Abstract
Background and aim
For those with a centrally located HCC, the two types of liver sectionectomy that can be performed are extended hepatectomy (EH) and central hepatectomy (CH). This meta-analysis aimed to compare the short- and long-term outcomes between patients treated with CH and patients treated with EH for those with centrally located HCC.
Method
We searched PubMed, Scopus, Web of Science, and Cochrane library for eligible studies from inception to 1 April 2022 and a systematic review and meta-analysis were done to compare the outcomes between the two groups.
Results
we included 9 studies with a total of 1674 patients in this study. The pooled results in this meta-analysis showed equal long-term overall survival, Disease-free survival, recurrence and mortality between the two groups (5-year OS, RR = 1.14, 95% CI = 0.96–1.35, P = 0.12; I2 = 56%), (5-year DFS, RR = 0.81, 95% CI = 0.61–1.08, P = 0.15; I2 = 60%), (Recurrence, RR = 1.04, 95% CI = 0.94–1.15, P = 0.45; I2 = 27%), and (Mortality, RR = 0.55, 95% CI = 0.26–1.15, P = 0.11; I2 = 0%). In addition to that, no significant difference could be detected in the overall incidence of complications between the two groups (Complications, RR = 0.94, 95% CI = 0.76–1.16, P = 0.57; I2 = 0%). However, CH is associated with a remarkable increase in the rate of biliary fistula (Biliary fistula, RR = 1.90, 95% CI = 1.07–3.40, P = 0.03; I2 = 0%). And Liver cell failure was higher in the case of EH (LCF, RR = 0.47, 95% CI = 0.30–0.76, P = 0.002; I2 = 0%). Regarding the operative details, CH is associated with longer operative time (Time of the operation, Mean difference = 0.82, 95% CI = 0.36, 1.27, P = 0.0004; I2 = 57%).
Conclusion
No significant difference in the short and long-term survival and recurrence between CH and MH for CL-HCC. However, CH is associated with greater future remnant liver volume that decreases the incidence of LCF and provides more opportunities for a repeat hepatectomy after tumour recurrence.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12893-022-01891-7.
Keywords: Centrak hepatectomy, Major hepatectomy, Hepatocellular carcinoma, Centrally located HCC
Introduction
Hepatocellular carcinoma (HCC) is the fifth-most common cancer globally and the third-highest cause of cancer-related death exceeded only by cancers of the lung and stomach [1]. It is estimated that 782,000 new cases are diagnosed with HCC annually and 600,000 die of this tumour globally each year [2]. treatment modalities are available for patients with local disease including ablation, liver resection, and liver transplantation (LT). However, for those with respectable tumors and tumours underlying liver disease, liver resection offers the best treatment. [3]
Based on Couinaud’s segmental anatomy of the liver, centrally located HCC is defined as tumours located in the middle part of the liver (segments IV, V, or VIII ± I) [4]. For those with a centrally located HCC, the two types of liver sectionectomy that can be performed are, firstly: a major hepatectomy (MH) or an extended hepatectomy (EH) which includes a right/left hemihepatectomy or right/left trisectionectomy and secondly: a central hepatectomy (CH) which involves a left medial sectionectomy, right anterior sectionectomy, or central bisectionectomy (mesohepatectomy).
On one hand, Traditionally, Hemi- or extended hepatectomy is suggested for the treatment of CL-HCC [5]. However, This modality includes the excision of 60–85% of liver parenchyma [6, 7]. Which in turn increases the risk of postoperative liver failure and is associated with higher mortality and morbidity rates [8, 9]. On the other hand, central hepatectomy allows up to 35% parenchymal sparing compared to EH [10]. However, CH has been associated with biliary fistula [11], significant blood loss [6, 12], a longer operative time [6, 13]. This could be explained by the presence of technical challenges related to the presence of two significant parenchymal transection planes in proximity to the hilar bifurcation.
This meta-analysis aimed to compare the short- and long-term outcomes including overall survival, recurrence rate and complications between patients treated with CH and patients treated with Hemi-/extended hepatectomy for those with centrally located HCC.
Patients and methods
Search strategy
We searched the database including (PubMed, Scopus, the Cochrane Library and Web of Science) from inception to 1 April 2022 using the following search terms: major hepatectomy and Mesohepatectomy or central Hepatectomy and hepatocellular carcinoma. In addition to that Google Scholar was searched to detect the presence of any missing articles. All the studies that met our inclusion criteria were included and the manuscripts were fully reviewed. All the included studies were reviewed by two authors independently (Gadallah, E. A. & Elkomos, B. E.).
Inclusion and exclusion criteria
The eligible studies included the following: (1) randomized controlled trials and prospective or retrospective cohort studies; (2) the target population were patients with hepatocellular carcinoma; (3) studies designed to compare central hepatectomy versus extended hepatectomy for hepatocellular carcinoma; (4) studies providing a sufficient description of the methods and baseline characteristics, and (5) the main outcomes were patient overall survival, disease-free survival for both central and major hepatectomy. The following types of studies were not included in our study: (1) unrelated or in vitro studies; (2) reviews, case reports and case series; (3) patients diagnosed with liver cancers other than hepatocellular carcinoma: (3) studies missing a comparison group.
Outcomes of interest
We assessed overall survival for central and extended hepatectomy for hepatocellular carcinoma as a primary outcome (1, 2, 3, 4, 5-year OS). in addition to that, we assessed 5 secondary outcomes including disease-free survival (1, 2, 3, 4, 5-year DFS), recurrence, early postoperative mortality, complications (liver cell failure, biliary fistula, wound infection and ascites), operative details (the time of the operation, the blood loss during the operation, blood transfusion and hospital stay after operation).
Data extraction
We extracted data on study characteristics (author, year of publication, country of operation, type of study and sample size), patient characteristics (age, sex, child score, virology and cirrhosis), tumour biology (tumour size, tumour number and vascular invasion), operative details (the time of the operation, the blood loss during the operation, blood transfusion, hospital stay after operation and resection margin), Patients outcome (overall survival, disease-free survival, recurrence and mortality) and complications (overall incidence of complications, liver cell failure, biliary fistula and wound infection). The data were extracted by 2 investigators (Gadallah, E. A. & Elkomos, B. E.) independently.
Statistical analysis
Cochrane Handbook of Systematic Reviews of Interventions [14] which is recommended by the Cochrane Collaboration was used as a guide while conducting this meta-analysis. For all the results included, the pooled risk ratios (RRs) and their corresponding 95% confidence intervals (CIs) were calculated with fixed effects models. However, if there was moderate or considerable heterogeneity (I2 > 40), random effects models were used to solve the heterogeneity between studies. Review Manager 5.4 (Cochrane Collaboration, Oxford, United Kingdom) was used for all calculations in this meta-analysis.
Assessment of publication bias and heterogeneity
Funnel plots were generated so that we could visually inspect for publication bias. Statistical heterogeneity was assessed with forest plots and the inconsistency statistic (I2). An I2 value of 40% or less corresponded to low heterogeneity. Statistical significance was considered at P < 0.05.
Results
Characteristics and quality assessment of eligible studies
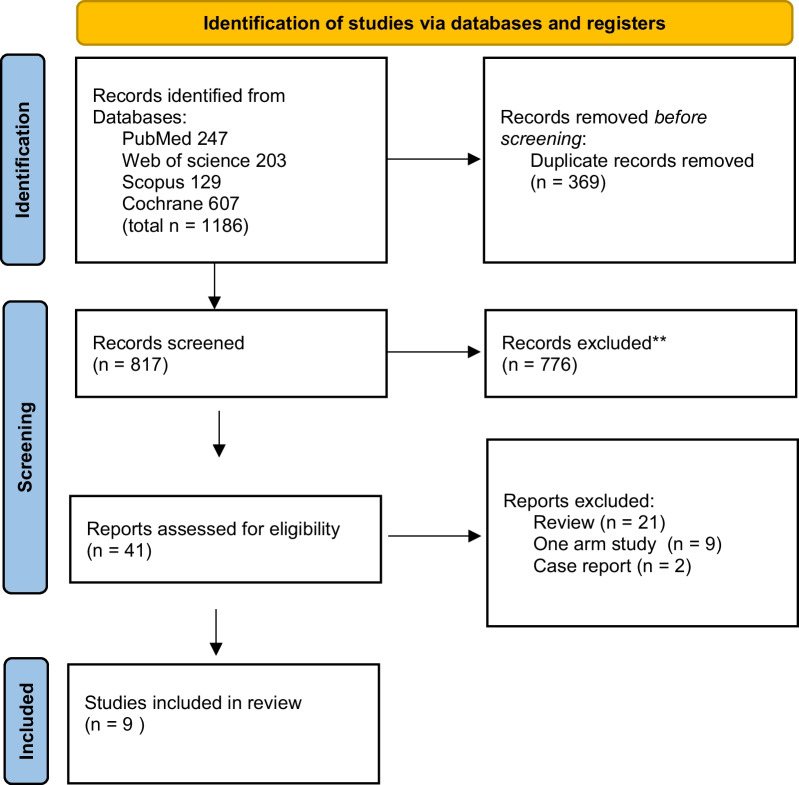
As shown in the flow diagram (Fig. 1),1186 articles were revealed using a combination of the following words: major hepatectomy and Mesohepatectomy or central Hepatectomy and hepatocellular carcinoma. After careful selection based on our eligibility criteria, 9 studies with 1674 patients were included in the meta-analysis. All the included studies were cohort studies. The studies were conducted in four different countries (China, Taiwan, Japan and Mongolia).
Fig. 1.
PRISMA flow diagram
Patients’ characteristics (age, sex, child score, virology and cirrhosis), and tumour biology (tumour size, tumour number and vascular invasion) were comparable between the two groups in all studies (Table 1).
Table 1.
Basic data of the included studies
| Author and Publication year | Country | Study design | Study period | Arm | Sample size (n) | Age (yr) | Gender: M/F(n) | Child score(A/B/C) | Virology (HBV/HCV/Both) | Cirrhosis (n/%) | ICG-R15 (%) | Tumor size (cm) | Tumor number (S/M) | Vascular invasion (%) | Resection margin (< 1 cm/ > 1 cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wu, 1999 [15] | Taiwan | Retrospective Cohort | 1987–1997 | CH | 15 | 53.0 ± 11.6 a | 15/0 | 14/1/0 | 13/2/0 | 11– | N/A | 12.8 | N/A | N/A | N/A |
| EH | 25 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Hu, 2003 [12] | Taiwan | Cohort | 1993–1999 | CH | 52 | N/A | N/A | N/A | 24(HCV) | 24 | 17.4 ± 1.5a | < 9 | 47/5 | 12 | 22/30 |
| EH | 63 | N/A | N/A | N/A | 13(HCV) | 24 | 12.4 ± 2.2a | < 9 | 50/13 | 26 | 16/47 | ||||
| Cheng, 2012 [16] | Taiwan | Cohort | 1999–2005 | CH | 63 | 58(50–66)b | 50/13 | 56/7/0 | 43/13/0 | 30 (47.6%) | 8.57 (5.20–13.53)b | 6.50 (5.50–8.50)b | N/A | 30 (47.6%) | 50//4 |
| EH | 41 | 61(50–68.5)b | 32/9 | 37/3/0 | 27/8/0 | 15 (36.6%) | 8.14 (5.88–14.48)b | 8.00 (5.50–10.25)b | N/A | 19 (46.3%) | 30//8 | ||||
| Chen, 2014 [17] | China | Cohort | 2002–2008 | CH | 118 | 56.4 ± 12.3a | 96/22 | 6.7 ± 1.2 | 100/15/0 | 106 | 5.3 ± 2.0 | 8.6 ± 2.4 | N/A | 30 | 39/79 |
| EH | 80 | N/A | 67/13 | N/A | 69/10/0 | 68 | N/A | N/A | N/A | 24 | 13/67 | ||||
| Yang, 2014 [18] | China | Retrospective Cohort | 2002–2012 | CH | 350 | N/A | 298/52 | 298/52/0 | 315 (HBV) | 281 | N/A | N/A | 161/189 | 195 | 144/206 |
| EH | 346 | 47.5 ± 11.8a | 289/57 | 284/62/0 | 303 (HBV) | 272 | N/A | 8.1 ± 5.3a | 141/205 | 194 | 133/213 | ||||
| Chinburen, 2015 [19] | Mongolia | Retrospective Cohort | 2003–2012 | CH | 45 | 59.8 ± 8.5a | 23/22 | 30/0/0 | 14/21/0 | N/A | N/A | 5.9 ± 2.2a | 37/8 | N/A | N/A |
| EH | 24 | 55.4 ± 9.2 a | 12//12 | 14/1/0 | 8/7//0 | N/A | N/A | 7.2 ± 2.1 a | 21/3 | N/A | N/A | ||||
| Chen, 2017 [20] | Taiwan | Retrospective Cohort | 2007–2010 | CH | 15 | 62 ± 14a | 12//3 | N/A | 10/3//1 | 3 | 8.94 ± 5.08a | 4.71 ± 2.25a | N/A | 5 | N/A |
| MH | 33 | 60 ± 13a | 22/11 | N/A | 18//5//2 | 15 | 8.34 ± 4.31a | 5.28 ± 4.17a | N/A | 15 | N/A | ||||
| Li, 2018 [13] | China | Retrospective Cohort | 200–2016 | CH | 87 | 53.0 ± 7.8a | 63/24 | N/A | 74 (HBV) | 80 | 7.3 ± 1.9a | 4.2 ± 0.9a | N/A | 10 (11.5%) | N/A |
| MH | 84 | 54.4 ± 9.2a | 66/18 | N/A | 70 (HBV) | 67 | 7.3 ± 2.3a | 4.1 ± 1.0 a | N/A | 10 (11.9%) | N/A | ||||
| Orimo, 2021 [21] | Japan | Retrospective Cohort | 200–2019 | CH | 132 | 68 (39–86)b | 112/20 | N/A | 40/39/0 | N/A | 11.6 (2.9–86.7)b | 4.5 (1.2–15.2)b | 91/41 | N/A | N/A |
| MH | 101 | 65 (33–85) b | 85/16 | N/A | 45/22/0 | N/A | 10.8 (1.4–54.0)b | 6.5 (0.6–22.5) b | 65/36 | N/A | N/A |
aThe results are presented as means and standard deviation
bThe results are presented as median and range
Table 2 summarizes the outcomes for CH and EH for HCC.
Table 2.
Outcomes for central and extended hepatectomy for hepatocellular carcinoma
| Outcomes | Studies (n) | Patients (n) | Effect estimate [RR/MD (95% CI)] | Heterogeneity | Test for overall effect | Favour group |
|---|---|---|---|---|---|---|
| Overall survival | ||||||
| 1-year | 8 | 1634 | 1.00 [0.96, 1.04] | I2 = 16% (P = 0.31) | Z = 0.10 (P = 0.92) | None |
| 2-year | 6 | 855 | 1.14 [1.06, 1.23] | I2 = 21% (P = 0.27) | Z = 3.46 (P = 0.0005) | CH |
| 3-year | 7 | 1530 | 1.13 [0.97, 1.33] | I2 = 71% (P = 0.002) | Z = 1.56 (P = 0.12) | None |
| 4-year | 5 | 765 | 1.31 [1.16, 1.48] | I2 = 6% (P = 0.36) | Z = 4.38 (P < 0.0001) | CH |
| 5-year | 7 | 1565 | 1.14 [0.96, 1.35] | I2 = 56% (P = 0.03) | Z = 1.54 (P = 0.12) | None |
| Disease free survival | ||||||
| 1-year | 8 | 1605 | 1.03 [0.92, 1.15] | I2 = 50% (P = 0.05) | Z = 0.49 (P = 0.63) | |
| 2-year | 5 | 765 | 1.04 [0.90, 1.20] | I2 = 29% (P = 0.23) | Z = 0.48 (P = 0.63) | |
| 3-year | 7 | 1447 | 1.19 [0.91, 1.56] | I2 = 74% (P = 0.0007) | Z = 1.26 (P = 0.21) | |
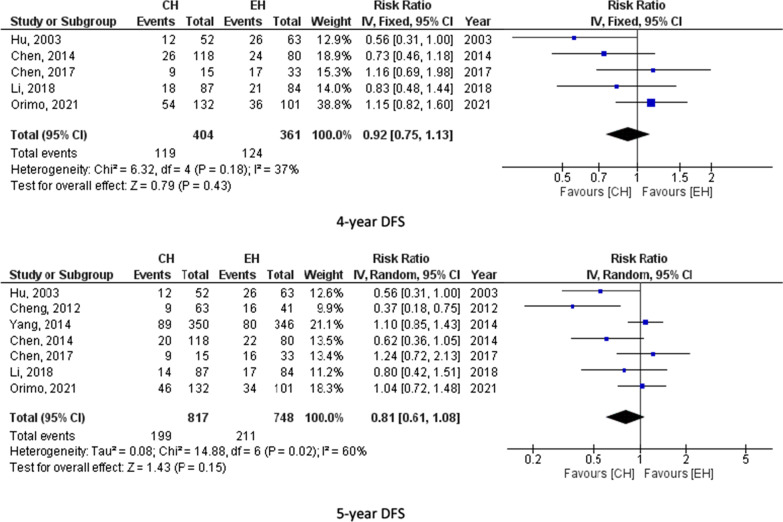
| 4-year | 5 | 765 | 0.92 [0.75, 1.13] | I2 = 37% (P = 0.18) | Z = 0.79 (P = 0.43) | |
| 5-year | 7 | 1565 | 0.81 [0.61, 1.08] | I2 = 60% (P = 0.02) | Z = 1.43 (P = 0.15) | |
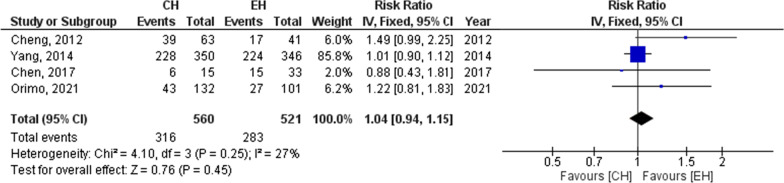
| Recurrence | 4 | 1081 | 1.04 [0.94, 1.15] | I2 = 27% (P = 0.25) | Z = 0.76 (P = 0.45) | |
| Mortality | 8 | 1626 | 0.55 [0.26, 1.15] | I2 = 0% (P = 0.91) | Z = 1.58 (P = 0.11) | |
| Complications | ||||||
| Overall | 7 | 1270 | 0.94 [0.76, 1.16] | I2 = 0% (P = 0.48) | Z = 0.57 (P = 0.57) | |
| Liver cell failure | 6 | 1415 | 0.47 [0.30, 0.76] | I2 = 0% (P = 0.52) | Z = 3.10 (P = 0.002) | CH |
| Biliary fistula | 7 | 1455 | 1.90 [1.07, 3.40] | I2 = 0% (P = 0.85) | Z = 2.18 (P = 0.03) | EH |
| Ascites | 4 | 1084 | 1.95 [1.00, 3.78] | I2 = 0% (P = 0.88) | Z = 1.97 (P = 0.05) | None |
| Wound infection | 5 | 1282 | 0.77 [0.39, 1.52] | I2 = 0% (P = 0.79) | Z = 0.76 (P = 0.44) | |
| Operative details | ||||||
| Time of the operation | 4 | 328 | 0.82 [0.36, 1.27] | I2 = 57% (P = 0.07) | Z = 3.53 (P = 0.0004) | |
| Blood loss | 3 | 280 | 40.87 [− 8.81, 90.54] | I2 = 13% (P = 0.32) | Z = 1.61 (P = 0.11) | |
| blood transfusion | 3 | 224 | 269.54 [− 169.28, 708.35] | I2 = 78% (P = 0.01) | Z = 1.20 (P = 0.23) | |
| Hospital stay | 3 | 280 | − 2.17 [− 5.56, 1.22] | I2 = 83% (P = 0.003) | Z = 1.25 (P = 0.21) |
Primary outcome
Overall survival
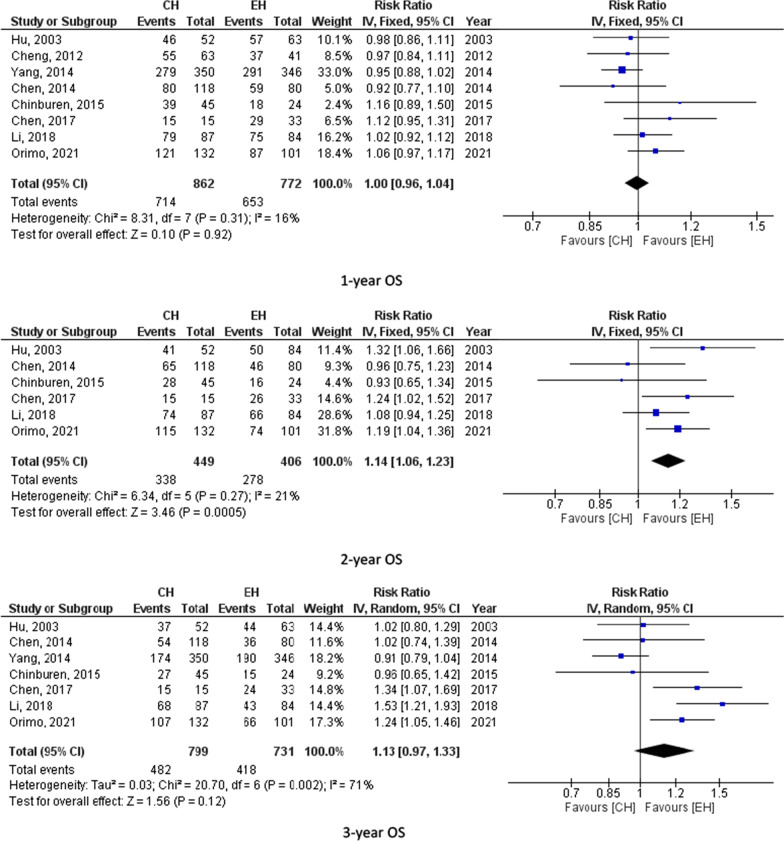
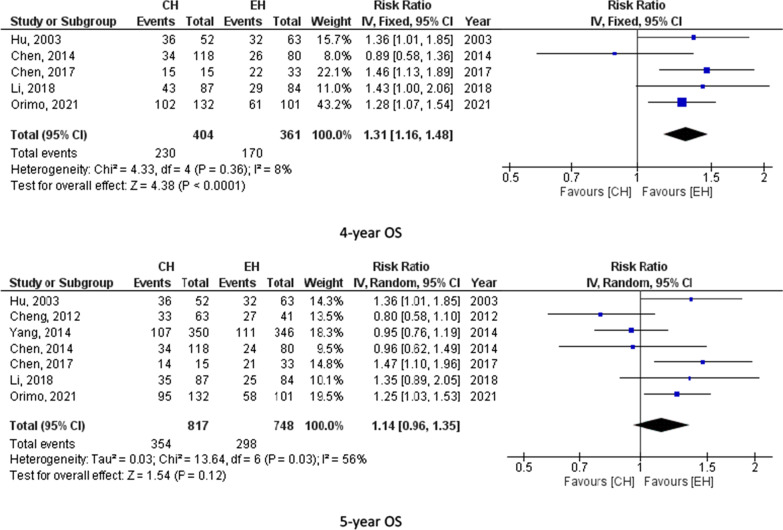
Eight studies (1634 participants) assessed 1-year OS, 7 studies (1530) reported 3-year OS and 7 studies (1565) calculated 5 year-OS. The pooled results from these studies showed equal overall survival for those who underwent central hepatectomy and extended hepatectomy as follows (1-year OS, RR = 1.00, 95% CI = 0.96–1.04, P = 0.92; I2 = 16%), (3-year OS, RR = 1.13, 95% CI = 0.97–1.33, P = 0.12; I2 = 71%) and (5-year OS, RR = 1.14, 95% CI = 0.96–1.35, P = 0.12; I2 = 56%). 5-year OS was 43.3% for CH and 39.8% for EH. However, the pooled results for the 2 and 4-year overall survival showed possible improvement in the overall survival for those who underwent extended hepatectomy (2-year OS, RR = 1.14, 95% CI = 1.06–1.23, P = 0.0005; I2 = 21%) and (4-year OS, RR = 1.31, 95% CI = 1.16–1.48, P < 0.0001; I2 = 8%) as shown in 6 studies (855) for 2-year OS and 5 studies (765) for the 4-year OS. 4-year OS was 56.9% for CH and 47% for EH. Figure 2 summarizes 1-, 2-, 3-, 4-and 5-year OS for CH and EH recipients.
Fig. 2.
overall survival for CH and EH
Secondary outcomes
Disease free survival
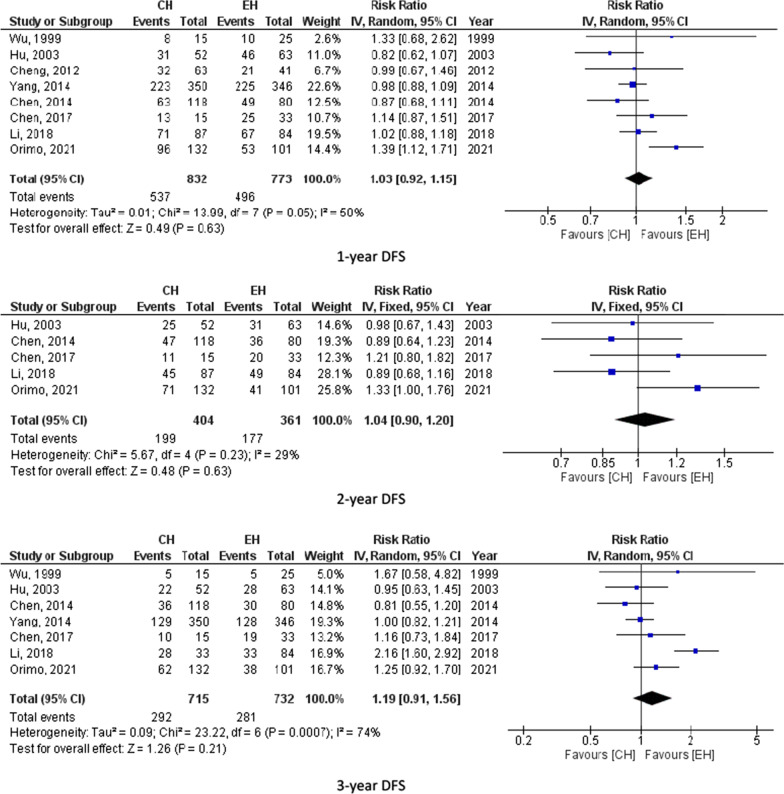
Eight studies (1605 participants) reported 1-year DFS, 5 studies (765 participants) assessed 2-year DFS, 7 studies (1447 participants) reported 3-year DFS, 5 studies (765 participants) calculated 4-year DFS and 7 studies (1565 participants) assessed 5-year DFS. The pooled results from these studies showed no significant difference between CH and EH. For instance, 5-year DFS was 24.4% for CH and 28.2% for EH. Figure 3 summarizes 1-, 2-, 3-, 4- and 5-year DFS for CH and EH recipients.
Fig. 3.
Disease free survival for CH and EH
Recurrence
Regarding the recurrence, after hepatectomy as reported by 4 studies (1081 patients), no significant difference could be detected between the groups (Recurrence, RR = 1.04, 95% CI = 0.94–1.15, P = 0.45; I2 = 27%) Fig. 4. According to the pooled results of these studies, the recurrence was 56.4% for those who underwent CH and 54.3% for those who underwent EH.
Fig. 4.
Recurrence for CH and EH
Mortality
In addition to that, as reported by 8 studies (1626), the early post-operative mortality during the first three months after surgery was nearly equal for the two modalities. It was an average of 2% for both groups. (Mortality, RR = 0.55, 95% CI = 0.26–1.15, P = 0.11; I2 = 0%) Additional file 1: Fig. S1.
Complications
Turning to post-operative complications, no remarkable difference in the total incidence of postoperative complications in the two groups. As reported by 7 studies (1270 patients), the pooled results showed a 19.9% complication rate for CH and 19.8% for EH. (Complications, RR = 0.94, 95% CI = 0.76–1.16, P = 0.57; I2 = 0%) Additional file 2: Fig. S2.
Liver cell failure
On one hand, liver cell failure was calculated for the two groups in 6 studies (1415 participants) and its incidence was higher in the EH group 5.3% in comparison to 3% in the CH group. (LCF, RR = 0.47, 95% CI = 0.30–0.76, P = 0.002; I2 = 0%) Additional file 3: Fig. S3.
Biliary fistula
On the other hand, the biliary fistula was reported in 7 studies (1455 patients) and the rate was higher in CH group 5% in comparison to 2.5% for EH group. (Biliary fistula, RR = 1.90, 95% CI = 1.07–3.40, P = 0.03; I2 = 0%) Additional file 4: Fig. S4.
Ascites
According to 4 studies (1084 participants), no significant difference could be detected in the rate of post-operative ascites in the two groups. (Ascites, RR = 1.95, 95% CI = 1.00–3.78, P = 0.05; I2 = 0%) Additional file 5: Fig. S5.
Wound infection
Moreover, as reported by 5 studies (1282 patients), no remarkable difference in the incidence of wound infection for the two modalities. (Wound infection, RR = 0.77, 95% CI = 0.39–1.52, P = 0.44; I2 = 0%) Additional file 6: Fig. S6.
Operative details
Time of operation
Turning to the duration of the operation, according to 4 studies (328 patients), the average time for surgery was longer in CH than in EH. (Time of the operation, Mean difference = 0.82, 95% CI = 0.36, 1.27, P = 0.0004; I2 = 57%). Additional file 7: Fig. S7.
Blood loss during operation
According to three studies (280 participants), no remarkable difference could be detected in the blood loss during the operation for both methods (Blood loss, Mean difference = 40.87, 95% CI = − 8.81, 90.54, P = 0.11; I2 = 13%). Additional file 8: Fig. S8.
Blood transfusion
In addition to that, as reported by three of the included studies (224 participants), the average amount of blood transfusion was similar for the two groups. (Blood transfusion, Mean difference = 269.54, 95% CI = − 169.28, 708.35, P = 0.78; I2 = 23%). Additional file 9: Fig. S9.
Hospital stay
In addition to that, according to the pooled results from three studies (280 participants), the two groups had the equal time of hospitalization. (Hospital stay, Mean difference = − 2.17, 95% CI = − 5.56, 1.22, P = 0.21; I2 = 83%). Additional file 10: Fig. S10.
Publication bias assessment
The funnel plot analysis demonstrated a symmetrical appearance. However, it was not reliable because only nine studies were included.
Discussion
Hepatocellular carcinoma (HCC) is the primary liver cancer derived from hepatocytes and accounts for 85–90% of all primary liver cancers [2]. Liver resection, ablation and transplantation are the curable treatment options for this tumour and according to BCLC treatment recommendations for HCC, liver resection offers the best treatment for HCC without underlying liver disease [3]. The two types of liver resection that can be performed for centrally located HCC are major hepatectomy (MH) or extended hepatectomy (EH) and central hepatectomy (CH) which was first performed by McBride and Wallace 1972 as a treatment for gall bladder cancer and intended as en bloc excision of the Couinaud’s segments 4, 5, 8 ± 1.
To begin with overall survival after hepatectomy, according to a recent systematic review, the 5-year OS after hepatectomy for HCC ranged from 30% to 61.4% [22]. On one hand, some studies reported better overall survival for those who underwent central hepatectomy [20, 21]. This has been explained by the increased liver volume preservation which might be associated with favourable OS [20] and as reported by Lee SY, the 5-year OS for those who underwent CH for HCC ranged from 31.7% to 66.8% [7]. However, on the other hand, other studies said that the overall survival was equal between the two modalities [17, 19]. In our meta-analysis a trivial improvement could be detected in the 2- and 4-years OS for those who underwent CH. However, no significant difference could be detected in the 5-year OS between the two modalities with a 5-year OS of 43.3% for CH and 39.8% for EH. In addition to that, DFS was similar in the two groups.
Although the early postoperative mortality rate of liver resection has been reduced to a few per cent in recent case series, its overall morbidity rate is reported to range from 4.1% to 47.7% [23, 24]. The causes for early post-operative mortality are haemorrhage, liver failure leading to ascites and hepatic encephalopathy, pulmonary infection/ pleural effusion/empyema, urinary tract infection, sepsis, upper gastrointestinal bleeding, renal failure, stroke, deep vein thrombosis, wound infection, intra-abdominal abscess and intestinal perforation. In our study, the incidence of this mortality was similar in the two groups.
Regarding the recurrence rate of HCC, as reported by some studies, central hepatectomy increases the chance of a future repeat resection. [25, 26] However, according to the pooled results from the included studies, no remarkable difference could be detected in the recurrence rate between the two types of liver resection. In addition to that as reported by Orimo et al. [21]. hepatectomy that was more in the CH group not because liver recurrence was more common in the CH group, but because the sufficient remnant liver that was preserved in the CH group could be removed after recurrence.
Post-liver resection complications tend to be severe and the risk factors for complications after liver resection depend on the pathological background of the liver itself [27]. These complications include liver cell failure, biliary fistula, ascites, surgical site infection, pneumonia and respiratory distress. Our meta-analysis showed that the overall incidence of complications was comparable between the two modalities.
To begin with liver cell failure, is the most serious complication after liver resection and can be life-threatening [28]. with estimated mortality ranging between 60 and 80% depending on the cause and the experience of the clinical department to which the patient is referred [29, 30]. And as reported by Van Den Broek et al., the incidence of post-resection liver cell failure after partial hepatic resection ranges from 0.7 to 9.1% and the key events in the pathogenesis are inadequate quantity or quality of residual liver mass [31]. According to the pooled results of the included studies, the incidence of postoperative liver cell failure was significantly higher in those who underwent major hepatectomy. This could be attributed to the fact that major hepatectomy is associated with the removal of 60–85% of liver parenchyma. [6, 7]
Regarding bile leakage after liver resection, it is one of the most frequently reported intra-abdominal complications [32]. And according to the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP), the incidence of biliary leakage after liver resection has been reported approximately 7% [33, 34]. In our study, a higher incidence of the biliary fistula was detected for those who underwent central hepatectomy. This has been explained by the presence of two transection planes and exposure of the hepatic hilum [35].
Ascites, which means pathological accumulation of fluid within the abdominal cavity and the word “ascites” is derived from the Greek word “asks,” which means a bag or sack [36], is a common complication in patients who exhibit liver dysfunction or cirrhosis after liver resection [37]. This complication has been explained by the increase in portal flow resistance at the sinusoidal level due to a reduction in the volume of the portal vascular bed [38]. And the acute phase after liver resection causes oedema in the interstitial organ space, which leads to increased portal flow resistance [37]. According to the pooled results of included studies, no significant difference could be detected in the rate of post-operative ascites in the two groups.
surgical site infections are common after all types of surgery and are classified into superficial, deep incisional, and organ/space surgical site infections [39]. According to the CDC, SSIs are infections that occur within 30 days of surgery or one year if an implant is present [40]. In our study, no difference in the incidence of SSI could be detected between the two types of liver resection.
Turning to the operative details, many studies reported that central hepatectomy is associated with greater operative blood loss and the need for operative blood transfusion [6, 12] and this was explained by technical complexity, which is the result from the presence of two parenchymal transection planes in proximity to the hilar bifurcation [5, 15]. As a result, it requires challenging handling of the right hepatic vein exposed along the right section plane, middle hepatic vein at its distal end, biliary confluence, and first- and second-order portal pedicles. However, the pooled results showed no remarkable difference between CH and MH in terms of blood loss and blood transfusion during the operation. It is worth mentioning that the operative time was longer in the case of central hepatectomy and as we mentioned earlier, this is because of the technical complexity that is associated with central hepatectomy.
To our knowledge it is the largest meta-analysis to compare the two types of resection for HCC as all the studies that were comparing the outcomes between the two modalities were included. However, we have to admit that all the included studies were cohort studies which are considered a limitation in our study because no randomized controlled trials could be found.
Conclusion
This study showed no significant difference in the short and long-term survival and recurrence between CH and MH for CL-HCC. However, CH is associated with greater future remnant liver volume that decreases the incidence of LCF and provides more opportunities for a repeat hepatectomy after tumour recurrence.
Supplementary Information
Additional file 1: Fig. S1. Mortality for CH and EH.
Additional file 2: Fig. S2. Post-operative complications for CH and EH.
Additional file 3: Fig. S3. Liver cell failure for CH and EH.
Additional file 4: Fig. S4. Biliary fistula for CH and EH.
Additional file 5: Fig. S5. Ascites for CH and EH.
Additional file 6: Fig. S6. Wound infection for CH and EH.
Additional file 7. Fig. S7. Operative time for CH and EH.
Additional file 8. Fig. S8. Blood loss for CH and EH.
Additional file 9. Fig. S9. Blood transfusion for CH and EH.
Additional file 10: Fig. S10. Hospital stay for CH and EH.
Acknowledgements
This research would not have been possible without the exceptional support and effort of our supervisor, Prof. Dr Amr Abdelaal. Prof Dr Ahmed Khalil, Dr Fawzi Salah.
Author contributions
EAG: Data gathering and data extraction. BEE: Data gathering, data extraction. Figure 1 and all tables. AK: Done the statistical analysis of the meta-analysis of all figures except No. 1. AA: writing the manuscript. FSF: helped write the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No fund was taken from any organization to conduct this meta-analysis.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional files (all the studies that were included in this meta-analysis are included in Additional files).
Declarations
Ethics approval and consent to participate
The meta-analysis does not collect deeply personal, sensitive or confidential information from participants, it collects the data from other studies that already have their ethical approval and consent. 'Not applicable'.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Edward Atef Gadallah, Email: edward.attef@med.asu.edu.eg.
Beshoy Effat Elkomos, Email: Beshoyafet0100304@med.asu.edu.eg, Email: beshoy3ft@gmail.com.
Ahmed Khalil, Email: ahmed_abdelrazek@med.asu.edu.eg.
Fawzy Salah fawzy, Email: fawzi.salah@yahoo.com.
Amr Abdelaal, Email: aaabdelaal@med.asu.edu.eg.
References
- 1.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Clin Liver Dis. 2019;13(1):1. doi: 10.1002/cld.802. [DOI] [PubMed] [Google Scholar]
- 2.Ozakyol A. Global epidemiology of hepatocellular carcinoma (HCC epidemiology) J Gastrointest Cancer. 2017;48(3):238–240. doi: 10.1007/s12029-017-9959-0. [DOI] [PubMed] [Google Scholar]
- 3.Reig M, Forner A, Rimola J, Ferrer-Fábrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2021. [DOI] [PMC free article] [PubMed]
- 4.Yu W, Wang W, Rong W, Wang L, Xu Q, Wu F, et al. Adjuvant radiotherapy in centrally located hepatocellular carcinomas after hepatectomy with narrow margin (< 1 cm): a prospective randomized study. J Am Coll Surg. 2014;218(3):381–392. doi: 10.1016/j.jamcollsurg.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Scudamore CH, Buczkowski AK, Shayan H, Ho SG, Legiehn GM, Chung SW, et al. Mesohepatectomy. Am J Surg. 2000;179(5):356–360. doi: 10.1016/S0002-9610(00)00374-3. [DOI] [PubMed] [Google Scholar]
- 6.Qiu J, Wu H, Bai Y, Xu Y, Zhou J, Yuan H, et al. Mesohepatectomy for centrally located liver tumours. J Br Surg. 2013;100(12):1620–1626. doi: 10.1002/bjs.9286. [DOI] [PubMed] [Google Scholar]
- 7.Lee SY. Central hepatectomy for centrally located malignant liver tumors: a systematic review. World J Hepatol. 2014;6(5):347. doi: 10.4254/wjh.v6.i5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo CH, Qiu XX, Ouyang YZ, Zhang D, Xiao H, Mo SC, et al. Mesohepatectomy for the treatment of patients with centrally located hepatocellular carcinoma. Mol Clin Oncol. 2014;2(5):833–838. doi: 10.3892/mco.2014.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machado MA, Kalil AN. Glissonian approach for laparoscopic mesohepatectomy. Surg Endosc. 2011;25(6):2020–2022. doi: 10.1007/s00464-010-1483-2. [DOI] [PubMed] [Google Scholar]
- 10.Mehrabi A, Mood Z, Roshanaei N, Fonouni H, Müller SA, Schmied BM, et al. Mesohepatectomy as an option for the treatment of central liver tumors. J Am Coll Surg. 2008;207(4):499–509. doi: 10.1016/j.jamcollsurg.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita YI, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K, Shimada M, et al. Bile leakage after hepatic resection. Ann Surg. 2001;233(1):45. doi: 10.1097/00000658-200101000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu RH, Lee PH, Chang YC, Ho MC, Yu SC. Treatment of centrally located hepatocellular carcinoma with central hepatectomy. Surgery. 2003;133(3):251–256. doi: 10.1067/msy.2003.102. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Li L, Minigalin D, Wu H. Anatomic mesohepatectomy versus extended hepatectomy for patients with centrally located hepatocellular carcinoma. HPB. 2018;20(6):530–537. doi: 10.1016/j.hpb.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. New York: Wiley; 2019. [Google Scholar]
- 15.Wu CC, Ho WL, Chen JT, Tang CS, Yeh DC, Liu TJ, et al. Mesohepatectomy for centrally located hepatocellular carcinoma: an appraisal of a rare procedure. J Am Coll Surg. 1999;188(5):508–515. doi: 10.1016/S1072-7515(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 16.Cheng CH, Yu MC, Wu TH, Lee CF, Chan KM, Chou HS, et al. Surgical resection of centrally located large hepatocellular carcinoma. Chang Gung Med J. 2012;35(2):178–191. doi: 10.4103/2319-4170.106153. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Li B, He W, Wei YG, Du ZG, Jiang L. Mesohepatectomy versus extended hemihepatectomy for centrally located hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2014;13(3):264–270. doi: 10.1016/S1499-3872(14)60253-8. [DOI] [PubMed] [Google Scholar]
- 18.Yang LY, Chang RM, Lau WY, Ou DP, Wu W, Zeng ZJ. Mesohepatectomy for centrally located large hepatocellular carcinoma: indications, techniques, and outcomes. Surgery. 2014;156(5):1177–1187. doi: 10.1016/j.surg.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Chinburen J, Gillet M, Yamamoto M, Enkh-Amgalan T, Taivanbaatar E, Enkhbold C, et al. Impact of Glissonean pedicle approach for centrally located hepatocellular carcinoma in mongolia. Int Surg. 2015;100(2):268–274. doi: 10.9738/INTSURG-D-14-00006.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CH, Huang TH, Chang CC, Li WF, Lin TL, Wang CC. Central hepatectomy still plays an important role in treatment of early-stage centrally located hepatocellular carcinoma. World J Surg. 2017;41(11):2830–2837. doi: 10.1007/s00268-017-4096-2. [DOI] [PubMed] [Google Scholar]
- 21.Orimo T, Kamiyama T, Kakisaka T, Shimada S, Nagatsu A, Asahi Y, et al. Central hepatectomy versus major hepatectomy for centrally located hepatocellular carcinoma: a propensity score matching study. Ann Surg Oncol. 2021;28(11):6769–6779. doi: 10.1245/s10434-021-09751-z. [DOI] [PubMed] [Google Scholar]
- 22.Xie QS, Chen ZX, Zhao YJ, Gu H, Geng XP, Liu FB. Systematic review of outcomes and meta-analysis of risk factors for prognosis after liver resection for hepatocellular carcinoma without cirrhosis. Asian J Surg. 2021;44(1):36–45. doi: 10.1016/j.asjsur.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Mizuguchi T, Kawamoto M, Meguro M, Shibata T, Nakamura Y, Kimura Y, et al. Laparoscopic hepatectomy: a systematic review, meta-analysis, and power analysis. Surg Today. [DOI] [PubMed]
- 24.Spolverato G, Ejaz A, Hyder O, Kim Y, Pawlik TM. Failure to rescue as a source of variation in hospital mortality after hepatic surgery. Br J Surg. 2014;101:836–846. doi: 10.1002/bjs.9492. [DOI] [PubMed] [Google Scholar]
- 25.Wu CC, Yeh DC, Ho WM, Yu CL, Cheng SB, Liu TJ, et al. Occlusion of hepatic blood inflow for complex central liver resections in cirrhotic patients: a randomized comparison of hemihepatic and total hepatic occlusion techniques. Arch Surg. 2002;137(12):1369–1376. doi: 10.1001/archsurg.137.12.1369. [DOI] [PubMed] [Google Scholar]
- 26.Chouillard E, Cherqui D, Tayar C, Brunetti F, Fagniez PL. Anatomical bi-and trisegmentectomies as alternatives to extensive liver resections. Ann Surg. 2003;238(1):2. doi: 10.1097/01.sla.0000075058.37052.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuguchi T, Nagayama M, Meguro M, Shibata T, Kaji S, Nobuoka T, et al. Prognostic impact of surgical complications and preoperative serum hepatocyte growth factor in hepatocellular carcinoma patients after initial hepatectomy. J Gastrointest Surg. 2009;13(2):325–333. doi: 10.1007/s11605-008-0711-8. [DOI] [PubMed] [Google Scholar]
- 28.Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369(26):2525–2534. doi: 10.1056/NEJMra1208937. [DOI] [PubMed] [Google Scholar]
- 29.Katoonizadeh A, Decaestecker J, Wilmer A, Aerts R, Verslype C, Vansteenbergen W, et al. MELD score to predict outcome in adult patients with non-acetaminophen-induced acute liver failure. Liver Int. 2007;27(3):329–334. doi: 10.1111/j.1478-3231.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- 30.Kremers WK, van Ijperen M, Kim WR, et al. MELD score as a predictor of pretransplant and posttransplant survival in OPTN/UNOS status 1 patients. Hepatology. 2004;39:764–769. doi: 10.1002/hep.20083. [DOI] [PubMed] [Google Scholar]
- 31.Van Den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malagó M, Jalan R, Saner FH. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28(6):767–780. doi: 10.1111/j.1478-3231.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 32.Ishii H, Ochiai T, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Nakanishi M, Ichikawa D, Fujiwara H, Okamoto K. Risk factors and management of postoperative bile leakage after hepatectomy without bilioenteric anastomosis. Digest Surg. 2011;28(3):198–204. doi: 10.1159/000324042. [DOI] [PubMed] [Google Scholar]
- 33.Brauer DG, Nywening TM, Jaques DP, Doyle MM, Chapman WC, Fields RC, et al. Operative site drainage after hepatectomy: a propensity score matched analysis using the American College of Surgeons NSQIP targeted hepatectomy database. J Am Coll Surg. 2016;223(6):774–783. doi: 10.1016/j.jamcollsurg.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spolverato G, Ejaz A, Kim Y, Hall BL, Bilimoria K, Cohen M, et al. Patterns of care among patients undergoing hepatic resection: a query of the National Surgical Quality Improvement Program-targeted hepatectomy database. J Surg Res. 2015;196(2):221–228. doi: 10.1016/j.jss.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Ishii H, Ochiai T, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, et al. Risk factors and management of postoperative bile leakage after hepatectomy without bilioenteric anastomosis. Dig Surg. 2011;28(3):198–204. doi: 10.1159/000324042. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds TB. Hepatology: a century of progress. Ascites Clin Liver Dis. 2000;4:151–168. doi: 10.1016/S1089-3261(05)70101-X. [DOI] [PubMed] [Google Scholar]
- 37.Senousy BE, Draganov PV. Evaluation and management of patients with refractory ascites. World J Gastroenterol WJG. 2009;15(1):67. doi: 10.3748/wjg.15.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salerno F, Guevara M, Bernardi M, Moreau R, Wong F, Angeli P, et al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int. 2010;30(7):937–947. doi: 10.1111/j.1478-3231.2010.02272.x. [DOI] [PubMed] [Google Scholar]
- 39.Wilson AP. Postoperative surveillance, registration and classification of wound infection in cardiac surgery—experiences from Great Britain. APMIS. 2007;115(9):996–1000. doi: 10.1111/j.1600-0463.2007.00831.x. [DOI] [PubMed] [Google Scholar]
- 40.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606–608. doi: 10.2307/30148464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Mortality for CH and EH.
Additional file 2: Fig. S2. Post-operative complications for CH and EH.
Additional file 3: Fig. S3. Liver cell failure for CH and EH.
Additional file 4: Fig. S4. Biliary fistula for CH and EH.
Additional file 5: Fig. S5. Ascites for CH and EH.
Additional file 6: Fig. S6. Wound infection for CH and EH.
Additional file 7. Fig. S7. Operative time for CH and EH.
Additional file 8. Fig. S8. Blood loss for CH and EH.
Additional file 9. Fig. S9. Blood transfusion for CH and EH.
Additional file 10: Fig. S10. Hospital stay for CH and EH.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Additional files (all the studies that were included in this meta-analysis are included in Additional files).