Abstract
Background
Immune checkpoint inhibitor (ICI) therapy combined with conventional therapies is being broadly applied in non-small cell lung cancer (NSCLC) patients. However, the risk of interstitial pneumonitis (IP) following a combined regimen is incompletely characterized.
Methods
A total of 46,127 NSCLC patients were extracted for disproportionality analyses of IP from the Food and Drug Administration’s Adverse Event Reporting System (FAERS) database. A total of 1108 NSCLC patients who received ICI treatment at Nanfang Hospital of Southern Medical University were collected and utilized for real-world validation.
Results
Of the 46,127 patients with NSCLC, 3830 cases (8.3%; 95% confidence interval [CI], 8.05–8.56) developed IP. Multivariable logistic regression analyses revealed that the adjusted ROR of ICI combined with radiation (RT) was the highest (121.69; 95% CI, 83.60–184.96; P < 0.0001) among all therapies, while that of ICI combined with chemotherapy (CHEMO) or targeted therapy (TARGET) was 0.90 (95% CI, 0.78–1.04; P = 0.160) and 1.49 (95% CI, 0.95–2.23; P = 0.065), respectively, using ICI monotherapy as reference. Furthermore, analyses from our validation cohort of 1108 cases showed that the adjusted odds ratio of ICI combined with RT was the highest (12.25; 95% CI, 3.34–50.22; P < 0.01) among all the therapies, while that of ICI combined with CHEMO or TARGET was 2.32 (95% CI, 0.89–7.92; P = 0.12) and 0.66 (95% CI, 0.03–4.55; P = 0.71), respectively, using ICI monotherapy as reference.
Conclusions
Compared with ICI monotherapy, ICI combined with RT, rather than with CHEMO or TARGET, is associated with a higher risk of IP in NSCLC patients. Hence, patients receiving these treatments should be carefully monitored for IP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02713-6.
Keywords: Interstitial pneumonitis, Non-small cell lung cancer, Immune checkpoint inhibitors, Radiation therapy, Conventional therapy
Background
Immune checkpoint inhibitor (ICI) therapy has been a breakthrough therapy that launched a treatment paradigm for non-small cell lung cancer (NSCLC). It mainly refers to programmed cell death-1 (PD-1) pathway inhibitors including monoclonal antibodies that target either PD-1 or PD-1 ligand (PD-L1). The combination regimen of ICI and conventional therapies, including chemotherapy, molecularly targeted therapy, and radiotherapy, further expands the entire landscape for treating NSCLC [1]. These different interventions manage to work on various steps in the cancer immunity cycle to affect the tumor microenvironment and modulate the existing activated antitumor T cell immune response so as to re-regulate the immune response in cancer as a series of group events and reach optimal killing against cancer cells [1–3]. However, such a regimen for NSCLC, unfortunately, has been accompanied with increasing concerns about safety profile, especially for interstitial pneumonitis (IP).
IP, also known as pneumonitis or interstitial lung disease, is one of the most prevalent and serious treatment-related side effects for NSCLC patients [4–6]. ICI-related IP has been extensively reported in previous studies [7, 8]. The incidence of IP was recently reported as high as 14.5% [9]. Moreover, prior data revealed that IP had led to a 17.5% death rate for patients treated with ICI [10] and accounted for 35% of anti-PD-1/PD-L1-related fatalities [5]. In addition to ICI, conventional treatments had also been verified to cause treatment-related IP in NSCLC patients receiving radiotherapy, molecularly targeted therapy, and chemotherapy [11–19]. Thus, not to our surprise, the combination of a conventional regimen with ICI had resulted in worsening rates of such side effects in some studies [20, 21]. These pooled data warrant risk assessment of IP prior to initiation of therapy in order to reach early prevention and timely intervention. Despite the clear evidence of IP associated with single therapeutic modalities exhibited in previous studies, whether combination regimen of conventional therapy with ICI would augment the risk of IP has not been completely depicted yet and still needs more reliable large data for solid validation. In this study, we hypothesized that combination regimen of conventional therapy with ICI would increase the risk of IP, compared to ICI or conventional therapy alone.
Immunotherapy-related adverse events (irAEs) are characterized by immense heterogeneity, bringing great challenges to clinical studies of these toxicities with ample cohort size. In the past few years, some alternative tools well-suitable for the investigation of potential mechanisms and clinical manifestations of irAEs have been demonstrated [22]. The US Food and Drug Administration Adverse Event Reporting System (FAERS) database [23] is a program run by the FDA to monitor the safety profile of various medications. It is a public website that encourages healthcare professionals, consumers, pharmaceutical firms, or the general population to report adverse reactions through the MedWatch program, and such data are open for access. The FAERS database has been widely used to investigate irAEs. Here, we aim to fully utilize the FAERS database to analyze the association of IP between different treatment regimens. Moreover, as such big data obtained from an open database do have limitations, it usually provides more of a big picture with trends and indicates certain risks, therefore requiring more comprehensive and reliable observational studies from the real world for further confirmation. Thus, we also performed additional analysis on NSCLC patients treated in Nanfang Hospital of Southern Medical University in order to conduct a further observational study using real-world data for external validation.
Methods
Study design
This real-world, retrospective, observational, pharmacovigilance study was conducted using the FDA FAERS database. Since data in FAERS are anonymized and publicly available, the requirements for obtaining informed consent and institutional review board approval were waived. As to validation, the medical records of NSCLC patients treated with ICI were retrospectively reviewed with approval from the institutional review board of the Nanfang Hospital of Southern Medical University.
Patients
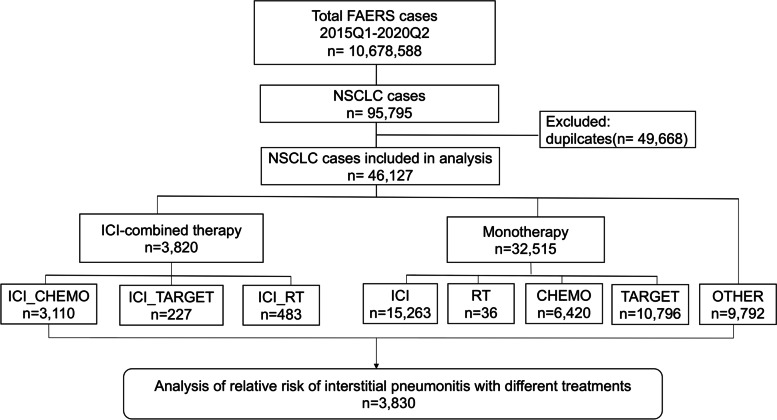
In this study, 10,678,588 reports were retrieved from the FAERS database covering a period from the first quarter of 2015 to the second quarter of 2020 (Fig. 1). For further investigation, 95,795 patients of NSCLC were extracted using NSCLC-related Medical Dictionary for Regulatory Activities (MedDRA) preferred terms (Additional file 1: Table S1). According to the FDA’s recommendations, a deduplication procedure was performed resulting in a reduction in the number of NSCLC patients to 46,127. The procedure of retrieving IP cases from the FAERS database was conducted using IP-related MedDRA preferred terms (Additional file 1: Table S2) according to a previous study [24]. Reports with any one of the IP-related terms were considered as IP cases. Meanwhile, all other reports were considered as non-IP cases. For this study, the following data were retrieved from FAERS: patient’s sex and age, the year when and country where the reports were retrieved, the type of reporter (healthcare professional or not), and the type of treatment used (Additional file 1: Table S3–S6).
Fig. 1.
Flow chart of the study population. In this study, 10,678,588 reports were retrieved from the FAERS database during the first quarter of 2015 to the second quarter of 2020. By using NSCLC-related MedDRA preferred terms, 95,795 reports were selected. Omitting the 49,668 duplicates, 46,127 reports of NSCLC which consist of 3830 IP cases and 42,297 non-cases were enrolled finally
For the validation cohort, a total of 1108 patients who were pathologically diagnosed with NSCLC and received at least one ICI treatment at the Nanfang Hospital of Southern Medical University between January 2015 and September 2022 were retrospectively enrolled in the present study (Additional file 2: Fig. S1). For retrieving IP cases in the validation cohort, it was based on the electric medical record which was diagnosed by the clinicians according to the history and findings on imaging, coupled with the exclusion of competing diagnoses [25]. The toxicity grades for IP according to the common terminology criteria for adverse events (CTCAE) were obtained from the medical records and the related imaging records. The patients’ characteristics and the treatment regimen were obtained from the medical records.
Statistics analysis
A descriptive analysis was used to summarize the characteristics of the NSCLC patients with different treatments. In a pharmacovigilance study, disproportionality occurs when a specific adverse event is associated with a given drug or treatment. Reporting odds ratio (ROR) (Additional file 1: Table S7) is widely used for assessing disproportionality between cases and non-cases and is currently employed by various reporting agencies and the World Health Organization [26]. In this study, ROR was used to compare the odds ratio of the number of IP events related to different treatment strategies (for the validation cohort, the odds ratio of IP events related to different treatment strategies was compared directly by using logistic regression models). It was defined as a significant signal if the lower limit of the 95% confidence interval (CI) exceeded 1, with at least three cases [27]. All RORs were a point estimate calculated as crude or adjusted for age and sex using a logistic regression model (cases with missing data were omitted by default in the model) by adopting a method described in the previous study [24]. Data mining manipulation and statistical analyses were both performed using the R software (version 3.6.1, R Foundation) [28]. The patient dataset is presented in Additional file 3: Table S1.
Results
Patient characteristics
A total of 46,127 NSCLC patients were included (mean [SD] age, 65.6 [11.1] years; 17,517 females [38.0%]). Commonly used therapeutic methods for NSCLC patients were taken into consideration, including monotherapy like chemotherapy, targeted therapy, ICI, RT, and corresponding ICI with conventional therapies. The clinical characteristics of patients with or without IP were described in Table 1. The mean age of patients with ICI with conventional therapies was comparable to that of patients with monotherapies, and near or more than 50% of patients receiving ICI monotherapy or ICI with conventional therapies were male. Most of the cases were reported by healthcare professionals in the recent 2 years, regardless of receiving monotherapy or ICI with conventional therapies. The majority of cases treated with ICI combined with RT were reported by Japanese healthcare professionals, with an overwhelmingly high proportion of IP.
Table 1.
Characteristics of 46,127 non-small cell lung cancer patients
| Variables | ICI with conventional therapies | Monotherapies | OTHER (N = 9792) | |||||
|---|---|---|---|---|---|---|---|---|
| ICI+CHEMO (N= 3110) | ICI+TARGET (N = 227) | ICI+RT (N = 483) | ICI (N = 15,263) | CHEMO (N = 6420) | TARGET (N = 10,796) | RT (N = 36) | ||
| Age | 65.6 (9.62) | 66.0 (11.5) | 68.3 (9.59) | 66.9 (10.1) | 64.0 (10.7) | 67.0 (12.0) | 58.9 (11.3) | 63.0 (11.9) |
| Sex | ||||||||
| Female | 931 (29.9) | 107 (47.1) | 117 (24.2) | 4569 (29.9) | 1856 (28.9) | 5837 (54.1) | 20 (55.6) | 4080 (41.7) |
| Male | 1954 (62.8) | 102 (44.9) | 337 (69.8) | 9527 (62.4) | 3153 (49.1) | 3744 (34.7) | 14 (38.9) | 4424 (45.2) |
| Not reported | 225 (7.2) | 18 (7.9) | 29 (6.0) | 1167 (7.6) | 1411 (22.0) | 1215 (11.3) | 2 (5.6) | 1288 (13.2) |
| Country | ||||||||
| Germany | 378 (12.2) | 5 (2.2) | 7 (1.4) | 684 (4.5) | 846 (13.2) | 303 (2.8) | 2 (5.6) | 479 (4.9) |
| France | 216 (6.9) | 14 (6.2) | 14 (2.9) | 1173 (7.7) | 616 (9.6) | 441 (4.1) | 0 (0) | 980 (10.0) |
| UK | 119 (3.8) | 3 (1.3) | 2 (0.4) | 278 (1.8) | 348 (5.4) | 1468 (13.6) | 1 (2.8) | 587 (6.0) |
| Japan | 896 (28.8) | 95 (41.9) | 392 (81.2) | 5384 (35.3) | 914 (14.2) | 2570 (23.8) | 3 (8.3) | 1854 (18.9) |
| USA | 641 (20.6) | 55 (24.2) | 22 (4.6) | 3651 (23.9) | 1636 (25.5) | 2413 (22.4) | 13 (36.1) | 2538 (25.9) |
| Others | 860 (27.7) | 55 (24.2) | 46 (9.5) | 4093 (26.8) | 2060 (32.1) | 3601 (33.4) | 17 (47.2) | 3354 (34.3) |
| Reporter occupation | ||||||||
| Healthcare professional | 1917 (61.6) | 161 (70.9) | 384 (79.5) | 10,342 (67.8) | 4730 (73.7) | 6573 (60.9) | 24 (66.7) | 7620 (77.8) |
| Non-healthcare professional | 1185 (38.1) | 60 (26.4) | 45 (9.3) | 4730 (31.0) | 1655 (25.8) | 3465 (32.1) | 12 (33.3) | 1999 (20.4) |
| Not reported | 8 (0.3) | 6 (2.6) | 54 (11.2) | 191 (1.3) | 35 (0.5) | 758 (7.0) | 0 (0) | 173 (1.8) |
| Year | ||||||||
| 2015 | 21 (0.7) | 3 (1.3) | 2 (0.4) | 576 (3.8) | 1113 (17.3) | 1446 (13.4) | 7 (19.4) | 1452 (14.8) |
| 2016 | 84 (2.7) | 26 (11.5) | 11 (2.3) | 1880 (12.3) | 837 (13.0) | 1735 (16.1) | 9 (25.0) | 1479 (15.1) |
| 2017 | 271 (8.7) | 45 (19.8) | 21 (4.3) | 2779 (18.2) | 1146 (17.9) | 2266 (21.0) | 6 (16.7) | 1419 (14.5) |
| 2018 | 609 (19.6) | 58 (25.6) | 68 (14.1) | 3952 (25.9) | 1527 (23.8) | 1898 (17.6) | 4 (11.1) | 2021 (20.6) |
| 2019 | 1275 (41.0) | 53 (23.3) | 251 (52.0) | 4163 (27.3) | 1223 (19.0) | 2370 (22.0) | 5 (13.9) | 2270 (23.2) |
| 2020 Q1–2 | 850 (27.3) | 42 (18.5) | 130 (26.9) | 1913 (12.5) | 574 (8.9) | 1081 (10.0) | 5 (13.9) | 1151 (11.8) |
| IP | ||||||||
| With IP | 290 (9.3) | 30 (13.2) | 446 (92.3) | 1579 (10.3) | 320 (5.0) | 591 (5.5) | 13 (36.1) | 561 (5.7) |
| Without IP | 2820 (90.7) | 197 (86.8) | 37 (7.7) | 13,684 (89.7) | 6100 (95.0) | 10,205 (94.5) | 23 (63.9) | 9231 (94.3) |
Classified variable data are shown as n (%)
IP Interstitial pneumonitis, RT Radiation therapy, CHEMO Chemotherapy, ICI Immune checkpoint inhibitor therapy, TARGET Targeted therapy, OTHER Other therapies not mentioned
Relative risk of IP under different therapies in NSCLC patients
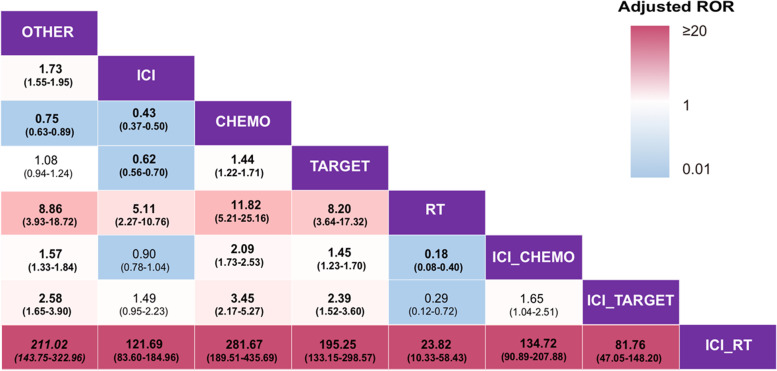
To verify our hypothesis that ICI with conventional therapies increase the risk of IP, multivariable logistic regression analyses were performed to calculate the adjusted ROR of IP under different therapies in NSCLC patients. The results revealed that using the ICI monotherapy as a reference, the adjusted ROR of ICI combined with RT was the highest (121.69; 95% CI, 83.60–184.96; P < 0.0001) among all the therapies (Fig. 2), while that of ICI combined with CHEMO or TARGET was 0.90 (95% CI, 0.78–1.04; P = 0.160) and 1.49 (95% CI, 0.95–2.23; P = 0.065), respectively. These results showed that a combination of RT and ICI may be associated with a higher risk of IP compared with ICI alone, while a combination of ICI and chemotherapy or targeted therapy was related to a risk of IP comparable to that of ICI alone. Besides, compared to ICI combined with chemotherapy or targeted therapy, the adjusted ROR of IP for ICI combined with RT was 134.72 (95% CI, 90.89–207.88; P < 0.0001) and 81.76 (95% CI, 47.05–148.20; P < 0.0001), respectively, indicating a possibly higher pulmonary toxicity in a combination of ICI and RT as compared to the other two combined patterns.
Fig. 2.
Relative risk of interstitial pneumonitis under different therapies in non-small cell lung cancer patients. Each cell contains the adjusted ROR and its 95% confidence interval of IP under treatment in the rows using that in the column as a reference. All of the numbers in bold were statistically significant. The P-value for the adjusted ROR was calculated using a multivariable logistic regression model and was adjusted by using the “p.adjusted()” command in R. Abbreviations: ROR, reporting odds ratio; OTHER, all the other therapies except the listed therapies; RT, radiation therapy; ICI, immune checkpoint inhibitor therapy; CHEMO, chemotherapy; TARGET, targeted therapy
In general, these results suggested that ICI combined with RT may increase the risk of IP, while the combination of ICI and chemotherapy or targeted therapy may not enhance this risk, partially supporting our hypothesis.
A synergistic interaction between ICI and RT associated with increased risk of IP in patients with NSCLC
As mentioned above, ICI combined with RT may potentially increase the risk of IP in NSCLC patients, but it was unknown whether this effect resulted from the toxicity overlapping of ICI and RT or an interaction of both treatments. To deal with this question, a multivariable logistic regression analysis was conducted to explore the interaction between ICI and RT treatment. The adjusted RORs of IP with RT and ICI treatment were 9.15 (95% CI, 4.07–19.25; P < 0.0001) and 1.77 (95% CI, 1.67–1.92; P < 0.0001), respectively. The adjusted ROR for the interaction effect was 13.44 (95% CI, 5.81–33.07; P < 0.0001), indicating the existence of an interaction (Table 2). This interaction effect between ICI and RT was also confirmed by a likelihood ratio test (P < 0.0001). However, there was no significant interaction between ICI and CHEMO/TARGET (Table 2). Furthermore, we implemented multivariable logistic regression analyses of subgroups stratified by RT or ICI treatment for IP. The relative crude RORs for ICI with RT versus ICI and RT monotherapy were 106.01 (95% CI, 76.67–151.23; P < 0.0001) and 21.33 (95% CI, 10.15–46.7; P < 0.0001), respectively. These results supported that an interaction between ICI and RT may account for an increased risk of IP in NSCLC patients following a combination therapy of ICI and RT.
Table 2.
A synergistic interaction between ICI and RT associated with an increased risk of IP in patients with non-small cell lung cancer
| Variables | Cases, N = 46,127 | IP, N = 3830 | Proportion (%), 95% CI | Crude ROR, 95% CI | Adjusteda ROR, 95% CI | P-value |
|---|---|---|---|---|---|---|
| Age (mean/SD) | 65.6 (11.1) | 1.02 (1.02–1.03) | 1.02 (1.02–1.03) | < 0.0001 | ||
| Sex | ||||||
| Female | 17,517 | 1022 | 5.83 (5.49–6.19) | 1 [reference] | 1 [reference] | |
| Male | 23,255 | 2,387 | 10.26 (9.88–10.66) | 1.85 (1.71–1.99) | 1.54 (1.41–1.68) | < 0.0001 |
| Not reported | 5355 | 421 | 7.86 (7.16–8.62) | 1.38 (1.22–1.55) | 0.95 (0.67–1.32) | 0.773 |
| Treatment options | ||||||
| ICI | 19,083 | 2345 | 12.29 (11.83–12.76) | 2.41 (2.25–2.58) | 1.77 (1.64–1.92) | < 0.0001 |
| CHEMO | 9530 | 610 | 6.4 (5.92–6.92) | 0.71 (0.65–0.77) | 0.71 (0.61–0.83) | < 0.0001 |
| TARGET | 11,023 | 621 | 5.63 (5.21–16.08) | 0.59 (0.54–0.65) | 1.18 (1.04–1.33) | 0.008 |
| RT | 519 | 459 | 88.44 (85.30–91.00) | 95.85 (73.71–126.95) | 9.15 (4.07–19.25) | < 0.0001 |
| ICIaCHEMOb | 3110 | 290 | 9.32 (8.34–10.41) | 0.97 (0.79–1.19) | 0.771 | |
| ICIaTARGETb | 227 | 30 | 13.22 (9.23–18.49) | 1.02 (0.65–1.55) | 0.929 | |
| ICIaRTb | 483 | 446 | 92.34 (89.50–94.48) | 13.44 (5.81–33.07) | < 0.0001 | |
IP Interstitial pneumonitis, ROR Reporting odds ratio, SD Standard deviation, RT Radiation therapy, ICI Immune checkpoint inhibitor therapy, CHEMO Chemotherapy, TARGET Molecular targeted therapy
aAge increment was per year for reporting the odds ratio of multivariable logistic regression
bThis row shows the result of the interaction between interstitial pneumonitis and 2 therapies. The P-value for adjusted ROR was calculated using a multivariable logistic regression model. P < 0.05 indicates a significant difference
Relative risk of IP under different ICI drugs in NSCLC patients treated with RT
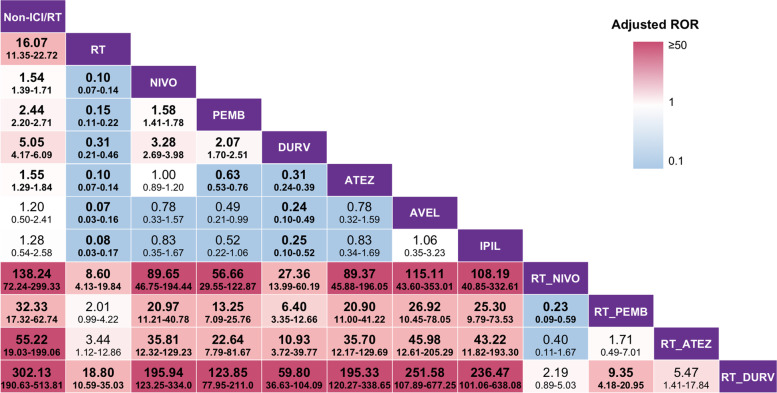
To further demonstrate our hypothesis, we next attempted to determine whether different ICI drugs combined with RT would make a difference in triggering IP. Some frequently employed ICI drugs, including nivolumab, pembrolizumab, durvalumab, and atezolizumab, were included in the multivariable logistic regression analyses. With regard to monotherapy, durvalumab earned the second highest adjusted ROR of IP, following RT. Moreover, the results showed that RT combined with nivolumab, pembrolizumab, durvalumab, or atezolizumab resulted in a higher adjusted ROR of IP than that of ICI alone. Of note, the adjusted ROR of IP for patients receiving RT combined with durvalumab treatment was the highest (302.13; 95% CI, 190.63–513.81; P < 0.0001, using non-ICI/RT as reference), compared with those of other treatments (Fig. 3, more details in Additional file 2: Table S1). These data indicated that RT combined with ICI may result in an increased risk of IP, regardless of the kind of ICI drugs, and compared with other ICI drugs, durvalumab may have the largest potential to induce IP when combined with RT.
Fig. 3.
Relative risk of interstitial pneumonitis under different ICI drugs in non-small cell lung cancer patients. Each cell contains the adjusted ROR and its 95% confidence interval of IP under treatment in the rows using that in the column as a reference. All of the number in bold were statistically significant. Since only one case received IPIL combined with RT and no case received AVEL combined with RT, these regimens were not invested further. The P-value for adjusted ROR was calculated using a multivariable logistic regression model and was adjusted by using the “p.adjusted()” command in R. Abbreviations: ROR, reporting odds ratio; RT, radiation therapy; ICI, immune checkpoint inhibitor therapy; NIVO, nivolumab; PEMB, pembrolizumab; DURV, durvalumab; ATEZ, atezolizumab; AVEL, avelumab; IPIL, ipilimumab
Validation of relative risk of IP under different therapies in NSCLC patients from an external cohort
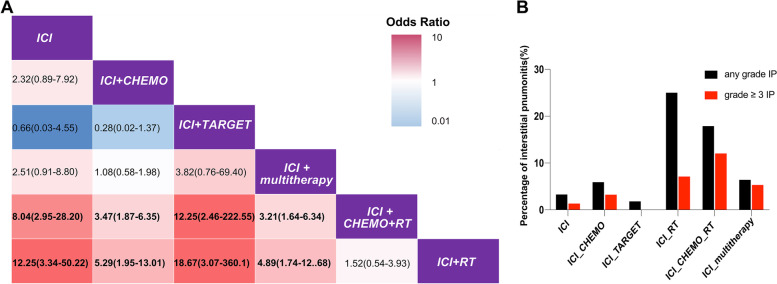
In order to validate the risk of IP in NSCLC patients receiving ICI combined with RT therapy, we reviewed the development of IP in 1108 patients who were pathologically diagnosed with NSCLC and received at least once ICI treatment at the Nanfang Hospital of Southern Medical University between January 2015 and September 2022. In total, 80 cases with IP events of any grade happened, including 48 cases with grade 3 or higher IP (Table 3, Additional file 2: Fig. S1 and Table S2). Consistently multivariable logistic regression analyses were performed to calculate the adjusted odds ratio of IP under different therapies in NSCLC patients. The results showed that using the ICI monotherapy as a reference, the adjusted odds ratio of ICI combined with RT was the highest (12.25; 95% CI, 3.34–50.22; P < 0.01) among all the therapies, and ICI combined with CHEMO+RT was 8.04 (95% CI, 2.95–28.20; P < 0.01), while ICI combined with CHEMO, TARGET, and CHEMO+multitherapy was 2.32 (95% CI, 0.89–7.92; P = 0.12), 0.66 (95% CI, 0.03–4.55; P = 0.71), and 2.51 (95% CI, 0.91–8.80; P = 0.10), respectively (Fig. 4A). This result from our cohort indicated a possibly higher IP risk in a combination of ICI and RT as compared to the other two combined patterns. Furthermore, we analyzed the difference of IP accidence of any grade or grades 3–5 among different therapies. We found that there was not only a higher incidence of IP (25%), but also a higher incidence of severe IP (7.1%) for patients receiving combination therapy of ICI and RT, especially a higher incidence of grade 3–5 IP (12%) in patients receiving ICI+CHEMO+RT therapy (Fig. 4B). Infection played an important role in interstitial pneumonitis of lung cancer patients [29]. Therefore, we further analyzed the CRP and procalcitonin (PCT) of 239 patients who received ICI combined with RT in the validation cohort. The result showed that the positive rate of CRP was significantly higher in IP patients than in non-IP patients, instead of PCT which was related to bacterial infection (Additional file 2: Table S3–S4). Thus, our cohort validated that there was a possibly higher pulmonary toxicity in a combination of ICI and RT as compared to the other two combined patterns.
Table 3.
Characteristic of 1108 NSCLC patients with ICI from Nanfang Hospital cohort
| Variables | ICI-combined therapy, N = 956(%) | ICI monotherapy, N = 152(%) | P-value |
|---|---|---|---|
| Age | 61.4 | 61.5 | |
| Sex | 0.67 | ||
| Female | 239 (25.0) | 41 (27.0) | |
| Male | 717 (75.0) | 111 (73.0) | |
| Clinical Stage (AJCC 8th edition) | 0.002 | ||
| I–III | 274 (28.7) | 25 (16.4) | |
| IV | 682 (71.3) | 127 (83.6) | |
| Smoking status | 0.220 | ||
| Ever smoking | 590 (61.7) | 84 (55.3) | |
| Never smoking | 315 (33.0) | 61 (40.1) | |
| Unknown | 51 (5.3) | 7 (4.6) | |
| Pathology subtypes | 0.725 | ||
| Squamous cell carcinoma | 337 (35.3) | 50 (32.9) | |
| Adenocarcinoma | 506 (52.9) | 81 (53.3) | |
| Others | 113 (11.8) | 21 (13.8) | |
| IP | 0.065 | ||
| With IP | 75 (7.8) | 5 (3.3) | |
| Without IP | 881 (92.2) | 147 (96.7) |
IP Interstitial pneumonitis
P < 0.05 indicates a significant difference
Fig. 4.
Relative risk of interstitial pneumonitis under different therapies in non-small cell lung cancer patients from a validation cohort. A Each cell contains the odds ratio and its 95% confidence interval of IP under treatment in the rows using that in the column as a reference. All of the numbers in bold were statistically significant. B The black bar stands for all patients with any grade IP under different therapies and the red bar for patients with grade 3 or higher IP and treated by certain therapy. The P-value for adjusted ROR was calculated using a multivariable logistic regression model and was adjusted by using the “p.adjusted()” command in R. Abbreviations: RT, radiation therapy; ICI, immune checkpoint inhibitor therapy; CHEMO, chemotherapy; TARGET, targeted therapy; poly, multiple kinds of treatments; multitherapy, all the other multimodal therapies except the listed therapies
Discussion
The introduction of immunotherapy has revolutionized modern cancer treatment. Along with it, also come concerns for its complications, especially IP, as it is a clinically significant and potentially life-threatening treatment-related adverse event for NSCLC patients [5]. The risk of IP has already been extensively demonstrated in single-agent studies with ICI or other conventional interventions. With the increasing need for more intensive cancer-killing therapy, the combination regimen with ICI and conventional therapy has also been more widely applied, thus raising worsening suspicion that it would exacerbate the odds of developing IP. Although some independent studies of small size have reported that ICI with conventional therapies increases the risk of IP, tremendous heterogeneity within irAEs poses limits on the cohort size studying these toxicities, thus entailing an alternative method more suitable for investigation of irAEs in order to better reveal the association between specific drugs and adverse events. In this study, we analyzed real-world big data and specifically focused on identifying the association between the risk of IP and the ICI with conventional therapies. We confirmed that combination treatment of ICI and RT, rather than the combination with chemotherapies or molecularly targeted therapies, is more likely to augment the risk of IP, urging clinicians to pay closer attention in such clinical scenarios. However, current data regarding ICI with conventional therapies and IP are limited to small-size studies, and no big data are available to fully evaluate the association. To the best of our knowledge, this is the largest real-world study to investigate the risk of IP for NSCLC patients following different ICI with conventional therapies, based on the FAERS pharmacovigilance database with real-world validation.
First of all, we have verified our hypothesis that ICI with conventional therapies may augment the risk of IP, compared to ICI monotherapy. Upon further analysis, we realized that different combination regimens exhibited various results. For instance, ICI combined with chemotherapy or targeted therapy may not enhance the risk of IP while ICI combined with RT was associated with an elevated risk of IP. This is in concordance with the previous report that the rate of ICI-related IP was not significantly different between patients receiving chemotherapy and chemo-naive patients [30]. On the other hand, in terms of the association of ICI and molecularly targeted therapy, a real-world observational study revealed a potentially increased risk of IP when nivolumab was combined with EGFR-TKI [24]. It is understandable to have such inconsistency between this study and our finding, since we included not only EGFR-TKI but also other molecularly targeted medications like VEGFR antibodies in our group, which may influence the final results.
A review of pooled current data on RT had presented controversial results. There were previous clinical trials which reported that combined therapy of ICI and RT did not induce a higher risk of treatment-related pneumonitis as compared to a single therapeutic modality in NSCLC patients [21, 31–34]. However, our study demonstrated a different view. While analyzing clinical data of a larger sample size with a total of 46,127 NSCLC patients from the real world, we found that the combination of RT and ICI is associated with an increased risk of IP in patients with NSCLC. The discrepancy of the findings between our real-world study and the clinical trials may be partially attributed to the different patient population enrolled. Patients treated with ICI with conventional therapies in our group were older than those of single therapeutic modality groups, and age is a widely acknowledged risk factor of IP. Besides, it is reported that Japanese patients are more susceptible to drug-related lung disease [35], and a considerably higher proportion of Japanese patients were included in the ICI with conventional treatment group, compared to that of the RT group and ICI group. Our finding was also supported by several other retrospective studies [36–38]. In a single-centered study from Korea [39], the rate of radiation pneumonitis of any grade from the durvalumab + RT group and RT group was 89.0% (17/21) and 37.5% (15/40), respectively, and the rate of grade 2 and higher radiation pneumonitis was 42.9% (9/21) and 20% (8/40), respectively. This was in line with our hypothesis that the addition of radiation to immunotherapy would add an unwarranted risk of IP.
The mechanism behind such a phenomenon is complex and not fully understood. Radiation-induced pneumonitis played an important role in the interstitial changes at the radiated field. The classic mechanism of RT-related IP is that radiation directly damaged vascular endothelial cells and alveolar epithelial cells. These cellular injuries led to cytokine release and immune cell recruitment resulting in acute pneumonitis and pulmonary fibrosis [40]. Immune-related interstitial pneumonitis has been rare but also been widely discussed since the introduction of ICI. One important mechanism of IP induced by ICI was the T cell-mediated inflammation [25, 41]. These two pathologies share some similarities in the clinical picture but also are two distinct processes. Moreover, the pathophysiology of IP could also be potentially related to infection, inflammation, aspiration, etc. More research is needed for deeper investigation.
This study, along with previous reports, alerts us to be more cautious when providing patients with combined therapy of ICI and RT and be prepared for early intervention if IP is in doubt. Moreover, during subgroup analysis among different ICI drugs, we found that different classes of ICI, when combined with RT, might have various degrees of harmful effects. Durvalumab may pose a higher risk of IP in patients who received RT than any other ICI drugs did. Similarly, in a recent meta-analysis of comparing the efficacy and safety of PD1/PDL1 for advanced NSCLC patients, durvalumab was considered to be the most toxic agent among several ICI regimens (durvalumab, nivolumab, atezolizumab, and pembrolizumab) in terms of SAEs or respiratory and thoracic disorders in the second-line or further-line settings [42]. Last, but not least, apart from utilizing the big database from FAERS, we took one step further and looked into our own real-world data attempting external validation, as we hope to testify whether ICI combined with RT would lead to a higher risk of IP events in our cohort, and our data showed that a combination of ICI and RT may result in not only a higher incidence of IP, but also a higher proportion of severe IP for NSCLC patients.
We do have several limitations in our study. First of all, the FAERS database relies on spontaneous reports from anyone including healthcare professionals, pharmaceutical companies, and patients for adverse events data collection [23]. Therefore, not all events would be reported resulting in under-reporting or over-reporting as duplicated data collected from different involved aspects. Furthermore, crucial details regarding treatment-related adverse events like important comorbidities, prior related treatments, duration of suspected therapy, and dosage might be missing. So, this FAERS database has either missing or incomplete information on the patients’ clinical data (such as the grade of IP, stage of NSCLC, details of radiation, underlying diseases, or previous treatment). Moreover, any of the reported events reported by non-healthcare professionals might be associated with limited verification as they might lack standardized clinical confirmation. Hence, there is no absolute certainty that an adverse event was caused by specific drugs. All these contribute to inevitable bias leading to sometimes inconsistent data of certain odds ratio, despite the consistent trends. However, this bias itself represents the intrinsic disadvantage of using big data from open public access.
Second, although verified by using an external validation cohort, this study was a retrospective, observational study with inevitable bias, and the sample size of our validation cohort remained relatively small considering the relatively low incidence of IP. Third, some of the key risk factors for developing ICI-related IP, such as the diagnosis of melanoma, combination immunotherapy, and previous ≥ grade 2 immune-mediated toxicity, were not included in this study [43], and ICI-treated patients in an emergency were not further analyzed in this study, which has been reported that a majority of acutely unwell patients treated with ICI therapy presenting with respiratory symptoms did not have an immune-mediated pathology [44]. Due to these limitations, our analysis refers more to a trend indicating a potentially increased risk of IP associated with the use of a particular medication and requires further confirmation.
Conclusions
Compared with ICI monotherapy, ICI combined with RT, rather than with CHEMO or TARGET, is associated with a higher risk of IP in NSCLC patients. Furthermore, there is a synergistic interaction between ICI and RT associated with an increased risk of IP in patients with NSCLC. Among different classes of ICI, durvalumab, when combined with RT, may potentially pose a significant threat to the development of IP in NSCLC patients; hence, patients receiving these treatments should be carefully monitored for IP.
Supplementary Information
Additional file 1: Table S1. Non-small cell lung cancer (NSCLC) associated PTs used. Table S2. Interstitial pneumonitis (IP) associated PTs used. Table S3. Immune checkpoint inhibitor (ICI) associated PTs used. Table S4. Chemotherapy (CHEMO) associated PTs used. Table S5. Targeted therapy (TARGET) associated PTs used. Table S6. Radiotherapy (RT) associated PTs used. Table S7. Fourfold table for measure of disproportionality.
Additional file 2: Fig. S1. Flow chart of the validation cohort from Nanfang Hospital. Table S1. Relative risk of IP with different ICI drugs in NSCLC patients from FAERS database (detailed numbers). Table S2. Relative risk of IP with different ICI combined therapies in NSCLC patients from Nanfang hospital cohort (detailed numbers). Table S3. The CRP positive rate of patients received ICI with RT in validation cohort. Table S4. The PCT positive rate of patients received ICI with RT in validation cohort.
Additional file 3: Table S1. Original data of NSCLC patients retrieved from FAERS database. (CSV 3899 kb)
Acknowledgements
Not applicable.
Abbreviations
- CHEMO
Chemotherapy
- CTCAE
Common terminology criteria for adverse events
- FAERS
Food and Drug Administration’s Adverse Event Reporting System
- ICI
Immune checkpoint inhibitors
- IP
Interstitial pneumonitis
- MedDRA
Medical Dictionary for Regulatory Activities
- NSCLC
Non-small cell lung cancer
- PD-1
Programmed cell death-1
- PD-L1
PD-1 ligand
- ROR
Reporting odds ratio
- RT
Radiation therapy
- TARGET
Targeted therapy
Authors’ contributions
JG, DHW, and ZYD had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. XJG and ZYD contributed to the conception and design. DHW, ZYD, XJG, XTC, YPZ, ZXR, YXW, XB, and JW contributed to the analysis and interpretation of the data. XJG, XTC, YPZ, ZXR, YXW, LLL, SCM, ZQG, and XRT contributed to the drafting of the original article. XJG, ZYD, DHW, JG, LL, QJF, JW, and XRT contributed to the critical revision of the article for important intellectual content. DHW, XJG, ZYD, and ZXR contributed to the final approval of the article. XJG and QJF contributed to the statistical analysis. DHW and ZYD obtained the funding. DHW, JG, and ZYD contributed to the administrative, technical, or logistic support. XJG, XTC, YXW, LLL, SCM, ZQG, YPZ, ZXR, XB, and JW contributed to the collection and assembly of the data. All authors have reviewed the manuscript and approved the final version.
Funding
This study was supported by the National Natural Science Foundation for Young Scientists of China (grant no. 81802863) and the National Natural Science Foundation of China (grant no. 81872399 and 82272820).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the FAERS database (https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard). Other data relevant to the study are included in the article or uploaded as supplementary information. Any additional data pertaining to this manuscript are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Since data in FAERS are anonymized and publicly available, the requirements for obtaining informed consent and institutional review board approval were waived. As the validation cohort, this retrospective, observational study was approved by the institutional review board of the Nanfang Hospital of Southern Medical University with exemption from informed consent (NFEC-2021-003).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xue-Jun Guo, Xiao-Ting Cai, Zi-Xuan Rong and Yan-Pei Zhang contributed equally to this work.
Contributor Information
Jian Guan, Email: guanjian5461@163.com.
Zhong-Yi Dong, Email: dongzy1317@foxmail.com.
De-Hua Wu, Email: 18602062748@163.com.
References
- 1.Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B, et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17(5):286–301. doi: 10.1038/nrc.2017.17. [DOI] [PubMed] [Google Scholar]
- 2.Sanmamed M, Chen LJC. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175(2):313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen D, Mellman IJI. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Rashdan S, Minna JD, Gerber DE. Diagnosis and management of pulmonary toxicity associated with cancer immunotherapy. Lancet Respir Med. 2018;6(6):472–478. doi: 10.1016/s2213-2600(18)30172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors. JAMA. Oncol. 2018;4(12). 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed]
- 6.Sears CR, Peikert T, Possick JD, Naidoo J, Nishino M, Patel SP, et al. Knowledge gaps and research priorities in immune checkpoint inhibitor–related pneumonitis. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2019;200(6):e31–e43. doi: 10.1164/rccm.201906-1202ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med. 2015;373(3):288–290. doi: 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor–related pneumonitis in patients with advanced cancer. JAMA Oncol. 2016;2(12):1607–1610. doi: 10.1001/jamaoncol.2016.2453. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki Y, Karayama M, Uto T, Fujii M, Matsui T, Asada K, et al. Assessment of immune-related interstitial lung disease in patients with NSCLC treated with immune checkpoint inhibitors: a multicenter prospective study. J Thorac Oncol. 2020;15(8):1317–1327. doi: 10.1016/j.jtho.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Moey MYY, Gougis P, Goldschmidt V, Johnson DB, Lebrun-Vignes B, Moslehi J, et al. Increased reporting of fatal pneumonitis associated with immune checkpoint inhibitors: a WHO pharmacovigilance database analysis. Eur Respir J. 2020;55(6). 10.1183/13993003.00038-2020. [DOI] [PubMed]
- 11.Palma DA, Senan S, Tsujino K, Barriger RB, Rengan R, Moreno M, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85(2):444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marks LB, Bentzen SM, Deasy JO, Kong F-MS, Bradley JD, Vogelius IS, et al. Radiation dose–volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3):S70–SS6. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding PN, Lord SJ, Gebski V, Links M, Bray V, Gralla RJ, et al. Risk of treatment-related toxicities from EGFR tyrosine kinase inhibitors: a meta-analysis of clinical trials of gefitinib, erlotinib, and afatinib in advanced EGFR-mutated non-small cell lung cancer. J Thorac Oncol. 2017;12(4):633–643. doi: 10.1016/j.jtho.2016.11.2236. [DOI] [PubMed] [Google Scholar]
- 14.Inoue A, Saijo Y, Maemondo M, Gomi K, Tokue Y, Kimura Y, et al. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003;361(9352):137–139. doi: 10.1016/s0140-6736(03)12190-3. [DOI] [PubMed] [Google Scholar]
- 15.Porta C, Osanto S, Ravaud A, Climent MA, Vaishampayan U, White DA, et al. Management of adverse events associated with the use of everolimus in patients with advanced renal cell carcinoma. Eur J Cancer. 2011;47(9):1287–1298. doi: 10.1016/j.ejca.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti–programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709–717. doi: 10.1200/jco.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genestreti G, Di Battista M, Trisolini R, Denicolò F, Valli M, Lazzari-Agli LA, et al. A commentary on interstitial pneumonitis induced by docetaxel: clinical cases and systematic review of the literature. Tumori. 2018;101(3):e92–ee5. doi: 10.5301/tj.5000275. [DOI] [PubMed] [Google Scholar]
- 18.Poole BB, Hamilton LA, Brockman MM, Byrd DC. Interstitial pneumonitis from treatment with gemcitabine. Hosp Pharm. 2014;49(9):847–850. doi: 10.1310/hpj4909-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jules-Elysee K, White DA. Bleomycin-induced pulmonary toxicity. Clin Chest Med. 1990;11:1. doi: 10.1016/S0272-5231(21)00668-7. [DOI] [PubMed] [Google Scholar]
- 20.Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903. doi: 10.1016/s1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theelen WSME, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts JGJV, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5(9):1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jing Y, Yang J, Johnson DB, Moslehi JJ, Han L. Harnessing big data to characterize immune-related adverse events. Nat Rev Clin Oncol. 2022:1-12. doi: 10.1038/s41571-021-00597-8 [DOI] [PubMed]
- 23.FDA’s Adverse Event Reporting System (FAERS). https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard. Accessed 4 Oct 2020.
- 24.Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-associated interstitial pneumonitis in nivolumab-treated patients with non-small cell lung cancer. JAMA Oncol. 2018;4(8):1112–1115. doi: 10.1001/jamaoncol.2017.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon VR, Anderson R, Blidner A, Choi J, Cooksley T, Dougan M, et al. Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of immune-related adverse events: pulmonary toxicity. Supportive Care in Cancer. 2020;28(12):6145–6157. doi: 10.1007/s00520-020-05708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. 2013;10(7):796–803. doi: 10.7150/ijms.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 28.RStudio Team (2020). RStudio: integrated development for R. RStudio, PBC, Boston http://www.rstudio.com/. Accessed 4 Apr 2020.
- 29.Hamashima R, Uchino J, Morimoto Y, Iwasaku M, Kaneko Y, Yamada T, et al. Association of immune checkpoint inhibitors with respiratory infections: a review. Cancer Treat Rev. 2020;90:102109. doi: 10.1016/j.ctrv.2020.102109. [DOI] [PubMed] [Google Scholar]
- 30.Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930–1939. doi: 10.1016/j.jtho.2018.08.2035. [DOI] [PubMed] [Google Scholar]
- 31.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 32.Anouti B, Althouse S, Durm G, Hanna N. Prognostic variables associated with improved outcomes in patients with stage III NSCLC treated with chemoradiation followed by consolidation pembrolizumab: a subset analysis of a phase II study from the Hoosier Cancer Research Network LUN 14-179. Clin Lung Cancer. 2020;21(3):288–293. doi: 10.1016/j.cllc.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Peters S, Felip E, Dafni U, Belka C, Guckenberger M, Irigoyen A, et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer—the ETOP NICOLAS trial. Lung Cancer. 2019;133:83–87. doi: 10.1016/j.lungcan.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Lin SH, Lin Y, Yao L, Kalhor N, Carter BW, Altan M, et al. Phase II trial of concurrent atezolizumab with chemoradiation in unresectable non-small cell lung cancer. J Thorac Oncol. 2019:1–31. 10.1016/j.jtho.2019.10.024. [DOI] [PubMed]
- 35.Shah RR. Tyrosine kinase inhibitor-induced interstitial lung disease: clinical features, diagnostic challenges, and therapeutic dilemmas. Drug Saf. 2016;39(11):1073–1091. doi: 10.1007/s40264-016-0450-9. [DOI] [PubMed] [Google Scholar]
- 36.Pozzessere C, Bouchaab H, Jumeau R, Letovanec I, Daccord C, Bourhis J, et al. Relationship between pneumonitis induced by immune checkpoint inhibitors and the underlying parenchymal status: a retrospective study. ERJ Open Res. 2020:1–11. 10.1183/23120541.00165-2019. [DOI] [PMC free article] [PubMed]
- 37.Voong KR, Hazell SZ, Fu W, Hu C, Lin CT, Ding K, et al. Relationship between prior radiotherapy and checkpoint-inhibitor pneumonitis in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2019;20(4):e470–e4e9. doi: 10.1016/j.cllc.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaverdian N, Beattie J, Thor M, Offin M, Shepherd AF, Gelblum DY, et al. Safety of thoracic radiotherapy in patients with prior immune-related adverse events from immune checkpoint inhibitors. Ann Oncol. 2020;31(12):1719–1724. doi: 10.1016/j.annonc.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung HA, Noh JM, Sun JM, Lee SH, Ahn JS, Ahn MJ, et al. Real world data of durvalumab consolidation after chemoradiotherapy in stage III non-small-cell lung cancer. Lung Cancer. 2020;146:23–29. doi: 10.1016/j.lungcan.2020.05.035. [DOI] [PubMed] [Google Scholar]
- 40.Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-induced lung injury: assessment and management. Chest. 2019;156(1):150–162. doi: 10.1016/j.chest.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delaunay M, Cadranel J, Lusque A, Meyer N, Gounant V, Moro-Sibilot D, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017:1–13. 10.1183/13993003.00050-2017. [DOI] [PubMed]
- 42.Liang J, Li M, Sui Q, Hu Z, Bian Y, Huang Y, et al. Compare the efficacy and safety of programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) inhibitors for advanced non-small cell lung cancer: a Bayesian analysis. Transl Lung Cancer Res. 2020;9(4):1302–1323. doi: 10.21037/tlcr-20-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nahm SH, Heywood R, Callaghan S, Serra-Bellver P, Gupta A, Cooksley T, et al. Patient and treatment characteristics of emergency presentations due to immune-mediated toxicities. Eur J Cancer. 2022;164:62–69. doi: 10.1016/j.ejca.2021.12.033. [DOI] [PubMed] [Google Scholar]
- 44.Cooksley T, Gupta A, Al-Sayed T, Lorigan P. Emergency presentations in patients treated with immune checkpoint inhibitors. Eur J Cancer. 2020;130:193–197. doi: 10.1016/j.ejca.2020.02.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Non-small cell lung cancer (NSCLC) associated PTs used. Table S2. Interstitial pneumonitis (IP) associated PTs used. Table S3. Immune checkpoint inhibitor (ICI) associated PTs used. Table S4. Chemotherapy (CHEMO) associated PTs used. Table S5. Targeted therapy (TARGET) associated PTs used. Table S6. Radiotherapy (RT) associated PTs used. Table S7. Fourfold table for measure of disproportionality.
Additional file 2: Fig. S1. Flow chart of the validation cohort from Nanfang Hospital. Table S1. Relative risk of IP with different ICI drugs in NSCLC patients from FAERS database (detailed numbers). Table S2. Relative risk of IP with different ICI combined therapies in NSCLC patients from Nanfang hospital cohort (detailed numbers). Table S3. The CRP positive rate of patients received ICI with RT in validation cohort. Table S4. The PCT positive rate of patients received ICI with RT in validation cohort.
Additional file 3: Table S1. Original data of NSCLC patients retrieved from FAERS database. (CSV 3899 kb)
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the FAERS database (https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard). Other data relevant to the study are included in the article or uploaded as supplementary information. Any additional data pertaining to this manuscript are available from the corresponding author upon reasonable request.