures-a review and network meta-analysis of randomized controlled trials. J Gastroenterol. 2017;52(6):663–676. doi: 10.1007/s00535-017-1335-0. [DOI] [PubMed] [Google Scholar]
- 12.van Reijn-Baggen DA, Elzevier HW, Putter H, Pelger RCM, Han-Geurts IJM (2022) Pelvic floor physical therapy in patients with chronic anal fissure: a randomized controlled trial. Tech Coloproctol (1128–045X (Electronic)) [DOI] [PMC free article] [PubMed]
- 13.van Reijn-Baggen DA, Elzevier HW, Pelger RCM, Han-Geurts IJM. Pelvic floor physical therapy in the treatment of chronic anal fissure (PAF-study): study protocol for a randomized controlled trial. Contemp Clin Trials Commun. 2021;24(2451–8654):100874. doi: 10.1016/j.conctc.2021.100874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frawley H, Shelly B, Morin M, Bernard S, Bo K, Digesu GA, et al. An International Continence Society (ICS) report on the terminology for pelvic floor muscle assessment. Neurourol Urodyn. 2021;40(5):1217–1260. doi: 10.1002/nau.24658. [DOI] [PubMed] [Google Scholar]
- 15.Rao SSC. Rectal exam: yes, it can and should be done in a busy practice! Am J Gastroenterol. 2018;113(5):635–638. doi: 10.1038/s41395-018-0006-y. [DOI] [PubMed] [Google Scholar]
- 16.Tantiphlachiva K, Rao P, Attaluri A, Rao SS. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8:955–960. doi: 10.1016/j.cgh.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Vander Mijnsbrugge GJ, Molenaar C, Buyl R, Westert G, van der Wees PJ (2020) How is your proctology patient really doing? Outcome measurement in proctology: development, design and validation study of the Proctoprom. Tech Coloproctol 291–300 [DOI] [PubMed]
- 19.Vander Zee KI, Sanderman R, Heyink JW, de Haes H. Psychometric qualities of the RAND 36-Item Health Survey 1.0: a multidimensional measure of general health status. Int J Behav Med. 1996;3(2):104–122. doi: 10.1207/s15327558ijbm0302_2. [DOI] [PubMed] [Google Scholar]
- 20.Castor (2022) Castor Electronic Data Capture (EDC). https://www.castoredc.com
- 21.Bharucha AE, Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020;158(5):1232–49.e3. doi: 10.1053/j.gastro.2019.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao SS, Bharucha AE, Chiarioni G, Felt-Bersma R et al (2016) Functional anorectal disorders. Gastroenterology 130:1510–1518 [DOI] [PMC free article] [PubMed]
- 23.Rao SS, Valestin J, Brown CK, Zimmerman B, Schulze K. Long-term efficacy of biofeedback therapy for dyssynergic defecation: randomized controlled trial. Am J Gastroenterol. 2010;105(4):890–896. doi: 10.1038/ajg.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battaglia E, Serra AM, Buonafede G, Dughera L, Chistolini F, Morelli A, Emanuelli G, et al. Long-term study on the effects of visual biofeedback and muscle training as a therapeutic modality in pelvic floor dyssynergia and slow-transit constipation. Dis Colon Rectum. 2004;47:90–95. doi: 10.1007/s10350-003-0010-0. [DOI] [PubMed] [Google Scholar]
- 25.Patcharatrakul T, Rao SSC. Update on the pathophysiology and management of anorectal disorders. Gut Liver. 2018;12(4):375–384. doi: 10.5009/gnl17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everaert K, Devulder J, De Muynck M, Stockman S, Depaepe H, De Looze D, et al. The pain cycle: implications for the diagnosis and treatment of pelvic pain syndromes. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(1):9–14. doi: 10.1007/s001920170087. [DOI] [PubMed] [Google Scholar]
- 27.Rao SS, Tuteja AK, Vellema T, Kempf J, Stessman M. Dyssynergic defecation: demographics, symptoms, stool patterns, and quality of life. J Clin Gastroenterol. 2004;38(8):680–685. doi: 10.1097/01.mcg.0000135929.78074.8c. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara A, De Jesus S, Gallagher JT, Williamson PR, Larach SW, Pappas D, et al. Time-related decay of the benefits of biofeedback therapy. Tech Coloproctol. 2001;5(3):131–135. doi: 10.1007/s101510100014. [DOI] [PubMed] [Google Scholar]
- 29.Borello-France D, Burgio KL, Goode PS, Ye W, Weidner AC, Lukacz ES, et al. Adherence to behavioral interventions for stress incontinence: rates, barriers, and predictors. Phys Ther. 2013;93(6):757–773. doi: 10.2522/ptj.20120072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garg P (2022) High recurrence rate after nonsurgical treatment of chronic anal fissure: can it be prevented? Dis Colon Rectum (1530–0358 (Electronic)) [DOI] [PubMed]
- 31.Jensen SL. Maintenance therapy with unprocessed bran in the prevention of acute anal fissure recurrence. J R Soc Med. 1987;80(5):296–298. doi: 10.1177/014107688708000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta PJ. A study of suppurative pathologies associated with chronic anal fissures. Tech Coloproctol. 2005;9(2):104–107. doi: 10.1007/s10151-005-0206-5. [DOI] [PubMed] [Google Scholar]
- 33.Wilson MZ, Swarup A, LR TW, Ivatury SJ (2018) The effect of nonoperative management of chronic anal fissure and hemorrhoid disease on bowel function patient-reported outcomes. Dis Colon Rectum 61(10):1223–1227. 10.1097/DCR.0000000000001193 [DOI] [PubMed]
- 34.Bjorner JB, Wallenstein GV, Martin MC, Lin P, Blaisdell-Gross B, Tak Piech C, Mody SH et al (2007) Interpreting score differences in the SF-36 vitality scale: using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin (1473–4877 (Electronic)) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available and applicable.