Abstract
Heterogeneous catalysis of alkenes to alkanes is of great importance in chemical industry, but more efficient and reusable heterogeneous catalysts are still demanded. Here, we report a metallically gradated composite of a silicon nanowire array and palladium nanoparticles which are reused for the hydrogenation of an alkene. The catalyst promotes the hydrogenation of stilbene with atmospheric hydrogen (0.1 MPa) to give diphenylethane quantitatively. The recovered catalyst can be reused, and mediates the reaction without loss of yield more than one hundred times, whereas the stability of Pd/C degrades rapidly over 10 cycles of reuse. The catalyst allows the hydrogenation of a variety of alkenes, including tetra-substituted olefins. Structural investigation reveals that palladium nanoparticles are metallically gradated onto the silicon nanowire array under mild conditions by agglomeration of palladium silicide, as confirmed by XAFS and XPS together with argon-ion sputtering. This means of metal agglomeration immobilization may be applicable to the preparation of a variety of metal nanoparticle catalysts.
Subject terms: Heterogeneous catalysis, Synthetic chemistry methodology, Nanowires
Degradation of heterogeneous catalysts limits their cost efficiency and sustainability. Here a hydrogenation catalyst that is stable over a hundred cycles of reuse is reported, with stability attributed to the formation of an agglomeration at the palladium/silicon interface.
Introduction
If catalysts worked perpetually with high catalytic activity, the chemical industry and catalytic science would benefit. Toward achieving this ultimate goal of catalytic science, researchers have developed highly active and reusable heterogeneous catalysts. Hydrogenation is a very important reaction in chemical processes, and thus, the development of highly reusable catalysts for hydrogenation is highly important and challenging1–4. Heterogeneous catalysts have been prepared mainly in two ways: the first is the immobilization of the metal species on solid supports through surface adsorption. Pd/C and Pd on metal oxides (SiO2, Al2O3, MgO, CeO2, etc.) are typical examples of this type (Fig. 1a)5–14. The other is the immobilization of metal species in solid matrices of polymers, dendrimers, and organic frameworks via coordinative interactions, where the metal species are stabilized through the steric bulk of the frameworks and/or the coordinative interactions with heteroatoms (e.g. N, O, P, and S) (Fig. 1b)15–25. For example, we have developed convoluted polymeric metal catalysts that promote various cross-couplings and Huisgen cycloadditions at mol ppm level loadings26–31. However, the adsorption and coordination interactions in these catalysts are generally weaker than those resulting from covalent and metal bonds, and may thus cause metal leaching problems, particularly in industrial applications.
Fig. 1. Morphology of metal-support stabilization in solid-supported metal nanoparticle catalysts.

a Surface adsorption. b Steric bulk and/or coordination. c Gradient agglomeration of metal nanoparticle and support material (this work).
To address these challenges, we envisioned the immobilization of metal nanoparticles on supports via bonding with the alloy/agglomeration of the metal nanoparticles and supports. In such an approach, the metal nanoparticles would be strongly anchored to the support via metallic bonds (Fig. 1c), and should overcome the traditional issues and improve catalysis.
The surface structure of support materials is known to significantly affect the catalytic activity and selectivity of a transformation. Nanostructured materials are attractive candidates as supports for transition metal nanoparticles for preparation of catalysts for sustainable and efficient chemical processes32. We have been interested in nanoscale reactions on support materials for the development of catalytic reactions for efficient organic transformations. Recently, we reported the development of a silicon nanowire array and palladium nanoparticle hybrid (SiNA-Pd) for catalytic organic transformations33,34. Here we investigate the reusability of this system for the hydrogenation of olefins, where the catalyst can be readily reused more than one hundred times without loss of catalytic activity. In contrast, the catalytic activity of Pd/C for the same transformation decreases during its reuse, and afforded much lower catalytic activity. Further investigation revealed that the palladium nanoparticles (PdNPs) were anchored to the silicon as palladium silicides via Pd–Si metallic bonds, and gradually form an agglomerated Pd/Si alloy layer as shown in Fig. 1c.
In this communication, we present the high reusability and catalytic activity of SiNA-Pd for the hydrogenation of olefins, including tetra-substituted alkenes. We also show evidence of the immobilization mode of the metal nanoparticles on the supports through gradated alloy formation/agglomeration of the metal nanoparticles and the supports.
Results and discussion
High reusability for hydrogenation
SiNA-Pd was reproducibly prepared in accordance with our previous report (Fig. 2), where palladium nanoparticles were installed into SiNA by reduction of Pd(II) on the SiNA surface carrying terminal hydrogens (see Supplementary method for details)35–41. The hydrogenation of trans-stilbene (1a) was carried out with SiNA-Pd corresponding to 0.12 mol% of Pd under 0.1 MPa hydrogen gas conditions and afforded bibenzyl (2a) in quantitative yield. The recovered catalyst was reused without of loss of catalytic activity 150 times more in the same reaction and afforded 2a quantitatively in all conversions (Fig. 3). Notably, the turnover number of the catalyst in the consecutive reactions reached 125000. No Pd was detected in the reaction mixture using ICP-MS analysis (detection limit: 0.021 ppb), which indicated the lack of Pd leaching during the catalytic transformation. In contrast, the catalytic activity of Pd/C decreased significantly during its consecutive reuse, which is in-line with the literature reports42,43.
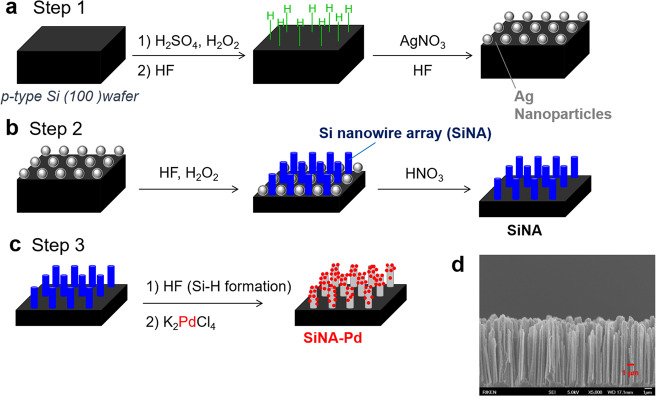
Fig. 2. Schematic illustration for preparation of SiNA-Pd.
a Hydrogen-termination of Si(100) surface, followed by deposition of silver nanoparticles. b Formation of SiNA by metal-assisted chemical etching. c Preparation of SiNA-Pd through reductive deposition of palladium species. d SEM image of SiNA-Pd.
Fig. 3. Hydrogenation of trans-stilbene (1a) for comparison with catalytic activity of SiNA-Pd and Pd/C.
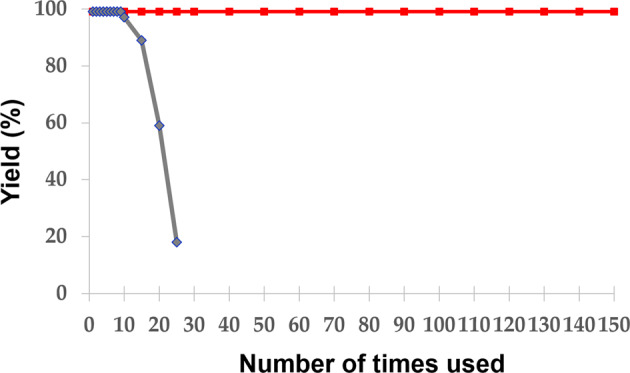
trans-stilbene (1a) (0.5mmol), catalyst (0.12mol%), ethanol (2.0mL) under H2 (0.1MPa) at 70°C for 24h. Red: SiNA-Pd; blue: Pd/C.
Hydrogenation of various alkenes
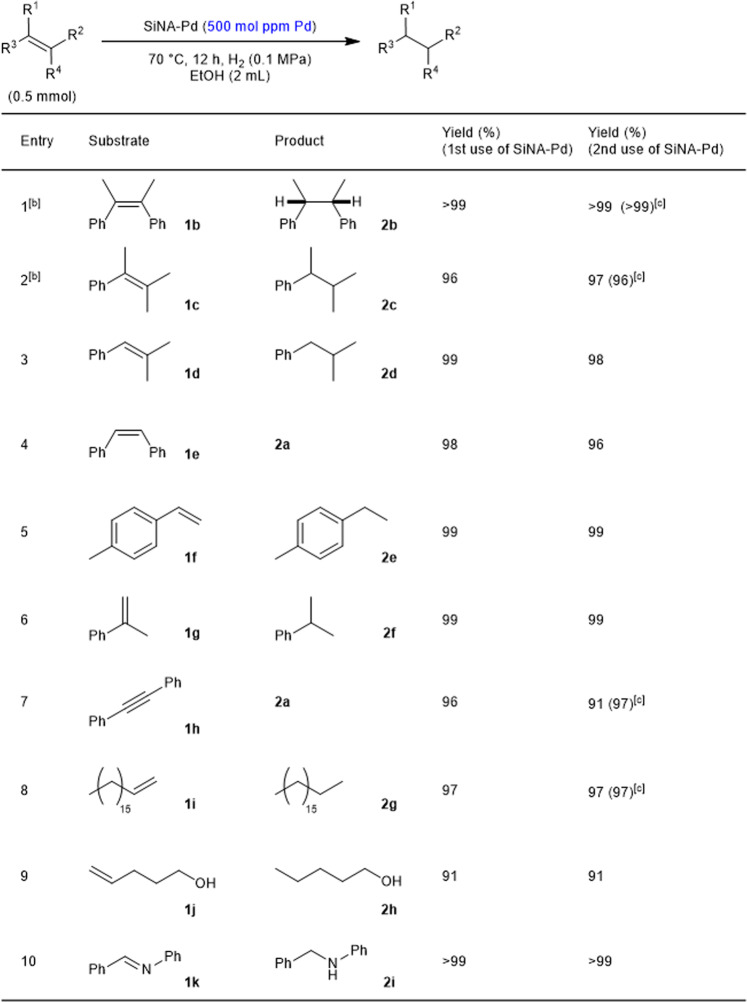
With a highly reusable catalyst in hand, the hydrogenation of various alkenes was evaluated with 500 mol ppm (0.05 mol%) Pd of SiNA-Pd (1/SiNA-Pd = 2000/1 (mol/mol)) (Fig. 4). In all of the reactions presented in Fig. 4, the catalyst was reused to show the generality of its reusability. When the reaction of the less reactive tetra-substituted alkenes 1b-c was carried out (Supplementary Data 1), we were pleased to find that the reaction proceeded smoothly to give the corresponding alkane 2b–c in >99% and 96% yields, respectively. The recovered catalyst promoted the reaction to give 2b in >99% and 97% yields (2nd use of SiNA-Pd), and in >99% and 96% yields (3rd use of SiNA-Pd), respectively (Entries 1 and 2) (Supplementary Data 2). Tri- and di-substituted alkenes, including aliphatic olefins, as well as an alkyne and an imine (1b–k) were readily converted by the fresh and recovered SiNA-Pd under similar conditions to the corresponding products 2b–i in 91–99% yields (Entries 3–10).
Fig. 4. Hydrogenation of alkenes by using fresh and used SiNA-Pd under hydrogen atmosphere (0.1 MPa).
a Conditions: 1 (0.5 mmol), H2 (0.1 MPa), SiNA-Pd (500 mol ppm Pd), EtOH (2 mL), 70 °C, 12 h. b The reaction time: 24 h. c Yield in the 3rd use of SiNA-Pd.
SiNA-Pd was applied to the hydrogenation of fatty acids and triolein under neat conditions (Fig. 5). Full conversion of unsaturated fatty acids to saturated fatty acids is important for preventing the formation of trans-fatty acids44–49. The hydrogenation of oleic acid (1l) was performed and gave stearic acid (2j), a fatty acid which activates high density lipoprotein (HDL) and decreases low density lipoprotein (LDL)50, in >99% yield (Fig. 5a). SiNA-Pd was reused under similar conditions and gave 2j in >99% yield. Linoleic acid (1m) was also converted to 2j in >99% yield (Fig. 5b). Triolein (1n) also underwent hydrogenation to give glyceryl tristearate (2k) in 99% yield (Fig. 5c).
Fig. 5. Hydrogenation of fatty acids.
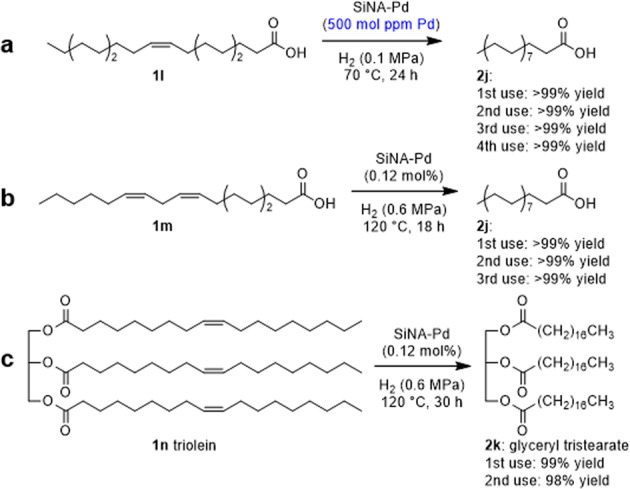
a Oleic acid (0.5 mmol), SiNA-Pd (500 mol ppm), H2 (0.1 MPa), 70 °C, 24 h. b Linoleic acid (0.5 mmol), SiNA-Pd (0.12 mol%) H2 (0.6 MPa), 120 °C, 18 h. c Triolein (0.5 mmol), H2 (0.6 MPa), 120 °C, 30 h.
In SiNA-Pd, the palladium nanoparticles are immobilized by the reduction with the silanes on the silicon surface. We hypothesized that the palladium nanoparticles were metallically gradated onto the silicon nanowire array via strong metal bonding (alloy/ agglomeration) between the palladium and silicon. Although we attempted studying the metal bonding in SiNA-Pd to gain insight into the immobilization mode of the metal nanoparticles on supports, the spectroscopic analysis was not feasible due to the high steric crowding of SiNA-Pd with palladium nanoparticles and silicon nanowires. To address this limitation, we prepared less crowded silicon-nanostructured palladium nanoparticles under similar conditions for the following investigation.
Elucidation of stable Pd–Si nanoparticle formation in the catalyst
X-ray photoelectron spectroscopy (XPS) allows relatively high surface-sensitive spectroscopic analysis, because the mean free path of photoelectrons in solid materials is within several nanometers from the outermost surface, and the technique is often utilized in combination with sputtering techniques for the purpose of depth analysis51. Remarkably, a series of XPS spectra of the silicon-nanostructured palladium nanoparticles for Pd 3d core level exhibited the presence of palladium silicide52 besides Pd(0) (Fig. 6). Ar-ion-sputtering was performed with 3-keV kinetic energy in vacuum. The XPS spectra were recorded at a take-off angle of 90˚. The as-prepared silicon-nanostructured palladium nanoparticles were etched by sputtering for up to 16 min in vacuum, and the nominal etching rate was determined to be 0.5 nm/min of SiO2 using a SiO2/Si standard. With the increase in the sputtering time, the peak intensity of Pd(0) (ca. 335 eV for Pd 3d5/2) decreased, whereas the intensity in the higher binding energy region (similarly 336–338 eV) increased. The surface oxides on PdNPs (usually described as Pd(II)O and Pd(IV)O2 (It is known that palladium dioxide synthesized at a high oxygen pressure decomposes at temperatures above 340 K)53,54) should be removed from the surface of the sample after the etching. Indeed, a weak and very broad peak with a significantly high binding energy (>338 eV for Pd 3d5/2) almost completely disappeared after a sputtering time of 1 min, which is attributed to the surface PdO2 localized within the block boundaries of PdO55. We suppose that the reason for the increased intensity in the high binding energy region in the 335–338 eV range for Pd 3d5/2 is due to the depth distribution of the palladium species. Thus, the higher binding energy species localize behind the Pd(0) nanoparticles. Furthermore, the binding energy of the main peak near 336 eV in the spectra of the etched samples was not consistent with that of PdO in the surface-oxidized palladium foil (336.9 eV)55. Over a period of the sputtering, the intensity of the broad peak derived from SiO2 near 102.6 eV in the spectra for Si 2p core level decreased monotonically relative to that of bulk silicon, which indicates the etching of the SiO2 surface along with that of the palladium oxide and bulk palladium. In addition, the peak shape of the crystalline silicon (split into 2p1/2 and 2p3/2 due to spin–orbit coupling) in the 98–100 eV range was continuously deformed during the etching. As is the case with a series of Pd 3d spectra, this indicated that the contribution of palladium silicide increased in the early stage of the etching, which affected the peak shape around 99 eV in the Si 2p spectra due to the removal of Pd(0).
Fig. 6. Study of Pd 3d5/2 and Si 2p XPS spectra.
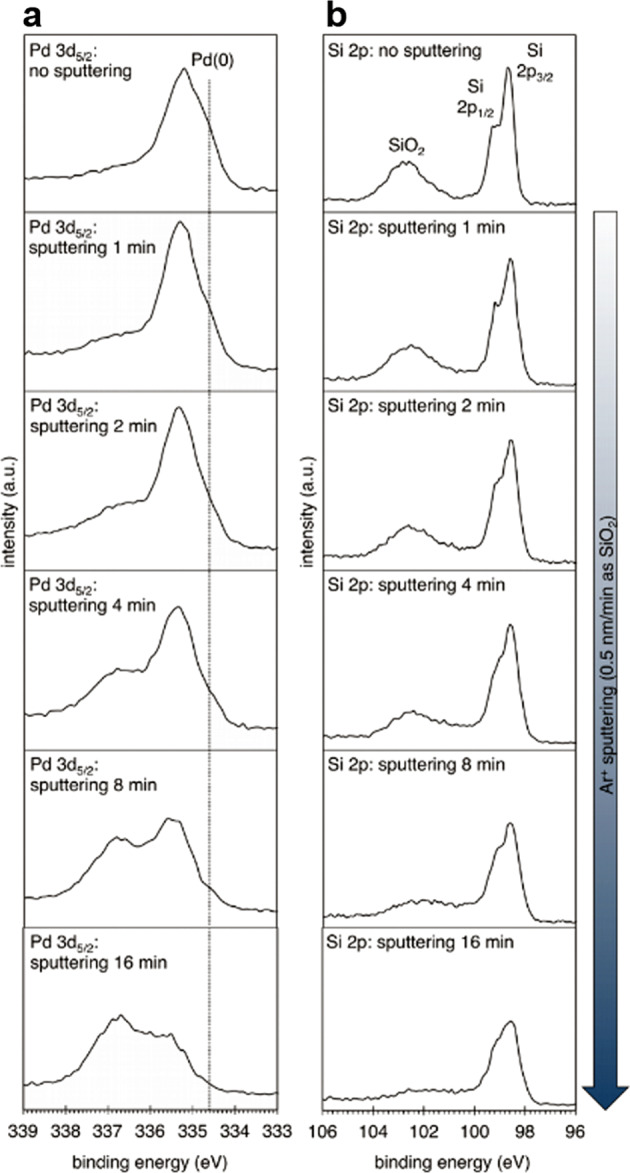
Pd 3d5/2 (a) and Si 2p (b) XPS spectra of the silicon-nanostructured palladium nanoparticles at a take-off angle of 90˚ with Ar-ion-sputtering with 3-keV kinetic energy. The nominal etching rate was 0.5nm/min as SiO2. The binding energy of Pd(0) in Pd 3d5/2 core level is displayed as dotted line at 334.6eV based on the curve fitting result for the spectrum at the sputtering time of 4min (Fig. 7).
We were successful in separating the peak components of Pd(0), and palladium silicide (Pd4Si, Pd3Si, Pd2Si, and PdSi) in the Pd 3d spectrum at a sputtering time of 4 min (Fig. 7). The envelope was fitted at 334.6/339.8 eV (3d5/2/3d3/2) for Pd(0), 335.4/340.6 eV for Pd4Si, 336.1/341.3 eV for Pd3Si, 336.9/342.1 eV for Pd2Si, and 337.8/343.08 eV for PdSi. The values of full width at half maximum (FWHM) were within the range of 0.9–1.3 eV, and these values of binding energy and FWHM were consistent with those reported in earlier research55. According to the observations in Fig. 6, in which the population of Pd 3d5/2 peaks gradually shifted toward the higher binding energy region with an increase in the etching time, the more silicon-rich silicide was distributed far from the outermost surface than the palladium-rich silicide and Pd(0). We also performed curve fitting on the Si 2p spectrum at the sputtering for 4 min to separate the peak components of crystalline silicon (2p1/2 and 2p3/2), palladium silicide, SiOx (suboxide), and SiO2. The envelope was fitted at 98.4 eV for 2p3/2, 99.2 eV for 2p1/2, 99.3 eV for PdxSi, 101.0 eV for SiOx, and 102.6 eV for SiO2. The values of FWHM are 0.7, 0.7, 2.3, 1.7, and 1.7 eV, respectively. Due to the very close chemical shifts of palladium silicide56–58, we let a somewhat broad peak (FWHM is 2.3) represent the sum of palladium silicides. The peak deformation near the crystalline silicon during the etching is possibly attributed to the increasing contribution of the palladium silicide due to the removal of Pd(0) from the surface toward the silicide phase.
Fig. 7. Pd 3d XPS spectrum of silicon-nanostructured palladium nanoparticles.
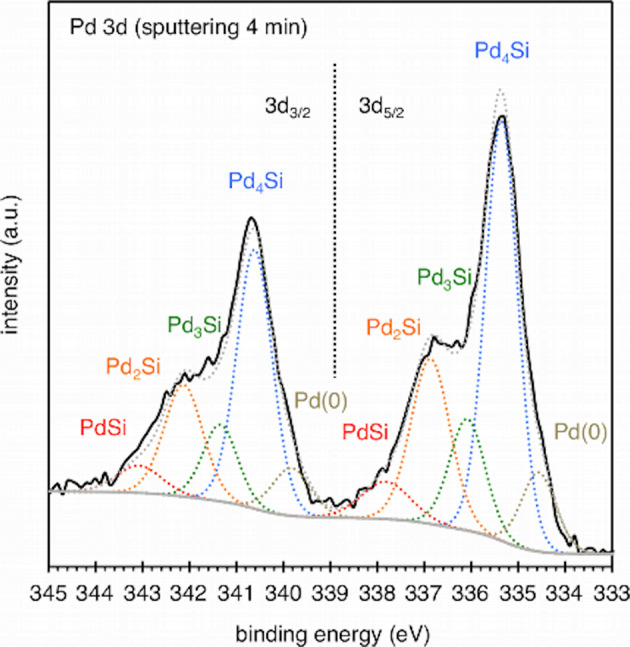
The black solid line shows the result of the sputtering time of 4 min, and the colored dotted lines do the curve fitting results for Pd(0), Pd4Si, Pd3Si, Pd2Si, and PdSi.
We evaluated the chemical state of the palladium in silicon-nanostructured palladium nanoparticles by Pd L3-edge X-ray absorption near-edge structure (XANES), which is often used to probe the unfilled d orbitals of transition metals (Fig. 8)59–61. (The surface of Pd foil was cleaned by Ar-ion-sputtering prior to the measurement to remove the surface oxide layer of palladium. The samples were transported for analysis at BL-13 beamline of Ritsumeikan SR Center (Shiga, Japan) without air-exposure.) The curve fitting for the near-edge region in the spectrum of silicon-nanostructured palladium nanoparticles was also performed to distinguish the chemical species in the higher-energy region to Pd(0) in the Pd L3-edge spectrum. Two pseudo-Voigt functions and one error function were applied to the two peaks (one of them is Pd(0)) and baseline (edge-jump of Pd L3-edge), respectively. The best fit with two peaks (E0 = 3174.2 and 3177.6 eV, where E0 is the centroid energy of line) showed good agreement (R-factor: 0.4%) with the experimental line shape of the silicon-nanostructured palladium nanoparticles in the range 3165–3180 eV. The E0 value of 3174.2 eV (fixed during fitting) is identical to that obtained from the fitting result for the spectrum of Pd foil. As expected, another peak (optimized for all parameters) appeared at a significantly higher E0 (3177.6 eV) and was slightly broader compared with Pd(0); this peak was assigned to the sum of palladium silicides. Additionally, the IR experiments indicated that after the formation of palladium nanoparticles on the HF-treated silicon surface, the surface Si–H were completely consumed and an oxidized surface of silicon was generated (Supplementary Fig. 1).
Fig. 8. Curve fitting for the white line in the Pd L3-edge XANES spectrum of silicon-nanostructured palladium nanoparticles.
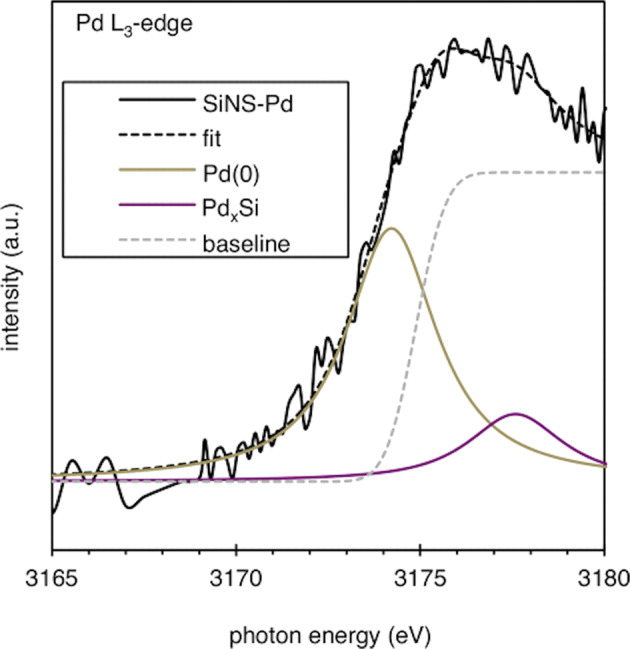
Two pseudo-Voigt functions are applied to Pd(0) and PdxSi.
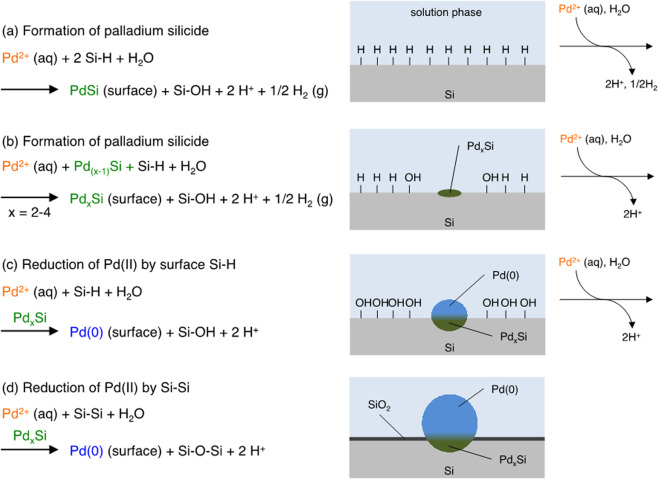
Taking all these observations into account, we proposed an immobilization mechanism for the reduction of Pd(II) species onto the silicon surface comprising terminal hydrogens (Fig. 9). The initial step in the formation of PdNPs is the generation of palladium silicides, which are formed in the reaction of Pd(II) with the surface Si–Hx. Subsequently, more palladium-rich silicide species (Pd2Si, Pd3Si, and Pd4Si) are also generated, and the PdNPs grow on the silicides associated with the reduction of Pd(II) by the surface Si–H and Si–Si bonds. The latter pathway (reduction of Pd(II) by the cleavage of Si–Si bond) can explain the reason for the mismatch between the amounts of surface hydrides and PdNPs. Thus, Pd(II) species are reduced by not only the surface Si–H but also Si–Si bonds near the surface. The silicon substrate serves as a reducing agent and the electrons are provided through the palladium silicide layer to the outermost surface of PdNPs at which the reduction of Pd(II) to Pd(0) occurs. Overall, the immobilization of palladium onto silicon is so robust and stable that the catalyst is reusable more than one hundred times without loss of catalytic activity.
Fig. 9. Plausible mechanism and illustration for immobilization of Pd nanoparticles on silicon surface bridged by palladium silicide layer.
a Initial formation of palladium silicide. b Consecutive formation of palladium silicide. c Reduction of Pd(II) by surface Si–H. d Reduction of Pd(II) by Si–Si.
We found that our silicon nanowire array-stabilized palladium nanoparticle catalyst (SiNA-Pd) can be reused more than one hundred times without loss of catalytic activity in alkene hydrogenation, and affords the corresponding alkanes quantitatively. A variety of alkenes including tetra-substituted olefins were readily converted to alkanes in high yield by fresh and reused SiNA-Pd. Metallically gradated Pd–Si nanoparticles formed on SiNA-Pd provided perpetual heterogeneous catalysis of hydrogenation. Since this methodology for immobilizing metal nanoparticles onto silicon nanowire arrays is promising, we are developing other metal catalysts for organic transformations by utilizing our methodology.
Methods
General information
General procedures
Characterization
X-ray structure determination
See Supplementary Fig. 29 and Supplementary Fig. 30. CIFs are available in Supplementary Data 1–2.
IR Experiments
See Supplementary Fig. 1.
Preparation of a SiNA-stabilized Pd nanoparticle catalyst
A boron doped p-type Si (100) wafer (0.1–100 Ω cm; Φ = 2 inch; 1.5 g; SBET 4 cm2/cm2 (Kr)) was immersed in a mixture of conc. 95% H2SO4 and 30% H2O2 (15 mL: 5 mL, v/v) for 15 min, and then, washed with H2O and dried with N2 blow. The washed Si wafer was treated with 5% aqueous HF (10 mL) for 3 min, washed with H2O, dried with N2 blow. One side of Si wafer was masked with a urethane mask sheet (Kokuyo, Co. Ltd). Masked Si wafer was placed into a mixture of 46% HF (15 mL) and AgNO3 (53.2 mg) in H2O (47.6 mL), which was slowly stirred for 1 min. The Ag-coated Si wafer was washed with H2O, and dried with N2 blow. The urethane mask sheet was peeled off. The Ag-coated Si wafer was placed into a mixture of 46%HF (4.0 mL) and 30% H2O2 (0.9 mL) in H2O (19.0 mL) at 60 °C for 3 min, and Si wafer was washed with H2O and dried with N2 blow. The etched Si wafer was immersed twice in 50% aqueous HNO3 (30 mL) for 3 min, washed with H2O, dried with N2 blow to give the Si nanowire array (SiNA). After SiNA was placed into 5% aqueous HF (10 mL) for 1 min, washed with H2O, dried with N2 blow, it was immersed in a mixture of 50 mM aqueous K2PdCl4 (4.5 mL) and acetone (1.5 mL) for 5 min. The Si wafer was washed with H2O and acetone, and dried with N2 blow to give SiNA-stabilized Pd nanoparticle catalyst (SiNA-Pd). The loading of Pd was determined by ICP-MS.
General procedure for the hydrogenation of various substrates
To a 10 mL glass vessel was added SiNA-Pd (0.00025 mmol, 0.05 mol% Pd), each substrate (0.5 mmol), and ethanol (2 mL). The reaction was carried out with H2 (0.1 MPa, balloon) at 70 °C for 12–24 h under the vortex mixing conditions by a vortex mixer with a temperature controller. After cooling to room temperature, the reaction mixture was analyzed with GC (HP-1) with dodecane or mesitylene as an internal standard to determine the yield.
Catalyst reuse experiments
To a 10 mL glass vessel was added SiNA-Pd (0.00025 mmol, 0.05 mol% Pd), trans-stilbene (0.5 mmol, 90 mg), ethanol (2 mL), and dodecane as an internal standard. The reaction was carried out with H2 (0.1 MPa, balloon) at 70 °C for 24 h under vortex mixer. After cooling to room temperature, the reaction mixture was analyzed with GC (HP-1) to determine the yield. The catalyst was recovered by picking up with a tweezer and washed with EtOAc/acetone/water. After drying with N2 blow, the catalyst was used for the next reaction.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The authors would like to thank Dr. Daisuke Hashizume (Materials Characterization Support Team, CEMS, RIKEN, Japan) for X-ray crystallography, Dr. Tetsuo Honma (JASRI) for hard X-ray XAFS experiment, and Ms. Aya Ohno (our team) for ICP-MS analysis. We are also very grateful to Prof. Toshiaki Ohta and Dr. Keisuke Yamanaka (SR Center, Ritsumeikan University) for soft X-ray XAFS experiments. Mass spectral data were acquired at the mass spectrometry facility run by Molecular Structure Characterization Unit (RIKEN CSRS, Wako). We gratefully acknowledge financial support from the JST ACT-C (#JPMJCR12ZC), the JST ACCEL (#JPMJAC1401), the JSPS (#24550126, #20655035, and #15K05510), AMED (#19ak0101115h), the Takeda Science Foundation, the Naito Foundation, and RIKEN.
Author contributions
Y.M.A.Y. and Y.U. conceived the work and designed the experiments. H.B. carried out the catalytic reactions. A.N. conducted the XPS measurements. T.S. prepared the silicon Pd devices for XPS measurements and the spectroscopic measurements. All the authors contributed to intellectual insights of this work. Y.M.A.Y., H.B., and T.S. wrote the paper, and all authors edited the paper.
Data availability
All data supporting the findings of this study are available within the paper as well as the Supplementary Information file, or available from the corresponding authors on reasonable request. The X-ray crystallographic coordinates for structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC1939498 and CCDC1939526. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s42004-020-0332-z.
References
- 1.Bonrath, W., Medlock, J., Schutz, J., Wustenberg, B. & Netscher, T. in Hydrogenation (ed Karam, I.) (InTech, Rijeka, Croatia, 2012).
- 2.Rylander, P. N. Hydrogenation and Dehydrogenation, Ulmanns’s Encyclopedia of Industrial Chemistry (Wiley-VCH, Weinheim, 2005).
- 3.Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (Wiley, New York, 2001).
- 4.Rylander, P. N. Hydrogenation Methods (Academic Pres, New York, 1985).
- 5.Liu H, Jiang T, Han B, Liang S, Zhou Y. Selective phenol hydrogenation to cyclohexanone over a dual supported Pd-lewis acid catalyst. Science. 2009;326:1250–1252. doi: 10.1126/science.1179713. [DOI] [PubMed] [Google Scholar]
- 6.Mastalir Á, Király Z, Berger F. Comparative study of size-quantized Pd-montmorillonite catalysts in liquid-phase semihydrogenations of alkynes. Appl. Catal. A. 2004;269:161–168. doi: 10.1016/j.apcata.2004.04.012. [DOI] [Google Scholar]
- 7.Papp A, Molnár Á, Mastalir Á. Catalytic investigation of Pd particles supported on MCM-41 for the selective hydrogenations of terminal and internal alkynes. Appl. Catal. A. 2005;289:256–266. doi: 10.1016/j.apcata.2005.05.007. [DOI] [Google Scholar]
- 8.Webb JD, MacQuarrie S, McEleney K, Crudden CM. Mesoporous silica-supported Pd catalysts: an investigation into structure, activity, leaching and heterogeneity. J. Catal. 2007;252:97–109. doi: 10.1016/j.jcat.2007.09.007. [DOI] [Google Scholar]
- 9.Piccolo L, et al. Tuning the shape of nanoparticles to control their catalytic properties: selective hydrogenation of 1,3-butadiene on Pd/Al2O3. Phys. Chem. Chem. Phys. 2008;10:5504–5506. doi: 10.1039/b809651a. [DOI] [PubMed] [Google Scholar]
- 10.Domínguez-Domínguez S, Berenguer-Murcia Á, Linares-Solano Á, Cazorla-Amorós D. Inorganic materials as supports for palladium nanoparticles: application in the semi-hydrogenation of phenylacetylene. J. Catal. 2008;257:87–95. doi: 10.1016/j.jcat.2008.04.008. [DOI] [Google Scholar]
- 11.Cai G, et al. Synthesis of a highly stable Pd@CeO2 catalyst for methane combustion with the synergistic effect of urea and citric acid. ACS Omega. 2018;3:16769–16776. doi: 10.1021/acsomega.8b02556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senftle TP, van Duin ACT, Janik MJ. Role of site stability in methane activation on PdxCe1-xOδ surfaces. ACS Catal. 2015;5:6187–6199. doi: 10.1021/acscatal.5b00741. [DOI] [Google Scholar]
- 13.Liu X, et al. Catalytic partial oxidation of cyclohexane by bimetallic Ag/Pd nanoparticles on magnesium oxide. Chem. Eur. J. 2017;23:11834–11842. doi: 10.1002/chem.201605941. [DOI] [PubMed] [Google Scholar]
- 14.Augustine, R. L. Catalytic Hydrogenation (Marcel Dekker, New York, 1965).
- 15.Thomas JM, Johnson BFG, Raja R, Sankar G, Midgley PA. High-performance nanocatalysts for single-step hydrogenations. Acc. Chem. Res. 2003;36:20–30. doi: 10.1021/ar990017q. [DOI] [PubMed] [Google Scholar]
- 16.Das DD, Sayari A. Applications of pore-expanded mesoporous silica 6. novel synthesis of monodispersed supported palladium nanoparticles and their activity for Suzuki reaction. J. Catal. 2007;246:60–65. doi: 10.1016/j.jcat.2006.11.020. [DOI] [Google Scholar]
- 17.Ren N, Yang Y-H, Zhang Y-H, Wang Q-R, Tang Y. Heck coupling in zeolite microcapsular reactor: a test for encaged quasi-homogeneous catalysis. J. Catal. 2007;246:215–222. doi: 10.1016/j.jcat.2006.11.028. [DOI] [Google Scholar]
- 18.Karakanov EA, Zolotukhina AV, Ivanov AO, Maximov AL. Dendrimer-encapsulated Pd nanoparticles, immobilized in silica pores, as catalysts for selective hydrogenation of unsaturated compounds. Chem. Open. 2019;8:358–381. doi: 10.1002/open.201800280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z, et al. Pd nanoparticles confined in the porous graphene-like carbon nanosheets for olefin hydrogenation. Langmuir. 2018;34:12809–12814. doi: 10.1021/acs.langmuir.8b02785. [DOI] [PubMed] [Google Scholar]
- 20.Budroni G, Corma., Garcia H, Primo A. Pd nanoparticles embedded in sponge-like porous silica as a Suzuki-Miyaura catalyst: similarities and differences with homogeneous catalysts. J. Catal. 2007;251:345–353. doi: 10.1016/j.jcat.2007.07.027. [DOI] [Google Scholar]
- 21.Polshettiwar V, Molnár Á. Silica-supported Pd catalysts for Heck coupling reactions. Tetrahedron. 2007;63:6949–6976. doi: 10.1016/j.tet.2007.04.023. [DOI] [Google Scholar]
- 22.Wisher AC, Bronstein I, Chechik V. Thiolated PAMAM dendrimer-coated CdSe/ZnSe nanoparticles as protein transfection agents. Chem. Commun. 2006;15:1637–1639. doi: 10.1039/b518115a. [DOI] [PubMed] [Google Scholar]
- 23.Niu Y, Yeung LK, Crooks RM. Size-selective hydrogenation of olefins by dendrimer-encapsulated palladium nanoparticles. J. Am. Chem. Soc. 2001;123:6840–6846. doi: 10.1021/ja0105257. [DOI] [Google Scholar]
- 24.Gopidas KR, Whitesell JK, Fox MA. Synthesis, characterization, and catalytic application of a palladium-nanoparticle-cored dendrimer. Nano Lett. 2003;3:1757–1760. doi: 10.1021/nl0348490. [DOI] [Google Scholar]
- 25.Tang Z. Core-shell palladium nanoparticle@metal-organic frameworks as multifunctional catalysts for cascade reactions. J. Am. Chem. Soc. 2014;136:1738–1741. doi: 10.1021/ja411468e. [DOI] [PubMed] [Google Scholar]
- 26.Yamada YMA. Development of batch and flow immobilized catalytic systems with high catalytic activity and reusability. Chem. Pharm. Bull. 2017;65:805–821. doi: 10.1248/cpb.c17-00349. [DOI] [PubMed] [Google Scholar]
- 27.Hu H, et al. Self-assembled polymeric pyridine copper catalysts for Huisgen cycloaddition with alkynes and acetylene gas: application in synthesis of tazobactam. Org. Proc. Res. Dev. 2019;23:493–498. doi: 10.1021/acs.oprd.8b00429. [DOI] [Google Scholar]
- 28.Hudson R, et al. Poly(meta-phenylene oxides) for the design of a tunable, efficient, and reusable catalytic platform. Chem. Commun. 2018;54:2878–2881. doi: 10.1039/C8CC00774H. [DOI] [PubMed] [Google Scholar]
- 29.Yamada YMA, Sarkar SM, Uozumi Y. Amphiphilic self-assembled polymeric copper catalyst to parts per million levels: click chemistry. J. Am. Chem. Soc. 2012;134:9285–9290. doi: 10.1021/ja3036543. [DOI] [PubMed] [Google Scholar]
- 30.Yamada YMA, Sarkar SM, Uozumi Y. Self-assembled poly(imidazole-palladium): highly active, reusable catalyst at parts per million to parts per billion levels. J. Am. Chem. Soc. 2012;134:3190–3198. doi: 10.1021/ja210772v. [DOI] [PubMed] [Google Scholar]
- 31.Sarkar SM, Uozumi Y, Yamada YMA. A highly active and reusable self-assembled poly(imidazole/palladium) catalyst: allylic arylation/alkenylation. Angew. Chem. Int. Ed. 2011;50:9437–9441. doi: 10.1002/anie.201103799. [DOI] [PubMed] [Google Scholar]
- 32.Chng LL, Erathodiyil N, Ying JY. Nanostructure catalysts for organic transformations. Acc. Chem. Res. 2013;46:1825–1837. doi: 10.1021/ar300197s. [DOI] [PubMed] [Google Scholar]
- 33.Yamada YMA, et al. A palladium-nanoparticle and silicon-nanowire-array hybrid: a platform for catalytic heterogeneous reactions. Angew. Chem. Int. Ed. 2014;53:127–131. doi: 10.1002/anie.201308541. [DOI] [PubMed] [Google Scholar]
- 34.Baek H, et al. Production of bio hydrofined diesel, jet fuel, and carbon monoxide from fatty acids using a silicon nanowire array-supported rhodium nanoparticle catalyst under microwave conditions. ACS Catal. 2020;10:2148–2156. doi: 10.1021/acscatal.9b04784. [DOI] [Google Scholar]
- 35.Casiello M, et al. Catalytic activity of silicon nanowires decorated with gold and copper nanoparticles deposited by pulsed lase ablation. Nanomaterials. 2018;8:78–95. doi: 10.3390/nano8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun X–H, et al. Reductive self-assembling of Pd and Rh nanoparticles on silicon nanowire templates. Chem. Mater. 2004;16:1143–1152. doi: 10.1021/cm035112e. [DOI] [Google Scholar]
- 37.Schmidt V, Wittemann JV, Gösele U. Growth, thermodynamics, and electrical properties of silicon nanowires. Chem. Rev. 2010;110:361–388. doi: 10.1021/cr900141g. [DOI] [PubMed] [Google Scholar]
- 38.Niwano M, Miura T, Kimura Y, Tajima R, Miyamoto N. Real-time, in situ infrared study of etching of Si (100) and (111) surfaces in dilute hydrofluoric acid solution. J. Appl. Phys. 1996;79:3708–3713. doi: 10.1063/1.361203. [DOI] [Google Scholar]
- 39.Niwano M, Takeda Y, Ishibashi Y, Kurita K, Miyamoto N. Morphology of hydrofluoric acid and ammonium fluoride-treated silicon surfaces studied by surface infrared spectroscopy. J. Appl. Phys. 1992;71:5646–5649. doi: 10.1063/1.350497. [DOI] [Google Scholar]
- 40.Trucks GW, Raghavachari K, Higashi GS, Chabal YJ. Mechanism of HF etching of silicon surfaces: a theoretical understanding of hydrogen passivation. Phys. Rev. Lett. 1990;65:504–507. doi: 10.1103/PhysRevLett.65.504. [DOI] [PubMed] [Google Scholar]
- 41.Zhang M-L, et al. Preparation of large-area uniform silicon nanowires arrays through metal-assisted chemical etching. J. Phys. Chem. C. 2008;112:4444–4450. doi: 10.1021/jp077053o. [DOI] [Google Scholar]
- 42.Yang S, et al. Nanoscale magnetic stirring bars for heterogeneous catalysis in microscopic systems. Angew. Chem. Int. Ed. 2015;54:2661–2664. doi: 10.1002/anie.201410360. [DOI] [PubMed] [Google Scholar]
- 43.Wei F, et al. In situ facile loading of noble metal nanoparticles on polydopamine nanospheres via galvanic replacement reaction for multifunctional catalysis. Sci. China Chem. 2017;60:1236–1242. doi: 10.1007/s11426-017-9042-x. [DOI] [Google Scholar]
- 44.Besson M, Gallezot P, Pinel C. Conversion of biomass into chemicals over metal catalysts. Chem. Rev. 2014;114:1827–1870. doi: 10.1021/cr4002269. [DOI] [PubMed] [Google Scholar]
- 45.Pews-Davtyan A, et al. Biomolecule-derived supported cobalt nanoparticles for hydrogenation of industrial olefins, natural oils and more in water. Green. Chem. 2019;21:5104–5112. doi: 10.1039/C9GC01276A. [DOI] [Google Scholar]
- 46.McArdle S, Girish S, Leahy JJ, Curtin T. Selective hydrogenation of sunflower oil over noble metal catalysts. J. Mol. Catal. A: Chem. 2011;351:179–187. doi: 10.1016/j.molcata.2011.10.004. [DOI] [Google Scholar]
- 47.Mäki-Arvela P. atalytic hydrogenation of linoleic acid to stearic acid over different Pd- and Ru-supported catalysts. Appl. Catal., A: Gen. 2008;345:201–212. doi: 10.1016/j.apcata.2008.04.042. [DOI] [Google Scholar]
- 48.Roach C, et al. Comparison of cis and trans fatty acid containing phosphatidylcholines on membrane properties. Biochemistry. 2004;43:6344–6351. doi: 10.1021/bi049917r. [DOI] [PubMed] [Google Scholar]
- 49.Emken EA. Nutrition and biochemistry of trans and positional fatty acid isomers in hydrogenated oil. Rev. Nutr. 1984;4:339–376. doi: 10.1146/annurev.nu.04.070184.002011. [DOI] [PubMed] [Google Scholar]
- 50.Mozaffarian D, et al. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006;354:1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 51.Hofmann S. Compositional depth profiling by sputtering. Prog. Surf. Sci. 1991;36:35–87. doi: 10.1016/0079-6816(91)90013-T. [DOI] [Google Scholar]
- 52.Chen L-Y, Hunter GW, Neudeck PG, Knight D. X-ray photoelectron spectroscopy study of the heating effects on Pd/6H-SiC Schottky structure. J. Vac. Sci. Technol., A. 1998;16:2890–2895. doi: 10.1116/1.581436. [DOI] [Google Scholar]
- 53.Lidiya, L. S., Stadnichenko, A. I., Koscheev, S. V., Zaikovskii, V. I. & Boronin, A. I. Highly oxidized palladium nanoparticles comprising Pd4+ Species: spectroscopic and structural aspects, thermal stability, and reactivity J. Phys. Chem. C116, 19342–19348 (2012).
- 54.Shaplugin IS, Aparnikov GL, Lazarev VB. Preparation of palladium dioxide at high pressure. Zh. Neorg. Khim. 1978;23:884–887. [Google Scholar]
- 55.Kibis LS, Titkov AI, Stadnichenko AI, Koscheev SV, Boronin AI. X-ray photoelectron spectroscopy study of Pd oxidation by RF discharge in oxygen. Appl. Surf. Sci. 2009;255:9248–9254. doi: 10.1016/j.apsusc.2009.07.011. [DOI] [Google Scholar]
- 56.Grunthaner PJ, Grunthaner FJ, Madhukar A, Mayer JW. metal/silicon interface formation: the Ni/Si and Pd/Si systems. J. Vac. Sci. Technol. 1981;19:649–656. doi: 10.1116/1.571079. [DOI] [Google Scholar]
- 57.Atzrodt V, Wirth TH, Lange H. Investigation of NiSi and Pd3Si thin films by AES and XPS. Phys. Status Solidi (a) 1980;62:531–537. doi: 10.1002/pssa.2210620222. [DOI] [Google Scholar]
- 58.Wagner CD, Taylor JA. Generation of XPS auger lines by bremsstrahlung. J. Electron Spectrosc. Relat. Phenom. 1980;20:83–93. doi: 10.1016/0368-2048(80)85008-0. [DOI] [Google Scholar]
- 59.Chen JG. NEXAFS investigations of transition metal oxides, nitrides, carbides, sulfides and other interstitial compounds. Surf. Sci. Rep. 1997;30:1–152. doi: 10.1016/S0167-5729(97)00011-3. [DOI] [Google Scholar]
- 60.Mason MG. Electronic structure of supported small metal clusters. Phys. Rev. B. 1983;27:748–762. doi: 10.1103/PhysRevB.27.748. [DOI] [Google Scholar]
- 61.Kubota T, Kitajima Y, Asakura K, Iwasawa Y. Pd L3-edge XANES spectra of supported Pd particles induced by the adsorption and the absorption of hydrogen. Bull. Chem. Soc. Jpn. 1999;72:673–681. doi: 10.1246/bcsj.72.673. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data supporting the findings of this study are available within the paper as well as the Supplementary Information file, or available from the corresponding authors on reasonable request. The X-ray crystallographic coordinates for structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC1939498 and CCDC1939526. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.