Abstract
Objectives:
While guidelines currently acknowledge that lung cancer screening should not be offered or should be discontinued in individuals with health problems that substantially limit life expectancy, there has been little guidance and evidence to inform whether patients should pursue screening. We used data from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial to examine the impact of self-reported chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), stroke, and diabetes mellitus (DM) on diagnostic complications in lung cancer screening evaluation.
Methods:
In our analysis, we included individuals from the usual care and intervention (annual chest x-ray) of the lung cancer screening trial with equal or greater than 55 years of age with a 20 pack-year smoking history who had undergone an invasive procedure. We performed multivariate logistic regression analysis to estimate the association of comorbidity on procedure complication. Our primary outcome was the incidence of major or moderate complications.
Results:
Features associated with high-risk complication included older age (OR=1.03 per year, p=0.001), history of CAD (OR=1.40, p=0.03), history of DM (OR=0.41, p<0.001, current smoking status (OR=1.46, p=<0.001), surgical biopsy (OR=7.39, p<0.001), needle biopsy (OR=1.94, p<0.001), and other invasive procedure (OR=1.58, p<0.001). We did not find any associated with complication and history of stroke (OR=0.84, p=0.53) or COPD (OR=1.27, p=0.06).
Conclusions:
Patient and procedure-level factors may alter the benefits of lung cancer screening. Data concerning individual risk factors and high-risk complications should therefore be incorporated into diagnostic algorithms to optimize clinical benefit and minimize harm. Further study and validation of the risk factors identified herein are warranted.
Keywords: Lung Neoplasms, Early Detection of Cancer, Diagnostic Screening Programs, Comorbidities, Biopsy
MicroAbstract
We studied 3,032 patients from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial to examine the impact of comorbidities on diagnostic complications in lung cancer screening evaluation. Positive associations included older age and history of coronary artery disease. These findings contribute to the developing literature on the impact of comorbidities on benefit and harm of lung cancer screening
Introduction
Lung cancer is the leading cause of cancer mortality in the United States.1 Lung cancer screening has been shown to decrease lung cancer mortality, and is currently recommended by the US Preventative Services Task Force (USPSTF) for adults aged 50 to 80 years with a 20 pack-year smoking history who currently smoke or have quit within the past 15 years.2,3 The National Lung Screening Trial (NLST) provides much of the evidence for the USPSTF lung cancer screening (LCS) recommendation. However, the NLST, like most clinical trials, is comprised of a patient cohort that differs substantially from the general population. Individuals in the NLST are younger, more educated, and have fewer comorbidities than the general population which the USPSTF recommendation targets.4 Underrepresentation of patients with comorbidities in lung cancer screening trials has led to uncertainty of screening benefits in the general population, and may be a contributing factor to the tepid adoption of lung cancer screening.5
Smoking history and age are key variables in the LCS recommendation as they significantly increase lung cancer risk. However, individuals who smoke also have increased rates of other major tobacco-related comorbidities including chronic obstructive pulmonary disease (COPD), cardiovascular disease (CAD), stroke, and diabetes mellitus (DM).4 Of these four major comorbidities, COPD remains the most common co-occurring condition in lung cancer with cross-sectional studies noting prevalence ranging from 40–70%.6,7 These comorbidities complicate the harm-to-benefit ratio of LCS as they increase the risk of treatment- and diagnosis-related complications that may lead to significant disability, decreased quality of life, and limited life expectancy. For example, patients with advanced COPD in particular are more likely to have complications during needle biopsy evaluations of pulmonary nodules.8 The American Thoracic Society notes the urgency of examining the harm-to-benefit ratio of screening in these types of patients as well.9 In a study evaluating lung cancer screening implementation in eight Veterans Administration hospitals, physicians excluded ~ 15% of USPSTF eligible individuals due to their comorbidities.10 Currently, USPSTF recommendations are vague for these patients, only noting that screening should be discontinued if a person develops a health problem that substantially limits life expectancy or ability or willingness to have curative lung surgery.2
In this study, we examine data from the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial to examine the association of COPD, CAD, stroke, and DM on diagnostic complications in lung cancer screening evaluation. Compared to the NLST trial, the PLCO trial had 3-fold larger enrollment, a significantly longer study period, less restrictive exclusion criteria, and no specific inclusion criteria for smoking status.11
Materials and Methods
Data Source
The PLCO Cancer Screening trial is a large, randomized trial designed and sponsored by the National Cancer Institute (NCI). Between November 8, 1993 to July 2, 2001, a total of 154,900 men and women were enrolled to investigate whether various screening interventions could reduce mortality in prostate, lung, colorectal, or ovarian cancer.12 Individuals with a history of a PLCO cancer were excluded. Screened patients were ages 55–74 at age of entry into the trial and were recruited in ten screening centers and were subsequently followed up by the same screening centers through 2011.12 Baseline morbidity characteristics data were collected through epidemiological questionnaires administered at baseline; detailed methodology, ethical approval and diagnostic testing hierarchy is detailed elsewhere.13
All actions and procedures in the PLCO trial were in accordance with the ethical standards of the national research committee and with the 1964 Declaration of Helsinki or comparable ethical standards. Ethical approvals were obtained from respective institutional review boards of all participating centers in the study. Informed consent was obtained from all participants included in the study.
Cohort
The primary data source for the study was the lung cancer screening sub-cohort within the PLCO trial, in which individuals in the intervention arm were screened with a chest x-ray. Data of 154,897 participants were available and downloaded after approval from the NCI. Only patients with baseline questionnaire information that included risk factors and health history were included. Patients with comorbidities were identified using data labels and codes provided by the NCI data dictionary. Comorbidity variables were defined using participant self-reported data and a composite comorbidity score was calculated by taking the sum of conditions that were reported (e.g. arthritis, osteoporosis, diabetes, COPD, history of MI, history of stroke). Within this cohort, we restricted eligibility to individuals greater than 55 years old with at least a 20 pack-year smoking history (Figure 1) who had one or more diagnostic procedures, defined as surgical biopsy (including thoracotomy, thoracoscopy, and resection), needle biopsy (including thoracentesis), bronchoscopy (with or without biopsy), mediastinoscopy, and ‘other’ procedure. ‘Other’ invasive procedure included cytology (sputum, bronchial, washing/brushing), open biopsy, lymphadenectomy, and others elaborated in Supplemental Table 3. Our final cohort consisted of 3,032 patients.
Figure 1:
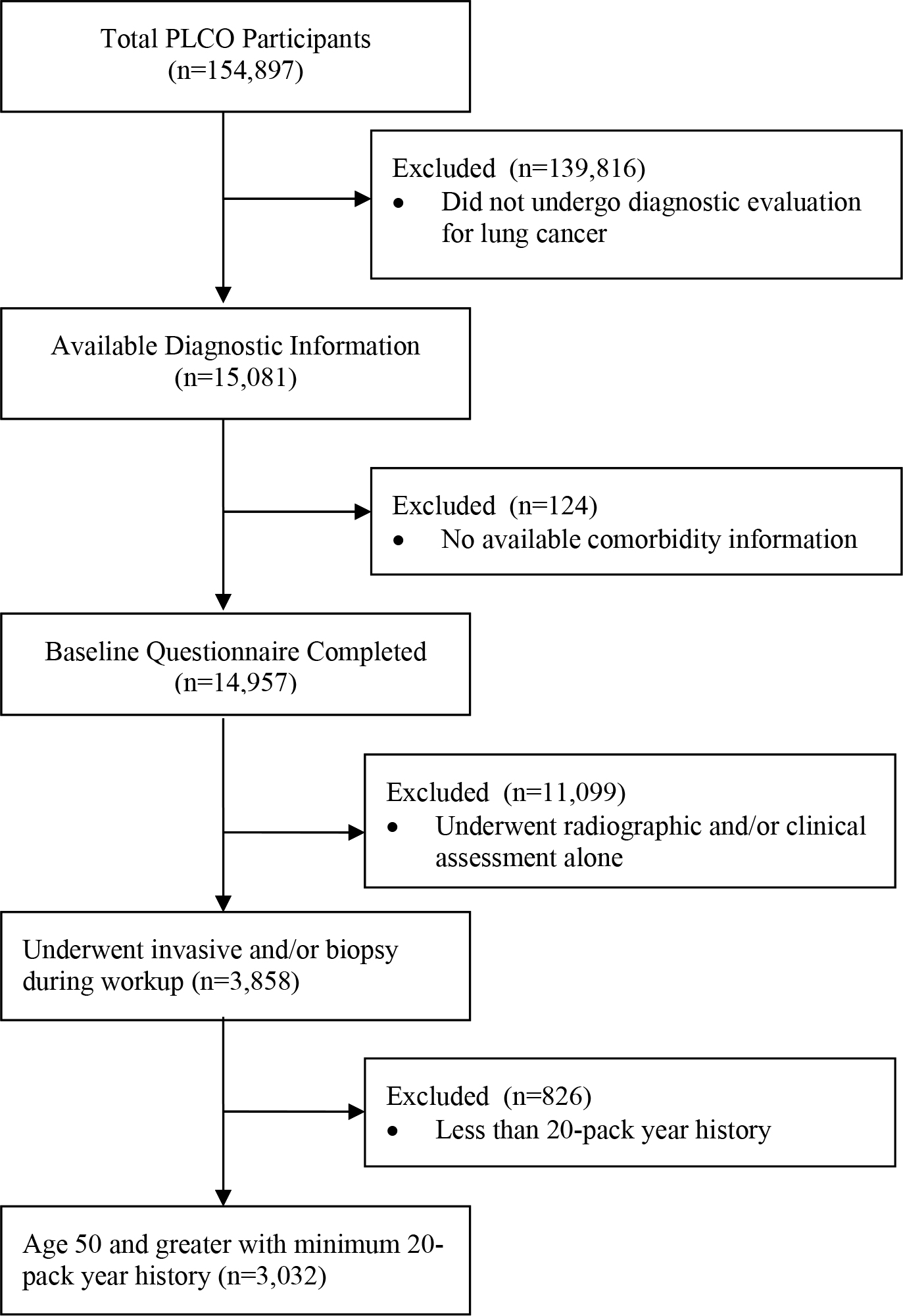
Flow Diagram of Cohort Identification
Outcomes
Primary outcomes were complications defined as those that occurred within 14 days of needle biopsy and bronchoscopy and within 60 days of mediastinoscopy and surgical biopsy.8,14–16 Complications occurring after diagnostic evaluation were systematically captured by PLCO study procedures in all participants with a positive screening test as well as in individuals who had a negative screening test but who subsequently had a diagnosis of lung cancer. We grouped complications into four major categories: respiratory, cardiac, infectious, or other. Respiratory complications included acute respiratory failure, chronic respiratory failure, respiratory arrest, pneumothorax, hemothorax, bronchospasm, atelectasis, bronchopulmonary fistula, and hemoptysis. Cardiac complications encompassed cardiac arrhythmia, myocardial infarction, cardiac arrest, congestive heart failure, or cerebrovascular accident. Infectious complications included fever requiring antibiotics, infection, infection and fever, wound infection, pneumonia, and pneumonia and fever. Finally, other complications included transfusion requirement, hospitalization, rib fracture, deep vein thrombosis/pulmonary embolism, wound dehiscence, vocal paralysis, and pain requiring specialist. These complications were also stratified based on severity of complication: major, intermediate, and minor (Supplemental Table 1). Major complications included acute / chronic respiratory failure, bronchopulmonary fistula, cardiac arrest, cerebrovascular accident, congestive heart failure, hemothorax, myocardial infarction, respiratory arrest, pulmonary embolism, or wound dehiscence. Intermediate complications were transfusion requirement, cardiac arrhythmia, fever requiring antibiotics, hospitalization, need for pain specialist, pneumothorax, rib fracture, vocal paralysis, infection with fever, wound infection, deep vein thrombosis, hemoptysis, bleeding, pneumonia, and pneumonia with fever. Minor complications included allergy, bronchospasm, atelectasis, hypotension, hypokalemia, urinary complications and other.
Statistical Analysis
Descriptive statistics were prepared with contingency tables analysis using chi-squared test. Multivariate logistic regression models were used to estimate odds ratios (OR) and 95% confidence intervals (CI) of the primary outcomes (complications). Predictor variables included age, race, smoking history, reported diabetes, COPD, history of MI, or stroke, and procedure type. Statistics were prepared using SAS 9.4 (SAS, Cary, NC).
Results
Patient characteristics
Our final study cohort consisted of 3,032 patients. Selection method is summarized in Figure 1. The overall mean age at randomization was 64.2; 63.4% (1,922/3,032) of participants were male. (Table 1). The cohort was 89.9% white, non-Hispanic (2,725/3,032). The mean pack-year total was 61.8. In terms of comorbidities—18.4% of participants (558/3,032) had COPD; 8.2% (248/3,032) had diabetes; 14.4% (435/3,032) had history of myocardial infarction; and 3.6% (109/3,032) had a history of stroke. The mean comorbidity sum among the cohort was 1.4.
Table 1.
Patient demographic and procedure categories (n=3032)
| Characteristic | Patients |
|---|---|
|
| |
| Clinical Features | |
| Age (mean, SD) | 64.2 (5.1) |
| Male | 1922 (63.4%) |
| Race | |
| White, Non-Hispanic | 2725 (89.9%) |
| Black | 197 (6.5%) |
| Other | 110 (3.6%) |
| BMI (≥30) | 583 (19.5%) |
| Pack Years (mean, SD) | 61.8 (31.3) |
| Pack Years (≥30) | 2688 (88.7%) |
| Smoking Status | |
| Current Smoker | 1427 (47.1%) |
| Former Smoker | 1605 (52.9%) |
| Comorbidities | |
| COPD | 558 (18.4%) |
| Diabetes | 248 (8.2%) |
| History of Myocardial Infarction | 435 (14.4%) |
| Hypertension | 1047 (34.5%) |
| History of Stroke | 109 (3.6%) |
| Liver Disease | 136 (4.5%) |
| Any History of Cancer | 182 (6.0%) |
| DCCI** | |
| 0 | 2117 (69.8%) |
| 1 | 742 (24.5%) |
| ≥2 | 173 (5.7%) |
| Complications | |
| Major | 158 (5.2%) |
| Intermediate | 570 (18.8%) |
| Minor | 144 (4.75%) |
| Any Complication | 691 (22.8%) |
| Respiratory | 458 (15.1%) |
| Cardiac | 169 (5.6%) |
| Infectious | 137 (4.5%) |
BMI=Body Mass Index
COPD=Chronic Obstructive Pulmonary Disease
Deyo Charleson Comorbidity Index
Individual procedure characteristics and diagnostic evaluation outcomes
A total of 5,886 diagnostic biopsy procedures were performed. In brief—36.3% of patients underwent surgical biopsy (1,099/3,032); 55.4% of patients underwent bronchoscopy (1,679/3,032); 50.8% underwent needle biopsy (1,541/3,032); 12.9% underwent mediastinoscopy (392/3,032); and 38% (1,175/3,032) underwent other invasive procedures (Table 2). Individual procedure characteristics and patient profiles are further detailed in Table 3.
Table 2.
Diagnostic biopsy procedure totals (n= 3032)
| Biopsy Type | Frequency | Percent |
|---|---|---|
|
| ||
| Surgical Biopsy | 1099 | 36.3% |
| Bronchoscopy | 1679 | 55.4% |
| Needle Biopsy | 1541 | 50.8% |
| Mediastinoscopy | 392 | 12.9% |
| Other Procedure | 1175 | 38.0% |
Table 3.
Clinical features and outcomes stratified by procedure type
| Characteristic | Surgical Bx (n=1099) | Bronchoscopy (n=1679) | Needle Bx (n=1583) | Mediastinoscopy (n=392) |
|---|---|---|---|---|
|
| ||||
| Clinical Features | ||||
| Age (mean, SD) | 63.9 (5.1) | 64.17 (5.1) | 63.77 (5.0) | 63.95 (5.1) |
| Male | 670 (61.0%) | 1046 (62.3%) | 1007 (63.6%) | 248 (63.3%) |
| BMI (≥30) | 204 (18.6%) | 308 (18.3%) | 311 (19.6%) | 62 (15.8%) |
| Pack years (mean, SD) | 60.66 (30.6) | 62.79 (31.1) | 61.33 (30.0) | 61.82 (29.1) |
| Pack Years (≥30) | 976 (88.8%) | 1513 (90.1%) | 1371 (86.6%) | 362 (92.3%) |
| Smoking Status | ||||
| CS | 480 (43.7%) | 804 (47.9%) | 710 (44.9%) | 173 (44.1%) |
| FS | 619 (56.3%) | 875 (52.1%) | 873 (55.1%) | 219 (55.9%) |
| Comorbidities | ||||
| COPD | 200 (18.2%) | 307 (18.3%) | 280 (17.7%) | 63 (16.1%) |
| Diabetes | 74 (6.7%) | 134 (8.0%) | 124 (7.8%) | 23 (5.9%) |
| History of Myocardial Infarction | 144 (13.1%) | 259 (15.4%) | 222 (14.0%) | 63 (16.1%) |
| Hypertension | 381 (34.7%) | 570 (33.9%) | 543 (34.3%) | 121 (30.9%) |
| History of Stroke | 46 (4.2%) | 68 (4.1%) | 60 (3.8%) | 18 (4.6%) |
| Liver Disease | 44 (1.5%) | 68 (2.2%) | 77 (2.5%) | 9 (0.3%) |
| Any History of Cancer | 67 (2.2%) | 109 (3.6%) | 83 (2.74%) | 15 (0.5%) |
| DCCI** | 0.34 (0.59) | 0.38 (0.62) | 0.37 (0.63) | 0.33 (0.59) |
| Screening / Diagnostic | ||||
| Screening Protocol | ||||
| X-Ray Arm | 616 (56.1%) | 904 (53.8%) | 803 (50.7%) | 202 (51.5%) |
| Control Arm | 483 (43.9%) | 775 (46.2%) | 738 (46.6%) | 190 (48.5%) |
| Lung Cancer Status | ||||
| Confirmed | 1010 (91.9%) | 1560 (92.9%) | 1416 (89.5%) | 377 (96.2%) |
| No Cancer | 78 (7.1%) | 108 (6.4%) | 118 (7.5%) | 14 (3.6%) |
| Other / Unconfirmable | 11 (1.0%) | 11 (0.7%) | 49 (3.1%) | 1 (0.3%) |
| Symptomatic | 479 (43.6%) | 1045 (62.2%) | 926 (58.5%) | 216 (55.1%) |
() Indicates percentage for categorical variables and SD for linear variables.
Deyo Charleson Comorbidity Index
FS = Former Smoker
CS = Current Smoker
Among patients undergoing invasive diagnostic evaluation, 22.8% (692/3,032) experienced a complication as a result of diagnostic evaluation. In terms of complication severity—5.2% of patients (158/3,032) experienced a major complication; 18.8% (570/3,032) experienced an intermediate complication; and 4.75% (144/3,032) of patients experienced a minor complication. Procedure outcomes were further analyzed by complication type—15.1% of patients (458/3,032) experienced a respiratory complication; 5.6% (169/3,032) experienced a cardiac complication; and 4.5% (137/3,032) experienced an infectious complication. Complication category definitions are outlined in Supplemental Table 2.
Features associated with diagnostic complications
We separately analyzed features associated with high-risk complications (major and intermediate) as well as with complication category (respiratory, cardiac, infectious) (Tables 4 & 5). Features associated with high-risk complication in our multivariate analysis included older age (OR=1.03 [1.01–1.06], p=0.001), Current smoking status (OR=1.46 [1.19–1.79], p<0.001), prior history of MI (OR=1.40 [1.03–1.91], p=0.03), surgical biopsy (OR=7.39 [5.84–9.34], p<0.001), needle biopsy (OR=1.94 [1.59–2.38], p<0.001), and other invasive procedure (OR=1.58 [1.22–2.05], p<0.001) (Table 4). Features associated with respiratory complication under multivariate conditions included current smoking status (OR=1.57 [1.26–1.96], p=<0.001), COPD (OR=1.32 [1.01–1.73], p=0.04), surgical biopsy (OR=6.31 [4.83–8.24], p<0.001) and needle biopsy (OR=2.15 [1.72–2.69], p<0.001). Features associated with cardiac complication under multivariate conditions included older age (per year) (OR=1.05 [1.02–1.09], p=0.003), male sex (OR=1.58 [1.09–2.30], p=0.02), surgical biopsy (OR=9.06 [5.64–14.56], p<0.001), and other invasive procedure (OR=1.74 [1.03–2.94], p=0.04). Finally, features associated with infectious complication included male sex (OR=2.40 [1.55–3.73], p<0.001), current smoking status (OR=1.52 [1.05–2.20], p=0.03), COPD (OR=1.55 [1.02–2.37], p=0.04), surgical biopsy (OR=6.16 [3.82–9.94], p<0.001), mediastinoscopy (OR=1.83 [1.22–2.77], p=0.004), and other invasive procedure (OR=1.99 [1.11–3.55], p=0.02).
Table 4.
Features associated with major and intermediate complication
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
|
|
|||
| Variable | OR (95%CI) | P-value | OR (95%CI) | P-value |
|
| ||||
| Age (per year) | 1.01 (1.00 – 1.03) | 0.13 | 1.03 (1.01 – 1.06) | 0.001 |
| Sex (male) | 1.08 (0.90 – 1.29) | 0.43 | 1.21 (0.98 – 1.50) | 0.07 |
| Pack Years | 1.00 (1.00 – 1.003) | 0.94 | 1.00 (1.00 – 1.00) | 0.79 |
| Current smoker | 1.12 (0.94 – 1.33) | 0.22 | 1.44 (1.18 – 1.76) | <0.001 |
| Diabetes | 0.42 (0.27 – 0.63) | <0.001 | 0.41 (0.26 – 0.64) | <0.001 |
| History of MI | 1.16 (0.91 – 1.48) | 0.23 | 1.39 (1.05 – 1.84) | 0.02 |
| History of Stroke | 1.12 (0.71 – 1.77) | 0.62 | 0.84 (0.50 – 1.41) | 0.50 |
| COPD | 1.21 (0.97 – 1.50) | 0.09 | 1.27 (0.99 – 1.62) | 0.06 |
| Procedure | ||||
| Surgical Bx | 7.61 (6.25 – 9.28) | <0.001 | 7.39 (5.84 – 9.34) | <0.001 |
| Needle Bx | 1.27 (1.06 – 1.51) | 0.01 | 1.95 (1.59 – 2.38) | <0.001 |
| Mediastinoscopy | 2.03 (1.61 – 2.55) | <0.001 | 1.23 (0.94 – 1.60) | 0.13 |
| Other Procedure | 3.81 (3.08 – 4.72) | <0.001 | 1.59 (1.23 – 2.06) | <0.001 |
Multivariate model inclusive to all listed variables
Bronchoscopy is the reference procedure.
OR=Odds Ratio
CI=Confidence Interval
Table 5.
Features associated with diagnostic complication (complication category)
| Respiratory Complication | Cardiac Complication | Infectious Complication | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Variable | OR (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value |
|
| ||||||
| Age (per year) | 1.02 (1.00 – 1.04) | 0.09 | 1.05 (1.02 – 1.09) | 0.004 | 1.04 (1.00 – 1.08) | 0.052 |
| Sex (male) | 0.90 (0.72 – 1.17) | 0.39 | 1.63 (1.13 – 2.35) | 0.01 | 2.38 (1.52 – 3.60) | <0.001 |
| Pack Years | 1.00 (1.00 – 1.01) | 0.55 | 1.00 (1.00 – 1.01) | 0.45 | 1.00 (1.00 – 1.01) | 0.42 |
| Current Smoker | 1.56 (1.25 – 1.94) | <0.001 | 1.15 (0.82 – 1.61) | 0.41 | 1.51 (1.05 – 2.19) | 0.03 |
| Diabetes | 0.47 (0.28 – 0.79) | 0.004 | 0.50 (0.22 – 1.10) | 0.08 | 0.27 (0.08 – 0.86) | 0.03 |
| History of MI | 1.27 (0.93 – 1.74) | 0.13 | 1.26 (0.80 – 1.98) | 0.32 | 0.81 (0.47 – 1.40) | 0.45 |
| History of Stroke | 1.09 (0.64 – 1.87) | 0.76 | 0.47 (0.17 – 1.35) | 0.16 | 0.98 (0.40 – 2.39) | 0.97 |
| COPD | 1.31 (1.003 – 1.71) | 0.0475 | 0.98 (0.64 – 1.50) | 0.92 | 1.58 (1.03 – 2.41) | 0.04 |
| Procedure | ||||||
| Surgical Bx | 6.29 (4.83 – 8.21) | <0.001 | 9.05 (5.63 – 14.52) | <0.001 | 6.17 (3.82 – 9.95) | <0.001 |
| Needle Bx | 2.16 (1.73 – 2.69) | <0.001 | 1.14 (0.82 – 1.58) | 0.44 | 1.37 (0.96 – 1.96) | 0.09 |
| Mediastinoscopy | 1.07 (0.80 – 1.43) | 0.66 | 1.39 (0.94 – 2.05) | 0.10 | 1.86 (1.22 – 2.77) | 0.003 |
| Other Procedure | 1.28 (0.96 – 1.71) | 0.09 | 1.73 (1.02 – 2.93) | 0.04 | 1.96 (1.10 – 3.51) | 0.02 |
Multivariate model inclusive to all listed variables
Bronchoscopy is the reference procedure.
OR=Odds Ratio
CI=Confidence Interval
Surprisingly, we identified a robust decreased risk for almost all groups of complications in patients with diabetes mellitus (DM). We investigated possible confounding relationships to explain such a result (Table 6). We noted that patients with diabetes underwent fewer procedure categories (1.96 vs. 2.16; p=0.0094) and were less likely to undergo surgical biopsy (29.8% vs. 36.8%; p=0.0285). Our study was inclusive to all participants who underwent invasive biopsy in the chest x-ray screening cohort, however in the control group, only individuals who underwent invasive biopsy that led to a diagnosis of cancer were included. As cancer itself is a risk factor for complication we investigated this relationship (Supplemental Table 4). These data provide useful context in interpreting our results compared to current screening strategies utilizing low-dose CT and the Lung-RADS criteria, which has led to a decrease of invasive testing of benign nodules.24
Table 6.
Clinical and procedure characteristics stratified by patient population
| Characteristic | DM (n=248) | Non-DM (n=2784) | P-Value |
|---|---|---|---|
|
| |||
| Clinical Features | |||
| °°Age (mean, SD) | 64.76 (5.06) | 64.15 (5.11) | 0.07 |
| Male | 189 (76.2%) | 1733 (62.3%) | <0.001 |
| BMI (≥30) | 98 (39.5%) | 485 (17.4%) | <0.001 |
| °°Pack Years (mean, SD) | 63.52 (32.45) | 61.66 (31.24) | 0.37 |
| Pack Years (≥30) | 223 (89.9%) | 2465 (88.5%) | 0.51 |
| Smoking Status | |||
| CS | 77 (31.0%) | 1350 (49.1%) | <0.001 |
| FS | 171 (69.0%) | 1434 (52.2%) | |
| Comorbidities | |||
| COPD | 47 (19.0%) | 511 (18.4%) | 0.82 |
| History of Myocardial Infarction | 72 (29.0%) | 363 (13.0%) | <0.001 |
| Hypertension | 146 (58.9%) | 901 (32.4%) | <0.001 |
| History of Stroke | 13 (5.24%) | 96 (3.45%) | 0.15 |
| Liver Disease | 7 (2.8%) | 129 (4.6%) | 0.19 |
| Any History of Cancer | 15 (6.1%) | 167 (6.0%) | 0.97 |
| DCCI** | 1.43 (0.63) | 0.27 (0.52) | <0.001 |
| Procedure | |||
| Surgical Biopsy | 74 (29.8%) | 1025 (36.8%) | 0.03 |
| Bronchoscopy | 134 (54.0%) | 1545 (55.5%) | 0.66 |
| Needle Biopsy | 124 (50.0%) | 1417 (50.9) | 0.77 |
| Mediastinoscopy | 23 (9.3%) | 369 (13.3%) | 0.07 |
| Other Procedure | 132 (53.2) | 1643 (59.0%) | 0.08 |
| °°Procedure category sum | 1.9637 (1.0848) | 2.1548 (1.111) | 0.01 |
| Complications | |||
| Major | 9 (3.6%) | 149 (5.4%) | 0.24 |
| Intermediate | 23 (9.3%) | 547 (19.6%) | <0.001 |
| Minor | 7 (2.8%) | 137 (4.9%) | 0.14 |
| Any Complication | 30 (12.1%) | 661 (23.7%) | <0.001 |
| Respiratory | 19 (7.7%) | 439 (15.8%) | 0.001 |
| Cardiac | 7 (2.8%) | 162 (5.8%) | 0.0487 |
| Infectious | 3 (1.2%) | 134 (4.8%) | 0.01 |
() Indicates percentage for categorical variables and SD for linear variables.
Deyo Charleson Comorbidity Index
Pooled-t test was employed. For all other variables chi-squared test was applied.
Discussion
While guidelines currently acknowledge that screening should not be offered or should be discontinued in individuals with health problems that substantially limit life expectancy, there has been little guidance and evidence to inform both clinicians and persons with comorbidities on whether patients should pursue screening. In this study, we found that older patients, current smoking status, and those with history of MI had the greatest risk of developing a high-risk complication during diagnostic workup. Patients with COPD and current smoking status had the greatest risk of developing pulmonary and infectious complications during workup, while male sex was associated with an increased risk of cardiac and infectious complications. Overall diagnostic complication rate was associated with procedure invasiveness. Diabetes and history of stroke were not associated with increased complication risk. Cumulatively, our data suggests that patient comorbidities have a limited role in informing patient and provider decision-making.
We first assessed the role of patient demographics and comorbidities in predicting high-risk diagnostic complications (major and moderate) controlling for procedure-type. Unsurprisingly, we found advanced age was associated with high-risk complications.17 While age is a well-known marker of increased morbidity following surgery, it should not be the only reason a patient is denied a given procedure. Given that history of MI and current smoking status were the other variables identified for this endpoint, these three factors make an attractive index for identifying patients at high-risk of diagnostic complication. These findings represent a potential future direction for investigations regarding the risks of pursuing biopsy.
We subsequently investigated the association between patient characteristics and respiratory, cardiac, and infectious complications (Supplemental Table 1; Table 5). COPD may be the most important comorbidity we examined, as COPD co-occurs in nearly 35% of LCS eligible individuals.4,6,19 We found that COPD had an increased association with both respiratory and infectious complications (Table 5). Past investigations give useful context that validate our findings as Wiener et al identified COPD as well as age as risk factors for pneumothorax after CT guided biopsy.8 Similarly, among lung cancer diagnostic procedures, the highest risk of iatrogenic pneumothorax is following CT-guided biopsy with 12% of patients requiring a chest tube.20,21 With regards to an increased risk of infection, Gupta et al showed COPD was an independent risk factor for increased mortality and pneumonia in patients undergoing any type of surgery.22 While we examined less-invasive procedures used in diagnostic workup, our findings may indicate a risk for COPD patients irrespective of invasiveness. In addition, patient smoking status nearly followed COPD as a significant risk factor for high-risk complications and both respiratory and infectious complications, an unsurprising result given the robust literature.23
The optimal diagnostic strategy for lung cancer is not well established, particularly in individuals with comorbidities. Considerations for diagnostic approach include the location of the nodule, the presence/absence of suspected nodal disease, the invasiveness of the procedure, the cost of the procedure, and whether tumor tissue is needed for molecular profiling, histological subtyping, and/or clinical trial inclusion. While Chen et al. have proposed an algorithm for tumor biopsy,25 and CHEST guidelines can be used to establish the diagnosis,26 these tools do not account for complication risks or comorbid conditions. These comorbidities complicate the harm-to-benefit ratio of LCS as they increase the risk of treatment- and diagnosis-related complications that may lead to significant reduction in the effectiveness of a screening program. Our findings provide evidence of how comorbidities impact the harms of screening, as the presence of CAD and COPD increased risk of multiple types of complications.
A primary limitation of this study is the effect of healthier volunteers in the PLCO cohort. Our particularly low percentage of patients with DM (<10%), may explain our contradictory finding that DM was associated with a decreased risk of complications. Furthermore, comorbidities were self-reported in the PLCO trial, which may have introduced some classification error. Nodule location (peripheral vs central) was not included in the data, information that contributes to the type of diagnostic procedure performed.
Conclusion
In summary, our investigation highlights the increased risk of complications in LCS eligible patients who are older and have COPD or a history of myocardial infarction and no increased risk with the presence of diabetes and history of stroke. These findings contribute to the developing literature on the impact of comorbidities on benefit and harm of lung cancer screening and provide encouraging reassurance for providers considering lung cancer screening in their patients with diabetes and stroke. However, additional population-based data to determine comorbidity impact, as well as functional status risk stratification, and competing risk of death are needed.
Supplementary Material
Clinical Practice Points.
Population-based studies have identified COPD as a risk factor for pneumothorax and procedure-related hemorrhage following needle biopsy and bronchoscopy. Other comorbidities remain unexamined. Our investigation highlights the increased risk of complications in LCS eligible patients who are older and have COPD or a history of myocardial infarction and no increased risk with the presence of diabetes and history of stroke. These findings contribute to the developing literature on the impact of comorbidities on benefit and harm of lung cancer screening and provide encouraging reassurance for providers considering lung cancer screening in their patients with diabetes and stroke
Acknowledgements
The authors thank the National Cancer Institute for access to NCI’s data collected by the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by NCI.
Funding:
This work was supported by the American Cancer Society (RSG 1911801) and the NIH (R01 MD014890). The funding sources had no role in the design, conduct, or analysis of the study or in the decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest: Dr. Wisnivesky has received consulting honorarium from Sanofi, Glaxosmithkline and Banook and research grants from Sanofi and Quorum.
Works Cited
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 2.Moyer VA. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Annals of internal medicine. 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
- 3.Reese TJ, Schlechter CR, Potter LN, et al. Evaluation of Revised US Preventive Services Task Force Lung Cancer Screening Guideline Among Women and Racial/Ethnic Minority Populations. JAMA network open. 2021;4(1):e2033769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard DH, Richards TB, Bach PB, Kegler MC, Berg CJ. Comorbidities, smoking status, and life expectancy among individuals eligible for lung cancer screening. Cancer. 2015;121(24):4341–4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kee D, Wisnivesky J, Kale MS. Lung Cancer Screening Uptake: Analysis of BRFSS 2018. Journal of general internal medicine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen-Heijnen ML, Schipper RM, Razenberg PP, Crommelin MA, Coebergh JW. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: a population-based study. Lung cancer (Amsterdam, Netherlands). 1998;21(2):105–113. [DOI] [PubMed] [Google Scholar]
- 7.Loganathan RS, Stover DE, Shi W, Venkatraman E. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest. 2006;129(5):1305–1312. [DOI] [PubMed] [Google Scholar]
- 8.Wiener RS, Schwartz LM, Woloshin S, Welch HG. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 2011;155(3):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivera MP, Tanner NT, Silvestri GA, et al. Incorporating Coexisting Chronic Illness into Decisions about Patient Selection for Lung Cancer Screening. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2018;198(2):e3–e13. [DOI] [PubMed] [Google Scholar]
- 10.Kinsinger LS, Anderson C, Kim J, et al. Implementation of Lung Cancer Screening in the Veterans Health Administration. JAMA internal medicine. 2017;177(3):399–406. [DOI] [PubMed] [Google Scholar]
- 11.Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. The New England journal of medicine. 2013;368(8):728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 Suppl):273S–309S. [DOI] [PubMed] [Google Scholar]
- 13.Hocking WG, Tammemagi MC, Commins J, et al. Diagnostic evaluation following a positive lung screening chest radiograph in the Prostate, Lung, Colorectal, Ovarian (PLCO) Cancer Screening Trial. Lung cancer (Amsterdam, Netherlands). 2013;82(2):238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Itzstein MS, Gupta A, Mara KC, Khanna S, Gerber DE. Increasing Numbers and Reported Adverse Events in Patients with Lung Cancer Undergoing Inpatient Lung Biopsies: A Population-Based Analysis. Lung. 2019;197(5):593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamel MK, Lee B, Harrison S, et al. Do the surgical results in the National Lung Screening Trial reflect modern thoracic surgical practice? J Thorac Cardiovasc Surg. 2019;157(5):2038–2046.e2031. [DOI] [PubMed] [Google Scholar]
- 16.Van’t Westeinde SC, Horeweg N, De Leyn P, et al. Complications following lung surgery in the Dutch-Belgian randomized lung cancer screening trial. Eur J Cardiothorac Surg. 2012;42(3):420–429. [DOI] [PubMed] [Google Scholar]
- 17.Polanczyk CA, Marcantonio E, Goldman L, et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Annals of internal medicine. 2001;134(8):637–643. [DOI] [PubMed] [Google Scholar]
- 18.Tanner NT, Dai L, Bade BC, Gebregziabher M, Silvestri GA. Assessing the Generalizability of the National Lung Screening Trial: Comparison of Patients with Stage 1 Disease. American journal of respiratory and critical care medicine. 2017;196(5):602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. The European respiratory journal. 2009;34(2):380–386. [DOI] [PubMed] [Google Scholar]
- 20.Hiraki T, Mimura H, Gobara H, et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy-guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9-year period. AJR American journal of roentgenology. 2010;194(3):809–814. [DOI] [PubMed] [Google Scholar]
- 21.Heyer CM, Reichelt S, Peters SA, Walther JW, Muller KM, Nicolas V. Computed tomography-navigated transthoracic core biopsy of pulmonary lesions: which factors affect diagnostic yield and complication rates? Acad Radiol. 2008;15(8):1017–1026. [DOI] [PubMed] [Google Scholar]
- 22.Gupta H, Ramanan B, Gupta PK, et al. Impact of COPD on postoperative outcomes: results from a national database. Chest. 2013;143(6):1599–1606. [DOI] [PubMed] [Google Scholar]
- 23.Grønkjær M, Eliasen M, Skov-Ettrup LS, et al. Preoperative smoking status and postoperative complications: a systematic review and meta-analysis. Annals of surgery. 2014;259(1):52–71. [DOI] [PubMed] [Google Scholar]
- 24.American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS). https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf. Accessed 8 June 2021
- 25.Chen HJ, Yang JJ, Xu CR, et al. Principles of biopsy in suspected lung cancer: priority still based on invasion in the era of targeted therapy? J Thorac Dis. 2013;5(3):E93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e142S–e165S. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


