Abstract
Steatotic liver grafts tolerate ischemia–reperfusion (I/R) injury poorly, contributing to poor survival following transplantation. However the molecular mechanisms leading to I/R injury still remain to be defined. We have previously reported that the protective effect of bortezomib towards inhibiting cold induced I/R injury in obese rat liver transplant model is through NF-κB down modulation. In this report using an orthotopic liver transplant (OLT) model in Zucker rats (from obese, leptin deficient donor, to lean recipient) we defined the mechanisms of steatotic liver injury, and characterized the role of bortezomib in inhibiting MMP activation and YKL-40, both of which are involved in extracellular matrix deposition and fibrosis, the key pathological features of liver allograft failure. Obese donor rats were treated with bortezomib (i.v., 0.1 mg/kg immediately prior to liver procurement) to assess the role of MMP and YKL-40 in steatotic liver I/R injury. I/R injury in steatotic livers resulted in significant increases in expression of YKL-40 (9 fold), and activation of MMP-2 (15 fold)/MMP-9 (12 fold). Bortezomib treatment reduced the expression of YKL-40 and MMP to basal levels. Bortezomib also inhibited the pro-fibrotic (VEGF, HGF, bFGF, TGF-β) and pro-inflammatory (IL-1β, TNF-α and IFN-γ) cytokines significantly in comparison to untreated animals with I/R injury. These results demonstrate that I/R injury in steatotic livers following transplantation are associated with MMP activation and YKL-40 upregulation resulting in pro-fibrotic and pro-inflammatory cytokine release. Administration of the proteosomal inhibitor, bortezomib, effectively attenuated the I/R injury by inhibiting MMP and YKL-40 expression and therefore support the clinical utility of this drug in donor management for preventing I/R injury and its sequelae.
Keywords: Bortezomib, Matrix metalloproteinases, YKL-40, Liver transplantation, I/R injury
1. Introduction
Liver transplantation is considered as a viable therapeutic option for end stage liver diseases. However, the shortage of available organs contributes to the waiting list mortality [1]. Hepatic steatosis remains one of the major reasons for liver organ discards with a prevalence of 13–50% [2,3]. The current prevalence of obesity and metabolic syndrome seems to be the major risk factor for the increased incidence of hepatic steatosis in potential donors [4]. Steatotic livers are more susceptible to ischemia/reperfusion (I/R) injury induced by both ischemic periods from organ procurement to engraftment, which is directly proportional to higher graft failure rate [5-7]. Severe steatosis, with greater than 60%, is generally viewed as a contraindication for liver transplantation [6,7], while livers with moderate steatosis (30–60% ) are sometimes used for transplantation due to limited availability of the donor organs [2]. These grafts usually have a higher incidence of I/R injury [6,7]. However the molecular mechanisms that contribute to increased susceptibility of steatotic grafts to I/R injury remain poorly understood.
Ischemia reperfusion (I/R) injury of the liver consists of direct ischemic damage during organ procurement and the subsequent reperfusion injury during engraftment. The activation of Kupffer cells in I/R injury induces proinflammatory cytokines including tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β) [8,9]. These mediators facilitate neutrophil sequestration in the ischemic liver and induce subsequent neutrophil-dependent organ dysfunction through the release of reactive oxygen species and proteases from the neutrophils [10]. Matrix metalloproteinases (MMPs) are proteolytic enzymes that induce the extracellular matrix deposition and play an important role in inflammation, and fibrotic damage [11]. MMPs play an important role in I/R injury in the liver, lung, and heart [12-14]. Along with MMP activation, our previous studies have demonstrated elevated fibrotic biomarker YKL-40, a chitinase like glycoprotein, in the sera of patients with liver diseases, including hepatic fibrosis associated with hepatitis C virus [15]. Serum concentrations of YKL-40 correlated with extracellular matrix (ECM) products secreted by hepatic stellate cells (HSCs) and fibroblasts in hepatic fibrosis [16]. Therefore, YKL-40 and MMPs appear to be two important mediators of tissue remodeling in the setting of liver injury [17].
Bortezomib (PS-341), a selective inhibitor of 26S proteasome is an FDA approved drug for the treatment of multiple myeloma and mantle cell lymphoma [18]. We have previously reported that bortezomib mediated blockade of NF-κB activation attenuated the I/R injury in rat steatotic liver transplant model [19]. In the current study we used the leptin deficient Zucker rat liver transplant model which exhibits uniform macrovesicular steatosis that closely resembles the clinical setting of human fatty livers employed in transplantation. Using this well established rat model of cold I/R injury, we demonstrate that the activation MMPs and the upregulation of YKL-40 are the major downstream mechanisms responsible for steatotic liver I/R injury and this can be abrogated with the administration of the proteosome inhibitor bortezomib to donors prior to liver procurement.
2. Objective
The overall objective of the study is to characterize the early molecular events mediated by pro-fibrotic biomarkers, matrix metalloproteinases (MMPs) and YKL-40, that contribute to the exacerbated I/R injury using an established rat steatotic liver transplantation in the steatotic liver transplantation model and to determine the ability of chemical small molecule bortezomib (PS-341) to ameliorate I/R injury in the steatotic liver transplantation model.
3. Materials and methods
3.1. Animal model
Zucker rats represent a well-characterized model of leptin receptor deficiency induced obesity [20] and were used in this study. Animals were housed in pathogen-free conditions with 12-hour diurnal light cycle and access to standard rodent chow and water ad libitum. Surgical procedures were performed under aseptic conditions approved by the Washington University Animals Studies Committee and in accordance with the National Institutes of Health guidelines in “The Guide for the Care and Use of Laboratory Animals”. Obese Zucker rats aged 9–11 weeks and weighing 250–350 g were chosen as donors with weight-matched heterozygous (lean) rats used as recipients. Donor–recipient pairs were matched for sex and weight as controls.
Drugs: bortezomib (PS-341, i.v., 0.5 mL of 0.1 mg/kg) (Millenium Pharmaceuticals, Boston, MA) was given to obese donors immediately prior to liver procurement, while control animals received intravenous saline.
3.2. Orthotopic liver transplantation and cold I/R injury
Orthotopic liver transplantation was performed under isofluorane (Baxter, IL) inhalational anesthesia using Kamada's modified cuff technique [21]. Prior to organ procurement, 1 mL of saline containing 200 units of heparin was given intravenously, and the donor liver perfused via portal vein with 10 mL of cold physiological saline, followed by 10 mL of cold University of Wisconsin (UW) solution Following cuff preparation, the graft was stored in UW solution at 4 °C for 2 h. Following storage, the graft was slowly flushed with 20 mL of cold saline prior to transplantation.
3.3. MMP2 and MMP9 determination
The MMP2 and MMP9 activity in the engrafted livers collected 2 h and 24 h following transplantation was determined by gelatin zymography [22-24]. The protein concentration of the supernatants was determined using the BCA protein assay (Pierce, Rockford, IL), and 5 μg of supernatant proteins were resolved by non-reducing 10% SDS-PAGE through Novex Tris–Glycine gels containing 0.1% gelatin (Invitrogen, Carlsbad, CA). The gels were then developed as per manufacturer's instructions (Invitrogen, Carlsbad, CA). The gelatinolytic activity of the MMPs were quantitatively analyzed by the optical density of the bands using the Kodak image analysis system (Gel Logic 100 System; Kodak, Inc., Rochester, NY).
The MMP2 and MMP9 activity is further quantitated using gelatinase substrate activity [25] (MMP-2/RPN2631, MMP-9/RPN2634, biotrack activity assay kit, GE Healthcare, Pittsburgh, PA) as per manufacturer protocols. Briefly, MMPs were captured by specific antibodies precoated microplate, the activity of which was eventually measured using chromogenic peptide substrate read at 405 nm. The concentration of active MMP is interpolated from a standard curve obtained using the manufacturer provided standard.
The MMP2 and MMP9 mRNA level expressions were analyzed using the FAM-labeled RT-PCR primers (Applied Biosystems, Foster City, CA) as per the manufacturer's recommendation. Briefly, total RNA was extracted from liver using TRIzol reagent (Sigma-Aldrich). The RNA was reverse-transcribed and Real-time PCR was performed in a final reaction volume of 20 μL using iCycler 480 Probes Master (Roche Diagnostics). Each sample was analyzed in triplicate. Cycling conditions consisted of an initial denaturation of 95 °C for 15 min, followed by 40 cycles of 95 °C for 30 s, followed by 61 °C for 1 min [26].
3.4. YKL-40 determination
Protein levels of YKL-40 were analyzed using the Western blot. The retrieved livers were lysed using 4% SDS cell lysis buffer supplemented with protease inhibitor cocktail and EDTA. The lysates were boiled for 20 min in sample buffer (200 mmol/L Tris (pH6.8), 20% glycerol, 2% SDS, 0.1% bromophenol blue, and 10% β-mercaptoethanol) and centrifuged for 30 min and run on 4–12% gradient Bis–Tris denaturing gel (Invitrogen). The gel was transferred onto nitrocellulose membrane, probed with appropriate primary and secondary antibodies, developed and analyzed on using Bio-Rad Universal Hood II (Hercules, CA). Densitometric analysis was done using the software provided by the company [27].
3.5. Growth factor and cytokine gene expression analysis
Expression profiles of intracellular inflammatory cytokine (IL-1β, IL-10, TNF-α and IFNγ), pro-fibrotic (VEGF, HGF, TGF-β and bFGF), and YKL-40 signal genes in the retrieved livers were analyzed using the FAM-labeled RT-PCR primers (Applied Biosystems, Foster City, CA) as per the manufacturer's recommendation. Briefly, total RNA was extracted from the liver using TRIzol reagent (Sigma-Aldrich). The RNA was reverse-transcribed and Real-time PCR was performed in a final reaction volume of 20 μL using iCycler 480 Probes Master (Roche Diagnostics). Each sample was analyzed in triplicate. Cycling conditions consisted of an initial denaturation of 95 °C for 15 min, followed by 40 cycles of 95 °C for 30s, followed by 61 °C for 1 min [26].
3.6. Statistical analysis
All the molecular studies were performed from the tissues obtained from independent experiments performed on animals (n = 5) from each cohorts. The data were analyzed using Graphpad prism 4 software, statistical difference between groups were analyzed by ANOVA, and when significant differences were observed Bonferroni's multiple comparison was performed. Results are presented as mean ± standard error, and considered significant when p < 0.05.
4. Results
4.1. Inhibition of MMP activation by PS341 following IR injury
The activation of matrix metalloproteinases (MMP) has been implicated in the I/R injury leading to poor steatotic graft survival [28]. We performed gelatin zymography to determine the role of bortezomib on MMP activation. As shown in Fig. 1, cold I/R injury of the steatotic liver induced the activation of MMP 9 and MMP 2 when steatotic livers were transplanted to lean recipients (2 h and 24 h following transplantation). However, following treatment with bortezomib there is significant reduction in the densitometry based zymographic activation (Fig. 1A-C) of MMP 9 (MMP9 to pro-MMP9 ratio at 2 h: without bortezomib 9.3 ± 3.7 fold vs. with bortezomib 0.8 ± 0.3 fold, p < 0.05; 24 h without 12.2 ± 2.4 fold and with bortezomib 1.8 ± 0.5 fold, p < 0.05) and MMP2 (MMP2 to pro-MMP2 ratio at 2 h: without bortezomib 12.8 ± 5.1 fold vs. with bortezomib 1.8 ± 0.5 fold, p < 0.05; 24 h without 17.1 ± 3.8 fold and with bortezomib 0.6 ± 0.4 fold, p < 0.05). Further to more quantitatively demonstrate the changes in MMP 2 and MMP9 activity, we have performed a chromogenic activity assay (Fig. 1D,E). Similar to zymographic analysis, chromogenic data demonstrate that MMP2 (MMP2 activity in blank control, untreated non-transplanted: 5.7 ± 2.1 U/μg of total protein; at 2 h: without bortezomib 31.4 ± 7.9 U/μg of total protein, vs. with bortezomib 9.8 ± 3.7 U/μg of total protein, p < 0.05; 24 h without 39.2 ± 8.3 U/μg of total protein and with bortezomib 11.6 ± 4.7 U/μg of total protein, p < 0.05) and MMP9 (MMP9 activity in blank control, untreated non-transplanted: 0.7 ± 0.3 U/μg of total protein; at 2 h: without bortezomib 6.2 ± 1.9 U/μg of total protein, vs. with bortezomib 1.4 ± 0.6 U/μg of total protein, p < 0.05; 24 h without 9.7 ± 2.3 U/μg of total protein and with bortezomib 1.8 ± 0.5 U/μg of total protein, p < 0.05) activity was reduced upon bortezomib treatment in our orthotopic liver transplant animal model. The MMP2 and MMP9 expression was quantitatively determined by RT-PCR (Fig. 1F). The ratio of MMP9 to MMP2 demonstrated that the expression of constitutively expressed MMP2 was significantly reduced over the inducibly expressed MMP9 [29,30] and thus increasing the numerical ratio of MMP9 to MMP2 following bortezomib treatment (MMP9 to MMP2 expression in blank control, untreated non-transplanted: 0.17 ± 0.05; at 2 h: without bortezomib 0.39 ± 0.11, vs. with bortezomib 0.75 ± 0.19, p < 0.05; 24 h without 0.37 ± 0.14 and with bortezomib 0.67 ± 0.15, p < 0.05). These results demonstrate that bortezomib has direct inhibitory effect on MMP and therefore ECM deposition in the transplanted livers.
Fig. 1.

Inhibition of MMP activation by bortezomib (PS341). (A) Gelatin zymography assay to determine the activity of MMP2 MMP2 (66 kDa) and MMP9 (97 kDa) at 2 h and 24 h following cold I/R injury with and without treatment of bortezomib; (B and C) Densitometric analysis of the ratio of activated over pro-form, MMP9 and 2 at 2 h and 24 h following cold I/R injury with and without treatment of bortezomib, respectively; (D, E) Gelatinase substrate activity assay of MMP2 (D), and MMP9 (E) were performed in the above mentioned cohorts; (F) Ratio of MMP9 to MMP2 expression determined by RT-PCR, performed in the above mentioned cohorts. All the studies were performed from the tissues obtained from independent experiments performed on animals (n = 5) from each cohorts. The significance, (**) p < 0.05, determined by between groups was analyzed by ANOVA, and when significant differences were observed, Bonferroni's multiple comparison was performed.
4.2. Inhibition of YKL-40 expression following PS341 treatment
Increased expression of the pro-fibrotic and pro-inflammatory signaling molecule, YKL-40, has been demonstrated in hepatic injury related disease states [31]. As shown in Fig. 2, we demonstrate that cold I/R injury of the steatotic liver increases the expression of YKL40 following obese to lean liver transplants at 2 h and 24 h following I/R injury. However, following treatment of donors with bortezomib prior to procurement and transplantation, there is decreased expression of YKL-40 at 2 h (6.7 ± 1.4 fold vs. 1.9 ± 0.7 fold, p < 0.05) and 24 h (8.9 ± 1.7 fold vs. 1.3 ± 0.5 fold, p < 0.05). These results suggest that the proteasomal inhibitor bortezomib can decrease the inflammatory and fibrotic injury by inhibition of YKL-40 protein.
Fig. 2.
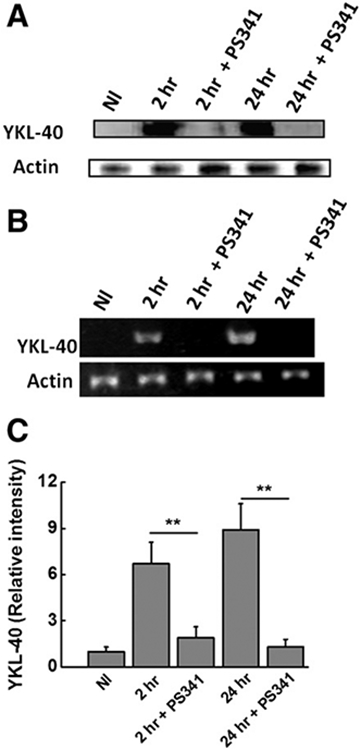
Inhibition of YKL-40 expression by bortezomib (PS341). (A) Western-blot analysis to determine the hepatic expression of YKL-40 at 2 h and 24 h following cold IR injury with and without treatment of bortezomib; (B) mRNA expression analysis to determine the hepatic expression of YKL-40 at 2 h and 24 h following cold IR injury with and without treatment of bortezomib; (C) RT-PCR analysis to determine the mRNA expression analysis to determine the hepatic expression of YKL-40 at 2 h and 24 h following cold I/R injury with and without treatment of bortezomib. All the studies were performed from the tissues obtained from independent experiments performed on animals (n = 5) from each cohorts. The significance, (**) p < 0.05, determined by between groups was analyzed by ANOVA, and when significant differences were observed, Bonferroni's multiple comparison was performed.
4.3. Inhibition of pro-inflammatory cytokines with PS341 treatment
Poor outcome of steatotic liver allografts have been shown to be associated with increased pro-inflammatory cytokines in the circulation [19]. To determine the effect of bortezomib on the pro-inflammatory cytokines in the serum in the cold I/R injury of the steatotic liver we performed LUMINEX assay on the expression of IL-1β, IL-10, TNF-α and IFN-γ following obese to lean liver transplant As shown in Fig. 3, IL-1β serum concentration increased upon 2 h and 24 h warm I/R injury. However, bortezomib treatment significantly decreased pro-inflammatory cytokine, IL-1β. on 2 h 983 ± 177 pg/mL vs. 271 ± 67 pg/mL, p < 0.05; blank control, untreated non-transplanted was 93 ± 26 pg/mL and 24 h (1187 ± 237 pg/mL vs. 139 ± 41 pg/mL, p < 0.05). Similarly, other pro-inflammatory cytokines, TNF-α (2 h: 594 ± 178 pg/mL vs. 158 ± 81 pg/mL, p < 0.05; and 24 h: 643 ± 91 pg/mL vs. 197 ± 48 pg/mL, p < 0.05; blank control, untreated non-transplanted was 87 ± 23 pg/mL), and IFN-γ (2 h: 472 ± 66 pg/mL vs. 103 ± 54 pg/mL, p < 0.05; and 24 h: 513 ± 49 pg/mL vs. 129 ± 48 pg/mL, p < 0.05; blank control, untreated non-transplanted was 69 ± 18 pg/mL) were also inhibited by bortezomib. Conversely, serum concentration of the anti-inflammatory cytokine, IL-10 increased (2 h: 234 ± 76 pg/mL vs. 567 ± 108 pg/mL, p < 0.05; and 24 h: 145 ± 56 vs. 637 ± 152 pg/mL, p < 0.05; blank control, untreated non-transplanted was 887 ± 132 pg/mL) following bortezomib treatment These results strongly suggest that bortezomib correlates with the inhibitory effect on the induction of pro-inflammatory cytokines along with an increase in the anti-inflammatory cytokine, IL-10.
Fig. 3.

Hepatic expression of inflammatory associated cytokines IL-1β (A), IL-10 (B), TNF-α (C) and IFN-γ (D) at 2 h and 24 h following cold IR injury with and without treatment of bortezomib. All the studies were performed from the tissues obtained from independent experiments performed on animals (n = 5) from each cohorts.
4.4. Inhibition of pro-fibrotic cytokine profile with PS341 treatment in I/R injury
To determine the effect of bortezomib on the pro-inflammatory and pro-fibrotic growth factor expression in the warm IR injury of the steatotic liver we have performed quantitative RT-PCR assay on the expression of VEGF, HGF, TGF-β and bFGF in the obese to lean liver transplant with and without bortezomib administration. As shown in Fig. 4, VEGF expression increased in 2 h and 24 h cold IR injury. However, bortezomib treatment of donors prior to procurement decreased the expression of VEGF on 2 h (8.1 ± 3.3 fold vs. 1.6 ± 0.8 fold, p < 0.05) and 24 h (9.4 ± 2.9 fold vs. 2.6 ± 1.7 fold, p < 0.05; fold increase determined as ratio expression over blank control, untreated, non-transplant) warm perfusion. Similarly, other fibrotic growth factors, HGF (2 h: 6.2 ± 2.1 fold vs. 1.2 ± 0.7 fold, p < 0.05; and 24 h: 7.2 ± 2.8 fold vs. 1.8 ± 1.1 fold, p < 0.05), TGF-β (2 h: 5.8 ± 2.4 fold vs. 1.5 ± 0.6 fold, p < 0.05; and 24 h: 6.4 ± 1.8 fold vs. 1.4 ± 0.9 fold, p < 0.05) and bFGF (2 h: 8.9 ± 2.4 fold vs. 2.1 ± 0.9 fold, p < 0.05; and 24 h: 11.4 ± 3.9 fold vs. 3.2 ± 1.8 fold, p < 0.05) were all inhibited by bortezomib. These results demonstrate that bortezomib strongly correlates with the inhibitory effect on the fibrotic growth factors.
Fig. 4.

Hepatic expression of pro-fibrotic growth factors VEGF (A), HGF (B), TGF-β (C) and bFGF (D) at 2 h and 24 h following cold IR injury with and without treatment of bortezomib. All the studies were performed from the tissues obtained from independent experiments performed on animals (n = 5) from each cohorts. The fold increase in the expression was presented as a ratio of expression of the pro-fibrotic growth factor over the blank negative control (untreated, non-transplanted animal cohort).
5. Discussion
Obesity and metabolic syndrome, major risk factors for hepatic steatosis are one of the major hurdles in the usage of marginally steatotic livers for transplantation [4-7]. The lack of understanding of the mechanisms of injury in the steatotic liver remains a major challenge in the inability to initiate specific therapeutic targeting of the steatotic liver to improve its function. Previously, we have reported the protective effect of proteosomal inhibitor, bortezomib, towards reducing I/R injury in the setting of steatosis by p65 NF-κB down regulation [19]. In the present study we demonstrate that MMP activation and YKL-40 upregulation resulting in the induction of pro-inflammatory and pro-fibrotic cytokines appear to play a significant role in initiating I/R injury following transplantation of steatotic livers which is efficiently abrogated by bortezomib.
Matrix metallo-proteinases (MMPs) have been associated with increased ECM turnover and fibrosis. Using murine MMP knock out models Hamada et al. [28] have demonstrated that MMP deficiency has a protective effect on hepatic I/R injury. Further MMP activation has also been demonstrated in various vascular and inflammatory disease models [32]. Further, reports by Shirhane et al. [33] have suggested that MMP inihibitor (ONO-4817) prevented hepatic I/R injury in non-steatotic rat liver transplant models [33]. These studies have also determined that this MMP inhibitor (ONO-4817) also reduced serum pro-inflammatory cytokine including TNF-α and IL-1β [33]. Transcription factors, especially NF-κB, induce IL-1β and TNF-α, have been shown to promote expression of other transcriptional factors [34]. Previous studies from our lab have demonstrated that bortezomib (PS-341) prevents hepatic I/R injury by suppression of p65 NF-κB activity [19]. Results presented in the current report demonstrate that bortezomib also inhibits MMP activity (Fig. 1) and NF-κB transcription factor expression [19], which play a significant role in inhibiting key downstream pro-inflammatory cytokines (Fig. 3) such as (IL-1β, TNF-α and IFN-γ). It is of interest that along with reduction in pro-inflammatory cytokines noted following bortezomib administration to the donors it also up regulated the expression of cytokine IL-10 (Fig. 3) which is known to have strong anti-inflammatory properties. These results are in good agreement with reports by Yoshidome et al. [35] which demonstrate a protective effect of IL-10 cytokine administration in preventing hepatic I/R injury following liver transplantation in a murine model [35]. These cytokine data need to be further studied by functional characterization studies such as Luciferase promoter assays.
Several studies have found a correlation between serum YKL-40 levels and liver fibrosis [36,37]. Fibrous deposition is a major reason for hepatic allograft failure [38]. YKL-40 is considered to be an important fibrotic growth factor that stimulates proliferation of cells that produce ECM proteins [39]. Although liver fibrosis and cirrhosis are characterized by inflammatory infiltration, a process in which a great number of cells participate, analysis using immunochemistry have demonstrated increased YKL-40 expression in fibrotic areas in the liver tissue effected by alcohol and viral hepatitis associated injury [16]. Our results demonstrate that YKL-40 expression increased in the liver allografts following cold I/R injury and the expression was significantly elevated at both 2 h (6.5 fold) and 24 h (9 fold) following the insult (Fig. 1), and specific RNA levels began to increase at 12 h, with a maximum peak at 24 h (Fig. 2). Along with YKL-40, we also noted increased expression of other downstream pro-fibrotic growth factors following the cold I/R injury. An important finding in our study is that the administration of bortezomib to the donor rats prior to procurement of steatotic livers for transplantation resulted in the return of expression levels of fibrotic biomarker YKL-40 (Fig. 2) to basal levels along with significant reduction in down stream pro-fibrotic signaling molecules. Although the attenuation of hepatic fibrosis by bortezomib (PS-341) has not yet described in literature, its role in inhibiting lung and skin fibrosis has been documented [40]. The results presented in Figs. 2 and 4 clearly demonstrate that bortezomib has an inhibitory role towards attenuation of fibrotic cascade in the I/R injury model. As YKL-40 induction has been correlated with NF-κB signaling in other hepatitis models [15] we propose that similar signaling inhibition might play a critical role in our finding of bortezomib induced inhibition of YKL-40 and other pro-fibrotic signaling molecules which needs to be further characterized.
In summary, our data demonstrate an important role for MMP and YKL-40 in the pathogenesis of I/R injury in steatotic livers. The current study demonstrates that proteasome inhibitor bortezomib, decreased MMP activation and YKL-40 expression which in collaboration with NF-κB inhibition downregulates the pro-inflammatory (IL-1β, TNF-α and IFN-γ) and pro-fibrotic (VEGF, TGF-β, HGF, bFGF) cascade of destructive events and thus ultimately leading to the reduction in I/R associated graft injury in fatty liver. Defining the mechanisms by which MMP activation and YKL-40 signaling leads to upregulation of pro-inflammatory and pro-fibrotic cascade may provide important therapeutic targets to prevent I/R injury in following transplantation of steatotic livers in clinical settings.
Acknowledgments
This work was supported by NIH P01 HL66196 (WCC).
Footnotes
Conflict of interest
None.
References
- [1].Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med 2007;356:1545–59. [DOI] [PubMed] [Google Scholar]
- [2].Trevisani F, Colantoni A, Caraceni P, Van Thiel DH. The use of donor fatty liver for liver transplantation: a challenge or a quagmire? J Hepatol 1996;24:114–21. [DOI] [PubMed] [Google Scholar]
- [3].Halon A, Patrzalek D, Rabczynski J. Hepatic steatosis in liver transplant donors: rare phenomenon or common feature of donor population? Transplant Proc 2006;38:193–5. [DOI] [PubMed] [Google Scholar]
- [4].Wicklow BA, Wittmeier KD, Macintosh AC, Sellers EA, Ryner L, Serrai H, et al. Metabolic consequences of hepatic steatosis in overweight and obese adolescents. Diabetes Care 2012;35:905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Todo S, Demetris AJ, Makowka L, Teperman L, Podesta L, Shaver T, et al. Primary nonfunction of hepatic allografts with preexisting fatty infiltration. Transplantation 1989;47:903–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis 2001;21:105–13. [DOI] [PubMed] [Google Scholar]
- [7].Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, et al. Risk factors for primary dysfunction after liver transplantation—a multivariate analysis. Transplantation 1993;55:807–13. [DOI] [PubMed] [Google Scholar]
- [8].Pevni D, Frolkis I, Schwartz D, Schwartz I, Chernichovski T, Kramer A, et al. New evidence for the role of TNF-alpha in liver ischaemic/reperfusion injury. Eur J Clin Invest 2008;38:649–55. [DOI] [PubMed] [Google Scholar]
- [9].Hato S, Urakami A, Yamano T, Uemura T, Ota T, Hirai R, et al. Attenuation of liver and lung injury after hepatic ischemia and reperfusion by a cytokine-suppressive agent, FR167653. Eur Surg Res 2001;33:202–9. [DOI] [PubMed] [Google Scholar]
- [10].Jaeschke H Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol 2006;290:G1083–8. [DOI] [PubMed] [Google Scholar]
- [11].Muller-Quernheim J MMPs are regulatory enzymes in pathways of inflammatory disorders, tissue injury, malignancies and remodelling of the lung. Eur Respir J 2011; 38:12–4. [DOI] [PubMed] [Google Scholar]
- [12].Soccal PM, Gasche Y, Pache JC, Schneuwly O, Slosman DO, Morel DR, et al. Matrix metalloproteinases correlate with alveolar–capillary permeability alteration in lung ischemia-reperfusion injury. Transplantation 2000;70:998–1005. [DOI] [PubMed] [Google Scholar]
- [13].Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia–reperfusion injury in the heart Circulation 2000;101:1833–9. [DOI] [PubMed] [Google Scholar]
- [14].Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 1998;29:1020–30. [DOI] [PubMed] [Google Scholar]
- [15].Sarma NJ, Tiriveedhi V, Subramanian V, Shenoy S, Crippin JS, Chapman WC, et al. Hepatitis C virus mediated changes in miRNA-449a modulates inflammatory biomarker YKL40 through components of the NOTCH signaling pathway. PLoS One 2012;7:e50826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Johansen JS, Christoffersen P, Moller S, Price PA, Henriksen JH, Garbarsch C, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol 2000;32:911–20. [DOI] [PubMed] [Google Scholar]
- [17].Hottinger AF, Iwamoto FM, Karimi S, Riedel E, Dantis J, Park J, et al. YKL-40 and MMP-9 as serum markers for patients with primary central nervous system lymphoma. Ann Neurol 2011;70:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets 2011;11:239–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ramachandran S, Liaw JM, Jia J, Glasgow SC, Liu W, Csontos K, et al. Ischemia–reperfusion injury in rat steatotic liver is dependent on NFkappaB P65 activation. Transpl Immunol 2012;26:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Argiles JM. The obese Zucker rat: a choice for fat metabolism 1968–1988: twenty years of research on the insights of the Zucker mutation. Prog Lipid Res 1989;28:53–66. [DOI] [PubMed] [Google Scholar]
- [21].Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat Surgery 1983;93:64–9. [PubMed] [Google Scholar]
- [22].Woessner JF Jr. Quantification of matrix metalloproteinases in tissue samples. Methods Enzymol 1995;248:510–28. [DOI] [PubMed] [Google Scholar]
- [23].Campbell LG, Ramachandran S, Liu W, Shipley JM, Itohara S, Rogers JG, et al. Different roles for matrix metalloproteinase-2 and matrix metalloproteinase-9 in the pathogenesis of cardiac allograft rejection. Am J Transplant 2005;5:517–28. [DOI] [PubMed] [Google Scholar]
- [24].Tiriveedhi V, Angaswamy N, Brand D, Weber J, Gelman AG, Hachem R, et al. A shift in the collagen V antigenic epitope leads to T helper phenotype switch and immune response to self-antigen leading to chronic lung allograft rejection. Clin Exp Immunol 2012;167:158–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vandooren J, Geurts N, Martens E, Van den Steen PE, Opdenakker G. Zymography methods for visualizing hydrolytic enzymes. Nat Methods 2013;10:211–20. [DOI] [PubMed] [Google Scholar]
- [26].Tiriveedhi V, Angaswamy N, Weber J, Mohanakumar T. Lipid raft facilitated ligation of K-alpha1-tubulin by specific antibodies on epithelial cells: Role in pathogenesis of chronic rejection following human lung transplantation. Biochem Biophys Res Commun 2010;399:251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tiriveedhi V, Gelman AE, Mohanakumar T. HIF-1alpha signaling by airway epithelial cell K-alpha1-tubulin: role in fibrosis and chronic rejection of human lung allografts. Cell Immunol 2012;273:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology 2008;47:186–98. [DOI] [PubMed] [Google Scholar]
- [29].Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 2007;8:221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res 2006;69:562–73. [DOI] [PubMed] [Google Scholar]
- [31].Pizano-Martinez O, Yanez-Sanchez I, Alatorre-Carranza P, Miranda-Diaz A, Ortiz-Lazareno PC, Garcia-Iglesias T, et al. YKL-40 expression in CD14( + ) liver cells in acute and chronic injury. World J Gastroenterol 2011;17:3830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov 2007;6:480–98. [DOI] [PubMed] [Google Scholar]
- [33].Shirahane K, Yamaguchi K, Koga K, Watanabe M, Kuroki S, Tanaka M. Hepatic ischemia/reperfusion injury is prevented by a novel matrix metalloproteinase inhibitor, ONO-4817. Surgery 2006;139:653–64. [DOI] [PubMed] [Google Scholar]
- [34].Bradham CA, Schemmer P, Stachlewitz RF, Thurman RG, Brenner DA. Activation of nuclear factor-kappaB during orthotopic liver transplantation in rats is protective and does not require Kupffer cells. Liver Transpl Surg 1999;5:282–93. [DOI] [PubMed] [Google Scholar]
- [35].Yoshidome H, Kato A, Edwards MJ, Lentsch AB. Interleukin-10 suppresses hepatic ischemia/reperfusion injury in mice: implications of a central role for nuclear factor kappaB. Hepatology 1999;30:203–8. [DOI] [PubMed] [Google Scholar]
- [36].Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology 2006;43:S54–62. [DOI] [PubMed] [Google Scholar]
- [37].Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res 2005;29:166S–71S. [DOI] [PubMed] [Google Scholar]
- [38].Demetris AJ, Adeyi O, Bellamy CO, Clouston A, Charlotte F, Czaja A, et al. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology 2006;44:489–501. [DOI] [PubMed] [Google Scholar]
- [39].Bigg HF, Wait R, Rowan AD, Cawston TE. The mammalian chitinase-like lectin, YKL-40, binds specifically to type I collagen and modulates the rate of type I collagen fibril formation. J Biol Chem 2006;281:21082–95. [DOI] [PubMed] [Google Scholar]
- [40].Mutlu GM, Budinger GR, Wu M, Lam AP, Zirk A, Rivera S, et al. Proteasomal inhibition after injury prevents fibrosis by modulating TGF-beta(1) signalling. Thorax 2012;67:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


