Abstract
This study demonstrates that pretreatment of macrophages with phosphatidylinositol, of either soya bean or mycobacterial origin, results in a down-regulation of the binding and uptake of Mycobacterium tuberculosis by the phagocytes. We also describe the novel observation that cardiolipin induces an increase in the binding and uptake of M. tuberculosis by macrophages. Neither phospholipid interacts with macrophages via the 2F8 epitope of scavenger receptor A, and treatment of macrophages with either phospholipid results in a down-regulation of CR3 function and tumor necrosis factor alpha production by the phagocyte. We have also shown that the ability of macrophages to interact with mycobacteria is greatly affected by an as yet unidentified product from the interaction of chloroform and polypropylene tubes.
Mycobacterium tuberculosis is a major human pathogen that resides in the host lung as a facultative intracellular pathogen and is found primarily in mononuclear phagocytic cells. Interactions between M. tuberculosis and host macrophages (Mφ) will therefore be of paramount importance in defining the pathogenesis of the bacterium. Since bronchoalveolar fluid is considered to contain insufficient amounts of serum opsonins to mediate phagocytosis (34, 38) and alveolar macrophages (Mφ) do not express significant levels of receptors for serum opsonins (2, 7, 10, 33, 48), serum-independent (nonopsonic) ingestion by Mφ is considered to be essential in the host defense of the lung. We have shown previously that nonopsonic binding of M. tuberculosis to mouse Mφ is partly mediated via an epitope within CR3 (CD11b/CD18, Mac-1) that is distinct from that which binds iC3b (46).
In contrast to our improved understanding of the receptors involved in the nonopsonic uptake of mycobacteria, little is known of the mycobacterial molecules involved in nonopsonic interactions with Mφ. The mycobacterial lipoglycans lipoarabinomannan (LAM), lipomannan (LM), and phosphatidylinositol mannosides (PIMs) are abundant molecules in the cell envelope of mycobacteria and have been proposed as having a role in the receptor-mediated uptake of mycobacteria (28, 42), even though it is uncertain whether these molecules are exposed at the surface of the bacteria and can therefore act as ligands. However, these molecules are thought to be released into the extracellular environment (15, 49), where they may have numerous effects on the host's immune system (15), including the inhibition of mycobacterial uptake by Mφ (47). LAM is a large molecule with extensively branched arabinan and mannan chains, PIMs refer to molecules with 2 to 6 mannose residues, while LM is essentially a long PIM, with about 20 mannose residues, and may be regarded as a precursor of LAM, lacking the branched arabinan (9, 12, 15). All of these molecules possess a common phosphatidylinositol (PI) anchor which could be inserted in the plasma membrane (15, 29) or an outer lipid bilayer shown to exist in mycobacteria (32). A plasma membrane location would allow the glycosylated portion of LAM and LM, but not the shorter PIMs, to be exposed on the outer surface of the envelope. However, the observation that PIMs can be released preferentially from the mycobacterial surface by gentle mechanical treatments indicates that such molecules may be located on the outside of the envelope and thus exposed at the surface of the mycobacterial cell (36).
Although LAM, LM, and PIM have widely different glycosylation patterns, all three molecules have been found to inhibit binding of M. tuberculosis to Mφ when added in a cell-free form (47). This, along with the observation that deacylation of LAM abrogates the capacity for LAM to down-regulate binding of M. tuberculosis to Mφ (47), indicates that the common PI anchor is the inhibitory component. In support of this contention, commercially prepared PI from soybean was shown to down-regulate binding of M. tuberculosis to Mφ in a dose-dependent manner (47). However, the fatty acyl groups of soya PI are palmitoyl and linoleoyl, whereas those of mycobacterial PI are palmitoyl and tuberculostearoyl. Thus, it is possible that PI from mycobacteria would not have the same inhibitory property that soya PI has. The purpose of the present study was, therefore, first to investigate whether mycobacterial PI acts on Mφ in a similar fashion as does soya PI and second to further elucidate the role of mycobacterial lipidated moieties in the interaction of mycobacteria with Mφ.
MATERIALS AND METHODS
Mycobacteria.
M. tuberculosis strain Erdman (Trudeau Mycobacterial Collection 107, American Type Culture Collection [Manassas, Va.] 35801) was grown and stored as previously described (46). Mycobacterium smegmatis 607 was grown in Sauton's medium in a fermentor.
Commercial Phospholipids.
Diphosphatidyl glycerol (cardiolipin; CL) and soya PI were purchased from Sigma-Aldrich Canada Ltd. (Oakville, Ontario, Canada).
Preparation of phospholipids of mycobacterial origin.
M. smegmatis 607 was grown and washed sequentially with 0.05% Tween 80 and distilled water to give a pellet of 550 g (wet weight). Phospholipids were prepared as acetone-ethanol-insoluble material (3 g) and further purified by chromatography on a DEAE-cellulose DE52 (acetate form) column (3). In a scaled-up version of the chromatography method, the DE52 (Whatman, Clifton, N.J.) was packed as a 4-cm-diameter- by 32-cm (400-ml) column. The void volume, determined with azulene (Aldrich, Gillingham, Dorset, United Kingdom), was 417 ml. Thus, the 3 g of glycolipid was applied in 60 ml of elution solvent (chloroform-methanol-water; 20/9/1 by volume). First, 600 ml of elution solvent was applied, then a 0 to 0.1 M ammonium acetate gradient in elution solvent was applied over 1.5 liter, and then the remaining phospholipids were eluted in 0.1 M ammonium acetate in elution solvent. Fractions (30 ml) were monitored for their lipid content by thin-layer chromatography (TLC). The material that eluted at 750 to 900 ml (0.025 to 0.05 M ammonium acetate) from the DE52 column contained all of the PI. However, it also included some PIM and CL about equal in quantity to PI. This single batch of material was used throughout this study and is referred to as pool 2.
Purification of PI by TLC fractionation of pool 2.
Up to 12 mg of pool 2 was applied as a 15-cm streak to a 20- by 20-cm Silica Gel 60 TLC plate (Merck, Darmstadt, Germany), placed in a nitrogen-filled tank, and developed twice in chloroform-methanol-water (65/25/4 by volume). The plate was air dried and then sprayed with MilliQ water to visualize opalescent bands of lipid. The band corresponding with PI was scraped, recovered in chloroform-methanol (2/1 by volume), filtered through a 4-mm PTFE (Whatman) high-pressure liquid chromatography (HPLC) filter, and partitioned into the upper phase of a butan-1-ol–water two-phase system (1 ml) in which any residual, colloidal silica collected on the interface. About 0.6 mg of pure (purity and quantity estimated on high-performance TLC [HPTLC] plates [Merck 13727]) PI was obtained from 12 mg of pool 2. Plate controls were obtained by scraping an area of the plate developed with solvent but containing no lipid.
Two directional TLC (2D-TLC; see below) was not suitable for monitoring preparative chromatography; for this purpose, fractions were applied to the concentration zone of 10- by 10-cm silica gel HPTLC plates and developed once in chloroform-methanol-water (65/25/4 by volume). Plates were air dried, then sprayed with 5% (wt/vol) molybdophosphoric acid in ethanol-water (95/5 by volume), and charred at 200°C for up to 10 min, until deep blue spots representing all lipids appeared.
Purification of PI by HPLC fractionation of pool 2.
Normal-phase HPLC of pool 2 was performed using a Partisil 5 WCS (Whatman) 4.6- by 250-mm column. Quantities injected were 2.4 mg in 50 μl, followed by isocratic elution at 0.4 ml/min with chloroform-methanol-water-glacial acetic acid (65/25/4/0.4 by volume). Fractions (0.25 ml) were collected, and 25-μl portions were analyzed by HPTLC (UV absorption was not a reliable way of monitoring eluant). PI eluted between 12.5 and 16 min, but from 14.5 min PIMs were also eluted. The 12.5- to 14.5-min material was collected from two runs, pooled, and rechromatographed to remove trace CL. The rechromatographed material was judged to be 100 μg of pure PI by HPTLC. This pure mycobacterial PI was shown to be tuberculostearoyl, palmitoyl PI ([M-H]− = 851) by negative electrospray mass spectrometry (MS) and MS-MS (Keith Brinded, personal communication).
2D-TLC analysis of phospholipids.
Phopholipids were prepared for 2D-TLC by resuspension in 2:1 chloroform-methanol (Omnisolve, EM Science, Gibbstown, N.J.). Approximately 40 μg of material was spotted onto the lower left corner of a 6.6-cm-square aluminum-backed TLC plate (Merck), 1 cm in from both edges. The plate was set at a 90° angle in chloroform-methanol-distilled water (dH2O) (60:30:6) for the first direction. When the solvent front reached the top of the plate, it was air dried, rotated another 90°, and placed in chloroform-acetic acid-methanol-dH2O (80:15:12:4) for the second direction. The plate was then air dried, sprayed with 5% molybdophosphoric acid in 70% ethanol, and charred at 200°C for 10 min.
Preparation of lipids for Mφ studies.
Initially, experiments were done using phospholipids that had been resuspended in chloroform and stored long-term in polypropylene microcentrifuge tubes (Sarstedt, Numbrecht, Germany) at −20°C. On the day of the experiment, these phospholipids were dried under N2, resuspended in binding medium (138 mM NaCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, 2.7 mM KCl, 0.6 mM CaCl2, 1 mM MgCl2, 5.5 mM d-glucose [45]), and dispersed by horn sonication for 90 s, using a micro-cup horn and Vibra-cell ultrasonic processor (Sonics & Materials, Danbury, Conn.).
A procedure was developed for the preparation of commercially prepared lipids that would simulate the preparation of the purified mycobacterial phospholipids described above. All purchased phospholipids were first prepared in 2:1 (vol/vol) chloroform-methanol (Omnisolve) and dried under N2, resuspended in 1:1 (vol/vol) ether (ACS grade; Fisher Scientific, Nepean, Ontario, Canada)- ethanol (LC grade; Sigma-Aldrich, St. Louis, Mo.), aliquoted into glass vials with Teflon-lined lids (Fisher Scientific), dried again under N2, and stored at −20°C. For preparation of solvent controls, equivalent amounts of solvents with no phospholipids were dried in glass vials. On the day of the experiment, phospholipids and control vials were reconstituted with binding medium and horn sonicated for 90 s as described above. Microscopic observation of these lipid preparations revealed that both phospholipids were insoluble and formed a mixture of small (0.5- to 2-μm) and large (2- to 6-μm) lamellar vesicles.
In vitro assay for binding of particles to Mφ.
Binding of resident murine peritoneal Mφ to M. tuberculosis in the absence of serum was assayed as previously described (46). Briefly, washings from the peritoneal cavity of BALB/c female mice were recovered using 5 ml of supplemented RPMI (RPMI 1640 medium plus 10% [vol/vol] fetal calf serum, 10 mM l-glutamine, 10 mM sodium pyruvate [Life Technologies, Grand Island, N.Y.]). Washings from several mice were pooled, counted, and plated onto glass coverslips in 24-well plates (Becton-Dickinson, Lincoln Park, N.J.) at 106 cells/ml, 1 ml per well. They were incubated at 37°C in 5% CO2) for 4 h. Nonadherent cells (approximately 60% of the cells added) were then removed with the overlay, 1 ml of fresh supplemented RPMI was added to each well, and the cells were returned to 37°C and 5% CO2 for overnight incubation before use in binding studies. Adherent Mφ were then washed three times in binding medium. When appropriate, 250 μl of binding medium containing phospholipids or antibodies was added to Mφ monolayers, and the cells were incubated for 10 min at 37°C in 5% CO2 (control cells received binding medium alone). Mycobacteria were pelleted and resuspended in binding medium by passage through a 25-gauge needle 10 times to break up clumps, and 250 μl of the suspension was added to each well to give a multiplicity of infection of approximately 50 bacteria:1 Mφ:
Red blood cells coated with the iC3b component of complement (EIgMC′) were used to assess the expression of functional complement receptors. EIgMC′ were prepared, pelleted, resuspended in binding medium, and added to Mφ in 250 μl of binding medium to give an EIgMC′:Mφ ratio of 20:1 as previously described (46). Latex beads (0.8 μm; Sigma-Aldrich) were used as a nonspecific probe for Mφ phagocytic function.
Mφ were allowed to interact with the particles for 3 h (1 h on a rocking platform and 2 h stationary) at 37° in 5% CO2 in the presence of any added antibodies and/or phospholipids. Visual inspection of the various experimental groups indicated that the aggregation of the mycobacteria was not affected by any added reagent. In all groups some aggregates formed, but these did not appear to be ingested by Mφ. We have previously investigated whether pretreatment of Mφ with PI affects the ability of the Mφ to interact with M. tuberculosis. In these experiments, Mφ were incubated with 40 μg of soya PI per ml overnight, washed, and tested for the ability to associate with M. tuberculosis as described here. The association of the mycobacteria with the pretreated Mφ was inhibited, but only at 50% of the levels of inhibition seen when PI was present during the interaction of Mφ and mycobacteria (unpublished observations). This led us to conduct the current series of experiments with the phospholipids present during the assay.
Following the 3-h incubation period, the monolayers were washed gently three times with binding medium and then fixed and stained. The distribution of acid-fast bacilli, latex, or EIgMC′ within the Mφ population was estimated as previously described (46). Although in this study we made no attempt to differentiate attachment from ingestion, previous studies from our laboratory, in which the association of M. tuberculosis with Mφ was assessed after 3 h of incubation at either 37°C (attached and ingested) or 4°C (attached only) in 5% CO2, demonstrated that >90% of the bacteria associated with Mφ at 3 h were ingested (unpublished observations).
To test the effect of solvents on Mφ, 100 μl of chloroform or methanol was added to polypropylene microcentrifuge tubes, which were then dried under N2. After addition of 1 ml of binding medium to each tube, the tubes were horn sonicated for 90 s. Either 250 μl of binding medium from these solvent-treated tubes or 250 μl of binding medium containing 2% chloroform or methanol was added to each well. The Mφ were incubated for 10 min at 37° in 5% CO2 before addition of bacteria.
To test whether CL or PI acted on Mφ via scavenger receptor A, (SR-A), Mφ were treated with PI (40 μg/ml) or CL (60 μg/ml) alone or preceded by a 10-min incubation with 2F8 (20 μg/ml), a monoclonal antibody (MAb) that recognizes SR-A (25). The Mφ were incubated for a further 10 min at 37° in 5% CO2 before addition of bacteria.
TNF-α assay.
Mφ were incubated with M. tuberculosis for 3 h as described above, either alone or in the presence of soya PI or CL. The overlay from each monolayer was removed, filter sterilized, and stored at −20°C. Tumor necrosis factor alpha (TNF-α) content of each sample was measured using an enzyme-linked immunosorbent assay (ELISA)-based kit (R&D Systems, Minneapolis, Minn.) as instructed by the manufacturer. Control experiments demonstrated that CL and PI did not interfere with the detection of TNF-α in this assay. Mφ were incubated with M. tuberculosis for 3 h and the supernatants were collected and filter sterilized. Then CL or PI in 10 μl of binding medium was added to replicate samples to give a final concentration of 60 or 40 μg/ml, respectively. Untreated supernatants received 10 μl of binding medium alone. The TNF-α in these samples was then assessed using the ELISA kit. The TNF-α present in CL- and PI-treated samples did not differ significantly from that in the untreated controls (P = 0.4 and 0.6, respectively).
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEM). When applicable, Student's t test for independent means was used to evaluate binding data. Differences were considered significant at P < 0.05.
RESULTS
Effect of phospholipid storage protocols on their subsequent effect on the binding of M. tuberculosis to Mφ.
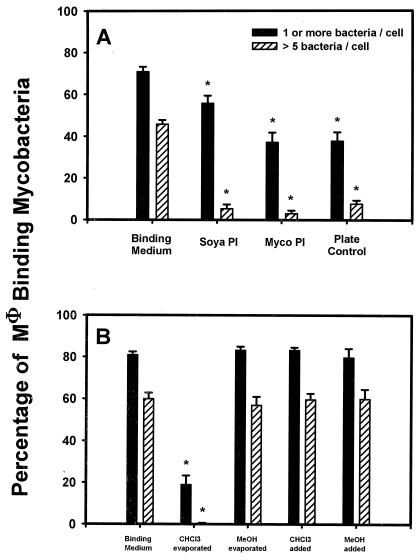
Soya PI and TLC-purified mycobacterial PI that had been stored in chloroform in polypropylene tubes were added to Mφ at 40 μg/ml to assess their effect on binding of M. tuberculosis (Fig. 1A). Both phospholipids inhibited binding significantly (P < 0.05) compared to the control, for both percentage of Mφ binding one or more bacteria and percentage of Mφ binding more than five bacteria. However, the plate control also inhibited significantly (P < 0.05) compared to the control. Since this was an unexpected result, we suspected the integrity of the materials used and therefore investigated our storage methods, in particular, the use of chloroform and methanol with polypropylene tubes.
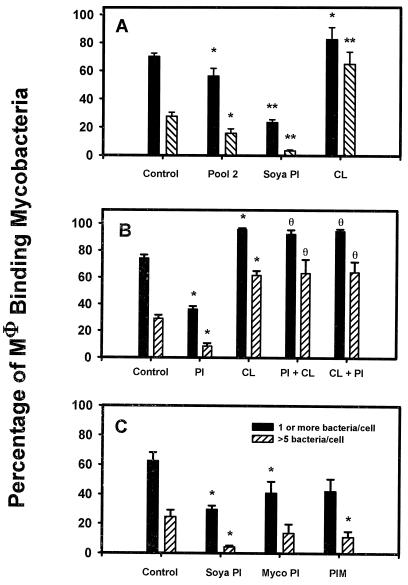
FIG. 1.
Effect of phospholipid preparation and solvents on the interaction of M. tuberculosis with Mφ. (A) Effect of soya PI and mycobacterial (myco)PI at 40 μg/ml on binding of M. tuberculosis to Mφ compared to binding medium alone. Plate Control refers to material from blank plate scrapings that had been processed identically to mycobacterial PI. The percentage of Mφ binding ≥1 and >5 M. tuberculosis is shown. The mean ± SEM is shown for four experiments, each with triplicate coverslips. ∗, P < 0.05 compared with control containing binding medium only. (B) The binding of M. tuberculosis to Mφ in the presence of 1% chloroform (CHCI3 added) or 1% methanol (MeOH added) compared to that in the presence of binding medium sonicated in polypropylene tubes in which 100 μl of either chloroform (CHCl3 evaporated) or methanol (MeOH evaporated) had been added and then evaporated under N2. Control wells contained binding medium only. The percentage of the Mφ population binding ≥1 and >5 bacteria is shown. The mean ± SEM is shown for one experiment done in triplicate. ∗, P < 0.05 compared with control.
To test if chloroform or methanol alone could account for the inhibition, they were added directly to Mφ at a final concentration of 1% to assess the effect of possible residual contamination in lipid preparations (Fig. 1B). There was no inhibition of binding of M. tuberculosis to Mφ in the presence of either solvent. When binding medium that had been horn sonicated in solvent-treated polypropylene tubes was added to Mφ, there was no inhibition mediated by medium from methanol-treated tubes, but there was significant inhibition mediated by medium from chloroform-treated tubes (P < 0.05 compared to the control). This indicated the presence of an unknown contaminant resulting from the interaction of chloroform and polypropylene.
From this information we developed a storage system whereby all phospholipids, including commercial material, were reconstituted, dried under N2, and stored in glass vials with Teflon-lined lids as described in Materials and Methods.
Effect of CL and PI on binding of Mφ to M. tuberculosis.
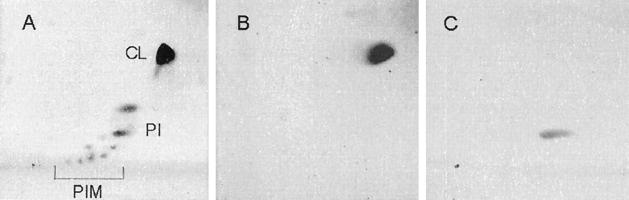
A subsequent source of mycobacterial PI, pool 2, was prepared and stored according to our new methods. TLC analysis of pool 2 (Fig. 2) indicated the presence of CL and PIMs as well as PI.
FIG. 2.
2D-TLC run on 40 μg of pool 2 (A), 10 μg of CL (B), and 10 μg of soya PI (C), all resuspended in chloroform-methanol (2:1). The first direction (horizontal) was run in chloroform-methanol-dH2O (60:30:6); the second, vertical direction was run in chloroform-acetic acid-methanol-dH2O (80:15:12:4).
Solvent controls prepared using procedures identical to those used for the phospholipids had no effect on Mφ binding of M. tuberculosis compared to controls with binding medium alone (data not shown), and so we were confident that data obtained with phospholipids stored using the new methods were meaningful.
We tested the effect of pool 2 on the interaction of M. tuberculosis with Mφ (Fig. 3A). Pool 2 contained approximately 60% CL and PIMs along with 40% PI. As previous studies (47) had shown that 40 μg of soya PI per ml resulted in strong inhibition of the association of M. tuberculosis with Mφ, pool 2 was added at a concentration of 100 μg/ml to give 40 μg of mycobacterial PI per ml. Therefore, soya bean PI was added at 40 μg/ml and CL was added at 60 μg/ml for comparison. Pool 2 significantly inhibited the interaction of M. tuberculosis with Mφ compared to controls (P < 0.05), though not to the same extent as did soya PI which, when added to Mφ at 40 μg/ml, was inhibitory compared with both the control and pool 2 (P < 0.05). Pure CL enhanced the binding of M. tuberculosis to Mφ, with more than twice the number of Mφ binding >5 bacteria compared to either the control or pool 2 (P < 0.05). We had determined that both CL and PI form lamellar vesicles in aqueous media, which indicated that differences in the physical presentation of the lipids to Mφ could not explain the different effects of CL and PI on the interaction of M. tuberculosis and Mφ.
FIG. 3.
Effect of phospholipids on the binding and uptake of M. tuberculosis by Mφ. (A) Pool 2 added to Mφ at a final concentration of 100 μg/ml was compared with CL at 60 μg/ml and soya PI at 40 μg/ml. Control wells contained binding medium only (Control). The percentage of the Mφ population binding ≥1 and >5 bacteria is shown. The mean ± SEM is shown for five experiments, each with triplicate coverslips. ∗, P < 0.05 compared with control; ∗∗, P < 0.05 compared with control or pool 2. (B) PI and CL were added to Mφ at 40 μg/ml (PI) or 60 μg/ml (CL). In other groups, Mφ received PI first and then 10 min later received CL (PI + CL) or received CL first followed by PI (CL + PI). Control wells contained binding medium only (Control). All groups were then assessed for binding of M. tuberculosis. The percentage of the Mφ population binding ≥1 and >5 bacteria is shown. The mean ± SEM is shown for two experiments, each with triplicate coverslips. ∗, P < 0.05 compared with control; θ, P > 0.05 compared with CL alone. (C) PI from M. smegmatis (mycobacterial [myco] PI) or soya PI and PIM were added at 40 μg/ml to Mφ, which were then assessed for binding of M. tuberculosis. Control wells contained binding medium only (Control). The percentages of the Mφ population binding ≥1 and >5 bacteria are shown. The mean ± SEM is shown for three experiments, each with triplicate coverslips. ∗, P < 0.05 compared with control.
The observation that pool 2 did not inhibit the interaction of M. tuberculosis with Mφ as much as did PI alone suggested that two opposing effects may be present in pool 2: a stimulatory effect of the CL portion and an inhibitory effect of the PI portion. We tested this by adding CL and PI sequentially to Mφ and testing the effect on the binding of M. tuberculosis to Mφ (Fig. 3B). As before, PI significantly inhibited the binding of M. tuberculosis to Mφ (P < 0.05), whereas CL significantly enhanced binding of bacteria to Mφ (P < 0.05). The effect of CL was dominant over that of PI, as the addition of CL, either before or after PI, always resulted in enhanced binding. This result did not explain the inhibitory effect of pool 2. However, pool 2 also contained PIM and other lipids (Fig. 2), which may have contributed to its overall inhibitory action.
Effect of PI from different sources.
A subsequent source of PI purified from pool 2 by HPLC (containing no CL) was added to Mφ at 40 μg/ml (Fig. 3C) and compared with purified PIM, also from pool 2, and with soya PI, both at 40 μg/ml. All three phospholipids inhibited the association of M. tuberculosis with Mφ. The mycobacterial PI and soya PI (P = 0.042 and 0.00007, respectively), but not the PIM (P = 0.058), inhibited binding of M. tuberculosis significantly compared to the solvent control in the Mφ population binding ≥1 bacterium. The soya PI and PIM (P = 0.0003 and 0.044, respectively), but not the mycobacterial PI (P = 0.16), inhibited binding significantly compared to the solvent control in the Mφ population binding >5 bacteria. There was no significant difference in inhibition between mycobacterial PI, soya PI, and PIM. This result demonstrated that soya PI was more inhibitory than the mycobacterial phospholipids and indicated that mycobacterial PI acted more to reduce the number of Mφ able to bind mycobacteria at all, whereas PIM acted more to reduce the number of bacteria binding to an individual Mφ.
Investigations into the mode of action of CL and PI on Mφ.
SR-A has been implicated in binding a wide range of lipids, including lipoteichoic acid of gram-positive bacteria (20) and lipopolysaccharide (LPS) from gram-negative bacteria (26). We considered the possibility that either PI or CL was acting via binding to SR-A. A MAb (2F8) recognizing SR-A had no effect on the binding of M. tuberculosis to Mφ when added alone (Fig. 4) and did not affect the modulation of M. tuberculosis binding to Mφ mediated by PI or CL (Fig. 4), demonstrating that neither PI or CL acts via binding to the 2F8 epitope of SR-A.
FIG. 4.
Effect of an antibody recognizing SR-A on the binding of M. tuberculosis to Mφ treated with PI or CL. PI and CL were added to Mφ at 40 μg/ml (PI) or 60 μg/ml (CL). In addition, the anti-SR-A MAb 2F8 was added to Mφ at 20 μg/ml either alone (a-SR) or 10 min before the addition of PI (a-SR + PI) or CL (a-SR + CL). Control wells contained binding medium only (Control). Following a further 10-min incubation, all Mφ were assessed for the ability to bind M. tuberculosis. The percentages of the Mφ population binding ≥1 and >5 bacteria are shown. The mean ± SEM is shown for three experiments, each with triplicate coverslips. ∗, P < 0.05 compared with control; ∗∗, P > 0.05 compared to PI; θ, P > 0.05 compared to CL.
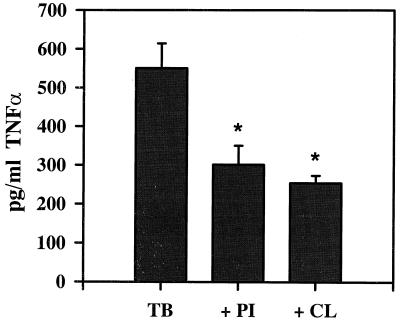
The binding of mycobacteria and mycobacterial lipoglycans to Mφ is known to stimulate TNF-α production (11, 16, 23, 35). TNF-α is also known to modulate the expression and function of CR3 (17, 31), which is a major receptor involved in the binding of M. tuberculosis (18, 41, 46). Thus, we investigated the possibility that CL or PI affected Mφ TNF-α production, thereby affecting the association of Mφ with M. tuberculosis (Fig. 5). As expected, M. tuberculosis binding induced TNF-α production. However, pretreatment of the Mφ with either CL or PI inhibited the subsequent TNF-α production in response to the bacteria, suggesting that the different effects of CL and PI on M. tuberculosis binding and uptake by Mφ could not be attributed to different effects on TNF-α production.
FIG. 5.
TNF-α production by Mφ in response to M. tuberculosis in the absence (TB) and presence of PI (40 μg/ml; + PI) or CL (60 μg/ml; + CL), measured by ELISA and quantitated by reference to a standard curve. Background levels of TNF-α production by unstimulated cells is between 35 and 100 pg/ml. The mean ± SEM is shown for two experiments, each with triplicate wells. P < 0.05 compared with TB group.
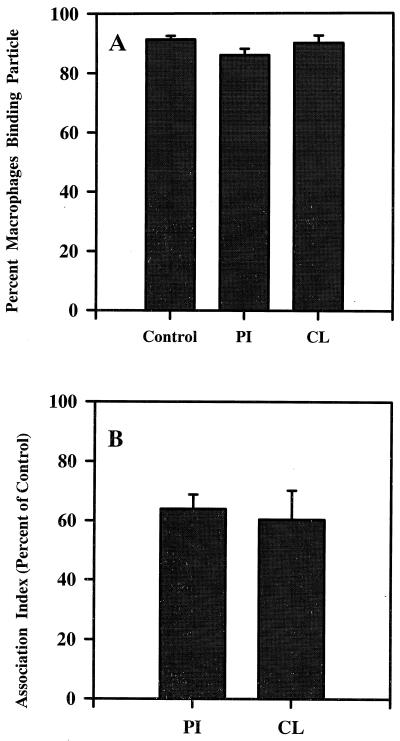
We next investigated the effect of CL or PI on the association of latex beads or EIgMC′ with Mφ. Latex beads are comparable in size to mycobacteria and were used to identify effects on particle uptake. EIgMC′ are used to identify CR3 function. This is a major receptor for M. tuberculosis, even in the absence of complement (18, 46), and its function has been shown to be affected by lipoglycans (47). The association of latex with Mφ was unaffected by PI (Table 1), whereas CL treatment resulted in a significant reduction in both the percentage of Mφ associated with latex and the number of beads associated with each Mφ (Table 1). Neither PI nor CL had any notable effect on the percentage of Mφ able to bind at least one EIgMC′ (Fig. 6A). However, both PI and CL treatment reduced the number of EIgMC′ binding to the Mφ by about one-third (Fig. 6B).
TABLE 1.
Effect of CL and PI on the association of latex beads with Mφa
| Treatment | % Mφ with ≥1 latex bead | Mean no. of latex beads/Mφ |
|---|---|---|
| Control | 97.2 ± 1.1 | 18.0 ± 1.4 |
| CL | 88.3 ± 3.6∗ | 6.0 ± 0.1∗∗ |
| PI | 99.0 ± 0.6 | 14.6 ± 0.7 |
Mφ were incubated with 0.8-μm diameter latex beads in the presence of binding medium alone (control), CL (60 μg/ml), or (PI) (40 μg/ml). After 3 h, the monolayers were processed and the values shown (means ± SEM from two experiments, each with triplicate coverslips) were determined. ∗, P < 0.05. ∗∗, P < 0.001.
FIG. 6.
Binding of EIgMC′ to Mφ in the absence (Control) or presence of PI (40 μg/ml) or CL (60 μg/ml), assessed as percentage of Mφ binding at least one EIgMC′ (A) or the number of EIgMC′ bound to 100 Mφ (association index) as a percentage of the control (B) (the mean ± SEM association index for the control was 1,180 ± 171). The mean ± SEM is shown for three experiments, each with triplicate wells.
DISCUSSION
The results of this study demonstrate that mycobacterial PI, like soya PI, inhibits the binding and uptake of M. tuberculosis by Mφ. We also describe, for the first time, how CL can increase the binding and uptake of M. tuberculosis by Mφ. In addition, we show that the association of Mφ with mycobacteria (and probably other particles) is greatly affected by an as yet unidentified product from the interaction of chloroform and polypropylene.
When we initially tested phospholipids that had been stored in chloroform, it was clear that there was a component present in both our phospholipids and our plate controls that inhibited binding. Since we saw evidence of the inhibitor only following the interaction of chloroform with polypropylene, it may be derived from the plastic, possibly a plastene. However, we did not pursue its identity and thus cannot rule out a volatile contaminant such as phosgene, an extremely toxic breakdown product of chloroform which condenses at 0°C and is soluble in most hydrocarbons, though its volatility suggests that it would dissipate from the phospholipids. A further caution is that contaminants such as those mentioned above might not appear on a TLC plate, commonly used as proof of purity of phospholipids.
Regardless of what the unknown component was, there are likely many areas of scientific investigation where the effect of using lipids stored in this manner would not be apparent. Mφ, in particular, are extremely sensitive to small amounts of potential stimulators or inhibitors of cell-signaling systems, and care should be taken to ensure purity of reagents used in all Mφ handling. We show in this work that lipids to be used in experiments with Mφ, should be dried under N2 immediately upon reconstitution with solvents, glass vials with Teflon-lined lids should be used, and solvent controls should be prepared simultaneously in an identical fashion. Stocks of phospholipids may be held as solutions in solvents, but they should be stored in the dark at −20°C or lower and flushed with N2 every time portions are taken. Furthermore, we recommend HPLC in preference to preparative TLC because it lessens the exposure of the phospholipids to air and light and obviates the necessity of a butan-1-ol extraction step to remove colloidal silica derived from the TLC plate, a step that results in losses in the yield of phospholipid.
We here demonstrate that mycobacterial PI is able to inhibit the association of M. tuberculosis with Mφ as had been previously shown for soya PI (47). However, it appears that mycobacterial PI is not as potent an inhibitor as is soya PI, probably due to the different acyl groups in the two molecules; both have palmitoyl but mycobacteria have tuberculostearoyl instead of linoleoyl moieties. This finding confirms the importance of PI as a potential regulator of Mφ interactions with mycobacteria and probably explains much of the activity of mycobacterial lipoglycans. LAM and, to a lesser extent LM and PIM have been implicated as having numerous biological activities, which has led to the suggestion that LAM is a mycobacterial virulence factor (1, 4, 13, 14, 16, 19, 35, 39, 43). Whenever it has been tested, the majority of studies have shown that deacylation of LAM destroys its biological activity (14, 16, 35, 43, 44, 47), suggesting that the PI end of the molecule is essential for its activity. Using PI as a paradigm for PI and PI-based lipoglycans, our demonstration of the differential effects dependent on the acyl groups of the PI provides further evidence for the importance of the hydrophobic termini of these molecules in their interaction with Mφ. Nevertheless, it should be remembered that when the acyl groups are intact, the biological activity of LAM is affected by the nature of the glycosylation of the branched carbohydrate portion of the molecule (13, 16, 35).
The mode of action of cell-free PI upon Mφ-mycobacterium interactions is unknown. It is tempting to think that the action is simple competitive inhibition for a receptor recognizing PI. The current model for the mycobacterial cell wall places the PI component of LAM as anchored in the plasma membrane or an outer lipid bilayer, where it would not be available to bind to receptors for PI. However, the precise location of PIMs is still uncertain. They could be buried under a glycan matrix or exposed at the surface (21), but PIMs, like LAM, are likely to escape from the mycobacterial envelope; indeed, their recognition by antibodies and presentation by CD1 indicates that the host immune system sees all of these molecules (22, 27, 44). As free phospholipids and lipophosphoglycans, they would be able to interact with Mφ.
While the pretreatment of Mφ with PI results in a 50% inhibition of the subsequent interaction of the Mφ with M. tuberculosis, maximal inhibition occurs only when the PI is present during the interaction of Mφ with mycobacteria. This suggests that at least two mechanisms of inhibition are acting: one long-term effect on the Mφ, and one more transient effect on the Mφ or on the mycobacteria. The fact that PI inhibits the interaction of Mφ with mycobacteria, EIgMC′, EIgG, and zymosan but not latex (reference 47 and this report) suggests an effect on several receptors but not a general inhibition of Mφ phagocytic function.
Cell-free LAM can bind to the Mφ mannose receptor (42) or CD14 (8, 37, 52), presumably by virtue of the glycosylated portion of the molecule. However, LAM can also integrate via its PI anchor into specialized plasma membrane domains (30). We also considered the possibility that SR-A could bind PI but did not obtain evidence to support this theory. Thus, while free PI would not be expected to bind to either the mannose receptor or CD14, current evidence suggests that it would integrate into the plasma membrane. How the PI then acts on the Mφ to inhibit the binding and uptake of M. tuberculosis and other particles remains unclear. Studies by Bate et al. (5, 6) indicate that the PI component of exoantigens from Plasmodium yoelii, a malarial parasite, is necessary for induction of TNF-α by murine peritoneal Mφ. As TNF-α can induce CR3 expression (17, 24), which in turn is important for binding and uptake of mycobacteria in a serum-free environment (18, 46), we investigated the activity of PI on TNF-α production and CR3 function and found it to be inhibitory of both parameters. Thus, we have evidence suggesting that PI may inhibit the interaction of Mφ with mycobacteria by inhibiting CR3 function, either independently or through the inhibition of TNF-α production.
The treatment of Mφ with CL resulted in a significant increase in the binding and uptake of M. tuberculosis. This novel finding was unexpected and explained why pool 2 did not inhibit mycobacterium-Mφ interactions as strongly as did PI, as this mixture of mycobacterial lipids contained the inhibitory molecules PI and PIM and the stimulatory molecule CL. CL, also known as diphosphatidyl glycerol, is commonly found in microorganisms, and current literature reports many and diverse effects of this negatively charged phospholipid, especially in patients with antiphospholipid syndrome. However, its effect on Mφ is less well studied. We found that CL did not bind to Mφ via the 2F8 epitope of SR-A; perhaps, like PI, it integrates with the plasma membrane. CL has been shown to induce Mφ growth in vitro (50) and, at low doses, to augment TNF-α production by Mφ stimulated with LPS (51). More pertinent to our study, pretreatment with CL at high doses (40 μg/ml or more) has been shown to inhibit TNF-α production by Mφ stimulated with LPS (40, 51). Our observations are comparable in that treatment with CL inhibited the induction of TNF-α production by Mφ stimulated with M. tuberculosis. We also found that CL inhibited CR3 function. These two observations do not concur with our contention that decreased CR3 function will lead to decreased uptake of M. tuberculosis (as seen for PI). However, CR3 is a promiscuous receptor with more than one binding site, such that it is possible to inhibit the nonopsonic binding of M. tuberculosis with a MAb recognizing an epitope distal from the iC3b binding site, whereas a MAb recognizing the iC3b site has little effect (46). In addition, treatment of Mφ with phorbol myristate acetate will increase binding of EIgMC′ but not M. tuberculosis to CR3 (46), again demonstrating the diverse activity of CR3. It is therefore possible that CL, while inhibiting the iC3b binding activity of CR3, enhances the M. tuberculosis binding epitope. It is equally possible that CL acts to inhibit CR3 and to enhance another, unidentified receptor that mediates the uptake of mycobacteria. These possibilities await further investigation.
It was unlikely that either PI or CL had a global affect on the ability of Mφ to associate with particles, as PI inhibits the interaction of Mφ with M. tuberculosis, EIgMC′, EIgG, and zymosan but not latex particles, whereas CL inhibits Mφ interactions with latex and EIgMC′ but enhances the uptake of M. tuberculosis. Both phospholipids appear to act separately on specific receptor-ligand interactions by acting on either the Mφ or the particle.
In conclusion, we have demonstrated that the two phospholipids PI and CL have significant effects on the binding and uptake of M. tuberculosis; PI is inhibitory, whereas CL is stimulatory. Neither molecule interacts with Mφ via the 2F8 epitope of SR-A, and treatment of Mφ with either phospholipid results in a down-regulation of CR3 function and TNF-α production by the phagocyte. Definition of the mode of action of PI and CL on Mφ awaits further investigation.
ACKNOWLEDGMENTS
We thank S. Gordon for the kind gift of MAb 2F8 and K. Brinded for the negative electrospray MS and MS-MS analysis of mycobacterial PI.
This work was supported by funding from the Network Centres of Excellence (Canadian Bacterial Diseases Network), Glaxo Wellcome Action TB, and the B.C. Tuberculosis and Chest Disabled Veterans' Association. M.G.S. is the recipient of a Medical Research Council of Canada Ph.D. studentship. R.W.S. is a BC Lung Association/Medical Research Council of Canada Scholar.
REFERENCES
- 1.Adams L B, Fukutomi Y, Krahenbuhl J L. Regulation of murine macrophage effector functions by lipoarabinomannan from mycobacterial strains with different degrees of virulence. Infect Immun. 1993;61:4173–4181. doi: 10.1128/iai.61.10.4173-4181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert R K, Embree L J, McFeely J E, Hickstein D D. Expression and function of β2 integrins on alveolar macrophages from human and nonhuman primates. Am J Respir Cell Mol Biol. 1992;7:182–189. doi: 10.1165/ajrcmb/7.2.182. [DOI] [PubMed] [Google Scholar]
- 3.Ballou C E. Biosynthesis of mannophosphoinositides in by Mycobacterium phlei. Methods Enzymol. 1971;28:493–500. [Google Scholar]
- 4.Barnes P F, Chatterjee D, Abrams J S, Lu S H, Wang E, Yamamura M, Brennan P J, Modlin R L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan—relationship to chemical structure. J Immunol. 1992;149:541–547. [PubMed] [Google Scholar]
- 5.Bate C A W, Kwiatkowski D. A monoclonal antibody that recognises phosphatidylinositol inhibits induction of tumor necrosis factor alpha by different strains of Plasmodium falciparum. Infect Immun. 1994;62:5261–5266. doi: 10.1128/iai.62.12.5261-5266.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bate C A W, Taverne J, Bootsma H J, Mason R C S, Skalko N, Gregoriadis G, Playfair J H L. Antibodies against phosphatidylinositol and inositol monophosphate specifically inhibit tumour necrosis factor induction by malaria exoantigens. Immunology. 1992;76:35–41. [PMC free article] [PubMed] [Google Scholar]
- 7.Berger M, Norvell T M, Tosi M F, Emancipator S E, Konstan M W, Schreiber J R. Tissue-specific Fcγ and complement receptor expression by alveolar macrophages determines relative importance of IgG and complement in promoting phagocytosis of Pseudomonas aeruginosa. Paediatr Res. 1993;35:68–77. doi: 10.1203/00006450-199401000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Bernardo J, Billingslea A M, Blumenthal R L, Seetoo K F, Simons E R, Fenton M J. Differential responses of human mononuclear phagocytes to mycobacterial lipoarabinomannans—role of CD14 and the mannose receptor. Infect Immun. 1998;66:28–35. doi: 10.1128/iai.66.1.28-35.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besra G S, Morehouse C B, Rittner C M, Waechter C J, Brennan P J. Biosynthesis of mycobacterial lipoarabinomannan. J Biol Chem. 1997;272:18460–18466. doi: 10.1074/jbc.272.29.18460. [DOI] [PubMed] [Google Scholar]
- 10.Bilyk N, Holt P G. The surface phenotypic characterization of lung macrophages in C3H/HeJ mice. Immunology. 1991;74:645–651. [PMC free article] [PubMed] [Google Scholar]
- 11.Bradbury M G, Moreno C. Effect of lipoarabinomannan and mycobacteria on tumour necrosis factor production by different populations of murine macrophages. Clin Exp Immunol. 1993;94:57–63. doi: 10.1111/j.1365-2249.1993.tb05977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan P J, Hunter S W, McNeil M, Chatterjee D, Daffe M. Reappraisal of the chemistry of mycobacterial cell walls, with a view to understanding the roles of individual entities in disease processes. In: Ayoub E M, Cassell G H, Branche C W C, Henry T J, editors. Microbial determinants of virulence and host response. Washington, D.C.: American Society for Microbiology; 1990. pp. 55–75. [Google Scholar]
- 13.Brown M C, Taffet S M. Lipoarabinomannans derived from different strains of Mycobacterium tuberculosis differentially stimulate the activation of NF-κB and KBF1 in murine macrophages. Infect Immun. 1995;63:1960–1968. doi: 10.1128/iai.63.5.1960-1968.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan J, Fan X, Hunter S W, Brennan P J, Bloom B R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatterjee D, Khoo K H. Mycobacterial lipoarabinomannan—an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology. 1998;8:113–120. doi: 10.1093/glycob/8.2.113. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee D, Roberts A D, Lowell K, Brennan P J, Orme I M. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect Immun. 1992;60:1249–1253. doi: 10.1128/iai.60.3.1249-1253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins H L, Bancroft G J. Cytokine enhancement of complement-dependent phagocytosis by macrophages—synergy of tumor necrosis factor-alpha and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur J Immunol. 1992;22:1447–1454. doi: 10.1002/eji.1830220617. [DOI] [PubMed] [Google Scholar]
- 18.Cywes C, Godenir N L, Hoppe H C, Scholle R R, Steyn L M, Kirsch R E, Ehlers M W R. Nonopsonic binding of Mycobacterium tuberculosis to human complement receptor type 3 expressed in Chinese hamster ovary cells. Infect Immun. 1996;64:5373–5383. doi: 10.1128/iai.64.12.5373-5383.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahl K E, Shiratsuchi H, Hamilton B D, Ellner J J, Toossi Z. Selective induction of transforming growth factor beta in human monocytes by lipoarabinomannan of Mycobacterium tuberculosis. Infect Immun. 1996;64:399–405. doi: 10.1128/iai.64.2.399-405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunne D W, Resnick D, Greenberg J, Krieger M, Joiner K A. The type I macrophage scavenger receptor binds to Gram-positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci USA. 1994;91:1863–1867. doi: 10.1073/pnas.91.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehlers M R W, Daffé M. Interactions between Mycobacterium tuberculosis and host cells: are mycobacterial sugars the key? Trends Microbiol. 1998;6:328–335. doi: 10.1016/s0966-842x(98)01301-8. [DOI] [PubMed] [Google Scholar]
- 22.Ernst W A, Maher J, Cho S G, Niazi K R, Chatterjee D, Moody D B, Besra G S, Watanabe Y, Jensen P E, Porcelli S A, Kronenberg M, Modlin R L. Molecular interaction of CD1b with lipoglycan antigens. Immunity. 1998;8:331–340. doi: 10.1016/s1074-7613(00)80538-5. [DOI] [PubMed] [Google Scholar]
- 23.Falcone V, Bassey E B, Toniolo A, Conaldi P G, Collins F M. Differential release of tumor necrosis factor-alpha from murine peritoneal macrophages stimulated with virulent and avirulent species of mycobacteria. FEMS Immunol Med Microbiol. 1994;8:225–232. doi: 10.1111/j.1574-695X.1994.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 24.Fan S T, Edgington T S. Integrin regulation of leukocyte inflammatory functions—CD11b/CD18 enhancement of the tumor necrosis factor-α responses of monocytes. J Immunol. 1993;150:2972–2980. [PubMed] [Google Scholar]
- 25.Gordon S. Macrophage-restricted molecules: role in differentiation and activation. Immunol Lett. 1999;65:5–8. doi: 10.1016/s0165-2478(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 26.Hampton R Y, Golenbock D T, Penman M, Krieger M, Raetz C R. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 27.Hetland G, Wiker H G, Hogasen K, Hamasur B, Svenson S B, Harboe M. Involvement of antilipoarabinomannan antibodies in classical complement activation in tuberculosis. Clin Diagn Lab Immunol. 1998;5:211–218. doi: 10.1128/cdli.5.2.211-218.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoppe H C, Dewet B J M, Cywes C, Daffe M, Ehlers M R W. Identification of phosphatidylinositol mannoside as a mycobacterial adhesin mediating both direct and opsonic binding to nonphagocytic mammalian cells. Infect Immun. 1997;65:3896–3905. doi: 10.1128/iai.65.9.3896-3905.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter S W, Brennan P J. Evidence for the presence of a phosphatidylinositol anchor on the lipoaribinomannan and lipomannan of Mycobacterium tuberculosis. J Biol Chem. 1990;265:9272–9279. [PubMed] [Google Scholar]
- 30.Ilangumaran S, Arni S, Poincelet M, Theler J M, Brennan P J, Nasir-Ud-Din, Hoessli D C. Integration of mycobacterial lipoarabinomannans into glycosylphosphatidylinositol-rich domains of lymphomonocytic cell plasma membranes. J Immunol. 1995;155:1334–1342. [PubMed] [Google Scholar]
- 31.Limb G A, Hamblin A S, Wolstencroft R A, Dumonde D C. Rapid cytokine up-regulation of integrins, complement receptor-1 and HLA-DR on monocytes but not on lymphocytes. Immunology. 1992;77:88–94. [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Barry III C E, Nikaido H. Cell wall: physical structure and permiability. In: Ratledge C, Dale J W, editors. Mycobacteria—molecular biology and virulence. Oxford, United Kingdom: Blackwell Science; 1999. pp. 220–39. [Google Scholar]
- 33.Maestrelli P, De Fina O, Bertin T, Papiris S, Ruggieri M P, Saetta M, Mapp C E, Fabbri L M. Integrin expression on neutrophils and mononuclear cells in blood and induced sputum in stable asthma. Allergy. 1999;54:1303–1308. doi: 10.1034/j.1398-9995.1999.00337.x. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell T G, Perfect J. Cryptococcosis in the era of AIDS—100 years after the dicovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreno C, Taverne J, Mehlert A, Bate C A, Brealey R J, Meager A, Rook G A, Playfair J H. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989;76:240–245. [PMC free article] [PubMed] [Google Scholar]
- 36.Ortalo-Magne A, Andersen A B, Daffe M. The outermost capsular arabinomannans and other mannoconjugates of virulent and avirulent tubercle bacilli. Microbiology. 1996;142:927–935. doi: 10.1099/00221287-142-4-927. [DOI] [PubMed] [Google Scholar]
- 37.Pugin J, Heumann D, Tomasz A, Kravchenko V V, Akamatsu Y, Nishijima M, Glauser M P, Tobias P S, Ulevitch R J. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds H Y, Newball H N. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974;84:559–573. [PubMed] [Google Scholar]
- 39.Roach T I A, Barton C H, Chatterjee D, Blackwell J M. Macrophage activation—lipoarabinomannan from avirulent and virulent strains of Mycobacterium tuberculosis differentially induces the early genes c-fos, KC, JE, and tumor necrosis factor-alpha. J Immunol. 1993;150:1886–1896. [PubMed] [Google Scholar]
- 40.Salageanu A, Ceacareanu B, Istrate N, Szegli G. Experimental studies on the bacterial product CANTASTIM derived from Pseudomonas aeruginosa. III. Suppression of lipopolysaccharide-induced tumor necrosis factor alpha: are the lipid components involved? Roum Arch Microbiol Immunol. 1997;56:27–35. [PubMed] [Google Scholar]
- 41.Schlesinger L S, Bellinger-Kawahara C G, Payne N R, Horwitz M A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 42.Schlesinger L S, Hull S R, Kaufman T M. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- 43.Sibley L D, Hunter S W, Brennan P J, Krahenbuhl J L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect Immun. 1988;56:1232–1236. doi: 10.1128/iai.56.5.1232-1236.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sieling P A, Chatterjee D, Porcelli S A, Prigozy T I, Mazzaccaro R J, Soriano T, Bloom B R, Brenner M B, Kronenberg M, Brennan P J, Modlin R L. CD-1 restricted T cell recognition of microbial lipoglycan antigens. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 45.Smith R J, Iden S S. Properties of calcium ionophore-induced generation of superoxide anion by human neutrophils. Inflammation. 1981;5:177–192. doi: 10.1007/BF00914442. [DOI] [PubMed] [Google Scholar]
- 46.Stokes R W, Haidl I D, Jefferies W A, Speert D P. Mycobacteria-macrophage interactions. Macrophage phenotype determines the nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J Immunol. 1993;151:7067–7076. [PubMed] [Google Scholar]
- 47.Stokes R W, Speert D P. Lipoarabinomannan inhibits nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J Immunol. 1995;155:1361–1369. [PubMed] [Google Scholar]
- 48.Stokes R W, Thorson L M, Speert D P. Nonopsonic and opsonic association of Mycobacterium tuberculosis with resident alveolar macrophages is inefficient. J Immunol. 1998;160:5514–5521. [PubMed] [Google Scholar]
- 49.Xu S M, Cooper A, Sturgillkoszycki S, Vanheyningen T, Chatterjee D, Orme I, Allen P, Russell D G. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]
- 50.Yui S, Yamazaki M. Induction of macrophage growth by negatively charged phospholipids. J Leukoc Biol. 1986;39:713–716. doi: 10.1002/jlb.39.6.713. [DOI] [PubMed] [Google Scholar]
- 51.Yui S, Yamazaki M. Augmentation and suppression of release of tumor necrosis factor from macrophages by negatively charged phospholipids. Jpn J Cancer Res. 1991;82:1028–1034. doi: 10.1111/j.1349-7006.1991.tb01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Doerfler M, Lee T C, Guillemin B, Rom W N. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by mycobacterium tuberculosis components. J Clin Investig. 1993;91:2076–2083. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]