Abstract
Background:
As the waves of coronavirus disease 2019 (COVID-19) pandemic continues, the current treatment modalities emphasize the use of antiviral agents to save the human lives. Even though remdesivir is one of the current recommended modalities, data on the efficacy of remdesivir in reducing the rate of 28-day mortality are still not concurrent in all the reports.
Aim:
The present study aimed to determine the effectiveness of remdesivir in reducing the rate of mortality in a tertiary care hospital as retrospective comparative analysis.
Setting and Design:
The present study is a retrospective, comparative analysis of accurate and well-documented case files.
Methods:
Data (n = 262) of COVID-19–infected patients admitted and treated with remdesivir (Gp R; n = 160) and without remdesivir (Gp NR: n = 102) between June 1, 2021, and November 30, 2021, were collected and analyzed to obtain the results.
Statistical Analysis:
The data from individual case files were transferred to excel files (Microsoft office, Redmond, WA, USA) and then analyzed using Statistical Package for the Social Studies (SPSS, IBM, Armonk, NY, USA). The descriptive statistical values were expressed as mean ± standard deviation and number, frequencies/percentages. Student's t-test, Chi-square test, and ANOVA were employed for comparative statistics. P < 0.05 was considered statistically significant.
Results:
On analysis of the extracted data, the age, Acute Physiology and Chronic Health Evaluation-IV score, and predicted mortality rate between two groups have not shown significant difference (P > 0.05, ANOVA) and were comparable (P > 0.05, ANOVA). Furthermore, the 28-day mortality rate was significantly reduced (P < 0/001) in the Gp R where the rate of mortality was found to be 6.87%, whereas in Gp NR, it was 29.41%.
Conclusion:
Treatment with remdesivir was able to significantly increase the rate of survival of the patients and reduction in day-28 mortality when compared with the patients who had undergone treatment without remdesivir. Therefore, the results of the current retrospective, observational analysis from a tertiary care hospital could also be a piece of remarkable information to a significant number of existing data globally.
Keywords: 28-day mortality rate, antiviral therapy, coronavirus disease 2019, remdesivir
INTRODUCTION
The year 2020 will be marked as the black year of this decade, with massive crises setting with the spread of the coronavirus disease 2019 (COVID-19). It is determined by the newly recognized beta-coronavirus named as SARS-CoV-2 in December 2019,[1] a main culprit, which is causing the disease chiefly related to respiratory tract infection. Since then, the outbreak of this infectious disease has been found to spread very rapidly across the world. As of March 11 2022, almost 452,052,304 infected people with a death of 6,027,059 have been recorded globally.[2] Especially in India, as of now, 42,984,261 cases have been identified and recorded as COVID-19 infected.[3] Despite continuing spread of the coronavirus with different variants, to save the human life, the entire world is considering to get vaccinated to prevent themselves from such communicable disease. Even though vaccination is currently available globally with the expectation of reducing the incidence of COVID-19 infection,[4,5,6] the world is still struggling with the mystery puzzle.
Several studies have reported that patients with COVID-19 infection, a pandemic widely affecting several million of peoples across the world are, mostly asymptomatic or presenting with mild clinical symptoms.[7,8] However, it is reported that COVID-19 infection is increasing the risk of life-threatening illness in aged people and patients having one or more comorbid conditions.[9,10] Furthermore, a recent study from western India has reported that the mortality rate in COVID-19 infection is raised to be 16% in an intensive care unit (ICU) including healthcare workers.[11] It is much critical to determine the best chances of successful treatment;[12] therefore, it is important to consider various variables including the diagnosis upon admission, and patient's comorbidities, harshness of the organ failure, age, and health status before admission to achieve a better rate of success. Therefore, the estimation of the mortality risk of patients in the ICU is one of the greatest challenges for clinicians. Although several scoring systems are available for ready to use, Acute Physiology and Chronic Health Evaluation-IV (APACHE-IV) is one of the best choices as reported by Alsaei et al., 2021.[13]
Even though, in the early days of the epidemic, only supportive care was available, the wide spread of the infection has increased a desperate need for the invention of an antiviral agent that could be active against SARS-CoV-2. Many countries have involved in controlled trials to study the safety and efficacy of several antiviral agents including the remdesivir, ritonavir/lopinavir, and their combination, as well as immunomodulating therapies such as tocilizumab, convalescent plasma, and interferon. In spite of all the above agents, at present, there are no antiviral therapies with proven effectiveness in treating severely ill COVID-19–infected patients. However, to manage and combat the disease, remdesivir, favipiravir, lopinavir, ritonavir, and oseltamivir are being used during the course of disease.[14,15,16] Among all, remdesivir is reported as a potential candidate drug that shows promising results and has been regarded as a “molecule of hope” for the treatment of COVID-19.[17] Furthermore, remdesivir is declared as the first United States Food and Drug Administration (FDA)–approved drug for the treatment of severely infected COVID-19 patients.[18] However, the efficacy of the drug is still doubtful.[19]
Remdesivir (previously GS-5734; chemical formula C27H35N6O8P) is a monophosphoramidate prodrug of a C-adenosine nucleoside analogue. Remdesivir terminates viral RNA synthesis by inhibiting viral RNA-dependent RNA polymerase. The active form, remdesivir triphosphate, competes with native adenosine triphosphate for chain inclusion, resulting in delayed chain termination. Remdesivir exhibits broad in vitro antiviral action against zoonotic and human pathogens from multiple virus families.[20,21] Several other research groups have conducted various trials and studies in different countries with so many diverse statistics from all over the world regarding the effectiveness of remdesivir.[22,23,24,25]
Owing the above-stated facts in mind, a retrospective analysis of the COVID-19 patients in a tertiary care teaching hospital at Ghaziabad was conducted to evaluate the effectiveness of remdesivir in reduction of 28-day mortality rate in patients with moderate-to-severe COVID illness.
METHODS
The present study is a retrospective, comparative analysis of accurate and well-documented case files; the authors assure that it was a well-controlled number and information. Case based monitoring and data collection of Remdesivir efficacy in reducing the day 28 mortality rate of moderate and severe COVID-19 infection was conducted at our hospital. Before the start of the study, a proper approval from the institutional ethics committee of Santosh Medical College and Hospital (SANTOSH DEEMED TO BE UNIVERSITY) Ethical committee clearance no- SU/2022/679[3] was obtained with wavier of the informed consent. Required information about the patients admitted to the L-3 COVID center (declared by the state government) at our hospital between June 1, 2020, and November 30, 2020, was collected from the medical records division of the hospital.
Patients eligibility
Patients fulfilling the following eligibility criteria were included in the study- COVID 19 patients diagnosed by Reverse transcriptase polymerase chain reaction (RT-PCR) of age >18 years of old, patients with Oxygen saturation <90% at room air, those patients who required High Flow Nasal Canula (HFNC), patients on Non Invasive Ventilation (NIV) or Mechanical Ventilation and patients with identified bilateral pulmonary infiltrates on examination of chest X-ray.
Medical records of COVID-19 patients admitted to the ICU of our hospital were collected and carefully checked. A total of 262 patients’ data that are in line with the eligibility criteria for the current study were extracted carefully and analyzed to attain the objective of the study.
Data extraction
Patients’ information such as age and sex, clinical condition at the time of admission, APACHE IV[13,26] scored based on severity of illness and predicted mortality rate, oxygen therapy, history of the drugs usage and doses, and outcome of the treatment was collected from electronic medical records including nursing and clinical records and progress notes.
For the current comparative retrospective analysis, the patients’ information was grouped into two as follows:
Group R (n = 160): Patients received the antiviral agent remdesivir as intravenous (i.v.) dose of 200 mg on day 1 and 100 mg i.v. dose for the following 4 days (a total of 5 days), infused over 30–60 min,[27] as per the guidelines of the Central Health Ministry of India
Group NR (n = 102): Patients who have not received remdesivir.
The rest of the treatment, i.e. the antibiotic cover, steroid, low molecular weight heparin, multivitamins, plasma therapy, and oxygen therapy, and the rest of the supportive treatments were same in both groups.
Statistical analysis
The data from individual case files were transferred to excel files (Microsoft office, Redmond, Wash., USA) and then analyzed using Statistical Package for the Social Studies (SPSS, IBM, Armonk, NY, USA). The descriptive statistical values were expressed as mean ± standard deviation (SD) and number, as well as frequencies/percentages. Student's t-test, Chi-square test, and ANOVA were employed for comparative statistics. P < 0.05 was considered statistically significant.
RESULTS
Flow diagram shown in Figure 1 explains the flow and grouping of patients’ data as per the requirement of our current study. A total of 262 patients’ data with laboratory confirmation for SARS-CoV-2 and treated (received remdesivir (n = 160) and without remdesivir (n = 102) at our hospital during the period of June 2021 to November 2021 were collected and utilized for the current retrospective analysis.
Figure 1.
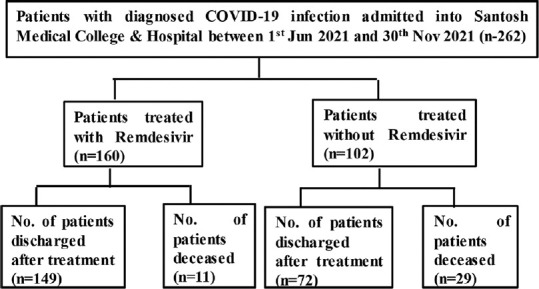
Flowchart of the study pattern
All the data utilized in the current analysis were of patients’ age >18 years. The average age of the patients grouped under Gp R and Gp NR was presented as mean ± SD [Figure 2]. Statistical analysis showed no significant difference (P > 0.05) between both groups. Furthermore, the gender of the patients in both the study groups [Figure 3] also did not show any difference based on the percentile analysis.
Figure 2.
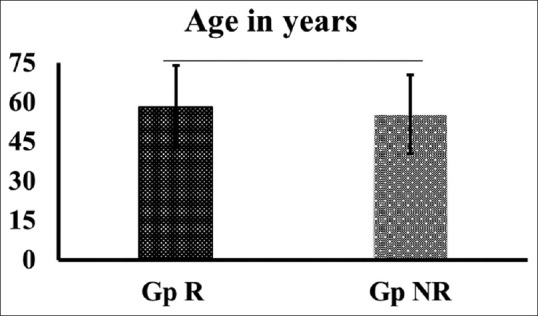
Distribution of patients based on the demographic variables such as age. Group R (patients who received Remdesivir) and Group NR (patients who 9 doesn’t received Remdesivir)
Figure 3.
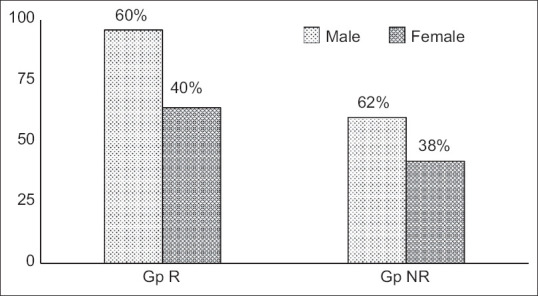
Gender distribution of patients (values are percentage of intragroup) Group R (patients who received Remdesivir) and Group NR (patients who 9 doesn’t received Remdesivir)
Comparison of the APACHE IV score and predicted mortality schematized in [Figure 4] showed that both groups did not exhibit a statistically significant difference (P > 0/05). Because both groups had not shown any significant difference on statistical analysis of the age, gender, APACHE IV score, and predicted mortality at the time of their admission, the data of those patients were utilized to determine the efficacy of remdesivir in reducing the rate of day-28 mortality in this current analysis.
Figure 4.
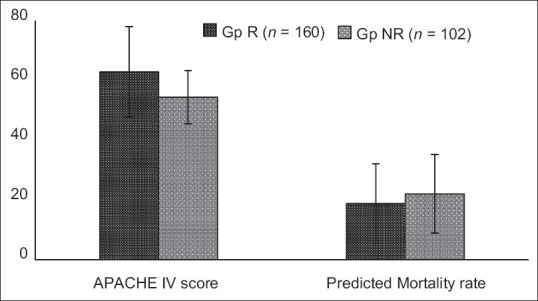
APACHE IV score and predicted mortality of Group R (patients who received Remdesivir) and Group NR (patients who doesn’t received Remdesivir)
The survival and death rates among the two groups were compared using the Chi-square test and are given in Table 1 along with the percentage as well. The results showed a significant difference (P < 0.001) between both groups. More specifically, it was observed that the rate of 28-day mortality (6.87%) was significantly reduced in on the treatment with remdesivir and thereby increased the rate of successful (93.12%) treatment (number of patients discharged) when compared with the data of the patients who had not treated with remdesivir. The rate of 28-day mortality and the number of patients got discharged after successful treatment in Gp NR were found to be 29.41% and 70.58%, respectively.
Table 1.
Outcome of treatment with and without remdesivir
| Group R (n=160), n (%) | Group NR (n=102), n (%) | |
|---|---|---|
| Number of patients discharged | 149 (93.12) | 72 (70.58) |
| Number of deaths | 11 (6.87) | 30 (29.41) |
| 28-day mortality rate (%) | 6.87 | 29.41 |
DISCUSSION
Safe guarding the human life against the bio-war in the form of life-threatening communicable disease called COVID-19 is being a greater challenge across the world. Antiviral therapies are now booming up as one of the major choices of treatment to save life of the SARS-CoV-19–infected patients. Several studies have reported that during the course of the disease, many antiviral agents such as remdesivir, favipiravir, lopinavir, ritonavir, and oseltamivir were used to combat the disease.[14,15,16] However, using such treatment modalities is also not showing promising results. Based on the available reports, the use of antiviral therapies including Remdesivir for the treatment of COVID-19 infection shows a significant level of discrepancies. Therefore, to add a piece of information to the puzzle of available literature, a single-centered, retrospective, observational analysis was performed in a tertiary care hospital with the main focus of determining the efficacy of remdesivir in reducing the rate of day-28 mortality.
Demographic data such as age and gender of the COVID-19–infected patients had not shown a significant difference between two groups namely Gp R (n = 160) and Gp NR (n = 102) that were randomized in the current study for comparison. Furthermore, the ACPACHE IV score and the predicted mortality determined based on the clinical day on the day of admission also did not show any significant difference between both groups that were grouped in the present study. It is quite interesting that, in our study, we found that the rate of survival and discharge was significantly higher, i.e. 93.12% in Gp R (patients treated with remdesivir therapy) and 70.58% in Gp NR where patients had undergone treatment without remdesivir. The results were found to be statistically significant (P < 0.001) on comparison between the two groups.
In addition, data on the rate of day-28 mortality also show a significant difference (P < 0.001) with a percentage value of 6.87% and 29.41% of Gp R and Gp NR, respectively. Results of our current analysis shows a good line of correlation with several other recent studies.[23,24,28] Wang et al.[23] conducted a study with 237 patients, out of which 158 assigned to remdesivir and 79 to placebo, and showed that remdesivir was able to improve the health of the COVID-19–infected patients in a short time (21 days) compared with placebo (23 days) control group. In addition to the above, Holshue et al. showed that i.v. administration of remdesivir showed a promising results in the treatment of patients with COVID-19 recovering from pneumonia without any harmful side effects.[24] Similar to the above findings, results obtained from the data analysis in our current study are also in agreement on treatment with remdesivir; the rate of mortality was greatly reduced, thereby increasing the rate of survival.
Amble evidence is also supporting that remdesivir has been effective when tested against members of the Filoviridae, Paramyxoviridae, Pneumoviridae, and Coronaviridae. Among HCoV, remdesivir inhibits three of the endemic strains associated with respiratory illness (HCoV-OC43, 229E, and NL63), as well as the less common MERS-CoV, SARS-CoV, and novel SARS-CoV-2.[20,21,22] In view with the above-given preliminary results about remdesivir, the FDA issued an Emergency Use Authorization on May 1, 2020 (modified on August 28, 2020), to permit the use of remdesivir for the treatment in adults and children hospitalized with suspected or laboratory-confirmed COVID-19. Alongside, remdesivir also received full or conditional approval in several other countries since that time. The Adaptive COVID-19 Treatment Trial in a preliminary study with 1063 patients has reported a 31% faster time to recovery for the remdesivir group compared to placebo (median time 11 days vs. 15 days; P < 0.001), with additional survival benefit of the remdesivir group compared to placebo (mortality rate 8.0% vs. 11.6%; P = 0.059).[25] Similarly, Beigel et al. showed that patients who received remdesivir had a median recovery time of 10 days compared with 15 days among those who received placebo.[29]
CONCLUSION
The current study suggests that, among the patients with moderate-to-severe COVID-19 infection, remdesivir was highly associated with a significant increase (P < 0.001) in the rate of recovery and discharge from the hospital. Furthermore, treatment with remdesivir showed a significant increase (P < 0.001) in the clinical status of the patients and reduction in day-28 mortality when compared with the patients’ undergone treatment without the remdesivir. Although the effectiveness of remdesivir is still under the scanner, the results of the current retrospective observational analysis from a tertiary care hospital also add a piece of remarkable information to the significant number of existing data globally. However, a more number of data in controlled settings are needed to further prove remdesivir as a potential drug candidate for the treatment of COIVD-19 infection and thereby to safeguard the human life.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Team NCPERE. Vital surveillances: The epidemiological characteristics of an outbreak of 2019 novelcoronavirus diseases (COVID-19)-China. China CDC Wkly. 2020;2:113–22. [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus (COVID-19) Dashboard. [Last accessed on 2022 Mar 11]. Available from: https://covid19.who.int .
- 3.Ministry of Health and Family Affair. [Last accessed on 2022 Mar 11]. Available from: https://www.mohfw.gov.in .
- 4.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–16. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–83. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen PA, Hall LE, John JN, Rapoport AB. The early natural history of SARS-CoV-2 infection: Clinical observations from an Urban, ambulatory COVID-19 clinic. Mayo Clin Proc. 2020;95:1124–6. doi: 10.1016/j.mayocp.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020;395:1763–70. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla U, Chavali S, Mukta P, Mapari A, Vyas A. Initial experience of critically ill patients with COVID19 in Western India: A case series. Indian J Crit Care Med. 2020;24:50913. doi: 10.5005/jp-journals-10071-23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidet B, de Lange DW, Boumendil A, Leaver S, Watson X, Boulanger C, et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: The VIP2 study. Intensive Care Med. 2020;46:57–69. doi: 10.1007/s00134-019-05853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alsaei A, Onyambu S, Mateen P, Javed MA, Khan A, Sadaka F. Predicting mortality in COVID-19: Comparison of APACHE-IV, mews, and MSOFA. Crit Care Med. 2021;49(Suppl 1):102. [Google Scholar]
- 14.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–99. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, et al. Favipiravir versus arbidol for COVID-19: A randomized clinical trial. medRxiv. 2020 [doi: 10.1101/2020.03.17.20037432] [Google Scholar]
- 16.Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saha A, Sharma AR, Bhattacharya M, Sharma G, Lee SS, Chakraborty C. Probable molecular mechanism of remdesivir for the treatment ofCOVID19: Need to know more. Arch Med Res. 2020;51:5856. doi: 10.1016/j.arcmed.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FDA Approves First Treatment for COVID-19. [Last accessed on 2020 Oct 22]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 .
- 19.Dyer O. COVID- 19: Remdesivir has little or no impact on survival, WHO trial shows. BMJ. 2020;371:m4057. doi: 10.1136/bmj.m4057. [DOI] [PubMed] [Google Scholar]
- 20.Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging Viruses. J Med Chem. 2017;60:1648–61. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 21.Ko WC, Rolain JM, Lee NY, Chen PL, Huang CT, Lee PI, et al. Arguments in favour of remdesivir for treating SARS-CoV-2 infections. Int J Antimicrob Agents. 2020;55:105933. doi: 10.1016/j.ijantimicag.2020.105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo MK, Jordan R, Arvey A, Sudhamsu J, Shrivastava-Ranjan P, Hotard AL, et al. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci Rep. 2017;7:43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–71. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–36. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute of Allergy and Infectious Diseases (NIAID). NIH Clinical Trial Shows Remdesivir Accelerates Recovery from Advanced COVID-19. National Institutes of Health. 2020 [Google Scholar]
- 26.Ko M, Shim M, Lee SM, Kim Y, Yoon S. Performance of APACHE IV in Medical Intensive Care Unit Patients: Comparisons with APACHE II, SAPS 3, and MPM0 III. Acute Crit Care. 2018;33:216–21. doi: 10.4266/acc.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garibaldi BT, Wang K, Robinson ML, Zeger SL, Bandeen-Roche K, Wang MC, et al. Comparison of time to clinical improvement with vs. without remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw Open. 2021;4:e213071. doi: 10.1001/jamanetworkopen.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of Remdesivir vs. standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA. 2020;324:1048–57. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19-Final report. N Engl J Med. 2020;383:1813–26. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]


