Abstract
Introduction
Recent studies reported a differential expression of both ACE2 and CD147 in pregnant women associated to SARS-CoV-2 placental infection. The aim of this study is to further investigate the placental SARS-CoV-2 infection and the potential effect on protein expression (ACE2, CD147, HLA-G and CD56).
Methods
The study was on three subgroups: i) 18 subjects positive for SARS-CoV-2 swab at delivery; ii) 9 subjects that had a positive SARS-CoV-2 swab during pregnancy but resulted negative at delivery; iii) 11 control subjects with physiological pregnancy and with no previous or concomitant SARS-CoV-2 swab positivity. None of the subjects were vaccinated for SARS-CoV-2 infection. The placenta samples were analyzed for SARS-CoV-2 NP (Nucleocapsid protein) positivity and the expression of ACE2, CD147, HLA-G and CD56.
Results
We observed a higher percentage of SARS-CoV-2 NP positive placenta samples in the group of SARS-CoV-2 PCR positive at delivery in comparison with SARS-CoV-2 PCR negative at delivery. The localization of SARS-CoV-2 NP positivity in placenta samples was mainly in syncytiotrophoblast (ST) of SARS-CoV-2 PCR positive at delivery group and in extra-villous trophoblast (EVT) of SARS-CoV-2 PCR negative at delivery group. CD147, HLA-G positivity was higher in ST of SARS-CoV-2 PCR positive at delivery group, while CD56-expressing immune cells were decreased in comparison with control subjects.
Discussion
We confirmed the ability of SARS-CoV-2 to infect placenta tissues. The simultaneous SARS-CoV-2 swab positivity at delivery and the positivity of the placenta tissue for SARS-CoV-2 NP seems to create an environment that modifies the expression of specific molecules, as CD147 and HLA-G. These data suggest a possible impact of SARS-CoV-2 infection during pregnancy, that might be worthy to be monitored also in vaccinated subjects.
Keywords: SARS-CoV-2, Placental infection, ACE2, CD147, HLA-G
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE-2, Angiotensin Converting Enzyme 2; TMPRRS2, Transmembrane serine protease 2; COVID-19, Coronavirus disease 2019; HLA, Human Leukocyte Antigen; NK, Natural killer; KIR, Killer-cell immunoglobulin-like receptor; ILT, Ig-like transcript
1. Introduction
After severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic outbreak in December 2019, most of studies concerning SARS-CoV-2 infection have been focused on coronavirus disease 2019 (COVID-19) severe respiratory disease, highlighting as main cellular receptor for viral entry the Angiotensin Converting Enzyme (ACE) 2 [1].
Then, several studies also investigated the possible multi-tissue tropism of SARS-CoV-2, basing on the wide expression of ACE2 in sites other than lungs [2]. It is known that SARS-CoV-2 cell entry via ACE2 engagement by the viral Spike protein also involved the proteases TMPRRS2 [3]. Placenta tissues are characterized by a lower TMPRSS2 expression compared to other tissues, like lungs, with a preferential expression at trophoblasts [4]. A few placental cells and chorioamniotic membranes co-express ACE2 and TMPRSS2 throughout gestation [5,6], suggesting a possible effect on SARS-CoV-2 placental and transplacental infection, as the co-presence of both these receptors, facilitating SARS-CoV-2 cell entry. For this reason, the presence of alternative receptors for SARS-CoV-2 entry into syncytiotrophoblast cells has been suggested [7] such as DPP4 (CD26) and CD147 [8,9]. CD147 is characterized by a diffuse tissue expression [10], in concomitance with the emerging data reporting SARS-CoV-2 infection in different body sites [11]. A recent work of Dong et al. reported a differential expression of both ACE2 and CD147 in a small cohort of pregnant women associated to SARS-CoV-2 placental infection compared to non-COVID19 infected pregnant women, with no direct evidence of viral transmission to the newborns [12].
Moreover, SARS-CoV-2 infection is associated to different clinical outcomes, that ranges from severe to asymptomatic disease [8]. This clinical heterogeneity may be correlated to a differential expression of susceptibility factors, as ACE2 and CD147, but also to peculiar escape mechanisms that the virus could exploit in some individuals, as for example the induction of the immunotolerogenic molecule Human Leukocyte Antigen (HLA)-G [[13], [14], [15]].
HLA-G is a non-classical HLA class I molecule firstly described in pregnancy, where it allows the protection of the semi-allogenic fetus from the maternal immune system through the interaction with specific inhibitory receptors [16].
HLA-G placenta expression during pregnancy is characterized by peculiar changes, with high levels of the molecules in the first trimester, which are reducing as they approach the birth, in order to promote the typical inflammatory environment needed to induce the delivery [17]. The presence of placental infections might affect HLA-G expression, leading to placental and fetal disorders, as recently reported for HHV-6 infection in association with IUGR condition [18].
Recently, the ability of SARS-CoV-2 to induce HLA-G has been described in bowel tissues [[19], [20], [21]], supporting the involvement of this molecule in its immune escape process. In particular, Natural Killer (NK) cells, that represent the first line of defense during viral infections, have been reported to be anergic in COVID-19 patients [22]. NK cells are also key players during pregnancy, representing the main immune cell type in endometrial tissues where they act to allow a correct decidualization and placentation process [23] and to kill target infected cells. Decidual natural killer cells (dNK) are the predominant leukocyte population in normal human endometrium [24]. Their content varies throughout the normal menstrual cycle, likely due to recruitment of peripheral NK cells (pNK) and/or in utero proliferation/differentiation of stem uNK cells. They represent 40% of the total leukocyte population during the proliferative phase which increases to 60% by mid-secretory phase and up to 75% in early pregnancy. At the end of pregnancy, dNKs show a different protein and gene expression profile which is associated to an increase degranulation response, suggesting their key role in counteract possible vertical transmission of infection to the fetus [25]. NK cell activity is controlled by the interaction between specific receptors (KIR (Killer immunoglobulin like receptor), ILT (Ig-like transcript)) with surface proteins. HLA-G molecules interact with ILT2 and ILT4 receptors, inducing an inhibitory signal in NK cells [26].
The evaluation of the effect of SARS-CoV-2 infection during pregnancy has raised interest. Even if virus vertical transmission is still controversial [27], several researches have evaluated the possible different susceptibility to SARS-CoV-2 infection, analyzing the placenta tissues. It has been demonstrated that SARS-CoV-2 can infect the placenta [28], documenting congenital SARS-CoV-2 infection during the first trimester of pregnancy with fetal organs, such as lung and kidney, as targets for coronavirus [29].
The aim of this study is to further investigate the placental SARS-CoV-2 infection and the potential effect on protein expression as ACE2, CD147, HLA-G and CD56, as a marker of CD56 positive immune cells (NK cells, T cells), in placental tissues from subjects positive or negative for SARS-CoV-2 infection at delivery.
2. Materials and methods
2.1. Study population
The study was conducted on one prospective cohort of 38 patients enrolled at the time of childbirth at the Gynecology and Obstetrics Department of the Sant'Anna University Hospital in Ferrara, during the period from March to December 2020. Three subgroups were identified, namely, a cohort made up of 18 subjects positive for SARS-CoV-2 infection at delivery and 9 subjects that were positive for SARS-CoV-2 infection during pregnancy but resulted negative for SARS-CoV-2 infection at delivery and a control group made up of 11 subjects with physiological pregnancy and with no previous or concomitant SARS-CoV-2 infection, that will be referred as control group. None of the subjects were vaccinated for SARS-CoV-2 infection. SARS-CoV-2 infection was tested with a molecular swab (RT-PCR) test for the presence of SARS-CoV-2 RNA and identified to be sustained by wild type Wuhan Hu-1 isolates. Clinical data were collected for the cohort. All subjects gave their informed consent for inclusion in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the local Ethics Committee (Comitato Etico di Area Vasta Emilia Centro della Regione Emilia-Romagna, CE-AVEC). All the data were anonymized and no connection with the patient identity was possible.
2.2. Placenta sample collection
The placenta samples were collected after delivery and sent to the Pathology Lab for examination as previously described [18]. They were initially examined in the unfixed state then the membranes were removed by trimming them along the placental margin and the umbilical cord was removed after recording the length and site of insertion. The placental disk weights and three-dimensional measures were recorded, and the parenchyma was sectioned at 1–2 cm intervals on the maternal surface; after formalin fixation, a full-thickness section from the central portion of the placenta was sampled for histology.
2.3. Immunohistochemical analysis
Placenta slides were deparaffined by using Toluene, rehydrated in decreasing ethanol concentration [[19], [20], [21]]. Heat antigen retrieval in Citrate Buffer pH6.1 with 0,05% Tween20 was performed. Slides were stained using the Ultratek kit (Histoline) with the following antibody: SARS-CoV-2 NP (Nucleocapsid protein) (NB100-56576, Novus Biologicals, Centennial, 1:250 dilution), Human Leukocyte Antigen-G (HLA-G) antibody (MEM-G2, Exbio, dilution 1:400), CD147 (clone MEM-M6/1, dilution 1:100, Novus Biologicals), CD56 (clone 123C3.D5, dilution 1:100, Novus Biologicals), ACE2 (clone EPR4435-2, 1:250 dilution, Abcam).
After immunohistochemical staining, the slides were scanned using the Philips Ultra Fast Scanner version 1.6, digitalized slides were converted in BigTIFF format and were then imported into QuPath software for further image analysis. First, the Simple tissue detection tool was used to create annotation of the tissue region to be analyzed. The Cell detection tool was used to detect every cell in the tissue by using a built‐in cell segmentation algorithm. To enable distinction between positively and negatively stained cells, the Intensity feature in the Detection classifier was used. The tissues were scored based on number of positively stained cells/mm2. An H-score between 0 and 300 was obtained where 300 was equal to 100% of cells strongly stained (3+) [[19], [20], [21]].
2.4. Statistical analysis
Frequencies were analyzed by Fisher's exact test, biological variables and H-Score comparisons were evaluated by Mann-Whitney U test. The statistical analysis was performed by GraphPad Software version 6.
3. Results
3.1. SARS-CoV-2 gestational infection is associated to clinical alterations
The prospective cohort was subdivided into three groups, characterized by a positive swab test for SARS-CoV-2 infection at the delivery (SARS-CoV-2 PCR positive at delivery) by a positive swab test for SARS-CoV-2 infection during pregnancy but negativized at the delivery (SARS-CoV-2 PCR negative at delivery) or without any positive swab test for SARS-CoV-2 during pregnancy and at delivery (controls). The subjects were matched for age, BMI and gestational period at delivery, to avoid confounding variables (Table 1 ). No subjects presented comorbidities, both pregnancy related, such as preeclampsia and gestational diabetes mellitus, and preexisting, such as chronic hypertension, pregestational diabetes, and heart disease (Table 1). The SARS-CoV-2 PCR positive at delivery group were all affected by the SARS-CoV-2 wild type Wuhan Hu-1 isolate, which includes most of the Italian sequences during the enrollment period. The SARS-CoV-2 PCR positive at delivery group presented hyperpyrexia (body temperature above 38°Celsius) and/or dyspnea (subjective difficulty in breathing) that needed oxygen therapy (p = 2.1 × 10-7; Fisher exact test). Two subjects with a positive SARS-CoV-2 swab at delivery needed to be transferred to intensive care units for supportive cares. None of the newborns from the SARS-CoV-2 PCR positive at delivery groups resulted positive for SARS-CoV-2 swab testing at childbirth. The SARS-CoV-2 PCR positive and negative at delivery groups differed for the gestational period of infection, that was 39.2 ± 1.6 weeks for SARS-CoV-2 PCR positive at delivery group and 22.2 ± 3.5 weeks for SARS-CoV-2 PCR negative at delivery group (p = 0.012; Mann-Whitney U test).
Table 1.
Demographic and clinical characteristics.
| Clinical data | SARS-CoV-2 PCR positive at deliverya (N = 18) | SARS-CoV-2 PCR negative at deliverya (N = 9) | Control (N = 11) | p-value |
|---|---|---|---|---|
| Age; years (mean ± SD) | 29.9 ± 6.9 | 31.8 ± 2.8 | 34.9 ± 5.5 | |
| BMI (mean ± SD) | 23.4 ± 3.5 | 27.2 ± 7.3 | 21.7 ± 2.1 | |
| Comorbidities (N) | 0 | 0 | 0 | |
| Gestational period of infection; weeks (mean ± SD) | 39.2 ± 1.6 | 22.2 ± 3.5 | – | 0.012c |
| Severity of infection (N) | Severeb: 2 | Severeb: 0 | – | 0.54d |
| Mild: 16 | Mild: 9 | |||
| Gestational period of delivery; weeks (mean ± SD) | 39.2 ± 1.8 | 38.9 ± 2.1 | 39.5 ± 2.7 | |
| Hyperpyrexia and/or dyspnea (N) | 18 | 0 | – | 2.1 × 10−7d |
| Weight of fetus at birth; g (mean ± SD) | 3353.6 ± 445.3 | 3192.2 ± 423.8 | 3343.6 ± 230.4 | 1vs2 0.35c |
| 1vs3 0.56c | ||||
| 2vs30.36c | ||||
| Apgar 5 min (mean ± SD) | 9.9 ± 0.2 | 10.0 ± 0.0 | 9.9 ± 1.3 | 1vs2 0.95c |
| 1vs3 0.98c | ||||
| 2vs30.96c | ||||
| Newborn gender (Male; Female) | Male:61 | Male:22 | Male:53 | 1vs2 0.68d |
| Female:6 | 1vs3 0.69d | |||
| Female:12 | Female:7 | 2vs30.37d | ||
| Newborn swab result (N) | Positive: 0 | Positive: 0 | – | 1d |
| Negative: 18 | Negative: 9 |
Swab results at delivery.
intensive care units for supportive cares.
Mann-Whitney U test.
Fisher exact test.
3.2. SARS-CoV-2 PCR positive at delivery group showed a ST positivity for SARS-CoV-2 NP
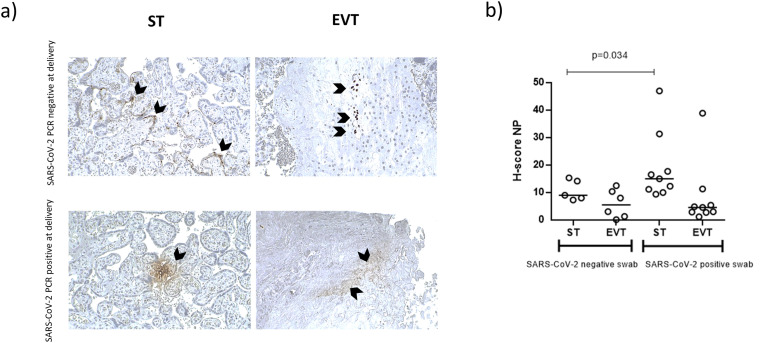
Placenta samples obtained from the three groups were analyzed for SARS-CoV-2 NP presence by IHC. The 78% of the placenta samples from the SARS-CoV-2 PCR positive at delivery group were positive for SARS-CoV-2 NP expression in comparison with the 39% of the SARS-CoV-2 PCR negative at delivery group (p < 0.0001, Fisher's exact test). The control group placenta samples were all negative for SARS-CoV-2 NP staining.
SARS-CoV-2 NP positivity showed a different localization between syncytiotrophoblast (ST) and extra-villous trophoblast (EVT). SARS-CoV-2 NP positivity was preferentially found in the ST of SARS-CoV-2 PCR positive at delivery group samples (100% vs 57%, p < 0.01, Fisher exact test), while the infection was at EVT level in SARS-CoV-2 PCR negative at delivery group (86% vs 71%, p < 0.01, Fisher exact test) (Fig. 1 a). The analysis of the staining for SARS-CoV-2 NP revealed a higher intensity in the ST of SARS-CoV-2 PCR positive at delivery group compared to the SARS-CoV-2 PCR negative at delivery group (Fig. 1b, p = 0.0316, Mann-Whitney U test).
Fig. 1.
a) Immunohistochemical staining for SARS-CoV-2 Nucleocapsid protein (NP) of the placenta tissues from SARS-CoV-2 PCR positive at delivery group (SARS-CoV-2 positive swab) or SARS-CoV-2 PCR negative at delivery group (SARS-CoV-2 negative swab). Magnifications are 10×. ST: syncytiotrophoblast; EVT: extra-villous trophoblast. b) H-score values. p values were obtained by Mann-Whitney test.
3.3. ACE2, CD147 and HLA-G are differentially expressed in placental samples
We investigated the expression and localization of these three molecules to identify a possible implication in placental SARS-CoV-2 infection susceptibility.
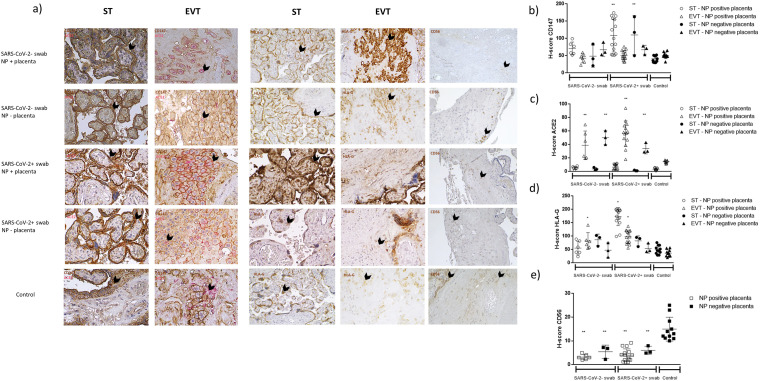
Both receptors were expressed in all the placental samples. CD147 diffuse expression and higher H score were found in the ST (Fig. 2 a and b) of all the placenta samples, with the strongest staining (H score) in SARS-CoV-2 PCR positive at delivery group, independently to placental SARS-CoV-2 NP positivity (p < 0.001; Mann-Whitney U test). CD147 membrane staining was present in EVT, with no significant differences between the placenta samples (Fig. 2a and b).
Fig. 2.
a) Immunohistochemical staining for CD147, ACE2, HLA-G and CD56 of the placenta tissues from SARS-CoV-2 PCR positive at delivery group (SARS-CoV-2+ swab) or SARS-CoV-2 PCR negative at delivery group (SARS-CoV-2- swab), both with (NP + placenta) or without (NP - placenta) positive staining for SARS-CoV-2 Nucleocapsid protein (NP) in placenta tissues, and SARS-CoV-2 negative swab during pregnancy and at delivery group (control). Magnifications are 10×. ST: syncytiotrophoblast; EVT: extra-villous trophoblast. b) H-score values. p values were obtained by Mann-Whitney test.
ACE2 is expressed by EVT of all the placental samples, but with a different cellular distribution (Fig. 2a, c). SARS-CoV-2 NP negative placenta presented a diffused expression of ACE2, while SARS-CoV-2 NP positive placenta, independently from the SARS-CoV-2 positivity or negativity of swab at delivery, showed a membrane-associated staining (Fig. 2a, c). Control placenta samples showed a slight intracellular ACE2 staining in comparison with SARS-CoV-2 PCR positive or negative at delivery groups (Fig. 2a, c; p < 0.001; Mann-Whitney U test).
Since we have previously shown that HLA-G molecules are modulated during SARS-CoV-2 infection in the intestinal villi during thromboembolic events [[19], [20], [21]], we evaluated HLA-G expression in placenta samples taking into consideration the implication of HLA-G molecules during viral infection in pregnancy [18].
HLA-G expression was evident in ST and EVT of all the placental samples, with a significant increase in ST of SARS-CoV-2 PCR positive at delivery group with SARS-CoV-2 NP positive placenta samples (Fig. 2a, d). HLA-G expression was also evident in EVT, with an induced expression in SARS-CoV-2 PCR positive at delivery group with SARS-CoV-2 NP positive placenta samples and in SARS-CoV-2 PCR negative at delivery group with SARS-CoV-2 NP positive placenta samples (Fig. 2d; p < 0.01; Mann Whitney U test).
3.4. SARS-CoV-2 placental infection influences CD56 positive immune cell presence
We analyzed placental tissues for the presence of CD56 positive immune cells, that account mainly to decidual NK (dNK) cells. We observed the presence of CD56 positive immune cells in the endometrial tissues (Fig. 2a; e). All the placenta samples of SARS-CoV-2 PCR positive and negative at delivery groups showed a significant decrease in CD56 positive cells compared to controls (Fig. 2e; p < 0.001; Mann Whitney U test).
4. Discussion
The immune system is modified during pregnancy to protect the growth of a semi-allogeneic fetus in the body of the pregnant woman, resulting in a peculiar immune response to microbial infections during pregnancy [24]. SARS-CoV-2 infection modifies the immune response of the host, mainly in those subjects affected by a severe disease [22,30]. The immune deregulation involves an increase in blood leukocytes (leukocytosis), decrease in lymphocytes (lymphopenia) and an increase in neutrophil-to-lymphocyte ratio (NLR).
Recent studies reported the presence of SARS-CoV-2 mRNA or virions in ST [29]. Whilst the possibility of vertical transmission of SARS-CoV-2 from pregnant woman to fetus during pregnancy is suggested, the role of the placenta in infection with the virus has not yet been fully understood. We evaluated the placental SARS-CoV-2 infection and the potential effect on protein expression as ACE2, CD147, HLA-G and CD56, as a marker of CD56 expressing immune cells (mainly NK cells, but also alpha beta T cells, gamma delta T cells, dendritic cells, and monocytes) [31], in placental tissues from pregnant women, positive or negative for SARS-CoV-2 infection at delivery.
We observed a higher percentage of SARS-CoV-2 positive placenta samples in SARS-CoV-2 PCR positive at delivery group in comparison with SARS-CoV-2 PCR negative at delivery group. The localization of SARS-CoV-2 positivity in placenta samples was mainly in ST of SARS-CoV-2 PCR positive at delivery group and in EVT of SARS-CoV-2 PCR negative at delivery group. CD147 and HLA-G positivity was higher in SARS-CoV-2 PCR positive at delivery group and mainly localized in ST. These results support previous observations on the presence of CD147 as protein complexes on the plasma membrane of primary human ST, suggesting a role of CD147 in trophoblast function [32], and a role for HLA-G molecules as immune escape mechanism of the virus. HLA-G expression is normally reduced during delivery. The increase in SARS-CoV-2 infected tissues sustains a possible induction by SARS-CoV-2 infection, as reported in other tissues [[19], [20], [21]].
ACE2 staining was evidenced in EVT of all the placental samples, with a diffused expression in SARS-CoV-2 negative placenta, and a membrane-associated staining in SARS-CoV-2 positive placenta. These results agree with previous data on the prevalent localization of ACE2 in the physiologic interface between pregnant woman and fetus, rather than in other placental villous cells [33]. The increased ACE2 membrane expression suggests a possible re-localization of ACE2 at the cell membrane after the internalization during SARS-CoV-2 infections via a clathrin-mediated endocytosis [34], in which ACE2 is recycled back to the cell surface and the virus is further replicated in the cell. However, this study has several limitations: i) the cohort was enrolled two years ago; ii) all the subjects were infected with wild type SARS-CoV-2 strain; iii) no subjects were vaccinated. It would be important to analyze a larger cohort, infected with different SARS-CoV-2 strains and with or without a protocol of vaccination. The confirmation of our results would clarify the effect of SARS-CoV-2 infection in placenta tissues. Interestingly, placenta samples delivered from SARS-CoV-2 positive subjects showed a decreased expression of two trophoblast genes (PSG3, with immunoregulatory and angiogenic functions and CGB3, essential for pregnancy maintenance) and increased expression of three immune genes (CXCL10, pro-inflammatory chemokine secreted in response to interferon gamma, TLR3 and DDX58, involved in recognizing double-stranded RNA) [35]. These data support our data and the role of SARS-CoV-2 infection in modifying immune and trophoblast gene expression [35].
We observed a decrease in CD56 expressing immune cells. The expression of CD56 is most stringently associated with, but certainly not limited to, NK cells, where upon activation, CD56dim NK cells can adopt a CD56bright-like immunophenotype or upregulate their CD56 expression in general [36]. Endometrial NK cells are CD56bright NK cells, that act as cytokine producing cells, that control embryo implantation and placentation. Numerical and functional deficiencies and phenotypic alterations of CD56 positive immune cells might modify the placental homeostasis during SARS-CoV-2 infection, with possible clinical implications.
In conclusion, these results confirm the ability of SARS-CoV-2 to infect placenta tissues, as previously reported [37,38]. The simultaneous swab SARS-CoV-2 positivity at delivery and the NP positivity of the placenta tissue seems to create a peculiar environment that modifies the expression of specific molecules, as SARS-CoV-2 infection related molecules (ACE2, CD147), immune regulators (HLA-G) and immune cells (CD56). There is a clear modification in the immune environment, with an increase in the immune-tolerogenic molecule HLA-G, that can act as immune-escape mechanism for SARS-CoV-2, and a decrease in CD56 expressing immune cells, suggesting that SARS-CoV-2 infection might influence the expression of CD56 and consequently cell behavior. The decrease in CD56 expression induces a cytotoxic phenotype, that might alter the immune tolerogenic status at the fetal-maternal interface.
These results sustain the need of further studies to determine whether such changes in the immune system result in higher susceptibility or are protective against SARS-CoV-2 infection during pregnancy.
Funding
This work was supported by Crowdfunding grant of the University of Ferrara.
Author contributions
Giovanna Schiuma, Daria Bortolotti, Pantaleo Greco, Angelina Passaro, Roberta Rizzo: conceived and designed the analysis; Giovanna Schiuma, Silvia Beltrami, Daria Bortolotti, Roberta Rizzo: wrote the paper; Erica Santi, Gennaro Scutiero, Juana Maria Sanz, Chiara Marina Semprini: collected the samples and data; Ines Zidi: contributed data or analysis tools; Mercedes Fernandez, Sabrina Rizzo, Silvia Beltrami, Roberta Gafà: performed the analysis.
Declaration of competing interest
None of the authors declared any conflict of interest.
Acknowledgements
We thank Iva Pivanti for the technical support.
References
- 1.Galan M., Jimenez-Altayo F. Small resistance artery disease and ACE2 in hypertension: a new paradigm in the context of COVID-19. Front Cardiovasc Med. 2020;7 doi: 10.3389/fcvm.2020.588692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meng-Yuan L., Lin L., Zhang Y., Xiao-Sheng W. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;28(1):45. doi: 10.1186/s40249-020-00662-x.3. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senapati S., Banerjee P., Bhagavatula S., Kushwaha P.P., Kumar S. Contributions of human ACE2 and TMPRSS2 in determining host–pathogen interaction of COVID-19. J. Genet. 2021;100:12. doi: 10.1007/s12041-021-01262-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ouyang Y., Bagalkot T., Fitzgerald W., Sadovsky E., Chu T., Martínez-Marchal A., Brieño-Enríquez M., Su E.J., Margolis L., Sorkin A., Sadovsky Y. Term human placental trophoblasts express SARS-CoV-2 entry factors ACE2, TMPRSS2, and furin. mSphere. 2021;6 doi: 10.1128/mSphere.00250-21. e00250-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pique-Regi R., Romero R., Tarca A.L., Sendler E.D., Xu Y., Garcia-Flores V., Leng Y., Luca F., Hassan S.S., Gomez-Lopez N. Single cell transcriptional signatures of the human placenta in term and preterm parturition. Elife. 2019;8 doi: 10.7554/eLife.52004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pique-Regi R., Romero R., Tarca A.L., Luca F., Xu Y., Alazizi A., Leng Y., Hsu C.D., Gomez-Lopez N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? Elife. 2020;9 doi: 10.7554/eLife.58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon D.E., Jang G.M., Bouhaddou M., Xu J., Obernier K., White K.M., O'Meara M.J., Rezelj V.V., Guo J.Z., Swaney D.L., et al. A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. bioRxiv. 2020;583:459–568. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portela L.M., Santos S.A., Constantino F.B., Camargo A.C., Colombelli K.T., Fioretto M.N., Barquilha C.N., Périco L.L., Hiruma-Lima C.A., Scarano W.R., et al. Increased oxidative stress and cancer biomarkers in the ventral prostate of older rats submitted to maternal malnutrition. Mol. Cell. Endocrinol. 2021;523 doi: 10.1016/j.mce.2020.111148. [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Zhang Z., Yang L., Lian X., Xie Y., Li S., Xin S., Cao P., Lu J. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 spike. iScience. 2020;23:6 101160. doi: 10.1016/j.isci.2020.101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yurchenko V., Constant S., Bukrinsky M. Dealing with the family: CD147 interactions with cyclophilins. Immunology. 2006;117:301–309. doi: 10.1111/j.1365-2567.2005.02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia L., Yufeng L., Qian L., Qun Y., Xi W., Huanyu Z., Rong C., Liang R., Juan M., Fei D., Bing Y., Liang L., Zhihong H., Manli W., Yiwu Z. SARS-CoV-2 cell tropism and multiorgan infection. Cell Discovery. 2021;7:17. doi: 10.1038/s41421-021-00249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong L., Pei S., Ren Q., Fu S., Yu L., Chen H., Chen X., Yin M. Evaluation of vertical transmission of SARS-CoV-2 in utero: nine pregnant women and their newborns. Placenta. 2021;111:91–96. doi: 10.1016/j.placenta.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bortolotti D., LeMaoult J., Trapella C., Di Luca D., Carosella E.D., Rizzo R. Pseudomonas aeruginosa quorum sensing molecule N-(3-Oxododecanoyl)-L-Homoserine-Lactone induces HLA-G expression in human immune cells. Infect. Immun. 2015;83:3918–3925. doi: 10.1128/IAI.00803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeMaoult J., Zafaranloo K., Le Danffand C., Carosella E.D. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. Faseb. J. 2005;19:662–664. doi: 10.1096/fj.04-1617fje. [DOI] [PubMed] [Google Scholar]
- 15.Eliassen E., Di Luca D., Rizzo R., Barao I. The interplay between natural killer cells and human herpesvirus-6. Viruses 9. 2017;12:367. doi: 10.3390/v9120367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizzo R., Bortolotti D., Bolzani S., Fainardi E. HLA-G molecules in autoimmune diseases and infections. Front. Immunol. 2014;5:592. doi: 10.3389/fimmu.2014.00592.eCollection2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizzo R., Stignani M., Amoudruz P., Nilsson C., Melchiorri L., Baricordi O., Sverremark-Ekström E. Allergic women have reduced sHLA-G plasma levels at delivery. Am. J. Reprod. Immunol. 2009;61(5):368–376. doi: 10.1111/j.1600-0897.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- 18.Bortolotti D., Gentili V., Santi E., Taliento C., Vitagliano A., Schiuma G., Beltrami S., Rizzo S., Lanza G., Rizzo R., Gafà R., Greco P. Late-onset intrauterine growth restriction and HHV-6 infection: a pilot study. J. Med. Virol. 2021;93(11):6317–6322. doi: 10.1002/jmv.27138. [DOI] [PubMed] [Google Scholar]
- 19.Rizzo R., Neri L.M., Simioni C., Bortolotti D., Occhionorelli S., Zauli G., Secchiero P., Semprini C.M., Laface I., Sanz J.M., Lanza G., Gafà R., Passaro A. SARS-CoV-2 nucleocapsid protein and ultrastructural modifications in small bowel of a 4-week-negative COVID-19 patient. Clin. Microbiol. Infect. 2021;27(6):936–937. doi: 10.1016/j.cmi.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bortolotti D., Simioni C., Neri L.M., Rizzo R., Semprini C.M., Occhionorelli S., Laface I., Sanz J.M., Schiuma G., Rizzo S., Varano G., Beltrami S., Gentili V., Gafà R., Passaro A. Relevance of VEGF and CD147 in different SARS-CoV-2 positive digestive tracts characterized by thrombotic damage. FASEB J 35. 2021;12 doi: 10.1096/fj.202100821RRR. [DOI] [PubMed] [Google Scholar]
- 21.Traina L., Mucignat M., Rizzo R., Gafà R., Bortolotti D., Passaro A., Zamboni P. COVID-19 induced aorto duodenal fistula following evar in the so called "negative" patient. Vascular. 2021;17 doi: 10.1177/17085381211053695. [DOI] [PubMed] [Google Scholar]
- 22.Bortolotti D., Gentili V., Rizzo S., Rotola A., Rizzo R. SARS-CoV-2 spike 1 protein controls natural killer cell activation via the HLA-E/NKG2A pathway. Cells. 2020;9 doi: 10.3390/cells9091975. 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caselli E., Bortolotti D., Marci R., Rotola A., Gentili V., Soffritti I., D'Accolti M., Lo Monte G., Sicolo M., Barao I., Di Luca D., Rizzo R. HHV-6A infection of endometrial epithelial cells induces increased endometrial NK cell-mediated cytotoxicity. Front. Microbiol. 2017;8:2525. doi: 10.3389/fmicb.2017.02525.eCollection2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marci R., Gentili V., Bortolotti D., Lo Monte G., Caselli E., Bolzani S., Rotola A., Di Luca D., Rizzo R. Presence of HHV-6A in endometrial epithelial cells from women with primary unexplained infertility. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0158304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Mendonça Vieira R., Meagher A., Crespo Â.C., Kshirsagar S.K., Iyer V., Norwitz E.R., Strominger J.L., Tilburgs T. Human term pregnancy decidual NK cells generate distinct cytotoxic responses. J. Immunol. 2020;204(12):3149–3159. doi: 10.4049/jimmunol.1901435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favier B., Lemaoult J., Lesport E., Carosella E.D. ILT2/HLA-G interaction impairs NK-cell functions through the inhibition of the late but not the early events of the NK-cell activating synapse. Faseb. J. 2010;3:689–699. doi: 10.1096/fj.09-135194. [DOI] [PubMed] [Google Scholar]
- 27.Mirbeyk M., Saghazadeh A., Rezaei N. A systematic review of pregnant women with COVID-19 and their neonates. Arch. Gynecol. Obstet. 2021;1:5–38. doi: 10.1007/s00404-021-06049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valdespino-Vázquez M.Y., Helguera-Repetto C.A., León-Juárez M., Villavicencio-Carrisoza O., Flores-Pliego A., Moreno-Verduzco E.R., Díaz-Pérez D.L., Villegas-Mota I., et al. Fetal and placental infection with SARS-CoV-2 in early pregnancy. J. Med. Virol. 2021;93(7):4480–4487. doi: 10.1002/jmv.26965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Argueta L.B., Lacko L.A., Bram Y., Tada T., Carrau L., Figueiredo Rendeiro A., Zhang T., et al. Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy. iScience. 2022;25(5) doi: 10.1016/j.isci.2022.104223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caccuri F., Messali S., Bortolotti D., Di Silvestre D., De Palma A., Cattaneo C., Bertelli A., Zani A., Milanesi M., Giovanetti M., Campisi G., Gentili V., Bugatti A., Filippini F., Scaltriti E., Pongolini S., Tucci A., Fiorentini S., d'Ursi P., Ciccozzi M., Mauri P., Rizzo R., Caruso A. Competition for dominance within replicating quasispecies during prolonged SARS-CoV-2 infection in an immunocompromised host. Virus Evolution. 2022:veac042. doi: 10.1093/ve/veac04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Acker H.H., Capsomidis A., Smits E.L., Van Tendeloo V.F. CD56 in the immune system: more than a marker for cytotoxicity? Front. Immunol. 2017;8:892. doi: 10.3389/fimmu.2017.00892.eCollection2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheuk-Lun L., Lam M.P.Y., Lam K.K.W., Leung C.O.N., Pang R.T.K., Chu I.K., Wan T.H.L., Chai J., Yeung W.S.B., Chiu P.C.N. Identification of CD147 (basigin) as a mediator of trophoblast functions. Hum. Reprod. 2013;28(11):2920–2929. doi: 10.1093/humrep/det355. [DOI] [PubMed] [Google Scholar]
- 33.Taglauera E., Benarrochc Y., Ropd K., Barnett E., Sabharwal V., Yarringtone C., Wachman E.M. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta. 2020;100:69–74. doi: 10.1016/j.placenta.2020.08.015. 10.1016/j.placenta.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayati A., Kumar R., Francis V., McPherson P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lesseur C., Jessel R.H., Ohrn S., Ma Y., Li Q., Dekio F., et al. Gestational SARS-CoV-2 infection is associated with placental expression of immune and trophoblast genes. Placenta. 2022;126:125–132. doi: 10.1016/j.placenta.2022.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poli A., Michel T., Thérésine M., Andrès E., Hentges F., Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patanè L., Morotti D., Giunta M.R., Sigismondi C., Piccoli M.G., Frigerio L., et al. Vertical transmission of COVID-19: SARS-CoV-2 RNA on the fetal side of the placenta in pregnancies with COVID-19 positive mothers and neonates at birth. Am. J. Obstet. Gynecol. 2020;2(3) doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Algarroba G., Rekawek P., Vahanian S.A. Visualization of SARS-CoV-2 virus invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020;223(2):275–278. doi: 10.1016/j.ajog.2020.05.0. [DOI] [PMC free article] [PubMed] [Google Scholar]