Abstract
Head and neck cancer is a malignant tumour with a high mortality rate characterized by late diagnosis, high recurrence and metastasis rates, and poor prognosis. Head and neck squamous cell carcinoma (HNSCC) is the most common type of head and neck cancer. Various factors are involved in the occurrence and development of HNSCC, including external inflammatory stimuli and oncogenic viral infections. In recent years, studies on the regulation of cell death have provided new insights into the biology and therapeutic response of HNSCC, such as apoptosis, necroptosis, pyroptosis, autophagy, ferroptosis, and recently the newly discovered cuproptosis. We explored how various cell deaths act as a unique defence mechanism against cancer emergence and how they can be exploited to inhibit tumorigenesis and progression, thus introducing regulatory cell death (RCD) as a novel strategy for tumour therapy. In contrast to accidental cell death, RCD is controlled by specific signal transduction pathways, including TP53 signalling, KRAS signalling, NOTCH signalling, hypoxia signalling, and metabolic reprogramming. In this review, we describe the molecular mechanisms of nonapoptotic RCD and its relationship to HNSCC and discuss the crosstalk between relevant signalling pathways in HNSCC cells. We also highlight novel approaches to tumour elimination through RCD.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is one of the most common malignancies worldwide, with an annual incidence of more than 780,000 cases. In the past few decades, the treatment of HNSCC, including surgery, radiotherapy, and chemotherapy, has made significant progress, but the mortality rate is still as high as 40–50% [1–3]. HNSCC is highly invasive and involves lesions in the oral cavity, oropharynx, larynx, and hypopharynx. Common risk factors are smoking, excessive alcohol consumption, and human papillomavirus (HPV) infection [4, 5]. Because the early symptoms of HNSCC are not obvious or specific, patients are often in an advanced state at the initial stage of diagnosis [6]. Therefore, it is necessary to gain a more systematic understanding of the molecular mechanisms and signalling pathways of cell death in HNSCC and to explore new treatment regimens to prolong the survival rate of patients.
Cell death is the irreversible cessation of life phenomenon and the end of life. Cell death often occurs in normal tissues and is necessary to maintain tissue function and morphology [7]. On the one hand, cell death is an essential physiological process that maintains tissue homeostasis and ensures normal development of the body. On the other hand, it is also the primary pathogenic mechanism that leads to local or systemic inflammation and disrupts normal organ function [8]. The modes of cell death can be divided into two main categories: accidental cell death (ACD) and regulatory cell death (RCD) [9]. The former is a passive biological process not regulated by life activities. At the same time, the latter occurs as a programmed regulation characterized by controlled signalling pathways and precise effector mechanisms [10]. RCD can be subdivided into apoptosis, necroptosis, pyroptosis, ferroptosis, autophagy, and the recently discovered cuproptosis (Table 1; Fig. 1). Tumours are diseases characterized by an imbalance in cell proliferation, differentiation, and cell death. Selective elimination of cancer cells without damaging nonmalignant cells is an ideal treatment strategy for most cancers [11] Death resistance is an essential characteristic of tumour cells, and inducing cell death is a necessary strategy for the nonsurgical treatment of tumours [12]. Different lethal substances in the RCD process affect tumour development and therapeutic effects. RCD processes involve signalling cascades of effector molecules and have unique biochemical characteristics, morphological characteristics, and immunological consequences. Apoptosis, necroptosis, pyroptosis, autophagy, ferroptosis, and cuproptosis have been the most widely studied RCDs in recent years, and different RCDs have unique molecular mechanisms [13].
Table 1.
Morphological, biochemical,immune features and major regulators of apoptosis,necroptosis,pyroptosis,ferroptosis,autophagy and cuproptosis
| Type | Morphological features | Biochemical features | Immune features |
Core regulators |
|---|---|---|---|---|
| Apoptosis | Cell shrinkage,chromatin condensation, cell fragmentation, apoptotic body formation | Activation of caspases,DNA fragmentation, formation of apoptosome |
TCD or ICD |
Positive:AIF,APAF1,BAX,BAK1,BBC3(PUMA),BID,PMAIP1(NOXA) and TP53 Negative: BCL-2, BCL-XL and MCL1 |
| Necroptosis | Cells swelling, pore formation on cells membranes,plasma membranes rupture, and moderate chromatin condensation | Caspase-independent,RIPK1/RIPK3-mediated phosphorylation of MLKL, formation of necrosome | ICD |
Positive: MLKL, RIPK1 and RIPK3 Negative:AURKA and ESCRT-III |
| Pyroptosis | Cell membrane perforation, cellswelling,osmotic lysis, release of contents | Caspase-dependent ,GSDMD or GSDME cleavage,formation of inflammasome | ICD |
Positive:Caspase-1,Caspase-4, Caspase-5,Caspase-11,GSDMD and GSDME Negative: ESCRT-III and GPX4 |
| Ferroptosis | Cell swelling, pore formation on cell membrane, smaller mitochondria, reduced mitochondria crista, elevated mitochondrial membrane densities | Caspase-independent, iron accumulation, lipid peroxidation, damage to the antioxidant system, inhibition of system Xc–/GSH/GPX4 signalling | ICD |
Positive:ACC,ACSL4,ALOX, CDKN1A,DPP4,LPCAT3, NCOA4 and TFRC Negative:GPX4, HIF1α and SLC7A11 |
| Autophagy | Phagosomes, autophagosomes, autolysosomes | Caspase-independent, LC3 lipidation, formation of autophagosome, elevated autophagic flux, and lysosomal activity | ICD |
Positive: AMPK,ULK1 and PI3K Negative: mTOR |
| Cuproptosis | Caspase-independent,copper accumulation,aggregation of lipoacylated proteins | ICD |
Positive:FDX1,LIAS,LIPT1,DLD,DLAT,PDHA1 and PDHB Negative:MTF,GLS and CDKN2A |
Fig. 1.
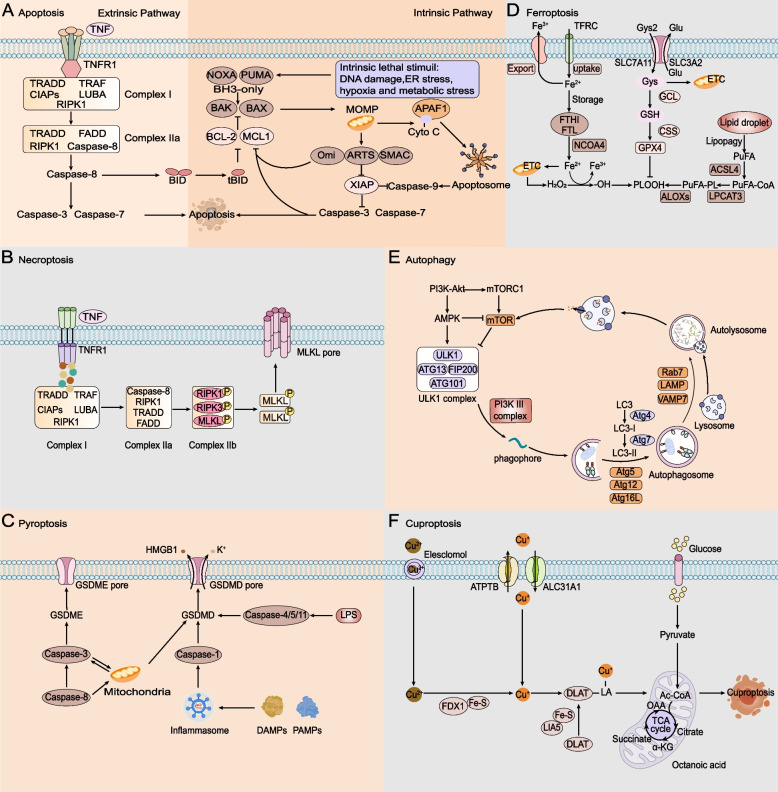
Core molecular mechanisms of apoptosis, necroptosis, pyroptosis, ferroptosis, autophagy and cuproptosis. A In the exogenous apoptosis pathway, TNF interacts with TNFR1, and TNFR1 begins to recruit downstream protein molecules to form complexes I and IIa, which promote the activation of caspase-8 and then activate caspase-3 and caspase-7. In the endogenous apoptotic pathway, activation of BH3-only proteins leads to Bax and BAK activity, triggering MOMP. Cyto C is released from mitochondria and forms a multiapoptotic complex with APAF1, which activates caspase-9 and then caspase-3 and caspase-7, leading to apoptosis. B TNF interacts with TNFR1; when caspase 8 is inhibited, activated RIPK1 promotes RIPK3 recruitment and MLKL phosphorylation, forms complex IIb, promotes inflammatory signal secretion, and promotes necroptosis. C PAMP and DAMP stimulate inflammasome activation, which leads to caspase-1 cleavage, and LPS can bind to caspase-4/5/11 to lyse GSDMD. Potassium efflux triggers the release of HMGB1 and K+. Caspase-3 can also be activated through the mitochondrial endogenous pathway and death receptor pathway to lyse GSDME and trigger pyroptosis. D Iron accumulation is achieved by increasing iron uptake by the TF-TFRC complex, limiting iron efflux by iron export transporters, and reducing iron storage by ferritosis. Cells obtain cysteine and exchange it for glutamate through the XC − antitransporter system. The ACSL4-LPCAT3-AlOXs pathway promotes iron death by activating lipid peroxidation to produce PLOOH from polyunsaturated fatty acids. E AMPK and mTORC1 act on mTOR, the ULK1 complex is phosphorylated, and the PI3K complex interacts with autophagosomes. Lc3 is modified to form LC3-II and ATG5-ATG12-ATG16L complexes to promote the formation and maturation of autophagic vesicles and binds to lysosomes under the action of LAMP, Rab7 and VAMP7. The recovered product is released into the cytosol for reuse. F Elesclomol binds extracellular copper (Cu2+) and transports it into the cell. FDX1 reduces Cu2 + to Cu + and promotes lipolylation (LA) and aggregation of DLAT involved in the mitochondrial TCA cycle. Copper importers (e.g., SLC31A1) and exporters (e.g., ATP7B) modulate copper sensitivity by affecting intracellular copper ion levels
Mechanisms of cell death in HNSCC
Apoptosis
Apoptosis is a cell death induced by cysteine aspartate aminotransferase (caspase) bound by the mitochondrial outer membrane permeability (MOMP) [14]. The morphology is characterized by cell shrinkage, chromatin condensation, and cell fragmentation to form so-called apoptotic bodies, which are phagocytosed by neighbouring or specific cells [15]. Apoptosis occurs mainly through two pathways. One pathway, called the endogenous pathway, is initiated by mitochondria. Cytochrome C release is activated by proapoptotic members, such as the apoptosis regulators BAX, BCL-2 homologous antagonist/killer 1 (BAK1), BCL-2-binding component 3 (BBC3; also known as PUMA), BH3 interacting domain death agonist (BID) and BH3-only proteins, and is stimulated by the apoptosis regulators BCL-2 (BCL-2), BCL-2-like 1 (BCL-2L1; also known as BCL-XL), BCL-2L2 (also known as BCLW) and MCL1 of the BCL-2 protein family to alter the permeability of the mitochondrial outer membrane [16, 17]. Cyto C released from mitochondria binds to the apoptotic peptidase activating factor 1 (APAF1), induces the recruitment, oligomerization, and activation of the apoptotic initiation protein caspase-9 in the conformational complex of the endoplasmic reticulum, and subsequently cleaves and activates the effector proteins caspase-3 and caspase-7 to induce apoptosis. Inhibition of caspase-9 is mediated by X-linked inhibitor of apoptosis (XIAP), which is degraded by the inhibitor of apoptosis (IAP) antagonists SMAC, Omi, and ARTS during cell death [15]. The other pathway is exogenous and is initiated through cell-surface death receptors of the tumour necrosis factor (TNF) family. TNF interacts with tumour necrosis factor receptor 1 (TNFR1), and TNFR1 begins to recruit downstream proteins, such as cytosolic adaptor protein receptor-interacting serine/threonine-protein kinase 1 (RIPK1), cellular IAPs (cIAPs), TNFRassociated factors (TRAFs) and linear ubiquitin chain assembly complex (LUBAC) proteins, to form complex I, which is involved in nuclear factor B (NF-B)-dependent survival gene expression. [18] TRADD, FAS-associated death domain (FADD), caspase-8, and RIPK1 constitute complex IIa and activate the apoptosis-initiating protein caspase-8, which cleaves and activates the effector proteins caspase-3 and caspase-7 to induce apoptosis [15, 19].
MCL-1 and XIAP are antiapoptotic proteins that induce apoptosis through caspase-activated cleavage inactivation [20]. G-protein-coupled receptor kinase-interacting protein 1 (GIT1) is upregulated in HNSCC to inhibit the apoptosis pathway through the PI3K/AKT/mTOR pathway, thereby promoting the development of HNSCC cells. Therefore, we believe that GIT1 may be a carcinogen of HNSCC and promote the occurrence and development of tumour, or may be a potential target for HNSCC therapy by inhibiting GIT1. [21, 22]. NF-κB is a key protein in regulating apoptosis, both to protect cells from apoptosis and to promote apoptosis in HNSCC cells in other cases. By inhibiting the activity of NF-κB in tumour cells, apoptosis procedures can be activated, so modulating the nuclear factor κB pathway may provide a new therapeutic strategy for HNSCC [23, 24]. WEE-1 is a serine/threonine kinase that inactivates cyclin-dependent kinase 1 (CDK1) by phosphorylation of the Tyr15 residue, resulting in G2/M phase arrest. Therefore, AZD-1775, an inhibitor of WeE-1, can overturn G2 cell cycle arrest and cause DNA damage by disrupting genome replication and can then promote the apoptosis of HNSCC [25–27]. The combination of cisplatin and AZD-1775 can produce persistent DNA damage in HPV + HNSCC cancer cells, downregulate MCL-1 and XIAP, and induce apoptosis in HPV + tumour cells [28, 29].
Necroptosis
For several years, apoptosis has been considered the simplest form of RCD, even as necroptosis seems to occur in an ACD manner. Genetic, biochemical, and functional evidence, as well as the discovery of specific chemical inhibitors of necrosis, redefine this process as a form of molecular-controlled regulation of cell death [30]. In terms of pathological functions, necroptosis does not involve apoptotic bodies; there is no great change in chromatin, the integrity of the cell membrane is destroyed, and cells and organelles are swollen, inflicting an excessive inflammatory response [31, 32]. The common pathway of necroptosis is initiated by activating receptor-interacting protein kinase-1 (RIP1). After the interaction between TNF and TNFR1, TNFR1 begins to recruit the downstream protein molecules TRADD, RIPK1, cIAPs, TRAF, and LUBAC to form complex I. Complex IIa is composed of TRADD, FADD, caspase-8, and RIPK1. When caspase-8 is inhibited, activated RIPK1 promotes RIPK3 recruitment and mixed lineage kinase domain-like (MLKL) phosphorylation, forms complex IIb, promotes proinflammatory signals such as DAMP secretion, and promotes necroptosis [33–35].
Chemotactic cells can promote the migration and invasion of HNSCC cells by means of freeing damage-related molecular patterns (DAMPs) and RIPK1, activating the nuclear factor-κB (NF-κB) pathway in HNSCC, and hence increasing the migration, invasion, and proliferation of tumour cells [36]. The determinant of the necrotic reaction is the RIPK3 receptor interaction. Cells expressing RIPK3 can be necrotic under the action of cytokines of the TNF family [37]. In patients with HNSCC, the loss of RIPK1 and RIPK3 function caused by hypermethylation of the promoter is closely related to metastatic disease and poor prognosis [38, 39]. Under normal circumstances, necroptosis is considered to back apoptosis because caspase-8 is a potent inhibitor of this process. However, some researchers have proven that caspase-8 is one of the genes with the highest mutation frequency in HNSCC, and it is also an essential factor leading to apoptosis resistance in HNSCC cells. Therefore, targeted necroptosis may provide a new strategy for HNSCC to bypass apoptosis resistance and eliminate tumour cells [40]. There is growing evidence that necroptosis plays a crucial role in regulating tumorigenesis and cancer progression, but it seems to be a double-edged sword [41]. Inducing necroptosis of cancer cells is a method for treating cancer, but necroptosis is also involved in the pathogenic process of tumour cells. The linear ubiquitin chain assembly complex (LUBAC) can also mediate NF-κB signalling and induce tumour cell death resistance [42]. Necroptosis can promote extravasation and migration of tumour cells. For example, tumour cells can induce necroptosis of endothelial cells through death receptor 6 (DR6) on endothelial cells, which leads to extravasation and metastasis of tumour cells mediated by the expression of amyloid precursor protein [43].
Pyroptosis
Pyroptosis is a programmed cell death mediated by cysteine aspartate protease 1 (caspase-1) and is characterized by cell membrane perforation, cell swelling, osmotic lysis, and content release [44, 45]. Unlike apoptosis, pyroptosis induces inflammation by releasing proinflammatory cytokines (IL-1β and IL-18) and cellular contents. Gasdermin (GSD) family proteins are the main executors of pyroptosis and mediate the formation of cell membrane perforation [46, 47]. Depending on the activated cysteine aspartate aminotransferase, typical and atypical signalling pathways may trigger pyroptosis [48]. The classical cellular pyroptosis pathway is mediated by caspase-1. [49] The inflammatory body is a kind of intracellular multiprotein signal complex that is usually assembled around the pattern recognition receptors (PRRs) [50]. Pathogen-associated molecular patterns (PAMPs) and DAMPs bind to adapter proteins on apoptosis-related protein (ASC) to activate caspase-1. Then, GSDMD is cleaved as a cutting substrate, resulting in cellular pyroptosis [51, 52]. The nonclassical pathway relies on caspase-11 (mouse) and caspase-4/5 (human). Pattern recognition receptors recognize the binding of Gram-negative bacterial lipopolysaccharide (LPS) to caspase-4/5/11 to cleave GSDMD, and potassium efflux-regulated pyroptosis triggers the release of high mobility group box 1 (HMGB1) and K+, leading to pyroptosis [43, 53, 54].
Inflammation is one of the causes of HNSCC, and the development and function of tumour stem cells may be related to chronic inflammation [55]. Inflammatory bodies and gasdermin family proteins are critical substrates leading to pyroptosis. At present, it has been found that these two proteins are involved in inhibiting tumour cell growth and promoting tumour cell death in a variety of system tumours. In addition, some studies also suggest that pyroptosis can promote tumour growth in different kinds of tumour cells [56–58]. This information shows that pyroptosis has dual roles of promoting and inhibiting tumours, and it is necessary to further study the relationship between pyroptosis and tumorigenesis and progression. The occurrence and development of HNSCC are inseparable from inflammation. NOD-like receptor 3 (NLRP3) inflammatory bodies are mainly composed of the receptor protein NLRP3, junction protein ASC, and effector protein caspase-1, which represents the most characteristic inflammatory body [59, 60]. It has been found that the expression of NLRP3 inflammatory bodies is upregulated in HNSCC. At the same time, blocking NLRP3 inflammatory bodies can reduce the number of tumour stem cells (CSCs) in HNSCC cell lines and in the TGFBR1/Pten2cKO mouse HNSCC model. Therefore, NLRP3 inflammatory bodies can be used as a potential target for CSCs in HNSCC therapy [61]. High expression of NLRP3 is also associated with the growth, invasion, and metastasis of HNSCC, with poor clinical prognosis in patients with oral squamous cell carcinoma (OSCC) treated with 5-FU, and NLRP3 gene knockout increases pyroptosis of 5-FU-induced OSCC cells [61, 62]. Gasdermin agonists may improve the efficacy of cancer immunotherapy. Gasdermin is directly activated by Phe-BF3-mediated desalinization, which provides a robust system for understanding the antitumour immunity stimulated by immunogenic cell pyroptosis [63]. Caspase-1 is upregulated in HNSCC tissues and may be a protumour gene in HNSCC tumour tissues. GSDME expression in HNSCC tumour tissue is upregulated and may be a biomarker for poor prognosis [64–66]. IL-6 is a proinflammatory cytokine secreted by cancer cells that is involved in regulating the proliferation and differentiation of cancer cells. The expression level in HNSCC tumour tissue is low, and inhibition of the IL-6 downstream signalling pathway may enhance the therapeutic effect on tumours with elevated IL-6 levels [66–68].
Ferroptosis
Ferroptosis is a regulated form of cell death characterized by intracellular iron accumulation, increased reactive oxygen species (ROS), decreased reduced glutathione (GSH), and lipid peroxidation, resulting in an imbalance between oxidation and antioxidation systems. Presently, ferroptosis mainly involves three key mechanisms, including iron accumulation, lipid peroxidation, and the destruction of the antioxidant system [12, 19, 69]. When ferroptosis occurs, a large amount of free Fe2 + accumulates in the cells. Free Fe2 + is highly oxidizing and easily undergoes the Fenton reaction with H2O2, which produces hydroxyl radicals that can cause oxidative damage to DNA, protein, and membrane lipids and promote lipid peroxidation to the damaged cell membrane, leading to cell death [19, 70, 71]. Glutathione peroxidase 4 (GPX4) is the most critical anti-lipid peroxidase and the core regulator of ferroptosis. System Xc- is a heterodimer composed of solute carrier family 7 member 11 (SLC7A11) and solute carrier family 3 member 2 (SLC3A2) on the cell membrane, which allows the passage of cystine and glutamate into and out of the cell. System Xc- transports extracellular cystine into cells, which is then converted into cysteine and becomes the raw material for synthesizing GSH [12, 19]. Because GSH is a necessary cofactor for the GPX4 enzyme to decompose peroxides, System Xc- affects the sensitivity of ferroptosis by affecting intracellular cystine metabolism and the GSH/GPX4 pathway, and inhibiting the expression of System Xc-, especially SLC7A11, can enhance the sensitivity of cells to ferroptosis. Therefore, inhibiting GPX4 and consuming GSH in cells is a potential anticancer strategy [71–73].
Acyl coenzyme A (CoA) synthase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) regulate polyunsaturated fatty acid (PUFA) synthesis [74, 75]. ACSL4 catalyses the ligation of free PUFAs with CoA to form PUFA-CoAs, which are then esterified to form PUFA-PL. Acetyl-CoA carboxylation to malonyl-CoA requires the catalysis of acetyl-CoA carboxylase (ACC). Therefore, the inactivation of ACSL4, LPCAT3, or ACC inhibits the progression of tumour cell ferroptosis [76–78]. Recent studies have shown that in HNSCC, the expression levels of the ACSL1 and TFRC genes are closely related to the occurrence of tumour cell ferroptosis and the prognosis of patients and are potential targets for the occurrence, development and treatment of HNSCC [79]. The high expression of acyl-CoA synthetase long-chain family member 1 (ACSL1) inhibits the progression of thyroid cancer cells and activates fatty acid metabolism reprogramming during cancer cell metastasis, thereby achieving an antagonizing effect of ferroptosis on tumour cells [79–81]. TFRCs are key transporters of intracellular iron and play a key role in tumour cell ferroptosis through their regulation by the NF2-YAP signalling axis. High expression of TFRC is associated with poor prognosis in patients with HNSCC [79, 82]. Compared with the levels in normal tissues, the ferroptosis driver SOCS1 and inhibitor FTH1 in HNSCC were positively correlated and upregulated. In other words, ferroptosis of HNSCC can be induced by increasing the expression of SOCS1 or decreasing the expression of FTH1 [83].
Autophagy
Autophagy is an intracellular lysosomal pathway that plays a crucial role in maintaining the dynamic balance of various physiological processes. Autophagy involves the sequential formation of three unique membrane structures: phagosomes, autophagosomes, and autolysosomes. More than 40 autophagy-related genes/proteins (Atgs) play a vital role in the dynamics and process of the autophagy membrane [84–87]. Under stress conditions, phagocytic vesicles or isolation membranes can be formed around damaged organelles, abnormally folded proteins, viruses, and bacteria in the cytoplasm, which are isolated into autophagosomes with bilayer membrane structures, and autophagy is induced by AMP-activated protein kinase (AMPK) and mTOR complex 1 (mTORC1) acting on the mechanistic target of rapamycin kinase (mTOR) [85, 88]. The phosphorylation of the UNC-51-like kinase 1 (ULK1) complex (including ULK1, ATG13, FIP200 and ATG101) interacts with the phosphatidylinositol 3-phosphate kinase (PI3K) complex on autophagosomes. Light chain 3 (LC3) modification forms LC3-II and the ATG5-ATG12- ATG16L complex to promote the formation and maturation of autophagic vesicles. After the formation of the autophagosome, it is transported to the vicinity of the lysosome under the action of lysosomal-associated membrane protein (LAMP), Rab7, and VAMP7 [85, 89]. The outer membrane of an autophagosome fuses with a lysosome to form an autophagy‒lysosome, and its contents are degraded by lysosome-related hydrolase. The degradation products of autophagy‒lysosome can be recycled in the cell to meet the cells’ metabolic needs [90–92].
Autophagy can play a major role in cell survival under adverse conditions, which means that autophagy can promote the growth of tumour cells. Autophagy supports tumorigenesis by maintaining mitochondrial metabolic function and energy levels, and may be necessary for tumour cell growth by inhibiting oxidative damage and maintaining metabolic homeostasis [93, 94]. The purpose of inhibiting the growth of tumour cells can be achieved by inducing tumour cells to activate autophagy. Hypoxia upregulated the expression of circCDR1as and induced autophagy in OSCC. Studies have shown that circCDR1as, as an oncogene, encapsulates miR-671-5p by sponging, inhibits mTOR activity, upregulates the AKT and ERK signalling pathways, and induces autophagy in OSCC cells [95, 96]. Erythroblast macrophage protein (MAEA) is a new type of E3 ubiquitin ligase that regulates autophagy through the AMPK and PI3K/AKT pathways. In HNSCC, TBC1D14 inhibits autophagy by downregulating the expression of MAEA. Overexpression of TBC1D14 inhibits autophagy by reducing the level of Lc3-II protein, increasing the level of p62 protein, and inhibiting the formation of autolysosomes, thus playing a role in anti-HNSCC metastasis [97–99]. Circ-PKD2 can increase the sensitivity of oral squamous cell carcinoma to cisplatin by inhibiting miR-646. Circ-PKD2 increases the level of Atg13, inhibits tumour proliferation and activates autophagy in OSCC cells by promoting the formation of autophagosomes and the activation of LC3II/I and other proteins [100, 101].
Cuproptosis
Copper ions are an indispensable cofactor for all organisms, but when their concentration exceeds a certain threshold, they will have a toxic effect on the body [102]. Tsvetkov P et al. discovered a new method of cell death that is dependent on copper ions and is regulated in the cell body, termed cuproptosis; copper ions directly bind to the fatty acylation components in the tricarboxylic acid cycle, resulting in abnormal accumulation of rich acylated proteins and loss of iron-sulfur cluster proteins, resulting in a protein toxic stress reaction and eventually leading to cell death [103]. This is different from the death mechanism that we know. Heavy metal ions are essential micronutrients for the human body, but insufficient or excessive metal abundance will lead to cell death [104]. At present, we have identified seven critical genes in the regulatory mechanism of cuproptosis, namely, ferredoxin 1 (FDX1), lipoic acid synthase (LIAS), lipoyl transferase-1 (LIPT1), dihydrolipoamide dehydrogenase (DLD), dihydrolipoamide-acetyltransferase (DLAT), pyruvate dehydrogenase E1 alpha (PDHA1) and pyruvate dehydrogenase β subunit gene (PDHB), and three harmful regulatory genes, namely, metal-regulated transcription factor (MTF), glutaminase (GLS) and cyclin-dependent kinase inhibitor 2 A (CDKN2A) [105]. Mitochondrial lipoylation is a posttranslational modification of lysine, which allows entry into the tricarboxylic acid (TCA) cycle through regulation by dihydrothiooctylamide branched chain transferase E2 (DBT), glycine lytic system protein H (GCSH), dihydrothiooctylamine-succinyltransferase (DLST) and DLAT. Copper directly binds to DLAT to promote the disulfide bond-dependent aggregation of lipid-based DLAT [106, 107].
In the Wilson disease mouse model, the loss of the copper exporter ATPase copper transporting beta (ATP7B) leads to excessive accumulation of copper and copper damage in the ageing liver, suggesting that drug inhibition of mitochondrial respiration may be a strategy against the disease [103]. Some experiments have shown that compared with normal oral tissues, the tissues of HNSCC patients express high levels of ATP7B, ATP7B in mitochondria and excrete copper from the cytoplasm to the extracellular space [108, 109]. In the differential expression analysis of oral squamous cell carcinoma and adjacent tissues, LIAS and PDHB were inhibited in cancer tissues, while GLS and CDKN2A were promoted in cancer tissues. This shows that the cuproptosis of tumour cells is inhibited, and the growth of tumour cells is promoted in OSCC [110]. Intratumor copper can regulate the expression of PD-L1, affect the immune escape of tumours, and then affect the efficacy of immunotherapy [111]. Mutations in the mitochondrial ribosomal gene MRPS7 affect mitochondrial respiratory chain dysfunction, and high expression of MRPS7 is associated with poor prognosis in patients with HNSCC [112, 113]. Studies have shown that high-dose Cu2 + exposure induces oxidative stress by increasing the levels of ROS and protein carbonyl compounds and reducing the GSH content and mRNA and enzyme activities, resulting in mitochondrial membrane potential depolarization, Cyto C release, cleavage of caspase-9 and caspase-3, increased BAK and BAX levels, decreased BCL2 levels and induction of tumour cell apoptosis [114]. The effect of elesclomol (ES) on cancer cells is to increase the level of Cu2 + in mitochondria, reduce the expression of ATP7A, lead to Cu2 + retention and the accumulation of ROS, promote SLC7A11 degradation, further enhance oxidative stress in cells and thereby induce tumour cell cuproptosis [115].
Modulation of cell death in HNSCC
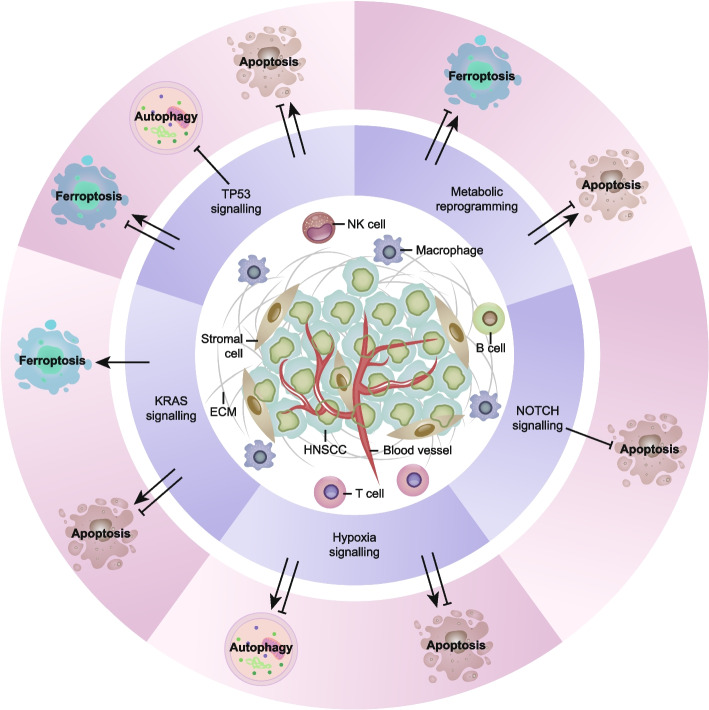
During the growth of HNSCC cells, a complex signal communication network has emerged to promote a collaborative environment that supports tumour proliferation and spread [116]. The TME is a complex ecosystem composed of cellular components (such as malignant cells, immune cells, and interstitial cells) and noncellular components (such as extracellular matrix, blood vessels, cytokines, chemokines, and growth factors) [117]. Various oncogenes or tumour suppressor signals in the TME determine the sensitivity of cell death patterns in HNSCC (Fig. 2). In this section, we focus on some of the most common HNSCC functions that affect cell death signals.
Fig. 2.
Modulation of cell death pathways in HNSCC. The tumour microenvironment of HNSCC is a complex ecosystem composed of cellular components (such as malignant cells, immune cells and stromal cells), extracellular matrix and interstitial fluid (such as blood vessels, cytokines, chemokines and growth factors). The interaction between cancer cells and invasive immune cells determines the progression and therapeutic effect of the tumour. ECM, extracellular matrix; NK, natural killer; TP53, tumour protein p53
TP53 signalling
The inactivation of tumour suppressor genes and the activation of oncogenes are key to the occurrence and development of HNSCC [118]. To date, the TP53 gene is the most commonly mutated gene in human cancer [119]. The mutation rate of the TP53 gene in human tumour cells has increased significantly from 10% (haematopoietic stem cell malignant tumour) to 50–70% (ovarian, colorectal, and head and neck cancer) [120]. Once the TP53 gene is mutated, the tumour suppression function of the wild-type TP53 gene will be lost, and many individual oncogenes will be active [121]. The TP53 gene encodes a tumour suppressor protein with transcriptional activation, DNA binding, and oligomerization domains. It is a tumour suppressor gene and can induce cell cycle arrest, apoptosis, and senescence. Mutation of the TP53 gene is related to many kinds of human cancers [122]. Cancer can be detected earlier and more accurately by extracting DNA from plasma to detect TP53 mutations [123]. Some studies have shown that high expression of TP53 plays a promoting role in the initial carcinogenesis of oral cancer [124].
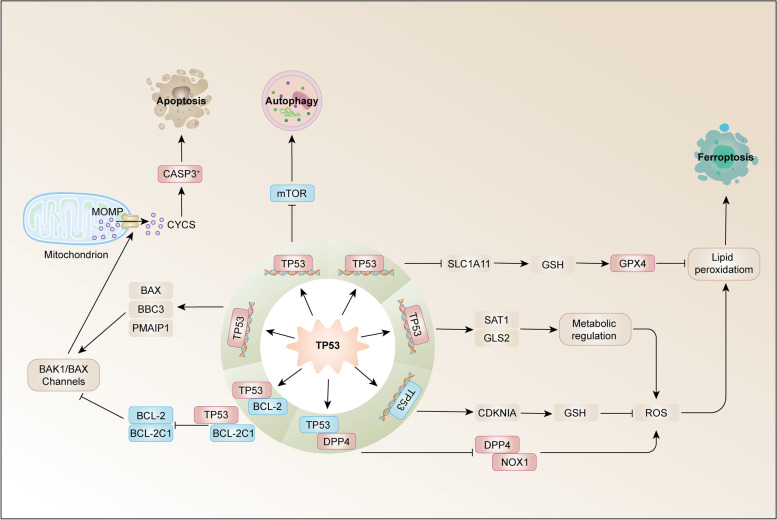
TP53 mutation occurs in the early stage of cancer. HPV-negative HNSCC has a typical TP53 mutation, while in HPV-positive cases, TP53 is downregulated by HPV oncogene E6 and usually does not mutate [125, 126]. During DNA damage and oncogene activation (carcinogenic stress), TP53 transcription factor, as a cell stress receptor, is activated, along with cyclin-dependent kinase inhibitor 1 A (CDKN1A, coding p21), which triggers cell cycle arrest and senescence, as well as BCL2-related X, apoptosis regulatory factor (Bax), TP53-upregulated apoptosis regulator (PUMA; also known as BBC3) and NOXA (also known as PMAIP1), thus triggering apoptosis [127–129] (Fig. 3). In malignant lesions, part of the TP53 protein is located in mitochondria. MOMP inhibits the antiapoptotic members Bcl2, Bcl-xl, and Mcl-1 and activates the proapoptotic Bak and Bax proteins of the Bcl2 family, thus triggering TP53-dependent apoptosis [130, 131]. P53 can also downregulate the mTOR signalling pathway to promote autophagy and induce the expression of genes, such as DRAM, PUMA, BAX, ISG20L1, and EI24, to promote autophagy [132, 133].
Fig. 3.
TP53 in HNSCC cell death. Tumour protein p53 (TP53) acts on mitochondria. MOMP inhibits BCL-2 and BCL-2L1, as antiapoptotic members, and activates BAX, BBC3 (also known as PUMA) and PMAIP1 (also known as NOXA), which are proapoptotic members of the Bcl2 family, thus triggering TP53-dependent apoptosis. P53 can also downregulate the mTOR signalling pathway to promote autophagy. TP53 induces iron death by inhibiting the expression of SLC7A11 or directly acting on diamine acetyltransferase SAT1 and mitochondrial glutaminase GLS2. TP53 inhibits iron death by inhibiting the activities of dipeptidyl peptidase 4 (DPP4) and NADPH oxidase 1 (NOX1) or inducing the expression of cyclin-dependent kinase inhibitor 1 A (CDKN1A). BAK1, BCL-2 homologous antagonist/killer 1; BBC3, BCL-2-binding component 3; CDKN1A, cyclin-dependent kinase inhibitor 1 A; CYCS, cytochrome C, somatic; GLS2, glutaminase 2; GPX4, glutathione peroxidase 4; GSH, glutathione; mTOR, mechanistic target of rapamycin kinase; PMAIP1, phorbol-12–myristate-13 acetate-induced protein 1; ROS, reactive oxygen species; SLC7A11, solute carrier family 7 member 11; SAT1, spermidine/spermine N1-acetyltransferase 1
TP53 causes apoptosis, and the activation of TP53 plays a vital role in inducing ferroptosis in some tumour cells [134]. The activation of TP53 can effectively reduce the expression of SLC7A11 at the mRNA and protein levels, while knockout of the p53 gene can relieve the inhibition of SLC7A11. [135] Inhibiting the expression of SLC7A11 by activating p53 can reduce the activity of System Xc- and eventually lead to ferroptosis [136]. In addition to inhibiting the movement of the System Xc- system, TP53 can directly act on diamine acetyltransferase SAT1 and mitochondrial glutaminase GLS2, regulate the metabolic process of glutamine and indirectly induce ferroptosis [137, 138]. Recent studies have found that erastin can activate TP53 and promote ferroptosis in cells. In the process of ferroptosis caused by erastin, the intracellular ROS produced can initiate TP53, resulting in a significant increase in the level of TP53 transcripts, thus further increasing the level of intracellular ROS [139]. However, in some cases, TP53 can also reduce the sensitivity of cells to ferroptosis. TP53 can activate p21 and delay ferroptosis in a transcription-dependent manner [140]. TP53 can also inhibit ferroptosis by inhibiting the activities of dipeptidyl peptidase 4 (DPP4) and NADPH oxidase 1 (NOX1) or inducing the expression of cyclin-dependent kinase inhibitor 1 A/p21 (CDKN1A/p21) [140, 141]. TP53 is at the core of the ferroptosis signalling network. On the one hand, it can be activated by erastin to enhance the sensitivity of cells to ferroptosis and inhibit tumorigenesis. On the other hand, TP53 protects normal cells from various stress factors. When some metabolic stress occurs, TP53 can reduce the sensitivity of cells to ferroptosis and maintain normal physiological function. Generally, the role of TP53 in the ferroptosis signalling network is very complex, and its specific mechanism in cancer treatment needs to be further studied [142].
KRAS signalling
Kirsten rat sarcoma virus oncogene (KRAS) mutation is one of many tumours’ most common carcinogenic factors. The KRAS gene encodes the KRAS protein. KRAS is a small GTP enzyme that acts as a molecular switch for various cellular processes by coupling membrane growth factor receptors with intracellular signalling pathways and transcription factors [143]. When KRAS is mutated, it loses the GTP hydrolase activity and then continues to be activated, promoting cell proliferation and carcinogenesis. We have been looking for drugs that can interfere with the binding of KRAS to GTP to block the carcinogenic effect of the mutant KRAS gene. Nevertheless, because of the particularity of the KRAS protein structure, there is no effective drug to treat KRAS mutant tumours thus far. Epidermal growth factor receptor (EGFR) is the upstream signalling pathway of KRAS [144, 145]. Through the study of targeted EGFR inhibitors, the upstream signalling pathway can be blocked to block the mutant expression of KRAS. EGFR is overexpressed in 80–90% of HNSCC and is associated with poor prognosis and treatment outcomes. Activation of KRAS signalling can promote metabolic reprogramming and autophagy in tumour cells to maintain proliferation and inhibit apoptosis [146–148]. Cancer cells with mutant RAS (including KRAS), such as HNSCC cells, are sensitive to the induction of ferroptosis, which may be due to the mutated expression of iron metabolism genes mediated by RAS, such as transferrin receptor TFRC, FTH1 and FTL. In HNSCC patients with overexpression of EGFR and cyclin D1, the combined effect of afatinib and palbociclib induces ageing by reducing the ATP pool, reducing antioxidant enzymes, and increasing the production of ROS, thus causing metabolic changes and enhancing the combined antitumour effect [149]. EGFR inhibitors are the only approved targeted drugs, but their efficacy is limited, and intrinsic and acquired drug resistance mechanisms remain unresolved [150].
NOTCH signalling
In HNSCC, NOTCH signalling pathway genes are changed at the transcriptional level, and NOTCH1 has been identified as a tumour suppressor gene [151, 152]. The NOTCH pathway transmits signals through transcriptional activation of the target genes HES1 and HEY1. The prognosis of patients with NOTCH1 mutation is poor, in which the direct downstream targets HES1 and HEY1 are overexpressed [153, 154]. HES, the target gene of the classical Notch signalling pathway, inhibits the expression of PTEN. When the NOTCH signal is suppressed, it downregulates the expression of HES, and HES suppresses PTEN and upregulates the expression of PTEN, which inhibits PI3K-AKT-mediated survival signal transduction and leads to tumour cell apoptosis [155, 156]. The activity of Notch is related to the inhibition of HPV E6 and E7 protein expression, which may provide additional selection pressure for the loss of NOTCH in HPV + HNSCC [157, 158]. In mature epithelium, the expression of p63 is the highest in basal epithelial cells, where it acts as an inhibitor of NOTCH1 expression and is downregulated during terminal differentiation, consistent with the upregulation of NOTCH1 [159]. ADAM10 and AJUBA inhibit HNSCC by promoting NOTCH receptor signal transduction in a haploid-deficient manner [160]. CD44 + cells overexpress HIF-1α in HNSCC. Inhibiting the expression of HIF-1α can inhibit the expression of NOTCH1 and block the metastasis rate and chemotherapy resistance of HNSCC cells. Therefore, NOTCH1 plays an essential role in maintaining the characteristics of HNSCC cells, promoting the malignancy of HNSCC cells and drug resistance to chemotherapy and radiation [161].
Hypoxia signalling
Hypoxia can aggravate tumour formation and treatment resistance. In HNSCC, hypoxia may lead to adverse reactions to radiotherapy and poor prognosis [162]. In solid tumours, tumour cells maintain their adaptation to hypoxia mainly by regulating hypoxia-inducible factor (HIF). The HIF family consists of three members, namely, HIF-1, HIF-2, and HIF-3, which can sense the changes in oxygen concentration in the microenvironment and regulate the pathway for cells to adapt to hypoxia [163, 164]. HIF-1 is a heterodimeric protein composed of the HIF-1α and HIF-1β subunits. Under normoxic conditions, HIF-1α is easily degraded by the ubiquitin protease system. At the same time, the degradation of HIF-1α is inhibited during hypoxia, which leads to its continuous accumulation, which makes cancer cells adapt to a hypoxic environment and provides conditions for the proliferation, invasion, and metastasis of cancer cells [164]. HIF-1 also interacts with P53 to promote caspase-3-dependent apoptosis [165, 166].
Hypoxia has a direct effect on a variety of cell death mechanisms and regulates the interaction between different pathways. During hypoxic stress, Bcl-2/adenovirus E1B 19-kDa protein interacting protein 3 (BNIP3) may induce autophagy by isolating Bcl2 or xl from the Bcl2 or Xl-Beclin1 complex, and then the shifted Beclin-1 can trigger autophagy [167]. In addition, caspase-mediated Beclin-1 cleavage inhibits autophagy and enhances apoptosis by promoting the mitochondrial release of proapoptotic factors and enhancing caspase-9 activity, a joint event in HNSCC [168, 169].
Metabolic reprogramming
Considering the massive demand for energy and biomass for cell survival, cancer cells show unique metabolic characteristics compared with normal cells. By participating in many core metabolic pathways, including glycolysis, mitochondrial metabolism, and the anabolism/catabolism of fats and amino acids, external signals are transmitted into cells to support cell survival [170, 171]. In other words, a series of changes cause tumour cells to reregulate adenine nucleoside triphosphate (ATP) production or increase glucose uptake to promote energy production. Under sufficient oxygen, tumour cells promote the rapid energy supply of glycolysis due to the change in the microenvironment, which is called the “Warburg effect” [172, 173]. Metabolic reprogramming in the TME was confirmed by analysing the metabolic-related gene prognostic index (MRGPI) in HNSCC.
System Xc- reverses glutamate transport and transduces cystine into cells. GPX4 catalyses the sulfhydryl binding of lipid peroxides (L-OHO) to reduce glutathione and converts harmful substances into nontoxic fatty alcohols (L-OH), thus preventing the ROS chain reaction and avoiding ferroptosis [174–176]. The upregulation of System Xc- expression may be related to chemotherapy resistance and tumour growth [177]. The absence of GPX4 increases phospholipid hydrogen peroxide and promotes lipoxygenase-mediated lipid peroxidation and eventually leads to ferroptosis [178]. Ferroptosis inhibitor protein 1 (FSP1) is an oxidoreductase that reduces coenzyme Q10 and produces a lipophilic free radical capture antioxidant to prevent lipid peroxidation and ferroptosis [179]. The tumour suppressor BAP1 enhances ferroptosis by regulating the expression of cystine transporter SLC7A11, thus improving the control of tumour growth [180, 181]. The concentration of glutathione (GSH) measured in metastatic tumours was higher than that of the corresponding primary tumours, indicating that GSH metabolism may affect the formation of HNSCC metastasis. In HPV-negative HNSCC, nonfunctional p53 leads to a decrease in mitochondrial OXPHOS and the prioritizing of glycolysis. Therefore, HPV-positive HNSCC may be more sensitive to mitochondrially targeted therapies, such as mitogens. Thus, the HPV oncoprotein E7 enhances ceramide-mediated mitosis and lethal mitosis to cope with chemotherapy-induced mitochondrial damage [182–184].
Therapy options in HNSCC
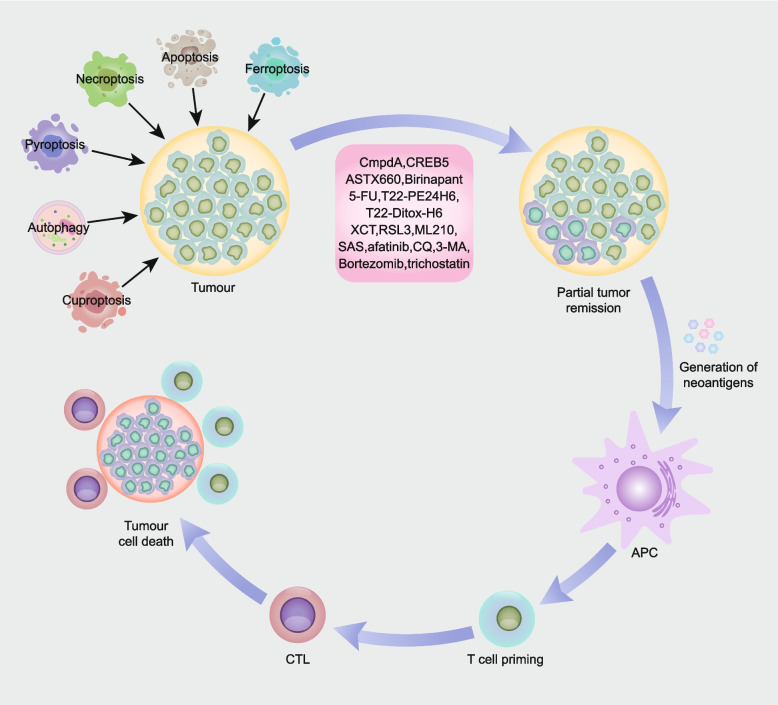
Surgical resection, radiotherapy, and chemotherapy are the main treatments for HNSCC, and identifying the most effective treatment and optimizing function preservation are our ideal goals. Immunogenic cell death (ICD) is a process whereby tumour cell death is induced and activates the body’s immune system to fight against dead cell antigens and endow dead tumour cells with immunogenicity [185, 186]. When ICD occurs, dead cells produce new antigenic epitopes and release DAMPs. Antigen-presenting cells (APCs) recognize and phagocytize dead cell antigens and present them to T cells, promoting the formation of antigen-specific cytotoxic T lymphocytes (CTLs) and activating the adaptive immune response to recognize and clear tumour antigens, resulting in long-term antitumour immune effects [187, 188] (Fig. 4).
Fig. 4.
Induction of cell death by therapeutic regimens evokes antitumour immune responses. Therapeutic modalities, including apoptosis, necroptosis, pyroptosis, ferroptosis, autophagy, and cuproptosis, induce death in cancer cells. Apoptosis, necroptosis, pyroptosis, ferroptosis, autophagy and cuproptosis in cancer generate abundant neoantigens, which are processed by antigen-presenting cells to promote the formation of antigen-specific cytotoxic T lymphocytes (CTLs), thereby evoking antitumour immunity. Agents that target apoptosis, necroptosis, pyroptosis, ferroptosis, and autophagy are noted in the figure (apoptosis inhibitor: CmpdA, CREB5; necroptosis inducer: ASTX660, birinapant; pyroptosis inducer: 5-FU, T22-PE24H6, T22-Ditox-H6; ferroptosis inducer: RSL3, ML210, SAS; ferroptosis inhibitor: XCT; autophagy inhibitor: afatinib, CQ, 3-MA, bortezomib, trichostatin)
Apoptosis in targeted therapy
The transcription factor NF-κB can regulate apoptosis and chemotherapy resistance [189]. NF-κB signal transduction can promote cisplatin resistance and increase the sensitivity of cancer cells to cisplatin therapy. Active NF-κB signalling in HNSCC cells can promote histone deacetylation and heterochromatin production to induce drug resistance to chemotherapy. Furthermore, targeted inhibition of histone deacetylase to destroy tumour drug resistance caused by NF-κB may be a therapeutic strategy [190, 191]. Other studies have shown that NF-κB phosphorylation at the serine 536 position plays a crucial role in regulating cisplatin resistance [192]. IKK phosphorylated NF-κB at serine 536, and NF-κB p65 gene knockout or overexpression of the NF-κB p65-serine 536 A mutant made drug-resistant cells sensitive to cisplatin. Therefore, blocking the activity of IKK/NF-κB with the IKK β inhibitor CmpdA may also be a strategy to improve the efficacy of cisplatin in HNCSS [193]. When APAF1 gene knockout or CRISPR-mediated caspase-9 deletion occurs, mitochondrial-dependent caspase activity is blocked. MOMP induces the activation of NF-κB and the production of TNF by degrading the IAP protein and upregulating the expression of NF-κB-induced kinase. Moreover, MOMP combined with caspase inhibition can transfer mitochondrial DNA to the cytoplasm through Bak1-Bax macropores, activate signalling in the cytoplasm, and promote the secretion of type I interferon, thus triggering the innate immune response [194–196]. CREB5 increases mitochondrial activity and ATP production through TOP1MT, promotes the expression of the antiapoptotic protein Bcl2, and increases the Bcl2/Bax ratio, thus inhibiting the mitochondrial apoptotic pathway and promoting cisplatin resistance in HNSCC cells. Dual targeting of CREB5 and TOP1MT is also a promising strategy to combat cisplatin resistance [197].
Necroptosis in targeted therapy
Approximately half of the necrosis in HNSCC can be attributed to necroptosis [36]. TNFα, TRAIL, or other death agonists bind to their receptors, activate RIPK1 and MLK1 and induce necroptosis, resulting in cell death [198]. RIP3 is a crucial regulator of necroptosis caused by TNF. Loss of RIP3 expression may reduce the sensitivity of cells to necroptosis stimulation, which in turn promotes cell survival and tumorigenesis [199]. Fumaric acid is a tumour metabolite. In many cancers, fumaric acid accumulation is caused by fumarate hydratase (FH) mutations [200]. The expression of EB virus (LMP1) in HNSCC leads to a decrease in fumaric acid and α-KG and inhibits the expression of RIP3 by DNA methylation, thus inhibiting necroptosis. This approach provides a new choice for the diagnosis and treatment of HNSCC [39]. CIAP/XIAP can inhibit cell death mediated by the mitochondrial and caspase-3 pathways, while the IAP1/XIAP antagonist ASTX660 can increase the sensitivity of HNSCC cells to TNF-α and TRAIL by promoting the expression of TP53 protein and induce necrotic apoptosis of HPV (-) cells by the cleavage of caspase-3 and caspase-8 [201, 202]. At the same time, IAP1/XIAP antagonists combined with radiotherapy had a more significant effect. This finding suggests that IAP1/XIAP antagonists, especially in combination with radiotherapy, are a potential treatment for HNSCC [203]. Studies have shown that FADD amplification is more common in HNSCC than in other cancers, with upregulation of FADD in HNSCC cell lines and minimal expression of BIRC3. CIAP can inhibit TNFα-FADD signalling to promote necroptosis [204]. The Smac analogue birinapant combined with TNFα promoted the degradation of CIAP1, the product of BIRC2, and activated caspase-8-mediated apoptosis and MLKL-mediated necroptosis in HNSCC. At the same time, birinapant plus radiotherapy may aggravate the death of HNSCC, which is a new direction in the treatment strategy [204].
Pyroptosis in targeted therapy
Among the pyroptosis pathways, the executive protein GSDME may be associated with chemoresistance in HNSCC [205, 206]. As a tumour suppressor, GSDME enhances antitumour immunity by activating cellular pyroptosis. In mice, the upregulation of GSDME can enhance the phagocytosis of macrophages and increase the number and function of tumour-infiltrating natural killer cells and CD8 + T lymphocytes [207]. In OSCC, tumours with high expression of GSDME increased the number of infiltrating CD8 + T cells, granzyme B, and M1 phenotypic macrophages. At the same time, cellular pyroptosis induced by GSDME-mediated chemotherapy played an essential role in the antitumour response [206, 208]. The activation of pyroptosis can trigger the efficacy of various antineoplastic drugs. Paclitaxel and cisplatin can activate GSDME and release inflammatory bodies, transforming the cell death pathway from apoptosis to pyroptosis, thus inhibiting the proliferation of cancer cells [209–211]. 5-Fluorouracil (5-FU) converts caspase-3-dependent apoptosis induced by chemotherapy drugs to pyroptosis by triggering the caspase-3/GSDME signalling pathway [212]. In the HNSCC mouse model with high expression of CXCR4, T22-PE24H6 and T22-Ditox-H6 nanotoxins showed strong antitumour effects by inducing caspase-3/GSDME-dependent pyroptosis. At the same time, recurrent or metastatic HNSCC may be sensitive to nanotoxin therapy. This mechanism of cell death, which triggers alternative apoptosis, is also a new therapeutic strategy for chemotherapy and radiotherapy resistance in patients with HNSCC [213–216].
Ferroptosis in targeted therapy
Ferroptosis inducers can act with more traditional drugs, such as cisplatin, to inhibit tumour growth in mouse models of head and neck cancer [217]. SLC7A11 (XCT) is a Na+-dependent cysteine/glutamate exchanger encoded by the SLC7A11 and SLC3A2 genes that can promote the synthesis of glutathione [218]. Inhibiting the activity of XCT in drug-resistant HNSCC cells induces glutathione depletion, thus promoting ferroptosis and enhancing the cytotoxicity of cisplatin in drug-resistant HNSCC cells [217, 219]. IFNγ released by CD8 + T cells induces ferroptosis of tumour cells by activating the JAK-STAT1 pathway, inhibiting cysteine uptake, reducing LOOH to nontoxic LOH, and downregulating the expression of SLC3A2 and SLC7A11 [220–222]. For example, GPX4 inhibitory molecules such as RSL3 and ML210 help to inhibit enzyme activity, resulting in excessive lipid peroxidation, leading to ferroptosis in tumour cells. [223] System Xc- inhibitors such as sulfasalazine and erastin can block the activity of System Xc- and entry of cystine into cells, thus inducing ferroptosis in tumour cells [19]. HMGB1 is related to the immunogenicity of tumour cell ferroptosis. Tumour cells that produce ferroptosis acetylate HMGB1 and release HMGB1 through autophagy. At the same time, a large amount of HMGB1 was found in the serum and tissues of HNSCC, indicating that HMGB1 stimulates the chemotaxis of regulatory T cells (Tregs) and promotes their immunosuppressive function [224, 225]. Ferroptosis can also induce the expression of cyclooxygenase-2 and the release of prostaglandin E2 (PGE2). In addition, the release of PGE2 is positively correlated with the immune escape of tumour cells [226].
Autophagy in targeted therapy
In HNSCC, IFNα has the function of immune regulation and inhibition of tumour cell proliferation [227]. On the one hand, IFNα can resist expansion and induce apoptosis and can also activate autophagy in human nasopharyngeal carcinoma cells. This autophagy has a cytoprotective effect and can promote IFNα-mediated apoptosis. On the other hand, IFNα combined with the autophagy inhibitors wortmannin and HCQ can increase the antitumour activity of IFNα, thus inhibiting autophagy, which is also a new strategy for the treatment of nasopharyngeal carcinoma [228, 229]. Afatinib is a second-generation tyrosine kinase inhibitor (TKI) that exerts its antitumour effect by inhibiting mTORC1-mediated autophagy in HNSCC [230]. Afatinib induced upregulation of Redd1 expression through ROS stimulation, resulting in downregulation of TSC1 and low conversion of LC3B-II, and then it inhibited mTORC1 to promote autophagy in HNSCC. [231] The autophagy inhibitors CQ and 3-MA block autophagy, significantly enhancing the sensitivity of tumour cells to afatinib-induced apoptosis. At the same time, the combination of afatinib and autophagy inhibitors may eliminate HNSCC tumour stem cells, which is also a potential clinical treatment [231, 232]. AMPK promotes autophagy by activating ULK1, while mTOR inhibits autophagy by phosphorylating ULK1 and disrupting the interaction between ULK1 and AMPK [233]. Inhibition of histone deacetylase 6 (HDAC6) activity can induce autophagy by affecting the activity of the autophagy promoter ULK1 in HNSCC cells through mTOR. Inhibition of HDAC6 can enhance the cytotoxicity of the proteasome inhibitor bortezomib to induce apoptosis. At the same time, the combination of bortezomib and the histone deacetylase inhibitor trichostatin inhibited HDAC6 activity, reduced autophagy induction, and significantly enhanced bortezomib-induced apoptosis of HNSCC cells. Therefore, targeting HDAC6 for chemotherapy-resistant HNSCC is a very effective treatment strategy [234, 235].
Cuproptosis in targeted therapy
It was found that the content of copper in serum and tumour tissues increased in patients with breast cancer, cervical cancer, ovarian cancer, lung cancer, gastric cancer, bladder cancer, thyroid cancer, oral cancer, and pancreatic cancer, which was closely related to the stage and progression of some cancers. [236–239]. The characteristics of copper enrichment in tumour tissues are becoming attractive targets in developing anticancer drugs. Copper chelators such as D-penicillamine, tetrathiomolybdate (TTM), and triamolybdate reduce the bioavailability of copper by directly combining with copper. Copper ion carriers such as clioquinol, 22-carbon hexanoic acid, thiosemicarbazone, and disulfiram (DSF) can increase intracellular copper levels. Through the production of ROS, proteasome inhibition and induction of apoptosis play antitumour roles [240]. Copper induces necroptosis and toxic damage through ROS-dependent DNA damage, and the necroptosis inhibitor Nec-1 or z-VAD-FMK can alleviate Cu2+-induced death [241]. Targeting the regulation of cellular ferroptosis induced by intracellular copper ions can be used to improve the sensitivity of the antitumour effect. Excess copper accumulation leads to cuproptosis by inducing DLAT aggregation and enhancing ROS and inactivating GPX4. Overloaded iron ions induce potent ferroptosis, which synergizes with cuproptosis and DOX-induced apoptosis to kill tumour cells [242]. The combination of DSF and antineoplastic drugs can enhance the antitumour effect and have a far-reaching impact on treating human cancer. When nasopharyngeal carcinoma cell lines and nonmalignant nasopharyngeal epithelial cells were treated with DSF and Cu2+, cell viability was significantly inhibited compared with that of DSF alone, and RNA-seq analysis showed that the ferroptosis pathway was involved in DSF/Cu-induced cell death. Seven ferroptosis-related genes were changed considerably, which confirmed the copper-dependent ferroptosis induction of DSF on nasopharyngeal carcinoma cells [243]. Although there are few studies on targeted therapy for HNSCC cuproptosis, with more in-depth research, there will be a new breakthrough in the treatment of HNSCC.
Conclusions and perspectives
Since an increasing number of RCDs have been discovered, the mechanism of RCD and its interaction with tumour cells have been gradually revealed. Many HNSCC treatment strategies induce cell death and prevent tumour growth. In this context, targeting apoptotic cell death appears to be an increasingly promising strategy to improve therapeutic outcomes in cancer therapy. However, the induction of tumour cell death is accompanied by the end of normal cells, and apoptosis, necroptosis, pyroptosis, autophagy, ferroptosis, and cuproptosis may play an antitumour role under different conditions and may also promote the growth of tumour cells. Therefore, we must explore a more targeted and personalized treatment to resist tumour cell proliferation. Fully understanding the development of HNSCC and apoptosis, necroptosis, pyroptosis, autophagy, and the mechanism of action of ferroptosis and cuproptosis for the treatment of HNSCC will provide new therapeutic targets and strategies that, combined with radiotherapy, chemotherapy, and targeted therapy, can effectively eliminate tumour cells and protect healthy cells at the same time, which may prolong the survival of HNSCC patients and is also a treatment direction of great research significance.
Acknowledgements
Not applicable.
Authors’ contributions
MXH、MSS and YZN conceived the manuscript. XYT wrote the manuscript. JXJ and WJR drew figures. ZYX participated in the revision of the manuscript. All authors have read and approved the article.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuting Xue, Xuejiao Jiang and Junrong Wang contributed equally to this work.
Contributor Information
Yuting Xue, Email: xueyuting4869@163.com.
Xuejiao Jiang, Email: 752439129@qq.com.
Junrong Wang, Email: wangjunrong1996@163.com.
Yuxuan Zong, Email: 1140895999@qq.com.
Zhennan Yuan, Email: yuanzhennan123@163.com.
Susheng Miao, Email: drmiaosusheng@126.com.
Xionghui Mao, Email: xhmao2022@163.com.
References
- 1.Mehanna H, Paleri V, West CML, Nutting C. Head and neck cancer-Part 1: Epidemiology, presentation, and prevention. BMJ-British Med J. 2010;341:6. doi: 10.1136/bmj.c4684. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA-Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbone M, Amelio I, Affar EB, Brugarolas J, Cannon-Albright LA, Cantley LC, et al. Consensus report of the 8 and 9th Weinman Symposia on Gene x Environment Interaction in carcinogenesis: novel opportunities for precision medicine. Cell Death Differ. 2018;25:1885–904. doi: 10.1038/s41418-018-0213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geng X, Zhang Y, Zeng Z, Zhu Z, Wang H, Yu W, et al. Molecular Characteristics, Prognostic Value, and Immune Characteristics of m(6)A Regulators Identified in Head and Neck Squamous Cell Carcinoma. Front Oncol. 2021;11:629718. doi: 10.3389/fonc.2021.629718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow LQM. Head and Neck Cancer. N Engl J Med. 2020;382:60–72. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Cai H, Li Z, Ren L, Ma X, Zhu H, et al. A tumor cell membrane-coated self-amplified nanosystem as a nanovaccine to boost the therapeutic effect of anti-PD-L1 antibody. Bioact Mater. 2023;21:299–312. doi: 10.1016/j.bioactmat.2022.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichim G, Gibert B, Adriouch S, Brenner C, Davoust N, Desagher S, et al. Keeping Cell Death Alive: An Introduction into the French Cell Death Research Network. Biomolecules. 2022;12:901. doi: 10.3390/biom12070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol Rev. 2019;99:1765–817. doi: 10.1152/physrev.00022.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SY, Ju MK, Jeon HM, Jeong EK, Lee YJ, Kim CH, et al. Regulation of Tumor Progression by Programmed Necrosis. Oxid Med Cell Longev. 2018;2018:3537471. doi: 10.1155/2018/3537471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Rev Clin Oncol. 2021;18:280–96. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 13.Bedoui S, Herold MJ, Strasser A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat Rev Mol Cell Biol. 2020;21:678–95. doi: 10.1038/s41580-020-0270-8. [DOI] [PubMed] [Google Scholar]
- 14.Bonora M, Giorgi C, Pinton P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat Rev Mol Cell Biol. 2022;23:266–85. doi: 10.1038/s41580-021-00433-y. [DOI] [PubMed] [Google Scholar]
- 15.Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17:395–417. doi: 10.1038/s41571-020-0341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalkavan H, Green DR. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018;25:46–55. doi: 10.1038/cdd.2017.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol. 2019;20:175–93. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15:738–52. doi: 10.1038/s41575-018-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santha S, Ling X, Aljahdali IAM, Rasam SS, Wang X, Liao J, et al. Mutant Kras as a Biomarker Plays a Favorable Role in FL118-Induced Apoptosis, Reactive Oxygen Species (ROS) Production and Modulation of Survivin, Mcl-1 and XIAP in Human Bladder Cancer. Cancers (Basel). 2020;12:3413. doi: 10.3390/cancers12113413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang WC, Chan SH, Jang TH, Chang JW, Ko YC, Yen TC, et al. miRNA-491-5p and GIT1 serve as modulators and biomarkers for oral squamous cell carcinoma invasion and metastasis. Cancer Res. 2014;74:751–64. doi: 10.1158/0008-5472.CAN-13-1297. [DOI] [PubMed] [Google Scholar]
- 22.Xu R, Xu R, Wang Y, Wang W, Jiang L, Gong S. G-Protein-Coupled Receptor Kinase-Interacting Protein 1 (GIT1) Promotes Head and Neck Squamous Cell Carcinoma Metastases via Activating the PI3K/AKT/mTOR Signal Pathway. Comput Math Methods Med. 2022;2022:6881932. doi: 10.1155/2022/6881932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Morgan EL, Chen Z, Van Waes C. Regulation of NFκB Signalling by Ubiquitination: A Potential Therapeutic Target in Head and Neck Squamous Cell Carcinoma? Cancers (Basel). 2020;12:2877. doi: 10.3390/cancers12102877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrà G, Lingua MF, Maffeo B, Taulli R, Morotti A. P53 vs NF-κB: the role of nuclear factor-kappa B in the regulation of p53 activity and vice versa. Cell Mol Life Sci. 2020;77:4449–58. doi: 10.1007/s00018-020-03524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Witt Hamer PC, Mir SE, Noske D, Van Noorden CJ, Würdinger T. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res. 2011;17:4200–7. doi: 10.1158/1078-0432.CCR-10-2537. [DOI] [PubMed] [Google Scholar]
- 26.Moser R, Xu C, Kao M, Annis J, Lerma LA, Schaupp CM, et al. Functional kinomics identifies candidate therapeutic targets in head and neck cancer. Clin Cancer Res. 2014;20:4274–88. doi: 10.1158/1078-0432.CCR-13-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirai H, Arai T, Okada M, Nishibata T, Kobayashi M, Sakai N, et al. MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor efficacy of various DNA-damaging agents, including 5-fluorouracil. Cancer Biol Ther. 2010;9:514–22. doi: 10.4161/cbt.9.7.11115. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka N, Patel AA, Wang J, Frederick MJ, Kalu NN, Zhao M, et al. Wee-1 Kinase Inhibition Sensitizes High-Risk HPV + HNSCC to Apoptosis Accompanied by Downregulation of MCl-1 and XIAP Antiapoptotic Proteins. Clin Cancer Res. 2015;21:4831–44. doi: 10.1158/1078-0432.CCR-15-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ow TJ, Thomas C, Fulcher CD, Chen J, Lopez A, Reyna DE, et al. Apoptosis signaling molecules as treatment targets in head and neck squamous cell carcinoma. Laryngoscope. 2020;130:2643–9. doi: 10.1002/lary.28441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negroni A, Colantoni E, Cucchiara S, Stronati L. Necroptosis in Intestinal Inflammation and Cancer: New Concepts and Therapeutic Perspectives. Biomolecules. 2020;10:1431. doi: 10.3390/biom10101431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2017;18:127–36. doi: 10.1038/nrm.2016.149. [DOI] [PubMed] [Google Scholar]
- 32.Galluzzi L, Kepp O, Chan FK, Kroemer G. Necroptosis: Mechanisms and Relevance to Disease. Annu Rev Pathol. 2017;12:103–30. doi: 10.1146/annurev-pathol-052016-100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan J, Wan P, Choksi S, Liu ZG. Necroptosis and tumor progression. Trends Cancer. 2022;8:21–7. doi: 10.1016/j.trecan.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, Dong G, Sheng C. Targeting necroptosis in anticancer therapy: mechanisms and modulators. Acta Pharm Sin B. 2020;10:1601–18. doi: 10.1016/j.apsb.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang T, Wang Y, Inuzuka H, Wei W. Necroptosis pathways in tumorigenesis. Semin Cancer Biol. 2022;86:32–40. doi: 10.1016/j.semcancer.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Huang S, Zeng L, Li K, Yang L, Gao S, et al. Necroptosis in head and neck squamous cell carcinoma: characterization of clinicopathological relevance and in vitro cell model. Cell Death Dis. 2020;11:391. doi: 10.1038/s41419-020-2538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh V, Huang E, Pathak V, Willard BB, Allende DS, Nagy LE. Phosphoproteomics identifies pathways underlying the role of receptor-interaction protein kinase 3 in alcohol-associated liver disease and uncovers apoptosis signal-regulating kinase 1 as a target. Hepatol Commun. 2022;6:2022–41. doi: 10.1002/hep4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormick KD, Ghosh A, Trivedi S, Wang L, Coyne CB, Ferris RL, et al. Innate immune signaling through differential RIPK1 expression promote tumor progression in head and neck squamous cell carcinoma. Carcinogenesis. 2016;37:522–9. doi: 10.1093/carcin/bgw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi F, Zhou M, Shang L, Du Q, Li Y, Xie L, et al. EBV(LMP1)-induced metabolic reprogramming inhibits necroptosis through the hypermethylation of the RIP3 promoter. Theranostics. 2019;9:2424–38. doi: 10.7150/thno.30941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uzunparmak B, Gao M, Lindemann A, Erikson K, Wang L, Lin E, et al. Caspase-8 loss radiosensitizes head and neck squamous cell carcinoma to SMAC mimetic-induced necroptosis. JCI Insight. 2020;5:e139837. [DOI] [PMC free article] [PubMed]
- 41.Qin X, Ma D, Tan YX, Wang HY, Cai Z. The role of necroptosis in cancer: A double-edged sword? Biochim Biophys Acta Rev Cancer. 2019;1871:259–66. doi: 10.1016/j.bbcan.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Taraborrelli L, Peltzer N, Montinaro A, Kupka S, Rieser E, Hartwig T, et al. LUBAC prevents lethal dermatitis by inhibiting cell death induced by TNF, TRAIL and CD95L. Nat Commun. 2018;9:3910. doi: 10.1038/s41467-018-06155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenbaum SR, Wilski NA, Aplin AE. Fueling the Fire: Inflammatory Forms of Cell Death and Implications for Cancer Immunotherapy. Cancer Discov. 2021;11:266–81. doi: 10.1158/2159-8290.CD-20-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–6. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 45.Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kovacs SB, Miao EA, Gasdermins Effectors of Pyroptosis. Trends Cell Biol. 2017;27:673–84. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson AG, Wein T, Mayer ML, Duncan-Lowey B, Yirmiya E, Oppenheimer-Shaanan Y, et al. Bacterial gasdermins reveal an ancient mechanism of cell death. Science. 2022;375:221–5. doi: 10.1126/science.abj8432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun P, Zhong J, Liao H, Loughran P, Mulla J, Fu G, et al. Hepatocytes Are Resistant to Cell Death From Canonical and Non-Canonical Inflammasome-Activated Pyroptosis. Cell Mol Gastroenterol Hepatol. 2022;13:739–57. doi: 10.1016/j.jcmgh.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–20. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 50.Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang Y, et al. Pyroptosis: A new frontier in cancer. Biomed Pharmacother. 2020;121:109595. doi: 10.1016/j.biopha.2019.109595. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–8. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. Embo j. 2016;35:1766–78. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minton K. Pyroptosis heats tumour immunity. Nat Rev Drug Discov. 2020;19:309. doi: 10.1038/d41573-020-00062-8. [DOI] [PubMed] [Google Scholar]
- 54.Shao F. Gasdermins: making pores for pyroptosis. Nat Rev Immunol. 2021;21:620–1. doi: 10.1038/s41577-021-00602-2. [DOI] [PubMed] [Google Scholar]
- 55.Uz U, Eskiizmir G. Association Between Interleukin-6 and Head and Neck Squamous Cell Carcinoma: A Systematic Review. Clin Exp Otorhinolaryngol. 2021;14:50–60. doi: 10.21053/ceo.2019.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu X, Xia S, Zhang Z, Wu H, Lieberman J. Channelling inflammation: gasdermins in physiology and disease. Nat Rev Drug Discov. 2021;20:384–405. doi: 10.1038/s41573-021-00154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newton K, Dixit VM, Kayagaki N. Dying cells fan the flames of inflammation. Science. 2021;374:1076–80. doi: 10.1126/science.abi5934. [DOI] [PubMed] [Google Scholar]
- 58.Minton K. Pyroptosis heats tumour immunity. Nat Rev Immunol. 2020;20:274–5. doi: 10.1038/s41577-020-0297-2. [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, Zhu D, Wang G, Wang D, Zhou H, Liu X, et al. Pro-Inflammatory Cytokine IL-1β Up-Regulates CXC Chemokine Receptor 4 via Notch and ERK Signaling Pathways in Tongue Squamous Cell Carcinoma. PLoS ONE. 2015;10:e0132677. doi: 10.1371/journal.pone.0132677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 61.Huang CF, Chen L, Li YC, Wu L, Yu GT, Zhang WF, et al. NLRP3 inflammasome activation promotes inflammation-induced carcinogenesis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36:116. doi: 10.1186/s13046-017-0589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Luo Q, Feng X, Zhang R, Li J, Chen F. NLRP3 promotes tumor growth and metastasis in human oral squamous cell carcinoma. BMC Cancer. 2018;18:500. doi: 10.1186/s12885-018-4403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W, et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579:421–6. doi: 10.1038/s41586-020-2079-1. [DOI] [PubMed] [Google Scholar]
- 64.De Schutter E, Croes L, Ibrahim J, Pauwels P, Op de Beeck K, Vandenabeele P, et al. GSDME and its role in cancer: From behind the scenes to the front of the stage. Int J Cancer. 2021;148:2872–83. doi: 10.1002/ijc.33390. [DOI] [PubMed] [Google Scholar]
- 65.Ibrahim J, De Schutter E, Op de Beeck K. GSDME: A Potential Ally in Cancer Detection and Treatment. Trends Cancer. 2021;7:392–4. doi: 10.1016/j.trecan.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 66.Li Z, Shen L, Li Y, Shen L, Li N. Identification of pyroptosis-related gene prognostic signature in head and neck squamous cell carcinoma. Cancer Med. 2022. 10.1002/cam4.4825. [DOI] [PMC free article] [PubMed]
- 67.Masjedi A, Hashemi V, Hojjat-Farsangi M, Ghalamfarsa G, Azizi G, Yousefi M, et al. The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer. Biomed Pharmacother. 2018;108:1415–24. doi: 10.1016/j.biopha.2018.09.177. [DOI] [PubMed] [Google Scholar]
- 68.Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553–72. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 69.Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 70.Yan B, Ai Y, Sun Q, Ma Y, Cao Y, Wang J, et al. Membrane Damage during Ferroptosis Is Caused by Oxidation of Phospholipids Catalyzed by the Oxidoreductases POR and CYB5R1. Mol Cell. 2021;81:355–69.e10. doi: 10.1016/j.molcel.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 71.Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22:381–96. doi: 10.1038/s41568-022-00459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friedmann Angeli JP, Krysko DV, Conrad M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat Rev Cancer. 2019;19:405–14. doi: 10.1038/s41568-019-0149-1. [DOI] [PubMed] [Google Scholar]
- 74.Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M, et al. Human Haploid Cell Genetics Reveals Roles for Lipid Metabolism Genes in Nonapoptotic Cell Death. ACS Chem Biol. 2015;10:1604–9. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–8. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li C, Dong X, Du W, Shi X, Chen K, Zhang W, et al. LKB1-AMPK axis negatively regulates ferroptosis by inhibiting fatty acid synthesis. Signal Transduct Target Ther. 2020;5:187. doi: 10.1038/s41392-020-00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L, et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol. 2020;22:225–34. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu F, Tang L, Li Q, Chen L, Pan Y, Yin Z, et al. Single-cell transcriptomics uncover the key ferroptosis regulators contribute to cancer progression in head and neck squamous cell carcinoma. Front Mol Biosci. 2022;9:962742. doi: 10.3389/fmolb.2022.962742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo L, Lu J, Gao J, Li M, Wang H, Zhan X. The function of SNHG7/miR-449a/ACSL1 axis in thyroid cancer. J Cell Biochem. 2020;121:4034–42. doi: 10.1002/jcb.29569. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Q, Zhou W, Yu S, Ju Y, To SKY, Wong AST, et al. Metabolic reprogramming of ovarian cancer involves ACSL1-mediated metastasis stimulation through upregulated protein myristoylation. Oncogene. 2021;40:97–111. doi: 10.1038/s41388-020-01516-4. [DOI] [PubMed] [Google Scholar]
- 82.Wu J, Minikes AM, Gao M, Bian H, Li Y, Stockwell BR, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:402–6. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu ZW, Wen YH, Ma RQ, Chen L, Zeng XL, Wen WP, et al. Ferroptosis Driver SOCS1 and Suppressor FTH1 Independently Correlate With M1 and M2 Macrophage Infiltration in Head and Neck Squamous Cell Carcinoma. Front Cell Dev Biol. 2021;9:727762. doi: 10.3389/fcell.2021.727762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakatogawa H. Mechanisms governing autophagosome biogenesis. Nat Rev Mol Cell Biol. 2020;21:439–58. doi: 10.1038/s41580-020-0241-0. [DOI] [PubMed] [Google Scholar]
- 85.Zhao YG, Codogno P, Zhang H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat Rev Mol Cell Biol. 2021;22:733–50. doi: 10.1038/s41580-021-00392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–64. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 87.Collier JJ, Guissart C, Oláhová M, Sasorith S, Piron-Prunier F, Suomi F, et al. Developmental Consequences of Defective ATG7-Mediated Autophagy in Humans. N Engl J Med. 2021;384:2406–17. doi: 10.1056/NEJMoa1915722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Strzyz P. Chaperoning solid aggregates for autophagy. Nat Rev Mol Cell Biol. 2022;23:387. doi: 10.1038/s41580-022-00482-x. [DOI] [PubMed] [Google Scholar]
- 90.Lőrincz P, Juhász G. Autophagosome-Lysosome Fusion. J Mol Biol. 2020;432:2462–82. doi: 10.1016/j.jmb.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 91.Mizushima N, Levine B. Autophagy in Human Diseases. N Engl J Med. 2020;383:1564–76. doi: 10.1056/NEJMra2022774. [DOI] [PubMed] [Google Scholar]
- 92.Ganley I. The Importance of Being Autophagic. N Engl J Med. 2021;384:2449–50. doi: 10.1056/NEJMe2107506. [DOI] [PubMed] [Google Scholar]
- 93.Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717–29. doi: 10.1101/gad.2016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wirawan E, Vanden Berghe T, Lippens S, Agostinis P, Vandenabeele P. Autophagy: for better or for worse. Cell Res. 2012;22:43–61. doi: 10.1038/cr.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao L, Dou ZC, Ren WH, Li SM, Liang X, Zhi KQ. CircCDR1as upregulates autophagy under hypoxia to promote tumor cell survival via AKT/ERK(½)/mTOR signaling pathways in oral squamous cell carcinomas. Cell Death Dis. 2019;10:745. doi: 10.1038/s41419-019-1971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meng X, Lou QY, Yang WY, Wang YR, Chen R, Wang L, et al. The role of non-coding RNAs in drug resistance of oral squamous cell carcinoma and therapeutic potential. Cancer Commun (Lond) 2021;41:981–1006. doi: 10.1002/cac2.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wei Q, Pinho S, Dong S, Pierce H, Li H, Nakahara F, et al. MAEA is an E3 ubiquitin ligase promoting autophagy and maintenance of haematopoietic stem cells. Nat Commun. 2021;12:2522. doi: 10.1038/s41467-021-22749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu H, Ding J, Köhnlein K, Urban N, Ori A, Villavicencio-Lorini P, et al. The GID ubiquitin ligase complex is a regulator of AMPK activity and organismal lifespan. Autophagy. 2020;16:1618–34. doi: 10.1080/15548627.2019.1695399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lu T, Li Y, Pan M, Yu D, Wang Z, Liu C, et al. TBC1D14 inhibits autophagy to suppress lymph node metastasis in head and neck squamous cell carcinoma by downregulating macrophage erythroblast attacher. Int J Biol Sci. 2022;18:1795–812. doi: 10.7150/ijbs.68992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao L, Zhang Q, Li S, Zheng J, Ren W, Zhi K. Circ-PKD2 promotes Atg13-mediated autophagy by inhibiting miR-646 to increase the sensitivity of cisplatin in oral squamous cell carcinomas. Cell Death Dis. 2022;13:192. doi: 10.1038/s41419-021-04497-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ding S, Abudupataer M, Zhou Z, Chen J, Li H, Xu L, et al. Histamine deficiency aggravates cardiac injury through miR-206/216b-Atg13 axis-mediated autophagic-dependant apoptosis. Cell Death Dis. 2018;9:694. doi: 10.1038/s41419-018-0723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Y, Zhang L, Zhou F. Cuproptosis: a new form of programmed cell death. Cell Mol Immunol. 2022;19:867–8. doi: 10.1038/s41423-022-00866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–61. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li SR, Bu LL, Cai L. Cuproptosis: lipoylated TCA cycle proteins-mediated novel cell death pathway. Signal Transduct Target Ther. 2022;7:158. doi: 10.1038/s41392-022-01014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tang D, Chen X, Kroemer G. Cuproptosis: a copper-triggered modality of mitochondrial cell death. Cell Res. 2022;32:417–8. doi: 10.1038/s41422-022-00653-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duan WJ, He RR. Cuproptosis: copper-induced regulated cell death. Sci China Life Sci. 2022;65:1680–2. doi: 10.1007/s11427-022-2106-6. [DOI] [PubMed] [Google Scholar]
- 107.Zhao J, Guo S, Schrodi SJ, He D. Cuproptosis and cuproptosis-related genes in rheumatoid arthritis: Implication, prospects, and perspectives. Front Immunol. 2022;13:930278. doi: 10.3389/fimmu.2022.930278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Inesi G. Molecular features of copper binding proteins involved in copper homeostasis. IUBMB Life. 2017;69:211–7. doi: 10.1002/iub.1590. [DOI] [PubMed] [Google Scholar]
- 109.Ryumon S, Okui T, Kunisada Y, Kishimoto K, Shimo T, Hasegawa K, et al. Ammonium tetrathiomolybdate enhances the antitumor effect of cisplatin via the suppression of ATPase copper transporting beta in head and neck squamous cell carcinoma. Oncol Rep. 2019;42:2611–21. doi: 10.3892/or.2019.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J, Chen S, Liao Y, Wang H, Zhou D, Zhang B. Arecoline Is Associated With Inhibition of Cuproptosis and Proliferation of Cancer-Associated Fibroblasts in Oral Squamous Cell Carcinoma: A Potential Mechanism for Tumor Metastasis. Front Oncol. 2022;12:925743. doi: 10.3389/fonc.2022.925743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Voli F, Valli E, Lerra L, Kimpton K, Saletta F, Giorgi FM, et al. Intratumoral Copper Modulates PD-L1 Expression and Influences Tumor Immune Evasion. Cancer Res. 2020;80:4129–44. doi: 10.1158/0008-5472.CAN-20-0471. [DOI] [PubMed] [Google Scholar]
- 112.Menezes MJ, Guo Y, Zhang J, Riley LG, Cooper ST, Thorburn DR, et al. Mutation in mitochondrial ribosomal protein S7 (MRPS7) causes congenital sensorineural deafness, progressive hepatic and renal failure and lactic acidemia. Hum Mol Genet. 2015;24:2297–307. doi: 10.1093/hmg/ddu747. [DOI] [PubMed] [Google Scholar]
- 113.Tang S, Zhao L, Wu XB, Wang Z, Cai LY, Pan D, et al. Identification of a Novel Cuproptosis-Related Gene Signature for Prognostic Implication in Head and Neck Squamous Carcinomas. Cancers (Basel). 2022;14:3986. doi: 10.3390/cancers14163986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu H, Guo H, Jian Z, Cui H, Fang J, Zuo Z, et al. Copper Induces Oxidative Stress and Apoptosis in the Mouse Liver. Oxid Med Cell Longev. 2020;2020:1359164. doi: 10.1155/2020/1359164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gao W, Huang Z, Duan J, Nice EC, Lin J, Huang C. Elesclomol induces copper-dependent ferroptosis in colorectal cancer cells via degradation of ATP7A. Mol Oncol. 2021;15:3527–44. doi: 10.1002/1878-0261.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18:269–82. doi: 10.1038/nrc.2018.11. [DOI] [PubMed] [Google Scholar]
- 117.Petroni G, Buqué A, Coussens LM, Galluzzi L. Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment. Nat Rev Drug Discov. 2022;21:440–62. doi: 10.1038/s41573-022-00415-5. [DOI] [PubMed] [Google Scholar]
- 118.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Soussi T, Wiman KG. TP53: an oncogene in disguise. Cell Death Differ. 2015;22:1239–49. doi: 10.1038/cdd.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feldser DM, Kostova KK, Winslow MM, Taylor SE, Cashman C, Whittaker CA, et al. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature. 2010;468:572–5. doi: 10.1038/nature09535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu J, Cao J, Topatana W, Juengpanich S, Li S, Zhang B, et al. Targeting mutant p53 for cancer therapy: direct and indirect strategies. J Hematol Oncol. 2021;14:157. doi: 10.1186/s13045-021-01169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18:89–102. doi: 10.1038/nrc.2017.109. [DOI] [PubMed] [Google Scholar]
- 123.East MP, Laitinen T, Asquith CRM. PIP5K1A: a potential target for cancers with KRAS or TP53 mutations. Nat Rev Drug Discov. 2020;19:436. doi: 10.1038/d41573-020-00067-3. [DOI] [PubMed] [Google Scholar]
- 124.Pickering CR, Zhang J, Yoo SY, Bengtsson L, Moorthy S, Neskey DM, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013;3:770–81. doi: 10.1158/2159-8290.CD-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, et al. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Castellsagué X, Alemany L, Quer M, Halec G, Quirós B, Tous S, et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J Natl Cancer Inst. 2016;108:djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 127.Baugh EH, Ke H, Levine AJ, Bonneau RA, Chan CS. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018;25:154–60. doi: 10.1038/cdd.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu Q, Chen Z, Liu M, Meng Y, Li X, Li B, et al. Exploring the potential targets of Sanshimao formula for hepatocellular carcinoma treatment by a method of network pharmacology combined with molecular biology. J Ethnopharmacol. 2022;297:115531. doi: 10.1016/j.jep.2022.115531. [DOI] [PubMed] [Google Scholar]
- 129.Jia X, Liu H, Ren X, Li P, Song R, Li X, et al. Nucleolar protein NOC4L inhibits tumorigenesis and progression by attenuating SIRT1-mediated p53 deacetylation. Oncogene. 2022;41:4474–4484. doi: 10.1038/s41388-022-02447-y. [DOI] [PubMed] [Google Scholar]
- 130.Comel A, Sorrentino G, Capaci V, Del Sal G. The cytoplasmic side of p53’s oncosuppressive activities. FEBS Lett. 2014;588:2600–9. doi: 10.1016/j.febslet.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 131.Blandino G, Valenti F, Sacconi A, Di Agostino S. Wild type- and mutant p53 proteins in mitochondrial dysfunction: emerging insights in cancer disease. Semin Cell Dev Biol. 2020;98:105–17. doi: 10.1016/j.semcdb.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 132.Jiang M, Li Z, Zhu G. The role of autophagy in the pathogenesis of periodontal disease. Oral Dis. 2020;26:259–69. doi: 10.1111/odi.13045. [DOI] [PubMed] [Google Scholar]
- 133.Hernandez GA, Perera RM. Autophagy in cancer cell remodeling and quality control. Mol Cell. 2022;82:1514–27. doi: 10.1016/j.molcel.2022.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Saint-Germain E, Mignacca L, Vernier M, Bobbala D, Ilangumaran S, Ferbeyre G. SOCS1 regulates senescence and ferroptosis by modulating the expression of p53 target genes. Aging. 2017;9:2137–62. doi: 10.18632/aging.101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gupta AK, Bharadwaj M, Kumar A, Mehrotra R. Spiro-oxindoles as a Promising Class of Small Molecule Inhibitors of p53-MDM2 Interaction Useful in Targeted Cancer Therapy. Top Curr Chem (Cham) 2017;375:3. doi: 10.1007/s41061-016-0089-0. [DOI] [PubMed] [Google Scholar]
- 136.Zhang W, Gai C, Ding D, Wang F, Li W. Targeted p53 on Small-Molecules-Induced Ferroptosis in Cancers. Front Oncol. 2018;8:507. doi: 10.3389/fonc.2018.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162–8. doi: 10.1016/j.freeradbiomed.2018.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang SJ, Li D, Ou Y, Jiang L, Chen Y, Zhao Y, et al. Acetylation Is Crucial for p53-Mediated Ferroptosis and Tumor Suppression. Cell Rep. 2016;17:366–73. doi: 10.1016/j.celrep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–79. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tarangelo A, Magtanong L, Bieging-Rolett KT, Li Y, Ye J, Attardi LD, et al. p53 Suppresses Metabolic Stress-Induced Ferroptosis in Cancer Cells. Cell Rep. 2018;22:569–75. doi: 10.1016/j.celrep.2017.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Xie Y, Zhu S, Song X, Sun X, Fan Y, Liu J, et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017;20:1692–704. doi: 10.1016/j.celrep.2017.07.055. [DOI] [PubMed] [Google Scholar]
- 142.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haigis KM. KRAS Alleles: The Devil Is in the Detail. Trends Cancer. 2017;3:686–97. doi: 10.1016/j.trecan.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Punekar SR, Velcheti V, Neel BG, Wong KK. The current state of the art and future trends in RAS-targeted cancer therapies. Nat Rev Clin Oncol. 2022;19:1–19. doi: 10.1038/s41571-022-00671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ceddia S, Landi L, Cappuzzo F. KRAS-Mutant Non-Small-Cell Lung Cancer: From Past Efforts to Future Challenges. Int J Mol Sci. 2022;23:9391. doi: 10.3390/ijms23169391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ravichandran M, Hu J, Cai C, Ward NP, Venida A, Foakes C, et al. Coordinated Transcriptional and Catabolic Programs Support Iron-Dependent Adaptation to RAS-MAPK Pathway Inhibition in Pancreatic Cancer. Cancer Discov. 2022;12:2198–219. doi: 10.1158/2159-8290.CD-22-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ferreira A, Pereira F, Reis C, Oliveira MJ, Sousa MJ, Preto A. Crucial Role of Oncogenic KRAS Mutations in Apoptosis and Autophagy Regulation: Therapeutic Implications. Cells. 2022;11:2183. doi: 10.3390/cells11142183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ohara Y, Valenzuela P, Hussain SP. The interactive role of inflammatory mediators and metabolic reprogramming in pancreatic cancer. Trends Cancer. 2022;8:556–69. doi: 10.1016/j.trecan.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chaudhary S, Pothuraju R, Rachagani S, Siddiqui JA, Atri P, Mallya K, et al. Dual blockade of EGFR and CDK4/6 delays head and neck squamous cell carcinoma progression by inducing metabolic rewiring. Cancer Lett. 2021;510:79–92. doi: 10.1016/j.canlet.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang H, Yun S, Batuwangala TD, Steward M, Holmes SD, Pan L, et al. A dual-targeting antibody against EGFR-VEGF for lung and head and neck cancer treatment. Int J Cancer. 2012;131:956–69. doi: 10.1002/ijc.26427. [DOI] [PubMed] [Google Scholar]
- 151.Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Islam SS, Qassem K, Islam S, Parag RR, Rahman MZ, Farhat WA, et al. Genetic alterations of Keap1 confers chemotherapeutic resistance through functional activation of Nrf2 and Notch pathway in head and neck squamous cell carcinoma. Cell Death Dis. 2022;13:696. doi: 10.1038/s41419-022-05126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao ZL, Zhang L, Huang CF, Ma SR, Bu LL, Liu JF, et al. NOTCH1 inhibition enhances the efficacy of conventional chemotherapeutic agents by targeting head neck cancer stem cell. Sci Rep. 2016;6:24704. doi: 10.1038/srep24704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wirth M, Jira D, Ott A, Piontek G, Pickhard A. High NOTCH1 mRNA Expression Is Associated with Better Survival in HNSCC. Int J Mol Sci. 2018;19:830. doi: 10.3390/ijms19030830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li X, Zou F, Lu Y, Fan X, Wu Y, Feng X, et al. Notch1 contributes to TNF-α-induced proliferation and migration of airway smooth muscle cells through regulation of the Hes1/PTEN axis. Int Immunopharmacol. 2020;88:106911. doi: 10.1016/j.intimp.2020.106911. [DOI] [PubMed] [Google Scholar]
- 156.Zhang Q, Zhu Z, Guan J, Hu Y, Zhou W, Ye W, et al. Hes1 Controls Proliferation and Apoptosis in Chronic Lymphoblastic Leukemia Cells by Modulating PTEN Expression. Mol Biotechnol. 2022;64:1419–1430. doi: 10.1007/s12033-022-00476-2. [DOI] [PubMed] [Google Scholar]
- 157.Nyman PE, Buehler D, Lambert PF. Loss of Function of Canonical Notch Signaling Drives Head and Neck Carcinogenesis. Clin Cancer Res. 2018;24:6308–18. doi: 10.1158/1078-0432.CCR-17-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Khelil M, Griffin H, Bleeker MCG, Steenbergen RDM, Zheng K, Saunders-Wood T, et al. Delta-Like Ligand-Notch1 Signaling Is Selectively Modulated by HPV16 E6 to Promote Squamous Cell Proliferation and Correlates with Cervical Cancer Prognosis. Cancer Res. 2021;81:1909–21. doi: 10.1158/0008-5472.CAN-20-1996. [DOI] [PubMed] [Google Scholar]
- 159.Wang J, Cao B, Zhao H, Gao Y, Luo Y, Chen Y, et al. Long noncoding RNA H19 prevents neurogenesis in ischemic stroke through p53/Notch1 pathway. Brain Res Bull. 2019;150:111–7. doi: 10.1016/j.brainresbull.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 160.Loganathan SK, Schleicher K, Malik A, Quevedo R, Langille E, Teng K, et al. Rare driver mutations in head and neck squamous cell carcinomas converge on NOTCH signaling. Science. 2020;367:1264–9. doi: 10.1126/science.aax0902. [DOI] [PubMed] [Google Scholar]
- 161.Byun JY, Huang K, Lee JS, Huang W, Hu L, Zheng X, et al. Targeting HIF-1α/NOTCH1 pathway eliminates CD44(+) cancer stem-like cell phenotypes, malignancy, and resistance to therapy in head and neck squamous cell carcinoma. Oncogene. 2022;41:1352–63. doi: 10.1038/s41388-021-02166-w. [DOI] [PubMed] [Google Scholar]
- 162.Codony VL, Tavassoli M. Hypoxia-induced therapy resistance: Available hypoxia-targeting strategies and current advances in head and neck cancer. Transl Oncol. 2021;14:101017. doi: 10.1016/j.tranon.2021.101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pérez-Sayáns M, Suárez-Peñaranda JM, Pilar GD, Barros-Angueira F, Gándara-Rey JM. García-García A. Hypoxia-inducible factors in OSCC. Cancer Lett. 2011;313:1–8. doi: 10.1016/j.canlet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 164.DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat Rev Immunol. 2021;21:785–97. doi: 10.1038/s41577-021-00541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nuñez-Hernandez DM, Felix-Portillo M, Peregrino-Uriarte AB, Yepiz-Plascencia G. Cell cycle regulation and apoptosis mediated by p53 in response to hypoxia in hepatopancreas of the white shrimp Litopenaeus vannamei. Chemosphere. 2018;190:253–9. doi: 10.1016/j.chemosphere.2017.09.131. [DOI] [PubMed] [Google Scholar]
- 166.Audero MM, Prevarskaya N, Fiorio Pla A. Ca(2+) Signalling and Hypoxia/Acidic Tumour Microenvironment Interplay in Tumour Progression. Int J Mol Sci. 2022;23:7377. doi: 10.3390/ijms23137377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qiu Y, Li P, Ji C. Cell Death Conversion under Hypoxic Condition in Tumor Development and Therapy. Int J Mol Sci. 2015;16:25536–51. doi: 10.3390/ijms161025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Basit F, Cristofanon S, Fulda S, Obatoclax GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ. 2013;20:1161–73. doi: 10.1038/cdd.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li X, Pan X, Zhang H, Lei D, Liu D, Xu F, et al. Overexpression of cFLIP in head and neck squamous cell carcinoma and its clinicopathologic correlations. J Cancer Res Clin Oncol. 2008;134:609–15. doi: 10.1007/s00432-007-0325-7. [DOI] [PubMed] [Google Scholar]
- 170.Wan M, Ding Y, Li Z, Wang X, Xu M. Metabolic manipulation of the tumour immune microenvironment. Immunology. 2022;165:290–300. doi: 10.1111/imm.13444. [DOI] [PubMed] [Google Scholar]
- 171.Davuluri GVN, Chan CH. Regulation of intrinsic and extrinsic metabolic pathways in tumour-associated macrophages. 2022. 10.1111/febs.16465. [DOI] [PMC free article] [PubMed]
- 172.Li Z, Li X, Ai S, Liu S, Guan W. Glucose Metabolism Intervention-Facilitated Nanomedicine Therapy. Int J Nanomedicine. 2022;17:2707–31. doi: 10.2147/IJN.S364840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu K, Yin N, Peng M, Stamatiades EG, Shyu A, Li P, et al. Glycolysis fuels phosphoinositide 3-kinase signaling to bolster T cell immunity. Science. 2021;371:405–10. doi: 10.1126/science.abb2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang C, Zhu L, Yuan W, Sun L, Xia Z, Zhang Z, et al. Diabetes aggravates myocardial ischaemia reperfusion injury via activating Nox2-related programmed cell death in an AMPK-dependent manner. J Cell Mol Med. 2020;24:6670–9. doi: 10.1111/jcmm.15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li N, Jiang W, Wang W, Xiong R, Wu X, Geng Q. Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol Res. 2021;166:105466. doi: 10.1016/j.phrs.2021.105466. [DOI] [PubMed] [Google Scholar]
- 176.Seibt TM, Proneth B, Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med. 2019;133:144–52. doi: 10.1016/j.freeradbiomed.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 177.Habib E, Linher-Melville K, Lin HX, Singh G. Expression of xCT and activity of system xc(-) are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol. 2015;5:33–42. doi: 10.1016/j.redox.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Imai H, Matsuoka M, Kumagai T, Sakamoto T, Koumura T. Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. Curr Top Microbiol Immunol. 2017;403:143–70. doi: 10.1007/82_2016_508. [DOI] [PubMed] [Google Scholar]
- 179.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–92. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Murphy MP. Metabolic control of ferroptosis in cancer. Nat Cell Biol. 2018;20:1104–5. doi: 10.1038/s41556-018-0209-x. [DOI] [PubMed] [Google Scholar]
- 181.Kim MJ, Yun GJ, Kim SE. Metabolic Regulation of Ferroptosis in Cancer. Biology (Basel). 2021;10:83. doi: 10.3390/biology10020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang DG, Zheng JN, Pei DS. P53/microRNA-34-induced metabolic regulation: new opportunities in anticancer therapy. Mol Cancer. 2014;13:115. doi: 10.1186/1476-4598-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cruz-Gregorio A, Aranda-Rivera AK, Aparicio-Trejo OE, Coronado-Martínez I, Pedraza-Chaverri J, Lizano M. E6 Oncoproteins from High-Risk Human Papillomavirus Induce Mitochondrial Metabolism in a Head and Neck Squamous Cell Carcinoma Model. Biomolecules. 2019;9:351. doi: 10.3390/biom9080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sandulache VC, Skinner HD, Ow TJ, Zhang A, Xia X, Luchak JM, et al. Individualizing antimetabolic treatment strategies for head and neck squamous cell carcinoma based on TP53 mutational status. Cancer. 2012;118:711–21. doi: 10.1002/cncr.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wang X, Wu S, Liu F, Ke D, Wang X, Pan D, et al. An Immunogenic Cell Death-Related Classification Predicts Prognosis and Response to Immunotherapy in Head and Neck Squamous Cell Carcinoma. Front Immunol. 2021;12:781466. doi: 10.3389/fimmu.2021.781466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Duan Q, Zhang H, Zheng J, Zhang L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer. 2020;6:605–18. doi: 10.1016/j.trecan.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 187.Chabanon RM, Rouanne M, Lord CJ, Soria JC, Pasero P, Postel-Vinay S. Targeting the DNA damage response in immuno-oncology: developments and opportunities. Nat Rev Cancer. 2021;21:701–17. doi: 10.1038/s41568-021-00386-6. [DOI] [PubMed] [Google Scholar]
- 188.Dai E, Zhu Z, Wahed S, Qu Z, Storkus WJ, Guo ZS. Epigenetic modulation of antitumor immunity for improved cancer immunotherapy. Mol Cancer. 2021;20:171. doi: 10.1186/s12943-021-01464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ryan SL, Beard S, Barr MP, Umezawa K, Heavey S, Godwin P, et al. Targeting NF-κB-mediated inflammatory pathways in cisplatin-resistant NSCLC. Lung Cancer. 2019;135:217–27. doi: 10.1016/j.lungcan.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 190.Almeida LO, Abrahao AC, Rosselli-Murai LK, Giudice FS, Zagni C, Leopoldino AM, et al. NFκB mediates cisplatin resistance through histone modifications in head and neck squamous cell carcinoma (HNSCC) FEBS Open Bio. 2014;4:96–104. doi: 10.1016/j.fob.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chang H, Xu Q, Li J, Li M, Zhang Z, Ma H, et al. Lactate secreted by PKM2 upregulation promotes Galectin-9-mediated immunosuppression via inhibiting NF-κB pathway in HNSCC. Cell Death Dis. 2021;12:725. doi: 10.1038/s41419-021-03990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhang L, Shao L, Creighton CJ, Zhang Y, Xin L, Ittmann M, et al. Function of phosphorylation of NF-kB p65 ser536 in prostate cancer oncogenesis. Oncotarget. 2015;6:6281–94. doi: 10.18632/oncotarget.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li Z, Yang Z, Lapidus RG, Liu X, Cullen KJ, Dan HC. IKK phosphorylation of NF-κB at serine 536 contributes to acquired cisplatin resistance in head and neck squamous cell cancer. Am J Cancer Res. 2015;5:3098–110. [PMC free article] [PubMed] [Google Scholar]
- 194.White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–62. doi: 10.1016/j.cell.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rongvaux A, Jackson R, Harman CC, Li T, West AP, de Zoete MR, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–77. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McArthur K, Whitehead LW, Heddleston JM, Li L, Padman BS, Oorschot V, et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science. 2018;359:eaao6047. doi: 10.1126/science.aao6047. [DOI] [PubMed] [Google Scholar]
- 197.Tong T, Qin X, Jiang Y, Guo H, Wang X, Li Y, et al. A novel CREB5/TOP1MT axis confers cisplatin resistance through inhibiting mitochondrial apoptosis in head and neck squamous cell carcinoma. BMC Med. 2022;20:231. doi: 10.1186/s12916-022-02409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS ONE. 2013;8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dionísio PA, Amaral JD, Rodrigues CMP. Molecular mechanisms of necroptosis and relevance for neurodegenerative diseases. Int Rev Cell Mol Biol. 2020;353:31–82. doi: 10.1016/bs.ircmb.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 200.Morin A, Letouzé E, Gimenez-Roqueplo AP, Favier J. Oncometabolites-driven tumorigenesis: From genetics to targeted therapy. Int J Cancer. 2014;135:2237–48. doi: 10.1002/ijc.29080. [DOI] [PubMed] [Google Scholar]
- 201.Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–74. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 202.Wicki S, Gurzeler U, Wei-Lynn Wong W, Jost PJ, Bachmann D, Kaufmann T. Loss of XIAP facilitates switch to TNFα-induced necroptosis in mouse neutrophils. Cell Death Dis. 2016;7:e2422. doi: 10.1038/cddis.2016.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xiao R, An Y, Ye W, Derakhshan A, Cheng H, Yang X, et al. Dual Antagonist of cIAP/XIAP ASTX660 Sensitizes HPV(-) and HPV(+) Head and Neck Cancers to TNFα, TRAIL, and Radiation Therapy. Clin Cancer Res. 2019;25:6463–74. doi: 10.1158/1078-0432.CCR-18-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Eytan DF, Snow GE, Carlson S, Derakhshan A, Saleh A, Schiltz S, et al. SMAC Mimetic Birinapant plus Radiation Eradicates Human Head and Neck Cancers with Genomic Amplifications of Cell Death Genes FADD and BIRC2. Cancer Res. 2016;76:5442–54. doi: 10.1158/0008-5472.CAN-15-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xia X, Wang X, Cheng Z, Qin W, Lei L, Jiang J, et al. The role of pyroptosis in cancer: pro-cancer or pro-“host”? Cell Death Dis. 2019;10:650. doi: 10.1038/s41419-019-1883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang S, Zhang MJ, Wu ZZ, Zhu SW, Wan SC, Zhang BX, et al. GSDME Is Related to Prognosis and Response to Chemotherapy in Oral Cancer. J Dent Res. 2022;101:848–58. doi: 10.1177/00220345211073072. [DOI] [PubMed] [Google Scholar]
- 207.Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–20. doi: 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lu L, Zhang P, Cao X, Guan M. Prognosis and Characterization of Immune Microenvironment in Head and Neck Squamous Cell Carcinoma through a Pyroptosis-Related Signature. J Oncol. 2022;2022:1539659. doi: 10.1155/2022/1539659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jiang M, Qi L, Li L, Li Y. The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 2020;6:112. doi: 10.1038/s41420-020-00349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 211.Segovia M, Russo S, Jeldres M, Mahmoud YD, Perez V, Duhalde M, et al. Targeting TMEM176B Enhances Antitumor Immunity and Augments the Efficacy of Immune Checkpoint Blockers by Unleashing Inflammasome Activation. Cancer Cell. 2019;35:767–81.e6. doi: 10.1016/j.ccell.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wu M, Wang Y, Yang D, Gong Y, Rao F, Liu R, et al. A PLK1 kinase inhibitor enhances the chemosensitivity of cisplatin by inducing pyroptosis in oesophageal squamous cell carcinoma. EBioMedicine. 2019;41:244–55. doi: 10.1016/j.ebiom.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kanno Y, Chen CY, Lee HL, Chiou JF, Chen YJ. Molecular Mechanisms of Chemotherapy Resistance in Head and Neck Cancers. Front Oncol. 2021;11:640392. doi: 10.3389/fonc.2021.640392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Raudenská M, Balvan J, Masařík M. Cell death in head and neck cancer pathogenesis and treatment. Cell Death Dis. 2021;12:192. doi: 10.1038/s41419-021-03474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rioja-Blanco E, Arroyo-Solera I, Álamo P, Casanova I, Gallardo A, Unzueta U, et al. CXCR4-targeted nanotoxins induce GSDME-dependent pyroptosis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2022;41:49. doi: 10.1186/s13046-022-02267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang L, Qin X, Liang J, Ge P. Induction of Pyroptosis: A Promising Strategy for Cancer Treatment. Front Oncol. 2021;11:635774. doi: 10.3389/fonc.2021.635774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Roh JL, Kim EH, Jang HJ, Park JY, Shin D. Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett. 2016;381:96–103. doi: 10.1016/j.canlet.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 218.Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21:648–57. doi: 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li S, Liu Y, Li J, Zhao X, Yu D. Mechanisms of Ferroptosis and Application to Head and Neck Squamous Cell Carcinoma Treatments. DNA Cell Biol. 2021;40:720–32. doi: 10.1089/dna.2021.0023. [DOI] [PubMed] [Google Scholar]
- 220.Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, et al. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001–2.e5. doi: 10.1016/j.cmet.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–4. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zou Y, Palte MJ, Deik AA, Li H, Eaton JK, Wang W, et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10:1617. doi: 10.1038/s41467-019-09277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113:E4966-75. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yamazaki T, Hannani D, Poirier-Colame V, Ladoire S, Locher C, Sistigu A, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ. 2014;21:69–78. doi: 10.1038/cdd.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–83. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 226.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fox LE, Locke MC, Lenschow DJ. Context Is Key: Delineating the Unique Functions of IFNα and IFNβ in Disease. Front Immunol. 2020;11:606874. doi: 10.3389/fimmu.2020.606874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ma H, Yang W, Zhang L, Liu S, Zhao M, Zhou G, et al. Interferon-alpha promotes immunosuppression through IFNAR1/STAT1 signalling in head and neck squamous cell carcinoma. Br J Cancer. 2019;120:317–30. doi: 10.1038/s41416-018-0352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yang W, Jiang C, Xia W, Ju H, Jin S, Liu S, et al. Blocking autophagy flux promotes interferon-alpha-mediated apoptosis in head and neck squamous cell carcinoma. Cancer Lett. 2019;451:34–47. doi: 10.1016/j.canlet.2019.02.052. [DOI] [PubMed] [Google Scholar]
- 230.Liu X, Lv Z, Zou J, Liu X, Ma J, Wang J, et al. Afatinib down-regulates MCL-1 expression through the PERK-eIF2α-ATF4 axis and leads to apoptosis in head and neck squamous cell carcinoma. Am J Cancer Res. 2016;6:1708–19. [PMC free article] [PubMed] [Google Scholar]
- 231.Liu X, Suo H, Zhou S, Hou Z, Bu M, Liu X, et al. Afatinib induces pro-survival autophagy and increases sensitivity to apoptosis in stem-like HNSCC cells. Cell Death Dis. 2021;12:728. doi: 10.1038/s41419-021-04011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hu X, Shi S, Wang H, Yu X, Wang Q, Jiang S, et al. Blocking autophagy improves the anti-tumor activity of afatinib in lung adenocarcinoma with activating EGFR mutations in vitro and in vivo. Sci Rep. 2017;7:4559. doi: 10.1038/s41598-017-04258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Chang I, Wang CY. Inhibition of HDAC6 Protein Enhances Bortezomib-induced Apoptosis in Head and Neck Squamous Cell Carcinoma (HNSCC) by Reducing Autophagy. J Biol Chem. 2016;291:18199–209. doi: 10.1074/jbc.M116.717793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol. 2011;2011:875824. [DOI] [PMC free article] [PubMed]
- 236.Sha S, Si L, Wu X, Chen Y, Xiong H, Xu Y, et al. Prognostic analysis of cuproptosis-related gene in triple-negative breast cancer. Front Immunol. 2022;13:922780. doi: 10.3389/fimmu.2022.922780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Song Q, Zhou R, Shu F, Fu W. Cuproptosis scoring system to predict the clinical outcome and immune response in bladder cancer. Front Immunol. 2022;13:958368. doi: 10.3389/fimmu.2022.958368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shan J, Geng R, Zhang Y, Wei J, Liu J, Bai J. Identification of cuproptosis-related subtypes, establishment of a prognostic model and tumor immune landscape in endometrial carcinoma. Comput Biol Med. 2022;149:105988. doi: 10.1016/j.compbiomed.2022.105988. [DOI] [PubMed] [Google Scholar]
- 239.Li Y. Copper homeostasis: Emerging target for cancer treatment. IUBMB Life. 2020;72:1900–8. doi: 10.1002/iub.2341. [DOI] [PubMed] [Google Scholar]
- 240.Babak MV, Ahn D. Modulation of Intracellular Copper Levels as the Mechanism of Action of Anticancer Copper Complexes: Clinical Relevance. Biomedicines. 2021;9:852. doi: 10.3390/biomedicines9080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Krumschnabel G, Ebner HL, Hess MW, Villunger A. Apoptosis and necroptosis are induced in rainbow trout cell lines exposed to cadmium. Aquat Toxicol. 2010;99:73–85. doi: 10.1016/j.aquatox.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 242.Xu W, Qian J, Hou G, Wang T, Wang J, Wang Y, et al. A Hollow Amorphous Bimetal Organic Framework for Synergistic Cuproptosis/Ferroptosis/Apoptosis Anticancer Therapy via Disrupting Intracellular Redox Homeostasis and Copper/Iron Metabolisms. Adv Funct Mater. 2022;1:2205013. doi: 10.1002/adfm.202205013. [DOI] [Google Scholar]
- 243.Li Y, Chen F, Chen J, Chan S, He Y, Liu W, et al. Disulfiram/Copper Induces Antitumor Activity against Both Nasopharyngeal Cancer Cells and Cancer-Associated Fibroblasts through ROS/MAPK and Ferroptosis Pathways. Cancers (Basel). 2020;12:138. doi: 10.3390/cancers12010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.