Abstract
Background
Chlamydia trachomatis and Mycoplasma infections have been regarded as severe challenges to public health worldwide because their potential risk of leading to serious reproductive complications. C. trachomatis is the most common sexually transmitted bacterial infections and the prevalence has been increasing in recent years. As a newly discovered pathogen, Mycoplasma genitalium has gradually been recognized as important sexually transmitted infection and even been called a “new chlamydia”. There are no official epidemiological data of M. genitalium in China especially in women with lower reproductive tract infection. This work aims to understand the prevalence and risk factors of M. genitalium and C. trachomatis in women with lower reproductive tract infections and to provide reference for the formulation of health policy in China.
Methods
This study was conducted in the gynecological clinics of 12 hospitals geographically located in different regions in China. Women with purulent cervical secretions or abnormal vaginal microecology were included as the research group, and those with normal vaginal microecology and cervical secretions were included as the control group. A total of 2190 participants were recruited in this project including 1357 of research group and 833 of control group. All participants were required to complete questionnaires, whose vaginal discharge were collected for vaginal microecology test and cervical discharge for detection of M. genitalium and C. trachomatis.
Results
The prevalence of C. trachomatis and M. genitalium were 7.1% (96/1357) and 3.8% (51/1357), respectively in research group. The prevalence of C. trachomatis and M. genitalium varied in different regions. Infection rates of C. trachomatis and M. genitalium were higher in women with abnormal vaginal microecology (C.t P = 0.038, M.g P = 0.043), especially in women with bacterial vaginosis and mixed vaginitis, of which C. trachomatis showed statistical differences (bacterial vaginosis, P = 0.035; mixed vaginitis, P = 0.0001) and M. genitalium was close to statistical differences (bacterial vaginosis, P = 0.057; mixed vaginitis, P = 0.081). Alcoholism and abnormal vaginal microecology were positively correlated with both C. trachomatis and M. genitalium infection. Increasing age, being married and multi-parity were negatively correlated with C. trachomatis infection. There is a positive correlation between multiple sexual partners, diversed styles of sex and C. trachomatis infection.
Conclusions
Women with lower genital dysbiosis have an increased risk of C. trachomatis and M. genitalium. The overall prevalence of M. genitalium is lower than that of C. trachomatis, while they have similarities in the characteristics of infection. Although M. genitalium is not routinely screened as C. trachomatis in young women, attention should be paid to M. genitalium infection in young women with abnormal vaginal microecology or having childbearing needs.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-022-07975-2.
Keywords: Mycoplasma genitalium, Chlamydia trachomatis, Lower reproductive tract infection, Prevalence, Risk factor
Introduction
In the last decades, chlamydial infections, caused by the obligate intracellular bacteria Chlamydia trachomatis (C. trachomatis), have been the most frequently reported sexually transmitted infections (STIs) worldwide [1]. In 2020, a total of 1,579,885 cases of C. trachomatis infection were reported to the CDC, which corresponds to a rate of 481.3 cases per 100,000 population. Over the past 20 years, rates of chlamydial infection have been steadily increasing in both men and women. Young age is a risk factor for C. trachomatis infection, particularly prevalent in those younger than 25 years old. In 2020, almost two-thirds (61%) of all reported chlamydia cases are among persons aged 15–24 years [1]. Infections caused by C. trachomatis are often asymptomatic so routine screening is essential for the detection of infection. Its asymptomatic nature facilitates transmission between sex partners [1]. In females, the cervix is the anatomic site most commonly infected, which can manifest as cervicitis, urethritis, pelvic inflammatory disease (PID), perihepatitis even proctitis. If untreated chlamydial infections in women increase the risk of infertility and ectopic pregnancy, leading to high medical costs [2]. If a woman was infected with C. trachomatis during pregnancy, infants born vaginally may develop conjunctivitis or pneumonia [3]. Nucleic acid amplification testing (NAAT) is the preferred [4] strategy for both symptomatic infections and routine screening, which is recommended by Centers for Disease Control and Prevention (CDC) in America for all sexually active women younger than 25, as well as older women of high risk group based on sexual practices [5]. The epidemiological investigation of C. trachomatis infection in China is limited because of small or poorly performed studies. Literature has reported that prevalence of C. trachomatis in healthy women of childbearing age in China is up to 5.4–13.4% and 10–13% in pregnant women [6]. In females with reproductive tract infection the prevalence of C. trachomatis may be higher.
C. trachomatis infection and its complications have been known for decades, while Mycoplasma genitalium (M. genitalium), another challenging STI pathogen, has been poorly understood for a long time because of its extremely difficult cultivated nature and limited screening. M. genitalium was first discovered and named by Tully in 1981 from the urethral secretions of two of thirteen male patients with non-gonococcal urethritis [7]. Although the pathogenic role of M. genitalium in male urethritis is clear, fewer studies have been conducted among women to determine its pathogenic role in the female reproductive tract. In the World Health Organisation (WHO) global estimates of curable STIs, no data are available for M. genitalium probably because of the absence of sufficient epidemiological data from many parts of the world [8]. Until now M. genitalium has not been included in CDC routine screening. PID is an important cause of infertility and ectopic pregnancy, and C. trachomatis and Neisseria gonorrhoeae (N. gonorrhoeae) are recognized causes. Recently emerging data demonstrate M. genitalium is associated with cervicitis and PID in 10–25% of women [9]. Prevalence of M. genitalium among women with PID ranges from 4 to 22% in 2021 CDC STI treatment guidelines [5]. Another systematic review and meta-analysis showed the summary prevalence of M. genitalium in general population was 1.3% in countries with higher levels of development and 3.9% in countries with lower levels [10]. However there have been no official epidemiological data of M. genitalium in China not only in general population but also in women with reproductive tract infection. It would be a serious situation if we have no effective epidemiological data because of the severe reproductive damage induced by M. genitalium. This work aims to understand the prevalence and risk factors of M. genitalium and C. trachomatis in the female population nationwide, and to provide reference and basis for the formulation of health strategies related to C. trachomatis and M. genitalium in China. Meanwhile we also want to understand the epidemiological characteristics of M. genitalium differ from C. trachomatis.
Methods
Study cohort, sample collection and detection
The study was conducted in accordance with the Declaration of Helsinki and its current amendments, and the protocol was approved by the medical ethics committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University. All subjects have provided written informed consent.
This study was conducted in the gynecological clinics of 12 hospitals in China. The 12 hospitals are geographically located in different regions of China from south to north, east to west, including provinces of Jilin, Beijing, Hebei, Shanxi, Shandong, Jiangsu, Shanghai, Guangdong and Chongqing etc. (Fig. 1). Majority of participants are from urban areas. All the women recruited are heterosexual. Females with purulent cervical secretions or abnormal vaginal microecology were included as the research group, and those with normal vaginal microecology and cervical secretions were included as the control group. A total of 2190 participants were recruited in this project including 1357 of research group and 833 of control group. Inclusion criteria: women of reproductive age over than 18 years old; having sexual experience; having regular menstruation, no usage of any medications within one week; no vaginal douching, cervical treatment or sexual intercourse within 72 h [11]. Exclusion criteria: women during pregnancy, lactation or menopause and women with chronic diseases who need long-term medication.
Fig. 1.
Geographical locations of twelve participating hospitals. Areas where these twelve hospitals are located are shown in purple including provinces of Jilin, Beijing, Hebei, Shanxi, Shaanxi, Shandong, Jiangsu, Shanghai, Guangdong and Chongqing etc.
All the recruited participants were required to complete questionnaires, including age, educational level, type of occupation, economic level, hygiene practices, number of sex partners, way of sex activity, age of first sex, number of gravidity and parity etc. Women recruited would undergo gynecological examination and vaginal microecological testing. Those with cervical purulent secretions or abnormal vaginal microecological conditions will be classified as the research group. Abnormal vaginal microecology were defined below.
Details of vaginal microecological testing: Swab of vaginal discharge was obtained from upper third of the vagina, and used for smearing, Gram staining and observing at oil lens (1000 ×) to evaluate the vaginal microecology with reference to “Evaluation of the vaginal microbiome in clinical diagnosis and management of vaginal infectious diseases” [12]. Vaginal microecology is different from vaginal microbiota. Vaginal microbiota is a general term for all the microbes in the vagina. While vaginal microecology is a comprehensive reflection of vaginal microorganisms, pH, white blood cells, vaginal cleanliness and bacterial secretion function etc. Women with the following data are considered to have a normal microecological vaginal condition [12]: pH values ranging from 3.8 to 4.5, large Gram-positive rods as predominant flora, Nugent score ≤ 3 and AV score < 3, and absence of pathogens and negative specific enzymes. The Nugent score was adopted to diagnose bacterial vaginosis (BV) (Nugent score 7–10 means BV, Nugent 4–6 means BV intermediate, Nugent 1–3 means normal). Vulvovaginal candidiasis (VVC) was indicated when hyphae or spores were discovered, and Trichomonas vaginitis (TV) was indicated when Trichomonas was seen under an oil lens. Clinical manifestations of aerobic vaginitis (AV) include purulent vaginal discharge, a strong inflammatory reaction and AV score ≥ 3. Flora abnormal means the dominant Lactobacillus in the vagina is replaced by other bacteria. Flora inhibition means that the bacterial species and quantity under the microscope of the secretory smear are significantly reduced, and no dominant bacteria exist. Vaginal cleanliness means the ratio of squamous epithelial cells to white blood cells in the vaginal discharge. If the number of squamous epithelial cells are more than white blood cells, we call it “vaginal cleanliness degree I”. If the number of squamous epithelial cells are similar to white blood cells, we call it degree II. If the number of white blood cells are more than squamous epithelial cells, we call it degree III. Degree III represents abnormal status.
Details of isothermal RNA amplification assay: Discharges of the cervix were collected and reserved with sterile cotton swabs and eppendorf (EP) tubes containing normal saline for M. genitalium and C. trachomatis detection. C. trachomatis and M. genitalium were tested using simultaneous amplification and testing (SAT) and isothermal RNA Amplification Assay (Rendu Biotecnology, Shanghai, China). Take N 1.5 ml clean EP tubes (N = number of samples to be tested), and add 400ul of samples to be tested. Add 100ul of nucleic acid extract into each tube (mix well before use), close the cover and shake for 30 s. Heat preservation at 60 °C for 5 min and stand at room temperature for 10 min. Put EP tube on the magnetic bead separation device, stand for 5 min, to be adsorbed on the wall of the magnetic beads, pipette suction tube cover and tube bubbles and liquid, retain the magnetic beads, after completion should be clear enough to see the magnetic beads. Add 1 ml of washing solution,wash twice, mix thoroughly and repeat the previous step. Move EP tubes away from the magnetic bead separation device, add 40 µl of expanded detection solution prepared before to each tube, shake and mix, and take 30 µl of magnetic bead suspension into a new microreaction tube. Negative and positive controls were also set up. Turn on the fluorescence detector and complete the preheating. The reaction procedure conditions of fluorescence detection instrument were set as follows: Each cycle was set at 42 °C, 1 min, for 40 cycles, and fluorescence signal was collected once every minute. Reaction system was 40 µl. Place the microreaction tube prepared above at 60 °C for 10 min, then immediately place it at 42 °C for 5 min, and preheat SAT filtrate to 42. Keep the microreaction tube at 42 °C, add 10 µl of preheated SAT enzyme solution to each tube, cover the tube immediately, shake at 1200 rpm for 15 s, mix well. Transfer the trace reaction tube to the fluorescence detection instrument quickly and start the reaction procedure immediately. Threshold setting: The threshold line was just above the highest point of the normal negative control amplification curve. Result Judgment: Decision value represents the abscissa reading at the intersection of the sample curve and the threshold line. Samples with decision value ≤ 35 are considered positive. 35 < decision value < 40 samples recommended for retesting. A sample with infinite value or 40 is considered negative.
Statistical analysis of data
SPSS 19.0 were used for data analysis. The prevalence of M. genitalium and C. trachomatis were compared by Chi-square test or Fisher’s exact test. The risk factors of M. genitalium and C. trachomatis infection were analyzed by univariate and multivariate analysis of Logistics regression. P < 0.05 was statistically significant.
Results
Prevalence of M. genitalium and C. trachomatis in different age groups
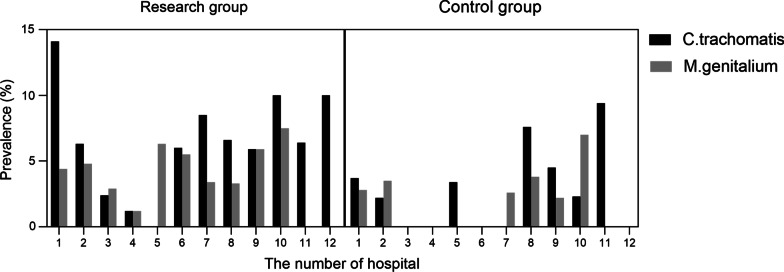
A total of 2190 valid samples were collected in this project including 1357 of research group and 833 of control group. The 12 hospitals participated are geographically located in different regions of China (Fig. 1). Enrollment ranged from 12 to 406 at different centers, with 8 hospitals each having more than 190 participants and the other 4 hospitals having fewer than 100 participants due to a late start. We have detected 74 M. genitalium (+) subjects (3.4%) and 123 C. trachomatis (+) subjects (5.6%) totally. There were respectively 96 and 27 women with C. trachomatis (+) in the research group and control group with statistical significance (96/1357, 7.1% vs 27/833, 3.2%, P < 0.0001), while no significance has been found in the prevalence of M. genitalium (51/1357, 3.8% vs 23/833, 2.8%, P = 0.210). The prevalence varies greatly among each centers. The prevalence of C. trachomatis in the research group at each hospital varied from 0.0% to 14.1%, exceeding 10.0% in three hospitals, while the prevalence of M. genitalium ranged from 0.0% to 7.5% (Fig. 2). In contrast, the prevalence of C. trachomatis in the control group varied from 0.0% to 9.4%, and the prevalence of M. genitalium ranged from 0.0% to 7.0%, with no M. genitalium detected in the control group of 6 hospitals (Fig. 2).
Fig. 2.
Prevalence of C. trachomatis and M. genitalium in each hospitals. Name of hospitals are listed following. 1. Peking University Shenzhen Hospital, 2. Capital Medical University Beijing Obstetrics and Gynecology Hospital, 3. The Second Affiliated Hospital of Hebei Medical University, 4. The Second Hospital of Jilin University, 5. The Second Affiliated Hospital of Shanxi Medical University, 6. Shaanxi Provincial People's Hospital, 7. Obstetrics and Gynecology Hospital of Fudan University, 8. The First Affiliated Hospital of Xi’an Jiaotong University, 9. The Second Hospital of Chongqing Medical University, 10. Nanjing Drum Tower Hospital, 11. Qilu Hospital of Shandong University, 12. Peking University First Hospital
We calculated the prevalence of M. genitalium and C. trachomatis in different age groups which were showed in Table 1. Due to the small number of people under the age of 20, we have not found M. genitalium infection in this part. The prevalence of M. genitalium has declined with increasing of age and showed bimodal distribution in 20–25 and 40–45 years old. In contrast, C. trachomatis infection is more common than that of M. genitalium and the infection rate is highest in people under 20 years old, but decreases gradually with increasing of age with no obvious bimodal distribution. The positive rate of C. trachomatis in the research group was significantly higher than that of the control group at the age of 20–25 years old (18.7% vs 4.3%, P = 0.019) while there was no statistical difference in other age groups. 45 women of unknown age were not included in the age group analysis.
Table 1.
Prevalence of M. genitalium and C. trachomatis in different age groups
| Age group | Number | M. genitalium prevalence | C. trachomatis prevalence | ||||
|---|---|---|---|---|---|---|---|
| Research | Control | P | Research | Control | P | ||
| [17,20) | 17 | 0/13 (0.0%) | 0/4 (0.0%) | – | 5/13 (38.5%) | 1/4 (25.0%) | 0.622 |
| [20,25) | 202 | 10/155 (6.5%) | 2/47 (4.3%) | 0.577 | 29/155 (18.7%) | 2/47 (4.3%) | 0.019 |
| [25,30) | 445 | 9/301 (3.0%) | 5/144 (3.5%) | 0.785 | 24 /301 (8.0%) | 5/144 (3.5%) | 0.099 |
| [30,35) | 517 | 15/335 (4.5%) | 4/182 (2.2%) | 0.188 | 22/335 (6.6%) | 5/182 (2.7%) | 0.065 |
| [35,40) | 404 | 4/216 (1.9%) | 4/188 (2.1%) | 0.843 | 8/216 (3.7%) | 7/188 (3.7%) | 0.992 |
| [40,45) | 274 | 8/159 (5.0%) | 5/115 (4.3%) | 0.793 | 4 /159 (2.5%) | 3/115 (2.6%) | 0.962 |
| ≥ 45 | 286 | 4/156 (2.6%) | 3/130 (2.3%) | 0.889 | 3/156 (1.9%) | 3/130 (2.3%) | 0.821 |
| Unknown | 45 | 1/22 (4.5%) | 0/23 (0.0%) | 0.301 | 1 /22 (4.5%) | 1/23 (4.3%) | 0.974 |
| Total | 2190 | 51/1357 (3.8%) | 23/833 (2.8%) | 0.210 | 96 /1357 (7.1%) | 27/833 (3.2%) | < 0.0001 |
The P value was calculated by Chi-square test or Fisher’s exact probability method
Vaginal microecology and M. genitalium/C. trachomatis infection
Prevalence of C. trachomatis was significantly higher in women with vaginal cleanliness over grade III (7.8% vs 3.8%, P < 0.0001), while prevalence of M. genitalium was not affected by vaginal cleanliness, which was showed in Table 2. The positive rates of M. genitalium and C. trachomatis in women with abnormal vaginal microecology were significantly higher than those with normal vaginal microecology (M.g, 4.2% vs 2.6%, P = 0.043; C.t, 7.1% vs 4.2%, P = 0.038), seen in Table 3. We made a more detailed classification of abnormal vaginal microecology and observed significantly higher C. trachomatis infection rates in women with mixed vaginitis and BV, while significant difference was nearly approached in M. genitalium infection. Infection rates of M. genitalium and C. trachomatis were slightly higher in women with other types of vaginitis, such as VVC, AV, TV, flora inhibition or flora abnormal compared to those with normal microecology, but the difference was not statistically significant. A total of 10 patients were co-infected with M. genitalium and C. trachomatis, including 5 cases with vaginal cleanliness over grade III, 1 case with BV intermediate, 1 case with BV, 1 case with VVC, 2 cases with abnormal flora, 2 cases with mixed vaginitis, 2 cases with normal microecology and 1 case with incomplete information. Among the 10 co-infected women, there were 9 persons aged 20–35 years old and 1 person aged 40–45 years old.
Table 2.
Prevalence of M. genitalium and C. trachomatis in women of different vaginal cleanliness
| Vaginal cleanliness | Number | M. genitalium prevalence | P | C. trachomatis prevalence | P | ||
|---|---|---|---|---|---|---|---|
| (+) | (−) | (+) | (−) | ||||
| < III | 1142 | 36 (3.2%) | 1106 (96.8%) | 0.508 | 43 (3.8%) | 1099 (96.2%) | < 0.0001 |
| ≥ III | 1008 | 37 (3.7%) | 971 (96.3%) | 79 (7.8%) | 929 (92.2%) | ||
| Total | 2150 | 73 (3.4%) | 23 (96.6%) | 122 (5.7%) | 1008 (94.3%) | ||
The P value was calculated by Chi-square test
Table 3.
Prevalence of M. genitalium and C. trachomatis in women of vaginitis
| Vaginal microbiology | Number | M. genitalium prevalence | P | C. trachomatis prevalence | P | ||
|---|---|---|---|---|---|---|---|
| (+) | (−) | (+) | (−) | ||||
| Normal | 1076 | 28 (2.6%) | 1048 (97.4%) | 45 (4.2%) | 1031 (95.8%) | ||
| Abnormal | 1077 | 45 (4.2%) | 1032 (95.8%) | 0.043 | 76 (7.1%) | 1001 (92.9%) | 0.038 |
| Mixed vaginitis | 181 | 9 (5.0%) | 172 (95.0%) | 0.081 | 20 (11.1%) | 161 (88.9%) | 0.0001 |
| VVC | 333 | 12 (3.6%) | 321 (96.4%) | 0.337 | 19 (5.7%) | 314 (94.3%) | 0.244 |
| AV | 63 | 2 (3.2%) | 61 (96.8%) | 0.783 | 4 (6.4%) | 59 (93.6%) | 0.410 |
| BV | 244 | 12 (4.9%) | 232 (95.1%) | 0.057 | 18 (7.4%) | 226 (92.6%) | 0.035 |
| BV intermediate | 36 | 2 (5.6%) | 34 (94.4%) | 0.282 | 1 (2.8%) | 35 (97.2%) | 0.677 |
| Flora inhibition | 22 | 1 (4.6%) | 21 (95.4%) | 0.574 | 1 (4.6%) | 21 (95.4%) | 0.933 |
| Flora abnormal | 189 | 6 (3.2%) | 183 (96.8%) | 0.654 | 12 (6.4%) | 177 (93.6%) | 0.186 |
| TV | 9 | 1 (11.1%) | 8 (88.9%) | 0.115 | 1 (11.1%) | 8 (88.9%) | 0.305 |
The P value was calculated by Chi-square test or Fisher’s exact test. All P values were calculated by comparing with those of normal vaginal microbiology
Risk factors for M. genitalium and C. trachomatis infection
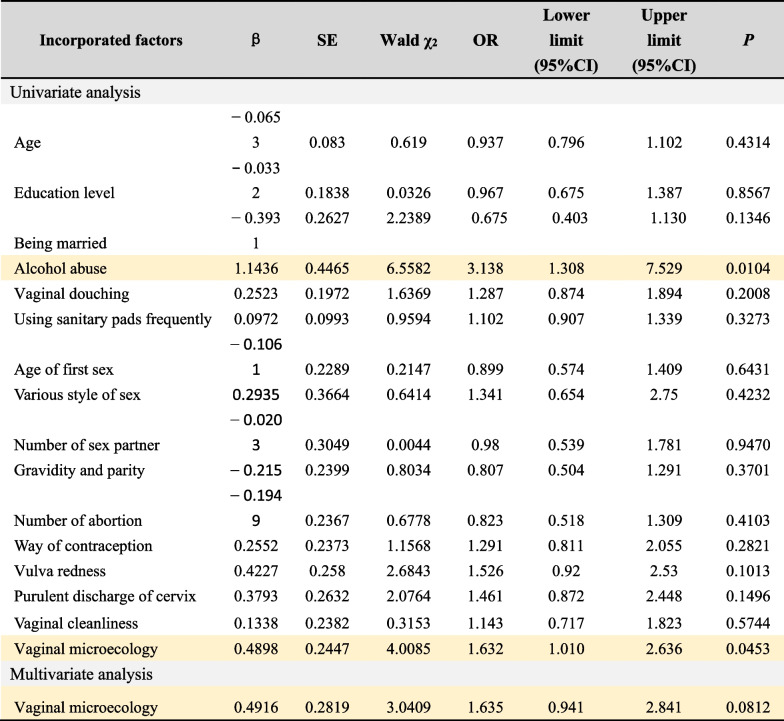
In order to understand the risk factors of M. genitalium and C. trachomatis infection, we used Logistics regression model analysis to conduct univariate and multivariate analysis on relevant data, and calculated OR value and 95% confidence interval (CI). Tables 4 and 5, respectively show the results of univariate and multivariate analysis for M. genitalium and C. trachomatis. Univariate analysis of M. genitalium infection showed that alcohol abuse (OR = 3.138, 95%CI 1.308–7.529, P = 0.0104) and abnormal vaginal microecology (OR = 1.632, 95%CI 1.010–2.636, P = 0.0453) were positively correlated with M. genitalium infection. Multivariate analysis further confirmed that abnormal vaginal microecology (OR = 1.635, 95%CI 0.941–2.841, P = 0.0812) was independent risk factors for M. genitalium infection.
Table 4.
Univariate and multivariate analysis for M. genitalium infection
Risk factors for M. genitalium infection are highlighted in yellow. Value assignment of incorporated factors are detailed in the Additional file 1. “Alcohol abuse” was defined as a state of dependence on alcohol due to prolonged or repeated drinking, which has maintained for more than 12 months
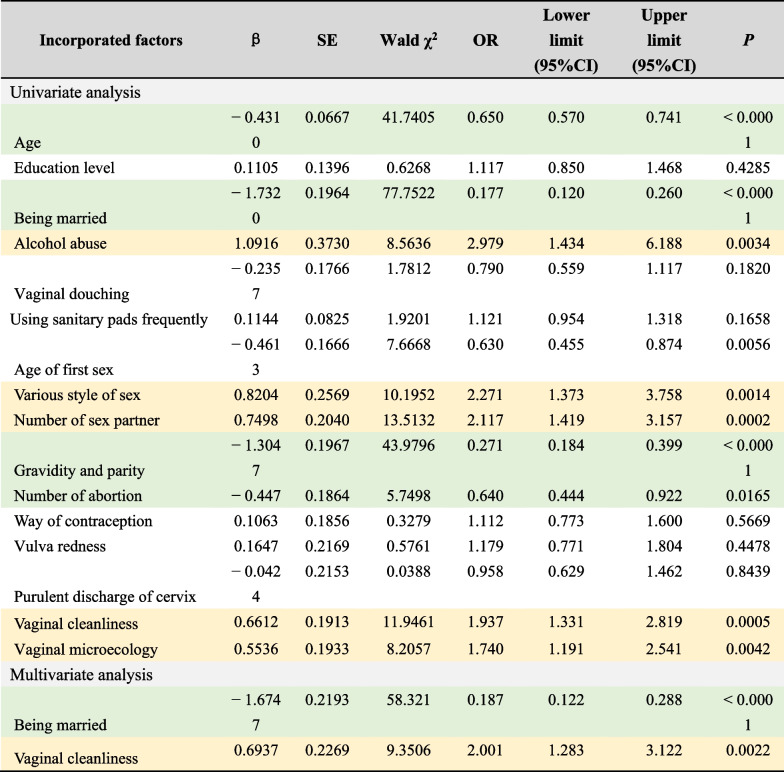
Table 5.
Univariate and multivariate analysis for C. trachomatis infection
Risk factors for C. trachomatis infection are highlighted in yellow, protective factors for C. trachomatis infection are highlighted in green. Value assignment of incorporated factors are detailed in the Additional file 1. “Alcohol abuse” was defined as a state of dependence on alcohol due to prolonged or repeated drinking, which has maintained for more than 12 months
Univariate analysis of C. trachomatis infection showed that increasing age (OR = 0.650, 95%CI 0.570–0.741, P < 0.0001), being married (OR = 0.177, 95%CI 0.120 ~ 0.260, P < 0.0001), gravidity and parity (OR = 0.271, 95%CI 0.184–0.399, P < 0.0001) and the number of abortion (OR = 0.640, 95%CI 0.444–0.922, P = 0.0165) was negatively correlated with C. trachomatis infection, while alcohol abuse (OR = 2.979, 95%CI 1.434–6.188, P = 0.0034), various style of sex (OR = 2.271, 95%CI 1.373–3.758, P = 0.0014), multiple sexual partners (OR = 2.117, 95%CI 1.419–3.157, P = 0.0002), poor vaginal cleanliness (OR = 1.937, 95%CI 1.331–2.819, P = 0.0005) and abnormal vaginal microecology (OR = 1.740, 95%CI 1.191–2.541, P = 0.0042) was positively correlated with C. trachomatis infection. Multivariate analysis showed that being married (OR = 0.187, 95%CI 0.122–0.288, P < 0.0001) was a protective factor and poor vaginal cleanliness (OR = 2.001, 95%CI 1.283–3.122, P = 0.0022) was an independent risk factor for C. trachomatis infection.
Discussion
Non-viral curable STIs such as C. trachomatis, N. gonorrhoeae, and Mycoplasma infections have been regarded as serious challenges to public health throughout the world because of their potential risk of leading to a range of serious reproductive complications like PID, ectopic pregnancy and infertility [13]. C. trachomatis is the most common STI worldwide with an estimated 105.7 million cases in adults aged 15–49 [14]. Prevalence of STIs has been increasing in recent years, with 90 million new cases of C. trachomatis occurring each year, many of which are asymptomatic, and hence undiagnosed and untreated [15]. As a newly discovered pathogen of Mycoplasma, M. genitalium has gradually been recognized as important STIs. M. genitalium has even been called a “new chlamydia” [10]. However, the epidemiological investigation, pathogenisis and mechanism of M. genitalium have not been fully studied, especially in female population and those female at high risk of reproductive tract infection. In a systematic review [16], the incidence of M. genitalium was 1.07 per 100 person-years in women in very highly developed countries. Existing data showed that prevalence of M. genitalium are higher in patients attending sexual health clinics which is estimated to be 10–35% [17]. Among female sex workers, women visiting STI clinic, or women with cervicitis and PID, infection rates of M. genitalium fluctuate between 10% and 19.2% [18]. There has been no available data in China. Therefore we conducted a multi-center epidemiological survey in over ten female reproductive tract infection clinics in China, systematically investigated the infection rate of M. genitalium and C. trachomatis in women with lower reproductive tract infectious diseases, which may provide a theoretical basis for making relevant health policies. Women with purulent cervical secretions or abnormal vaginal microecology were recruited as research group. The prevalence of C. trachomatis and M. genitalium varied in different regions. The prevalence of C. trachomatis was significantly higher in the research group than in the control group in general (7.1% vs 3.2%). Although the prevalence of M. genitalium was higher in the research group as compared to the control group (3.8% vs 2.8%) statistical significance was not reached. Among women with vaginal discharge cleanliness greater than degree III, infection rates of C. trachomatis and M. genitalium were 7.8% and 3.7%, respectively, only C. trachomatis showed significant differences. These data gave us the illusion that M. genitalium infection may be less common and severe than C. trachomatis.
However, when we divided the included women into more detailed age groups, we could see that the positive rate of M. genitalium in women under the age of 20–25 could reach 6.5%, who were at a critical age of reproductive health and fertility. Women in this age group are more likely to have abnormal vaginal microbiology due to active sex. We have found the positive rates of C. trachomatis and M. genitalium were higher in women with abnormal vaginal microecology, especially in women with BV and mixed vaginitis. Data of C. trachomatis showed statistical differences and data of M. genitalium was close to statistical differences.
In previous studies, no precise data suggest M. genitalium is associated with specific organisms that can cause vaginitis, such as Trichomonas vaginalis or Candida albicans. In contrast, evidence of a relationship with BV is inconsistent, with some studies showing decreased risk of BV in women with M. genitalium [19, 20], one showing increased risk [21], and other showing no association [22–24]. A recent longitudinal study found that infection rate of M. genitalium might be higher in the presence of BV [25], and C. trachomatis showed similar situations. M. genitalium alone, however, does not appear to be a cause of vaginitis, which may explain why M. genitalium is not routinely screened.
Although the infection rates of M. genitalium in this research is not as high as expected, we still can't ignore the detriment of M. genitalium to female reproductive system. Some basic studies have confirmed the damage of M. genitalium to female reproductive health. M. genitalium was significantly associated with a 70% increase in risk of cervicitis [26]. Acute M. genitalium infection of endocervical cells causes microvilli destruction and persistent M. genitalium infection may lead to resultant chronic inflammation. In vitro and animal researches have observed microscopic evidence of ciliary damage of human fallopian tube infected with M. genitalium, albeit the damage was more moderate than that seen with C. trachomatis or N. gonorrhoeae infection. Cilia damage leads to potential damage to ectopic pregnancy and infertility [27]. In fact most women with infertility due to fallopian tube damage do not have a history of acute PID [28], which suggests that subclinical infections may be more common than acute PID and could equally cause reproductive harm [29]. The association between M. genitalium infection and tubal infertility has been demonstrated, independent of chlamydial infection [30]. Our study did not include women with PID or a history of PID, so the prevalence of M. genitalium in our data was not as high as expected. We are now recruiting women with PID and the epidemiological data of M. genitalium in Chinese women will be more complete in the near future.
Logistic regression analysis showed that alcoholism and abnormal vaginal microecology were positively correlated with C. trachomatis and M. genitalium infection, which confirmed the above results. We also observed that increasing age, being married and multiple births were negatively correlated with C. trachomatis infection. Perhaps being married and having children means that sexual partners are relatively stable and reproductive function have not been impaired. After all, there is a clear correlation between multiple sexual partners/diverse sexual styles and C. trachomatis infection.
Associations between M. genitalium and most female reproductive tract syndromes have been demonstrated although the statistical significance of results is not always uniform. Fairly consistent evidence shows that women infected with M. genitalium are at increased risk of PID, infertility, and adverse pregnancy outcomes and it is probably an important pathogen. However, M. genitalium is not a “new chlamydia” due to their different characteristics of infection, as well as the possible differences in pathogenesis. Additional data will be required before routine screening can be recommended for M. genitalium. But this research prefers that attention should be paid to M. genitalium infection in young women with abnormal vaginal microecology while having childbearing needs.
Conclusion
We performed the current study to evaluated the prevalence and risk factors of M. genitalium and C. trachomatis in Chinese women with lower genital infection. Our results suggested that prevalence of C. trachomatis and M. genitalium were higher in women with abnormal vaginal microbiology especially in women with BV and mixed vaginitis. The overall prevalence of M. genitalium is lower than that of C. trachomatis, while they have similarities in the characteristics of infection. Although M. genitalium is not routinely screened as C. trachomatis in young women, attention should be paid to M. genitalium infection in young women with abnormal vaginal microecology or having childbearing needs. However, there are several limitations in this study which should be noted. First, the population sample size was still low compared to huge Chinese population. Second, it would be a limitation that we have not include female with PID and this part are still in research. Third, given the study population is highly selected, the prevalence rates given in this study may not be generalisable to the whole population. We are giving treatment and following up the infected women, hoping to know the average duration of M. genitalium in Chinese women and the changes of vaginal microbiology after clearance of pathogen. Hope for meaningful results in the future. At the same time, prospective studies evaluating whether screening programs and targeted treatment of M. genitalium improve reproductive outcomes in women are necessary to guide public health policy for this newly emerging pathogen.
Supplementary Information
Additional file 1: Table S1. Value assignmentof incorporated factors in univariate and multivariate analysis.
Acknowledgements
Not applicable.
Author contributions
ZZ, XZ and ZL wrote the main manuscript text and HB, LF and TL provided help in the literature search, patient recruitment and specimen collection. All authors have read and approved the final manuscript.
Funding
This work was supported by Beijing Natural Science Foundation (No. 7214230), Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital ‘Excellent Youth’ Plan Special Funds (NO.YQRC201906) and Beijing Obstetrics and Gynecology Hospital, Capital Medical University (NO.FCYY201916).
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and its current amendments, and the protocol was approved by the medical ethics committee of Beijing Obstetrics and Gynecology Hospital, Capital Medical University. All subjects are over than 18 years old and have provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhan Zhang and Xiaonan Zong contributed equally to this paper
References
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2020.Atlanta: U.S. Department of Health and Human Services; 2022.
- 2.Owusu-Edusei K, Chesson HW, Gift TL, Tao G, Mahajan R, Ocfemia MC, Kent CK. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis. 2013;40(3):197–201. doi: 10.1097/OLQ.0b013e318285c6d2. [DOI] [PubMed] [Google Scholar]
- 3.Tang W, Mao J, Li KT, Walker JS, Chou R, Fu R, Chen W, Darville T, Klausner J, Tucker JD. Pregnancy and fertility-related adverse outcomes associated with Chlamydia trachomatis infection: a global systematic review and meta-analysis. Sex Transm Infect. 2020;96(5):322–329. doi: 10.1136/sextrans-2019-053999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Workowski KA, Bolan GA. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 5.Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen XS, Peeling RW, Yin YP, Mabey DC. The epidemic of sexually transmitted infections in China: implications for control and future perspectives. BMC Med. 2011;6(9):111. doi: 10.1186/1741-7015-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tully JG, Taylor-Robinson D, Cole RM, Rose DL. A newly discovered mycoplasma in the human urogenital tract. Lancet. 1981;1(8233):1288–1291. doi: 10.1016/S0140-6736(81)92461-2. [DOI] [PubMed] [Google Scholar]
- 8.Wiesenfeld HC, Manhart LE. Mycoplasma genitalium in women: current knowledge and research priorities for this recently emerged pathogen. J Infect Dis. 2017;216(Suppl_2):S389–S395. doi: 10.1093/infdis/jix198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen JS, Cusini M, Gomberg M, Moi H, Wilson J, Unemo M. 2021 European guideline on the management of Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2022;36(5):641–650. doi: 10.1111/jdv.17972. [DOI] [PubMed] [Google Scholar]
- 10.Baumann L, Cina M, Egli-Gany D, Goutaki M, Halbeisen FS, Lohrer GR, Ali H, Scott P, Low N. Prevalence of Mycoplasma genitalium in different population groups: systematic review andmeta-analysis. Sex Transm Infect. 2018;94(4):255–262. doi: 10.1136/sextrans-2017-053384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C, Luo Y, Zhong R, Law PTY, Boon SS, Chen Z, Wong CH, Chan PKS. Role of polycyclic aromatic hydrocarbons as a co-factor in human papillomavirus-mediated carcinogenesis. BMC Cancer. 2019;19(1):138. doi: 10.1186/s12885-019-5347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li T, Liu ZH, Li K, Bai HH. Evaluation of the vaginal microbiome in clinical diagnosis and management of vaginal infectious diseases. Chin Med J (Engl) 2019;132(9):1100–1103. doi: 10.1097/CM9.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rumyantseva T, Golparian D, Nilsson CS, Johansson E, Falk M, Fredlund H, Van Dam A, Guschin A, Unemo M. Evaluation of the new AmpliSens multiplex real-time PCR assay for simultaneous detection of Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis. APMIS. 2015;123(10):879–886. doi: 10.1111/apm.12430. [DOI] [PubMed] [Google Scholar]
- 14.López-Corbeto E, González V, Lugo R, Rivaya B, Casabona J, Matas L, CT/NG Study Group; Investigators of the CT/NG Study Group Pooling of urine samples for molecular detection of Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium as a screening strategy among young adults in Catalonia. Enferm Infecc Microbiol Clin (Engl Ed) 2020;38(2):65–71. doi: 10.1016/j.eimc.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Gerbase AC, Rowley JT, Heymann DH, Berkley SF, Piot P. Global prevalence and incidence estimates of selected curable STDs. Sex Transm Infect. 1998;74(Suppl 1):S12–S16. [PubMed] [Google Scholar]
- 16.Cina M, Baumann L, Egli-Gany D, Halbeisen FS, Ali H, Scott P, Low N. Mycoplasma genitalium incidence, persistence, concordance between partners and progression: systematic review and meta-analysis. Sex Transm Infect. 2019;95(5):328–335. doi: 10.1136/sextrans-2018-053823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yueyue W, Feichen X, Yixuan X, Lu L, Yiwen C, Xiaoxing Y. Pathogenicity and virulence of Mycoplasma genitalium: Unraveling Ariadne's Thread. Virulence. 2022;13(1):1161–1183. doi: 10.1080/21505594.2022.2095741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ona S, Molina RL, Diouf K. Mycoplasma genitalium: an overlooked sexually transmitted pathogen in women? Infect Dis Obstet Gynecol. 2016;2016:4513089. doi: 10.1155/2016/4513089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandepitte J, Bukenya J, Hughes P, Muller E, Buvé A, Hayes R, Weiss HA, Grosskurth H. Clinical characteristics associated with Mycoplasma genitalium infection among women at high risk of HIV and other STI in Uganda. Sex Transm Dis. 2012;39(6):487–491. doi: 10.1097/OLQ.0b013e31824b1cf3. [DOI] [PubMed] [Google Scholar]
- 20.Napierala Mavedzenge S, Müller EE, Lewis DA, Chipato T, Morrison CS, Weiss HA. Mycoplasma genitalium is associated with increased genital HIV type 1 RNA in Zimbabwean women. J Infect Dis. 2015;211(9):1388–1398. doi: 10.1093/infdis/jiu644. [DOI] [PubMed] [Google Scholar]
- 21.Manhart LE, Critchlow CW, Holmes KK, Dutro SM, Eschenbach DA, Stevens CE, Totten PA. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis. 2003;187(4):650–657. doi: 10.1086/367992. [DOI] [PubMed] [Google Scholar]
- 22.Keane FE, Thomas BJ, Gilroy CB, Renton A, Taylor-Robinson D. The association of Mycoplasma hominis, Ureaplasma urealyticum and Mycoplasma genitalium with bacterial vaginosis: observations on heterosexual women and their male partners. Int J STD AIDS. 2000;11(6):356–360. doi: 10.1258/0956462001916056. [DOI] [PubMed] [Google Scholar]
- 23.Lawton BA, Rose SB, Bromhead C, Gaitanos LA, MacDonald EJ, Lund KA. High prevalence of Mycoplasma genitalium in women presenting for termination of pregnancy. Contraception. 2008;77(4):294–298. doi: 10.1016/j.contraception.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Cohen CR, Nosek M, Meier A, Astete SG, Iverson-Cabral S, Mugo NR, Totten PA. Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex Transm Dis. 2007;34(5):274–279. doi: 10.1097/01.olq.0000237860.61298.54. [DOI] [PubMed] [Google Scholar]
- 25.Lokken EM, Balkus JE, Kiarie J, Hughes JP, Jaoko W, Totten PA, McClelland RS, Manhart LE. Association of recent bacterial vaginosis with acquisition of Mycoplasma genitalium. Am J Epidemiol. 2017;186(2):194–201. doi: 10.1093/aje/kwx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis. 2015;61(3):418–426. doi: 10.1093/cid/civ312. [DOI] [PubMed] [Google Scholar]
- 27.Baczynska A, Funch P, Fedder J, Knudsen HJ, Birkelund S, Christiansen G. Morphology of human Fallopian tubes after infection with Mycoplasma genitalium and Mycoplasma hominis—in vitro organ culture study. Hum Reprod. 2007;22(4):968–979. doi: 10.1093/humrep/del455. [DOI] [PubMed] [Google Scholar]
- 28.Tubal infertility: serologic relationship to past chlamydial and gonococcal infection. World Health Organization Task Force on the Prevention and Management of Infertility. Sex Transm Dis. 1995;22(2):71–7. [PubMed]
- 29.Cates W, Jr, Joesoef MR, Goldman MB. Atypical pelvic inflammatory disease: can we identify clinical predictors? Am J Obstet Gynecol. 1993;169(2 Pt 1):341–346. doi: 10.1016/0002-9378(93)90085-W. [DOI] [PubMed] [Google Scholar]
- 30.Svenstrup HF, Fedder J, Kristoffersen SE, Trolle B, Birkelund S, Christiansen G. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility—a prospective study. Fertil Steril. 2008;90(3):513–520. doi: 10.1016/j.fertnstert.2006.12.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Value assignmentof incorporated factors in univariate and multivariate analysis.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.