Abstract
Objective:
The primary aim was to examine the change in left ventricular mass (LVM) in adults with overweight or obesity in response to a behavioral weight loss intervention, with variable physical activity (PA) prescriptions.
Methods:
383 adults were randomized to a 12-month intervention of diet modification (DIET), DIET plus 150 min/wk of PA (DIET+MODPA), or DIET plus prescription of 250 min/wk of PA (DIET+HIGHPA). LVM was measured with cardiac magnetic resonance imaging (CMR).
Results:
12-month weight loss was −10.2% (95% CI: −11.7, −8.8) in DIET, −11.0% (95% CI: −12.4, −9.5) in DIET+MODPA, and −10.3% (95% CI: −11.8, −8.9) in DIET+HIGHPA. LVM decreased at 12 months in DIET [−2.9 grams (95% CI: −5.2, −0.7); P=0.0114] with no change observed in DIET+MODPA [−0.8 grams (95% CI: −3.0, 1.5); P=0.4979] or DIET+HIGHPA [−1.1 grams (95% CI: −3.3, 1.1); P=0.3299].
Conclusions:
Weight loss through dietary modification resulted in reduced LVM, whereas when combined with at least 150 minutes per week of prescribed moderate-to-vigorous PA LVM is preserved. These may both be favorable adaptations to weight loss and physical activity in adults with overweight or obesity that warrant further investigation to understand the clinical implications of these changes on cardiovascular disease risk.
Keywords: obesity, physical activity, cardiovascular disease, weight loss
INTRODUCTION
Overweight (body mass index [BMI] ≥25 kg/m2) or obesity (BMI ≥30 kg/m2) are associated with chronic diseases, with a major concern being the association with cardiovascular disease.(1, 2) Approximately 70% of adults in the United States are overweight with 40% of adults obese.(3) Weight loss improves in cardiovascular disease (CVD) risk factors,(1) and weight loss of at least 10% is associated with reduced CVD.(4)
Left ventricular mass (LVM) of the heart is a clinically important prognostic indicator of CVD,(5, 6) and BMI is associated with greater LVM measured with echocardiography.(7) Studies using cardiac magnetic resonance imaging (CMR) have also shown larger LVM at higher levels of BMI,(8) and we have shown that LVM measured by CMR is positively associated with weight and BMI in adults with overweight or obesity.(9)
The larger LVM associated with excess weight may lead to maladaptive hypertrophy and potentially heart failure,(10) this may be a pathway by which overweight or obesity contribute to CVD. While weight loss has been shown to reduce CVD risk factors and potentially the onset of CVD.(4) However, it is unclear if the method of weight loss has differential effects on LVM measured by CMR.
Physical activity (PA), when added to modest dietary restriction, can enhance weight loss and may be predictive of improved weight loss maintenance.(11) PA also contributes to improvements in cardiorespiratory fitness (CRF) in the presence of weight loss,(12, 13) and CRF may influence health even with excess weight.(14, 15, 16) We reported that CRF is associated with LVM in adults with overweight or obesity, possibly suggesting a favorable adaptive response.(9) This is consistent with the known effects of PA on an adaptive response that results in increased LVM while retaining or enhancing cardiac function.(17, 18) However, it is unclear if the dose of PA combined with moderate dietary restriction to achieve weight loss results in differential effects on LVM compared to weight loss achieved with moderate dietary restriction alone.
This study examined the change in LVM in response to a weight loss intervention that compared modest dietary restriction (DIET), DIET plus a moderate dose of PA (DIET+MODPA), and DIET plus a high dose of PA (DIET+HIGHPA). Changes in end-systolic (ESV) and end-diastolic volume (EDV) and ejection fraction (EF), weight, body composition, CRF, and other CVD risk factors were also examined.
METHODS
Design
This was a university-based (Pittsburgh) clinical trial with randomization to 1 of 3 interventions, with all groups receiving a 12-month behavioral weight loss intervention (ClinicalTrials.gov NCT01500356). The groups were: 1) DIET received a prescribed energy reduced diet with no PA, 2) DIET+MODPA received a prescribed energy reduced diet plus PA to progress to 150 minutes per week, 3) DIET+HIGHPA received a prescribed energy reduced diet plus PA to progress to 250 minutes per week. The randomization scheme was stratified by sex and race (white or nonwhite) in randomly selected block sizes. The randomization scheme was developed by the statistician and implemented by the principal investigator. The primary outcome was change in LVM from baseline to 12 months.
Participants
Recruitment occurred between December 2011 and June 2015. Participants (N=383) were recruited through methods that included targeted mailing of post cards, email advertisements, research registries, and other referrals. Eligibility and exclusion criteria have been reported previously.(9) Eligibility included being 18 to 55 years and within a BMI range of 25 to <40 kg/m2. Ineligibility criteria included: 1) self-reporting ≥60 minutes per week of structured moderate-to-vigorous intensity PA (MVPA), 2) weight loss of ≥5% within the prior 6 months or a history of bariatric surgery, 3) history of cardiometabolic disease, diabetes mellitus, or cancer, 4) taking medication that could affect heart rate or blood pressure, 5) taking medication that could influence weight, 6) treatment for psychological conditions that included medication or counseling, 7) currently pregnant, pregnant within the prior 6 months, or planning a pregnancy within the next 12 months, 8) planning on geographical relocation outside of the region within 12 months, 9) inability to comply with the components of the interventions, 10) or had a contraindication that would prohibit MRI scanning. Participants provided written informed consent and procedures were approved by the University of Pittsburgh’s Institutional Review Board. Participants provided clearance from their personal physician prior to study participation.
Intervention
Intervention Contact
The interventions received the same prescribed contact across the 12-month intervention. Weekly intervention sessions were scheduled during months 1-6. Each intervention session was scheduled to last approximately 30-45 minutes in duration, with these including discussion of theory-based strategies to promote adherence to the weight loss behaviors.(19, 20, 21, 22) If an intervention session was missed, attempts were made to engage the participant in an in-person or telephonic make-up session. During months 7-12, the in-person sessions were decreased to approximately two sessions per month along with two brief individual telephonic sessions per month.
Dietary Intervention
All of the interventions received the same prescribed diet.(23, 24) Calorie intake was prescribed at 1,200 kcal/d, 1,500 kcal/d, and 1,800 kcal/d for participants who weighed <90.7 kg, ≥90.7 kg to <113.4 kg, and ≥113.4 kg, respectively. Dietary fat intake was prescribed at 20% to 30% of total calorie intake, with specific amounts of other nutrients (e.g., carbohydrates, protein, sodium, added sugars, etc.) not prescribed. Participants were instructed to record food intake in a diary that was reviewed by intervention staff with feedback provided. If weight loss was >6% during each 4-week period, or if BMI was reduced to ≤22 kg/m2, calorie intake was adjusted to slow the rate of weight loss.
Physical Activity
DIET was instructed to maintain their PA across the 12-month intervention. DIET+MODPA and DIET+HIGHPA were prescribed PA that started at 100 min/wk and progress by 25 min/wk in 4-week intervals until achieving a prescribed dose of 150 min/wk for DIET+MODPA or 250 min/wk for DIET+HIGHPA. All activity was non-supervised, and participants were instructed to engage in PA similar to brisk walking, or other modes that were consistent with MVPA, for periods of ≥10 minutes to achieve the prescribe amount of PA. DIET+MODPA and DIET+HIGHPA were instructed to record PA in a diary that was reviewed by intervention staff with feedback provided. Participants engaging in PA that was not consistent with their prescription were counseled by the intervention staff.
Outcome Measures
CMR Measures:
CMR was performed at baseline and 12 months to examine changes in LVM, which was the primary outcome. CMR also assessed ESV, EDV, and EF. CMR staff were blinded to randomized assignment. As previously reported,(9) subjects were scanned on a 1.5 Tesla Siemens Magnetom Espree scanner (Erlangen, Germany) with a 32-channel phased array cardiovascular coil. Standard long axis cines were acquired in the 2, 3, and 4 chamber orientations. Measurements without geometric assumptions from manual end-diastolic and end-systolic endocardial and epicardial traces of short axis stacks of cines acquired with 6 mm slice thickness using commercially available software were taken for LVM. There was no inter-slice gap to improve spatial resolution in the long axis direction and accurately measure left ventricular myocardium at the base of the heart. Papillary muscles were excluded from LVM measures. Typical parameters were: FOV 380 x 340 cm, matrix 256 x 144, 1.5 x 2.4 mm pixels, FA 50 degrees, temporal resolution 30-45 msec, 30 frames per cardiac cycle, TR/TE=2.9/1.2 msec, pixel bandwidth=930 Hz, parallel imaging factor 2 or 3 (GRAPPA).
Descriptive Characteristics:
Demographic characteristics (gender, race, ethnicity, education) were collected via questionnaire at baseline.
Weight, Height, BMI, and Body Surface Area (BSA):
Weight was assessed at baseline, 6, and 12 months with the subject clothed in a lightweight hospital gown and shoes removed. Weight was assessed using a calibrated digital scale to the nearest 0.1 kg with duplicate measures differing by ≤0.5 kg. Height was assessed at baseline with shoes removed using a wall-mounted stadiometer to the nearest 0.1 cm with duplicate measures differing by ≤0.5 cm. Weight and height were used to compute BMI (kg/m2). BSA was computed as BSA=0.0235×Height in cm0.42246×Weight in kg0.51456. Weight change (kg) and percent weight change were computed at 6 and 12 months compared to baseline.
Body Composition:
Body composition was measured as baseline, 6, and 12 months from a total body scan obtained from a dual-energy x-ray absorptiometer (DXA, GE Lunar iDXA, Madison, WI).(9)
Cardiorespiratory Fitness:
CRF was assessed at baseline, 6, and 12 months with a submaximal graded treadmill exercise test (9, 23). Indirect calorimetry was used to assess oxygen consumption (L·min−1 and ml·kg·min−1). The test was terminated when the participant achieved 85% of their age-predicted maximal heart rate.(23)
Resting Blood Pressure:
Resting blood pressure was assessed at baseline, 6, and 12 months. Duplicate measures were obtained following a 5-minute seated rest period with systolic blood pressure (SBP) measures differing by ≤10 mmHg and diastolic blood pressure (DBP) measures differing by ≤6 mmHg, with a third measurement taken if these criteria were not achieved.
Blood Samples:
Blood samples were collected using venipuncture at baseline, 6, and 12 months. Participants were instructed to fast for a period of 12 hours prior to sample collection. Samples were stored at −80° C until the time of analysis by a CLIA certified laboratory. Samples were analyzed for total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C) high-density lipoprotein cholesterol (HDL-C), glucose, insulin, and c-reactive protein (CRP).
Physical Activity:
PA was assessed at baseline, 6 months, and 12 months using both a self-reported questionnaire(25) and a wearable device as previously described.(23, 26) The questionnaire queried on walking, flights of stairs climbed, and other sport, recreational, and fitness activities over the past week. For the wearable device, subjects wore it for a one-week period at each assessment period. Data were considered valid if the participant wore the device for 10 or more hours per day on 4 or more days during the observation period. Data were used to identify minutes and metabolic equivalent task (MET)-minutes per week of MVPA (≥3.0 METs) and light-intensity PA (1.5 to <3.0 METs). Percent sedentary time (awake time <1.5 METs) was calculated as sedentary time divided by the activity monitor wear time.
Dietary Intake:
Dietary intake was assessed used the Diet History Questionnaire, with DietCalc software (version 1.5.0) used for analysis. Subjects reported their typical dietary patterns over the prior month.
Safety Monitoring:
An external data and safety monitoring board (DSMB) was appointed to assist with oversight of safety monitoring. Additionally, the investigators monitored a variety of safety measures (e.g., resting blood pressure, abnormal exercise test, etc.) and were referred to their primary care physician when appropriate. At each assessment period participants were queried regarding adverse events using a standardized questionnaire, and we observed no serious adverse events related to participation in this study.
Statistical Analysis
The primary outcome was LVM, and this study was designed to detect an effect size of 0.45 for the change in LVM from baseline to the end of the 12-month weight loss intervention. Applying the Bonferroni-adjustment (2-tailed significance level of 0.0167) to control for three pairwise comparisons, it was estimated that 104 participants per group were required to provide 80% power to detect an effect size of 0.45. To account for up to a 20% attrition rate, the projected sample size was increased from 312 to 390 subjects.
Analyses were performed using SAS version 9.4 (SAS Institute, Cary NC). Baseline characteristics of study participants were presented both overall and by randomization groups, with frequency (percentage) for categorical variables and mean ± standard deviation for continuous variables.
All randomized participants were included in intent-to-treat analyses. A linear regression model was fit to examine the effects of three interventions on the primary outcome, change in LVM from baseline to 12-months. Covariate adjustment in the model included randomization stratification factors of race and sex as well as baseline LVM. Adjusted least square mean (LSMEAN) for each intervention was obtained from the model and pairwise group comparison between the LSMEANs was tested at a significance level of 0.0167.
Changes from baseline in weight, BMI, body composition, CRF, fasting lipids, glucose, insulin, CRP, resting blood pressure, and PA measures were assessed at 6- and 12-month. These changes were analyzed by separate mixed effects models with two time points adjusting for race, sex, and baseline level of each outcome. Effects of interventions, time, and interventions by time interaction were tested at 0.05 significance level. Adjusted LSMEANs for each intervention at 6- or 12-month were obtained from the model. Since we considered these as exploratory analyses, each pairwise group comparison between the LSMEANs at 6- or 12-month was tested at a significance level of 0.0167. Missing data were assumed to be missing at random.
RESULTS
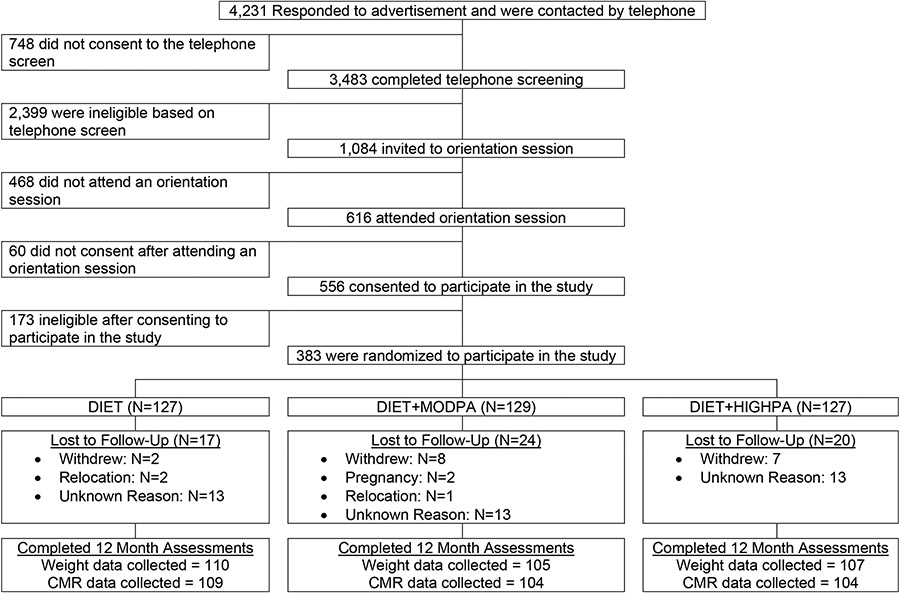
This study randomized 383 participants (DIET=127, DIET+MODPA=129, DIET+HIGHPA=127) with reasons for exclusion shown in Figure 1. Descriptive characteristics are shown in Table 1. At 12 months, weight data were available for 84.1% (322/383) of randomized participants (86.6% [110/127] in DIET, 80.6% [104/129] in DIET+MODPA, 81.9% [105/127] in DIET+HIGHPA) and CMR data were available for 82.8% (317/383) of randomized participants (85.8% [109/127] in DIET, 80.6% [104/129] in DIET+MODPA, 81.9% [104/127] in DIET+HIGHPA).
Figure 1.
Consort diagram.
Table 1.
Baseline characteristics of participants by intervention condition.
| Variable | Total (N=383) |
Randomized Group | ||
|---|---|---|---|---|
| DIET (N=127) |
DIET+MODPA (N=129) |
DIET+HIGHPA (N=127) |
||
| Age, years* | 45.6±8.0 | 44.3±8.0 | 46.8±7.6 | 45.6±8.1 |
| Gender** | ||||
| Male | N=79 (20.6%) | N=26 (20.5%) | N=27 (21.0%) | N=26 (20.5%) |
| Female | N=304 (79.4%) | N=101 (79.5%) | N=102 (79.1%) | N=101 (79.5%) |
| Race** | ||||
| White | N=273 (71.3%) | N=91 (71.7%) | N=93 (72.1%) | N=89 (70.1%) |
| Non-white | N=110 (28.7%) | N=36 (28.4%) | N=36 (28.0%) | N=38 (29.9%) |
| Hispanic/Latino** | ||||
| Yes | N=13 (3.4%) | N=5 (3.9%) | N=3 (2.3%) | N=5 (3.9%) |
| No | N=370 (96.6%) | N=122 (96.1%) | N=126 (97.7%) | N=122 (96.1%) |
| Weight, kg* | 90.9±13.7 | 91.8±14.5 | 89.9±13.5 | 91.0±13.1 |
| Body mass index, kg/m2 * | 32.4±3.8 | 32.6±3.5 | 32.3±3.8 | 32.2±4.0 |
| Education** | ||||
| High school graduate or Graduate Equivalency Degree | N=123 (32.1%) | N=32 (25.2%) | N=46 (35.7%) | N=45 (35.4%) |
| College graduate or higher | N=256 (66.8%) | N=93 (73.3%) | N=82 (63.6%) | N=81 (63.8%) |
| Relationship Status** | ||||
| Married | N=225 (58.8%) | N=73 (57.5%) | N=82 (63.6%) | N=70 (55.1%) |
| Separated/Divorced | N=54 (14.1%) | N=21 (16.5%) | N=16 (12.4%) | N=17 (13.4%) |
| Widowed | N=2 (0.5%) | N=1 (0.8%) | N=0 (0.0%) | N=1 (0.8%) |
| Single/Not Married | N=102 (26.6%) | N=32 (25.2%) | N=31 (24.0%) | N=39 (30.7%) |
| Household income** | ||||
| Less than $25,000 | N=68 (17.9%) | N=23 (18.3%) | N=27 (21.3%) | N=18 (14.3%) |
| $25,000 and more | N=300 (79.2%) | N=102 (81.0%) | N=96 (75.6%) | N=102 (81.0%) |
| Participant did not report income | N=11 (2.9%) | N=1 (0.8%) | N=4 (3.2%) | N=6 (4.8%) |
indicates data presented as Mean ± Standard Deviation
indicates data presented as N (%)
CMR Outcomes
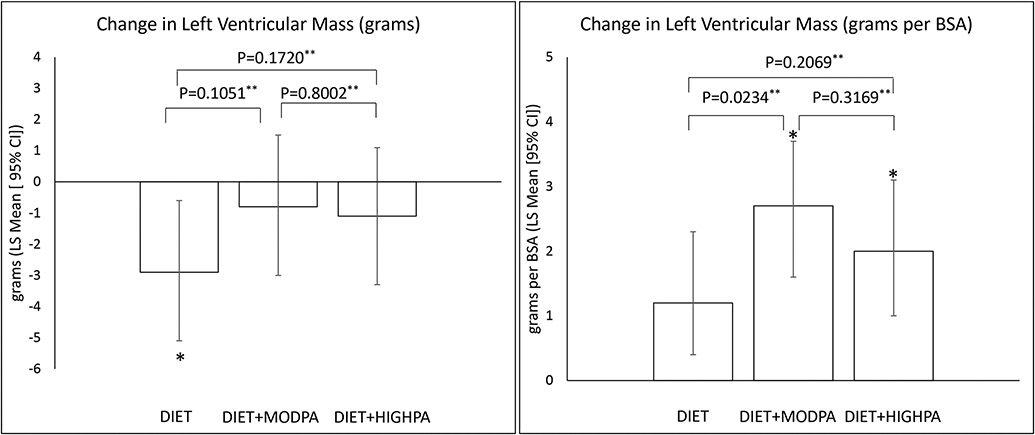
CMR outcomes are shown in Table 2 and Figure 2. The primary outcome was LVM, and there was a significant reduction observed at 12 months in DIET [−2.9 grams (95% CI: −5.2, −0.7); P=0.0114] with no change observed in DIET+MODPA [−0.8 grams (95% CI: −3.0, 1.5); P=0.4979] or DIET+HIGHPA [−1.1 grams (95% CI: −3.3, 1.1); P=0.3299]. LVM relative to BSA increased in all groups [DIET=1.2 grams/BSA (95% CI: 0.2, 2.3), P=0.0220; DIET+MODPA=2.7 grams/BSA (95% CI: 1.6, 3.7); P<0.0001; DIET+HIGHPA=2.0 grams/BSA (95% CI: 1.0, 3.1); P=0.0002]. All groups had significant increases in ESV and EDV, with no change in EF. Prespecified pairwise comparisons showed that there was no difference between the interventions for the change in LVM, ESV, EDV, or EF (Table 2, Figure 2).
Table 2.
Change in cardiac magnetic resonance imaging measures by intervention condition.
| Least-Squares Mean (95% CI)* | P value* | P values for pairwise comparisons*** | ||||
|---|---|---|---|---|---|---|
| Baseline | Change from baseline to 12 months |
DIET vs. DIET+MODPA |
DIET vs. DIET+HIGHPA |
DIET+MODPA vs. DIET+HIGHPA |
||
| Left Ventricular Mass (grams) | N = 383 | N = 317 | 0.2169 | 0.1051 | 0.1720 | 0.8002 |
| DIET | 90.9 (87.2, 94.6) | −2.9 (−5.2, −0.7) P = 0.0114** |
||||
| DIET+MODPA | 88.2 (84.2, 92.1) | −0.8 (−3.0, 1.5) P = 0.4979** |
||||
| DIET+HIGHPA | 87.7 (84.4, 91.1) | −1.1 (−3.3, 1.1) P = 0.3299** |
||||
| Left Ventricular Mass (grams per BSA) | N = 383 | N = 317 | 0.0752 | 0.0234 | 0.2069 | 0.3169 |
| DIET | 43.2 (42.0, 44.5) | 1.2 (0.2, 2.3) P = 0.0220** |
||||
| DIET+MODPA | 42.5 (41.0, 44.0) | 2.7 (1.6, 3.7) P <0.0001** |
||||
| DIET+HIGHPA | 42.0 (40.7, 43.2) | 2.0 (1.0, 3.1) P = 0.0002** |
||||
| Resting End-Systolic Volume | N = 383 | N = 317 | 0.5958 | 0.7457 | 0.3173 | 0.5053 |
| DIET | 54.2 (51.8, 56.6) | 3.6 (1.5, 5.7) P = 0.0010** |
||||
| DIET+MODPA | 54.3 (51.7, 56.9) | 3.2 (1.0, 5.3) P = 0.0042** |
||||
| DIET+HIGHPA | 55.4 (53.0, 57.8) | 2.3 (0.1, 4.5) P = 0.0441** |
||||
| Resting End-Diastolic Volume | N = 383 | N = 317 | 0.9994 | 0.9879 | 0.9845 | 0.9726 |
| DIET | 151.1 (145.9, 156.3) | 6.3 (3.4, 9.3) P <0.0001** |
||||
| DIET+MODPA | 145.8 (140.6, 151.0) | 6.3 (3.4, 9.3) P <0.0001** |
||||
| DIET+HIGHPA | 146.7 (142.5, 150.8) | 6.4 (3.4, 9.4) P <0.0001** |
||||
| Resting Ejection Fraction | N = 383 | N = 317 | 0.7725 | 0.5629 | 0.5129 | 0.9378 |
| DIET | 64.4 (63.6, 65.2) | −0.3 (−1.2, 0.7) P = 0.5758** |
||||
| DIET+MODPA | 63.3 (62.5, 64.1) | −0.6 (−1.6, 0.3) P = 0.2104** |
||||
| DIET+HIGHPA | 63.0 (62.3, 63.8) | −0.7 (−1.6, 0.3) P = 0.1827** |
||||
P-value comparing the change between groups. The model includes adjustment for sex, race, and baseline value.
P-value for change from baseline for each specific group, with the critical p-value set at ≤0.0167 to adjust for multiple comparisons.
P-value for comparison of groups for change from baseline, with the critical p-value set at ≤0.0167 to adjust for multiple comparisons.
Figure 2.
Change in left ventricular mass
*indicates significant change from baseline at p≤0.0167, **indicates p-value for pairwise comparison, BSA = body surface area
Weight and Body Composition
Weight and body composition are shown in Table 3. Weight decreased significantly (P<0.0001) across the 12-month intervention with no significant difference among interventions. Weight loss at 6 month was −9.3% (95% CI: −10.3, −8.2) in DIET, −10.3% (95% CI: −11.4, −9.2) in DIET+MODPA, and −10.3% (95% CI: −11.3, −9.2) in DIET+HIGHPA, and weight loss as 12 months was −10.2% (95% CI: −11.7, −8.8) in DIET, −11.0% (95% CI: −12.4, −9.5) in DIET+MODPA, and −10.3% (95% CI: −11.8, −8.9) in DIET+HIGHPA, with the weight loss at 6 and 12 months being significantly different (Time Effect P=0.0175). A similar pattern was observed for absolute change in weight, BMI, fat mass, and percent body fat. A modest reduction in lean mass was also observed in each group (P<0.0001), with no difference among interventions, and the change at 6 and 12 months did not differ.
Table 3.
Weight, BMI, body composition, and cardiorespiratory fitness by intervention condition.
| Least-Squares Mean (95% CI)* | P value |
P value for Pairwise Comparisons of Change at 6 Months**** |
P value for Pairwise Comparisons of Change at 12 Months**** |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change from Baseline to 6 months |
Change from Baseline to 12 months |
Group | Time** | Group x Time |
DIET vs. DIET+MODPA |
DIET vs. DIET+HIGHPA |
DIET+MODPA vs. DIET+HIGHPA |
DIET vs. DIET+MODPA |
DIET vs. DIET+HIGHPA |
DIET+MODPA vs. DIET+HIGHPA |
|
| Weight, kg | N = 383 | N = 340 | N = 322 | 0.5684 | 0.0152 | 0.2979 | 0.1643 | 0.2532 | 0.8017 | 0.4503 | 0.9928 | 0.4477 |
| DIET | 91.8 (90.0, 93.6) | −8.5 (9.5, −7.5)*** | −9.4 (−10.8, −8.0)*** | |||||||||
| DIET+MODPA | 89.9 (88.3, 91.6) | −9.5 (−10.5, −8.5)*** | −10.1 (−11.5, −8.8)*** | |||||||||
| DIET+HIGHPA | 91.0 (89.4, 92.6) | −9.3 (−10.3, −8.3)*** | −9.4 (−10.8, −8.0)*** | |||||||||
| Weight change from baseline, % | N = 340 | N = 322 | 0.6010 | 0.0175 | 0.2763 | 0.1843 | 0.1843 | 0.9931 | 0.4902 | 0.9182 | 0.5591 | |
| DIET | −---- | −9.3 (−10.3, −8.2)*** | −10.2 (−11.7, −8.8)*** | |||||||||
| DIET+MODPA | −---- | −10.3 (−11.4, −9.2)*** | −11.0 (−12.4, −9.5)*** | |||||||||
| DIET+HIGHPA | −---- | −10.3 (−11.3, −9.2)*** | −10.3 (−11.8, −8.9)*** | |||||||||
| Body Mass Index, kg/m2 | N = 383 | N = 340 | N = 322 | 0.4985 | 0.0133 | 0.3342 | 0.1275 | 0.1948 | 0.8198 | 0.3929 | 0.8985 | 0.4702 |
| DIET | 32.6 (32.0, 33.2) | −3.0 (−3.3, −2.6)*** | −3.3 (−3.8, −2.8)*** | |||||||||
| DIET+MODPA | 32.3 (31.6, 32.9) | −3.4 (−3.7, −3.0)*** | −3.6 (−4.1, −3.1)*** | |||||||||
| DIET+HIGHPA | 32.2 (31.5, 32.9) | −3.3 (−3.6, −3.0)*** | −3.3 (−3.8, −2.8)*** | |||||||||
| Fat mass (kg) | N = 383 | N = 339 | N = 316 | 0.5348 | 0.0076 | 0.4961 | 0.1164 | 0.1467 | 0.9056 | 0.5206 | 0.7303 | 0.7688 |
| DIET | 39.3 (38.4, 40.2) | −7.0 (−7.8, −6.2)*** | −7.8 (−8.9, −6.6)*** | |||||||||
| DIET+MODPA | 38.6 (37.6, 39.6) | −7.9 (−8.7, −7.1)*** | −8.3 (−9.5, −7.2)*** | |||||||||
| DIET+HIGHPA | 39.6 (38.5, 40.8) | −7.8 (−8.6, −7.0)*** | −8.1 (−9.2, −6.9)*** | |||||||||
| Lean mass (kg) | N = 383 | N = 339 | N = 316 | 0.5501 | 0.8615 | 0.6134 | 0.7688 | 0.8999 | 0.6063 | 0.3502 | 0.7478 | 0.2134 |
| DIET | 49.3 (48.1, 50.5) | −1.4 (−1.7, −1.1)*** | −1.4 (−1.7, −1.1)*** | |||||||||
| DIET+MODPA | 48.2 (47.2, 49.2) | −1.5 (−1.8, −1.2)*** | −1.6 (−2.0, −1.3)*** | |||||||||
| DIET+HIGHPA | 48.3 (47.3, 49.3) | −1.4 (−1.7, −1.1)*** | −1.3 (−1.7, −1.0)*** | |||||||||
| Percent body fat (%) | N = 383 | N = 339 | N = 316 | 0.5868 | 0.0004 | 0.3299 | 0.1384 | 0.0934 | 0.8450 | 0.6492 | 0.7967 | 0.8458 |
| DIET | 43.1 (42.5, 43.7) | −4.2 (−4.8, −3.7)*** | −5.1 (−5.9, −4.2)*** | |||||||||
| DIET+MODPA | 42.6 (41.8, 43.4) | −4.8 (−5.4, −4.3)*** | −5.3 (−6.2, −4.5)*** | |||||||||
| DIET+HIGHPA | 43.5 (42.7, 44.2) | −4.9 (−5.5, −4.4)*** | −5.2 (−6.1, −4.4)*** | |||||||||
| Cardiorespiratory Fitness (ml/kg/min) | N = 381 | N = 331 | N = 309 | 0.0081 | 0.4946 | 0.0612 | 0.0888 | 0.0008 | 0.0943 | 0.0120 | 0.0476 | 0.5958 |
| DIET | 23.1 (22.5, 23.6) | 2.0 (1.4, 2.5)*** | 2.1 (1.4, 2.9)*** | |||||||||
| DIET+MODPA | 22.3 (21.7, 22.9) | 2.7 (2.1, 3.2)*** | 3.3 (2.6, 3.7)*** | |||||||||
| DIET+HIGHPA | 22.6 (22.0, 23.1) | 3.4 (2.8, 3.9)*** | 3.0 (2.3, 3.7)*** | |||||||||
| Cardiorespiratory Fitness (L/min) | N = 381 | N = 331 | N = 309 | 0.0435 | 0.1680 | 0.1228 | 0.4208 | 0.0141 | 0.0983 | 0.0330 | 0.0597 | 0.7983 |
| DIET | 2.1 (2.1, 2.2) | −0.03 (−0.08, 0.01) | −0.06 (−0.11, −0.01)*** | |||||||||
| DIET+MODPA | 2.0 (1.9, 2.1) | −0.01 (−0.05, 0.04) | 0.01 (−0.04, 0.06) | |||||||||
| DIET+HIGHPA | 2.0 (2.0, 2.1) | 0.05 (0.00, 0.09) | 0.00 (−0.04, 0.05) | |||||||||
| Cardiorespiratory Fitness (termination time, minutes) | N = 383 | N = 337 | N = 312 | 0.0285 | 0.1598 | 0.5206 | 0.1630 | 0.0092 | 0.2306 | 0.0638 | 0.0631 | 0.09957 |
| DIET | 7.8 (7.5, 8.2) | 1.6 (1.2, 2.1)*** | 1.5 (1.0, 1.9)*** | |||||||||
| DIET+MODPA | 7.5 (7.1, 7.9) | 2.1 (1.6, 2.5)*** | 2.1 (1.6, 2.5)*** | |||||||||
| DIET+HIGHPA | 7.9 (7.5, 8.2) | 2.4 (2.0, 2.8)*** | 2.1 (1.6, 2.5)*** | |||||||||
Model adjusted for sex and race and baseline value.
P-value for comparison of change at 6 months to change at 12 months.
Indicates change from baseline is statistically significant at p-value ≤0.0167 to adjust for multiple comparisons.
P-value for comparison of groups at specific time points, with the critical p-value set at ≤0.0167 to adjust for multiple comparisons.
Cardiorespiratory Fitness
CRF is shown in Table 3. There was a significant increase in relative oxygen consumption (ml/kg/min) (P<0.0001), with a difference in the pattern of change among the three interventions (Group X Time: P=0.0081). A similar pattern was observed when CRF was assessed based on termination time on the graded exercise test. The change in absolute oxygen consumption (L/min) from baseline to 12 months significantly decreased in DIET (P=0.0106) with no change in either DIET+MODPA (P=0.6158) or DIET+HIGHPA (P=0.8883).
CVD Risk Factors
CVD risk factors are shown in Table 4. Total cholesterol decreased significantly in all groups at 6 months, but at 12 months was only significant for DIET (P=0.0004) and DIET+HIGHPA (P=0.0010). The decrease in LDL-C was significant at 6 (P’s<0.0001) and 12 months (P’s≤0.0059) for DIET and DIET+HIGHPA. HDL-C decreased significantly at 6 months for DIET and no significant change for DIET+MODPA or DIET+HIGHPA, with all groups showing a significant increase at 12 months (P≤0.0001). Triglycerides decreases significantly (P<0.0001) in all groups at both 6 and 12 months. Fasting glucose decreased significantly for all groups at 6 months, but at 12 months the decrease was only significant for DIET (P=0.0026) and DIET+HIGHPA (P=0.0010). Fasting insulin (P’s<0.0001) and CRP (P’s≤0.0107) decreased significantly in all groups at both 6 and 12 months. SBP significantly decreased at both 6 and 12 months in all groups (all P’s <0.0001), with a similar pattern observed for change in DBP.
Table 4.
Fasting lipids, glucose, insulin, CRP, and resting blood pressure by intervention condition.
| Least-Squares Mean (95% CI)* | P value |
P value for Pairwise Comparisons of Change at 6 Months**** |
P value for Pairwise Comparisons of Change at 12 Months**** |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change from Baseline to 6 months |
Change from Baseline to 12 months |
Group | Time** | Group x Time |
DIET vs. DIET+MODPA |
DIET vs. DIET+HIGHPA |
DIET+MODPA vs. DIET+HIGHPA |
DIET vs. DIET+MODPA |
DIET vs. DIET+HIGHPA |
DIET+MODPA vs. DIET+HIGHPA |
|
| Total Cholesterol (mg/dl) | N = 382 | N = 335 | N = 314 | 0.1091 | 0.0002 | 0.8536 | 0.0573 | 0.9489 | 0.0695 | 0.1576 | 0.8990 | 0.2026 |
| DIET | 197.2 (191.4, 203.0) | −13.5 (−17.9, −9.1)*** | −8.3 (−12.9, −3.7)*** | |||||||||
| DIET+MODPA | 199.9 (194.5, 205.4) | −7.4 (−11.9, −2.9)*** | −3.6 (−8.3, 1.0) | |||||||||
| DIET+HIGHPA | 198.3 (191.8, 204.9) | −13.3 (−17.8, −8.7)*** | −7.9 (−12.5, −3.2)*** | |||||||||
| LDL Cholesterol (mg/dl) | N = 382 | N = 335 | N = 314 | 0.1189 | 0.1437 | 0.8126 | 0.0827 | 0.7384 | 0.0410 | 0.2467 | 0.8706 | 0.1903 |
| DIET | 119.2 (114.0, 124.3) | −7.7 (−11.4, −3.9)*** | −5.7 (−9.8, −1.7)*** | |||||||||
| DIET+MODPA | 122.2 (117.6, 126.7) | −3.0 (−6.8, 0.8) | −2.3 (−6.4, 1.8) | |||||||||
| DIET+HIGHPA | 120.3 (115.2, 125.4) | −8.6 (−12.4, −4.8)*** | −6.2 (−10.3, −2.1)*** | |||||||||
| HDL Cholesterol (mg/dl) | N = 382 | N = 335 | N = 314 | 0.0267 | <0.0001 | 0.9672 | 0.0520 | 0.0206 | 0.7039 | 0.0680 | 0.0573 | 0.9351 |
| DIET | 53.6 (51.3, 56.0) | −1.5 (−2.6, −0.4) *** | 2.6 (1.3, 3.9)*** | |||||||||
| DIET+MODPA | 52.9 (50.9, 54.9) | 0.1 (−1.0, 1.3) | 4.3 (3.0, 5.6)*** | |||||||||
| DIET+HIGHPA | 53.2 (51.2, 55.2) | 0.4 (−0.7, 1.6) | 4.4 (3.0, 5.7)*** | |||||||||
| Triglycerides (mg/dl) | N = 382 | N = 335 | N = 314 | 0.7939 | 0.0052 | 0.9041 | 0.8752 | 0.6261 | 0.5233 | 0.8707 | 0.5767 | 0.6949 |
| DIET | 122.4 (109.4, 135.5) | −22.2 (−30.6, −13.9)*** | −27.6 (−33.7, −21.5)*** | |||||||||
| DIET+MODPA | 124.9 (112.5, 137.3) | −21.3 (−29.7, −12.8)*** | −28.3 (−34.5, −22.1)*** | |||||||||
| DIET+HIGHPA | 124.0 (112.6, 135.4) | −25.2 (−33.7, −16.6)*** | −30.1 (−36.3, −23.8)*** | |||||||||
| Fasting Glucose (mg/dl) | N = 382 | N = 335 | N = 314 | 0.7244 | 0.4840 | 0.3270 | 0.3110 | 0.1878 | 0.7586 | 0.6555 | 0.8124 | 0.4971 |
| DIET | 94.0 (92.0, 96.0) | −3.1 (−4.5, −1.7)*** | −2.7 (−4.5, −1.0)*** | |||||||||
| DIET+MODPA | 94.3 (92.0, 96.6) | −2.1 (−3.5, −0.7)*** | −2.2 (−3.9, −0.4) | |||||||||
| DIET+HIGHPA | 96.5 (94.1, 99.0) | −1.8 (−3.2, −0.4)*** | −3.0 (−4.8, −1.2)*** | |||||||||
| Fasting Insulin (mIU/mL) | N = 382 | N = 335 | N = 314 | 0.8412 | 0.7882 | 0.4091 | 0.4575 | 0.5594 | 0.8771 | 0.8919 | 0.5164 | 0.4346 |
| DIET | 17.5 (16.1, 18.8) | −3.1 (−4.2, −2.0)*** | −3.7 (−4.6, −2.8)*** | |||||||||
| DIET+MODPA | 15.3 (14.0, 16.6) | −3.7 (−4.9, −2.6)*** | −3.8 (−4.7, −2.8)*** | |||||||||
| DIET+HIGHPA | 16.3 (14.3, 18.3) | −3.6 (−4.8, −2.5)*** | −3.2 (−4.2, −2.3)*** | |||||||||
| CRP (mg/L) | N = 382 | N = 335 | N = 314 | 0.1888 | 0.8705 | 0.4596 | 0.6215 | 0.2192 | 0.4643 | 0.1208 | 0.1160 | 0.9802 |
| DIET | 4.3 (3.4, 5.3) | −1.5 (−2.0, −1.0)*** | −1.8 (−2.5, −1.1)*** | |||||||||
| DIET+MODPA | 4.1 (3.4, 4.8) | −1.3 (−1.8, −0.8)*** | −1.0 (−1.7, −0.2)*** | |||||||||
| DIET+HIGHPA | 4.5 (3.7, 5.3) | −1.0 (−1.5, −0.6)*** | −1.0 (−1.7, −0.2)*** | |||||||||
| Resting Systolic Blood Pressure (mmHg) | N = 383 | N = 340 | N = 321 | 0.9809 | 0.0452 | 0.8414 | 0.8694 | 0.8397 | 0.7166 | 0.8631 | 0.7590 | 0.8940 |
| DIET | 120.2 (118.2, 122.2) | −5.1 (−6.7, −3.4)*** | −4.3 (−6.0, −2.6)*** | |||||||||
| DIET+MODPA | 120.1 (118.1, 122.1) | −4.9 (−6.6, −3.2)*** | −4.1 (−5.8, −2.3)*** | |||||||||
| DIET+HIGHPA | 120.2 (118.0, 122.3) | −5.3 (−7.0, −3.6)*** | −3.9 (−5.7, −2.1)*** | |||||||||
| Resting Diastolic Blood Pressure (mmHg) | N = 383 | N = 340 | N = 321 | 0.1720 | 0.1483 | 0.5463 | 0.1347 | 0.7042 | 0.0635 | 0.6681 | 0.3075 | 0.1523 |
| DIET | 72.0 (70.5, 73.5) | −3.2 (−4.3, −2.1)*** | −2.3 (−3.4, −1.1)*** | |||||||||
| DIET+MODPA | 72.3 (70.8, 73.8) | −2.0 (−3.1, −0.8)*** | −1.9 (−3.1, −0.7)*** | |||||||||
| DIET+HIGHPA | 72.5 (70.8, 74.1) | −3.5 (−4.7, −2.4)*** | −3.1 (−4.3, −1.9)*** | |||||||||
Model adjusted for sex and race and baseline value.
P-value for comparison of change at 6 months to change at 12 months.
Indicates change from baseline is statistically significant at p-value ≤0.0167 to adjust for multiple comparisons.
P-value for comparison of groups at specific time points, with the critical p-value set at ≤0.0167 to adjust for multiple comparisons.
Dietary Intake
Total calorie intake significantly decreased at both 6 and 12 months in all groups (all P’s <0.0001), with a similar pattern observed for the change in percent of calories consumed as dietary fat (all P’s <0.0001) (Table 5). The percent of calories consumed as carbohydrates increased at both 6 and 12 months in all groups (all P’s ≤0.0035), with a similar pattern for the increase in the percent of calories consumed as protein (all P’s ≤0.0046). The change in total calorie intake and macronutrient composition did not differ between groups.
Table 5.
Dietary Intake by Intervention Condition.
| Least-Squares Mean (95% CI)* | P value |
P value for Pairwise Comparisons of Change at 6 Months**** |
P value for Pairwise Comparisons of Change at 12 Months**** |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change from Baseline to 6 months |
Change from Baseline to 12 months |
Group | Time** | Group x Time |
DIET vs. DIET+MODPA |
DIET vs. DIET+HIGHPA |
DIET+MODPA vs. DIET+HIGHPA |
DIET vs. DIET+MODPA |
DIET vs. DIET+HIGHPA |
DIET+MODPA vs. DIET+HIGHPA |
|
| Intake: Total calories (kcal/day) | N =382 | N = 332 | N = 302 | 0.1633 | 0.7737 | 0.3997 | 0.1288 | 0.3917 | 0.5075 | 0.1201 | 0.7232 | 0.0595 |
| DIET | 1867.4 (1696.1, 2038.7) | −544.6 (−631.5, −457.7)*** | −526.7 (−610.7, −442.7)*** | |||||||||
| DIET+MODPA | 1804.9 (1651.9, 1957.9) | −448.5 (−537.2 −359.7)*** | −431.2 (−517.8, −344.7)*** | |||||||||
| DIET+HIGHPA | 1765.0 (1621.4, 1908.6) | −490.7 (−578.8, −402.5)*** | −548.3 (−633.9, −462.8)*** | |||||||||
| Intake: % calories carbohydrates | N = 382 | N = 332 | N = 302 | 0.9781 | 0.3009 | 0.3001 | 0.6576 | 0.4949 | 0.8139 | 0.5221 | 0.3206 | 0.7301 |
| DIET | 48.2 (46.6, 49.8) | 3.9 (2.4, 5.4)*** | 2.3 (0.8, 3.9)*** | |||||||||
| DIET+MODPA | 47.7 (46.0, 49.4) | 3.4 (1.9, 5.0)*** | 3.1 (1.5, 4.7)*** | |||||||||
| DIET+HIGHPA | 46.7 (45.1, 48.3) | 3.2 (1.6, 4.7)*** | 3.5 (1.9, 5.1)*** | |||||||||
| Intake: % calories protein | N = 382 | N = 332 | N = 302 | 0.3885 | 0.1684 | 0.4449 | 0.1590 | 0.0992 | 0.8143 | 0.5732 | 0.9264 | 0.6409 |
| DIET | 15.5 (14.9, 16.1) | 0.9 (0.38, 1.4)*** | 0.9 (0.3, 1.5)*** | |||||||||
| DIET+MODPA | 15.8 (15.2, 16.4) | 1.5 (0.9, 2.0)*** | 1.1 (0.5, 1.7)*** | |||||||||
| DIET+HIGHPA | 15.8 (15.2, 16.3) | 1.6 (1.0, 2.1)*** | 0.9 (0.3, 1.5)*** | |||||||||
| Intake: % calories fat | N = 382 | N = 332 | N = 302 | 0.7443 | 0.0019 | 0.4207 | 0.3661 | 0.9141 | 0.4290 | 0.6751 | 0.3526 | 0.6165 |
| DIET | 36.2 (35.0, 37.4) | −5.3 (−6.5, −4.1)*** | −3.3 (−4.7, −2.0)*** | |||||||||
| DIET+MODPA | 36.9 (35.5, 38.3) | −4.5 (−5.8, −3.3)*** | −3.7 (−5.1, −2.4)*** | |||||||||
| DIET+HIGHPA | 37.3 (36.0, 38.5) | −5.2 (−6.4, −4.0)*** | −4.2 (−5.6, −2.9)*** | |||||||||
Model adjusted for sex and race and baseline value.
P-value for comparison of change at 6 months to change at 12 months.
Indicates change from baseline is statistically significant at p-value ≤0.0167 to adjust for multiple comparisons.
P-value for comparison of groups at specific time points, with the critical p-value set at ≤0.0167 to adjust for multiple comparisons.
Physical Activity
PA is shown in Table 6. Minutes of self-reported PA increased in DIET+MODPA and DIET+HIGHPA at both 6 and 12 months (P’s<0.0001), with the change not being statistically significant at either 6 or 12 months for DIET. Based on pairwise comparisons, the change in self-reported PA was significantly different between groups when compared at both 6 months (P’s <0.0001) and 12 months (P’s ≤0.0156). A similar pattern was observed for energy expenditure of self-reported PA; however, the change in self-reported PA did not differ between DIET+MODPA and DIET+HIGHPA at 12 months.
Table 6.
Physical Activity by Intervention Condition.
| Least-Squares Mean (95% CI)* | P value |
P value for Pairwise Comparisons of Change at 6 Months**** |
P value for Pairwise Comparisons of Change at 12 Months**** |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change from Baseline to 6 months |
Change from Baseline to 12 months |
Group | Time** | Group x Time |
DIET vs. DIET+MODPA |
DIET vs. DIET+HIGHPA |
DIET+MODPA vs. DIET+HIGHPA |
DIET vs. DIET+MODPA |
DIET vs. DIET+HIGHPA |
DIET+MODPA vs. DIET+HIGHPA |
|
| Self-Reported Physical Activity (kcal/wk) # | N = 383 | N = 338 | N = 317 | <0.0001 | 0.9476 | 0.0395 | <0.0001 | <0.0001 | 0.0001 | <0.0001 | <0.0001 | 0.2423 |
| DIET | 525.8 (453.7, 597.9) | 122.3 (0.6, 244.1) | 89.3 (−60.4, 238.9) | |||||||||
| DIET+MODPA | 595.6 (493.3, 697.8) | 580.2 (456.1, 704.4)*** | 743.7 (590.8, 896.6)*** | |||||||||
| DIET+HIGHPA | 638.3 (532.8, 743.8) | 993.4 (869.3, 1117.5)*** | 872.1 (720.3, 1024.0)*** | |||||||||
| Self-Reported Physical Activity (kcal/wk) $ | N = 383 | N = 338 | N = 317 | <0.0001 | 0.7290 | 0.0780 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0657 |
| DIET | 310.9 (241.8, 379.9) | 121.6 (3.9, 239.3) | 69.9 (−71.6, 211.5) | |||||||||
| DIET+MODPA | 371.1 (276.6, 465.6) | 562.6 (442.5, 682.7)*** | 685.6 (540.8, 830.3)*** | |||||||||
| DIET+HIGHPA | 404.6 (307.0, 502.2) | 994.7 (874.7, 1114.6)*** | 877.2 (733.5, 1020.8)*** | |||||||||
| Self-Reported Physical Activity (min/wk) $ | N = 383 | N = 338 | N = 317 | <0.0001 | 0.3911 | 0.2904 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0156 |
| DIET | 59.4 (46.3, 72.4) | 23.0 (3.3, 42.6) | 12.4 (−11.0, 35.8) | |||||||||
| DIET+MODPA | 69.3 (52.1, 86.4) | 105.3 (85.2, 125.4)*** | 114.8 (90.9, 138.7)*** | |||||||||
| DIET+HIGHPA | 75.7 (58.3, 93.0) | 174.0 (154.0, 194.1)*** | 156.4 (132.7, 180.2)*** | |||||||||
| Total MVPA (MET-min/wk)^ | N = 375 | N = 320 | N = 301 | <0.0001 | 0.0108 | 0.1192 | 0.0085 | <0.0001 | 0.0084 | 0.0081 | 0.0064 | 0.9557 |
| DIET | 1292.3 (1051.5, 1533.0) | 142.6 (−71.2, 356.4) | 407.1 (137.1, 677.1)*** | |||||||||
| DIET+MODPA | 1392.4 (1127.6, 1657.1) | 555.4 (335.7, 775.1)*** | 936.7 (654.3, 1219.0)*** | |||||||||
| DIET+HIGHPA | 1321.8 (1045.8, 1597.8) | 969.7 (754.8,1184.7)*** | 947.8 (669.7, 1226.0)*** | |||||||||
| Total MVPA (min/wk) ^ | N = 375 | N = 320 | N = 301 | <0.0001 | 0.0086 | 0.1302 | 0.0364 | <0.0001 | 0.0066 | 0.0157 | 0.0090 | 0.8595 |
| DIET | 346.2 (288.1, 404.3) | 25.8 (−22.7, 74.3) | 82.2 (23.0, 141.4)*** | |||||||||
| DIET+MODPA | 366.6 (301.0, 432.3) | 100.1 (50.2, 149.9)*** | 188.0 (126.1, 249.9)*** | |||||||||
| DIET+HIGHPA | 350.4 (279.5, 421.3) | 197.1 (148.3, 245.8)*** | 195.8 (134.8, 256.8)*** | |||||||||
| Total MVPA in >10 minute sessions (MET-min/wk) ^ | N = 375 | N = 320 | N =301 | <0.0001 | 0.5106 | 0.0126 | 0.0093 | <0.0001 | 0.0006 | 0.0165 | 0.0144 | 0.9758 |
| DIET | 926.8 (744.6, 1109.0) | 128.8 (−59.1, 316.6) | 317.2 (101.4, 532.9)*** | |||||||||
| DIET+MODPA | 1042.9 (834.8, 1250.9) | 487.2 (294.1, 680.4)*** | 700.3 (474.0, 926.5)*** | |||||||||
| DIET+HIGHPA | 957.1 (749.3, 1165.0) | 963.2 (774.4, 1152.2)*** | 705.2 (482.4, 928.0)*** | |||||||||
| Total MVPA in >10 minute sessions (min/wk) ^ | N = 375 | N = 320 | N =301 | <0.0001 | 0.4892 | 0.0108 | 0.0486 | <0.0001 | 0.0003 | 0.0282 | 0.0282 | 0.9516 |
| DIET | 244.3 (200.9, 287.6) | 21.0 (−20.6, 62.6) | 57.9 (10.9, 105.0)*** | |||||||||
| DIET+MODPA | 265.5 (215.6, 315.4) | 81.1 (38.3, 123.9)*** | 134.3 (85.0, 183.6)*** | |||||||||
| DIET+HIGHPA | 246.4 (193.2, 299.5) | 193.4 (151.6, 235.2)*** | 136.4 (87.9, 185.0)*** | |||||||||
| LPA (MET-min/wk) ^ | N = 375 | N = 320 | N = 301 | 0.0844 | 0.3343 | 0.2771 | 0.1149 | 0.1214 | 0.9662 | 0.0216 | 0.5744 | 0.0858 |
| DIET | 2773.3 (2530.3, 3016.3) | 202.3 (−4.4, 409.1) | 288.2 (106.6, 469.7)*** | |||||||||
| DIET+MODPA | 2829.4 (2618.8, 3040.1) | 440.3 (228.0, 652.6)*** | 596.0 (406.51, 785.4)*** | |||||||||
| DIET+HIGHPA | 2556.5 (2362.7, 2750.3) | 433.9 (225.9, 641.8)*** | 362.6 (175.9, 549.4)*** | |||||||||
| LPA (min/wk) ^ | N = 375 | N = 320 | N = 301 | 0.1031 | 0.3454 | 0.3397 | 0.1221 | 0.1539 | 0.8934 | 0.0289 | 0.6075 | 0.0982 |
| DIET | 1381.4 (1262.8, 1500.1) | 158.3 (49.4, 267.2)*** | 201.9 (105.7, 298.1)*** | |||||||||
| DIET+MODPA | 1429.9 (1320.3, 1539.6) | 281.2 (169.4, 393.1)*** | 357.0 (256.6, 457.3)*** | |||||||||
| DIET+HIGHPA | 1283.6 (1185.8, 1381.3) | 270.5 (161.0, 380.1)*** | 238.0 (139.1, 336.9)*** | |||||||||
| Sedentary (% of monitor wear time)^ | N = 375 | N = 320 | N = 301 | 0.0096 | 0.0768 | 0.1004 | 0.0478 | 0.0039 | 0.3776 | 0.0069 | 0.1699 | 0.1825 |
| DIET | 71.4 (68.8, 74.0) | −2.7 (−4.9, −0.6)*** | −4.3 (−6.4, −2.2)*** | |||||||||
| DIET+MODPA | 70.6 (68.1, 73.2) | −5.8 (−8.0, −3.6)*** | −8.6 (−10.8, −6.3)*** | |||||||||
| DIET+HIGHPA | 72.8 (70.3, 75.3) | −7.2 (−9.3, −5.1)*** | −6.4 (−8.6, −4.2)*** | |||||||||
| Sedentary (hr/day) ^ | N = 375 | N = 320 | N = 301 | 0.0748 | 0.1243 | 0.0975 | 0.0525 | 0.0357 | 0.8896 | 0.0587 | 0.9935 | 0.0654 |
| DIET | 10.4 (9.9, 10.8) | −0.4 (−0.7, −0.1) | −0.7 (−1.1, −0.4)*** | |||||||||
| DIET+MODPA | 10.3 (9.8, 10.7) | −0.9 (−1.2, −0.5)*** | −1.2 (−1.62, −0.9)*** | |||||||||
| DIET+HIGHPA | 10.5 (10.1, 10.9) | −0.9 (−1.3, −0.6)*** | −0.8 (−1.1, −0.4)*** | |||||||||
MET: metabolic equivalent; LPA: light-intensity physical activity (1.5 to <3.0 METs); MVPA: moderate-to-vigorous physical activity (≥3.0 METs).
Model adjusted for sex and race and baseline value.
P-value for comparison of change at 6 months to change at 12 months.
Indicates change from baseline is statistically significant at p-value ≤0.0167 to adjust for multiple comparisons.
P-value for comparison of groups at specific time points, with the critical p-value set at ≤0.0167 to adjust for multiple comparisons.
Indicates self-report and that the variable includes flights of stairs climbed when computing kcal/week.
Indicates self-report and that the variable excludes flights of stairs climbed when computing kcal/week or min/week.
Indicates objectively measured.
Objectively measured total minutes of MVPA significantly increased in DIET+MODPA and DIET+HIGHPA from baseline to 6 months (P’s≤0.0001) and in all groups at 12 months (P’s≤0.0032). Additional post-hoc comparisons showed that all groups differed from each other for the change at 6 months (P’s≤0.0085) with the change in MVPA being greater in both DIET+MODPA (P=0.0081) and DIET+HIGHPA (P=0.0064) compared to DIET at 12 months. A similar pattern was observed for the change in total minutes of MVPA.
Objectively measured MET-min/week of MVPA in ≥10-minute sessions significantly increased in DIET+MODPA and DIET+HIGHPA from baseline to 6 months (P’s<0.0001) and in all groups at 12 months (P’s≤0.0041). Additional post-hoc comparisons showed that all groups differed from each other for the change at 6 months (P’s≤0.0093) with the change in being greater in both DIET+MODPA (P=0.0165) and DIET+HIGHPA (P=0.0144) compared to DIET at 12 months. A similar pattern was observed for the change in min/wk of MVPA in ≥10-minute sessions at 6 months, but the groups did not differ at 12 months.
Objectively measured MET-min/wk of light-intensity PA significantly increased for both DIET+MODPA and DIET+HIGHPA at 6 months (P’s<0.0001) and in all groups at 12 months (P’s≤0.0020), with no difference between groups for the change at either 6 or 12 months. Similar findings were observed for min/wk of light-intensity PA. Sedentary behavior, based on percent of monitor wear time, significantly decreased in all groups at 6 months (P’s≤0.0123) and 12 months (P’s<0.0001); however, only DIET+HIGHPA differed from DIET at 6 months (P=0.0039) and DIET+MODPA differed from DIET at 12 months (P=0.0069).
DISCUSSION
This study was designed to examine changes in LVM with weight loss induced by moderate dietary restriction alone or in combination with a moderate or high amount of prescribed PA. Weight loss induced through dietary modification alone resulted in a significant reduction in LVM, whereas dietary modification combined with moderate-to-high amounts of PA resulted in non-significant reductions in LVM. By comparison, an observational study reported that weight loss was associated with reduction in LVM, however, that study did not report on whether weight loss was achieved with diet or the combination of diet and PA.(27) Weight loss induced by bariatric surgery has also been shown to reduce LVM.(28) The reduction in LVM observed in this study with weight loss in the absence of increased PA, which may be a favorable adaptive response that reduces LVM hypertrophy and cardiovascular risk,(27) appears to be consistent with the prior studies of weight loss.(29) Unique to this study is the finding that weight loss combined with PA may preserve LVM, which may also be a favorable adaptive response that reflects normal or enhanced cardiac function.(17, 18) However, the prespecified pairwise comparisons suggest that the observed changes from baseline may not differ between the interventions. Moreover, the clinical implications of reducing, preserving, or increasing LVM during periods of weight loss are not able to be determined from this study and warrant further investigation.
One could hypothesize potential benefits and clinical implications of reducing LVM with weight loss or potentially preserving LVM with weight loss combined with PA. The Look AHEAD Study found that an intensive lifestyle intervention focused on weight loss did not decrease composite measures of CVD compared to a diabetes support and education condition;(30) however, post-hoc analysis has shown that participants with a weight loss of at least 10% at one-year or an increase in CRF of at least 2 metabolic equivalents (METS) did have a reduction in cardiovascular outcomes.(4) Additional post-hoc analyses showed that an increased in PA was associated with reduced risk of cardiovascular outcomes.(31) While the mechanisms by which weight loss, CRF, and PA reduced the risk of CVD outcomes in Look AHEAD have not been determined, the results of the current study showed that weight loss of at least 10% in the absence of increased PA may result in an adaptive reduction in LVM, whereas PA when added to weight loss may result in an adaptive preservation of LVM. These may provide potential mechanistic pathways for the cardiovascular benefits of weight loss and PA within the context of obesity treatment that warrant further investigation. Future studies should also consider inclusion of extracellular volume (ECV) measures, which may provide valuable information on myocardial “quality” (i.e., extent of excess interstitial expansion, usually from myocardial fibrosis) beyond quantity (LVM).(32)
Weight loss is associated with improvements in numerous cardiometabolic risk factors.(1) The interventions in this study improved resting blood pressure, fasting lipids, fasting glucose, fasting insulin, and inflammation. PA, when added to weight loss, did not add to the improvements in these CVD risk factors. This may suggest that weight loss of the magnitude achieve in this study should be a focus of interventions to improve cardiometabolic health in adults with overweight or obesity. However, it is possible that when weight loss of a lesser magnitude is achieved, PA may have a greater influence on these cardiometabolic risk factors.
Strengths include a randomized design with retention of >80% of participants. The intervention successfully achieving mean weight loss of approximately 10% of baseline weight in all interventions by 6 months with this magnitude of mean weight loss maintained for an additional 6 months. There was randomization to different amounts of PA, and the intervention was successful at achieving different amounts of PA across the three groups. Moreover, this study included best practice assessment of LVM and other cardiometabolic profiles.
There are limitations to consider. Participants were age 18 to 55 years, primarily women who were white/Caucasian, and relatively healthy with no known medical conditions that would limit participation in this study (e.g., CVD, diabetes, etc.). It is unclear if similar results would be observed in populations with different characteristics or medical conditions that were excluded in this study. Moreover, objective measures of dietary intake and compliance were not included in this study. The lack of statistical significance between groups for the change in the primary outcome of LVM may be reflection of the sample size of this study, which should be considered for further studies of weight loss and its effects on LVM.
CONCLUSIONS
Weight loss achieved through dietary modification resulted in reduced LVM, whereas with the addition of PA LVM appears to be preserved, and both may be favorable adaptive responses that reduce cardiovascular risk. Findings suggest that weight loss should be considered as a potential treatment to reduce LVM and its association with cardiovascular health risk. Findings also support that PA within the context of a behavioral weight loss intervention may assist in preserving or enhancing cardiac structure in adults with overweight or obesity. This may be a favorable adaptive response, but this cannot be confirmed with this study. Further investigation is needed to understand the complexity of lifestyle on LVM and the clinical implications of the potential opposing, and potential beneficial, effects of weight loss and PA on cardiac structure in adults with overweight or obesity.
What is already known about this subject?
Left ventricular mass (LVM) of the heart is a clinically important prognostic indicator of cardiovascular disease.
Excess body weight is associated with a maladaptive larger LVM, which may contribute to hypertrophy and potential negative clinical outcomes such as heart failure, in adults with overweight or obesity.
Exercise can contribute to an adaptive response resulting in larger LVM accompanied by normal or enhanced cardiac function.
What are the new findings in your manuscript?
Weight loss achieved through dietary modification results in reduced LVM in adults with overweight or obesity.
Weight loss achieved through dietary modification combined with physical activity, prescribed at ≥150 minutes per week, preserved LVM in adults with overweight or obesity.
How might your results change the direction of research or the focus of clinical practice?
Diet induced weight loss reduces LVM in adults with overweight or obesity, which may be a favorable adaptive response to reduce cardiovascular risk.
Physical activity within a behavioral weight loss intervention may preserve cardiac structure in adults with overweight or obesity, and this may be a favorable adaptive response that reflects a normal or enhanced cardiac function.
Investigation is needed to understand the complex and potential opposing effects of lifestyle on LVM.
Acknowledgements
We recognize the contribution of the staff and graduate students at the Physical Activity and Weight Management Research Center at the University of Pittsburgh who contributed to this project.
Data sharing for this study may be available after publication of the main outcomes and only for requests that meet the approved process contained within the informed consent and other institutional policies. These data will not be provided in a public use data set but rather may require approval through a material transfer agreement, and only deidentified data may be made available. Investigators interested in data from this study should contact Dr Jakicic (John.Jakicic@AdventHealth.com), who is the principal investigator, for data sharing policies and procedures after publication.
FUNDING:
Support provided by grants R01 HL096770 and UL1 TR001857 from the National Institutes of Health.
DISCLOSURE:
Dr. Jakicic has received grant funding from the National Institutes of Health, is on the Scientific Advisory Board for Wondr Health, was formerly on the Scientific Advisory Board for WW International, Inc. and the Advisory Board for Spark360, serves on data and safety monitoring boards for U10HL131552 and P01AG052352, has received renumeration for professional presentations, and serves in as a volunteer in a professional leadership role to the American College of Sports Medicine. Dr. Rogers has received funding from the National Institutes of Health, is a consultant for Wondr Health, has received renumeration for professional presentations, and serves in as a volunteer in a professional leadership role to the American College of Sports Medicine. Dr. Gibbs has received funding from the National Institutes of Health and the American Heart Association, and serves on data and safety monitoring board for HSRP20153604. Dr. Schelbert serves on the Scientific Advisory Board for Hay Therapeutics. Dr. Lang, Ms. Yuan, and Dr. Fridman have no disclosures.
Footnotes
CLINICAL TRIAL REGISTRATION: ClinicalTrials.gov NCT01500356
Contributor Information
John M. Jakicic, AdventHealth, Translational Research Institute, Orlando, FL USA.
Renee J. Rogers, AdventHealth, Translational Research Institute, Orlando, FL USA.
Wei Lang, Center on Aging and Mobility, University Hospital Zurich and University of Zurich, Zurich, Switzerland.
Bethany Barone Gibbs, University of Pittsburgh, Department of Health and Human Development, Pittsburgh, PA USA.
Nalingna Yuan, University of Pittsburgh, Department of Psychology, Pittsburgh, PA USA.
Yaron Fridman, Asheville Cardiology Associates, Mission Health, Asheville, NC USA.
Erik B. Schelbert, University of Pittsburgh, School of Medicine, Pittsburgh, PA USA; Minneapolis Heart Institute East, Saint Paul, MN USA.
References
- 1.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63: 2985–3023. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health National Heart Lung and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults - The Evidence Report. Obes Res 1998;6(suppl.2). [PubMed] [Google Scholar]
- 3.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief. National Center for Health Statistics: Hyattsville, MD, 2020. [PubMed] [Google Scholar]
- 4.The Look AHEAD Research Group. Association of the magniture of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016;4: 913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med 1989;110: 101–107. [DOI] [PubMed] [Google Scholar]
- 6.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322: 1561–1566. [DOI] [PubMed] [Google Scholar]
- 7.Kishi S, Armstrong AC, Gidding SS, Colangelo LA, Yenkatesh BA, Jacobs DR, et al. Association between obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the CARDIA study (Coronary Artery Risk Development in Young Adults). JACC Heart Fail 2014;2: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, et al. The impact of obesity on the left ventricle. J Am Coll Cardiol Img 2010;3: 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers RJ, Schelbert EB, Lang W, Fridman Y, Yuan N, Jakicic JM. Association of fitness and body fatness with left ventricular mass: The Heart Health Study. Obes Sci Pract 2020;6: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO. Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis 2009;52: 153–167. [DOI] [PubMed] [Google Scholar]
- 11.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. ACSM position stand on appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009;42: 459–471. [DOI] [PubMed] [Google Scholar]
- 12.Ross R, Dagnone D, Jones PJH, Smith H, Paddags A, Hudson R, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. Ann Intern Med 2000;133: 92–103. [DOI] [PubMed] [Google Scholar]
- 13.Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women. A randomized trial. JAMA 2003;290: 1323–1330. [DOI] [PubMed] [Google Scholar]
- 14.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr 1999;69: 373–380. [DOI] [PubMed] [Google Scholar]
- 15.Barlow CE, Kohl HW, Gibbons LW, Blair SN. Physical activity, mortality, and obesity. Int J Obes 1995;19: S41–S44. [PubMed] [Google Scholar]
- 16.Jakicic JM, Egan CE, Fabricatore AN, Gaussoin SA, Glasser SP, Hesson L, et al. Change in cardiorespiratory fitness and influence on diabetes control and CVD risk factors in adults with type 2 diabetes: 4-year results from the Look AHEAD Trial. Diabetes Care 2013;36 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazzeroni D, Rimoldi O, Camici PG. From left ventricular hypertrophy to dysfunction and failure. Circ J 2016;80: 555–564. [DOI] [PubMed] [Google Scholar]
- 18.Lovic D, Narayan P, Pittaras A, Faselis C, Doumas M, Kokkinos P. Left ventricular hypertrophy in athletes and hypertensive patients. J Clin Hypertens 2017;19: 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandura A Social foundations of thought and action: a social cognitive theory. Prentice Hall: Englewood Cliffs, NJ, 1986. [Google Scholar]
- 20.Marlatt GA, Gordon JR. Relapse Prevention. Guilford Press: New York, 1985. [Google Scholar]
- 21.Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem solving therapy in the long-term management of obesity. J Consult Clin Psychol 2001;69: 722–726. [PubMed] [Google Scholar]
- 22.Rosenstock IM. Historical origins of the health belief model. Health Education Monograph 1974;2: 1–9. [Google Scholar]
- 23.Jakicic JM, Davis KK, Rogers RJ, King WC, Marcus MD, Helsel D, et al. Effect of wearable technology combined with a lifestyle intervention on long-term weight loss in the IDEA Study: a randomized clinical trial. JAMA 2016;316: 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakicic JM, Tate D, Davis KK, Polzien K, Rickman AD, Erickson K, et al. Effect of a stepped-care intervention approach on weight loss in adults: The Step-Up Study Randomized Trial. JAMA 2012;307: 2617–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakicic JM, King WC, Gibbs BB, Rogers RJ, Rickman AD, Davis KK, et al. Objective versus self-reported physical activity in overweight and obese young adults. J Phys Act Health 2015;12: 1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakicic JM, King WC, Marcus MD, Davis KK, Helsel D, Rickman AD, et al. Short-term weight loss with diet and physical activity in young adults: the IDEA Study. Obesity 2015;23: 2385–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonnebakken MT, Mancusi C, Losi MA, Gerdts E, Izzo R, Manzi MV, et al. Weight loss facilitates reduction of left ventricular mass in obese hypertensive patients: The Campania Salute Network. Nutr Metab Cardiovasc Dis 2019;29: 185–190. [DOI] [PubMed] [Google Scholar]
- 28.Mahajan R, Stokes M, Elliott A, Munawar DA, Khokhar KB, Thiyagarajah A, et al. Complex interaction of obesity, intenstional weight loss and heart failure: a systematic review and meta-analysis. Heart 2020;106: 58–68. [DOI] [PubMed] [Google Scholar]
- 29.Haufe S, Utz W, Engeli S, Kast P, Bohnke J, Pofahl M, et al. Left ventricular mass and function with reduced-fat or reduced-carbohydrate diets in overweight and obese subjects. Hypertension 2012;59: 70–75. [DOI] [PubMed] [Google Scholar]
- 30.Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Look AHEAD Study Group. Association between change in accelerometer-measured and self-reported physical activity and cardiovascular disease in the Look AHEAD Trial. Diabetes Care Online Ahead of Print January 10, 2022: doi.org/ 10.2337/dc2321-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schelbert EB, Sabbah HN, Butler J, Gheorghiade M. Employing extracellular volume cardiovascular magnetic resonance measures of myocardial fibrosis to foster novel therapeutics. Circ Cardiovasc Imaging 2017;10: e005619. [DOI] [PubMed] [Google Scholar]