Abstract
The stomach is a hostile environment for most microbes because strong gastric acid kills indigenous microorganisms. Thus, the mass of indigenous microbes detected by traditional culturing method in a highly acidic stomach is reported to be very small. However, in a stomach with less acidity due to atrophic changes of the gastric mucosa, the number of live gastric microbiota dramatically increases and their composition changes. A probiotic is defined as a live microorganism that, when administered in adequate amounts, confers a health benefit on the host. The administration of probiotics to the stomach has thus far been considered impractical, mainly due to the strong acidity in the stomach. The identification of candidate probiotic strains with sufficient resistance to acidity and the ability to achieve close proximity to the gastric mucosa could enable the application of probiotics to the stomach. The utilization of probiotics alone for Helicobacter pylori (H. pylori) infection significantly improves gastric mucosal inflammation and decreases the density of H. pylori on the mucosa, although complete eradication of H. pylori has not yet been demonstrated. The use of probiotics in combination with antimicrobial agents significantly increases the H. pylori eradication rate, especially when the H. pylori strains are resistant to antimicrobial agents. While H. pylori has been considered the most important pathogenic bacterium for the development of gastric cancer, bacteria other than H. pylori are also suggested to be causative pathogens that promote the development of gastric cancer, even after the eradication of H. pylori. Increased non-H. pylori Gram-negative bacteria in the stomach with weak acidity accompanying atrophic gastritis may perpetuate gastric mucosal inflammation and accelerate carcinogenic progression, even after H. pylori eradication. Probiotics restore the acidity in this stomach environment and may therefore prevent the development of gastric cancer by termination of Gram-negative bacteria-induced inflammation. Functional dyspepsia (FD) is defined as the presence of symptoms that are thought to originate in the gastroduodenal region in the absence of any organic, systematic or metabolic diseases. Accumulating evidence has pointed out the duodenum as a target region underlying the pathophysiology of FD. A randomized placebo-controlled clinical trial using a probiotic strain (LG21) demonstrated a significant improving effect on major FD symptoms. One of the possible mechanisms of this effect is protection of the duodenal mucosa from injurious intestinal bacteria through the resolution of small intestinal bacterial over growth.
Keywords: Stomach, Microbiota, Probiotics, Helicobacter pylori, Post-eradication gastric cancers, Functional dyspepsia
Core Tip: Gastric microbiota and application of probiotics to the gastroduodenal diseases have so far been unfamiliar because the mass of live microbes is so small in the stomach with high acidity. However, in the subject whose stomach is low acidity due to atrophic gastritis or proton pump inhibitor long-use, the number of live bacteria increases so much in the stomach thus they can significantly influence the pathophysiology of gastroduodenal diseases.
INTRODUCTION
The intestine is colonized by a complex and dynamic microbial ecosystem with a high density of bacteria whose cell number can reach as high as approximately 1012/g feces. Their total number is therefore estimated to be 10-times greater than the number of eukaryotic cells in the human body, and the genes of these microbes outnumber human genes more than 100-fold[1]. As the early definition of probiotics emphasized their effects on improving the ecology of intestinal microbiota, their actions on the intestinal tract and gut mucosal immunity have received a great deal of attention.
On the other hand, the size of gastric microbiota, in which probiotic bacteria exert their beneficial effects, is very small [around 103 colony-forming units (CFU)/mL gastric fluid (GF)[2]] because of the strong acidity and frequent peristalsis to the intestine in the stomach. Such high acidity due to secreted gastric acid kills probiotic strains as well as gastric commensal microbes. Therefore, the application of probiotics to the stomach or proximal small intestine has historically been considered impractical.
Based on the outline of microbiota and probiotics in the stomach, this article reviews Helicobacter pylori (H. pylori) infection and functional dyspepsia (FD). The former includes the pathogenicity of H. pylori, the suppressive mechanism of probiotics on this bacterium and the present status of the application of probiotics in eradication therapy. In addition, this review argues a possible role of probiotics in the prevention of post-H. pylori eradication gastric cancer. In the description, basic and clinical data reported by the author’s group are emphasized because they are indispensable for communicating the author’s ideas in relation to the theme of this review article.
MICROBIOTA IN THE STOMACH
Probiotics are considered to exert beneficial effects on the host by improving the indigenous microbiota[3]. Thus, a brief description of the gastric microbiota is necessary to understand the effects of probiotics in the stomach. The stomach is a hostile environment for most microbes because strong gastric acid kills indigenous microorganisms. Therefore, when examined using traditional culturing methods, bacterial numbers in the gastric mucosa-associated or GF are reported to contain only approximately 103 CFU per g or mL[2]. Moreover, this method can only detect microbes that are able to grow in the media components and the atmospheric conditions of the culturing assay. Thus, in the stomach-where the acidity is high enough to kill indigenous bacteria-the investigation of gastric microbiota by traditional culturing methods makes no sense as the stomach contains few live bacteria. However, the introduction of DNA sequencing using high performance next-generation sequencers has markedly enhanced the analysis of microbiomes in the stomach as well as in the intestine. In a stomach with less acidity due to the continuous use of acid-suppressive agents [e.g., proton-pump inhibitors (PPIs)] or atrophic change of the gastric mucosa, the number of bacteria in the live gastric bacterial mass dramatically increases and the composition changes, respectively[4,5]. In these low acidity settings, the gastric microbiota will exert a significant effect on the pathophysiology of upper gastrointestinal (GI) tract disorders.
The mouth is located at the entrance of the GI tract, and contains complex anatomical sites, including the teeth, gingiva and tongue. The oral microbiota flows downstream into the stomach by swallowing of saliva and mastication of foods. These are expected to exert a great influence on the microbiota in the stomach. While the gut harbors a very large and complex microbial community, it is conceivable that such intestinal microbiota can be significantly affected by the gastric microbiota through continuous inflow. This is especially true after a large increase in the bacterial mass in a stomach with low acidity. This raises the hypothesis that the GI tract has a common microbial ecosystem in which the gastric microbiota plays the role of a relay base between the oral and gut microbiotas. Therefore, in this chapter, the microbiota in the stomach is described and compared to the microbiotas of the oral cavity and gut. H. pylori is not an indigenous resident but a pathogen of exogenous origin. Accordingly, it is not described in the present chapter.
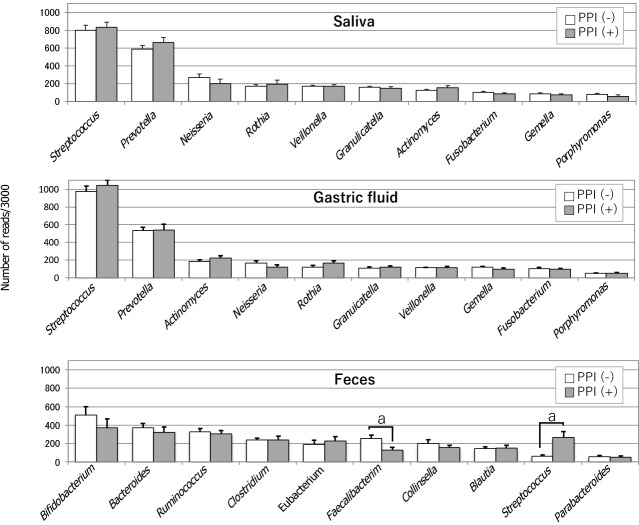
In 2006, Bik et al[6] identified 128 bacterial phenotypes based on a 16S rRNA gene analysis of gastric microbiomes with 1833 sequences obtained from 23 human gastric endoscopic biopsy specimens. A few years later, Li et al[7] also performed a 16S rRNA gene analysis using 1223 non-H. pylori sequences of 10 biopsy samples from the stomach, which were then classified into 133 phylotypes. Despite examining racially distinct populations (North America and China, respectively), both studies investigating the gastric mucosa-associated microbiomes revealed similar results. Streptococcus and Prevotella were the predominant genera, and they accounted for approximately half of the total detected species detected in their studies. In 2015, Tsuda et al[2] performed a meta-16S analysis of the gastric luminal microbiome with far greater sequencing depth. Their analysis was performed using GF samples obtained from Japanese subjects in a fasting state in the morning. They obtained roughly 40000 high-quality reads for the analysis from 45 GF specimens and also identified Streptococcus and Prevotella as the most prevalent genera, accounting for approximately 50% of all the species detected in the stomach (Figure 1). Moreover, in all three of these studies, Neisseria and Rothia ranked among the top 5 most prevalent genera. This similarity in the bacterial composition between the mucosa-associated[6,7] and luminal[2] samples suggested that the former bacteria moved back into the lumen, while the latter continuously colonized the mucosa. H. pylori is a predominant inhabitant of the mucous layer and also inhabits the gastric epithelial cells. Accordingly, it is mainly found in the mucosa-associated specimens.
Figure 1.
Comparison of the microbiota in the saliva, gastric fluid and feces, and the influence of proton-pump inhibitors. Bacterial compositions at the genus level in saliva (top), gastric fluid (median) and feces (bottom) are shown. The average of read numbers of the top 10 major genera are indicated in each group. Open and filled bars represent proton-pump inhibitor (PPI)-nonusers and PPI-users, respectively. Asterisks show a significant difference according to Student t-test. aP < 0.05. PPI: Proton-pump inhibitor. Citation: Tsuda A, Suda W, Morita H, Takanashi K, Takagi A, Koga Y, Hattori M. Influence of Proton-Pump Inhibitors on the Luminal Microbiota in the Gastrointestinal Tract. Clin Transl Gastroenterol 2015; 6: e89. Copyright ©Wolters Kluwer Health, Inc. 2015. Published by Wolters Kluwer Health, Inc.
Tsuda et al[2] also compared three different bacterial communities along the alimentary tract (oral cavity, stomach and colon) using stimulated saliva, GF, and feces specimens. There was no significant difference in the degree of species richness (α-diversity) among the three types of specimens. While the median log CFU bacterial number was only 3.4/mL (determined by culturing), the bacterial log genome copy number was as high as 7.8/mL (median) in GF samples. This large discrepancy between the CFU and genome copy numbers implied that > 99.9% of the GF bacteria were dead or viable but non-culturable. The analysis of bacterial genome copies also suggested that-if microbes were alive and metabolically active in the stomach with weak acidity-the mass size of the GF microbiota may be high enough to significantly affect the pathophysiology of the stomach and its downstream organs. A bacterial composition analysis at the genus level showed high similarity between the salivary and GF microbiota (Figure 1). Indeed, the five most prevalent genera in these microbiomes (in descending order) were as follows: Streptococcus, Prevotella, Neisseria, Rothia and Veillonella, and Streptococcus Prevotella, Actinomyces, Neisseria and Rothia. In contrast, the composition of the fecal microbiota was markedly different.
In a meta-16S analysis, which was performed to investigate the influence of gastric acidity on the microbiome composition in the stomach without atrophic change of the mucosa, PPI treatment was found to significantly increase the amount of Streptococcus in the mucosa-associated gastric microbiota[8]. This increase occurred independently of H. pylori infection. The compositions of other bacteria at the genus level showed no significant alteration. In another study, in which a bacterial DNA analysis study was conducted using GF samples, PPI-treated subjects whose GF acidity was > pH 4, showed lower α-diversity than subjects without PPI treatment[9]. No other significant changes in the GF microbiome composition were observed in GF samples with weak acidity. Furthermore, the presence of H. pylori was not associated with the difference in the microbiome composition. Moreover, in a study of PPI-users by Tsuda et al[2], no significant changes were observed in the bacterial composition (at the genus level) in the GF of PPI users, with the exception that Streptococcus tended to be more prevalent (Figure 1). In their study, the average pH values of GF samples obtained from the PPI-users and PPI-nonusers were 3.2 and 1.6, respectively. Of note, the average log copy number of bacterial cells (/mL) in these GF samples (measured by quantitative polymerase chain reaction) was almost the same in PPI-users (8.0) and PPI-nonusers (8.1), while the CFU number (determined by culturing) was > 1000-fold greater in PPI-users with weak acidity. This result implies that PPI-induced low acidity protected the gastric microbiota from strong acid.
Bacterial overgrowth in the stomach with weak acidity has been suggested to occur due to the restoration of active growth of relatively acid-resistant indigenous bacteria, which are kept alive (in small numbers) due to their suppressed growth in the strongly acidic stomach. However, the high similarity in the bacterial community structure between the GF and saliva, and the high similarity of their bacterial genome copy numbers, suggests that no “bacterial overgrowth” occurred. Instead, the bacteria that moved from the oral cavity to the stomach with weak acidity simply avoided being killed by gastric acid. Although PPI use was not associated with any significant alteration in the composition of the gastric microbiota, a significant decrease of Faecalibacterium and a significant increase of Streptococcus were found in the feces of PPI-users (Figure 1). A reduction in the abundance of Faecalibacterium was also reported in the feces of dogs treated with PPIs[10]. Whether the large increase of live bacteria induced by PPI treatment significantly influences the intestinal bacterial community structure remains to be elucidated.
PROBIOTICS FOR THE STOMACH
In 1989, Fuller defined probiotics as “a live microbial feed supplement that beneficially affects the host animals by improving its intestinal microbial balance”[3]. This early and influential definition was refined by a standard definition proposed by the Joint (Food and Agricultural Organizations of the United Nations)/World Health Organization Expert Consultation in 2001[11]: “a live microorganism that, when administered in adequate amounts, confers a health benefit on the host”. The International Scientific Association for Probiotics and Prebiotics consensus statements reported in 2014 exclusively retained the body of these definitions[12].
The main beneficial effects of probiotics on the host include inhibition of potential pathogens in the GI tract, modulation of immunity, and reinforcement of the mucosal barrier. Competition with bacteria for binding sites on the mucosa by probiotic strains is the dominant mechanisms underlying the protection of the host from pathogenic bacteria in the intestine. Organic acids secreted by probiotic strains (e.g., lactic acid) and short-chain fatty acids (e.g., acetic acid) are considered to exert a significant bactericidal effect on the pathogens. As underlined in the definitions of probiotics, a “living” state is indispensable for probiotics to protect the hosts from pathogens, because dead probiotic strains no longer have the ability to specifically bind to the mucosa or generate organic acids.
Because the early definition of probiotics emphasized their beneficial effects on improving intestinal microbial ecology, it has been considered that probiotics should be exclusively applied to the gut. Actually, the gut is colonized with a high density of bacteria, the cell number of which can reach as high as 1012/g feces[13]. In contrast, the size of bacterial mass in the stomach, in which probiotic strains could work, is small. The number of indigenous bacteria in the gastric fluid of healthy stomach-when examined by culturing methods- is at most 103/mL[2,4]. The strong acidity in the stomach due to secreted gastric acid, which causes a marked reduction in the size of such resident gastric bacteria also suppresses or terminates the beneficial effects exerted by probiotic strains soon after they move to the stomach. For this reason, the application of probiotics to the stomach or proximal small intestine has so far been considered impractical.
The proximity of probiotic strains to the mucosa-which will be achieved by bearing affinity to the surface mucus layer, or by adhering to the epithelial cell layers-is crucial for beneficial effects to be exerted in the GI tract[14]. Those effects include competitive inhibition of the pathogenic bacteria’s attachment to the mucosa, secretion of organic acids in effective concentrations, and contact-dependent immunomodulation. Peristaltic movements in the stomach are more frequent and active in comparison to the intestine; thus, it is difficult for probiotic strains to come into close proximity to the gastric mucosa. This factor also makes the use of gastric probiotics difficult.
From another point of view, the small size of resident microbiota in the stomach can easily be affected by the introduction of exogenous microorganisms. These transient bacteria (e.g., ingredients of foods or accidental contaminants) exert a much greater effect on the microbiota in the upper GI tract than on the gut microbiota due to its much smaller size. The same situation is encountered with the administration of probiotics to the upper GI tract, where they are not disturbed by large amounts of robust and resilient microbiota, which are present in the gut. The application of probiotics to the upper GI tract may therefore be more advantageous in comparison to application to the gut, as long as the candidate strains are able to resist the strong acidity and achieve close proximity to the gastric mucosa.
The stomach is a harsh environment for most microorganisms because strong gastric acid kills many ingested microbes. In a fasting state in the morning, GF has a peak acidity of pH 1-2. Such strong acidity is quite reasonable because one major physiological role of gastric acid is to kill exogenous pathogenic bacteria that move to the digestive tract through the mouth. At present, the most prevalent probiotic strains belong to genera Lactobacillus or Bifidobacterium[11]. In general, Lactobacilli and Bifidobacteria show considerably high resistance to acidity. While both bacterial groups can survive acidic conditions of approximately pH 3 to 4[15], this is not as strong as the peak gastric acidity (pH 1-2). Thus, screening using candidate probiotic strains is necessary to identify a suitable probiotic strain for the stomach that can tolerate approximately pH 2.
Genus Lactobacillus is one of the predominant resident bacterial groups found in the stomach (when examined by culturing methods)[16]. Accordingly, Lactobacillus strains might be the most appropriate for use as probiotics in the stomach. Furthermore, in adult mice with a specific pathogen-free (SPF) environment, no H. pylori infection was found in the stomach after oral inoculation of the bacteria[16]. In contrast, mice bred in a germ-free environment were easily infected by oral inoculation of H. pylori. In this animal study, H. pylori infection was prevented in SPF mice with a large number of indigenous lactobacilli in the stomach (> 108 CFU/g tissue). A representative probiotic strain that can be used for the stomach is Lactobacillus gasseri OLL2716 (LG21). This was selected out of approximately 2000 strains of lactobacilli[17]. The criteria for selection were both resistance to acidity and the ability to bind to the gastric mucosa. In the stationary growth phase, LG21 can survive in culture broth at pH 2.5, which is similar to the acidity of GF. LG21 has several defense mechanisms that enable it to withstand acid stress, including the up-regulation of the cation ATP-binding cassette transporter genes and the downregulation of the genes associated with transcription and protein synthesis[18]. The acid stress response is generally indispensable for lactobacilli, because they always secrete large amounts of organic acids to the external environment when they grow with metabolic activity. Without this defense mechanism, the acidic milieu induces the arrest of the growth of lactobacilli and it may even cause their death.
In a handful of trials, endoscopy directly demonstrated mucosal colonization by probiotics. Using biopsy samples obtained by upper GI endoscopy, LG21 strains administered through a yogurt drink were shown to be able to enter the mucous layer of the human stomach[19]. The laser-assisted micro-dissection and non-contact pressure catapulting method enabled this fine topical analysis.
H. PYLORI INFECTION
H. pylori and its pathogenicity
H. pylori is a gram-negative and microaerophilic bacterium that can move in the mucus layer on the surface epithelial cells of the stomach using several of flagella that are located at one end (Figure 2). As much as half of the people in the world are infected with H. pylori[20]. H. pylori infection causes inflammation of the gastric mucosa and then leads to a gradual loss of hydrochloric acid-secreting parietal cells of the stomach. This ultimately results in a condition known as atrophic gastritis. Atrophic gastritis is a chronic inflammatory and low gastric acidity state that has a high risk of progressing to gastric cancer[21].
Figure 2.
Observation of Helicobacter pylori by scanning electron microscopy. The bar at the bottom shows 1 μm.
H. pylori can tightly bind to epithelial cells by multiple bacterial-surface components. The best-characterized adhesin, BabA, is a 78 kD outer-membrane protein that binds to the fucosylated Lewis B blood group antigen on the host cell[22]. Firm contact between H. pylori and the host cell through adhesion is considered a prerequisite for the H. pylori to transport effecter molecules (e.g., CagA) into the host cell using the cag PAI-encoded type IV secretion system[23]. This event is regarded as the pathway leading to the generation of proinflammatory cytokines (e.g., IL-8 and IL-1β) by host cells. Thus, the adhesion of H. pylori to epithelial cells is a critical event in the development of an inflammatory response and the establishment of infection in the stomach.
H. pylori eradication
Mechanism of the suppressive effect of probiotics on H. pylori: The major mechanisms of probiotics against H. pylori infection are thought to be competition with H. pylori for binding sites on gastric epithelial cells, reinforcement of the mucosal barrier, and secretion of bactericidal organic acids (e.g., lactic acid). These are the principle anti-bacterial effects exerted by probiotics. As for the mechanism of competitive binding, L. reuteri is reported to inhibit the attachment of H. pylori on the epithelial cell surface by competitive binding to asialo-GM1 and surface receptors[24]. Moreover, other probiotic species (e.g., L. acidophilus, L. johnsonii and L. salivarius) were reported to prevent H. pylori colonization through specific adhesion molecules[25,26]. Specific binding of H. pylori to the host cell then induces the production of IL-8 through the type IV secretion system. Tamura et al[27] also demonstrated competition for binding sites between H. pylori and a probiotic strain LG21 using a coculture system with MKN45 cells (a human gastric epithelial cell line) and H. pylori. Large amounts of IL-8 were produced in the gastric epithelial cells cocultured with H. pylori (106 CFU) alone. When 106 CFU of non-treated live LG21 (equivalent to the number of H. pylori) was added to the coculture system, the amount of IL-8 secreted into the culture supernatant significantly decreased. However, UV- or heat-treated LG21 could not exert any suppressive effect on H. pylori-induced IL-8 production, even at 108 CFU (100 times the amount of non-treated LG21). An adherence assay in their study supported that LG21 competitively inhibited the binding of H. pylori to MKN45 cells, which suppressed the production of IL-8. Moreover, they demonstrated that the suppressive effect of LG21 also worked in the human stomach. The measurement of the IL-8 Level in gastric biopsy specimens from H. pylori-infected subjects also revealed that the oral intake of probiotic LG21 significantly suppressed the generation of IL-8 in the gastric mucosa[27].
Application of probiotics in the eradication therapy: The clinical application of probiotics in the treatment of H. pylori infection has been performed in many countries for more than 20 years. Now the utilization of probiotics alone for H. pylori infection has almost been settled. Both early and recent reviews[28,29] concluded that probiotics significantly improved gastric mucosal inflammation, and decreased the density of H. pylori on the mucosa. However, to our knowledge, the complete eradication of H. pylori colonizing the stomach by probiotic treatment alone has not been demonstrated. One representative trial of probiotics alone for the treatment of H. pylori infection was reported by Sakamoto et al[17] in 2001. In their study, 31 H. pylori-infected subjects (mean age 50 years) ingested yogurt containing 109 CFU of LG21 or placebo yogurt without LG21 every day for 8 wk. The results of 13C-urea breath tests (UBT) and assays of serum pepsinogens I and II (PGI/II) showed a significant clinical improvement after LG21 yogurt treatment. The 13C-UBT result and the PGI/II ratio are known to indirectly represent H. pylori density and the degree of mucosal inflammation in the stomach, respectively[30,31]. A bacterial examination of gastric mucosal biopsy specimens (by culturing) revealed 2-100-fold decreases in the number of H. pylori. However, there were no subjects in whom H. pylori was completely eliminated. Pantoflickova et al[32] reported the effects of the administration of L. johnsonii La1 (LC-1) to 50 H. pylori-positive healthy volunteers in a randomized controlled, double-blind study. The subjects received 125 g of fermented milk containing 106-107 CFU/g of LC-1 or placebo milk without LC-1 every day for 16 wk. The severity/activity of antral gastritis (assessed histologically) and the H. pylori density (assesses by a 13C-UBT) showed significant improvement. The histological examination of the mucous mucosa also revealed a significant increase in the mucous thickness in the LC-1-treated group. This suggested that the stabilization of the mucosal barrier by probiotics also enhanced the suppression of H. pylori.
Recently, the H. pylori eradication rate in patients treated using anti-microbial agents is decreasing. This is mainly due to antimicrobial resistance. In the early 1990s, the standard triple therapy achieved an eradication rate of > 90%. In contrast in the past decade, the effectiveness of this regimen often falls to < 70%[33,34]. According to an ITT analysis by Deguchi et al[35] in 2012, the successful eradication rate using the same regimen was just 69.3%. In those subjects, the rate of infection with clarithromycin-resistant strains of H. pylori was as high as 27.1%. This increase in resistance to antimicrobials like clarithromycin is thought to have reduced the eradication rate. Actually, the clarithromycin resistance rates of H. pylori isolated from children in North America and Europe were reported to be 10.6%-25% and 1.7%-23.4% respectively[36,37]. These studies also reported the increasing prevalence of H. pylori isolates that are resistant to metronidazole, which is frequently used in the first-line regimen.
The use of probiotics in combination with antimicrobial agents significantly increased the eradication rate, especially for bacteria with antimicrobial resistance. Both the suppressive effect on H. pylori by probiotics and the compliance-promoting effect of ameliorating the side effects of antimicrobials are thought to significantly increase the eradication rate. Actually, Deguchi et al[35] reported that a group treated with one week-triple therapy supplemented with LG21 yogurt and a group with triple therapy alone showed cure rates of 82.6% and 69.3%, respectively. The difference in the intention-to-treat analysis was statistically significant (P = 0.018). In their study, 112 g of yogurt containing 109 CFU of LG21 was given twice daily for 4 wk (3 wk of pretreatment and 1 wk during eradication therapy). According to a recent meta-analysis of 40 eligible studies with 8924 patients[29], the use of probiotics before and throughout the eradication treatment was associated with a superior eradication effect. Patients who received supplementary probiotics showed a higher eradication rate [relative risk (RR) 1.14, 95%CI: 1.10-1.18, P < 0.001] and lower incidence of total side effects (RR 0.47, 95%CI: 0.39-0.57, P < 0.001) in comparison to the control group without probiotics. In a sub-analysis, Lactobacillus was the best choice among the probiotic strains, and probiotics combined with bismuth quadruple regimen was suggested to be the best combination.
Possible role of probiotics in preventing post-eradication gastric cancer: The lifetime risk of gastric cancers in H. pylori-infected individuals is estimated to be 3%-5%[38]. In H. pylori-infected patients, colonization with H. pylori on the gastric mucosa is known to gradually decrease overtime and often becomes undetectable in patients who develop gastric cancer[39]. Furthermore, during long-term follow-up (up to approximately 20 years) of patients who had been cured of H. pylori infection at the start of observation, 0.35% of subjects developed gastric cancer per year. That is, 7% is estimated to have developed gastric cancer at 20 years[40]. These findings strongly suggest that there are some causative factors other than H. pylori can also promote the development of gastric cancer even after H. pylori eradication. However, H. pylori is currently considered the most important pathogen for the development of gastric cancer.
According to the Correa pathway[41], chronic H. pylori infection progresses over the decades through the following stages: chronic gastritis, atrophy, intestinal metaplasia, and cancer. Gastric adenocarcinomas are classified as both well-differentiated (intestinal-type) and undifferentiated (diffuse-type) ones[42]. The development of gastric atrophy is recognized as a critical step to the development of intestinal-type gastric cancer in the Correa pathway. Mucosal atrophy is usually accompanied by inflammation, and is thus recognized as atrophic gastritis. Accordingly, atrophic gastritis appears to be the strongest risk factor for gastric cancer[21]. The histological characteristics of the gastric mucosa (e.g., inflammation, atrophy, and intestinal metaplasia) were analyzed to identify risk factors for gastric cancer after H. pylori eradication[43]. The mucosal inflammation score of the group who developed gastric cancer after successful H. pylori eradication (n = 61) was significantly higher than the group without cancer after eradication (n = 122). The RR and 95%CI were 5.92 and 2.11-16.6, respectively (P < 0.01). Neither atrophy nor intestinal metaplasia itself was a direct risk factor for post-eradication cancer.
The gastric corpus and antrum predominantly contain acid-secreting parietal cells and gastrin-secreting G cells, respectively. Thus, the mucosal atrophy in the corpus caused by H. pylori infection rapidly leads to a reduction in the gastric acid production. In contrast, the production of gastrin (an acid secretion stimulating hormone) remains relatively unchanged. Of note, patients who develop H. pylori-associated duodenal ulcers seem to be somewhat protected from the occurrence of gastric cancers[44]. The predominant mechanism of this protection in patients with duodenal ulcer from the cancers appears to be a higher basal level of gastric acid secretion. On the contrary, it was reported that the long-term suppression of gastric acid secretion by proton pump inhibitors (PPIs) was associated with a significantly increased risk of gastric cancer in H. pylori-infected subjects[45]. During approximately 8 years of follow-up, Cheung et al[46] evaluated the gastric cancer risk in patients treated with PPI using a Cox proportional hazards model. The study population consisted of approximately 63000 subjects, who had received clarithromycin-based triple therapy for H. pylori eradication. The use of PPIs was associated with an increased gastric cancer risk (HR 2.44, 95%CI: 1.42-4.20). This result demonstrated that the long-term use of PPIs was still associated with an increased risk of gastric cancer, even after H. pylori eradication. Accordingly, the stomach with low acidity accompanied by gastritis with atrophy and/or intestinal metaplasia must be considered a high-risk environment that predisposes the gastric mucosa to the development of gastric cancer.
Gastric acid reduction invariably results in a marked increase in the number of non-H. pylori bacteria. Due to the low acidity, these bacteria are still viable and show metabolic activity in the stomach. It therefore appears likely that such an enlarged bacterial mass causes the development of gastric cancers even after H. pylori eradication. Recent studies on the characteristics of the gastric microbial changes associated with gastric carcinogenesis revealed a reduction of species richness, the enrichment of intestinal bacteria or an increase of bacterial species of oral cavity origin[47,48]. It seems unlikely that the deoxidization of dietary nitrates to nitrite by such dysbiotic bacteria could rapidly convert dietary amines into carcinogenic N-nitro compounds, because this conversion requires a sufficient amount of acid (which is not present in the stomach with mucosal atrophy)[49].
Sung et al[5] analyzed gastric microbes associated with gastric mucosal inflammation-which is considered to be the strongest risk factor for post-eradication gastric cancer-at one year after H. pylori eradication. They identified several of bacterial groups that were significantly associated with persistent inflammation. These bacteria included the genera Acientobacter, Ralstonia, Actinobacillus and Erwinia, which are all Gram-negative bacteria. Miyata et al[50] isolated several types of Gram-negative bacteria from the H. pylori-infected gastric mucosa, including Fusobacterium, Haemophilus, Neisseria and Veillonella species. Coculture of a gastric epithelial cell line with the lipopolysaccharide (LPS) specimens extracted from these bacterial groups stimulated a significant amount of IL-8 production. Sano et al[51] found high LPS activity in gastric fluid samples with weak acidity (pH > 4), whereas there was little or no activity in those with strong acidity (pH < 2). Spearman’s test demonstrated a close correlation between pH and LPS activity in their 136 samples (r = 0.872) (Figure 3). These findings suggested that LPS from such non-H. pylori Gram-negative bacteria may perpetuate gastric inflammation and accelerate neoplastic progression in the hypochlorhydric stomach after H. pylori eradication.
Figure 3.
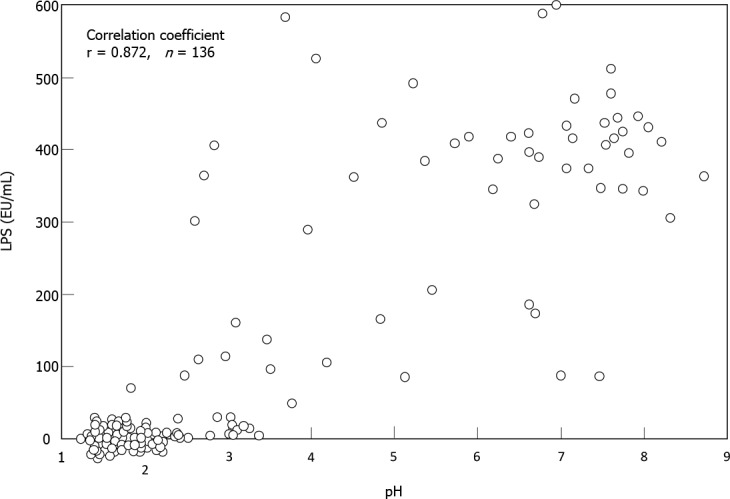
Correlation between pH and lipopolysaccharide activity in gastric fluid. The pH values and lipopolysaccharide activity of gastric fluid samples from 136 subjects were examined using a recombinant factor C assay kit. The correlation coefficients of the both parameters by Spearman test (r) is shown on the upper left. LPS: Lipopolysaccharide. Citation: Sano M, Uchida T, Igarashi M, Matsuoka T, Kimura M, Koike J, Fujisawa M, Mizukami H, Monma M, Teramura E, Yoshihara S, Sato H, Morimachi M, Ito A, Ueda T, Shiraishi K, Matsushima M, Suzuki T, Koga Y. Increase in the Lipopolysaccharide Activity and Accumulation of Gram-Negative Bacteria in the Stomach With Low Acidity. Clin Transl Gastroenterol 2020; 11: e00190. Copyright ©Wolters Kluwer Health, Inc. 2020. Published by Wolters Kluwer Health, Inc.
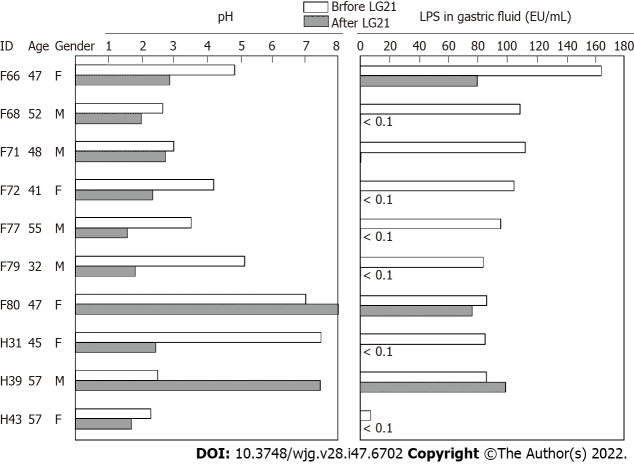
To examine a possible preventive effect of probiotics on post-eradication gastric cancer, we administered a probiotic LG21 strain to subjects with successful eradication who still suffered from atrophic gastritis. In a fasting state in the morning, the pH value and LPS activity of their gastric fluids’ samples were > 3.0 and > 10 EU/mL, respectively (Figure 4). Then, they received 109 CFU of LG21 in yogurt every day for 3 mo. In 8 of 10 subjects, the pH value considerably decreased after LG21 treatment. Lactic acid secreted by the probiotic LG21 strain is thought to restore acidity in the stomach with low acidity. Interestingly, the LPS activity of these subjects, in whom the gastric acidity partially recovered, almost disappeared or markedly decreased. The possible termination of LPS-induced inflammation by LG21 suggests a possible role of probiotics in preventing the development of gastric cancer after H. pylori eradication[52].
Figure 4.
Effect of LG21 administration on the pH and lipopolysaccharide activity in the gastric fluid. Ten subjects who had gastric fluid (GF) with low acidity and high lipopolysaccharide (LPS) activity consumed yogurt containing 109 CFU of LG21 every day for 3 mo. The pH value and LPS activity in the GF were measured before and after LG21 treatment. LPS: Lipopolysaccharide.
FD
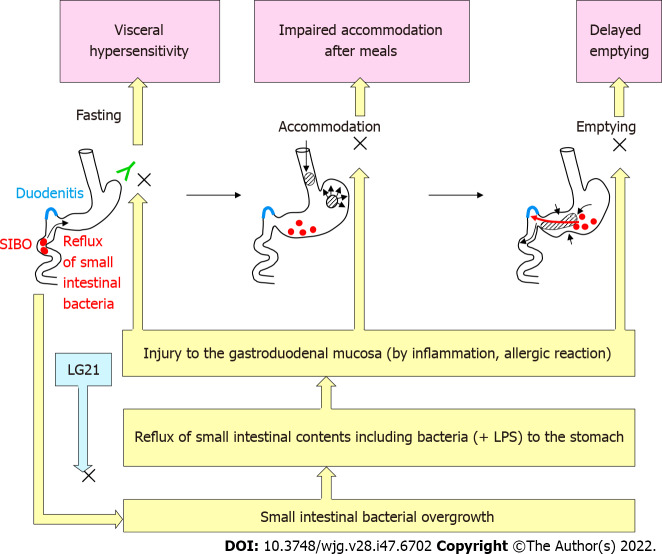
FD is defined as the presence of symptoms that are thought to originate in the gastroduodenal region, in the absence of any organic, systemic or metabolic disease that is likely explain the symptoms. Because of the high prevalence and recurrent nature of symptoms, FD is a clinical problem of considerable magnitude for healthcare. According to the Rome IV criteria[53], there are two subtypes of FD: Postprandial distress syndrome (PDS) with postprandial fullness or early satiation, and epigastric pain syndrome (EPS) with epigastric pain or epigastric burning. The symptoms must be severe enough to affect daily activities, and must be present for > 3 mo with the onset of symptoms at least 6 mo before the diagnosis. While the exact pathophysiology of FD remains to be clarified, gastric motility disturbance (e.g., impaired gastric accommodation and delayed gastric emptying) and visceral hypersensitivity have been suggested as critical underlying mechanisms (Figure 5)[54]. Recently, accumulating evidence supports that the duodenum is a target region underlying the pathophysiology of FD[55]. Impaired mucosal integrity and low-grade inflammation in the duodenum are thought to be associated with altered neuronal signaling and mucosal immune activation in this region. This eventually result in the uncontrolled motile and sensory mechanisms in FD. In addition, gastric acid, bile, food and microbiota are considered to induce and/or aggravate such underlying disorders in FD.
Figure 5.
Pathophysiology of functional dyspepsia and possible mechanisms of the effects of LG21 treatment. SIBO: Small intestinal bacterial overgrowth; LPS: Lipopolysaccharide.
There is evidence to suggest that dysbiosis of intestinal microbiota is involved in the pathogenesis of irritable bowel syndrome (IBS) [a functional gastrointestinal disorder (FGID) originating in the intestine][56]. However, the role of the gastroduodenal microbiota in the pathophysiology of FD (an FGID originating from the stomach and possibly the proximal small intestine) remains to be clarified. H. pylori infection had been considered to be involved in the pathogenesis of FD-like symptoms that are often observed in these subjects. While FD-like symptoms in some H. pylori-infected patients are alleviated by antimicrobial eradication therapy, the improvement of the symptoms might not be mediated by the elimination of H. pylori but by the effect of antimicrobials on non-H. pylori bacteria in the stomach and proximal small intestine[57]. Indeed, Miwa et al[58] demonstrated that the curative treatment of H. pylori infection in eradication therapy was not significantly accompanied by the improvement of symptoms in a double-blind placebo-controlled clinical test. The involvement of an H. pylori-independent mechanism in the pathogenesis of FD is also suggested by a clinical study of probiotics. When the effect of an LG21 strain on FD-like symptoms was examined in H. pylori-infected patients, the severity of PDS after LG21 treatment was significantly lower than that was before treatment, while laboratory tests indicating the number and activity of H. pylori colonizing the stomach showed no significant difference between before and after the treatment[59]. These results suggested that bacteria other than H. pylori, which are resident in the GI tract, play an important role in the pathophysiology of FD. Tan et al[60] reported that the oral administration of the antimicrobial refaximin to patients with FD induced adequate relief of PDS. This implied the involvement of dysbiotic microbiota in the pathogenesis of FD. Nakae et al[61] compared the structure of the microbiota in GF between 44 FD patients and 44 healthy control subjects. A PERMANOVA test showed that the overall bacterial community structures of the two groups were significantly different (P = 0.001). In the bacterial composition analysis using those samples[62], the accumulation of bacteria that usually colonize the intestine, such as Bacteroides, Bifidobacterium and Escherichia, was often found in the GF of FD patients. As bile acids are also detected in these GF samples, the reflux of small intestinal contents, including bile and proximal small intestinal bacteria, to the stomach was suggested to induce such changes in the bacterial composition. Small intestinal bacterial overgrowth (SIBO) is broadly defined as an increase in the number of bacteria in the proximal small intestine together with various types of GI symptoms[63]. More than 60% of Japanese patients with FD have been reported to have overlapping IBS, in which SIBO is considered a critical etiological factor[64]. Thus, FD patients, whose GF contained large numbers of intestinal-type bacteria, might also suffer from SIBO. It is likely that the duodenal mucosa is injured by bile and/or the bacteria-especially Gram-negative bacteria such as Escherichia-in the content that is refluxed from the small intestine. Such mucosal damage would cause both deranged duodenal mucosal integrity and low-grade inflammation in the mucosa (Figure 5). Their studies also showed that 12-wk treatment of FD patients with an LG21 strain was effective for significantly improving symptoms, and shifted the composition of the GF microbiota to that observed in healthy subjects, whose GF microbiota no longer included any intestinal-type bacteria[61,62]. Therefore, the disappearance of such dysbiotic intestinal-type bacteria may be attributable to the resolution of SIBO after LG21 treatment.
Interestingly, there was also a significant inverse correlation between the differential abundance of Prevotella and the improvement of PDS symptoms in the FD patients treated with LG21[61]. That is; a greater increase in the relative abundance of Prevotella in GF after treatment was associated with a higher degree of symptom improvement. A significantly higher abundance of Prevotella on the duodenal mucosa was also observed in healthy control subjects in comparison to patients with FD[65]. Given that the genus Prevotella is sensitive to bile[66], the increase in the abundance after the treatment in FD patients may reflect a lower frequency of bile reflux, while lower abundance in FD patients may reflect a higher frequency of bile reflux.
To evaluate the clinical efficacy of treatment with a probiotic LG21 strain in FD patients, a randomized placebo-controlled clinical trial was performed using 116 individuals without H. pylori infection[67]. Participants were assigned to ingest yogurt containing 109 CFU of LG21 (LG21 group) or LG21-free yogurt (placebo group) every day. According to a questionnaire on the severity of FD symptoms, a trend toward a positive overall ameliorative effect on FD symptoms was observed in the LG21 treatment group (P = 0.07). Moreover, after treatment, the elimination rate for 4 major FD symptoms (postprandial fullness, early satiety, epigastric pain and epigastric burning) was 17.3% in the placebo group and 35.3% in the LG21 group, respectively (P = 0.048).
Although the beneficial effect of LG21 on FD was demonstrated by the clinical trial, the underlying mechanism remains to be clarified. One possible mechanism is protection of the duodenal mucosa from injurious intestinal bacteria and bile by through the resolution of SIBO and/or frequent duodeno-gastric reflux, as mentioned above (Figure 5). Considering that acid-suppressive therapy is so effective and has thus been recommended as the first-line treatment for FD[68], another mechanism underlying the effects of LG21 treatment may be a reduction of gastric acid production. Nakae et al[61], reported that the mean pH values (IQR) of GF of FD patients (n = 44) before and after the treatment were 1.58 (1.43-1.85) and 1.84 (1.56-3.81), respectively. Although this difference was not so great, it was statistically significant (P = 0.012). Given that hypersensitivity of the gastroduodenal mucosa is a critical pathophysiology underlying FD, it is reasonable that even a small reduction of gastric acidity by LG21 treatment can attenuate the gastric sensory and motor disturbances, which would then lead to an improvement of PDS and EPS symptoms. Similarly, in addition to PPI treatment, H-2 receptor antagonist (H2RA) treatment is effective for FD, although the efficiency of acid suppressive by H2RA is considerably lower in comparison to PPIs[55]. The moderate decrease in the secretion of gastric acid observed with LG21 treatment may be attributable to a reduction in the expression of gastrin (an acid secretion stimulating hormone) as the oral administration of LG21 has been reported to reduce the gastrin exression in the murine system[69]. However, LG21 treatment did not reduce the serum gastrin concentration at all in long-term PPI users, who showed very high gastrin levels (> 200 pg/mL)[70]. This means that the suppressive effect of LG21 is no longer exerted in subjects with high gastrin levels such as PPI users and possibly patients with corpus-dominant atrophic gastritis, in whom the serum gastrin concentration is abnormally high due to the secondary response to very low intragastric acidity. Emerging data increasingly point toward the role of gastroduodenal microbiota in the pathophysiology of FD. Accordingly, the application of probiotics in the treatment of this regions is expected to be successful.
CONCLUSION
Probiotics for the stomach have been demonstrated to suppress H. pylori in the stomach, and thus improve eradication rate in patients who receive antimicrobial treatment. If probiotics strains are sufficiently resistant to the gastric acidity and able to achieve close proximity to the gastric mucosa, they are also expected to prevent the development of gastric cancer, even after H. pylori eradication, through the correction of the dysbiotic gastric microbiota. If a deranged gastric bacterial population is involved in the pathophysiology of functional dyspepsia, the use of such probiotics may be useful for the treatment of this functional gastroduodenal disorder.
Footnotes
Conflict-of-interest statement: No conflicts of interest to disclose.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: August 17, 2022
First decision: October 20, 2022
Article in press: November 25, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ji G, China; Neelapu NRR, India; Pan WS, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
References
- 1.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature . 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuda A, Suda W, Morita H, Takanashi K, Takagi A, Koga Y, Hattori M. Influence of Proton-Pump Inhibitors on the Luminal Microbiota in the Gastrointestinal Tract. Clin Transl Gastroenterol . 2015;6:e89. doi: 10.1038/ctg.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuller R. Probiotics in man and animals. J Appl Bacteriol . 1989;66:365–378. [PubMed] [Google Scholar]
- 4.Ruddell WS, Axon AT, Findlay JM, Bartholomew BA, Hill MJ. Effect of cimetidine on the gastric bacterial flora. Lancet. 1980;1:672–674. [PubMed] [Google Scholar]
- 5.Sung JJY, Coker OO, Chu E, Szeto CH, Luk STY, Lau HCH, Yu J. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut . 2020;69:1572–1580. doi: 10.1136/gutjnl-2019-319826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A . 2006;103:732–737. doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li XX, Wong GL, To KF, Wong VW, Lai LH, Chow DK, Lau JY, Sung JJ, Ding C. Bacterial microbiota profiling in gastritis without Helicobacter pylori infection or non-steroidal anti-inflammatory drug use. PLoS One . 2009;4:e7985. doi: 10.1371/journal.pone.0007985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paroni Sterbini F, Palladini A, Masucci L, Cannistraci CV, Pastorino R, Ianiro G, Bugli F, Martini C, Ricciardi W, Gasbarrini A, Sanguinetti M, Cammarota G, Posteraro B. Effects of Proton Pump Inhibitors on the Gastric Mucosa-Associated Microbiota in Dyspeptic Patients. Appl Environ Microbiol . 2016;82:6633–6644. doi: 10.1128/AEM.01437-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Rosenvinge EC, Song Y, White JR, Maddox C, Blanchard T, Fricke WF. Immune status, antibiotic medication and pH are associated with changes in the stomach fluid microbiota. ISME J . 2013;7:1354–1366. doi: 10.1038/ismej.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Mazcorro JF, Suchodolski JS, Jones KR, Clark-Price SC, Dowd SE, Minamoto Y, Markel M, Steiner JM, Dossin O. Effect of the proton pump inhibitor omeprazole on the gastrointestinal bacterial microbiota of healthy dogs. FEMS Microbiol Ecol . 2012;80:624–636. doi: 10.1111/j.1574-6941.2012.01331.x. [DOI] [PubMed] [Google Scholar]
- 11.Food and Agricultural Organization of the United Nations and World Health Organization. Evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. World Health Organization [Internet]. Available from: http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf .
- 12.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol . 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 13.Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology. 1984;86:174–193. [PubMed] [Google Scholar]
- 14.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25:716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 15.Marteau P, Minekus M, Havenaar R, Huis in't Veld JH. Survival of lactic acid bacteria in a dynamic model of the stomach and small intestine: validation and the effects of bile. J Dairy Sci . 1997;80:1031–1037. doi: 10.3168/jds.S0022-0302(97)76027-2. [DOI] [PubMed] [Google Scholar]
- 16.Kabir AM, Aiba Y, Takagi A, Kamiya S, Miwa T, Koga Y. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut . 1997;41:49–55. doi: 10.1136/gut.41.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakamoto I, Igarashi M, Kimura K, Takagi A, Miwa T, Koga Y. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J Antimicrob Chemother . 2001;47:709–710. doi: 10.1093/jac/47.5.709. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki Y. Investigation of acid-resistance mechanisms of an anti-H. pylori strain of Lactobacillus gasseri using a DNA microarray technique. Bioscience and Industry (Japanese) . 2004;62:17–20. [Google Scholar]
- 19.Fujimura S, Kato S, Oda M, Miyahara M, Ito Y, Kimura K, Kawamura T, Ohnuma M, Tateno H, Watanabe A. Detection of Lactobacillus gasseri OLL2716 strain administered with yogurt drink in gastric mucus layer in humans. Lett Appl Microbiol . 2006;43:578–581. doi: 10.1111/j.1472-765X.2006.02017.x. [DOI] [PubMed] [Google Scholar]
- 20.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med . 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 21.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest . 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guruge JL, Falk PG, Lorenz RG, Dans M, Wirth HP, Blaser MJ, Berg DE, Gordon JI. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proc Natl Acad Sci U S A . 1998;95:3925–3930. doi: 10.1073/pnas.95.7.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naumann M, Wessler S, Bartsch C, Wieland B, Covacci A, Haas R, Meyer TF. Activation of activator protein 1 and stress response kinases in epithelial cells colonized by Helicobacter pylori encoding the cag pathogenicity island. J Biol Chem. 1999;274:31655–62. doi: 10.1074/jbc.274.44.31655. [DOI] [PubMed] [Google Scholar]
- 24.Mukai T, Asasaka T, Sato E, Mori K, Matsumoto M, Ohori H. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med Microbiol . 2002;32:105–110. doi: 10.1111/j.1574-695X.2002.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 25.Canducci F, Armuzzi A, Cremonini F, Cammarota G, Bartolozzi F, Pola P, Gasbarrini G, Gasbarrini A. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment Pharmacol Ther . 2000;14:1625–1629. doi: 10.1046/j.1365-2036.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh PS, Tsai YC, Chen YC, Teh SF, Ou CM, King VA. Eradication of Helicobacter pylori infection by the probiotic strains Lactobacillus johnsonii MH-68 and L. salivarius ssp. salicinius AP-32. Helicobacter . 2012;17:466–477. doi: 10.1111/j.1523-5378.2012.00992.x. [DOI] [PubMed] [Google Scholar]
- 27.Tamura A, Kumai H, Nakamichi N, Sugiyama T, Deguchi R, Takagi A, Koga Y. Suppression of Helicobacter pylori-induced interleukin-8 production in vitro and within the gastric mucosa by a live Lactobacillus strain. J Gastroenterol Hepatol . 2006;21:1399–1406. doi: 10.1111/j.1440-1746.2006.04318.x. [DOI] [PubMed] [Google Scholar]
- 28.Gotteland M, Brunser O, Cruchet S. Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment Pharmacol Ther . 2006;23:1077–1086. doi: 10.1111/j.1365-2036.2006.02868.x. [DOI] [PubMed] [Google Scholar]
- 29.Shi X, Zhang J, Mo L, Shi J, Qin M, Huang X. Efficacy and safety of probiotics in eradicating Helicobacter pylori: A network meta-analysis. Medicine (Baltimore) . 2019;98:e15180. doi: 10.1097/MD.0000000000015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perri F, Clemente R, Pastore M, Quitadamo M, Festa V, Bisceglia M, Li Bergoli M, Lauriola G, Leandro G, Ghoos Y, Rutgeerts P, Andriulli A. The 13C-urea breath test as a predictor of intragastric bacterial load and severity of Helicobacter pylori gastritis. Scand J Clin Lab Invest . 1998;58:19–27. doi: 10.1080/00365519850186797. [DOI] [PubMed] [Google Scholar]
- 31.Samloff IM, Varis K, Ihamaki T, Siurala M, Rotter JI. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology. 1982;83:204–209. [PubMed] [Google Scholar]
- 32.Pantoflickova D, Corthésy-Theulaz I, Dorta G, Stolte M, Isler P, Rochat F, Enslen M, Blum AL. Favourable effect of regular intake of fermented milk containing Lactobacillus johnsonii on Helicobacter pylori associated gastritis. Aliment Pharmacol Ther . 2003;18:805–813. doi: 10.1046/j.1365-2036.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- 33.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut . 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guevara B, Cogdill AG. Helicobacter pylori: A Review of Current Diagnostic and Management Strategies. Dig Dis Sci . 2020;65:1917–1931. doi: 10.1007/s10620-020-06193-7. [DOI] [PubMed] [Google Scholar]
- 35.Deguchi R, Nakaminami H, Rimbara E, Noguchi N, Sasatsu M, Suzuki T, Matsushima M, Koike J, Igarashi M, Ozawa H, Fukuda R, Takagi A. Effect of pretreatment with Lactobacillus gasseri OLL2716 on first-line Helicobacter pylori eradication therapy. J Gastroenterol Hepatol . 2012;27:888–892. doi: 10.1111/j.1440-1746.2011.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elitsur Y, Lawrence Z, Rüssmann H, Koletzko S. Primary clarithromycin resistance to Helicobacter pylori and therapy failure in children: the experience in West Virginia. J Pediatr Gastroenterol Nutr . 2006;42:327–328. doi: 10.1097/01.mpg.0000214157.52822.40. [DOI] [PubMed] [Google Scholar]
- 37.Koletzko S, Richy F, Bontems P, Crone J, Kalach N, Monteiro ML, Gottrand F, Celinska-Cedro D, Roma-Giannikou E, Orderda G, Kolacek S, Urruzuno P, Martínez-Gómez MJ, Casswall T, Ashorn M, Bodanszky H, Mégraud F. Prospective multicentre study on antibiotic resistance of Helicobacter pylori strains obtained from children living in Europe. Gut . 2006;55:1711–1716. doi: 10.1136/gut.2006.091272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Björkholm B, Falk P, Engstrand L, Nyrén O. Helicobacter pylori: resurrection of the cancer link. J Intern Med. 2003;253:102–119. doi: 10.1046/j.1365-2796.2003.01119.x. [DOI] [PubMed] [Google Scholar]
- 39.Stewart OA, Wu F, Chen Y. The role of gastric microbiota in gastric cancer. Gut Microbes . 2020;11:1220–1230. doi: 10.1080/19490976.2020.1762520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Take S, Mizuno M, Ishiki K, Kusumoto C, Imada T, Hamada F, Yoshida T, Yokota K, Mitsuhashi T, Okada H. Correction to: Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J Gastroenterol . 2020;55:289–290. doi: 10.1007/s00535-019-01654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol . 1995;19 Suppl 1:S37–S43. [PubMed] [Google Scholar]
- 42.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand . 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 43.Obayashi Y, Kawano S, Sakae H, Abe M, Kono Y, Kanzaki H, Iwamuro M, Kawahara Y, Tanaka T, Yanai H, Okada H. Risk Factors for Gastric Cancer after the Eradication of Helicobacter pylori Evaluated Based on the Background Gastric Mucosa: A Propensity Score-matched Case-control Study. Intern Med . 2021;60:969–976. doi: 10.2169/internalmedicine.5486-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansson LE, Nyrén O, Hsing AW, Bergström R, Josefsson S, Chow WH, Fraumeni JF Jr, Adami HO. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med . 1996;335:242–249. doi: 10.1056/NEJM199607253350404. [DOI] [PubMed] [Google Scholar]
- 45.Tran-Duy A, Spaetgens B, Hoes AW, de Wit NJ, Stehouwer CD. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1706–1719.e5. doi: 10.1016/j.cgh.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 46.Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut . 2018;67:28–35. doi: 10.1136/gutjnl-2017-314605. [DOI] [PubMed] [Google Scholar]
- 47.Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, Costa JL, Carneiro F, Machado JC, Figueiredo C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut . 2018;67:226–236. doi: 10.1136/gutjnl-2017-314205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coker OO, Dai Z, Nie Y, Zhao G, Cao L, Nakatsu G, Wu WK, Wong SH, Chen Z, Sung JJY, Yu J. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut . 2018;67:1024–1032. doi: 10.1136/gutjnl-2017-314281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engstrand L, Graham DY. Microbiome and Gastric Cancer. Dig Dis Sci . 2020;65:865–873. doi: 10.1007/s10620-020-06101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyata N, Hayashi Y, Hayashi S, Sato K, Hirai Y, Yamamoto H, Sugano K. Lipopolysaccharides From Non-Helicobacter pylori Gastric Bacteria Potently Stimulate Interleukin-8 Production in Gastric Epithelial Cells. Clin Transl Gastroenterol . 2019;10:e00024. doi: 10.14309/ctg.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sano M, Uchida T, Igarashi M, Matsuoka T, Kimura M, Koike J, Fujisawa M, Mizukami H, Monma M, Teramura E, Yoshihara S, Sato H, Morimachi M, Ito A, Ueda T, Shiraishi K, Matsushima M, Suzuki T, Koga Y. Increase in the Lipopolysaccharide Activity and Accumulation of Gram-Negative Bacteria in the Stomach With Low Acidity. Clin Transl Gastroenterol . 2020;11:e00190. doi: 10.14309/ctg.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koga Y, Suzuki T and Matsushima M. Gastric microbiota and its role in the carcinogenesis in the stomach. Jpn J Clin Exp Med (Japanese) . 2021;98:107–114. [Google Scholar]
- 53.Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology . 2016;150:1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 54.Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology . 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 55.Wauters L, Talley NJ, Walker MM, Tack J, Vanuytsel T. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut . 2020;69:591–600. doi: 10.1136/gutjnl-2019-318536. [DOI] [PubMed] [Google Scholar]
- 56.Barbara G, Feinle-Bisset C, Ghoshal UC, Quigley EM, Santos J, Vanner S, Vergnolle N, Zoetendal EG. The Intestinal Microenvironment and Functional Gastrointestinal Disorders. Gastroenterology . 2016 doi: 10.1053/j.gastro.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 57.Blum AL, Talley NJ, O'Moráin C, van Zanten SV, Labenz J, Stolte M, Louw JA, Stubberöd A, Theodórs A, Sundin M, Bolling-Sternevald E, Junghard O. Lack of effect of treating Helicobacter pylori infection in patients with nonulcer dyspepsia. Omeprazole plus Clarithromycin and Amoxicillin Effect One Year after Treatment (OCAY) Study Group. N Engl J Med . 1998;339:1875–1881. doi: 10.1056/NEJM199812243392602. [DOI] [PubMed] [Google Scholar]
- 58.Miwa H, Hirai S, Nagahara A, Murai T, Nishira T, Kikuchi S, Takei Y, Watanabe S, Sato N. Cure of Helicobacter pylori infection does not improve symptoms in non-ulcer dyspepsia patients-a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2000;14:317–324. doi: 10.1046/j.1365-2036.2000.00706.x. [DOI] [PubMed] [Google Scholar]
- 59.Takagi A, Yanagi H, Ozawa H, Uemura N, Nakajima S, Inoue K, Kawai T, Ohtsu T, Koga Y. Effects of Lactobacillus gasseri OLL2716 on Helicobacter pylori-Associated Dyspepsia: A Multicenter Randomized Double-Blind Controlled Trial. Gastroenterol Res Pract . 2016;2016:7490452. doi: 10.1155/2016/7490452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan VP, Liu KS, Lam FY, Hung IF, Yuen MF, Leung WK. Randomised clinical trial: rifaximin versus placebo for the treatment of functional dyspepsia. Aliment Pharmacol Ther. 2017;45:767–776. doi: 10.1111/apt.13945. [DOI] [PubMed] [Google Scholar]
- 61.Nakae H, Tsuda A, Matsuoka T, Mine T, Koga Y. Gastric microbiota in the functional dyspepsia patients treated with probiotic yogurt. BMJ Open Gastroenterol . 2016;3:e000109. doi: 10.1136/bmjgast-2016-000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Igarashi M, Nakae H, Matsuoka T, Takahashi S, Hisada T, Tomita J, Koga Y. Alteration in the gastric microbiota and its restoration by probiotics in patients with functional dyspepsia. BMJ Open Gastroenterol . 2017;4:e000144. doi: 10.1136/bmjgast-2017-000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rao SSC, Bhagatwala J. Small Intestinal Bacterial Overgrowth: Clinical Features and Therapeutic Management. Clin Transl Gastroenterol . 2019;10:e00078. doi: 10.14309/ctg.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hori K, Matsumoto T, Miwa H. Analysis of the gastrointestinal symptoms of uninvestigated dyspepsia and irritable bowel syndrome. Gut Liver . 2009;3:192–196. doi: 10.5009/gnl.2009.3.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhong L, Shanahan ER, Raj A, Koloski NA, Fletcher L, Morrison M, Walker MM, Talley NJ, Holtmann G. Dyspepsia and the microbiome: time to focus on the small intestine. Gut . 2017;66:1168–1169. doi: 10.1136/gutjnl-2016-312574. [DOI] [PubMed] [Google Scholar]
- 66.Paster BJ, Dewhirst FE, Olsen I, Fraser GJ. Phylogeny of Bacteroides, Prevotella, and Porphyromonas spp. and related bacteria. J Bacteriol . 1994;176:725–732. doi: 10.1128/jb.176.3.725-732.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohtsu T, Takagi A, Uemura N, Inoue K, Sekino H, Kawashima A, Uchida M, Koga Y. The Ameliorating Effect of Lactobacillus gasseri OLL2716 on Functional Dyspepsia in Helicobacter pylori-Uninfected Individuals: A Randomized Controlled Study. Digestion . 2017;96:92–102. doi: 10.1159/000479000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moayyedi PM, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. Corrigendum: ACG and CAG Clinical Guideline: Management of Dyspepsia. Am J Gastroenterol. 2017;112:1484. doi: 10.1038/ajg.2017.238. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi H, Nakano Y, Matsuoka T, Kumaki N, Asami Y, Koga Y. Role of indigenous lactobacilli in gastrin-mediated acid production in the mouse stomach. Appl Environ Microbiol . 2011;77:6964–6971. doi: 10.1128/AEM.05230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Igarashi M, Nagano J, Tsuda A, Suzuki T, Koike J, Uchida T, Matsushima M, Mine T, Koga Y. Correlation between the Serum Pepsinogen I Level and the Symptom Degree in Proton Pump Inhibitor-Users Administered with a Probiotic. Pharmaceuticals (Basel) . 2014;7:754–764. doi: 10.3390/ph7070754. [DOI] [PMC free article] [PubMed] [Google Scholar]