Abstract
The purpose of the present study was to evaluate the effectiveness of an attenuated Salmonella enterica serovar Typhimurium vaccine strain expressing the saliva-binding region (SBR) of the Streptococcus mutans antigen I/II adhesin, either alone or linked with the mucosal adjuvant cholera toxin A2 and B subunits (CTA2/B) and under the control of the anaerobically inducible nirB promoter, in inducing a protective immune response against S. mutans infection. BALB/c mice were immunized by either the intranasal or the intragastric route with a single dose of 109 or 1010 Salmonella CFU, respectively. The Salmonella vaccine strain expressing an unrelated antigen (fragment C of tetanus toxin [TetC]) was also used for immunization as a control. Samples of serum and secretion (saliva and vaginal washes) were collected prior to and following immunization and assessed for antibody activity by enzyme-linked immunosorbent assay. Anti-SBR antibodies were detected in the serum and saliva of experimental animals by week 3 after immunization. A booster immunization at week 17 after the initial immunization resulted in enhanced immune responses to the SBR. The serum immunoglobulin G subclass profiles were indicative of T helper type 1 responses against both the vector and the SBR antigen. To determine the effectiveness of these responses on the protection against S. mutans infection, mice were challenged after the second immunization with a virulent strain of S. mutans which was resistant to tetracycline and erythromycin. Prior to the challenge, mice were treated for 5 days with tetracycline, erythromycin, and penicillin. S. mutans was initially recovered from all of the challenged mice. This bacterium persisted at high levels for at least 5 weeks in control TetC-immunized or nonimmunized mice despite the reappearance of indigenous oral organisms. However, mice immunized with Salmonella clones expressing SBR or SBR-CTA2/B demonstrated a significant reduction in the number of S. mutans present in plaque compared to the control groups. These results provide evidence for the effectiveness of the Salmonella vector in delivering the SBR antigen for the induction of mucosal and systemic immune responses to SBR. Furthermore, the induction of a salivary anti-SBR response corresponded with protection against S. mutans colonization of tooth surfaces.
Streptococcus mutans is the principal etiologic agent of human dental caries (17). The pathogenesis of this oral disease involves several steps, including attachment of this bacterium to the tooth surface and the demineralization of tooth surfaces caused by organic acids produced by microbial fermentation of dietary sugars (17, 19). Although caries is not a life-threatening disease, it is among the most prevalent and costly diseases in both developing and industrialized countries, and the development of a safe and effective vaccine is viewed as a beneficial preventive measure (for a review, see reference 8). The tropism of S. mutans for the saliva-coated tooth surfaces depends on the presence of the saliva-binding region (SBR) of antigen I/II (Ag I/II) located on the surface of this bacterium (30). Furthermore, the ability of this bacterium to synthesize water-insoluble glucan from sucrose via glucosyltransferases contributes to the formation of dental plaque (14, 26, 35).
The SBR is localized within the N-terminal one-third of AgI/II (4, 7). Human secretory immunoglobulin A (IgA) antibodies to the whole AgI/II molecule, as well as rabbit IgG antibodies to an AgI/II segment which contains the SBR, inhibit the adherence of S. mutans to saliva-coated hydroxyapatite (9, 36). The postulated involvement of the SBR in S. mutans colonization suggests that it is a reasonable immunogen for use in a caries vaccine. Our group has previously evaluated the 42-kDa SBR in soluble form in a caries immunization study (10). Specifically, intranasal (i.n.) immunization of rats with SBR genetically linked to the A2 and B subunits of cholera toxin (CT) and in the presence of adjuvant amounts of CT induced moderate protective immunity against S. mutans infection and caries formation (10).
Evidence from our group and others has shown that secretory IgA antibodies provide a major defense against microbial infection at mucosal surfaces, including the oral cavity (23). These antibodies are induced following immunization via a mucosal route. Vaccines administered via mucosal routes can induce not only mucosal responses via the common mucosal immune system but also systemic immune responses (20, 21). However, most soluble proteins are poor mucosal immunogens and may result in mucosal tolerance when given orally (22). To overcome this limitation of oral vaccination and the requirement for purification of the vaccine protein, we used an attenuated Salmonella enterica serovar Typhimurium vector expressing SBR, or SBR linked to A2/B subunits of CT, i.e., SBR-CTA2/B, under the control of T7 promoter, to immunize mice via mucosal routes (11). Salivary IgA antibodies against SBR were induced in BALB/c mice after mucosal immunizations with these Salmonella clones; however, we also observed hyperexpression of the protein which was associated with reduced viability of the vector (11). We have recently expressed the SBR and the SBR-CTA2/B in attenuated serovar Typhimurium under the control of the anaerobically inducible nirB promoter (13). We found that these vectors were able to colonize the nasal-associated lymphoid tissue (NALT) and gut-associated lymphoid tissue (GALT) for at least three weeks, during which time they expressed the immunogens (13). This finding is in agreement with previous reports on the use of the nirB promoter (2).
The objective of this study was to assess the ability of the attenuated serovar Typhimurium strains expressing SBR alone or SBR linked to the mucosal adjuvant CTA2/B, and under the control of nirB promoter, to induce specific mucosal and systemic immune responses against SBR when given by the i.n. or intragastric (i.g.) route. We also evaluated the effect of inducing a salivary IgA anti-SBR response on the colonization of murine tooth surfaces by S. mutans.
MATERIALS AND METHODS
Preparation of recombinant Salmonella clones for immunization.
Small freezer stocks of serovar Typhimurium BRD509(pSBRnirB), BRD509(pSBR-CTA2/BnirB), and BRD509(pTETnir15) were used to inoculate Luria-Bertani (LB) broth supplemented with 50 μg of carbenicillin per ml. The cultures were grown overnight at 37°C under aerobic conditions with shaking to prevent premature induction of protein expression. These cultures were used to inoculate 1 liter of LB broth in screw-cap glass bottles. The caps were closed tightly, and the cultures were grown overnight under anaerobic conditions at 30°C without shaking.
The bacteria were harvested by centrifugation. The cell pellets were suspended in sterile phosphate-buffered saline (PBS) for i.n. immunization or in a medium consisting of four parts Hank's balanced salt solution (Life Technologies, Inc., Grand Island, N.Y.) and one part 7.5% sodium bicarbonate (Life Technologies, Inc.) (intubation medium) to neutralize the stomach acids (11) for i.g. immunization. The concentration of the cell suspensions was adjusted so that a dose for i.n. immunization contained 109 CFU in 20 μl and a dose for i.g. immunization was 1010 CFU in 0.25 ml.
Immunizations.
Groups of five or six female BALB/c mice, 10 to 12 weeks old, were immunized once with the appropriate serovar Typhimurium vaccine clones. For i.n. immunization, groups were immunized with the bacteria in 20 μl of PBS, applied equally to both nostrils. An additional group of mice received 20 μg of AgI/II, supplemented with 1 μg of CT, and served as a positive control group for protection in the S. mutans infection study (9, 10). For i.g. immunization, mice received the bacteria in 0.25 ml of the intubation medium with the aide of a 22-gauge feeding tube (5, 29). Mice immunized with serovar Typhimurium BRD509(pTETnir15) clone, which expresses an unrelated antigen (fragment C of tetanus toxin [TetC]) served as a negative control for both the immunization study and the study on the inhibition of S. mutans infection. All groups of immunized mice were boosted using the same vaccine and immunization procedure at 17 weeks after the initial immunization. All animal work was performed according to the National Institutes of Health guidelines, and protocols were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Sample collection.
Serum, saliva, and vaginal wash samples were collected at day 0 (preimmune samples) and at weeks 3, 7, 15, 19, 21, and 26 (except for vaginal wash samples). The serum samples were obtained from blood collected from the retro-orbital plexus using a heparinized microhematocrit capillary tube (Fisher Scientific Co., Pittsburgh, Pa.). Saliva samples (ca. 100 μl) were collected after the induction of salivary flow by intraperitoneal injection of the animals with 5 μg of carbachol (Sigma Chemical Co., St. Louis, Mo.). Vaginal wash samples were collected by flushing the vagina twice with a 60-μl volume of PBS. Samples were stored at −70°C until assayed for antibody activity.
Evaluation of immune responses.
The levels of isotype-specific antibodies in serum, saliva, and vaginal wash samples and of total salivary and total vaginal IgA were determined by enzyme-linked immunosorbent assay (ELISA) (6). Microtiter plates (Nunc, Roskilde, Denmark) were coated with 1 μg of GM1 ganglioside (Sigma) per ml followed by 1 μg of CT per ml (List Biological Laboratories, Campbell, Calif.), 2 μg of purified SBR per ml, or 0.25 μg of goat anti-mouse IgA per ml by overnight incubation at 4°C. Plates were then blocked for 4 h at room temperature with 0.01 M phosphate buffer (pH 7.2) containing 0.5 M NaCl and 0.15% Tween 20. Serial twofold dilutions of the samples were then added to wells in duplicate, and the plates were incubated overnight at 4°C. The plates were developed by the addition to the wells of the appropriate horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulins (IgG or IgA, for serum samples or secretions, respectively) (Southern Biotechnology Associated, Inc., Birmingham, Ala.) and o-phenylenediamine substrate (Sigma) with H2O2 (6). IgG1 and IgG2a antibody responses were assayed using plates coated with goat anti-mouse IgG subclass-specific antibodies, and the responses were detected by using HRP-conjugated goat anti-mouse IgG subclass-specific antibodies (Southern Biotechnology Associated, Inc.). The concentrations of antibodies in the samples were determined by interpolation on standard curves generated using a mouse immunoglobulin reference serum and constructed by a computer program based on four parameter logistic algorithms (Softmax/Molecular Devices Corp., Menlo Park, Calif.). Data were logarithmically transformed, and statistical analysis (one-way analysis of variance in conjunction with the Tukey multiple-comparisons test) was performed by using the InStat program (Graphpad Software, San Diego, Calif.). The data were retransformed and presented as the geometric means ×/÷ standard deviations (SD) for ease of interpretation.
Mouse infection model.
S. mutans strain PC3379 (provided by P. J. Crowley and A. S. Bleiweis, Gainesville, Fla.), which is resistant to tetracycline and erythromycin, was used to infect the oral cavity of adult mice (15). S. mutans PC3379 is a serotype c strain and was constructed by spaP-complementation of the spaP mutant strain PC3370 (3). In this study, mice were challenged with S. mutans when the mean salivary IgA anti-SBR antibody level in immunized animals reached the value of a level of 1% specific IgA antibodies per total IgA. Briefly, 2 weeks after the boost immunization, which corresponded to week 19 after the initial immunization, mice were fed powdered diet 300, supplemented with 1% sucrose (24) and with 4 mg of tetracycline and 4 mg of erythromycin per g of diet for 5 days. Mice were also provided sterile, distilled drinking water containing 4,000 U of penicillin G per ml of water during the first 4 days (32). The level of bacteria in the oral flora was determined prior to and after the antibiotic treatment.
The S. mutans PC3379 was grown in Todd-Hewitt Broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.3% yeast extract and 10 μg of tetracycline and 10 μg of erythromycin per ml under anaerobic conditions in a screw-cap conical tube at 37°C overnight. Following antibiotic treatment, mice were challenged with S. mutans PC3379 by applying 2 × 109 CFU in 20 μl of sterile saline to the tooth surface of each animal using sterile Calgi applicators (Spectrum, Houston, Tex.) daily for five consecutive days. Oral swabs were taken at 1-week intervals for 5 weeks (weeks 21 to 25 after the initial immunization), beginning 1 week after the last day of S. mutans infection, to determine the level of bacteria present in plaque. Sterile Calgi applicators were used to swab the tooth surfaces of mice. The tip of each applicator was dissolved in 0.5 ml of sterile saline with shaking. Appropriate serial dilutions of bacterial suspension (0.1 ml per aliquot) were plated on mitis salivarius (MS) agar (Difco), which allowed the selection of streptococci; MS medium supplemented with tetracycline (10 μg/ml) and erythromycin (10 μg/ml), which allow the selection of the tetracyclin- and erythromycin-resistant S. mutans strain PC3379; or blood agar for the growth of the total oral flora. The plates were incubated anaerobically at 37°C overnight. The numbers of CFU were counted after 48 h.
RESULTS
Mucosal IgA antibody responses.
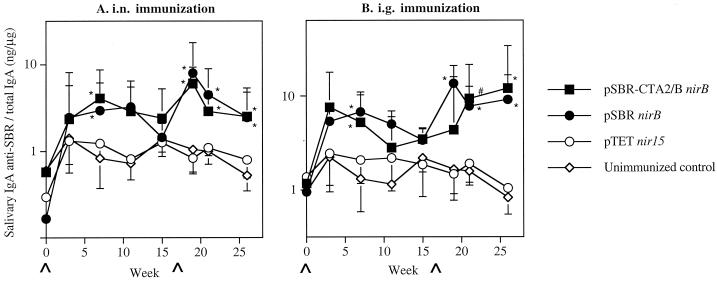
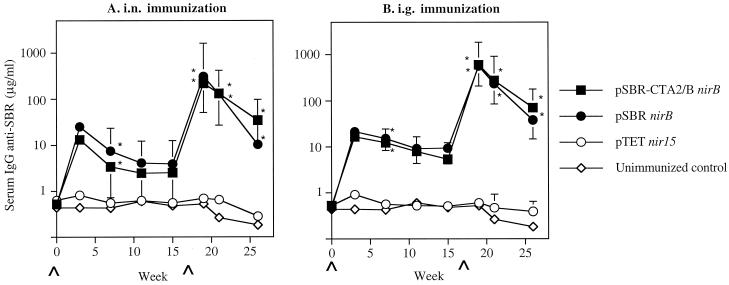
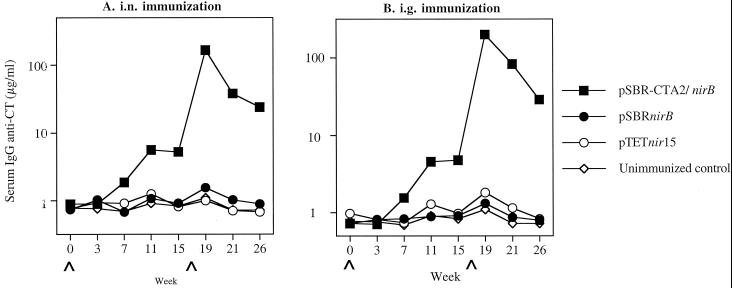
Specific salivary IgA responses against cloned immunogen SBR were induced in mice i.n. immunized with serovar Typhimurium clones expressing SBR or SBR-CTA2/B under the control of nirB promoter 7 weeks after immunization (Fig. 1A). These responses [0.65 or 0.47% of total salivary IgA activity for mice immunized with serovar Typhimurium BRD509(pSBR-CTA2/BnirB) or BRD509(pSBRnirB), respectively] were significantly (P < 0.01) higher than those of the TetC-immunized or unimmunized controls. A booster immunization at week 17 after the initial immunization resulted in enhanced salivary IgA responses against SBR, which were significantly higher (P < 0.01) in i.n. immunized mice than in the control groups of unimmunized mice (Fig. 1A). These responses reached peak levels of 0.95 and 1.26% for serovar Typhimurium BRD509(pSBR-CTA2/BnirB) and BRD509(pSBRnirB), respectively, of specific IgA antibodies against SBR per total salivary IgA. The salivary IgA antibody responses induced against SBR were also significantly (P < 0.01) higher in mice immunized by the i.g. route with either clone than in the control groups (Fig. 1B). Following the booster immunization by the i.g. route, the specific salivary IgA response against SBR was significantly enhanced (1.35% of total salivary IgA activity; P < 0.01) in mice immunized with serovar Typhimurium BRD509(pSBRnirB) (Fig. 1B). By weeks 21 and 26, significantly enhanced salivary IgA anti-SBR responses were observed in both groups of i.g. immunized mice compared to controls (Fig. 1B). After the booster immunization, salivary IgA antibodies against CT were induced as expected only in mice immunized with the Salmonella clone expressing SBR-CTA2/B via either the i.n. or the i.g. route (Fig. 2).
FIG. 1.
Salivary IgA anti-SBR responses in mice immunized with serovar Typhimurium BRD509 (pSBR-CTA2/BnirB) or BRD509(pSBRnirB) by the i.n. (A) or i.g. (B) route. The results are presented as the geometric means of the specific IgA responses/total IgA antibodies ×/÷ the standard deviation (SD) of five or six mice in one experimental group. Values significantly different from that of unimmunized controls at P <0.01 (∗) or P < 0.05 (#) are indicated.
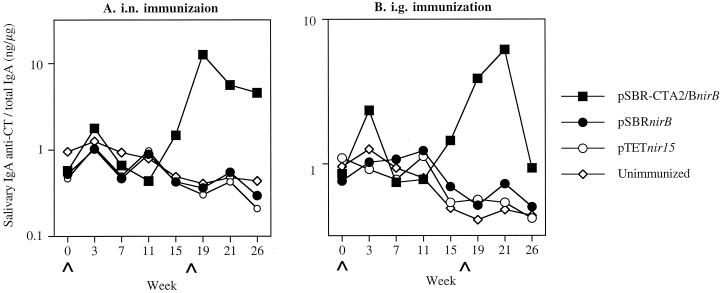
FIG. 2.
Salivary IgA anti-CT responses in mice immunized with serovar Typhimurium BRD509(pSBR-CTA2/BnirB) or BRD509(pSBRnirB) by the i.n. (A) or i.g. (B) route. The results are presented as the specific IgA responses/total IgA antibodies in pooled samples of five to six mice in one experimental group.
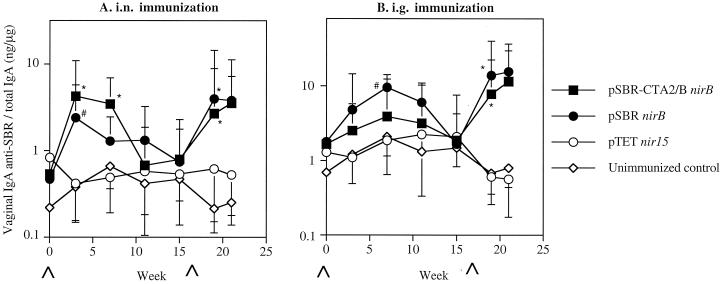
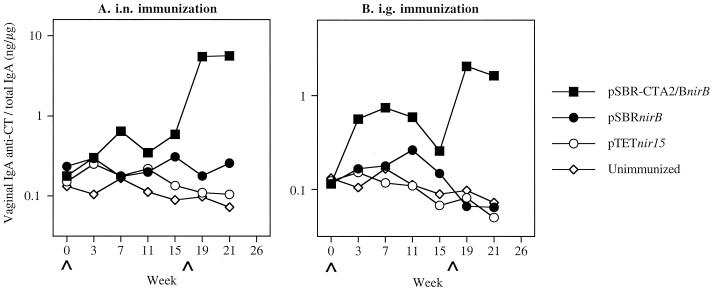
Specific IgA antibody responses against SBR were also observed in vaginal washes of mice immunized with either clones via the i.n. route (Fig. 3A). The responses induced in the immunized mice were significantly (P < 0.01) higher at week 3 after the initial immunization compared to the unimmunized controls (Fig. 3A). The responses then declined. Following the booster immunization, the secondary responses induced were significantly (P < 0.01) higher in the immunized mice than seen in the unimmunized controls. The secondary vaginal IgA antibody responses against SBR were also significantly (P < 0.01) higher in mice immunized by the i.g. route compared to those in the unimmunized controls (Fig. 3B). Higher IgA anti-CT responses were induced in mice immunized by the i.n. route with the clone expressing SBR-CTA2/B compared to that seen in the unimmunized controls (Fig. 4). Lower levels of vaginal IgA anti-CT antibody responses were seen in mice immunized by the i.g. route.
FIG. 3.
Vaginal IgA anti-SBR responses in mice immunized with serovar Typhimurium BRD509(pSBR-CTA2/BnirB) or BRD509(pSBRnirB) by the i.n. (A) or i.g. (B) route. The results are presented as the geometric means of the specific IgA responses/total IgA antibodies ×/÷ the SD of five or six mice per group. Values significantly different from that of unimmunized controls at P < 0.01 (∗) or P < 0.05 (#) are indicated.
FIG. 4.
Vaginal IgA anti-CT responses in mice immunized with serovar Typhimurium BRD509(pSBR-CTA2/BnirB) or BRD509(pSBRnirB) by the i.n. (A) or i.g. (B) route. The results are presented as the specific IgA responses/total IgA antibodies in pooled samples of five or six mice in each group.
Serum IgG antibody.
A peak in the IgG anti-SBR antibody response in serum was seen as early as week 3 in mice immunized with either the pSBR-CTA2/BnirB or the pSBRnirB clone by the i.n. or i.g. route and was significantly higher (P < 0.01) than in the unimmunized controls at week 7 (Fig. 5). The responses persisted through week 15. Following the i.n. or i.g. booster immunization in week 17, enhanced IgG anti-SBR responses in serum were seen by week 19, which were significantly higher (P < 0.01) than the primary response seen in these mice. These responses decreased but remained high through experimental week 26. Serum IgG responses against CT were induced in mice immunized with the clone expressing SBR-CTA2/B under the control of nirB promoter (Fig. 6). As expected, a serum IgG response against CT was not induced in mice immunized with the clone expressing only SBR.
FIG. 5.
Serum IgG anti-SBR responses in mice immunized with serovar Typhimurium BRD509(pSBR-CTA2/BnirB) or BRD509(pSBRnirB) by the i.n. (A) or i.g. (B) route. The results are presented as the geometric means of the specific IgG responses ×/÷ the SD of five or six mice in one experimental group. Values significantly different from those of unimmunized controls at P < 0.01 (∗) are indicated. The results from week 3 are from pooled samples of six mice per group.
FIG. 6.
Serum IgG anti-CT responses in mice immunized with serovar Typhimurium BRD509(pSBR-CTA2/BnirB) or BRD509(pSBRnirB) by the i.n. (A) or i.g. (B) route. The results are presented as the specific IgG responses in pooled samples of five or six mice per group.
Serum IgG antibody subclass distribution.
To better understand the nature of the response to the various vaccine antigens, the levels of the serum IgG subclass responses were determined following the initial and booster immunizations. In mice immunized with AgI/II and CT via the i.n. route, the ratios of the SBR-specific serum IgG2a to IgG1 responses were indicative of T helper 2 (Th2) type responses (Table 1). These ratios were significantly (P < 0.001) different from the ratios of IgG2a to IgG1 antibodies to Salmonella (11.5 to 20.9) and to SBR (7.07 to 126) in mice immunized with Salmonella vaccine strains via either the i.n. or the i.g. route (Table 1). In mice immunized with the Salmonella vaccine strains, serum responses to SBR, as well as the responses to the Salmonella vector, were predominantly of the IgG2a isotype. Interestingly, the SBR-specific IgG2a/IgG1 ratios were significantly (P < 0.001) lower in mice immunized by the i.n. route with Salmonella strain expressing SBR-CTA2/B than in mice immunized with the Salmonella clone expressing SBR alone. These results were observed at both weeks 7 and 21 (Table 1), as well as at other time points in the study (data not shown). When the Salmonella clones were administered to the mice via the i.g. route, the ratios of the IgG subclass responses to SBR were significantly different (P < 0.001) between the clone expressing the chimeric SBR-CTA2/B and the clone expressing SBR alone following the boost at week 17 (Table 1 and data not shown).
TABLE 1.
IgG2a/IgG1a antibody responses to antigen in serum
| Clones | Immunization route | Antigen | Mean IgG2a/IgG1 ratio ×/÷ SD at wk:
|
|
|---|---|---|---|---|
| 7 | 21 | |||
| AgI/II + CT | i.n. | SBR | 0.59 ×/÷ 2.75b | 0.91 ×/÷ 1.95b |
| pSBR-CTA2/B | i.n. | SBR | 39.8 ×/÷ 8.47c | 7.07 ×/÷ 4.47c |
| pSBR | i.n. | SBR | 126 ×/÷ 2.09 | 30.2 ×/÷ 4.27 |
| pSBR-CTA2/B | i.g. | SBR | 10.7 ×/÷ 4.68 | 8.71 ×/÷ 4.79c |
| pSBR | i.g. | SBR | 13.8 ×/÷ 7.24 | 23.4 ×/÷ 2.51 |
| pSBR-CTA2/B | i.n. | Salmonella | 20.0 ×/÷ 1.66 | 15.5 ×/÷ 3.98 |
| pSBR | i.n. | Salmonella | 18.6 ×/÷ 2.14 | 20.9 ×/÷ 2.00 |
| pSBR-CTA2/B | i.g. | Salmonella | 18.6 ×/÷ 1.51 | 15.1 ×/÷ 1.82 |
| pSBR | i.g. | Salmonella | 20.4 ×/÷ 1.95 | 11.5 ×/÷ 1.51 |
Ratio between IgG2a and IgG1 obtained from subclass-specific ELISA. Values are presented as the geometric means ×/÷ the SD of the IgG2a/IgG1 ratios for individual mice.
Values significantly different (P < 0.001) from those of SBR-specific and Salmonella-specific IgG2a/IgG1 ratios in mice immunized with Salmonella clones via either the i.n. or the i.g. route.
Values significantly different (P < 0.001) from those of SBR-specific IgG2a/IgG1 ratios in mice immunized with the Salmonella clone expressing SBR alone via the same route.
Inhibition of S. mutans colonization.
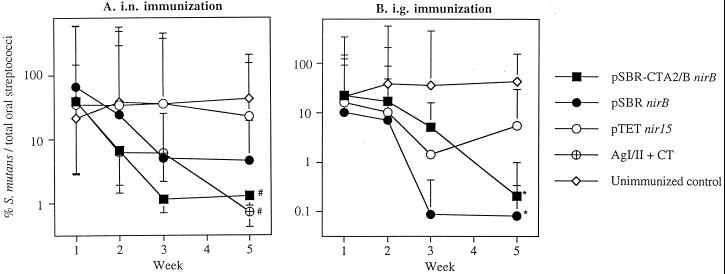
In order to determine the effectiveness of the anti-SBR antibody responses in protecting against S. mutans colonization of the oral cavity, groups of mice were challenged at week 21 with virulent S. mutans PC3379. In this study, the antibiotic treatment temporarily suppressed the indigenous oral flora and allowed the implantation of S. mutans. Furthermore, this bacterium persisted for at least 5 weeks on the tooth surface, despite the gradual reappearance of indigenous oral organisms (data not shown).
Following challenge, unimmunized mice retained high numbers of S. mutans for at least 5 weeks (Fig. 7). Sham-immunized mice that received serovar Typhimurium BRD509 (pTETnir15) also maintained high levels of S. mutans in the oral cavities throughout the 5-week period. In contrast, mice immunized by the i.n. route with serovar Typhimurium BRD509 (pSBR-CTA2/BnirB) or BRD509(pSBRnirB) showed a 97 or a 93% reduction (P < 0.05), respectively, in S. mutans colonization (Fig. 7A). The group of mice immunized by the i.n. route with AgI/II and CT showed a 98% reduction in levels of S. mutans per total streptococci (P < 0.05). Intragastric immunization of mice with serovar Typhimurium BRD509(pSBRnirB) or BRD509(pSBR-CTA2/BnirB) resulted in a 99% reduction (P < 0.05) in the level of S. mutans colonization in their oral cavities at week 5 (Fig. 7B).
FIG. 7.
(A) Percentage of S. mutans PC3379 per total streptococci in the oral cavity of i.n.-immunized or control mice which were challenged with 2 × 109 CFU of the PC3379 strain for 5 consecutive days. The results shown are geometric means ×/÷ the standard error of the mean (SEM) of five or six mice. Values significantly different from those of unimmunized controls at P < 0.05 (#) are indicated. (B) The percentage of S. mutans PC3379 per total streptococci in the oral cavity of i.g.-immunized or control mice which were challenged with 2 × 109 CFU of the PC3379 strain for 5 consecutive days. The results shown are geometric means ×/÷ the SEM of five or six mice. Values significantly different from those of unimmunized controls at P < 0.01 (∗) are indicated.
DISCUSSION
We have previously reported the construction of recombinant Salmonella clones expressing the S. mutans adhesin SBR alone or SBR linked to the A2/B subunits of CT under the control of the anaerobic inducible nirB promoter (13). These Salmonella clones were shown to persist for at least 21 days in Peyer's patches following i.g. administration of these attenuated bacteria. It was also shown that these Salmonella clones colonize and persist for at least 21 days in nasal lymphoid tissues, such as NALT, superficial lymph nodes, and internal jugular lymph nodes, as well as in the Peyer's patches and spleens of mice challenged by the i.n. route.
In the present study, we examined the mucosal immunogenicity and protective potential of serovar Typhimurium clones expressing the S. mutans adhesin SBR or SBR-CTA2/B under the control of the nirB promoter in a mouse model (15, 32). Specific salivary IgA anti-SBR responses were induced in mice immunized with the recombinant Salmonella clones after a single initial i.g. immunization, although a booster immunization further augmented the antibody levels. Furthermore, mice immunized with the Salmonella clone expressing SBR alone or SBR-CTA2/B via either the i.n. or the i.g. route exhibited almost complete inhibition of S. mutans colonization of the mouse oral cavities, whereas the sham-immunized and unimmunized control groups of mice showed consistent colonization by S. mutans.
An important aspect in the development of vaccines is the generation of persistent immune responses. In our study, a booster immunization at week 17 augmented the salivary IgA anti-SBR antibody responses. These responses persisted in the mice immunized with the Salmonella vaccine, although the level of augmentation was not as pronounced as that observed in another study using Salmonella clones expressing SBR or SBR-CTA2/B under the control of T7 promoter (11). In that report, when a low-level primary salivary IgA antibody response was induced, a secondary immunization was shown to induce a much higher response. However, when high levels of specific primary IgA antibodies (>1% of total IgA) were induced following the primary immunization, the salivary IgA antibody response induced after a second immunization was not higher than the primary response (11). It is possible that the high primary mucosal immune responses suppressed or delayed the effect of a booster immunization. In our current study, high primary salivary IgA anti-SBR antibody responses were induced by both clones after either the i.n. or i.g. immunization. Furthermore, enhanced secondary salivary IgA antibody responses were induced and persisted for up to 9 weeks after the booster immunization, demonstrating immunological memory in the mucosal compartment.
The results of the present study also indicate that the i.n. route is an effective way of immunization with Salmonella vaccine strains, especially for the induction of specific salivary IgA responses, and are in agreement with other reports (11, 31, 34). Previously, we have shown that serovar Typhimurium strains can invade and colonize the nasal tissues, possibly via mucosal inductive sites associated with the tissues, and can apparently disseminate through the draining lymph nodes (13). Antigens expressed by the Salmonella clones can be presented by professional antigen-presenting cells, which in turn can induce the activation and differentiation of lymphocytes and result in both mucosal and systemic immune responses. The Salmonella clones used in our study were also able to induce high levels of serum IgG anti-SBR responses. Although a higher number (10-fold) of salmonellae was administered to mice via the i.g. than by the i.n. route, the serum IgG anti-SBR responses were higher in the mice immunized via the i.n. route following the booster immunization. Furthermore, the serum anti-SBR response induced following the booster immunization was more pronounced compared to that seen in saliva. Differences in the magnitude of the systemic and mucosal secondary antibody results from different mechanisms of induction.
Previous studies by our group (11) have reported that CTA2/B is able to prolong the duration of the salivary IgA anti-SBR responses when expressed by Salmonella and under the control of the T7 promoter. Similar findings were made by Hirabayashi et al. (12). In the current study, we did not observe higher mucosal immune responses in mice immunized with Salmonella expressing SBR-CTA2/B compared to mice immunized with Salmonella expressing SBR alone. However, this study was terminated 9 weeks after the second immunization and a more long-term study may be necessary to demonstrate the adjuvant effect of CTB on host immune responses to SBR induced by our Salmonella vector system. In addition to the salivary responses, IgA antibody activity was also seen in vaginal washes of mice immunized with the Salmonella clones expressing SBR or SBR-CTA2/B, under the control of nirB promoter. Interestingly, the Salmonella clone expressing SBR-CTA2/B induced a higher IgA anti-SBR response than did the Salmonella clone expressing SBR alone following the initial immunization, when the immunization was administered i.n. but not via the oral route. This suggests that the potentiating effect of CTA2/B on the mucosal immune responses is more prominent when immunization is administered by the i.n. route.
Mosmann et al. (25) first reported the existence of different Th cell subsets. Gamma interferon and interleukin-12 (IL-12) promote the differentiation of Th1, whereas IL-4 has been found to be more important for the differentiation of Th2 cells. In mice, Th1 cells mediate the prominent production of IgG2a antibody responses, whereas IgG1 antibody production has been shown to be associated with type 2 responses (1). Salmonellae and other intracellular bacteria primarily induce host type 1 responses (16, 28). Soluble proteins administered with adjuvant via mucosal routes can induce type 2 responses (16). When foreign antigens are expressed in Salmonella vector, Th1-like responses are mainly induced to the cloned antigens, as well as to the vector (16, 33). However, recent studies have also reported mixed Th1 and Th2 responses (11) or biphasic Th responses to cloned antigens expressed by Salmonella (27). We report here the primary induction of a type 1 response to the Salmonella expressed SBR-CTA2/B or SBR, as well as to the vector. Interestingly, the responses induced in mice immunized with the Salmonella clone expressing the chimeric SBR-CTA2/B by the i.n. route showed a significant shift toward a type 2 response compared to the responses induced in mice immunized with the clone expressing SBR only. This finding indicates that the shift toward a type 2 response was influenced by the linked CTB. CT has been shown to promote IgG1 switch differentiation (18). The observed influence of CTB on the shift in the IgG2a/IgG1 ratio was more pronounced in the i.n.-immunized mice than in the i.g.-immunized mice, since there were no significant differences in mice immunized by the i.g. route.
The inhibition of S. mutans colonization on the tooth surface was in agreement with the enhanced salivary IgA anti-SBR antibodies detected in the mice. The reduction in the S. mutans levels was significant in mice immunized with clones expressing SBR-CTA2/B or SBR alone via either the i.n. or the i.g. route at 5 weeks postchallenge. In mice immunized with AgI/II and CT by the i.n. route, salivary IgA anti-SBR responses were induced and remained at high levels (1% specific IgA antibody against SBR/total salivary IgA antibodies; data not shown). Following challenge, these mice also showed a significant reduction in the level of S. mutans colonization on the tooth surfaces. The reduction in the colonization of S. mutans on the tooth surface of mice supports the effectiveness of salivary IgA antibodies against SBR in controlling S. mutans infection. Previous studies have reported the induction of salivary IgA antibodies against SBR or AgI/II and protection against caries formation caused by S. mutans on the tooth surface of rats after i.n. immunization with purified proteins (10). It has also been shown that i.n. immunization with a peptide segment of PAc, which is another designated name of AgI/II, coupled to CTB suppressed the colonization of S. mutans in a murine model (32). Moreover, the induction of salivary IgA antibodies against the glucan-binding region of glucosyltransferase produced by S. mutans was shown to correspond with protection against colonization by S. mutans and caries formation (15). Taken together, these results support the importance of specific salivary IgA antibodies against virulence factors involved in S. mutans attachment and colonization as a means to control the spread of this oral pathogen and the resulting caries formation. To our knowledge, this is the first study, which used an in vivo murine model to demonstrate the effectiveness of using serovar Typhimurium clones expressing cloned antigens of S. mutans in inducing a protective response against S. mutans colonization.
Previously, we reported that the Salmonella clones expressing antigens, under the control of the nirB promoter, colonize and persist in the host nasal tissues and intestinal tissues for at least 21 days. One can speculate that this allows the persistent expression of antigens to enhance the initial priming for the T helper cells as they express antigens over a period of time, thus prolonging the initial mucosal responses. On the contrary, clones expressing antigens under the control of T7 promoter produce a high antigen load at the initial stage of infection (5) but cannot persist in the host environment since they cannot be recovered even 1 day after infection (13). The initial high antigen load by the clones expressing antigens under the control of T7 promoter and the more persistent antigen presentation by the clones expressing antigens under the control of nirB promoter may result in a difference in the mechanism and timing of T-cell activation, costimulation, and B-cell activation and differentiation. It will be interesting to study the similarities and/or differences between the mechanisms of the mucosal T-cell priming driven by two distinct bacterial expression systems.
In the current study, the data suggest that Salmonella serovar Typhimurium clones expressing SBR or SBR-CTA2/B under the control of anaerobically inducible nirB promoter can induce a high level of salivary antibody response against SBR, which corresponds with the efficient control of S. mutans infection in the oral cavities of the immunized mice.
ACKNOWLEDGMENTS
We thank Cecily C. Harmon for excellent technical assistance. We thank Paula J. Crowley and Arnold S. Bleiweis of the Department of Oral Biology at the University of Florida for providing the S. mutans PC3379 strain. We also thank Terrence E. Greenway and Christina Jespersgaard for valuable advice.
This study was supported by USPHS grants DE08182, DE09081, and AI07051.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Chatfield S N, Charles I G, Makoff A J, Oxer M D, Dougan G, Pickard D, Slater D, Fairweather N F. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Bio/Technology. 1992;10:888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- 3.Crowley P, Brady L J, Michalek S M, Bleiweis A S. Virulence of a spaP mutant of Streptococcus mutans in a gnotobiotic rat model. Infect Immun. 1999;67:1201–1206. doi: 10.1128/iai.67.3.1201-1206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley P J, Brady L J, Piacentini D A, Bleiweis A S. Identification of a salivary agglutinin-binding domain within cell surface adhesin P1 of Streptococcus mutans. Infect Immun. 1993;61:1547–1552. doi: 10.1128/iai.61.4.1547-1552.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajishengallis G, Harokopakis E, Hollingshead S K, Russell M W, Michalek S M. Construction and oral immunogenicity of a Salmonella typhimurium strain expressing a streptococcal adhesin linked to the A2/B subunits of cholera toxin. Vaccine. 1996;14:1545–1548. doi: 10.1016/s0264-410x(96)00093-x. [DOI] [PubMed] [Google Scholar]
- 6.Hajishengallis G, Hollingshead S K, Koga T, Russell M W. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J Immunol. 1995;154:4322–4332. [PubMed] [Google Scholar]
- 7.Hajishengallis G, Koga T, Russell M W. Affinity and specificity of the interactions between Streptococcus mutans antigen I/II and salivary components. J Dent Res. 1994;73:1493–1502. doi: 10.1177/00220345940730090301. [DOI] [PubMed] [Google Scholar]
- 8.Hajishengallis G, Michalek S M. Current status of a mucosal vaccine against dental caries. Oral Microbiol Immunol. 1998;14:1–20. doi: 10.1034/j.1399-302x.1999.140101.x. [DOI] [PubMed] [Google Scholar]
- 9.Hajishengallis G, Nikolova E, Russell M W. Inhibition of Streptococcus mutans adherence to salivary-coated hydroxyapatite by human secretory immunoglobulin A (S-IgA) antibodies to cell surface protein antigen I/II: reversal by IgA1 protease cleavage. Infect Immun. 1992;60:5057–5063. doi: 10.1128/iai.60.12.5057-5064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajishengallis G, Russell M W, Michalek S M. Comparison of an adherence domain and a structural region of Streptococcus mutans antigen I/II in protective immunity against dental caries in rats after intranasal immunization. Infect Immun. 1998;66:1740–1743. doi: 10.1128/iai.66.4.1740-1743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harokopakis E, Hajishengallis G, Greenway T, Russell M W, Michalek S M. Mucosal immunogenicity of a Salmonella typhimurium-cloned heterologous antigen in the absence or presence of co-expressed cholera toxin A2/B subunits. Infect Immun. 1997;65:1445–1454. doi: 10.1128/iai.65.4.1445-1454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirabayashi Y, Kurata H, Funato H, Nagamine T, Aizawa C, Tamura S, Shimada K, Kurata T. Comparison of intranasal inoculation of influenza HA vaccine combined with cholera toxin B subunit with oral or parenteral vaccination. Vaccine. 1990;8:243–248. doi: 10.1016/0264-410x(90)90053-o. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Hajishengallis G, Michalek S M. Construction and characterization of a Salmonella enterica serovar Typhimurium clone expressing a salivary adhesin of Streptococcus mutans under control of the anaerobically inducible nirB promoter. Infect Immun. 2000;68:1549–1556. doi: 10.1128/iai.68.3.1549-1556.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenkinson H F, Lamont R J. Streptococcal adhesion and colonization. Crit Rev Oral Biol Med. 1997;8:175–200. doi: 10.1177/10454411970080020601. [DOI] [PubMed] [Google Scholar]
- 15.Jespersgaard C, Hajishengallis G, Huang Y, Russell M W, Smith D J, Michalek S M. Protective immunity against Streptococcus mutans infection in mice after intranasal immunization with the glucan-binding region of S. mutans glucosyltransferase. Infect Immun. 1999;67:6543–6549. doi: 10.1128/iai.67.12.6543-6549.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klimpel G R, Asuncion M, Haithcoat J, Niesel D W. Cholera toxin and Salmonella typhimurium induce different cytokine profiles in the gastrointestinal tract. Infect Immun. 1995;63:1134–1137. doi: 10.1128/iai.63.3.1134-1137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lycke N, Severinson E, Strober W. Cholera toxin acts synergistically with IL-4 to promote IgG1 switch differentiation. J Immunol. 1990;145:3316–3324. [PubMed] [Google Scholar]
- 19.Marcotte H, LaVoie M C. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol Mol Biol Rev. 1998;62:71–109. doi: 10.1128/mmbr.62.1.71-109.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mestecky J. The common mucosal immune system and current strategies for induction of immune response in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 21.Mestecky J, McGhee J R. Oral immunization: past and present. Curr Top Microbiol Immunol. 1989;146:3–11. doi: 10.1007/978-3-642-74529-4_1. [DOI] [PubMed] [Google Scholar]
- 22.Michalek S M, Childers N K. Development and outlook for a caries vaccine. Crit Rev Oral Biol Med. 1990;1:37–54. doi: 10.1177/10454411900010010401. [DOI] [PubMed] [Google Scholar]
- 23.Michalek S M, Childers N K, Dertzbaugh M T. Vaccination strategies for mucosal pathogens. In: Roth J A, Bolin C A, Brogden K A, Minion F C, Wannemuehler M J, editors. Virulence mechanisms of bacterial pathogens. 2nd ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 269–301. [Google Scholar]
- 24.Michalek S M, McGhee J R, Shiota T, Devenys D. Low sucrose levels promote extensive Streptococcus mutans-induced dental caries. Infect Immun. 1977;16:712–714. doi: 10.1128/iai.16.2.712-714.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. Two types of murine helper T cell clone. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 26.Munro C, Michalek S M, Macrina F L. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect Immun. 1991;59:2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pascual D W, Hone D M, Hall S, Van Ginkel F W, Yamamoto M, Walters N, Fujihashi K, Powell R J, Wu S, VanCott J L, Kiyono H, McGhee J R. Expression of recombinant enterotoxigenic Escherichia coli colonization factor antigen I by Salmonella typhimurium elicits a biphasic T helper cell response. Infect Immun. 1999;67:6249–6256. doi: 10.1128/iai.67.12.6249-6256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramarathinam L, Shaban R A, Niesel D W, Klimpel G R. Interferon gamma (IFN-γ) production by gut-associated lymphoid tissue and spleen following oral Salmonella typhimurium challenge. Microb Pathog. 1991;11:347–356. doi: 10.1016/0882-4010(91)90020-b. [DOI] [PubMed] [Google Scholar]
- 29.Redman T K, Harmon C C, Lallone R L, Michalek S M. Oral immunization with recombinant Salmonella typhimurium expressing surface protein antigen A of Streptococcus sobrinus: dose response and induction of protective humoral responses in rats. Infect Immun. 1995;63:2004–2011. doi: 10.1128/iai.63.5.2004-2011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell M W, Lehner T. Characterization of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23:7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- 31.Russell M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S M. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi I, Okahashi N, Matsushita K, Tokuda M, Kanamoto T, Munekata E, Russell M W, Koga T. Immunogenicity and protective effect against oral colonization by Streptococcus mutans of synthetic peptides of a streptococcal surface protein antigen. J Immunol. 1991;146:332–336. [PubMed] [Google Scholar]
- 33.VanCott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Coste M, Carter P B, Kiyono H, McGhee J R. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytoines following oral immunization with live recombinant Salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 34.Wu H-Y, Russell M W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita Y, Bowen W H, Burne R A, Kuramitsu H K. Role of Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu H, Nakano Y, Yamashita Y, Oho T, Koga T. Effects of antibodies against cell surface protein antigen PAc-glucosyltransferase fusion proteins on glucan synthesis and cell adhesion of Streptococcus mutans. Infect Immun. 1997;65:2292–2298. doi: 10.1128/iai.65.6.2292-2298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]