Abstract
Artificial light plays an essential role in information technologies such as optical telecommunications, data storage, security features, and the display of information. Here, we show a chiral lanthanide lumino-glass with extra-large circularly polarized luminescence (CPL) for advanced photonic security device applications. The chiral lanthanide glass is composed of a europium complex with the chiral (+)-3-(trifluoroacetyl)camphor ligand and the achiral glass promoter tris(2,6-dimethoxyphenyl)phosphine oxide ligand. The glass phase transition behavior of the Eu(III) complex is characterized using differential scanning calorimetry. The transparent amorphous glass shows CPL with extra-large dissymmetry factor of gCPL = 1.2. The brightness of the lumino-glass is one thousand times larger than that of Eu(III) luminophores embedded in polymer films of the same thickness at a Eu(III) concentration of 1 mM. The application of the chiral lanthanide lumino-glass in an advanced security paint is demonstrated.
Subject terms: Sensors and biosensors, Inorganic chemistry, Nonlinear optics, Glasses, Optical materials
Circularly polarized luminescence from chiral organic molecules is used in devices such as security tags, lasers, or data storage. Here, the authors use (+)-3-(trifluoroacetyl)camphor and a glass-promoting phosphine oxide ligand to achieve a chiral Eu(III) lumino-glass.
Introduction
Artificial light plays an essential role in information technologies such as optical telecommunications, data storage, security features, and the display of information1. Organic-based luminophores that allow precise control of information have been extensively studied for the development of light-based information technologies2,3. This precise control originates from the molecular quantum design of organic luminophores4–6. The combination of the molecules with quantum design and flexible amorphous materials is expected to result in advancements in photo-science and -technology for industrial applications such as flexible organic light-emitting diodes (OLED)7–9.
In this work, we focus on an optical information technology based on chiral luminescent molecules. Recently, circularly polarized luminescence (CPL) from chiral organic molecules has attracted considerable attention because of its applications in sophisticated photo-information technologies, such as security tags, lasers, data storage, and organic electroluminescent (EL) devices for three-dimensional displays10–16. The CPL phenomena are characterized by the differential emission of right- and left-handed circularly polarized light. The magnitude of CPL is given by the following equation17,18:
| 1 |
where IL and IR are the emission intensities of the left- and right-handed CPL, respectively.
In particular, lanthanide complexes containing chiral organic ligands exhibit dissymmetry factors (gCPL) approximately a thousand times larger than those of chiral organic molecules10–13,19–22. Parker prepared anion sensors using the CPL signals of mononuclear Eu(III) and Tb(III) complexes with chiral ligands incorporating an azaxanthone sensitizer23. Muller and colleagues24 observed an exceptionally large CPL in an Eu(III)-Cs(I) system with characteristic chiral β-diketonate ligands containing a camphor framework (tetrakis(3-heptafluorobutylryl-(+)-camphorato) (gCPL = −1.38)24, although the emission quantum yield was extremely low (Φtot < 1.0%)25. Recently, we found that phosphine oxide ligands improve the quantum emission yield by controlling the energy quenching state for a chiral Eu(III) complex with camphor (Φtot > 10%)26. The use of well-designed phosphine oxide ligands is a key strategy for the construction of chiral Eu(III) complexes with large gCPL and Φtot values for advanced CPL luminophore applications.
Herein, we prepare a bulky phosphine oxide ligand with six methoxy groups, tmpo (tris(2,6-dimethoxyphenyl)phosphine oxide, Fig. 1a), for the construction of complexes with large gCPL and Φtot values. The bulky ligand coordinates weakly to the Eu(III) ion; such weak coordination has been reported to be related to the enhancement of CPL22. The tmpo ligand is also expected to promote glass formation due to the multiple coordination sites provided by its phosphine oxide (P=O)26 and methoxy groups (O–Me)27, which is beneficial for the fabrication of transparent materials. The ligand (±)-3-(trifluoroacetyl)camphor (±tfc) is selected to achieve a chiral ligand arrangement around the Eu center (Fig. 1b). The chiral Eu(III) complexes with phosphine oxide ligands are prepared by casting Eu(±tfc)3(H2O)2 and tmpo ligands on a substrate. The glass phase transition behavior of the Eu(III) complex (Eu(±tfc)-tmpo) is characterized using differential scanning calorimetry. The resulting chiral Eu(III) lumino-glass exhibits extraordinary CPL intensity (gCPL = 1.2) and a relatively high emission quantum yield (Φtot = 13 %). Chiral Eu(III) complexes with other phosphine oxide ligands (Fig. 1c, L1: Tris(2-methoxyphenyl)phosphine oxide, L2: Tris(3-methoxyphenyl)phosphine oxide, L3: tris(4-methoxyphenyl)phosphine oxide, L4: tris(4-methylphenyl)phosphine oxide, L5: tris(2,4,6-trimethylphenyl)phosphine oxide, Supplementary Methods) are also prepared for comparison of their photophysical properties. Based on spectroscopic analyses, we determine that the extra-large gCPL of Eu(±tfc)-tmpo originates from its characteristic symmetric coordination structure with a strong crystal field. Using the chiral Eu(III) lumino-glass, the first application of CPL paint as a security ink (to display a sun or moon shape) are demonstrated. These glass-type chiral Eu(III) complexes are promising candidates for future devices with polarized light information.
Fig. 1. Molecular structures.
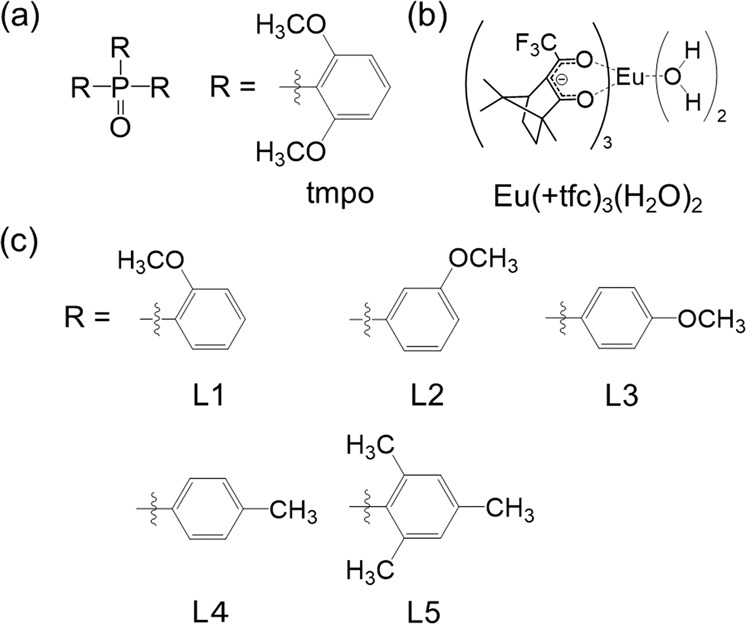
Chemical structures of tmpo ligand (a), Eu(+tfc)3(H2O)2 (b), L1, L2, L3, L4, and L5 ligands (c).
Results and discussion
Luminescence intensity of Eu(III) lumino-glass
Emission properties of Eu(III) lumino-glass (Fig. 2) were evaluated using emission spectra and time-resolved emission measurement. The emission spectra of the Eu(+tfc)-tmpo(n) films (n = 1, 2, 3, n: number of equivalents relative to Eu(+tfc)3) are shown in Fig. 3 (Supplementary Note 1 and Supplementary Figs. 1–3). The spectra were normalized using the integrated intensity of the magnetic dipole transition (MD: 5D0 → 7F1). Two peaks were observed at 584 and 594 nm for the MD transition (Fig. 3, inset). The strong emission bands near 612 nm were attributed to the electric dipole transition (ED: 5D0 → 7F2). The emission band of Eu(+tfc)-tmpo(1) was different from those of Eu(+tfc)-tmpo(2) and Eu(+tfc)-tmpo(3). The luminescence intensity ratio of the ED/MD transition (AED/AMD) decreased with increasing n (i.e., increasing number of tmpo molecules), indicating that the Eu(III) complex with tmpo ligands forms a symmetric coordination geometry28. The crystal field splitting in the MD transition was found to be 274 cm−1. Small AED/AMD and large crystal field splitting values were also observed in a comparative study with other Eu(III) complexes (Supplementary Note 2–3, Supplementary Figs. 4–7, and Supplementary Table 1). These results indicated that a symmetric coordination structure with a strong crystal field was formed by the tmpo ligands, which was attributed to the large steric hindrance and multiple interaction of tmpo ligands.
Fig. 2. Photographs of Eu(III) lumino-glass.
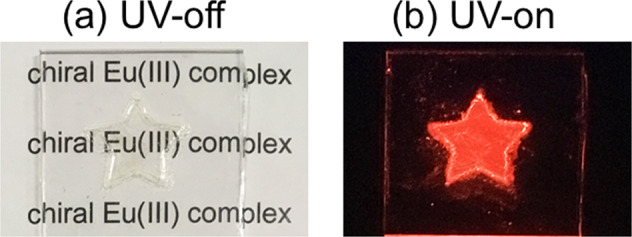
Photographs of Eu(III) lumino-glass (Eu(+tfc)-tmpo(2). UV-off (a), UV-on (b, λex = 365 nm)).
Fig. 3. Emission spectra.
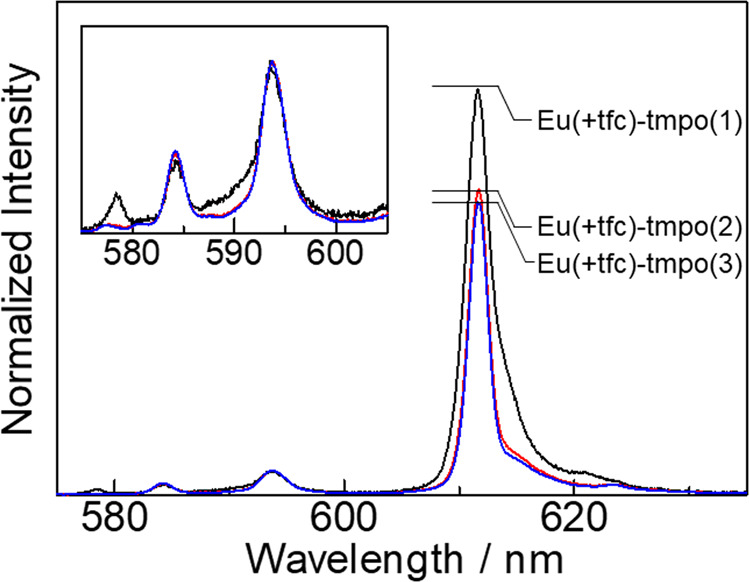
Emission spectra of Eu(+tfc)-tmpo(1) (black line), Eu(+tfc)-tmpo(2) (red line), and Eu(+tfc)-tmpo(3) (blue line).
To obtain information regarding the coordination structure around the luminescent Eu(III) center, the time-resolved emission profiles of the complexes were measured. The emission lifetimes were estimated using triple exponential decays (Table 1), and were consistent with the existence of several metastable structures. The relative lifetime of the τ3 component increased with the addition of more equivalents of tmpo. The emission decay phenomenon of Eu(+tfc)-tmpo(2) was similar to that of Eu(+tfc)-tmpo(3). The emission quantum efficiencies excited by the tfc ligands (Φtot) of the films are also shown in Table 1. The Φtot values also increased with the number of equivalents of tmpo added. The emission quantum yield of Eu(+tfc)-tmpo(2) (Φtot = 13 %) was much larger than that of previously reported Eu(III)-Cs(I) systems (Φtot < 1%) with a large gCPL value (−1.38) in solution24,25.
Table 1.
Photophysical properties of Eu(III) lumino-glass.
| τ1 (ms)a | τ2 (ms)a | τ3 (ms)a | Φtot (%)b | |
|---|---|---|---|---|
| Eu(+tfc)-tmpo(1) | 0.10 (11%) | 0.28 (44%) | 0.70 (45%) | 2.6 |
| Eu(+tfc)-tmpo(2) | 0.14 (6%) | 0.37 (34%) | 0.74 (60%) | 13 |
| Eu(+tfc)-tmpo(3) | 0.16 (5%) | 0.41 (34%) | 0.76 (61%) | 13 |
aEmission lifetime (λex = 356 nm, λem = 612 nm).
bΦtot (λex = 370 nm).
CPL properties of Eu(±tfc) with tmpo ligands
The CPL spectra of Eu(±tfc)-tmpo(2) are shown in Fig. 4 (Supplementary Note 4–6, Supplementary Figs. 8–15, and Supplementary Table 2). Large CPL signals were observed for the MD transition at 594 nm (Eu(+tfc)-tmpo(2): gCPL = −1.2, Eu(−tfc)-tmpo(2): gCPL = 1.2) and the ED transition at 612 nm (Eu(+tfc)-tmpo(2): gCPL = 0.15, Eu(−tfc)-tmpo(2): gCPL = −0.15). The gCPL value in the MD transition is almost same as that of Eu(+tfc)-tmpo(1) (gCPL = −1.0) and Eu(+tfc)-tmpo(3) (gCPL = −1.2; Supplementary Note 7 and Supplementary Fig. 16). The CPL spectrum of Eu(+tfc) with two equivalents of tmpo in solution also exhibited a large CPL signal (gCPL = −1.0) (Supplementary Notes 8 and 9, Supplementary Figs. 17–19, Supplementary Table 3, and Supplementary Data 1–3). The gCPL values at 594 nm (film: gCPL = −1.2, solution: gCPL = −1.0) were as large as that previously reported (gCPL = −1.38) for Eu(III)-Cs(I) systems24,25. These values were different from the small gCPL value of a mononuclear Eu(III) complex with tightly coordinated phosphine oxide ligands (Eu(+tfc)3(tppo)2, tppo: triphenylphosphine oxide, gCPL = 0.09) and a polynuclear Eu(III) complex ([Eu(+tfc)3dpbp]n, dpbp: 4,4-bis(diphenylphosphoryl)biphenyl, gCPL = 0.17)26. The gCPL value of Eu(+tfc)-tmpo(2) was also higher than those of the other Eu(III) lumino-glasses in a comparative study (Supplementary Fig. 7 and Supplementary Table 1). In the comparative compounds, the Eu(+tfc)-L1(2) showed a relatively large gCPL signal. We attributed the large gCPL value of the Eu(III) lumino-glass to the large J-mixing (J: total angular momentum)22 induced by the symmetric coordination geometry with a strong crystal field. In addition, the methoxy group in the ortho-position in the phosphine oxide ligands play an important role in the formation of the characteristic coordination field.
Fig. 4. CPL spectra.
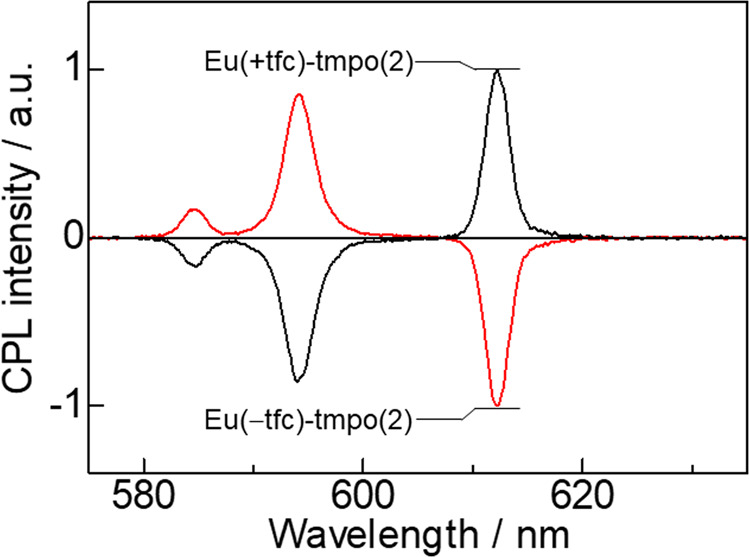
CPL spectra of Eu(+tfc)-tmpo(2) (black line) and Eu(−tfc)-tmpo(2) (red line).
Demonstration of a CPL application
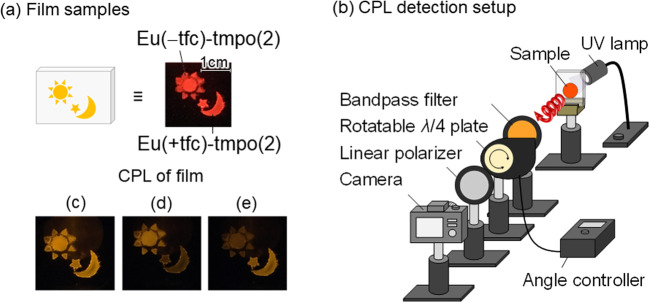
We also demonstrated a CPL paint application using the amorphous Eu(III) lumino-glass. The prepared samples are shown in Fig. 5a. Transparent glasses of Eu(+tfc)-tmpo(2) and its enantiomer (Eu(−tfc)-tmpo(2)) were prepared on a substrate using a casting method (Eu(+tfc)-tmpo(2): sun symbol; Eu(−tfc)-tmpo(2): moon symbol). The experimental setup for visualizing the CPL image is depicted in Fig. 5b. In the setup, the emission of the lumino-glasses is detected using a camera with a linear polarizer, a rotatable λ/4 plate, and a bandpass filter (594 nm). The left- or right-handed polarized light is detected by rotating the λ/4 plate 90° clockwise or anticlockwise using an angle controller. The photographs of the emission of the samples using total light (without the λ/4 plate), left-handed, and right-handed circularly polarized light are shown in Fig. 5c, d, and e, respectively. The total emission from the Eu(+) and Eu(−) lumino-glass is observed in photograph (Fig. 5c). Under clockwise and anticlockwise rotation, the sun and moon shapes of the Eu(+) and Eu(−) lumino-glasses were observed, respectively (photographs Fig. 5d, e). This is the first demonstration of naked-eye detection of a security paint based on the CPL phenomenon using amorphous Eu(III) lumino-glasses.
Fig. 5. CPL detection.
a Photographs and images of the films of Eu(+tfc)-tmpo(2) and Eu(−tfc)-tmpo(2) prepared on glasses under UV-light (λex = 365 nm). b The simple setup for CPL detection. Photographs of the emission (λem = 594 nm) related to c left and right, d left, or e right circularly polarized light from the samples.
To the best of our knowledge, the first lanthanide lumino-glass with an extra-large CPL and a relatively high emission quantum yield was reported. We revealed that symmetric coordination with a strong crystal field is a key factor for the enhancement of gCPL. We also successfully demonstrated a security paint based on the CPL phenomenon using Eu(III) lumino-glass for the first time. Our results provide new insights into the design of Eu(III) complexes with excellent CPL performance and high emission intensity, which should lead to new applications of CPL materials as security inks.
Methods
Materials
Europium(III) acetate n-hydrate, acetone-d6 (99.9%) and 28% ammonia solution were purchased from Wako Pure Chemical Industries Ltd. Tris(2,4,6-trimethylphenyl)phosphine, (+)-3-(trifluoroacetyl)camphor and (−)-3-(trifluoroacetyl)camphor were purchased from Sigma-Aldrich Co. Tris(2,6-dimethoxyphenyl)phosphine, tris(4-methoxyphenyl)phosphine, and tris(4-methylphenyl)phosphine were purchased from Tokyo Chemical Industry Co., Ltd. All other chemicals and solvents were of reagent grade and were used without further purification.
Apparatus
Electrospray ionization mass spectra (ESI-MS) were measured by using a Thermo Scientific Exactive instrument. Elemental analyses were performed on an Exeter Analytical CE440. Emission spectra and emission lifetimes were measured using a Horiba/Jobin-Yvon FluoroLog-3 spectrofluorometer. CPL spectra were measured using a JASCO CPL-300 spectrofluoropolarimeter. DSC measurements were recorded on a SII DSC 7020 heat flux meter. Proton nuclear magnetic resonance (1H NMR) spectra were recorded on an auto-NMR JEOL ECS 400 MHz.
Synthesis of Tris(2,6-dimethoxyphenyl)phosphine oxide
Tris(2,6-dimethoxyphenyl)phosphine (8.84 g, 0.02 mol) was dissolved in dichloromethane (30 mL) in a 100-mL flask, which was cooled in an ice bath. H2O2 solution (5 mL) was added slowly to the solution. The mixture was stirred for 3 h. The product was extracted with dichloromethane and saturated NaCl aqueous solution. The organic layer was dried with anhydrous MgSO4, and the solvent was evaporated. The obtained oil was precipitated with acetone and hexane. The precipitate was filtered and washed with acetone to afford white powder.
Yield: 3.95 g (41%). δ/ppm = 7.23 (t, 3H, J = 8 Hz, Ar), 6.48 (dd, 6H, J = 8 and 4.8 Hz, Ar), 3.51 (s, 18H, CH3). ESI-MS (m/z): [M+H]+ calculated for C24H28O7P, 459.15; found, 459.16. Elemental analysis: Calcd for C24H27O7P: C, 62.88%, H, 5.94%. Found: C, 62.55%, H, 5.88%.
Synthesis of Tris(3-trifluoroacetyl-(+)-camphorato)europium(III)
Europium(III) acetate n-hydrate (0.36 g) was dissolved in distilled water (150 mL). (+)-3-triflouroacetyl camphor (+tfc, 0.50 g, 2.0 mmol) in methanol (20 mL) was added to the solution. A few drops of 28% ammonia solution were added and the mixture was stirred for 3 h at room temperature. The obtained powder was washed with distilled water to afford yellow powder.
Yield: 0.42 g (68%). Elemental analysis: Calcd for C36H46EuF9O8: C, 46.51%, H, 4.99%. Found: C, 46.45%, H, 4.91%.
Synthesis of Tris(3-trifluoroacetyl-(−)-camphorato)europium(III)
[Eu(−tfc)3(H2O)2] was prepared using the same method for [Eu(+tfc)3(H2O)2], starting from (−)-3-triflouroacetyl camphor, yielding yellow powder.
Preparation of Eu(III) lumino-glass
Eu(±tfc)3(H2O)2 (3 mg, 0.003 mmol) and n equivalents of tmpo (1.5 mg, 3.0 mg, or 4.5 mg for n = 1, 2, or 3, respectively) were dissolved in dichloromethane (0.1 mL) (Supplementary Note 1 and Supplementary Fig. 1). The solution was casted onto a glass substrate and allowed to slowly evaporate, yielding a transparent film of the Eu(+tfc)-tmpo(n) (n = 1, 2, 3, n: number of equivalents relative to Eu(+tfc)3). The obtained film was characterized by ESI-MS, XRD (Supplementary Fig. 2), and DSC (Supplementary Fig. 3). ESI mass spectrometry revealed the existence of the Eu(III) complex [Eu(+tfc)2(tmpo)2]+ (cacld: 1563.41, found: 1563.38). The XRD signals of the transparent film were broad, similar to those of typical amorphous organic molecules. In the DSC curve, a characteristic endothermic peak with shoulders indicating a glass transition was observed at ~50 °C (Supplementary Fig. 3). The glass-typed Eu(III) complexes showed bright red luminescence (“Eu(III) lumino-glass”, ex. Eu(+tfc)-tmpo(2), Fig. 2).
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We are particularly grateful for experimental assistance by Professor H. Ito and Assistant Professor T. Seki of Hokkaido University. This work was supported by grant-in-aid for grant numbers 20H02748, 19H04556, 18H04497, 18H02041, 20H04653, and 20H05197. This work was also supported by the Institute for Chemical Reaction Design and Discovery (ICReDD), established by the World Premier International Research Initiative (WPI) of MEXT, Japan. This study was supported in part by grants-in-aids for regional R&D Proposal-Based Program from Northern Advancement Center for Science & Technology of Hokkaido, Japan. S.W. was also supported by The Ministry of Education, Culture, Sports Science and Technology through Program for Leading Graduate Schools (Hokkaido University ‘Ambitious Leader’s Program’).
Author contributions
Y.K. designed research and performed photophysical measurements of Eu(III) complexes. S.W. found the Eu-lumino glass with large CPL property. S.W. and M.D.J.I. performed synthesis of Eu(III) complexes and their measurements. M.G. and K.T. prepared spin-coat films and support CPL measurements. K.S. and S.M. calculated the structure of Eu(III) complex. Y.K., S.W., K.F., and Y.H. wrote the paper. All authors reviewed the manuscript.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yuichi Kitagawa, Satoshi Wada.
Contributor Information
Yuichi Kitagawa, Email: y-kitagawa@eng.hokudai.ac.jp.
Yasuchika Hasegawa, Email: hasegaway@eng.hokudai.ac.jp.
Supplementary information
Supplementary information is available for this paper at 10.1038/s42004-020-00366-1.
References
- 1.Miolo, G., Stair, J. L. & Zloh, M. Light In Forensic Science: Issues And Applications (RSC, 2018).
- 2.Xu S, Chen R, Zheng C, Huang W. Excited state modulation for organic afterglow: materials and applications. Adv. Mater. 2016;28:9920. doi: 10.1002/adma.201602604. [DOI] [PubMed] [Google Scholar]
- 3.Xu S, Duan Y, Liu B. Precise molecular design for high-performance luminogens with aggregation-induced emission. Adv. Mater. 2019;32:1903530. doi: 10.1002/adma.201903530. [DOI] [PubMed] [Google Scholar]
- 4.Liang X, Tu Z-L, Zheng Y-X. Thermally activated delayed fluorescence materials: Towards realization of high efficiency through strategic small molecular design. Chem. Eur. J. 2019;25:5623. doi: 10.1002/chem.201805952. [DOI] [PubMed] [Google Scholar]
- 5.Li L-K, et al. Strategies towards rational design of gold(III) complexes for high-performance organic light-emitting devices. Nat. Photon. 2019;13:185. doi: 10.1038/s41566-018-0332-z. [DOI] [Google Scholar]
- 6.Ma H, Peng Q, An Z, Huang W, Shuai Z. Efficient and long-lived room-temperature organic phosphorescence: Theoretical descriptors for molecular designs. J. Am. Chem. Soc. 2019;141:1010. doi: 10.1021/jacs.8b11224. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Jin M, Xie J, Malval J-P, Wan D. Molecular engineering of UV/Vis light-emitting diode (LED)-sensitive donor-π-acceptor-type sulfonium salt photoacid generators: Design, synthesis, and study of photochemical and photophysical properties. Chem. Eur. J. 2017;23:15783. doi: 10.1002/chem.201703414. [DOI] [PubMed] [Google Scholar]
- 8.Noda T, Ogawa H, Shirota Y. A blue-emitting organic electroluminescent device using a novel emitting amorphous molecular material, 5,5′-Bis(dimesitylboryl)-2,2′-bithiophene. Adv. Mater. 1999;11:283. doi: 10.1002/(SICI)1521-4095(199903)11:4<283::AID-ADMA283>3.0.CO;2-V. [DOI] [Google Scholar]
- 9.Hirata S, et al. Efficient persistent room temperature phosphorescence in organic amorphous materials under ambient conditions. Adv. Funct. Mater. 2013;23:3386. doi: 10.1002/adfm.201203706. [DOI] [Google Scholar]
- 10.Zinna F, et al. Design of lanthanide-based OLEDs with remarkable circularly polarized electroluminescence. Adv. Funct. Mater. 2017;27:1603719. doi: 10.1002/adfm.201603719. [DOI] [Google Scholar]
- 11.Muller G. Luminescent chiral lanthanide(III) complexes as potential molecular probes. Dalton Trans. 2009;44:9692. doi: 10.1039/b909430j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr R, Evans NH, Parker D. Lanthanide complexes as chiral probes exploiting circularly polarized luminescence. Chem. Soc. Rev. 2012;41:7673. doi: 10.1039/c2cs35242g. [DOI] [PubMed] [Google Scholar]
- 13.Zinna F, Di Bari L. Lanthanide circularly polarized luminescence: bases and applications. Chirality. 2015;27:1. doi: 10.1002/chir.22382. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Han J, Wang Y, Duan P. Photon-upconverting chiral liquid crystal: significantly amplified upconverted circularly polarized luminescence. Chem. Sci. 2019;10:172. doi: 10.1039/C8SC03806F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song F, et al. Highly efficient circularly polarized electroluminescence from aggregation-induced emission luminogens with amplified chirality and delayed fluorescence. Adv. Funct. Mater. 2018;28:1800051. doi: 10.1002/adfm.201800051. [DOI] [Google Scholar]
- 16.Sánchez-Carnerero EM, et al. Circularly polarized luminescence from simple organic molecules. Chem. Eur. J. 2015;21:13488. doi: 10.1002/chem.201501178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mason, S. F. Molecular Optical Activity And The Chiral Discriminations (Cambridge University Press, 1982).
- 18.Riehl JP, Richardson FS. Circularly polarized luminescence spectroscopy. Chem. Rev. 1986;86:1. doi: 10.1021/cr00071a001. [DOI] [Google Scholar]
- 19.Brittain HG, Richardson FS. Circularly polarized emission studies on the chiral nuclear magnetic resonance lanthanide shift reagent tris(3-trifluoroacetyl-d-camphorato)europium(III) J. Am. Chem. Soc. 1976;98:5858. doi: 10.1021/ja00435a018. [DOI] [Google Scholar]
- 20.Brittain HG, Richardson FS, Martin RB. Terbium(III) emission as a probe of calcium(II) binding sites in proteins. J. Am. Chem. Soc. 1976;98:8255. doi: 10.1021/ja00441a060. [DOI] [PubMed] [Google Scholar]
- 21.Spaulding L, Brittain HG. Complexation of amino-acids by terbium(III) ethylenediaminetetraacetate. Inorg. Chem. 1985;24:3692. doi: 10.1021/ic00216a044. [DOI] [Google Scholar]
- 22.Wada S, et al. Electronic chirality inversion of lanthanide complex induced by achiral molecules. Sci. Rep. 2018;8:16395. doi: 10.1038/s41598-018-34790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith DG, Pal R, Parker D. Measuring equilibrium bicarbonate concentrations directly in cellular mitochondria and in human serum using europium/terbium emission intensity ratios. Chem. Eur. J. 2012;18:11604. doi: 10.1002/chem.201201738. [DOI] [PubMed] [Google Scholar]
- 24.Lunkley JL, Shirotani D, Yamanari K, Kaizaki S, Muller G. Chiroptical spectra of a series of tetrakis((+)-3-heptafluorobutylrylcamphorato)lanthanide(III) with an encapsulated alkali metal ion: circularly polarized luminescence and absolute chiral structures for the Eu(III) and Sm(III) complexes. Inorg. Chem. 2011;50:12724. doi: 10.1021/ic201851r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar J, Marydasan B, Nakashima T, Kawai T, Yuasa J. Chiral supramolecular polymerization leading to eye differentiable circular polarization in luminescence. Chem. Commun. 2016;52:9885. doi: 10.1039/C6CC05022K. [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa Y, et al. Spiral Eu(III) coordination polymers with circularly polarized luminescence. Chem. Commun. 2018;54:10695. doi: 10.1039/C8CC05147J. [DOI] [PubMed] [Google Scholar]
- 27.Zhang WY, et al. Dramatic impact of the lattice solvent on the dynamic magnetic relaxation of dinuclear dysprosium single-molecule magnets. Inorg. Chem. Front. 2018;5:1575. doi: 10.1039/C8QI00266E. [DOI] [Google Scholar]
- 28.Binnemans K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015;295:1. doi: 10.1016/j.ccr.2015.02.015. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information.