Abstract
One means by which Bordetella bronchiseptica scavenges iron is through production of the siderophore alcaligin. A nonrevertible alcaligin mutant derived from the virulent strain 4609, designated DBB25, was constructed by insertion of a kanamycin resistance gene into alcA, one of the genes essential for alcaligin biosynthesis. The virulence of the alcA mutant in colostrum-deprived, caesarean-delivered piglets was compared with that of the parent strain in two experiments. At 1 week of age, piglets were inoculated with phosphate-buffered saline, 4609, or DBB25. Two piglets in each group were euthanatized on day 10 postinfection. The remainder were euthanatized at 21 days postinfection. Clinical signs, including fever, coughing, and sneezing, were present in both groups. Nasal washes performed 7, 14, and 21 days postinoculation demonstrated that strain DBB25 colonized the nasal cavity but did so at levels that were significantly less than those achieved by strain 4609. Analysis of colonization based on the number of CFU per gram of tissue recovered from the turbinate, trachea, and lung also demonstrated significant differences between DBB25 and 4609, at both day 10 and day 21 postinfection. Mild to moderate turbinate atrophy was apparent in pigs inoculated with strain 4609, while turbinates of those infected with strain DBB25 developed no or mild atrophy. We conclude from these results that siderophore production by B. bronchiseptica is not essential for colonization of swine but is required for maximal virulence. B. bronchiseptica mutants with nonrevertible defects in genes required for alcaligin synthesis may be candidates for evaluation as attenuated, live vaccine strains in conventionally reared pigs.
Bordetella bronchiseptica is an etiologic agent of atrophic rhinitis and pneumonia in swine. Despite vaccination rates of up to 42%, only 10.5% of swine herds in the United States are free from atrophic rhinitis (54). A more recent study indicates that although B. bronchiseptica vaccines continue to be widely utilized by swine producers in both the United States and Europe, their effectiveness is questionable (5). B. bronchiseptica is also one of the agents involved in porcine respiratory disease complex, a multifactorial disease state that has been of increasing concern to swine producers since the early 1990s (7). Many host-related environmental and management factors are known to influence the frequency and severity of disease associated with B. bronchiseptica. Considerably less is understood about the role of specific bacterial products in infection and disease, and this gap in knowledge hinders the design of more efficacious vaccines. Numerous studies have identified various bacterial proteins that may act as virulence factors (11, 41). However, the proposed adhesin pertactin is the only one that has been evaluated in a model system with direct relevance to swine (40).
Iron is an essential nutrient for nearly all living organisms, and its importance in regulation of virulence determinant expression has been well documented for a number of pathogens (34). Previously, it was shown that in response to iron limitation B. bronchiseptica synthesizes and excretes the siderophore alcaligin, a low-molecular-weight, dihydroxamate Fe-chelating compound (37, 39). Alcaligin is encoded by the alcABCDER operon (10, 23, 24, 29, 45). The genes alcA, alcB, and alcC show significant similarity to the Escherichia coli aerobactin biosynthesis genes iucD, iucB, and iucC, respectively (23, 24, 29). AlcE is predicted to be an iron-sulfur protein, while alcD has no homologs in the public databases which would suggest a function (45). The alcR locus encodes an AraC-type regulator that is essential for expression of the alc operon and the alcaligin receptor FauA (10, 13, 45). AlcR appears not to be essential for colonization of the mouse respiratory tract (45). Alcaligin can acquire iron from the iron-binding proteins lactoferrin and transferrin (3, 21), suggesting that alcaligin synthesis may be an important determinant of Bordetella pathogenesis. The goal of this study was to determine whether alcaligin production promotes the ability of B. bronchiseptica to colonize and cause disease in swine.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
B. bronchiseptica strain 4609 is a virulent isolate that causes respiratory disease in swine (1, 2). The isogenic mutant DBB25 was generated by replacing a segment of the alcA gene with a kanamycin resistance (KANr) gene. Plasmid pDLA5, a pGEM3Zf(+) (Promega) derivative containing a 4.7-kb B. bronchiseptica EcoRI fragment containing alcA, alcB, and the 5′ end of alcC (24), was digested with BglII to release a 297-bp fragment from alcA. This fragment was replaced by the KANr gene of Tn903, purified from a BamHI digest of pUC4K (Pharmacia). The entire B. bronchiseptica plasmid insert containing the KANr gene was released from the pGEM3Zf(+) vector by EcoRI digestion and ligated into pSS1129 (53), a plasmid designed for allelic replacement in B. bronchiseptica that contains a gentamicin resistance gene. The resulting construct, designated pSORT-AKB, was electroporated into E. coli strain SM10 and then transferred by conjugal mating to B. bronchiseptica strain 4609. The conjugation mix was plated onto Bordet Gengou agar (BGA) containing streptomycin (200 μg/ml), to which B. bronchiseptica is naturally resistant, for selection against E. coli. Streptomycin-resistant colonies of B. bronchiseptica were replica-plated onto BGA containing kanamycin (50 μg/ml) or gentamicin (40 μg/ml). Double-crossover mutants were identified as those strains capable of growth on BGA containing kanamycin and incapable of growth on BGA with gentamicin. The presence of the KANr gene inserted into alcA was confirmed by PCR and Southern blot analyses (data not shown). Loss of alcaligin production was confirmed by assaying for siderophore production using chrome azurol S and absorption spectra analyses, as previously described (3, 18, 50; data not shown). One mutant deficient in the ability to synthesize alcaligin was designated DBB25 and used for additional studies.
All bacteria were grown at 37°C for 18 to 36 h, except where otherwise indicated. Routine culturing of B. bronchiseptica strain 4609 was carried out on BGA. Culture conditions for strain DBB25 were identical, except that 50 μg of kanamycin per ml was included in the medium. When required, Fe-depleted Stainer-Scholte broth was prepared by treatment with Chelex, as previously described (3, 52). Cultures used for testing mouse lethality were grown in Fe-replete or Fe-depleted Stainer-Scholte broth, as indicated. Bacteria used to prepare inocula were subcultured twice in Fe-depleted Stainer-Scholte broth.
Phenotypic characterization of strain DBB25.
Alcaligin production was measured using an assay for the presence of hydroxamic acids (3, 18). Briefly, culture supernatants were filter sterilized and acid hydrolyzed for 30 min at 120 lb/in2. The free hydroxylamine was oxidized and measured colorimetrically using 1-napthylamine at an absorbance wavelength of 526 nm and was compared to a hydroxylamine standard curve.
Bacterial protein expression was evaluated by immunoblotting. Proteins from whole-cell extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% polyacrylamide gels, according to the method of Doucet et al. (19). Following electrophoretic transfer to nitrocellulose, immunoblotting was carried out as reported previously (46). Expression of filamentous hemagglutinin (FHA), pertactin, and adenylate cyclase toxin was detected using previously described monoclonal antibodies X3C (33), BPE3 (12), and 9D4 (27), respectively.
Lethality in mice was used as a correlate for expression and activity of the B. bronchiseptica heat-labile dermonecrotic toxin (30). Groups of three mice each were injected intraperitoneally with 0.5 ml of sterile, cell-free sonicates prepared from strain 4609 or DBB25, as previously described (2). Additional groups of two mice each were injected intraperitoneally with sonicates incubated at 56°C for 1 h prior to use. Death within 24 h was interpreted as a positive result. Surviving mice were monitored for 3 days, at which time the experiment was terminated.
Hemagglutination (HA) studies were carried out in 96-well V-bottom plates (Costar Corp.). Bacterial suspensions containing 5 × 109 CFU/ml were serially diluted in phosphate-buffered saline (PBS). Equal volumes of bovine, porcine, equine, or ovine red blood cells, washed 3 times in PBS and resuspended at a concentration of 1%, were mixed with the bacterial suspension in each well. Negative controls containing red blood cells in PBS were included on each plate. Plates were incubated at room temperature, undisturbed, for 2 h and then scored as follows: 3+, complete agglutination with little or no cell pellet; 2+, moderate to large aggregates but with a cell pellet clearly visible; 1+, small aggregates but with a cell pellet similar to the negative control; and 0, no aggregates and a cell pellet indistinguishable from the negative control. The HA titer was recorded as the reciprocal of the highest dilution giving a 2+ reaction. Samples from which only a 1+ reaction was obtained are designated +/−.
Infection protocol.
Infection with wild-type and mutant B. bronchiseptica was compared in a colostrum-deprived, caesarean-delivered swine model, as reported previously (1), in two identical experiments. In each experiment, groups of six 7-day-old piglets housed together in isolators were inoculated intranasally with PBS or with 106 CFU of strain 4609 or DBB25. All animals were evaluated at least twice daily for clinical signs. Rectal temperatures were acquired daily, beginning on the day of inoculation and continuing up to day 18 postinoculation. Weights were checked twice a week for the duration of the experiment. On day 10 postinoculation, two randomly selected pigs from each group were euthanatized. Remaining pigs were euthanatized at day 21 postinoculation. All procedures were conducted in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines for Animal Care and Use, as determined by the National Animal Disease Center Animal Care and Use Committee.
Assessment of colonization.
Nasal washes were performed on all surviving animals on days 7, 14, and 21 postinoculation by flushing the nasal cavity with 5 ml of sterile PBS and collecting the effluent. Colonization of tissues was evaluated using homogenates of ventral nasal turbinate, trachea, and lung tissue removed postmortem. The number of CFU of B. bronchiseptica per milliliter from nasal washes or CFU per gram from tissues was determined by culturing dilutions on sheep's blood agar supplemented with 20 μg of penicillin per ml, 10 μg of fungizone per ml, 10 μg of streptomycin per ml, and 10 μg of spectinomycin per ml. Four replicates per animal were obtained at each time point from every nasal wash or tissue homogenate. The dilutions chosen for culture permitted detection at levels of 10 CFU/ml of nasal wash and 100 CFU/g of tissue.
Presumptive identification of B. bronchiseptica from tissues and nasal washes was based on colony morphology and was confirmed using a previously described colony lift assay (47). Randomly chosen dilutions were plated in duplicate on medium containing kanamycin to confirm the expected phenotypes of strains 4609 (sensitive) and DBB25 (resistant).
Snout scores.
Pig snouts were cross-sectioned at the level of the first premolar, and each of four turbinate bones was assigned a score of 0 to 4 as follows: 0, no atrophy; 1, mild atrophy with less than half of the turbinate scroll bone missing: 2, moderate atrophy with half or more of the turbinate scroll bone missing; 3, severe atrophy in which the turbinate scroll is straight and only a small portion remains; and 4, complete atrophy with no turbinate scroll bone remaining. Septal deviation was scored on a scale of 0 to 2 as follows: 0, no deviation; 1, slight deviation; and 2, severe deviation. Snout scores are the sum of scores for atrophy and septal deviation, with a maximum possible value of 18. Scores, rounded to the nearest whole number, are interpreted as follows: 0 to 2, normal; 3 to 6, mild atrophy; 7 to 10, moderate atrophy; and 11 to 18, severe atrophy.
Pathology.
Gross pathology was evaluated in all animals postmortem. Samples of ventral nasal turbinate, trachea, and lung tissue were fixed in 10% neutral buffered formalin for histopathological examination. Turbinates were decalcified in EDTA Decalcifying Solution (Baxter Scientific Products) for an additional 24 h following fixation. Tissue sections were stained with hematoxylin and eosin.
Statistics.
Data were analyzed by using a two-tailed Student's t test. A P value of ≤0.05 was considered statistically significant.
RESULTS
Phenotypic analysis of DBB25.
Strain DBB25 failed to produce alcaligin regardless of the availability of iron in the growth medium, while strain 4609 produced increased amounts in response to iron depletion (data not shown).
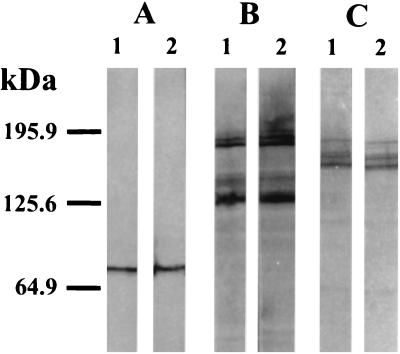
Comparison of immunoblottings carried out with whole-cell extracts of strain 4609 and DBB25 cells did not reveal either qualitative or quantitative differences in the production of FHA, pertactin, or adenylate cyclase toxin (Fig. 1).
FIG. 1.
Expression of FHA, pertactin, and adenylate cyclase toxin. Proteins contained in bacterial cell extracts prepared from strain 4609 (lanes 1) or strain DBB25 (lanes 2) were separated by SDS-PAGE and transferred to nitrocellulose. Pertactin, FHA, and adenylate cyclase toxin were detected by incubation with monoclonal antibodies BPE3 (A), X3C (B), and 9D4 (C), respectively.
Cell-free sonicates of both strains 4609 and DBB25 were lethal for all mice within 24 h of injection. Mice injected with sonicates that were heat-treated prior to administration remained healthy, with no clinical signs, for the duration of the observation period. Identical results were obtained when sonicates were prepared from cultures grown in iron-depleted medium.
The HA titers of strain 4609 were 1:16 (bovine), +/− (equine), 1:2 (porcine), and 0 (ovine). Titers of strain DBB25 were identical for all species of red blood cells tested.
Clinical signs in B. bronchiseptica-infected pigs.
In experiment 1, the number of fever days (total number of daily temperatures ≥40.0°C) was 20 for the group inoculated with strain 4609 and 2 for those infected with strain DBB25. The numbers of fever days for these groups in experiment 2 were 24 and 13, respectively. In both experiments, from day 1 to day 10 postinoculation, the mean daily temperature of strain 4609-infected piglets was higher than those inoculated with strain DBB25. Sneezing and coughing were noted in pigs of both groups, beginning at 3 days postinfection and lasting for approximately 10 days. None of the piglets inoculated with PBS demonstrated signs of respiratory disease.
In experiment 1, the cumulative weight gain of piglets infected with strain 4609 was 320 g less than that of piglets infected with strain DBB25. In experiment 2, the cumulative weight gain of strain 4609-infected piglets was 170 g less that of strain DBB25-infected piglets. Pigs inoculated with PBS had the highest cumulative weight gains in both experiments. However, a statistically significant difference was apparent only between groups inoculated with strains 4609 and DBB25 in experiment 1.
Colonization of the respiratory tract.
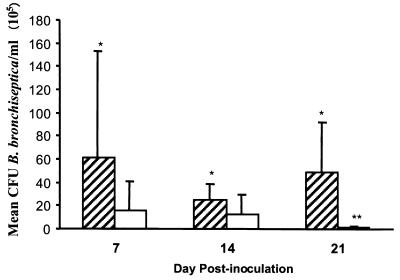
Results from analysis of nasal washes showed that strain 4609 was present in the nasal cavities of pigs in significantly higher numbers than was mutant strain DBB25 as early as 7 days postinoculation in both experiments 1 and 2 (P ≤ 0.029). A statistically significant difference between these groups persisted for the duration of the 21-day experiment. Results from experiment 2 are depicted in Fig. 2.
FIG. 2.
Colonization of the nasal cavity in pigs infected with strains 4609 (striped bars) or DBB25 (white bars) at 7, 14, and 21 days postinoculation. Solid lines represent the mean ± standard error. ∗, P ≤ 0.029 versus DBB25 at corresponding times postinoculation; ∗∗, P ≤ 0.006 versus DBB25 at day 7 postinoculation.
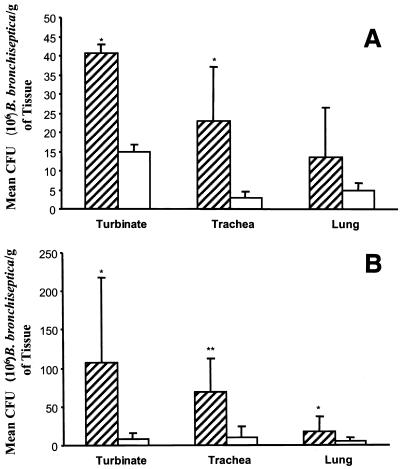
Assessment of colonization based on homogenized tissues of the upper and lower respiratory tracts also revealed differences in the levels of strains 4609 and DBB25 that were present. Results from experiment 2 are depicted in Fig. 3. At 10 days postinoculation, the levels of strain 4609 present in the turbinate and trachea were significantly higher than those of strain DBB25 (Fig. 3A). While more CFU of strain 4609 per gram was also recovered from the lung, the difference was just short of statistical significance (P = 0.052). By 21 days postinoculation, differences in the level of strains DBB25 and 4609 were present in all tissues examined (Fig. 3B). Generally similar results were observed in experiment 1, except that significant differences between strains 4609 and DBB25 occurred only in the trachea at 10 days postinoculation and only in the turbinate and trachea at 21 days postinfection.
FIG. 3.
Colonization of respiratory tract tissues from pigs infected with 4609 (striped bars) or DBB25 (white bars) at 10 days (A) or 21 days (B) postinoculation. Solid lines represent the mean ± standard error. ∗, P ≤ 0.008; ∗∗, P ≤ 0.0002.
In accord with previously published observations (1), strain 4609 colonized the turbinate to a significantly higher degree than the lung at 10 days postinoculation (P ≤ 0.02 in both experiments 1 and 2). This was also true for strain DBB25 (P ≤ 0.01). However, at 21 days postinfection, a strain-specific difference became apparent in both experiments. The turbinates of pigs infected with strain 4609 continued to yield higher numbers of B. bronchiseptica than the lungs (P ≤ 0.003), while the same tissues acquired from DBB25-infected pigs contained statistically equivalent levels of the organism.
Dilutions of nasal washes and homogenized tissues, chosen at random, were cultured on medium supplemented with kanamycin. Growth was apparent only from those dilutions expected to contain strain DBB25. No B. bronchiseptica was recovered at any time from nasal washes or tissues of pigs inoculated with PBS.
Snout scores.
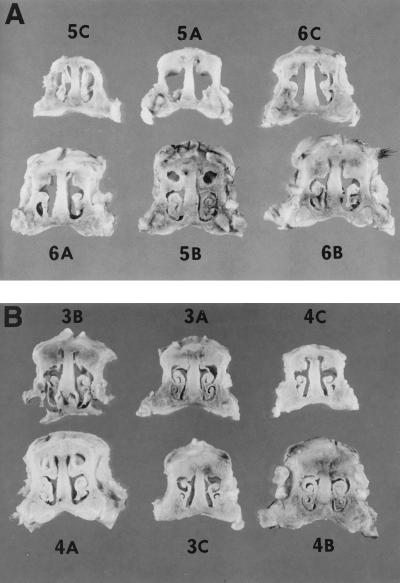
The mean snout scores of pigs in experiment 1 infected with strains 4609 or DBB25 were 8.3 (moderate atrophy) and 2.3 (normal), respectively (Table 1). Individual snout cross-sections from animals in this experiment are shown in Fig. 4. Within each experimental group, those pigs having the highest levels of B. bronchiseptica in the turbinate generally had the highest snout scores. However, there was not a straightforward relationship between turbinate colonization and the degree of atrophy, either within or between groups. Some pigs having similar numbers of 4609 or DBB25 CFU in the turbinate displayed disparate snout scores (compare pigs 5A versus 6B, 4A versus 4B, and 5A versus 3A in Table 1). In other cases, pigs with lower numbers of B. bronchiseptica CFU in the turbinate developed more severe atrophy than others in their group (compare 3A versus 4A). In the second experiment, a less pronounced difference was apparent between the two groups. The mean snout score of pigs infected with strain 4609 was 5.2 (mild atrophy), while a score of 3.0 (the lower limit of mild atrophy) was obtained from pigs infected with strain DBB25. Of the 12 piglets inoculated with PBS, 1 piglet had a snout score of 4; the snout scores of all others were zero.
TABLE 1.
Snout scores of pigs infected with B. bronchiseptica strains 4609 or DBB25
| Group and pig no. | 106 CFU/g (mean)b | Snout scoresa by group
|
||||||
|---|---|---|---|---|---|---|---|---|
| Left turbinate
|
Right turbinate
|
Septum | Total score | Mean group score | ||||
| Dorsal | Ventral | Dorsal | Ventral | |||||
| 4609 | 8.3 | |||||||
| 5Ac | 23.2 | 3 | 3 | 3 | 3 | 0 | 12 | |
| 5Bc | 2.8 | 1 | 1 | 1 | 1 | 0 | 4 | |
| 6Ac | 43.2 | 3 | 3 | 3 | 3 | 0 | 12 | |
| 6Bc | 24.5 | 1 | 1 | 2 | 2 | 0 | 6 | |
| 5Cd | 8.5 | 2 | 2 | 2 | 2 | 0 | 8 | |
| 6Cd | 50.5 | 2 | 2 | 2 | 2 | 0 | 8 | |
| DBB25 | 2.3 | |||||||
| 3Ac | 29.2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3Bc | 8.8 | 1 | 0 | 1 | 0 | 0 | 2 | |
| 4Ac | 2.2 | 1 | 1 | 1 | 1 | 0 | 4 | |
| 4Bc | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3Cd | 77.8 | 1 | 1 | 1 | 1 | 0 | 4 | |
| 4Cd | 45.5 | 1 | 1 | 1 | 1 | 0 | 4 | |
See text.
Mean number of B. bronchiseptica recovered per gram of turbinate.
Pigs euthanatized 21 days postinoculation.
Pigs euthanatized 10 days postinoculation.
FIG. 4.
Snout cross sections from pigs at 21 days postinfection inoculated with strain 4609 (A) or strain DBB25 (B). The pig from which each cross section was derived is indicated.
Pathology.
Pneumonic lung lesions, characterized by well-demarcated, red (at 10 days postinoculation) or tan (at 21 days postinoculation) consolidation with a cranial ventral distribution, were noted in nearly equal numbers of pigs infected with either strain 4609 or strain DBB25. Although the mean percentage of lung involvement at 21 days postinoculation was higher in pigs inoculated with strain 4609 (approximately 30% versus 12% in pigs infected with strain DBB25), the difference was not statistically significant. Values in both groups ranged from 0 to 50%. Those animals with the highest numbers of B. bronchiseptica CFU per gram of lung also had the highest percentages of lung involvement, for both pigs infected with strain 4609 and those infected with strain DBB25.
Histologic examination.
Tissue specimens of nasal turbinate, trachea, and lung were examined to detect pathologic changes. Pigs in the group inoculated with PBS did not have microscopic lesions at either 10 or 21 days postinoculation. Lesions typical of B. bronchiseptica infection were seen in the turbinate, trachea, and lung of pigs inoculated with either strain DBB25 or 4609 at both 10 and 21 days postinoculation. No qualitative or quantitative differences in the lesions were detected between the two infected groups. Lesions of the turbinate consisted of epithelial hyperplasia that forced the epithelium into undulations, loss of cilia, and infiltration of inflammatory cells (principally neutrophils) into the epithelium, occasionally resulting in microabscess formation. In addition, there was fibroplastic replacement of the osseous core of the turbinate beginning at the tips of the scrolls. Lesions of the trachea consisted mainly of inflammatory exudate of neutrophils and macrophages and occasional epithelial metaplasia and loss of cilia. Lesions of the lung consisted of suppurative bronchopneumonia with infiltrates in the airways (primarily neutrophils, with a few macrophages), multifocal alveolar hemorrhages, and fibrosis. The alveolar hemorrhages were most prevalent at 10 days postinoculation, whereas the fibrosis was more severe at 21 days postinoculation.
DISCUSSION
It was previously reported that most swine isolates of B. bronchiseptica, unlike a majority of isolates derived from other host species, synthesize alcaligin in response to iron deprivation independently of the global virulence regulator locus bvgAS (22). On the basis of that observation, it was proposed that alcaligin production may be required for colonization of swine by B. bronchiseptica. Results of the present study clearly establish that, although alcaligin is required for full virulence, it is not required for colonization of the respiratory tract. Nonetheless, comparison of the levels of B. bronchiseptica found in the nasal cavity and in tissues of the respiratory tract demonstrated significant differences between piglets infected with strains 4609 and DBB25 as early as 7 days postinoculation. Although comparative growth studies carried out in vitro revealed no difference in the rate of growth between strains 4609 and DBB25 (D. W. Dyer and T. F. Ducey, unpublished data), altered rates of growth in vivo cannot be ruled out and may be responsible for the colonization results observed. Synthesis and utilization of alcaligin appears to be a primary means by which B. bronchiseptica acquires iron (21), but alternative iron sources have been reported by various investigators (8, 9, 36, 38). Since DBB25 was readily detectable in the respiratory tract in numbers far exceeding those contained in the initial inocula, growth of the organism was apparently supported by one or more of these alternative sources. However, partial iron limitation might be expected to result from the defect in alcaligin synthesis which could, in turn, slow the rate of growth in vivo.
Alternatively, our observations may be explained by one or more alterations of the usual interaction between host cells and fully virulent B. bronchiseptica. Reduced levels of strain DBB25 could result if the mutant has a diminished capacity to attach to cells of the epithelium immediately following exposure. Although the specific bacterial products responsible for attachment to porcine nasal epithelium have not been definitively identified, others have reported a strong association between adherence to swine ciliated epithelial cells and the ability to agglutinate calf red blood cells (28, 49, 51). In the present study, no differences were detected in the HA titers of strains 4609 and DBB25 with either bovine cells or those of several other species. Additionally, results of immunoblotting with monoclonal antibodies did not reveal either qualitative or quantitative differences in the production of FHA and pertactin. These proteins are likely to function as adhesins in swine infection, based on studies in heterologous host species (17) and with the closely related pathogen B. pertussis (32, 48). Thus, it seems unlikely that the inability of the mutant strain DBB25 to colonize the respiratory tract at the level observed for strain 4609 is the result of a defect in adherence, although the data do not entirely eliminate this possibility.
There is increasing evidence that invasion and intracellular survival of B. bronchiseptica in cells of the immune system may be an important aspect of the disease process (6, 14, 20, 25). It can be hypothesized that lower numbers of strain DBB25 were present in the respiratory tract because of a reduction in its ability to avoid or neutralize the effects of phagocytes recruited to the site of infection. It is well established that Bordetella adenylate cyclase toxin inhibits the respiratory burst and other phagocyte functions (15, 42). Nonetheless, an adenylate cyclase toxin-related mechanism is not likely to account for our observations, since immunoblotting failed to detect differences in expression of this toxin by DBB25 as compared to that of 4609. Recently, it was reported that maximum survival of B. pertussis within macrophages is dependent on the availability of iron (35). Although B. bronchiseptica was not included in that study, the possibility exists that strain DBB25 may be killed more effectively than strain 4609 by phagocytes as a result of its defect in siderophore-mediated iron acquisition. However, it should also be noted that polar effects on genes downstream of alcA, unrelated to siderophore synthesis, cannot be ruled out as contributing to the phenotype of DBB25.
Based on results obtained using a mouse respiratory infection model, it was previously reported that a B. bronchiseptica alcaligen mutant and its isogenic parent were found in equal numbers in the lungs for up to 19 days postinfection (45). Other tissues of the respiratory tract were not evaluated. In contrast, this study demonstrated that at 21 days postinoculation, significantly higher numbers of the wild-type strain 4609 were found both in the lungs and in other respiratory tract tissues, as compared to the isogenic alcaligen mutant DBB25. One or more differences between these two studies, including the host species used for experimental infection and the species of origin of the B. bronchiseptica strain employed, may underlie the apparent discrepancy. Although frequently used as a convenient model system for B. pertussis, murine infection does not reproduce all features of natural infection. The relevance of B. bronchiseptica infection of mice to respiratory disease in swine is presently unclear.
Despite widespread use of B. bronchiseptica vaccines by swine producers throughout the world, respiratory disease associated with this agent remains a significant problem for the industry (5, 54). The development of vaccines with improved efficacy is hindered by the absence of definitive data related to virulence mechanisms of B. bronchiseptica in swine. In vitro studies have greatly aided the identification of likely virulence factors but cannot fully reveal the roles of these factors in induction and development of disease in vivo. Some investigators have used rodent or rabbit model systems to uncover important aspects of the pathogenesis of B. bronchiseptica and to define protective features of the immune response in these hosts (4, 16, 17, 26). Such information can be only tentatively applied to swine respiratory disease because of basic differences in the structure and organization of the respiratory tract among these animals. Primates are the only animals known to share with pigs the same anatomic structure of lymphoid tissues in the upper respiratory tract (43, 44). Rodents and rabbits lack the pharyngeal and palatine tonsils that act as inductive sites for secretory antibody responses in swine (31, 55). Evaluation in swine of the role of B. bronchiseptica virulence factors provides data directly applicable to the design of improved vaccines and therapeutic interventions.
Like the closely related human pathogen B. pertussis, B. bronchiseptica is thought to have a redundancy of adhesins and other virulence factors, suggesting that complete protection against infection and/or disease in swine will require multicomponent vaccines. Until a sufficient level of knowledge is attained to permit selection of appropriate vaccine immunogens, whole-cell vaccines are likely to provide the best available degree of protection. Our study demonstrates that fully virulent strains of B. bronchiseptica, partially attenuated by stable mutations in alcA and perhaps in conjunction with other iron-transport mutations, may be candidates for evaluation as vaccine strains in conventionally reared pigs. As shown for the virulent strain 4609, strain DBB25 colonized the turbinate in higher levels than the other tissues examined and also colonized the nasal cavity, trachea, and lung. Also like 4609, the level of DBB25 in the lung could be directly correlated with the degree of lung involvement, while the relationship between turbinate atrophy and turbinate colonization was somewhat variable. These observations suggest that many facets of the interaction between host and pathogen during infection with strain 4609 are likely to be reproduced during infection with DBB25, perhaps leading to a more fully protective immune response than that elicited by the relatively undefined bacterin or modified live vaccines currently licensed for use. An obvious concern related to any live vaccine possessing even low levels of virulence is whether undesirable side effects will result from its use. The data presented here suggest that infection with strain DBB25 is not likely to significantly affect weight gain, an important parameter of performance for swine producers. However, studies of longer duration, conducted under field conditions, are required to evaluate long-term comparative effects on weight gain and feed conversion in swine inoculated with siderophore-deficient mutants of B. bronchiseptica. One outcome of infection with strain DBB25 likely to be unacceptable for producers is the development of lung lesions at levels comparable to a fully virulent strain. It would be of interest to determine whether the same result occurs in conventionally reared swine, which possess a greater level of immunological fitness than the colostrum-deprived, caesarean-delivered pigs used in this study. Additional studies determining the degree of protection against challenge in pigs previously inoculated with strain DBB25 could directly address the suitability of such mutants as vaccine strains. A direct comparison of the nature and magnitude of immune responses in swine infected with strains 4609 or DBB25 will also help to identify those features that provide maximal protection.
ACKNOWLEDGMENT
The authors thank Tibor Magyar (Veterinary Medical Research Institute, Hungarian Academy of Sciences, Budapest, Hungary) for carrying out a portion of the DNT tests.
REFERENCES
- 1.Ackermann M R, Register K B, Gentry-Weeks C, Gwaltney S M, Magyar T. A porcine model for the evaluation of virulence of Bordetella bronchiseptica. J Comp Pathol. 1997;116:55–61. doi: 10.1016/s0021-9975(97)80043-6. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann M R, Rimler R B, Thurston J R. Experimental model of atrophic rhinitis in gnotobiotic pigs. Infect Immun. 1991;59:3626–3629. doi: 10.1128/iai.59.10.3626-3629.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agiato L A, Dyer D W. Siderophore production and membrane alterations by Bordetella pertussis in response to iron starvation. Infect Immun. 1992;60:117–123. doi: 10.1128/iai.60.1.117-123.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akerley B J, Cotter P A, Miller J F. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80:611–620. doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- 5.Bäckström L. Present uses of and experiences with swine vaccines. In: Schultz R D, editor. Veterinary vaccines and diagnostics. San Diego, Calif: Academic Press, Inc.; 1999. pp. 419–429. [DOI] [PubMed] [Google Scholar]
- 6.Banemann A, Gross R. Phase variation affects long-term survival of Bordetella bronchiseptica in professional phagocytes. Infect Immun. 1997;65:3469–3473. doi: 10.1128/iai.65.8.3469-3473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baysinger A. PRDC: is it new or déjà vu? Pork '99. 1999;19:64. [Google Scholar]
- 8.Beall B, Hoenes T. An iron-regulated outer-membrane protein specific to Bordetella bronchiseptica and homologous to ferric siderophore receptors. Microbiology. 1997;143:135–145. doi: 10.1099/00221287-143-1-135. [DOI] [PubMed] [Google Scholar]
- 9.Beall B, Sanden G N. A Bordetella pertussis fepA homologue required for utilization of exogenous ferric enterobactin. Microbiology. 1995;141:3193–3205. doi: 10.1099/13500872-141-12-3193. [DOI] [PubMed] [Google Scholar]
- 10.Beaumont F C, Kang H Y, Brickman T J, Armstrong S K. Identification and characterization of alcR, a gene encoding an AraC-like regulator of alcaligin siderophore biosynthesis and transport in Bordetella pertussis and Bordetella bronchiseptica. J Bacteriol. 1998;180:862–870. doi: 10.1128/jb.180.4.862-870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bemis D A, Burns E H., Jr . Bordetella. In: Gyles C L, Thoen C O, editors. Pathogenesis of bacterial infections in animals. Ames, Iowa: Iowa State University Press; 1993. pp. 201–215. [Google Scholar]
- 12.Brennan M J, Li Z M, Cowell J L, Bisher M E, Steven A C, Novotny P, Manclark C R. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect Immun. 1988;56:3189–3195. doi: 10.1128/iai.56.12.3189-3195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brickman T J, Armstrong S K. Essential role of the iron-regulated outer membrane receptor FauA in alcaligin siderophore-mediated iron uptake in Bordetella species. J Bacteriol. 1999;181:5958–5966. doi: 10.1128/jb.181.19.5958-5966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brockmeier S L, Register K B. Effect of temperature modulation and bvg mutation of Bordetella bronchiseptica on adhesion, intracellular survival and cytotoxicity for swine alveolar macrophages. Vet Microbiol. 2000;73:1–12. doi: 10.1016/s0378-1135(99)00201-1. [DOI] [PubMed] [Google Scholar]
- 15.Confer D L, Eaton J W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982;217:948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 16.Cotter P A, Miller J F. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotter P A, Yuk M H, Mattoo S, Akerley B J, Boschwitz J, Relman D A, Miller J F. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect Immun. 1998;66:5921–5929. doi: 10.1128/iai.66.12.5921-5929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csaky T Z. On the estimation of bound hydroxylamine in biological samples. Acta Chem Scand. 1948;2:450–454. [Google Scholar]
- 19.Doucet J P, Murphy B J, Tuana B S. Modification of a discontinuous and highly porous sodium dodecyl sulfate-polyacrylamide gel system for minigel electrophoresis. Anal Biochem. 1990;190:209–211. doi: 10.1016/0003-2697(90)90182-9. [DOI] [PubMed] [Google Scholar]
- 20.Forde C B, Parton R, Coote J G. Bioluminescence as a reporter of intracellular survival of Bordetella bronchiseptica in murine phagocytes. Infect Immun. 1998;66:3198–3207. doi: 10.1128/iai.66.7.3198-3207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster L A, Dyer D W. A siderophore production mutant of Bordetella bronchiseptica cannot use lactoferrin as an iron source. Infect Immun. 1993;61:2698–2702. doi: 10.1128/iai.61.6.2698-2702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giardina P C, Foster L A, Musser J M, Akerley B J, Miller J F, Dyer D W. bvg repression of alcaligin synthesis in Bordetella bronchiseptica is associated with phylogenetic lineage. J Bacteriol. 1995;177:6058–6063. doi: 10.1128/jb.177.21.6058-6063.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giardina P C, Foster L A, Toth S I, Roe B A, Dyer D W. Identification of alcA, a Bordetella bronchiseptica gene necessary for alcaligin production. Gene. 1995;167:133–136. doi: 10.1016/0378-1119(95)00659-1. [DOI] [PubMed] [Google Scholar]
- 24.Giardina P C, Foster L A, Toth S I, Roe B A, Dyer D W. Analysis of the alcABC operon encoding alcaligin biosynthesis enzymes in Bordetella bronchiseptica. Gene. 1997;194:19–24. doi: 10.1016/s0378-1119(97)00094-2. [DOI] [PubMed] [Google Scholar]
- 25.Guzman C A, Rohde M, Bock M, Timmis K N. Invasion and intracellular survival of Bordetella bronchiseptica in mouse dendritic cells. Infect Immun. 1994;62:5528–5537. doi: 10.1128/iai.62.12.5528-5537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvill E T, Cotter P A, Yuk M H, Miller J F. Probing the function of Bordetella bronchiseptica adenylate cyclase by manipulating host immunity. Infect Immun. 1999;67:1493–1500. doi: 10.1128/iai.67.3.1493-1500.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewlett E L, Gordon V M, McCaffery J D, Sutherland W M, Gray M C. Adenylate cyclase toxin from Bordetella pertussis: identification and purification of the holotoxin molecule. J Biol Chem. 1989;264:19379–19384. [PubMed] [Google Scholar]
- 28.Ishikawa H, Isayama Y. Bovine erythrocyte-agglutinin as a possible adhesin of Bordetella bronchiseptica responsible for binding to porcine nasal epithelium. J Med Microbiol. 1988;26:205–209. doi: 10.1099/00222615-26-3-205. [DOI] [PubMed] [Google Scholar]
- 29.Kang H Y, Brickman T J, Beaumont F C, Armstrong S K. Identification and characterization of iron-regulated Bordetella pertussis alcaligin siderophore biosynthesis genes. J Bacteriol. 1996;178:4877–4884. doi: 10.1128/jb.178.16.4877-4884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kume K, Nakai T, Samejima Y, Sugimoto C. Properties of dermonecrotic toxin prepared from sonic extracts Bordetella bronchiseptica. Infect Immun. 1986;52:370–377. doi: 10.1128/iai.52.2.370-377.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuper C F, Koornstra P J, Hameleers D M, Biewenga J, Spit B J, Duijvestijn A M, van Breda Vriesman P J C, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 32.Leininger E, Ewanowich C A, Bhargava A, Peppler M S, Kenimer J G, Brennan M J. Comparative roles of the Arg-Gly-Asp sequence present in the Bordetella pertussis adhesins pertactin and filamentous hemagglutinin. Infect Immun. 1992;60:2380–2385. doi: 10.1128/iai.60.6.2380-2385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leininger E, Probst P G, Brennan M J, Kenimer J G. Inhibition of Bordetella pertussis filamentous hemagglutinin-mediated cell adherence with monoclonal antibodies. FEMS Microbiol Lett. 1993;106:31–38. doi: 10.1111/j.1574-6968.1993.tb05931.x. [DOI] [PubMed] [Google Scholar]
- 34.Litwin C M, Calderwood S B. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahon B P, Mills K H. Interferon-gamma mediated immune effector mechanisms against Bordetella pertussis. Immunol Lett. 1999;68:213–217. doi: 10.1016/s0165-2478(99)00070-x. [DOI] [PubMed] [Google Scholar]
- 36.Menozzi F D, Gantiez C, Locht C. Identification and purification of transferrin- and lactoferrin-binding proteins of Bordetella pertussis and Bordetella bronchiseptica. Infect Immun. 1991;59:3982–3988. doi: 10.1128/iai.59.11.3982-3988.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore C H, Foster L A, Gerbig D G, Jr, Dyer D W, Gibson B W. Identification of alcaligin as the siderophore produced by Bordetella pertussis and B. bronchiseptica. J Bacteriol. 1995;177:1116–1118. doi: 10.1128/jb.177.4.1116-1118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicholson M L, Beall B. Disruption of tonB in Bordetella bronchiseptica and Bordetella pertussis prevents utilization of ferric siderophores, haemin and haemoglobin as iron sources. Microbiology. 1999;145:2453–2461. doi: 10.1099/00221287-145-9-2453. [DOI] [PubMed] [Google Scholar]
- 39.Nishio T, Tanaka N, Hiratake J, Katsube Y, Ishida Y, Oda J. Isolation and structure of the novel dihydroxamate siderophore alcaligin. J Am Chem Soc. 1988;110:8733–8734. [Google Scholar]
- 40.Novotny P, Kobisch M, Cownley K, Chubb A P, Montaraz J A. Evaluation of Bordetella bronchiseptica vaccines in specific-pathogen-free piglets with bacterial cell surface antigens in enzyme-linked immunosorbent assay. Infect Immun. 1985;50:190–198. doi: 10.1128/iai.50.1.190-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parton R. New perspectives on Bordetella pathogenicity. J Med Microbiol. 1996;44:233–235. doi: 10.1099/00222615-44-4-233. [DOI] [PubMed] [Google Scholar]
- 42.Pearson R D, Symes P, Conboy M, Weiss A A, Hewlett E L. Inhibition of monocyte oxidative responses by Bordetella pertussis adenylate cyclase toxin. J Immunol. 1987;139:2749–2754. [PubMed] [Google Scholar]
- 43.Perry M E, Mustafa Y, Licence S T, Smith D, Whyte A. Pig palatine tonsil as a functional model for the human. Clin Anat. 1997;10:358. [Google Scholar]
- 44.Pracy J P, White A, Mustafa Y, Smith D, Perry M E. The comparative anatomy of the pig middle ear cavity: a model for middle ear inflammation in the human? J Anat. 1998;192:359–368. doi: 10.1046/j.1469-7580.1998.19230359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pradel E, Guiso N, Locht C. Identification of AlcR, an AraC-type regulator of alcaligen siderophore synthesis in Bordetella bronchiseptica and Bordetella pertussis. J Bacteriol. 1998;180:871–880. doi: 10.1128/jb.180.4.871-880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Register K B, Ackermann M R. A highly adherent phenotype associated with virulent Bvg+-phase swine isolates of Bordetella bronchiseptica grown under modulating conditions. Infect Immun. 1997;65:5295–5300. doi: 10.1128/iai.65.12.5295-5300.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Register K B, Ackermann M R, Dyer D W. Nonradioactive colony lift-hybridization assay for detection of Bordetella bronchiseptica infection in swine. J Clin Microbiol. 1995;33:2675–2678. doi: 10.1128/jcm.33.10.2675-2678.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts M, Fairweather N F, Leininger E, Pickard D, Hewlett E L, Robinson A, Hayward C, Dougan G, Charles I G. Construction and characterization of Bordetella pertussis mutants lacking the vir-regulated P.69 outer membrane protein. Mol Microbiol. 1991;5:1393–1404. doi: 10.1111/j.1365-2958.1991.tb00786.x. [DOI] [PubMed] [Google Scholar]
- 49.Sakurai Y, Suzuki H, Terada E. Purification and characterisation of haemagglutinin from Bordetella bronchiseptica. J Med Microbiol. 1993;39:388–392. doi: 10.1099/00222615-39-5-388. [DOI] [PubMed] [Google Scholar]
- 50.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 51.Semjén G, Magyar T. A bovine haemagglutinin of Bordetella bronchiseptica responsible for adherence. Acta Vet Hung. 1985;33:129–136. [PubMed] [Google Scholar]
- 52.Stainer D W, Scholte M J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 53.Stibitz S. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 1994;235:458–465. doi: 10.1016/0076-6879(94)35161-9. [DOI] [PubMed] [Google Scholar]
- 54.Veterinary Services, Animal and Plant Health Inspection Service. Morbidity/mortality and health management of swine in the United States. U.S. Washington, D.C.: Department of Agriculture; 1991. [Google Scholar]
- 55.Wu H Y, Nguyen H H, Russell M W. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46:506–513. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]