Abstract
The octadentate siderophore analog 3,4,3-LI(1,2-HOPO), denoted 343-HOPO hereafter, is known to have high affinity for both trivalent and tetravalent lanthanide and actinide cations. Here we extend its coordination chemistry to the rare-earth cations Sc3+ and Y3+ and characterize fundamental metal–chelator binding interactions in solution via UV-Vis spectrophotometry, nuclear magnetic resonance spectroscopy, and spectrofluorimetric metal-competition titrations, as well as in the solid-state via single crystal X-ray diffraction. Sc3+ and Y3+ binding with 343-HOPO is found to be robust, with both high thermodynamic stability and fast room temperature radiolabeling, indicating that 343-HOPO is likely a promising chelator for in vivo applications with both metals. As a proof of concept, we prepared a 86Y-343-HOPO complex for in vivo PET imaging, and the results presented herein highlight the potential of 343-HOPO chelated trivalent metal cations for therapeutic and theranostic applications.
Subject terms: Inorganic chemistry, Bioinorganic chemistry, Coordination chemistry, Drug discovery and development
Radionuclide pairs 44Sc/47Sc and 86Y/90Y possess great promise in the development of theranostic agents, but realizing the full potential of these isotopes necessitates improving rare-earth chelation chemistry. Here the authors show that a hydroxypyridinone-based ligand is a promising candidate for applications in this arena.
Introduction
Nuclear medicine is a rapidly growing field based upon the clinical use of radionuclides for diagnostic and therapeutic purposes1. Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are two of the most common imaging modalities for diagnostic purposes, while targeted-radionuclide therapeutic efforts focus on inducing irreversible DNA damage via the emission of either α-particles, β− particles, or low-energy (Auger) electrons2. The full potential of nuclear medicine may be realized with theranostics, wherein a molecular targeting vector is labeled with both a diagnostic and a therapeutic radionuclide that are utilized for concomitant imaging and treatment3–6. Ideally, the employed radionuclides are a matched pair, where both are radioisotopes of the same chemical element; however, very few elements have isotope pairs with suitable nuclear decay properties. As a result, current efforts focus on perceived chemical homologs such as 68Ga as the PET imaging agent mimic of 177Lu or 90Y for β− therapy. However, this approach is hindered by the different ionic radii of these elements (CN = 6, 0.62 Å for Ga3+; CN = 8, 0.977 Å for Lu3+; CN = 8, 1.019 Å for Y3+)7,8, which leads to mismatches in coordination chemistry and varying in vivo behavior9–11. The identification of chemically identical (i.e., isotopic) pairs of radionuclides with complementary nuclear properties is valuable for the development of new theranostic agents, and 44Sc/47Sc and 86Y/90Y are two pairs with great promise in this arena1,12–14.
Both 44Sc and 86Y offer several advantages compared to the commonly utilized 68Ga as diagnostic pairs for 177Lu or 90Y, including longer half-lives (3.97 and 14.74 h, respectively), higher resolution PET images (Supplementary Table 2), and preferences for higher coordination numbers15,16. The latter is of particular importance as the current gold standard for metal ion chelation in nuclear medicine is the octadentate ligand 1,4,7,10-tetraazocyclododecane-1,4,7,10-tetraacetic acid (DOTA). Despite its frequent use as a radionuclide chelator for preclinical and clinical applications, DOTA has a number of limitations, including poor binding kinetics, which generally necessitate heating for radiochemical complexation17–19. An ideal chelator for theranostic applications would feature fast radiolabeling kinetics at room temperature, low toxicity, and in vivo stability (i.e., kinetic and thermodynamic inertness), and ligands featuring hydroxypyridinone moieties are known to meet these criteria20,21. The octadentate siderophore analog, 3,4,3-LI(1,2-HOPO), denoted 343-HOPO hereafter, is known to satisfy all three criteria described above, and is an especially effective theranostic chelator due to its propensity to bind with both trivalent and tetravalent metal cations22,23. In addition, density functional theory calculations have highlighted structural deformities for 343-HOPO complexes with endogenous metals, which minimizes in vivo competition for the rare-earth cations (Sc3+, Y3+) included in 343-HOPO chelates herein24.
Here we begin our investigations by looking at fundamental Sc3+-343-HOPO and Y3+-343-HOPO binding interactions in solution via UV-Vis spectrophotometry, nuclear magnetic resonance (NMR) spectroscopy, and spectrofluorimetric metal-competition titrations, and in the solid-state via single crystal X-ray diffraction. Subsequently, we conduct proof-of-concept in vivo PET imaging with a 86Y-343-HOPO complex. 86Y was selected over 44Sc for PET studies as 86Y3+ is a matched pair with 90Y and a better chemical match than either Ga3+ or Sc3+ as a diagnostic partner to 177Lu, both of which (90Y and 177Lu) are β− emitting isotopes included in treatments approved by the U.S. Food and Drug Administration25,26. Moreover, while Sc3+ and Y3+ are similar in hardness, with IA values of 10.49 and 10.64, respectively, they differ greatly in pKa values (4.3 for Sc3+ v. 7.7 for Y3+)1. This manifests in the pH where hydrolysis begins for the two rare-earth cations, with Sc3+ hydrolysis beginning at approximately pH 2.5 while Y3+ hydrolysis occurs at neutral pH or above27–29. This latter characteristic is desirable for radiolabeling processes, which typically require pH values between 4 and 5, where hydrolysis products in a Sc3+ system may limit the efficacy of a selected chelator30. Finally, the longer half-life of 86Y (t1/2 = 14.74 h) covers a much longer portion (~2 days) of the pharmacokinetics of relevant therapeutic targeting vectors compared to either 68Ga (t1/2 = 1.13 h) or 44Sc (t1/2 = 3.97 h), which is a significant advantage as the latter PET isotopes are limited to measuring only early therapeutic uptake kinetics31.
Results and discussion
Solution chemistry of rare earth complexes with 343-HOPO
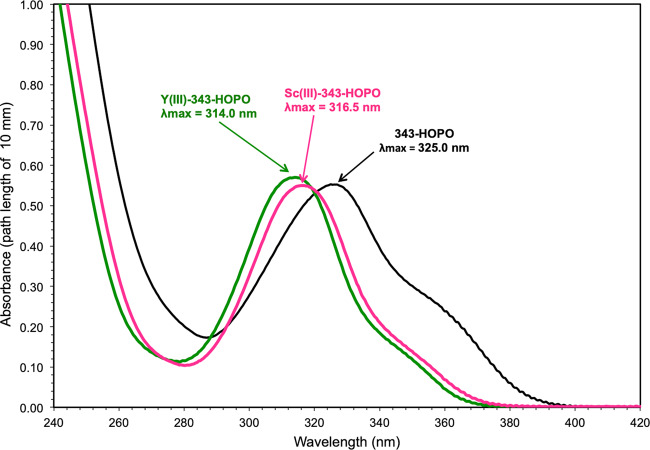
The UV-Visible absorbance spectra of 343-HOPO at pH 7.4 upon addition of Sc3+ or Y3+ are shown in Fig. 1. Compared to free 343-HOPO, the UV spectrum is blue shifted upon addition of the metal ions Sc3+ and Y3+, which do not absorb in the highlighted spectral window. Changes in UV-Vis spectra are due to the binding of Sc3+ or Y3+ ions to the 1,2-HOPO groups of the chelator, and such results are consistent with those previously reported for the binding of lanthanide ions (Ln3+, Ln = La3+ to Lu3+) to 343-HOPO and other 1,2-HOPO chelators22,32,33. The results depicted in Fig. 1 suggest that at physiological pH and at high metal concentrations, compared to radiopharmaceutical formulations, one equivalent of 343-HOPO is sufficient to form stable complexes with Sc3+ or Y3+ and prevent metal ion hydrolysis. Further confirmation of 343-HOPO complex stability can be seen in the 45Sc NMR spectrum (Supplementary Fig. 1) where the [Sc-343-HOPO]− chemical shift is observed downfield at ca. 62.5 ppm. The Sc3+-aqua ion in dilute HCl produces a sharp signal at ca. 4 ppm (Supplementary Fig. 1), and the large 45Sc chemical shift upon complexation indicates 343-HOPO effectively shields the 45Sc nucleus from solvent molecules (vide infra)13. The single peak in the 45Sc NMR spectrum for [Sc-343-HOPO]− also suggests no metal-centered isomerism on the NMR timescale, consistent with only a single species in solution.
Fig. 1. UV-Vis absorbance spectra of [Sc-343-HOPO]− and [Y-343-HOPO]−.
Samples contained 30 μM of 343-HOPO (black curve), 30 μM of 343-HOPO and 30 μM of Sc3+ ions (pink curve), or 30 μM of 343-HOPO and 30 μM of Y3+ ions (green curve). Path length: 10 mm. Background electrolyte: 25 mM HEPES buffer, pH = 7.4. Absorbance corrected from blank absorbance (25 mM HEPES). T = 25 °C.
Based on the solution thermodynamics of Ln3+ complexes with 343-HOPO22,34,35, the complexes that are likely formed in aqueous solutions with Sc3+ and Y3+ are: [M-343-HOPOH], [M-343-HOPO]−, and [M-343-HOPO(OH)]2− (where M = Sc3+ or Y3+). The protonated and hydroxylated complexes, [M-343-HOPOH] and [M-343-HOPO(OH)]2-, are only present in relatively narrow pH ranges for Ln3+ cations (0 < pH < 3 and 9 < pH < 11, respectively), thus the most relevant complex at physiological pH (7.4) is [M-343-HOPO]−. By analogy, the complexes [Sc-343-HOPO]− and [Y-343-HOPO]− are expected to be the predominant species in the pH range 3 to 9 (Supplementary Fig. 2). The proton-independent stability constants (log βmlh) for both complexes were determined via spectrofluorimetric metal-competition titration using the luminescent [Eu-343-HOPO]− complex as a reference22. Upon excitation at 325 nm, the europium complex exhibits an intense emission spectrum in the spectral window 500–800 nm. When another metal that competes with Eu3+ for 343-HOPO binding is added to the system, the intensity of the emission spectrum of [Eu-343-HOPO]− decreases based on Eq. (1).
| 1 |
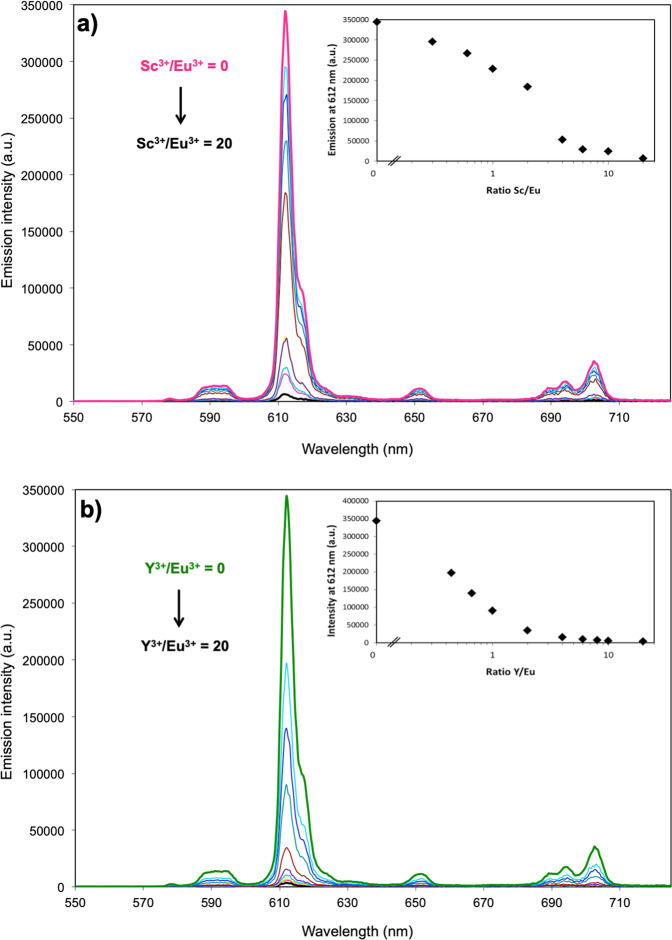
This equation can be applied if the competing M3+ ions (here Sc3+ and Y3+) do not form a luminescent complex with 343-HOPO or generate an emission spectrum different from that of [Eu-343-HOPO]−. 343-HOPO complexes of Sc3+ and Y3+ are not luminescent; consequently, upon addition of Sc3+ and Y3+ to a [Eu-343-HOPO]− solution, the emission intensity decreases until the concentration of [Eu-343-HOPO]− in the system is undetectable due to the formation of the spectroscopically silent [Sc-343-HOPO]− or [Y-343-HOPO]−. Examples of this type of spectroscopic titration with both Sc3+ and Y3+ are shown in Fig. 2.
Fig. 2. Spectrofluorimetric metal competition titrations.
[Eu-343-HOPO]− was competed against Sc3+ (a) and Y3+ (b). Insets: Emission intensity at 612 nm versus the ratio M/Eu (M = Sc3+ or Y3+). Ratio M/Eu = 0 to 20 equivalents. Background electrolyte: 2.5 mM HEPES + KCl. I = 0.1 M. pH = 7.4. T = 25°C.
Utilizing the known metal hydrolysis constants, ligand protonation constants, and log β110 value of [Eu-343-HOPO]−22,34, proton-independent stability constants values for [Sc-343-HOPO]− and [Y-343-HOPO]− were obtained using the method described above. Deconvolution of titration data were performed with HypSpec34,36, which yielded log β110 values of 25.16 ± 0.01 and 20.76 ± 0.09 for [Sc-343-HOPO]− and [Y-343-HOPO]−, respectively. Notably, [Sc-343-HOPO]− is the most stable trivalent-343-HOPO complex reported to date, with a log β110 value three orders of magnitude higher than its closest lanthanide or actinide analog (Table 1).
Table 1.
Summary of proton-independent stability constants with 343-HOPO and trivalent metal cations.
| Cation | log β110 | Reference(s) |
|---|---|---|
| Sc3+ | 25.16 ± 0.01 | this work |
| Y3+ | 20.76 ± 0.09 | this work |
| La3+ | 16.4(3) | 22 |
| Ce3+ | 17.4(5) | 35 |
| Pr3+ | 18.2(4) | 22 |
| Nd3+ | 18.7(1) | 22 |
| Sm3+ | 19.7(3) | 22 |
| Eu3+ | 20.2(2) | 22, 34 |
| Gd3+ | 20.5(1) | 22 |
| Tb3+ | 20.9(1) | 22 |
| Dy3+ | 21.2(1) | 22 |
| Ho3+ | 21.5(1) | 22 |
| Er3+ | 21.7(1) | 22 |
| Tm3+ | 22.0(1) | 22 |
| Yb3+ | 22.2(1) | 22 |
| Lu3+ | 21.2(1) | 22 |
| Am3+ | 20.4(2) | 54 |
| Cm3+ | 21.8(4) | 47 |
Structural chemistry of rare earth complexes with 343-HOPO
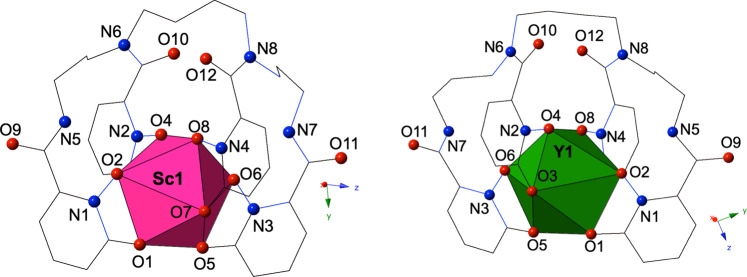
While the solution chemistry of 343-HOPO and its use in a variety of applications such as actinide decorporation and separations37,38, post-MRI chelation therapy39, or 89Zr PET imaging40 is increasingly well-characterized, the structural chemistry of this chelator, with trivalent metals in particular, remains largely unexplored. Daumann et al. reported the first crystal structure featuring 343-HOPO and Eu3+ in 201541, and this remained the only published structure featuring 343-HOPO and a trivalent metal until this study. Our group has made progress recently on the structural chemistry of 343-HOPO with tetravalent p-block and d-block metals42,43, and here we extend this knowledge to systems featuring Sc3+ and Y3+. Both complexes 1, K[Sc(343-HOPO)]•DMF•(H2O)x, and 2, K[Y(343-HOPO)]•DMF, crystallize in the space group P-1 upon slow evaporation from solutions of methanol containing 5% DMF. Complexes 1 and 2 both feature a single crystallographically unique rare-earth metal center with the Sc3+ and Y3+ cations adopting distorted square antiprismatic molecular geometries (Fig. 3). Sc3+ and Y3+ cations are each eight coordinate with all four 1,2-HOPO moieties of 343-HOPO binding in a bidentate manner and the average Sc3+ and Y3+–O bond distances are 2.213 Å and 2.347 Å, respectively. The asymmetric units for both complexes feature potassium cations for charge balancing purposes, as well as solvent molecules, which are significantly disordered for complex 1. Akin to other examples of crystallographically characterized metal complexes of 343-HOPO, we find the Sc3+ and Y3+ complexes (1 and 2) display handedness, as racemic mixtures of Δ(λ) and Λ(δ) isomers. This notation, which we have previously employed, describes the configuration of the 1,2-HOPO moieties surrounding the metal center as either Δ or Λ, and the relative configuration of the butylene diamine backbone as either λ or δ (Supplementary Fig. 3)43. We have proposed that the extremely high thermodynamic stabilities of 343-HOPO complexes may allow for the chromatographic separation or resolution of these Δ(λ) and Λ(δ) enantiomers, and while outside the scope of the current study, we are currently working to achieve this goal and delineate differing in vivo behaviors that result due to complex handedness43.
Fig. 3. Solid-state structures of Sc3+-343-HOPO and Y3+-343-HOPO complexes.
Polyhedral representations of complexes 1 and 2, where pink and green polyhedra are Sc3+ and Y3+ centers, respectively, and spheres represent oxygen atoms (red) and nitrogen atoms (blue). Hydrogen atoms, potassium cations, and solvent molecules are omitted for clarity.
343-HOPO complexation with 86Y and in vivo imaging experiments
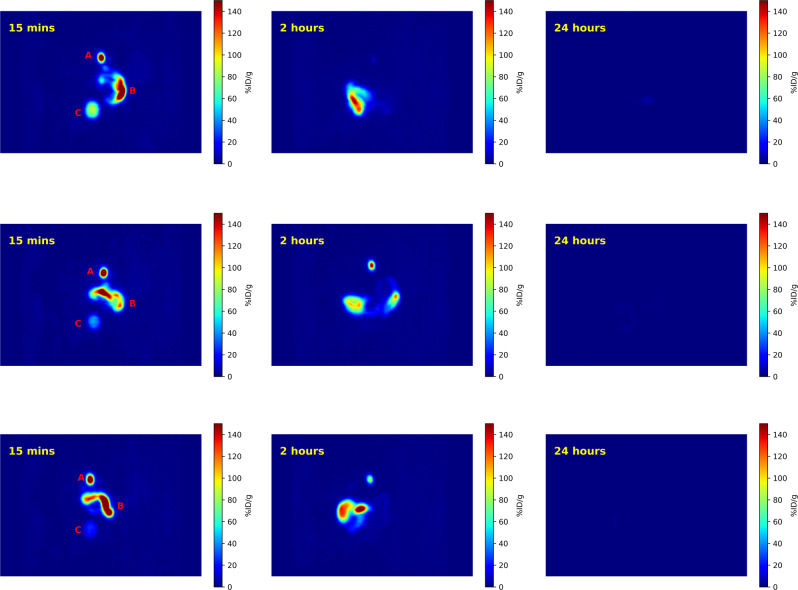
An advantage of working with 343-HOPO is that it has been evaluated for potential biomedical applications; thus, toxicology and pharmacology studies have already been completed44–46. In addition, the in vivo stability of metal complexes of 343-HOPO formed with both trivalent (Eu, Am, Cm) and tetravalent (Zr, Pu) metals has been investigated in multiple studies, using ex vivo radioanalytical techniques and in vivo PET imaging. All of these biodistribution studies have highlighted the unusual in vivo stability and quantitative, rapid hepatobiliary clearance of 343-HOPO complexes, as well as minimal or undetectable non-targeted uptake of metal ions, when compared to other commonly used chelators, including diethylenetriaminepentaacetic acid (DTPA) and desferrioxamine B (DFO)22,37,40,47. To assess the in vivo behavior and pharmacokinetics of the 86Y-343-HOPO complex, we administered the radiolabeled small molecule complex to young adult female Swiss Webster mice via intravenous injection. Mice were imaged at 15 min, 2 h, 24 h, and 48 h post-injection, and the results are highlighted in Fig. 4, Supplementary Fig. 4, and Supplementary Table 4, largely confirming biodistribution patterns previously noted with other metal ions. At 15 min, the vast majority of the radioactivity is observed in the gall bladder and gastrointestinal tract, indicating hepatic clearance is the main excretion pathway for 86Y-343-HOPO, with a small amount of activity appearing in the bladder, suggesting a minor renal clearance pathway as well. This is confirmed at 2 h when the majority of remaining radioactivity is found in the gastrointestinal tract and the liver. At 24 and 48 h, very little detectable radioactivity remains (Fig. 4, Supplementary Fig. 4, and Supplementary Table 4), which suggests the pharmacokinetics of the 86Y-343-HOPO complex are rapid. PET images at all four time points also display minimal kidney uptake, an important feature that may prevent dose-limiting nephrotoxicity (Fig. 4, Supplementary Fig. 4, and Supplementary Table 4), in contrast to 86Y complexes formed with DOTA or DTPA48. Moreover, the lack of radioactivity accumulating in the skeleton or other organs suggests that the 86Y complex remains intact over several hours in vivo12, thereby demonstrating that 343-HOPO is well suited for targeted in vivo applications with yttrium radioisotopes.
Fig. 4. Coronal PET images of 86Y-343-HOPO.
Three healthy mice were administered 86Y-343-HOPO (93.1 µCi [3.44 MBq] in 10x PBS) via tail vein injection and imaged between 15 min and 24 h after injection. The gall bladder (a), the gastrointestinal tract (b), and the bladder (c) can be visualized at the 15 min timepoint. The 86Y-343-HOPO complex primarily undergoes rapid hepatic clearance and no uptake of 86Y in the skeleton is observed.
Herein, we characterized the complexation of Sc3+ and Y3+ with 343-HOPO in solution via UV-Vis spectrophotometry, NMR spectroscopy, and spectrofluorimetric metal-competition titrations, and in the solid-state via single crystal X-ray diffraction. We experimentally observed high thermodynamic stability for the binding of 343-HOPO with Sc3+ and Y3+, and crystallographic analysis agrees with solution-state NMR results, indicating rare-earth cations are well shielded from solvent molecules. Proof of concept 86Y-343-HOPO PET imaging experiments revealed hepatic clearance, rapid pharmacokinetics, and no detectable radioactivity accumulating in bones or other organs, all of which are valuable for future work pairing 86Y with therapeutic isotopes of interest. In combination, these results indicate that 343-HOPO is likely a promising chelator for both the 44/47Sc and 86/90Y theranostic isotope pairs. Future studies will focus on expanding efforts to 343-HOPO conjugates49 and siderocalin fusion proteins50,51 using Sc3+ and Y3+, as well as lanthanides and actinides of theranostic interest.
Methods
Incremental spectrofluorimetric titrations
The spectrofluorimetric metal-metal competition titration method developed by Sturzbecher-Hoehne et al.22 was used to evaluate the thermodynamic stability of the Sc(III)-343-HOPO and Y(III)-343-HOPO complexes. This method used the luminescent [Eu-343-HOPO]− complex as a reference as this complex has been thoroughly characterized34,41, and typically, 10–12 samples of 2 mL were prepared per titration. Each sample contained 0.1 µM Eu(III), 0.1 µM 343-HOPO, and 0 to 20 equivalents of competing metal ions (here Sc3+ or Y3+). Samples were buffered with 0.1 M HEPES at pH 7.4, and after 24 h of equilibration in a thermostated bath at 25 °C, the emission spectra of the samples were acquired with a HORIBA Jobin Yvon IBH FluoroLog-3 spectrofluorimeter in steady state mode. Emission (550–725 nm–350 data points) was monitored perpendicular to the excitation pulse after excitation of the sample at 325 nm. Slits were set at 2.0 nm and 1.0 nm for the emission and excitation monochromators, respectively, and the integration time was 0.4 s per point.
343-HOPO radiolabeling with 86Y
A 2.86 mL 10× phosphate buffered saline (PBS) solution containing 5 µL of 343-HOPO (34.9 µM) in DMSO was added to the 86Y stock solution in 0.1 M HCl. To minimize radioactivity dose, the solution was not shaken and was instead allowed to equilibrate at room temperature inside a lead pig for 10 min. 86Y-343-HOPO binding was confirmed via a wild-type siderocalin (Scn) binding assay developed in-house, wherein an aliquot of 86Y-343-HOPO complex was combined with Scn, and upon Scn recognition, separation from free 86Y was done via spin filtration52. This technique has been established for charge-based separation of neighboring actinides, and was adapted here to qualitatively confirm 86Y complexation by 343-HOPO, with characterization done using an optimized Ludlum 2224-1 Alpha-Beta Scaler-Ratemeter53. An aliquot was also taken to determine the activity via γ-spectroscopy, wherein the 307.00, 443.13, 580.57, and 777.37 keV gamma lines of 86Y were measured on a P-Type High Purity Germanium γ-Spectrometer.
Small animal PET imaging
All procedures and protocols used in small animal PET imaging studies were reviewed and approved by the Institutional Animal Care and Use Committee at Lawrence Berkeley National Laboratory. Experiments were performed in compliance with guidelines from the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) in AAALAC accredited facilities. The animals used were healthy young adult (11–12 weeks old) female (32.6 ± 1.5 g) Swiss Webster mice (Simonsen Laboratories, Gilroy, CA, USA), which were given water and food ad libitum, and kept under a 12-h light cycle with controlled temperature (18–22 °C) and relative humidity (30–70%). Intravenous (iv) injections into a warmed lateral tail vein were performed under isoflurane anesthesia. Three mice were injected iv with a single 200 µL dose of 86Y-343-HOPO (3.44 MBq, 93.1 μCi, 2.18 nM), the preparation of which is described above. The mice were imaged at 15 min, 2 h, 24 h, and 48 h after injection on a Concorde microPET R4 which supports a transaxial resolution of 1.66 mm FWHM, in the head first supine position. During the scan, mice were anesthetized with a mixture of isoflurane and oxygen. An energy window of 350 − 650 keV and a coincidence timing window of 6 ns were used during image acquisition. Mice were subsequently euthanized under isoflurane anesthesia via cervical dislocation following the 48-h time point.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division at the Lawrence Berkeley National Laboratory under Contract DE-AC02-05CH11231. We acknowledge additional support from U.S. DOE Integrated University Program graduate research fellowships (T.D.L. and K.M.S.) and a Nuclear Regulatory Commission Faculty Development Grant (NRC-HQ-84-14-G-0052; R.J.A.). We gratefully recognize the U.S. DOE, Office of Science, National Isotope Development Center (NIDC) subprogram within the Office of Nuclear Physics for supplying 86Y. Use of the Advanced Light Source (A.L.S.) is supported by the U.S. DOE, Office of Science, Office of Basic Energy Sciences, under Contract DE-AC02-05CH11231. We thank Dr. Simon J. Teat for training and guidance throughout our crystallography experiments at the A.L.S.
Author contributions
All work was performed at the Lawrence Berkeley National Laboratory. K.P.C., G.J.-P.D., and R.J.A. designed the research. G.J.-P.D. and W.W.L. performed solution state experiments. K.P.C. and G.J.-P.D. grew crystals for X-ray diffraction (XRD) experiments. K.P.C. and T.D.L. collected and analyzed XRD data. K.P.C. and K.M.S. prepared the radiolabeled 86Y complex. T.A.B. and D.D.A. performed in vivo PET experiments. All authors discussed the experimental results and contributed to the manuscript.
Data availability
Additional experimental details (see Supplementary Methods), as well as X-ray crystallographic files in CIF format, ORTEP figures of both complexes, and additional figures and tables are included in the supplementary information. All data generated or analyzed during this study are included in this published article (or in the supplementary information). CCDC 1983231 and 1983232 contain the supplementary crystallographic information for each complex, which can be obtained free of charge from The Cambridge Crystallographic Data Center. The supplementary crystallographic information is also available as Supplementary Data 1 and 2.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Korey P. Carter, Gauthier J.-P. Deblonde.
Supplementary information
Supplementary information is available for this paper at 10.1038/s42004-020-0307-0.
References
- 1.Kostelnik TI, Orvig C. Radioactive main group and rare earth metals for imaging and therapy. Chem. Rev. 2019;119:902–956. doi: 10.1021/acs.chemrev.8b00294. [DOI] [PubMed] [Google Scholar]
- 2.Boros E, Packard AB. Radioactive transition metals for imaging and therapy. Chem. Rev. 2019;119:870–901. doi: 10.1021/acs.chemrev.8b00281. [DOI] [PubMed] [Google Scholar]
- 3.Rösch F, Baum RP. Generator-based PET radiopharmaceuticals for molecular imaging of tumours: on the way to THERANOSTICS. Dalton Trans. 2011;40:6104–6111. doi: 10.1039/c0dt01504k. [DOI] [PubMed] [Google Scholar]
- 4.Notni J, Wester H-J. Re-thinking the role of radiometal isotopes: towards a future concept for theranostic radiopharmaceuticals. J. Label. Compd. Radiopharmaceuticals. 2018;61:141–153. doi: 10.1002/jlcr.3582. [DOI] [PubMed] [Google Scholar]
- 5.Qaim SM, Scholten B, Neumaier B. New developments in the production of theranostic pairs of radionuclides. J. Radioanalytical Nucl. Chem. 2018;318:1493–1509. doi: 10.1007/s10967-018-6238-x. [DOI] [Google Scholar]
- 6.Mikolajczak R, van der Meulen NP, Lapi SE. Radiometals for imaging and theranostics, current production, and future perspectives. J. Label. Compd. Radiopharmaceuticals. 2019;62:615–634. doi: 10.1002/jlcr.3770. [DOI] [PubMed] [Google Scholar]
- 7.Shannon R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A. 1976;32:751–767. doi: 10.1107/S0567739476001551. [DOI] [Google Scholar]
- 8.Price EW, Cawthray JF, Adam MJ, Orvig C. Modular syntheses of H4octapa and H2dedpa, and yttrium coordination chemistry relevant to 86Y/90Y radiopharmaceuticals. Dalton Trans. 2014;43:7176–7190. doi: 10.1039/C4DT00239C. [DOI] [PubMed] [Google Scholar]
- 9.Deshmukh MV, et al. NMR studies reveal structural differences between the gallium and yttrium complexes of DOTA-D-Phe-Tyr3-octreotide. J. Medicinal Chem. 2005;48:1506–1514. doi: 10.1021/jm0496335. [DOI] [PubMed] [Google Scholar]
- 10.Weineisen M, et al. 68Ga- and 177Lu-Labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J. Nucl. Med. 2015;56:1169–1176. doi: 10.2967/jnumed.115.158550. [DOI] [PubMed] [Google Scholar]
- 11.Umbricht CA, et al. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617—preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017;7:9. doi: 10.1186/s13550-017-0257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzog H, et al. Measurement of pharmacokinetics of Yttrium-86 Radiopharmaceuticals with PET and radiation dose calculation of analogous Yttrium-90 radiotherapeutics. J. Nucl. Med. 1993;34:2222–2226. [PubMed] [Google Scholar]
- 13.Vaughn BA, et al. Chelation with a twist: a bifunctional chelator to enable room temperature radiolabeling and targeted PET imaging with scandium-44. Chem. Sci. 2020;11:333–342. doi: 10.1039/C9SC04655K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira CA, et al. 86/90Y-Labeled monoclonal antibody targeting tissue factor for pancreatic cancer theranostics. Mol. Pharmaceuti. 2020;17:1697–1705. doi: 10.1021/acs.molpharmaceut.0c00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez R, et al. 44Sc: an attractive isotope for peptide-based PET Imaging. Mol. Pharmaceutics. 2014;11:2954–2961. doi: 10.1021/mp500343j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qaim SM. Nuclear data for production and medical application of radionuclides: present status and future needs. Nucl. Med. Biol. 2017;44:31–49. doi: 10.1016/j.nucmedbio.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Breeman WAP, de Jong M, Visser TJ, Erion JL, Krenning EP. Optimising conditions for radiolabelling of DOTA-peptides with 90Y, 111In and 177Lu at high specific activities. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:917–920. doi: 10.1007/s00259-003-1142-0. [DOI] [PubMed] [Google Scholar]
- 18.Pniok M, et al. Thermodynamic and kinetic study of scandium(III) complexes of DTPA and DOTA: a step toward scandium radiopharmaceuticals. Chem. A Eur. J. 2014;20:7944–7955. doi: 10.1002/chem.201402041. [DOI] [PubMed] [Google Scholar]
- 19.Thiele NA, et al. An eighteen-membered macrocyclic ligand for actinium-225 targeted alpha therapy. Angew. Chem. Int. Ed. 2017;56:14712–14717. doi: 10.1002/anie.201709532. [DOI] [PubMed] [Google Scholar]
- 20.Cilibrizzi A, et al. Hydroxypyridinone journey into metal chelation. Chem. Rev. 2018;118:7657–7701. doi: 10.1021/acs.chemrev.8b00254. [DOI] [PubMed] [Google Scholar]
- 21.Deblonde GJP, et al. Solution thermodynamics and kinetics of metal complexation with a hydroxypyridinone chelator designed for thorium-227 targeted alpha therapy. Inorg. Chem. 2018;57:14337–14346. doi: 10.1021/acs.inorgchem.8b02430. [DOI] [PubMed] [Google Scholar]
- 22.Sturzbecher-Hoehne M, et al. 3,4,3-LI(1,2-HOPO): In vitro formation of highly stable lanthanide complexes translates into efficacious in vivo europium decorporation. Dalton Trans. 2011;40:8340–8346. doi: 10.1039/c1dt10840a. [DOI] [PubMed] [Google Scholar]
- 23.Sturzbecher-Hoehne M, Choi TA, Abergel RJ. Hydroxypyridinonate complex stability of group (IV) metals and tetravalent f-block elements: the key to the next generation of chelating agents for radiopharmaceuticals. Inorg. Chem. 2015;54:3462–3468. doi: 10.1021/acs.inorgchem.5b00033. [DOI] [PubMed] [Google Scholar]
- 24.Kelley MP, et al. Bond covalency and oxidation state of actinide ions complexed with therapeutic chelating agent 3,4,3-LI(1,2-HOPO) Inorg. Chem. 2018;57:5352–5363. doi: 10.1021/acs.inorgchem.8b00345. [DOI] [PubMed] [Google Scholar]
- 25.Milenic DE, Brady ED, Brechbiel MW. Antibody-targeted radiation cancer therapy. Nat. Rev. Drug Discov. 2004;3:488–499. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 26.Hennrich, U. & Kopka, K. Lutathera®: The first FDA- and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals12, 114 (2019). [DOI] [PMC free article] [PubMed]
- 27.Baes, C. F. & Mesmer, R. E. The Hydrolysis of Cations. (John Wiley and Sons, 1976).
- 28.Martell, A. E. & Hancock, R. D. Metal Complexes in Aqueous Solutions. (Springer, 1996). .
- 29.Lindqvist-Reis, P., Persson, I. & Sandström, M. The hydration of the scandium(III) ion in aqueous solution and crystalline hydrates studied by XAFS spectroscopy, large-angle X-ray scattering and crystallography. Dalton Trans.32, 3868–3878 (2006). [DOI] [PubMed]
- 30.Melson, G. A. Scandium:Its Occurrence, ChemistryPhysics, Metallurgy, Biology and Technology (eds. Horovitz, C. T. et al.) Ch. 6, 111–138 (Academic Press, 1975).
- 31.Rösch F, Herzog H, Qaim SM. The beginning and development of the theranostic approach in nuclear medicine, as exemplified by the radionuclide pair 86Y and 90Y. Pharmaceuticals. 2017;10:56. doi: 10.3390/ph10020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore EG, Jocher CJ, Xu J, Werner EJ, Raymond KN. An octadentateluminescent Eu(III) 1,2-HOPO chelate with potent aqueous stability. Inorg. Chem. 2007;46:5468–5470. doi: 10.1021/ic700364t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D’Aléo A, Moore EG, Xu J, Daumann LJ, Raymond KN. Optimization of the sensitization process and stability of octadentate Eu(III) 1,2-HOPO complexes. Inorg. Chem. 2015;54:6807–6820. doi: 10.1021/acs.inorgchem.5b00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abergel RJ, D’Aléo A, Ng Pak Leung C, Shuh DK, Raymond KN. Using the antenna effect as a spectroscopic tool: photophysics and solution thermodynamics of the model luminescent hydroxypyridonate complex [EuIII(3,4,3-LI(1,2-HOPO))]−. Inorg. Chem. 2009;48:10868–10870. doi: 10.1021/ic9013703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deblonde GJP, Sturzbecher-Hoehne M, Abergel RJ. Solution thermodynamic stability of complexes formed with the octadentate hydroxypyridinonate ligand 3,4,3-LI(1,2-HOPO): a critical feature for efficient chelation of lanthanide(IV) and actinide(IV) ions. Inorg. Chem. 2013;52:8805–8811. doi: 10.1021/ic4010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricano A, et al. Combinatorial design of multimeric chelating peptoids for selective metal coordination. Chem. Sci. 2019;10:6834–6843. doi: 10.1039/C9SC01068H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kullgren B, Jarvis EE, An DD, Abergel RJ. Actinide chelation: biodistribution and in vivo complex stability of the targeted metal ions. Toxicol. Mechanisms Methods. 2013;23:18–26. doi: 10.3109/15376516.2012.728641. [DOI] [PubMed] [Google Scholar]
- 38.Deblonde GJP, Ricano A, Abergel RJ. Ultra-selective ligand-driven separation of strategic actinides. Nat. Commun. 2019;10:2438. doi: 10.1038/s41467-019-10240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rees JA, et al. Evaluating the potential of chelation therapy to prevent and treat gadolinium deposition from MRI contrast agents. Sci. Rep. 2018;8:4419. doi: 10.1038/s41598-018-22511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deri MA, et al. Alternative chelator for 89Zr radiopharmaceuticals: radiolabeling and evaluation of 3,4,3-(LI-1,2-HOPO) J. Medicinal Chem. 2014;57:4849–4860. doi: 10.1021/jm500389b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daumann LJ, et al. New insights into structure and luminescence of EuIII and SmIII complexes of the 3,4,3-LI(1,2-HOPO) ligand. J. Am. Chem. Soc. 2015;137:2816–2819. doi: 10.1021/ja5116524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deblonde GJP, Lohrey TD, An DD, Abergel RJ. Toxic heavy metal-Pb, Cd, Sn-complexation by the octadentate hydroxypyridinonate ligand archetype 3,4,3-LI(1,2-HOPO) New. J. Chem. 2018;42:7649–7658. doi: 10.1039/C7NJ04559J. [DOI] [Google Scholar]
- 43.Deblonde GJP, Lohrey TD, Abergel RJ. Inducing selectivity and chirality in group IV metal coordination with high-denticity hydroxypyridinones. Dalton Trans. 2019;48:8238–8247. doi: 10.1039/C9DT01031A. [DOI] [PubMed] [Google Scholar]
- 44.Bunin DI, et al. Dose-dependent efficacy and safety toxicology of hydroxypyridinonate actinide decorporation agents in rodents: towards a safe and effective human dosing regimen. Radiat. Res. 2013;179:171–182. doi: 10.1667/RR3115.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi TA, et al. Biodistribution of the multidentate hydroxypyridinonate ligand [14C]-3,4,3-LI(1,2-HOPO), a potent actinide decorporation agent. Drug Dev. Res. 2015;76:107–122. doi: 10.1002/ddr.21246. [DOI] [PubMed] [Google Scholar]
- 46.Choi TA, et al. In vitro metabolism and stability of the actinide chelating agent 3,4,3-LI(1,2-HOPO) J. Pharm. Sci. 2015;104:1832–1838. doi: 10.1002/jps.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sturzbecher-Hoehne M, Kullgren B, Jarvis EE, An DD, Abergel RJ. Highly luminescent and stable hydroxypyridinonate complexes: a step towards new curium decontamination strategies. Chem. A Eur. J. 2014;20:9962–9968. doi: 10.1002/chem.201402103. [DOI] [PubMed] [Google Scholar]
- 48.Le Fur M, et al. Yttrium-86 is a positron emitting surrogate of gadolinium for noninvasive quantification of whole-body distribution of gadolinium-based contrast agents. Angew. Chem. Int. Ed. 2020;59:1474–1478. doi: 10.1002/anie.201911858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deri MA, et al. p-SCN-Bn-HOPO: a superior bifunctional chelator for 89Zr immunoPET. Bioconjugate Chem. 2015;26:2579–2591. doi: 10.1021/acs.bioconjchem.5b00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bandaranayake AD, et al. Daedalus: a robust, turnkey platform for rapid production of decigram quantities of active recombinant proteins in human cell lines using novel lentiviral vectors. Nucleic Acids Res. 2011;39:e143–e143. doi: 10.1093/nar/gkr706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finton KAK, et al. Autoreactivity and exceptional CDR plasticity (but not unusual polyspecificity) hinder elicitation of the anti-HIV antibody 4E10. PLoS Pathog. 2013;9:e1003639. doi: 10.1371/journal.ppat.1003639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deblonde GJP, et al. Chelation and stabilization of berkelium in oxidation state +IV. Nat. Chem. 2017;9:843–849. doi: 10.1038/nchem.2759. [DOI] [PubMed] [Google Scholar]
- 53.Strong, R. K., Rupert, P. B., Abergel, R. J., Captain, I. & Deblonde, G. J.-P. Chelating platform for delivery of radionuclides. International Patent WO 2018/044812 A2 (2018).
- 54.Sturzbecher-Hoehne M, Yang P, D’Aleo A, Abergel RJ. Intramolecular sensitization of americium luminescence in solution: shining light on short-lived forbidden 5f transitions. Dalton Trans. 2016;45:9912–9919. doi: 10.1039/C6DT00328A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Additional experimental details (see Supplementary Methods), as well as X-ray crystallographic files in CIF format, ORTEP figures of both complexes, and additional figures and tables are included in the supplementary information. All data generated or analyzed during this study are included in this published article (or in the supplementary information). CCDC 1983231 and 1983232 contain the supplementary crystallographic information for each complex, which can be obtained free of charge from The Cambridge Crystallographic Data Center. The supplementary crystallographic information is also available as Supplementary Data 1 and 2.