Abstract
Background
Immune-related adverse events (irAEs) are a common phenomenon in cancer patients treated with immune checkpoint inhibitors (ICIs). Surprisingly, the toxicity burdens of these irAEs have not been illustrated clearly. In this study, we analyzed irAEs for seven FDA-approved ICIs in cancer treatment to show the pattern of toxicity burden among cancer patients.
Methods
irAEs associated with seven FDA-approved ICIs, including three PD-1 inhibitors (cemiplimab, nivolumab and pembrolizumab), three PD-L1 inhibitors (atezolizumab, avelumab and durvalumab), and one CTLA-4 inhibitor (ipilimumab), were analyzed based on data from 149,303 reported cases (from January 1, 2015 to June 30, 2022) collected from the FDA Adverse Events Reporting System (FAERS) public dashboard. Proportions of serious irAEs and correlations with tumor type, age and sex were assessed via R package and GraphPad software.
Results
irAEs related to anti-PD-1 ICIs required less hospital care resources compared with anti-PD-L1 and anti-CTLA-4 ICIs. Patients treated with pembrolizumab had relatively fewer serious cases. Treatment with ICIs led to the highest probability of serious irAEs in patients with lung cancer. ‘Respiratory, thoracic and mediastinal disorders’ and ‘gastrointestinal disorders’ were the two most common groups of disorders caused by the seven ICIs studied. ‘Cardiac disorders’ was the main type of disorders caused by these ICIs in cancer patients aged 65–85, while ‘reproductive system and breast disease’ was the main type of disorder in cancer patients aged 18–64. ‘Respiratory, thoracic, mediastinal diseases’ and ‘reproductive system and breast diseases’ were the main types of disorders associated with treatment with these ICIs in male and female patients, respectively.
Conclusion
Tissue and organ toxicities of ICIs are age and sex specific. There are risks of respiratory and urinary system toxicity in male patients and reproductive system toxicity in female patients treated with the ICIs studied. Future studies on the toxicity burden of ICIs should incorporate age and sex differences to better understand the relevance of ICI toxicity burden to human immune function to develop appropriate tumor immune and therapeutic intervention strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13046-022-02568-y.
Keywords: immune checkpoint inhibitors, Immunotherapy, Adverse events, Toxicity burden, Therapeutic intervention
Background
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment landscape for multiple cancers, demonstrating effective and durable responses and becoming the standard of care for a variety of malignancies [1]. The Food and Drug Administration (FDA) has approved various ICIs for cancer therapy [2], including programmed death-1 (PD-1) receptor inhibitors (e.g., cemiplimab, nivolumab and pembrolizumab), programmed death ligand-1 (PD-L1) inhibitors (e.g., atezolizumab, avelumab and durvalumab), and cytotoxic T lymphocyte-associated antigen (CTLA-4) inhibitors (ipilimumab) [3–5]. Although these ICIs improve patient outcomes in various clinical settings, they pose concomitant risks of immune-related adverse events (irAEs) [6]. These irAEs are unique, delayed, and long-lasting, and can involve any tissue or organ system [7]. Given the increasing use of ICIs, there has been an increase in the burden of both clinical and financial toxicity. More common irAEs include skin toxicities, colitis, hepatitis, pneumonitis, nephritis, and endocrinopathies (i.e. thyroid abnormalities) [8]. Rare irAEs can have unique clinical presentations that can pose a serious issue if not identified promptly, such as encephalitis, myocarditis, and hematologic toxicities (i.e. hemolytic uremic syndrome). From a financial toxicity standpoint, patients, family members, healthcare systems, and insurance companies experience the significant economic weight of ICI treatment and irAEs [9]. Factors such as increased utilization of ICIs due to their expanding FDA approvals in several cancers, high drug expenses with high out-of-pocket expenditures, and costs associated with managing irAEs (e.g., hospitalizations and the use of biologic agents) all contribute to the exponentially increasing cost of cancer care with ICIs. As ICI prevalence continues to increase with immunotherapy being introduced into earlier stages of disease (neoadjuvant and adjuvant settings) and more combinations with ICIs being developed, clinical and financial toxicity will only become more problematic.
irAEs can range widely in severity from mild (grade 1) to life-threatening (grade 4) [2, 6, 7]. By targeting immune checkpoints, ICI-associated irAEs, characterized by T-cell infiltration to a number of organ systems, can occur [6, 10, 11]. The physical burden of irAEs is significant, as they can lead to hospitalizations, long term use of high dose steroids which have several AEs (e.g., hyperglycemia, increased risk for infections and bone loss/osteoporosis), or even permanent discontinuation of ICIs. In solid tumor patients, the incidence of any-grade irAEs in trials is 66% with PD-1/PD-L1 inhibitor monotherapy and 72% with ipilimumab monotherapy [4, 12]. Combined PD-1 and CTLA-4 blockade results in considerably higher rates of irAEs in comparison to anti-PD-1 alone (55%-60% vs 10%-20% high-grade events) [5, 13–15]. A retrospective meta-analysis conducted by Wang et al. reported immunotherapy toxicity-related fatality rates of 0.36% with anti-PD-1, 0.38% with anti-PD-L1, 1.08% with anti-CTLA-4, and 1.23% with combined anti-PD-1/anti-PD-L1 and anti-CTLA-4 [12]. The type of fatal irAEs varied depending on the regimen; the most common fatal irAE with anti-CTLA-4 treatment was colitis (70%), whereas the most common fatal irAEs with anti-PD-1/anti-PD-L1 treatment were pneumonitis (35%), hepatitis (22%), and neurotoxicity (15%). For combined anti-PD-1/anti-PD-L1 and anti-CTLA-4 treatment, the most common fatal irAEs were colitis (37%) and myocarditis (25%) [12]. Furthermore, ICIs have the potential to trigger immune-related endocrine diseases in tumor patients, such as thyroid and pituitary dysfunction, and these complications are relatively more frequent than expected (e.g., 11.8% in anti-PD-1 treatment, 13.4% in anti-PD-L1 treatment, 5% in anti-CTLA4 treatment, and 18.5% in sequential and/or combination treatment) [16–19].
Interrogating irAEs in cancer patients remains to be evaluated systemically. In the present study, we estimated the toxicity burden of seven FDA-approved ICIs by analyzing irAEs among treated patients in the United States. The proportions of total and serious irAEs for each ICI and the proportion of serious irAEs for each ICI by cancer type were calculated. Correlations between tissue or organ disease and patient demographics were also calculated for each ICI. Our comprehensive assessment of irAEs according to the latest data reported by the FDA summarizes the major types of risk factors correlated with irAEs, providing a reference for clinicians to predict the occurrence of irAEs resulting in a timely process in clinical practice.
Methods
Cases of irAEs associated with ICI treatment
All cases for this study were obtained from the FDA Adverse Events Reporting System (FAERS) public dashboard (https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard), and a total of 149,303 cases from January 1, 2015 to June 30, 2022 were analyzed for seven FDA-approved ICIs, including three PD-1 inhibitors (cemiplimab: 492 cases, nivolumab: 60,469 cases, and pembrolizumab: 34,962 cases), three PD-L1 inhibitors (atezolizumab: 16,117 cases, avelumab: 2,136 cases, and durvalumab: 6,974 cases), and one CTLA-4 inhibitor (ipilimumab: 28,153 cases). Serious AEs (cemiplimab: 472 cases; nivolumab: 55,027 cases; pembrolizumab: 29,379 cases; atezolizumab: 15,309 cases; avelumab: 1,898 cases; durvalumab: 6,486 cases; and ipilimumab: 24,675 cases) and deaths associated with these ICIs were downloaded and counted.
Proportional reporting ratio (PRR) analysis
A PRR was used to analyze the irAEs via the Chi-squared test, and the odds ratio (OR) was calculated for irAEs associated with each drug using retrospective case–control studies. First, all cases were divided into seven outcome groups (died, disabled, hospitalized, life-threatening, non-serious, required intervention, and other outcomes) depending on the MedDRA dictionary Preferred Term (PT) and the percentage of cases in each group was counted using R software. The ratios of total serious cases and deaths were calculated for each ICI. Then we focused on irAEs for each drug in each individual cancer type and counted the value for each tumor type via R software, including bladder cancer, breast cancer, colorectal cancer, endometrial cancer, glioma, head and neck cancer (HNC), hepatocellular carcinoma, lung cancer, melanoma, ovarian cancer, prostate cancer, renal cancer, and thyroid cancer. To assess the tumor-type-specificity of irAEs, correlations between irAEs and age (excluding 0–3 years old) and sex were evaluated for each ICI using R software in 18 tissues or organs (reproductive system and breast, cardiac, musculoskeletal and connective tissue, ear and urinary, respiratory, thoracic and mediastinal, renal and urinary, endocrine, skin and subcutaneous tissue, blood and lymphatic system, psychiatric, immune system, eye, hepatobiliary, gastrointestinal, nervous system, vascular, metabolism and nutrition, and neoplasms benign, malignant and unspecified). All ‘tissue or organ disorders’, as the one of the ‘reaction groups’, were defined by the Medical Dictionary for Regulatory Activities (MedDRA) (https://fis.fda.gov/sense).
Tissue-specific gene expression analysis
To attempt to evaluate irAEs with tumor-type specificity at the gene expression level, the expression levels of PD-1, PD-L1, and CTLA-4 in various tissues or organs (such as brain, heart, lung and skin) from male and female patients were analyzed in the GTEx portal (https://gtexportal.org/home/gene/). Gene expression levels were normalized using log10 (TMP + 1).
Statistics
Chi-squared test and case–control studies were used to evaluate irAEs associated with the seven drugs. An OR value > 1 indicated that the drug is a positive factor for irAEs, while OR value < 1 indicated the drug is a negative factor. P < 0.05 represented a statistically significant difference. Data statistics and correlation analysis were performed using the GraphPad prism 9 and R packages (Rmisc, corrplot, ggcorrplot, grDevices and vegan).
Results
irAEs related to pembrolizumab require less hospital care resources and are associated with relatively fewer serious cases
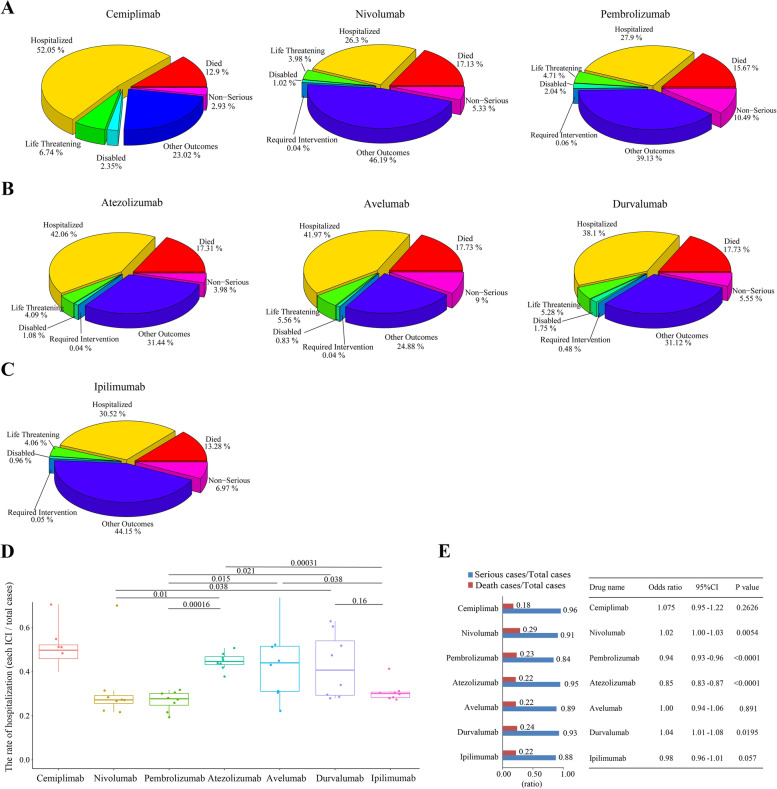
Referring to the FDA dataset, all annual irAE cases for each drug were divided into the 7 outcome groups separately (died, disabled, hospitalized, life-threatening, non-serious, required intervention, and other outcomes) (Fig. 1). Here, we calculated the proportion of each outcome group for each individual ICI per year (Supplementary Figure S1). To assess the weight of the individual groups for each ICI, we calculated the average probability of each group for each single drug (Fig. 2A-C) and found for all drugs that hospitalization was the most common outcome (except for the “other outcomes” group). The mean rate of hospitalization varied widely among patients treated with PD-1 inhibitors and experiencing irAEs: for instance, the average hospitalization rate for cemiplimab patients was 52.05% (from 48.42% to 70.59%) and was the highest among all irAE cases associated with the seven ICIs, while that for nivolumab was the lowest at 26.3% (21.66%—31.47%) and for pembrolizumab was the second lowest at 27.9% (21.6%—31.77%). The mean hospitalization proportion for the other four ICIs ranged 30% to 45%. The proportion of hospitalizations due to irAEs was significantly lower among patients treated with anti-PD-1 ICIs than with anti-PD-L1 or anti-CTLA4 ICIs (P < 0.05) (Fig. 2D). Therefore, irAEs related to nivolumab and pembrolizumab require less hospital care resources.
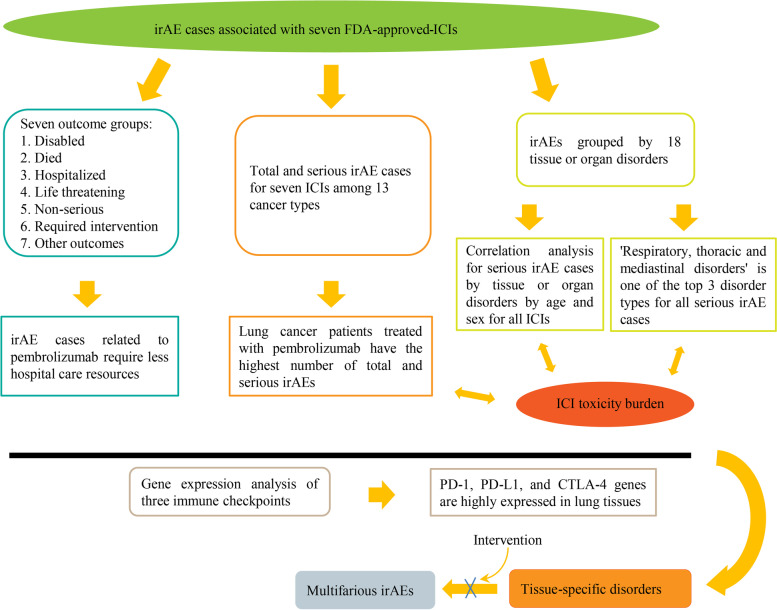
Fig. 1.
Flow chart of irAE studies for seven FDA-approved ICIs: ICI toxicity burden causing tissue or organ disorders may exacerbate ICI-associated irAEs
Fig. 2.
Distribution of irAE cases among patients treated with seven FDA-approved ICIs according to the FDA dataset in the past seven years. A-C irAE cases for each ICI were divided into seven outcome groups, including died, disabled, hospitalized, life-threatening, non-serious, required intervention and other outcomes. The total percentage for each outcome group is indicated for ICIs targeting PD1, PDL1 and CTLA4 in (A), (B) and (C), respectively. D Comparison of the rate of hospitalization among patients treated with each ICI. E Proportions of serious irAEs or deaths for each FDA-approved ICI in the FDA dataset (left) and summary of statistical analysis of odds ratio (OR) for seven FDA-approved ICIs (right)
To accurately evaluate the severity of irAEs, the proportion of serious irAEs (serious irAEs cases / total irAEs cases) and deaths (deaths / total irAEs cases) was calculated for each drug. All drugs were associated with a high proportion of serious irAEs (cemiplimab, 0.96; nivolumab, 0.91; pembrolizumab, 0.84; atezolizumab, 0.95; avelumab, 0.89; durvalumab, 0.93; and ipilimumab, 0.88), with pembrolizumab having the lowest proportion of serious irAE cases among all seven drugs (Fig. 2E). Next, we calculated the odds ratio value for these seven drugs, and found the OR values for pembrolizumab, atezolizumab and ipilimumab were 0.94 (P < 0.0001), 0.85 (P < 0.0001) and 0.98 (p = 0.57), respectively, while the OR for avelumab was 1 and other three drugs had ORs > 1. However, we noticed that the OR for atezolizumab was less than 1 but its irAEs ratio was high (0.95), and the OR for ipilimumab was 0.98 (< 1) but was not significant (P = 0.057 > 0.05).
Serious irAEs are most common among patients with lung cancer
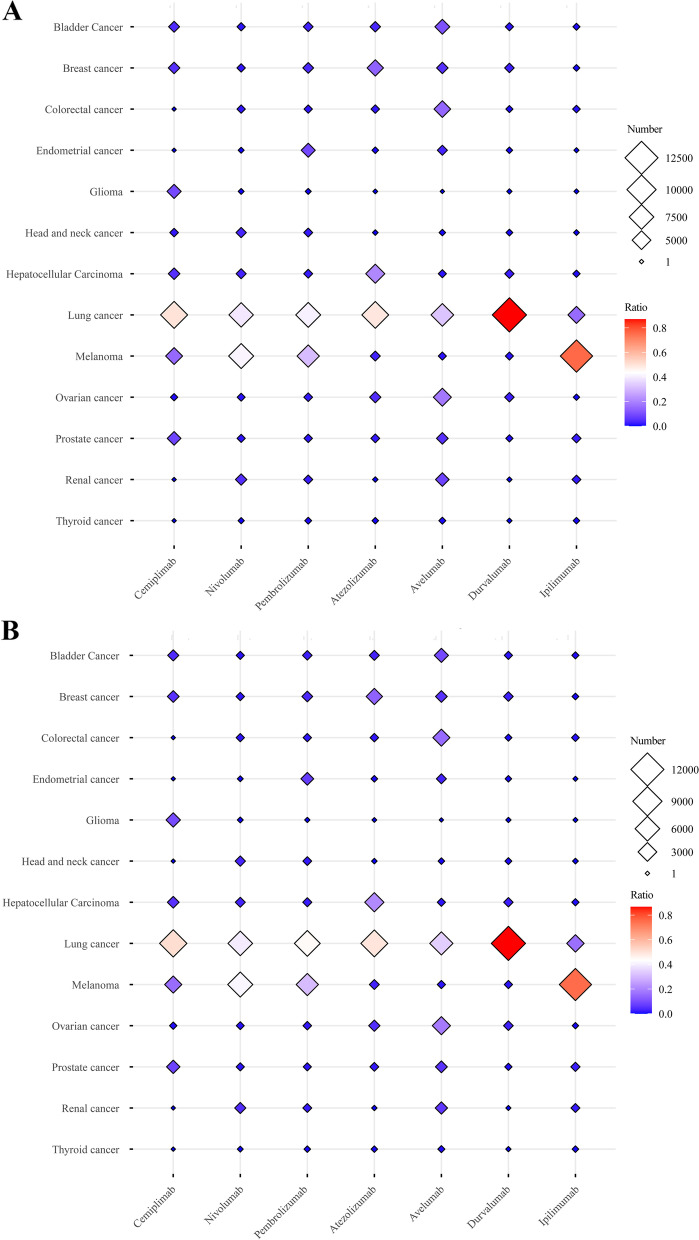
To evaluate which cancer type was most prone to irAEs with the seven drugs, 13 main cancer types were selected. First, the numbers of total and serious irAEs for each drug were calculated for each cancer type (Table 1 and Fig. 3A and B). For cemiplimab, the numbers of total irAEs (59 cases) and serious irAEs (58 cases) were higher in lung cancer patients than in the other 12 cancers, and the proportion of serious irAEs was 50% among all 13 cancer types (Supplementary Figure S2A). For nivolumab, melanoma (11,602 cases) and lung cancer (11,052 cases) patients were most prone to serious irAEs, with an incidence of 41% and 31%, respectively. Similarly, total and serious irAEs were most common among lung cancer (4,399 cases) and melanoma (6,335 cases) patients treated with pembrolizumab, at 41% and 39%, respectively. For all three PD-L1 inhibitors, the numbers of total and serious irAEs were higher among patients with lung cancer than the other 12 cancers: atezolizumab (49%), avelumab (33%) and durvalumab (87%) (Supplementary Figure S2B). From Fig. 3 and S2C (yellow), we also found that serious irAEs were most common among melanoma (76%) and lung cancer (16%) patients treated with ipilimumab among all 13 cancer types. In summary, patients are most likely to develop serious irAEs with the seven ICIs studied when treated for lung cancer.
Table 1.
Total irAE cases and serious irAE cases for FDA-approved ICIs among 13 common tumor types
| Bladder cancer | Breast cancer | Colorectal cancer | Endometrial cancer | Glioma | Head and neck cancer | Hepatocellular carcinoma | Lung cancer | Melanoma | Ovarian cancer | Prostate cancer | Renal cancer | Thyroid cancer | Total number | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number /ratio | Number /ratio | Number /ratio | Number /ratio | Number /ratio | Number /ratio | Number /ratio | Number ratio | Number /ratio | Number /ratio | Number /ratio | Number /ratio | Number /ratio | |||
| Cemiplimab | Total irAE cases | 5/ 0.0424 | 6/ 0.0508 | 0/ 0.0000 | 0/ 0.0000 | 11/ 0.0932 | 2/ 0.0169 | 6/ 0.0508 | 59/ 0.5000 | 18/ 0.1525 | 1/ 0.0084 | 10/ 0.0847 | 0/ 0.0000 | 0/ 0.0000 | 118 |
| Serious irAE cases | 5/ 0.0438 | 6/ 0.0526 | 0/ 0.0000 | 0/0.0000 | 11/ 0.0964 | 0/ 0.0000 | 6/ 0.0526 | 58/ 0.5087 | 18/ 0.1578 | 1/ 0.0087 | 9/ 0.0789 | 0/ 0.0000 | 0/ 0.0000 | 114 | |
| Nivolumab | Total irAE cases | 423/ 0.015 | 454/ 0.016 | 435/ 0.0153 | 72/ 0.0025 | 64/ 0.0023 | 966/ 0.0340 | 886/ 0.0312 | 11,130/0.3914 | 11,796/ 0.4149 | 356/ 0.0125 | 404/ 0.0142 | 1,351/ 0.0475 | 96/ 0.0034 | 28,433 |
| Serious irAE cases | 387/ 0.014 | 444/ 0.0159 | 413/ 0.0148 | 64/ 0.0023 | 62/ 0.0022 | 964/ 0.0346 | 845/0.0303 | 11,052/ 0.3967 | 11,602/ 0.4164 | 337/ 0.0121 | 391/ 0.0140 | 1237/ 0.0444 | 65/ 0.0023 | 27,863 | |
| Pembrolizumab | Total irAE cases | 467/ 0.0283 | 648/ 0.0392 | 250/ 0.0151 | 1,499/ 0.0908 | 42/ 0.0025 | 330/ 0.0200 | 330/ 0.0200 | 6,765/ 0.4101 | 5,154/ 0.3124 | 304/ 0.0184 | 259/ 0.0157 | 347/ 0.0210 | 101/ 0.0061 | 16,496 |
| Serious irAE cases | 367/ 0.0258 | 555/0.0390 | 220/ 0.0154 | 979/ 0.0688 | 12/ 0.0008 | 270/ 0.0190 | 304/ 0.0213 | 6,335/ 0.4454 | 4,399/ 0.3093 | 241/ 0.0169 | 195/ 0.0137 | 282/ 0.0198 | 64/ 0.0045 | 14,223 | |
| Atezolizumab | Total irAE cases | 365/ 0.0360 | 1,415/ 0.1396 | 167/ 0.0165 | 50/ 0.0049 | 2/ 0.0002 | 15/ 0.0015 | 2,123/ 0.2095 | 4,976/ 0.4910 | 306/ 0.0302 | 462/ 0.0456 | 195/ 0.0192 | 12/ 0.0012 | 45/ 0.0044 | 10,133 |
| Serious irAE cases | 301/ 0.0307 | 1,360/ 0.1388 | 163/ 0.0166 | 49/ 0.0050 | 2/ 0.0002 | 14/ 0.0014 | 2,095/ 0.2138 | 4,829/ 0.4928 | 289/ 0.0295 | 453/ 0.0462 | 189/ 0.0193 | 11/ 0.0011 | 43/ 0.0044 | 97,98 | |
| Avelumab | Total irAE cases | 49/ 0.1077 | 22/ 0.0490 | 66/ 0.1470 | 14/ 0.0311 | 0/ 0.0000 | 2/ 0.0045 | 6/ 0.0134 | 146/ 0.3251 | 6/ 0.0134 | 81/ 0.1804 | 23/ 0.0512 | 37/ 0.0824 | 3/ 0.0067 | 455 |
| Serious irAE cases | 40/ 0.0930 | 22/ 0.0519 | 66/ 0.1557 | 13/ 0.0307 | 0/ 0.0000 | 2/ 0.0047 | 6/ 0.0141 | 145/ 0.3420 | 6/ 0.0142 | 78/ 0.1839 | 23/ 0.0542 | 26/ 0.0613 | 3/ 0.0071 | 430 | |
| Durvalumab | Total irAE cases | 57/ 0.0145 | 102/ 0.0259 | 28/ 0.0071 | 17/ 0.0043 | 3/ 0.0008 | 23/ 0.0058 | 92/ 0.0234 | 3,427/ 0.8700 | 53/ 0.0135 | 100/ 0.0254 | 31/ 0.0079 | 3/ 0.0007 | 3/ 0.0008 | 3,939 |
| Serious irAE cases | 52/ 0.0139 | 99/ 0.0265 | 26/ 0.0070 | 17/ 0.0046 | 3/ 0.0008 | 23/ 0.0062 | 91/ 0.0243 | 3,235/ 0.8666 | 53/ 0.0142 | 99/ 0.0265 | 30/ 0.0080 | 2/ 0.0005 | 3/ 0.0008 | 3,733 | |
| Ipilimumab | Total irAE cases | 120/ 0.0070 | 90/ 0.0052 | 178/ 0.0105 | 10/ 0.0006 | 7/ 0.0004 | 33/ 0.0019 | 143/ 0.0084 | 2,660/ 0.1563 | 12,924/ 0.7593 | 66/ 0.0039 | 379/ 0.0222 | 333/ 0.0196 | 77/ 0.0045 | 17,020 |
| Serious irAE cases | 115/ 0.0071 | 87/ 0.0054 | 171/ 0.0106 | 8/ 0.0005 | 7/ 0.0004 | 31/ 0.0019 | 131/ 0.0081 | 2,645/ 0.1646 | 12,050/ 0.7497 | 63/ 0.0039 | 369/ 0.0230 | 321/ 0.0200 | 75/ 0.0047 | 16,073 | |
Fig. 3.
Total irAE cases and serious irAE cases for seven FDA-approved ICIs among various cancer types. A Total number of irAE cases for seven FDA-approved ICIs: patients with lung cancer ranked first or second for the highest number of total irAEs. B Number of serious irAEs for seven FDA-approved ICIs: patients with lung cancer also ranked first or second for serious irAEs
FDA-approved ICIs have greatest potential to induce ‘respiratory, thoracic and mediastinal disorders’ and ‘gastrointestinal disorders’
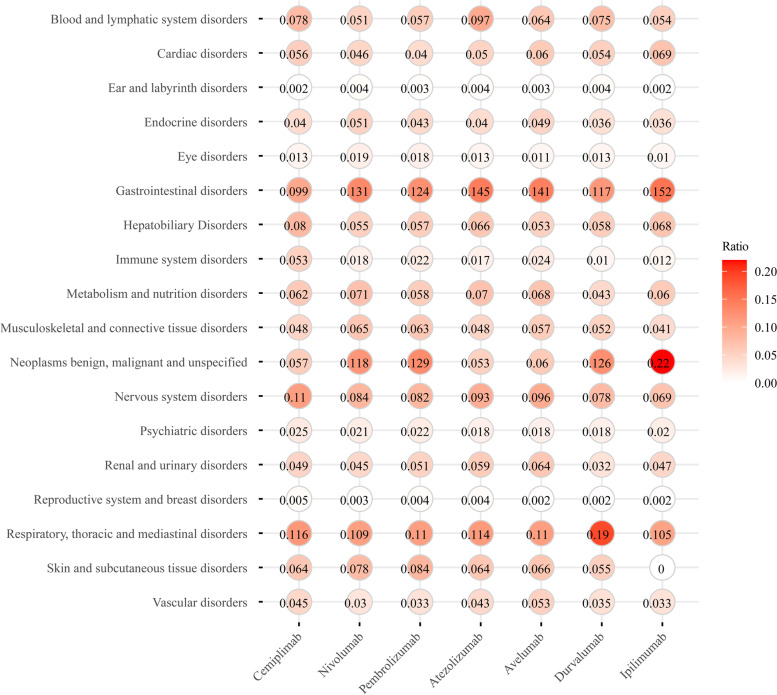
To understand why these ICIs lead to high rates of irAEs for specific cancer types, irAEs associated with each drug were grouped according to disorders of 18 tissues or organs (Table 2, Fig. 4 and Supplementary Figure S3). For the three PD-1 inhibitors, we found the three most common tissue or organ disorders among patients treated with cemiplimab were ‘respiratory, thoracic and mediastinal disorders’ (73 cases and 11.6% of the total), ‘nervous system disorders’ (69 cases, 10.99%) and ‘gastrointestinal disorders’ (62 cases, 9.87%) (Figure S3A); for nivolumab ‘gastrointestinal disorders’ (11,231 cases, 13.14%), ‘neoplasms benign, malignant and unspecified’ (10,113 cases, 11.83%) and ‘respiratory, thoracic and mediastinal disorders’ (9,317 cases, 10.90%); and for pembrolizumab ‘gastrointestinal disorders’ (6,764 cases, 12.92%), ‘neoplasms benign, malignant and unspecified’ (6,505 cases, 12.42%) and ‘respiratory, thoracic and mediastinal disorders’ (5,755 cases, 10.99%).
Table 2.
Number of irAEs grouped by 18 tissue or organ disorders for FDA-approved ICIs
| Cemiplimab | Nivolumab | Pembrolizumab | Atezolizumab | Avelumab | Durvalumab | Ipilimumab | |
|---|---|---|---|---|---|---|---|
| Number/ratio | Number/ ratio | Number/ ratio | Number/ ratio | Number/ratio | Number/ ratio | Number/ratio | |
| BL | 49/0.078 | 4,336/0.051 | 2,975/0.057 | 2,070/0.097 | 163/0.064 | 604/0.075 | 438/0.054 |
| CD | 35/0.056 | 3,947/0.046 | 2,117/0.040 | 1,074/0.050 | 151/0.060 | 435/0.054 | 561/0.069 |
| ELD | 1/0.002 | 382/0.004 | 177/0.003 | 93/0.004 | 7/0.003 | 29/0.004 | 18/0.002 |
| EnD | 25/0.040 | 4,346/0.051 | 2,234/0.043 | 848/0.040 | 124/0.049 | 289/0.036 | 289/0.036 |
| ED | 8/0.013 | 1,664/0.019 | 919/0.018 | 269/0.013 | 27/0.011 | 108/0.013 | 78/0.010 |
| GD | 62/0.099 | 11,231/0.131 | 6,505/0.124 | 3,093/0.145 | 357/0.141 | 939/0.117 | 1,233/0.152 |
| HD | 50/0.080 | 4,666/0.055 | 3,009/0.057 | 1,405/0.066 | 133/0.053 | 463/0.058 | 553/0.068 |
| ID | 33/0.053 | 1,539/0.018 | 1,151/0.022 | 366/0.017 | 60/0.024 | 84/0.010 | 98/0.012 |
| MnD | 39/0.062 | 6,095/0.071 | 3,012/0.058 | 1,494/0.070 | 173/0.068 | 342/0.043 | 490/0.060 |
| McD | 30/0.048 | 5,544/0.065 | 3,280/0.063 | 1,029/0.048 | 145/0.057 | 418/0.052 | 335/0.041 |
| NMU | 36/0.057 | 10,113/0.118 | 6,764/0.129 | 1,121/0.053 | 153/0.060 | 1,008/0.126 | 1,781/0.220 |
| NsD | 69/0.110 | 7,168/0.084 | 4,278/0.082 | 1,990/0.093 | 243/0.096 | 627/0.078 | 559/0.069 |
| PD | 16/0.025 | 1,836/0.021 | 1,136/0.022 | 381/0.018 | 45/0.018 | 148/0.018 | 159/0.020 |
| RuD | 31/0.49 | 3,827/0.045 | 2,661/0.051 | 1,261/0.059 | 162/0.064 | 259/0.032 | 382/0.047 |
| RB | 3/0.005 | 259/0.003 | 205/0.004 | 95/0.004 | 6/0.002 | 20/0.002 | 14/0.002 |
| RTM | 73/0.116 | 9,317/0.109 | 5,755/0.110 | 2,429/0.114 | 277/0.110 | 1,526/0.190 | 849/0.105 |
| SSD | 40/0.064 | 6,631/0.078 | 4,414/0.084 | 1,362/0.064 | 168/0.066 | 439/0.055 | 1/0.000 |
| VD | 28/0.045 | 2,550/0.030 | 1,743/0.033 | 918/0.043 | 135/0.053 | 283/0.035 | 271/0.033 |
| Total number cases | 628 | 85,451 | 52,335 | 21,298 | 2,529 | 8,021 | 8,109 |
Fig. 4.
Number of irAEs grouped by 18 tissue or organ disorders for FDA-approved ICIs. Nivolumab, pembrolizumab and atezolizumab had the highest case numbers of serious irAEs. “Respiratory, thoracic and mediastinal disorders” was consistently among the top 3 for each ICI
The three most common tissue or organ disorders after treatment with PD-L1 inhibitors were, for atezolizumab, ‘gastrointestinal disorders’ (3,093 cases, 14.52%), ‘respiratory, thoracic and mediastinal disorders’ (2429 cases, 11.40%), and ‘blood and lymphatic system disorders’ (2,070 cases, 9.72%) (Fig. 4 and Supplementary Figure S3B); for avelumab, ‘gastrointestinal disorders’ (357 cases, 14.12%), ‘respiratory, thoracic and mediastinal disorders’ (277 cases, 10.95%), and ‘nervous system disorders’ (243 cases, 9.61%); and for durvalumab, ‘respiratory, thoracic and mediastinal disorders’ (1,526 cases, 19.03%), ‘neoplasms benign, malignant and unspecified’ (1,008 cases, 12.57%) and ‘gastrointestinal disorders’ (939 cases, 11.71%).
For the CTLA-4 inhibitor ipilimumab, the three most common tissue or organ disorders were ‘neoplasms benign, malignant and unspecified’ (1,781 cases, 21.97%), ‘gastrointestinal disorders’ (1,233 cases, 15.21%) and ‘respiratory, thoracic and mediastinal disorders’ (849 cases, 10.47%) (Fig. 4 and Supplementary Figure S3C). In summary, ‘respiratory, thoracic and mediastinal disorders’ and ‘gastrointestinal disorders’ are the two major disorders caused by these seven ICIs.
Tissue or organ disorders caused by FDA-approved ICIs vary widely by age group
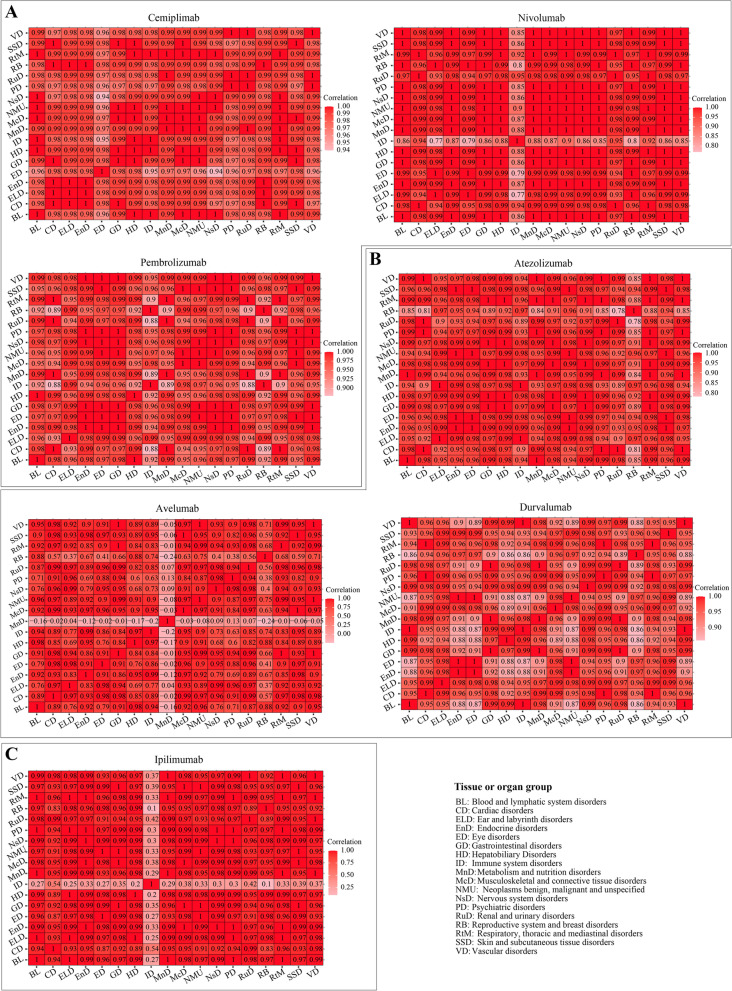
The proportions of tissue or organ disorders for these ICIs varied in different age groups (Supplementary Figure S3), shown in descending order of irAE rate in the 65–85 age group (left to right). We calculated the correlations between these tissue or organ disorders in patients treated with ICIs (Fig. 5 and Supplementary Table S1).
Fig. 5.
Correlation between irAEs and 18 tissue or organ disorders in different patient age groups. Correlations for ICIs targeting PD1, PDL1 and CTLA4 are shown in (A), (B) and (C), respectively
‘Nervous system disorders’ (17%) was the most common type of disorder caused by cemiplimab in patients with age 65–85 and was significantly correlated with ‘metabolism and nutrition disorders’ (Spearman value = 0.99, P = 0.004) and ‘psychiatric disorders’ (Spearman value = 0.99, P = 0.038). ‘Immune system disorders’ (15%) was the second most common disorder in patients aged 65–85 and was significantly correlated with ‘blood and lymphatic system disorders’ (Spearman value = 1, P < 0.0001), ‘endocrine disorders’ (Spearman value = 0.98, P = 0.02), ‘hepatobiliary disorders’ (Spearman value = 1, P = 0.002), and ‘musculoskeletal and connective tissue disorders’ (Spearman value = 0.99, P = 0.004). ‘Blood and lymphatic system disorders’ (14%) was the third most common disorder caused by cemiplimab in patients aged 65–85 and was significantly correlated with ‘endocrine disorders’ (Spearman value = 0.98, P = 0.009), ‘gastrointestinal disorders’ (Spearman value = 0.99, P = 0.001), ‘hepatobiliary disorders’ (Spearman value = 1, P = 0.004), ‘musculoskeletal and connective tissue disorders’ (Spearman value = 1, P < 0.0001) and ‘neoplasms benign, malignant and unspecified’ (Spearman value = 1, P = 0.027). The three most common disorders among patients aged 18–64 treated with cemiplimab were eye [(13%), and significantly correlated with ‘cardiac disorders’ (Spearman value = 0.98, P = 0.027)]; hepatobiliary [(10%), and significantly correlated with ‘blood and lymphatic system disorders’ (Spearman value = 1, P = 0.0004), ‘endocrine disorder’ (Spearman value = 0.99, P = 0.022), ‘immune system disorders’ (Spearman value = 1, P = 0.002) and ‘musculoskeletal and connective tissue disorders’ (Spearman value = 1, P = 0.014)]; and skin and subcutaneous tissue [(12%), and significantly correlated with ‘blood and lymphatic system disorders’ (Spearman value = 0.9, P = 0.012), ‘endocrine disorder’ (Spearman value = 0.99, P = 0.027), ‘gastrointestinal disorders’ (Spearman value = 0.99, P = 0.029), ‘immune system disorders’ (Spearman value = 0.96, P = 0.021), ‘musculoskeletal and connective tissue disorders’ (Spearman value = 1, P = 0.007) and ‘neoplasms benign, malignant and unspecified’ (Spearman value = 0.9, P = 0.003)].
For nivolumab and pembrolizumab, the three most common types of disorders among treated patients aged 65–85 were ‘cardiac disorders’, ‘renal and urinary disorders’ and ‘metabolism and nutrition disorders’, while ‘reproductive system and breast disorders’, ‘hepatobiliary disorders’ and ‘blood and lymphatic system disorders’ were the top 3 among patients aged 18–64; these were significantly correlated among these tissue or organ disorders (Spearman value > 0.8, p value < 0.05) (Supplementary Table S1).
Overall, our data show a highly varied influence of different ICIs on ‘reproductive system and breast disorders’. For ipilimumab patients (58 cases), breast pain was the most common AE among the reproductive system and breast disorders (17.2%), followed by scrotal edema (8.62%), female genital tract fistula (6.96%), pelvic pain (5.17%), and erectile dysfunction (5.17%). Among patients treated with atezolizumab (46 cases), prostatitis (15.2%) was the most common reproductive system/breast disorder followed by vaginal hemorrhage (13.04%), pelvic pain (13.04%), female genital tract fistula (13.04%) and erectile dysfunction (6.52%). For nivolumab patients (136 cases), pelvic pain (9.56%), breast pain (7.35%), vaginal hemorrhage (6.62%), erectile dysfunction (6.62%), and scrotal edema (4.41%) were the most common AEs among the reproductive system and breast disorders in cancer patients aged 18–64.
The three most common types of disorders among patients aged 65–85 treated with atezolizumab were ‘renal and urinary disorders’ (52%), ‘cardiac disorders’ (50%) and ‘metabolism and nutrition disorders’ (49%) (Figure S3B) and they were significantly correlated with other disorders (P value < 0.05). Among patients aged 18–64, the three most common disorders were ‘immune system disorders’ (43%), ‘ear and labyrinth disorders’ (42%), ‘reproductive system and breast disorders’ (41%) (a higher correlation among these disorders, except ‘immune system disorders’ (P = 0.065 > 0.05)).
For avelumab, the three most common types of disorders among treated patients aged 65–85 were ‘psychiatric disorder’ (71%, significantly correlated with other disorders except ‘metabolism and nutrition disorders’), ‘nervous system disorders’ (60%, significantly correlated with other disorders except ‘ear and labyrinth disorders’ and ‘metabolism and nutrition disorders’), ‘ear and labyrinth disorders’ (57%, significantly correlated with ‘immune system disorders’ and ‘neoplasms benign, malignant and unspecified’), while ‘reproductive system and breast disorders’ (67%, significantly correlated with ‘blood and lymphatic system disorders’, ‘hepatobiliary disorders’ and ‘psychiatric disorders’), ‘hepatobiliary disorders’ (42%, significantly correlated with other disorders expect ‘metabolism and nutrition disorders’) and ‘blood and lymphatic system disorders’ (42%, significantly correlated with other disorders except ‘ear and labyrinth disorders’) were the three most common disorders in patients aged 18–64.
Among patients aged 65–85 treated with durvalumab, the three most common types of disorders were ‘metabolism and nutrition disorders’ (49%, significantly correlated with other disorders except ‘psychiatric disorders’), ‘renal and urinary disorders’ (48%, significantly correlated with other disorders except ‘ear and labyrinth disorders’ and ‘psychiatric disorders’) and ‘cardiac disorders’ (47%, significantly correlated with other disorders except ‘ear and labyrinth disorders’ and ‘psychiatric disorders’).
For ipilimumab, ‘cardiac disorders’ (49%), ‘renal and urinary disorders’ (47%) and ‘vascular disorders (44%) were the three most common types of disorders (significantly correlated with other disorders expect ‘immune system disorders’) in patients with age 65–85; and ‘reproductive system and breast disorders’ (52%), ‘hepatobiliary disorders’ (47%) and ‘ear and labyrinth disorders (45%) were the three most common disorders (significantly correlated with other disorders expect ‘immune system disorders’) in patients aged 18–64.
Taken together, ‘cardiac disorders’ were the major type of disorder caused by these seven drugs among patients aged 65–85 and ‘reproductive system and breast disorders’ were the main type of disorder among patients aged 18–64.
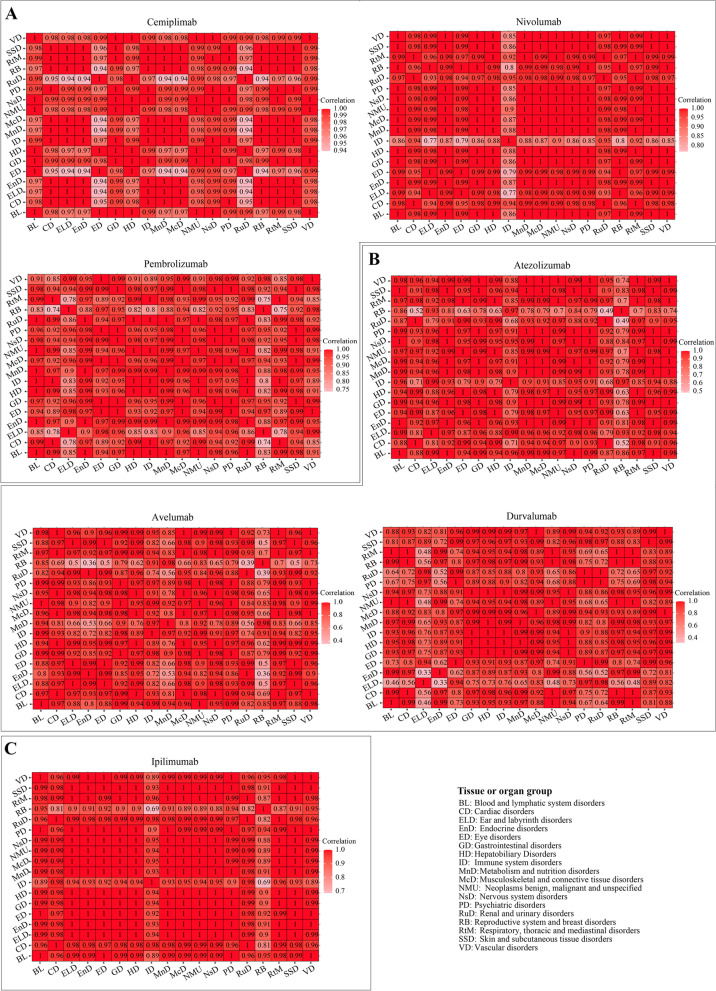
‘Respiratory, thoracic and mediastinal disorders’ is the major type of disorder caused by FDA-approved ICIs in male patients, and ‘reproductive system and breast disorders’ in female patients
Next, we determined proportion of serious irAEs among total irAEs grouped by 18 tissue or organ disorders and associated with patient sex. (Supplementary Figure S4). Correlations between these disorders were also calculated (Fig. 6 and Table 3). ‘Renal and urinary disorders’ was one of the three most common types of disorders in male patients cause by PD-1 inhibitors, and was significantly correlated with ‘cardiac disorders’, ‘endocrine disorders’, ‘hepatobiliary disorders’ and ‘nervous system disorders’ in patients treated with nivolumab, and significantly correlated with ‘blood and lymphatic system disorders’ and ‘endocrine disorders’ in patients treated with pembrolizumab (Fig. 6A). ‘Reproductive system and breast disorders’ was one of the three most common types of disorders (all correlation P value > 0.05) in females treated with nivolumab (37%, none) and pembrolizumab (52%, none). ‘Renal and urinary disorders’ (59%, none) was the most common disorder caused by atezolizumab in males and was significantly correlated with ‘cardiac disorders’ (P = 0.021), while ‘reproductive system and breast disorders’ (60%, P > 0.05) was the most common disorder in females (Fig. 6B). The three most common disorders caused by avelumab were ‘ear and labyrinth disorders’ (71%, significantly correlated with ‘eye disorder’), ‘respiratory, thoracic and mediastinal’ (64%, significantly correlated with ‘eye disorder’) and ‘vascular disorders’ (64%, significantly correlated with ‘nervous system disorder, P = 0.049’) in males respectively. ‘Reproductive system and breast disorders’ (50%, none), ‘metabolism and nutrition disorders’ (42%, none) and ‘immune system disorders’ (35%, none) were the three most common disorders in females (no correlation with other disorders, P > 0.05). Among patients treated with durvalumab, ‘blood and lymphatic system disorders’ (64%, significantly correlated with ‘cardiac disorders’, ‘metabolism and nutrition disorders’ and ‘reproductive system and breast disorders’), ‘respiratory, thoracic and mediastinal’ (61%, significantly correlated with ‘eye disorders’, ‘musculoskeletal and connective tissue disorders’, and ‘nervous system disorders’), and ‘reproductive system and breast disorders’ (60%, significantly correlated with ‘blood and lymphatic system disorders’ and ‘cardiac disorders’) were the three most common disorders in male patients, while ‘ear and labyrinth’ (52%, significantly correlated with ‘gastrointestinal disorders’), ‘psychiatric disorders’ (47%, none), and ‘renal and urinary disorders’ (46%, none) were the three most common disorders in females.
Fig. 6.
Correlation between irAEs and 18 tissue or organ disorders in different patient sex groups Correlations for ICIs targeting PD1, PDL1 and CTLA4 are shown in (A), (B) and (C), respectively
Table 3.
Number of irAEs grouped by 18 tissue or organ disorders in different patient sex groups
| Cemiplimab Number/ratio | Nivolumab Number/ratio | Pembrolizumab Number/ratio | Atezolizumab Number/ratio | Avelumab Number/ratio | Durvalumab Number/ratio | Ipilimumab Number/ratio | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | |
| BL | 0/0.00 | 10/0.20 | 1,485/0.34 | 2,468/0.57 | 1,093/0.27 | 1,681/0.57 | 902/0.44 | 979/0.47 | 53/0.33 | 92/0.56 | 157/0.26 | 385/0.64 | 673/0.37 | 993/0.55 |
| CD | 1/0.03 | 2/0.06 | 1,111/0.28 | 2,497/0.63 | 695/0.33 | 1,283/0.61 | 344/0.32 | 599/0.56 | 41/0.27 | 82/0.54 | 132/0.30 | 243/0.56 | 479/0.29 | 1,035/0.62 |
| ELD | 0/0.00 | 0/0.00 | 149/0.39 | 222/0.58 | 89/0.50 | 77/0.44 | 47/0.51 | 44/0.47 | 1/0.14 | 5/0.71 | 15/0.52 | 11/0.38 | 67/0.34 | 122/0.62 |
| EnD | 0/0.00 | 0/0.00 | 1,458/0.34 | 2,513/0.58 | 856/0.38 | 1,132/0.51 | 327/0.39 | 366/0.43 | 32/0.26 | 56/0.45 | 80/0.28 | 140/0.48 | 1,071/0.34 | 1,715/0.55 |
| ED | 0/0.00 | 2/0.25 | 620/0.37 | 912/0.58 | 421/0.46 | 446/0.49 | 94/0.35 | 136/0.51 | 6/0.22 | 15/0.56 | 46/0.43 | 46/0.43 | 321/0.35 | 511/0.56 |
| GD | 0/0.00 | 9/0.15 | 3,849/0.34 | 6,497/0.55 | 2,834/0.44 | 3,267/0.50 | 1,235/0.40 | 1,522/0.49 | 107/0.30 | 203/0.57 | 345/0.37 | 501/0.53 | 2,555/0.34 | 4,201/0.56 |
| HD | 2/0.4 | 11/0.22 | 1,539/0.33 | 2,686/0.58 | 1,092/0.36 | 1,696/0.56 | 494/0.35 | 727/0.52 | 33/0.25 | 75/0.56 | 164/0.35 | 234/0.51 | 881/0.34 | 1,491/0.57 |
| ID | 4/0.12 | 6/0.18 | 420/0.27 | 704/0.58 | 403/0.36 | 596/0.52 | 172/0.47 | 145/0.40 | 21/0.35 | 29/0.48 | 30/0.36 | 39/0.46 | 214/0.29 | 346/0.47 |
| MnD | 3/0.08 | 3/0.08 | 1,994/0.33 | 3,737/0.46 | 1,183/0.39 | 1,639/0.54 | 600/0.40 | 746/0.50 | 72/0.42 | 87/0.50 | 114/0.33 | 192/0.56 | 1,096/0.34 | 1,924/0.61 |
| McD | 3/0.10 | 3/0.10 | 1,863/0.34 | 3,252/0.61 | 1,410/0.43 | 1,634/0.50 | 411/0.40 | 494/0.48 | 40/0.28 | 74/0.51 | 163/0.39 | 202/0.48 | 741/0.33 | 1,304/0.58 |
| NMU | 1/0.03 | 7/0.19 | 3,225/0.32 | 6,146/0.59 | 2,466/0.36 | 3,816/0.56 | 424/0.38 | 593/0.53 | 48/0.31 | 91/0.59 | 281/0.28 | 582/0.58 | 1,338/0.33 | 2,293/0.57 |
| NsD | 3/0.04 | 12/0.17 | 2,439/0.34 | 4,238/0.61 | 1,797/0.42 | 2,215/0.52 | 847/0.43 | 941/0.47 | 58/0.24 | 148/0.61 | 225/0.36 | 332/0.53 | 1,145/0.33 | 2,089/0.60 |
| PD | 2/0.13 | 3/0.19 | 635/0.35 | 1,132/0.59 | 506/0.45 | 584/0.51 | 160/0.42 | 198/0.52 | 13/0.29 | 28/0.62 | 70/0.47 | 65/0.44 | 321/0.37 | 513/0.59 |
| RuD | 0/0.00 | 8/0.26 | 1,023/0.27 | 2,494/0.62 | 990/0.37 | 1,525/0.57 | 391/0.31 | 739/0.59 | 28/0.17 | 97/0.60 | 120/0.46 | 108/0.42 | 504/0.28 | 1,150/0.65 |
| RB | 0/0.00 | 0/0.00 | 97/0.37 | 152/0.65 | 106/0.52 | 87/0.42 | 57/0.60 | 34/0.36 | 3/0.50 | 3/0.50 | 6/0.30 | 12/0.60 | 49/0.47 | 52/0.50 |
| RTM | 5/0.07 | 10/0.14 | 2,782/0.30 | 5,763/0.59 | 1,900/0.33 | 3,454/0.60 | 901/0.37 | 1,225/0.50 | 69/0.64 | 178/0.64 | 416/0.27 | 934/0.61 | 1,059/0.32 | 2,051/0.61 |
| SSD | 3/0.08 | 5/0.13 | 2,244/0.34 | 3,842/0.58 | 1,831/0.41 | 2,214/0.50 | 537/0.39 | 593/0.44 | 40/0.52 | 88/0.52 | 177/0.40 | 196/0.45 | 1,189/0.34 | 1,979/0.57 |
| VD | 2/0.07 | 6/0.21 | 885/0.35 | 1,522/0.60 | 815/0.47 | 802/0.46 | 355/0.39 | 459/0.50 | 35/0.26 | 86/0.64 | 112/0.40 | 144/0.51 | 444/0.37 | 673/0.57 |
The most common disorders among male patients treated with ipilimumab were ‘renal and urinary disorders’ (65%, significantly correlated with ‘cardiac disorders’, ‘hepatobiliary disorders’ and ‘musculoskeletal and connective tissue disorders’), ‘ear and labyrinth disorders’ (62%, none) and ‘cardiac disorders’ (62%, significantly correlated with ‘hepatobiliary disorders’, ‘musculoskeletal and connective tissue disorders’ and ‘renal and urinary disorders’), ‘respiratory, thoracic and mediastinal disorders’ (61%, significantly correlated with ‘cardiac disorders’, ‘endocrine disorders’, ‘hepatobiliary disorders’ and ‘musculoskeletal and connective tissue disorders’, etc.). Among female patients treated with ipilimumab, the most common disorders were ‘reproductive system and breast disorders’ (47%, significantly correlated with ‘metabolism and nutrition disorders’, ‘nervous system disorders’, etc.), ‘blood and lymphatic system disorders’ (37%, significantly correlated with ‘metabolism and nutrition disorders’), ‘vascular disorders’ (37%, significantly correlated with ‘blood and lymphatic system disorders’ and ‘metabolism and nutrition disorders’) and ‘psychiatric disorders’ (37%, significantly correlated with ‘endocrine disorders’, ‘hepatobiliary disorders’, etc.), and ‘eye disorders’ (35%, significantly correlated with ‘endocrine disorders’, ‘hepatobiliary disorders’, etc.). In summary, ‘respiratory, thoracic and mediastinal disorders’ was the major type of disorder caused by these ICIs in male patients, and ‘reproductive system and breast disorders’ in female patients.
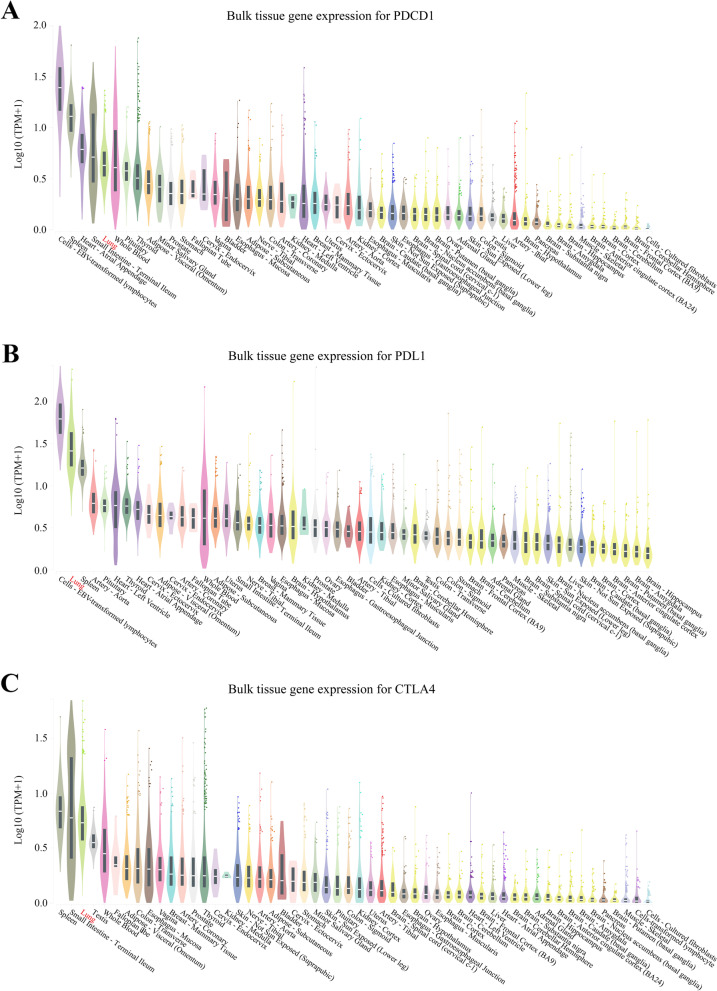
The molecular targets of FDA-approved ICIs are highly expressed in human lung
To better understand the effects of the seven drugs on tissue or organ disorders, expression levels of the PD-1, PD-L1, and CTLA-4 genes were analyzed in 30 human (male and female) tissues or organs, such as brain, adipose tissues, adrenal gland, bladder, blood vessel, breast, heart, kidney, liver, lung, ovary, spleen, uterus, etc., via the GTEx Portal dataset. The five tissues or organs with highest expression of the PD-1 gene were cells-EBV-transformed lymphocytes (median TPM value: male = 25.22, female = 22.57), spleen (median TPM value: male = 12.17, female = 11.40), heart-atrial appendage (median TPM value: male = 5.12, female = 5.14), small intestine-terminal ileum (median TPM value: male = 3.76, female = 5.01) and lung (median TPM value: male = 3.33, female = 3.16) (Fig. 7A). The five tissues or organs with highest expression of the PD-L1 gene were cells-EBV-transformed lymphocytes (median TPM value: male 66.74, female = 66.74), lung (median TPM value: male = 24.77, female = 23.88), spleen (median TPM value: male = 14.21, female = 15.29), artery (median TPM value: male = 5.21, female = 4.896) and pituitary (median TPM value: male = 4.66, female = 5.02) (Fig. 7B). The five tissues or organs with highest expression of the CTLA-4 gene were spleen (median TPM value: male = 5.60, female = 6.19), small intestine-terminal ileum (median TPM value: male = 4.56, female = 6.63), lung (median TPM value: male = 4.39, female = 4.13), testis (median TPM value: male = 2.53), whole blood (median TPM value: male = 1.78, female = 1.80) (Fig. 7C). In summary, the PD-1, PD-L1 and CTLA-4 genes were highly expressed in lungs in both males and females.
Fig. 7.
Expression of PDCD1 (encoding PD1), CD274 (encoding PD-L1) and CLTA4 in human tissues (including female and male). Tissue-specific gene expression for PDCD1, CD274 and CTLA4 are shown in (A), (B) and (C), respectively. These three genes are highly expressed in lung tissues regardless of sex
Discussion
Cancer immunotherapy attempts to boost the body’s own defense mechanism to kill cancer cells and defeat cancer. Over the past few decades, immunotherapy with T cell checkpoint inhibitors has promised to revolutionize cancer therapy [3]. ICIs have offered new hope for cancer patients, especially for those with immunoactive tumors classified as “hot tumors” [20]. However, a major limitation of these therapies is that they are effective in only a subset of patients. Furthermore, the use of ICIs involves a series of related complications, namely, irAEs [21–23]. Recent research data revealed that up to 69% of ICI-treated patients develop acute or short-term AEs (13% of which are severe or fatal) and up to 43% of ICI patients display chronic or long-term (lasting three month or longer) AEs [21, 24]. It is difficult to identify the current evidence in the literature regarding risk factors or biomarkers for the whole category of ICIs as studies are typically either disease-specific (e.g., lung cancer or melanoma), or ICI drug-specific (e.g., pembrolizumab, ipilimumab), or irAE-specific (e.g., pneumonia or gastritis) [25]. It is extremely important to determine whether the assessment of ICI toxicity is needed to predict the occurrence of irAEs or provide early treatment that can modulate the immune system to obtain lasting antitumor effects [26–29]. As a new class of targeted anticancer drugs, ICIs target the immune tolerance pathways of tumor cells, such as PD-1, PD-L1 and CTLA-4, to kill tumor cells [30]. Chemokines and their receptors exert essential functions in all aspects of immune processes involved in physiology (hematopoiesis, immune defense and tissue health) and pathophysiology (chronic inflammation, allergy, and cancer), suggesting that irAEs may limit the use of chemokine-based reagents including ICIs in cancer treatment [3]. There is a clear need to understand the pattern of drug response and toxicity for ICIs. In this study, we focused on interrogating irAEs associated with treatment with seven FDA-approved ICIs. These included three PD-1 inhibitors (cemiplimab, nivolumab and pembrolizumab), three PD-L1 inhibitors (atezolizumab, avelumab and durvalumab) and one CTLA-4 inhibitor (ipilimumab).
Treatment of ICIs may cause the acute occurrence or the toxicity of any organ system, producing clinical AEs, which have been widely concerned [31–34]. There is an urgent need to develop reliable toxicity diagnosis and management methods to meet clinical needs. Few studies have determined tissue or organ-specific irAEs as they are mainly a discrete toxicity caused by the nonspecific activation of the immune system, reversible and easily overlooked [3]. Our data indicated that respiratory and gastrointestinal system toxicity is a common irAE in patients treated with these seven FDA-approved ICIs. It appears that lung cancer patients receiving ICIs are prone to serious irAEs. There are several reasons for this. Firstly, from clinical and research data [35–39], ICIs act as first or second-line treatment for lung cancer, and thus the numbers of lung cancer patients treated with ICIs are higher than in other tumor types. Our data also reveals respiratory system disorders are one of the three most common disorders among irAEs in lung cancer patients with ICIs. For instance, cemiplimab, as a new tumor immunotherapy agent showing anti-tumor activity and an acceptable safety profile, has been reported to improve the overall survival (OS) and progression-free survival (PFS) in advanced non-small-cell lung cancer (NSCLC) [39, 40]. However, cemiplimab treatment-related irAEs occur in 50% of NSCLC patients, and are more serious than in other tumors [29, 41]. Nivolumab is a second-line treatment for lung cancer [35], and is well tolerated in most patients yet has a wide range of irAEs because of its unique toxicity [42]. Pembrolizumab is a first-line monotherapy ICI improving OS and PFS in lung cancer patients, but grade 3 or worse treatment-related irAEs have occurred [15]. Our data also showed the number and proportion of serious irAEs in lung cancer are higher than in other types of cancers. Pembrolizumab and atezolizumab not only lead to lobular hepatitis in patients, but also result in sclerosing cholangitis, lymphocyte duct damage and granulomatous hepatitis. These agents can interact to cause impaired cellular functions such as CD8 ( +) lymphocytes and macrophages [15]. For avelumab, despite this drug showing good clinical results and an acceptable safety profile in solid tumor treatment, severe irAEs occur [43, 44], with respiratory disorders ranking second. ‘Respiratory, thoracic and mediastinal disorders’ is the leading type of disorder caused by durvalumab. Despite durvalumab showing high efficacy in the treatment of lung cancer, this drug can cause irAEs such as interstitial lung disease [38, 45], leading to discontinuation. The three-year OS of nivolumab plus ipilimumab (58%) is higher than that with ipilimumab (34%) [14, 37], and acute renal failure, diarrhea, hepatotoxicity, hepatitis, pneumonia, sepsis with acute renal insufficiency and thrombocytopenia are common phenomenon with treatment with these two ICIs [46]. Taken together, respiratory and gastrointestinal system toxicity is among the most common types of irAEs associated with the seven ICIs examined in this study.
The human immune system function declines annually with age and disorders among different immune system components are manifest by an enhanced response to autoantigens and decreased defense against microbes and cancer. Signs of "immune system aging" in humans may reduce the safety and efficacy of immune system-based treatment strategies or approaches and may lead to the occurrence of cancer and increased respiratory disease [47–50]. Huang et al. reported that older patients displayed a higher percentage of pulmonary toxicity when treated with ICIs (anti-PD-1/L1) [51], while another study found that older patients better tolerate treatment with ICIs [52, 53]. However, information about the irAEs generated by ICIs, and ICIs in older patients is still relatively limited. In this study, we analyzed the irAEs of seven ICIs across different age groups, including ages 18–64, 65–85 and > 85 years, and focused on irAEs among patients aged 65–85. ‘Renal and urinary disorders’ and ‘cardiac disorders’ were the most common types of disorders with these seven ICIs in patients aged 65–85 years. Compared with older patients, ‘reproductive system and breast disorders’ were the main irAEs caused by ICIs in patients aged 18–64. Age-related changes in the immune system may affect the efficacy and toxicity of ICI drugs [51, 54]. Here we demonstrate that patients of age 65–85 are more susceptible to ICI-related renal and urologic toxicity, while those of age 18–64 have more reproductive toxicity.
ICIs appear to more commonly produce respiratory and urinary system toxicity in male patients and reproductive system toxicity in female patients. The immune systems differ significantly between men and women after adolescence, which have profound implications for health and disease [55–57]. which are also manifest in the response to ICIs [58]. As immune function changes with age, adult women demonstrate greater inflammation and responsiveness than adult men [20, 50]. In general, sex differences in immune responses are more pronounced in young adults, which is also evident in older men and women [50]. In addition, older women are more likely to develop autoimmune diseases than older men, while older men are more prone to develop tumors than older women [50, 55, 57]. In a variety of immune cells, sex hormones can bind to specific receptors to achieve immune function [59, 60]. Chen et al. reported that men are more likely to develop ICI-associated renal toxicity with a longer median time of onset and poor prognosis [59]. Our data revealed that ICIs show greater toxicity in respiratory and urinary systems than in other tissues or organs in men, and more intense reproductive toxicity in women. PD-1, PD-L1 and CTLA-4 are slightly different in male and female tissues, therefore, insight into their pathogenesis and interactions may help in better development of immunotherapy strategies to promote clinical care of patients [49, 50]. Moreover, an accurate assessment of the effects of cancer immunotherapy in males and females, and the assessment of the applicability of various tumor models for predicting the sex-dependent success of specific immunotherapies, is crucial [59].
ICIs developed to target immune checkpoint proteins have been successfully used to treat patients with metastatic melanoma, HNC, and NSCLC [61–64]. A high percentage of cancer patients treated with ICIs has ‘respiratory, thoracic, and mediastinal disorders’, suggesting the dysfunction of patients’ lungs. The expression levels of immune checkpoint-coding genes may implicate their importance in the corresponding tissues, and inhibiting their expression may lead to functional disturbance of these tissues, which could be a causative factor for organ-specific irAEs. By analyzing the GTEx dataset, we found higher levels of genes encoding PD-1, PD-L1 and CTLA in human lung tissues than in other organs. This may explain why lung-irAEs occur more frequently in ICI-treated patients. On the other hand, emerging evidence supports the notion that irAEs may be reflective of mechanism-based autoimmune or inflammatory reactions towards the ICI. Previous reports have described that irAEs can prolong OS and PFS by about 6 or 3 months, respectively [65, 66]. For example, the presence of overall irAEs was significantly associated with longer OS in melanoma or NSCLC patients treated with nivolumab [67, 68] Recently, a retrospective study of 156 patients who were treated with ICIs compared 82 patients with irAEs with 74 patients with non-irAEs, and indicated that PFS and OS in the irAE group were significantly longer than those in the non-irAE group [69]. Judo et al. reported that only low grade irAEs, but not high grade irAEs, are associated with better responses to anti-PD-1 antibodies in non-melanoma patients [70]. These findings suggest that irAEs could be used to predict more favorable clinical outcomes of ICI therapy given that irAEs are managed appropriately. The effect of irAEs on PFS and OS is evident; however, lung-irAEs can occur at a later phase than non-lung-irAEs (skin, endocrine, digestive tract) and seemed not to prolong OS and PFS. To this end, understanding the general aspect of lung-irAE is a critical issue for cancer researchers.
Our data may differ from previously reported data for several reasons. Different data sources may be one cause of discrepancies. For example, Baggi’s study analyzed data from 131 advanced and metastatic cutaneous squamous cell carcinoma cases [41], while our study included 492 total irAE cases across 13 common tumors. Another important factor is the difference in statistical objects. The rate of grade 3–4 irAEs was 9.2%, while treatment-related irAEs were seen in 42.7% of the total patients in Baggi’s study [41], which is very similar to the data we reported. As for specific ICIs, the rates of grade 3–4 treatment emergent AEs and serious irAEs were 44% and 29%, respectively, in advanced cutaneous squamous cell carcinoma patients with cemiplimab treatment [40], while the rates of grade 3 and 4 irAEs were 13.8% and 73.2%, respectively, in small-cell lung cancer patients treated with nivolumab [71]. These studies were conducted at a single institution with higher or lower medical comorbidities and reported cancer disparities compared to the rest of the country or only for a single ICI. Our current study estimated irAEs of seven FDA-approved ICIs in the United States. For example, the rate of cemiplimab treatment-associated AEs in lung cancer patients was 50% of the total cancer cases reported to the FDA. Thus, the resulting data should be more applicable to preserving quality of life and avoiding or minimizing the risk of irAE-related fatal outcomes.
As the major consequence of ICI treatment, antitumor immunity is activated with infiltration of immune cells, including T cells, into tumors. Increased T cell diversity in response to ICI treatment could also be a sign of immune response to normal tissues. Oh et al. found that initial broadening in the repertoire of circulating T cells occurred within 2 weeks in patients with metastatic castration-resistant prostate cancer treated with a combination of ipilimumab and granulocyte-monocyte colony-stimulating factor, which significantly preceded irAE onset and was correlated with the development of irAEs [72]. To the best of our knowledge, there are few relevant studies reporting the potential association between innate immune cells and irAEs, which will be an interesting research direction in the future.
Conclusions
In summary, our study focuses on the toxicity burden of seven FDA-approved ICIs in 13 common cancers and 18 tissues or organs of patients. ICIs have the highest probability of serious irAEs in patients with lung cancer, which may be associated with the respiratory toxicity of ICIs. Further, the tissue or organ toxicity of ICIs is age-and sex-specific. ICIs are associated with greater renal and urinary system toxicity among patients aged 65–85 years, reproductive toxicity in patients aged 18–64, respiratory and urinary system toxicity in males, and reproductive system toxicity in females. These differences in patients’ age and sex should be considered during ICI treatment. Further studies on the toxicity mechanism of ICIs are needed to provide more accurate basic research data for clinical practice.
Supplementary information
Additional file 2: Supplementary Figure S1. Proportion of irAE outcomes for seven ICIs reported by the FDA annually (January 1, 2015 to June 30, 2022). The irAE cases for each drug were divided into seven outcome groups, including died, disabled, hospitalized, life-threatening, non-serious, required intervention and other outcomes. Percentages of hospitalized and life-threatening outcomes are indicated for each drug every year. Data for ICIs targeting PD-1, PD-L1 and CTLA4 are shown in (A), (B) and (C), respectively. Supplementary Figure S2. Proportion of serious irAEs among total irAEs for various types of cancer. Data for ICIs targeting PD-1, PD-L1 and CTLA4 are shown in (A), (B) and (C), respectively. Supplementary Figure S3. Proportion of serious irAEs among total irAEs grouped by 18 tissue or organ disorders and associated with patient age. Data for ICIs targeting PD-1, PD-L1 and CTLA4 are shown in (A), (B) and (C), respectively. Supplementary Figure S4. Proportion of serious irAEs among total irAEs grouped by 18 tissue or organ disorders and associated with patient sex. Data for ICIs targeting PD-1, PD-L1 and CTLA4 are shown in (A), (B) and (C), respectively.
Acknowledgements
We would like to acknowledge Anthea Hammond for critical reading of this manuscript. Yong Teng is the inaugural recipient of the Wally Award from Winship Cancer Institute of Emory University.
Abbreviations
- CTLA-4
Cytotoxic T lymphocyte-associated antigen
- FAERS
FDA Adverse Events Reporting System
- HNC
Head and neck cancer
- irAEs
Immune-related adverse events
- ICIs
Immune checkpoint inhibitors
- NSCLC
Non-small cell lung cancer
- OR
Odds ratio
- OS
Overall survival
- PD-1
Programmed death-1
- PD-L1
Programmed death ligand-1
- PFS
Progression-free survival
- PT
MedDRA dictionary Preferred Term
- TPM
Transcripts Per Kilobase Million
Authors’ contributions
YL, ZQ, NFS and YT designed the experiments. YT supervised the study. FY, CS and CT performed the experiments. FY, CS, MA and YT wrote the manuscript. YT is the guarantor of all the results shown in the study. The author(s) read and approved the final manuscript.
Funding
This work was supported in part by NIH/NIDCR grants R01DE028351 and R03DE032084 (to YT), Winship Invest$ Team Science Award (to YT and NFS) and P30CA138292 (to Winship Cancer Institute). Research reported in this publication was also supported in part by Imagine, Innovate and Impact (I3) from the Emory School of Medicine, a gift from Woodruff Fund Inc., and through the Georgia CTSA NIH award (UL1-TR002378).
Availability of data and materials
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tang J, Hubbard-Lucey VM, Pearce L, O'Donnell-Tormey J, Shalabi A. The global landscape of cancer cell therapy. Nat Rev Drug Discovery. 2018;17(7):465–466. doi: 10.1038/nrd.2018.74. [DOI] [PubMed] [Google Scholar]
- 2.Thompson JA. New NCCN Guidelines: Recognition and Management of Immunotherapy-Related Toxicity. Journal of the National Comprehensive Cancer Network : JNCCN. 2018;16(5S):594–596. doi: 10.6004/jnccn.2018.0047. [DOI] [PubMed] [Google Scholar]
- 3.Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA, Lambotte O, Mariette X, Prat A, Suarez-Almazor ME. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xin YuJ, Hodge JP, Oliva C, Neftelinov ST, Hubbard-Lucey VM, Tang J. Trends in clinical development for PD-1/PD-L1 inhibitors. Nat Rev Drug Discovery. 2020;19(3):163–164. doi: 10.1038/d41573-019-00182-w. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021;9(6):e002435. [DOI] [PMC free article] [PubMed]
- 7.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moslehi J, Lichtman AH, Sharpe AH, Galluzzi L, Kitsis RN. Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms. J Clin Invest. 2021;131(5):e145186. [DOI] [PMC free article] [PubMed]
- 9.Marron TU, Ryan AE, Reddy SM, Kaczanowska S, Younis RH, Thakkar D, et al. Considerations for treatment duration in responders to immune checkpoint inhibitors. J Immunother Cancer. 2021;9(3):e001901. [DOI] [PMC free article] [PubMed]
- 10.Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of Immunotherapy for the Practitioner. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(18):2092–2099. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Shao C, Shi Y, Han W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol. 2018;11(1):31. doi: 10.1186/s13045-018-0578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, Shen C, Duma N, Vera Aguilera J, Chintakuntlawar A, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019;5(7):1008–1019. doi: 10.1001/jamaoncol.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoushtari AN, Friedman CF, Navid-Azarbaijani P, Postow MA, Callahan MK, Momtaz P, Panageas KS, Wolchok JD, Chapman PB. Measuring Toxic Effects and Time to Treatment Failure for Nivolumab Plus Ipilimumab in Melanoma. JAMA Oncol. 2018;4(1):98–101. doi: 10.1001/jamaoncol.2017.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, Lao CD, Wagstaff J, Schadendorf D, Ferrucci PF, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 16.Kassi E, Angelousi A, Asonitis N, Diamantopoulos P, Anastasopoulou A, Papaxoinis G, Kokkinos M, Giovanopoulos I, Kyriakakis G, Petychaki F, et al. Endocrine-related adverse events associated with immune-checkpoint inhibitors in patients with melanoma. Cancer Med. 2019;8(15):6585–6594. doi: 10.1002/cam4.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright JJ, Powers AC, Johnson DB. Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol. 2021;17(7):389–399. doi: 10.1038/s41574-021-00484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan Z, Hammer C, Guardino E, Chandler GS, Albert ML. Mechanisms of immune-related adverse events associated with immune checkpoint blockade: using germline genetics to develop a personalized approach. Genome medicine. 2019;11(1):39. doi: 10.1186/s13073-019-0652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309–5337. doi: 10.1016/j.cell.2021.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Dai Z, Wu W, Wang Z, Zhang N, Zhang L, Zeng WJ, Liu Z, Cheng Q. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. Journal of experimental & clinical cancer research : CR. 2021;40(1):184. doi: 10.1186/s13046-021-01987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Britto Evangelista GF, Figueiredo AB, de Barros ESMJ, Gollob KJ: Balancing the good and the bad: controlling immune-related adverse events versus anti-tumor responses in cancer patients treated with immune checkpoint inhibitors. Immunotherapy advances 2022, 2(1):ltac008. [DOI] [PMC free article] [PubMed]
- 22.Chennamadhavuni A, Abushahin L, Jin N, Presley CJ, Manne A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front Immunol. 2022;13:779691. doi: 10.3389/fimmu.2022.779691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sengul Samanci N, Cikman DI, Oruc K, Bedir S, Celik E, Degerli E, Derin S, Demirelli FH, Ozguroglu M. Immune-related adverse events associated with immune checkpoint inhibitors in patients with cancer. Tumori. 2021;107(4):304–310. doi: 10.1177/0300891620953468. [DOI] [PubMed] [Google Scholar]
- 24.Patrinely JR, Jr, Johnson R, Lawless AR, Bhave P, Sawyers A, Dimitrova M, Yeoh HL, Palmeri M, Ye F, Fan R, et al. Chronic Immune-Related Adverse Events Following Adjuvant Anti-PD-1 Therapy for High-risk Resected Melanoma. JAMA Oncol. 2021;7(5):744–748. doi: 10.1001/jamaoncol.2021.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds KL, Arora S, Elayavilli RK, Louv WC, Schaller TH, Khandelwal A, et al. Immune-related adverse events associated with immune checkpoint inhibitors: a call to action for collecting and sharing clinical trial and real-world data. J Immunother Cancer. 2021;9(7):e002896. [DOI] [PMC free article] [PubMed]
- 26.Chowell D, Yoo SK, Valero C, Pastore A, Krishna C, Lee M, Hoen D, Shi H, Kelly DW, Patel N, et al. Improved prediction of immune checkpoint blockade efficacy across multiple cancer types. Nat Biotechnol. 2022;40(4):499–506. doi: 10.1038/s41587-021-01070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardena-Gutierrez A, Lopez Barahona M. Predictive Biomarkers of Severe Immune-Related Adverse Events With Immune Checkpoint Inhibitors: Prevention, Underlying Causes, Intensity, and Consequences. Front Med. 2022;9:908752. doi: 10.3389/fmed.2022.908752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuma M, Uojima H, Hattori N, Arase Y, Fukushima T, Hirose S, Kobayashi S, Ueno M, Tezuka S, Iwasaki S, et al. Safety and efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma in early clinical practice: A multicenter analysis. Hepatology research : the official journal of the Japan Society of Hepatology. 2022;52(3):269–280. doi: 10.1111/hepr.13732. [DOI] [PubMed] [Google Scholar]
- 29.Valentin J, Gerard E, Ferte T, Prey S, Dousset L, Dutriaux C, Beylot-Barry M, Pham-Ledard A. Real world safety outcomes using cemiplimab for cutaneous squamous cell carcinoma. Journal of geriatric oncology. 2021;12(7):1110–1113. doi: 10.1016/j.jgo.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 30.Xiang Z, Li J, Zhang Z, Cen C, Chen W, Jiang B, Meng Y, Wang Y, Berglund B, Zhai G, et al. Comprehensive Evaluation of Anti-PD-1, Anti-PD-L1, Anti-CTLA-4 and Their Combined Immunotherapy in Clinical Trials: A Systematic Review and Meta-analysis. Front Pharmacol. 2022;13:883655. doi: 10.3389/fphar.2022.883655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson DB, Reynolds KL, Sullivan RJ, Balko JM, Patrinely JR, Cappelli LC, Naidoo J, Moslehi JJ. Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. Lancet Oncol. 2020;21(8):e398–e404. doi: 10.1016/S1470-2045(20)30107-8. [DOI] [PubMed] [Google Scholar]
- 32.Guidon AC, Burton LB, Chwalisz BK, Hillis J, Schaller TH, Amato AA, et al. Consensus disease definitions for neurologic immune-related adverse events of immune checkpoint inhibitors. J Immunother Cancer. 2021;9(7):e002890. [DOI] [PMC free article] [PubMed]
- 33.Jia XH, Geng LY, Jiang PP, Xu H, Nan KJ, Yao Y, Jiang LL, Sun H, Qin TJ, Guo H. The biomarkers related to immune related adverse events caused by immune checkpoint inhibitors. Journal of experimental & clinical cancer research : CR. 2020;39(1):284. doi: 10.1186/s13046-020-01749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Z, Chen Q, Qu L, Li M, Wang L, Mir MC, Carbonara U, Pandolfo SD, Black PC, Paul AK, et al. Adverse Events of Immune Checkpoint Inhibitors Therapy for Urologic Cancer Patients in Clinical Trials: A Collaborative Systematic Review and Meta-analysis. Eur Urol. 2022;81(4):414–425. doi: 10.1016/j.eururo.2022.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Chen R, Tao Y, Xu X, Shan L, Jiang H, Yin Q, Pei L, Cai F, Ma L, Yu Y. The efficacy and safety of nivolumab, pembrolizumab, and atezolizumab in treatment of advanced non-small cell lung cancer. Discov Med. 2018;26(143):155–166. [PubMed] [Google Scholar]
- 36.Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, Audigier-Valette C, Pardo Aranda N, Juan-Vidal O, Cheng Y, et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331() Annals of oncology : official journal of the European Society for Medical Oncology. 2021;32(5):631–641. doi: 10.1016/j.annonc.2021.01.071. [DOI] [PubMed] [Google Scholar]
- 37.Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 38.Sato J, Nakano K, Shimizu T, Uchida M. Evaluation of Durvalumab-induced Lung Toxicity Using a Spontaneous Reporting Database. Anticancer Res. 2022;42(7):3575–3582. doi: 10.21873/anticanres.15844. [DOI] [PubMed] [Google Scholar]
- 39.Sezer A, Kilickap S, Gumus M, Bondarenko I, Ozguroglu M, Gogishvili M, Turk HM, Cicin I, Bentsion D, Gladkov O, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397(10274):592–604. doi: 10.1016/S0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- 40.Migden MR, Khushalani NI, Chang ALS, Lewis KD, Schmults CD, Hernandez-Aya L, Meier F, Schadendorf D, Guminski A, Hauschild A, et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21(2):294–305. doi: 10.1016/S1470-2045(19)30728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baggi A, Quaglino P, Rubatto M, Depenni R, Guida M, Ascierto PA, Trojaniello C, Queirolo P, Saponara M, Peris K, et al. Real world data of cemiplimab in locally advanced and metastatic cutaneous squamous cell carcinoma. Eur J Cancer. 2021;157:250–258. doi: 10.1016/j.ejca.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 42.Reddy CA, Schneider BJ, Brackett LM, Tai AW. Nivolumab-induced large-duct cholangiopathy treated with ursodeoxycholic acid and tocilizumab. Immunotherapy. 2019;11(18):1527–1531. doi: 10.2217/imt-2019-0121. [DOI] [PubMed] [Google Scholar]
- 43.Kelly K, Infante JR, Taylor MH, Patel MR, Wong DJ, Iannotti N, Mehnert JM, Loos AH, Koch H, Speit I, et al. Safety profile of avelumab in patients with advanced solid tumors: A pooled analysis of data from the phase 1 JAVELIN solid tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer. 2018;124(9):2010–2017. doi: 10.1002/cncr.31293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, Boccia R, Lippman ME, Somer R, Smakal M, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671–686. doi: 10.1007/s10549-017-4537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murakami S. Durvalumab for the treatment of non-small cell lung cancer. Expert Rev Anticancer Ther. 2019;19(12):1009–1016. doi: 10.1080/14737140.2019.1699407. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Sun L, Yu J, Shan F, Zhang K, Pang X, Ma C, Zhang Y, Shen M, Ma S, et al. Comparison of Immune Checkpoint Inhibitors between Older and Younger Patients with Advanced or Metastatic Lung Cancer: A Systematic Review and Meta-Analysis. Biomed Res Int. 2019;2019:9853701. doi: 10.1155/2019/9853701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y, Su GH, Ma D, Xiao Y, Shao ZM, Jiang YZ. Technological advances in cancer immunity: from immunogenomics to single-cell analysis and artificial intelligence. Signal Transduct Target Ther. 2021;6(1):312. doi: 10.1038/s41392-021-00729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang JH, Bluestone JA, Young A. Predicting and Preventing Immune Checkpoint Inhibitor Toxicity: Targeting Cytokines. Trends Immunol. 2021;42(4):293–311. doi: 10.1016/j.it.2021.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Ye Y, Jing Y, Li L, Mills GB, Diao L, Liu H, Han L. Sex-associated molecular differences for cancer immunotherapy. Nat Commun. 2020;11(1):1779. doi: 10.1038/s41467-020-15679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gubbels Bupp MR, Potluri T, Fink AL, Klein SL. The Confluence of Sex Hormones and Aging on Immunity. Front Immunol. 2018;9:1269. doi: 10.3389/fimmu.2018.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang X, Tian T, Zhang Y, Zhou S, Hu P, Zhang J. Age-Associated Changes in Adverse Events Arising From Anti-PD-(L)1 Therapy. Front Oncol. 2021;11:619385. doi: 10.3389/fonc.2021.619385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nebhan CA, Cortellini A, Ma W, Ganta T, Song H, Ye F, Irlmeier R, Debnath N, Saeed A, Radford M, et al. Clinical Outcomes and Toxic Effects of Single-Agent Immune Checkpoint Inhibitors Among Patients Aged 80 Years or Older With Cancer: A Multicenter International Cohort Study. JAMA Oncol. 2021;7(12):1856–1861. doi: 10.1001/jamaoncol.2021.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pawelec G. Does patient age influence anti-cancer immunity? Seminars in immunopathology. 2019;41(1):125–131. doi: 10.1007/s00281-018-0697-6. [DOI] [PubMed] [Google Scholar]
- 54.Wong SK, Nebhan CA, Johnson DB. Impact of Patient Age on Clinical Efficacy and Toxicity of Checkpoint Inhibitor Therapy. Front Immunol. 2021;12:786046. doi: 10.3389/fimmu.2021.786046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gubbels Bupp MR. Sex, the aging immune system, and chronic disease. Cell Immunol. 2015;294(2):102–110. doi: 10.1016/j.cellimm.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 56.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 57.Mitchell E, Graham AL, Ubeda F, Wild G. On maternity and the stronger immune response in women. Nat Commun. 2022;13(1):4858. doi: 10.1038/s41467-022-32569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Neal-Perry G, Nejat E, Dicken C. The neuroendocrine physiology of female reproductive aging: An update. Maturitas. 2010;67(1):34–38. doi: 10.1016/j.maturitas.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen C, Zhang C, Jin Z, Wu B, Xu T. Sex differences in immune-related adverse events with immune checkpoint inhibitors: data mining of the FDA adverse event reporting system. Int J Clin Pharm. 2022;44(3):689–697. doi: 10.1007/s11096-022-01395-7. [DOI] [PubMed] [Google Scholar]
- 60.Jing Y, Zhang Y, Wang J, Li K, Chen X, Heng J, Gao Q, Ye Y, Zhang Z, Liu Y, et al. Association Between Sex and Immune-Related Adverse Events During Immune Checkpoint Inhibitor Therapy. J Natl Cancer Inst. 2021;113(10):1396–1404. doi: 10.1093/jnci/djab035. [DOI] [PubMed] [Google Scholar]
- 61.Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad H, Ladwa R, O'Byrne K, Kulasinghe A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol. 2022;29(5):3044–3060. doi: 10.3390/curroncol29050247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luoma AM, Suo S, Wang Y, Gunasti L, Porter CBM, Nabilsi N, Tadros J, Ferretti AP, Liao S, Gurer C et al: Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell 2022, 185(16):2918–2935 e2929. [DOI] [PMC free article] [PubMed]
- 63.Lisi L, Lacal PM, Martire M, Navarra P, Graziani G. Clinical experience with CTLA-4 blockade for cancer immunotherapy: From the monospecific monoclonal antibody ipilimumab to probodies and bispecific molecules targeting the tumor microenvironment. Pharmacol Res. 2022;175:105997. doi: 10.1016/j.phrs.2021.105997. [DOI] [PubMed] [Google Scholar]
- 64.Zhao Z, Wang X, Qu J, Zuo W, Tang Y, Zhu H, Chen X. Immune-Related Adverse Events Associated With Outcomes in Patients With NSCLC Treated With Anti-PD-1 Inhibitors: A Systematic Review and Meta-Analysis. Front Oncol. 2021;11:708195. doi: 10.3389/fonc.2021.708195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teraoka S, Fujimoto D, Morimoto T, Kawachi H, Ito M, Sato Y, Nagata K, Nakagawa A, Otsuka K, Uehara K, et al. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. J Thorac Oncol. 2017;12(12):1798–1805. doi: 10.1016/j.jtho.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 66.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018;4(3):374–378. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tucci M, Passarelli A, Mannavola F, Stucci LS, Ascierto PA, Capone M, Madonna G, Lopalco P, Silvestris F. Serum exosomes as predictors of clinical response to ipilimumab in metastatic melanoma. Oncoimmunology. 2018;7(2):e1387706. doi: 10.1080/2162402X.2017.1387706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res. 2016;22(4):886–94. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirata A, Saraya T, Kobayashi F, Noda A, Aso K, Sakuma S, Kurokawa N, Inoue M, Mikura S, Oda M, et al. Immune-related adverse events with immune checkpoint inhibitors: Special reference to the effects on the lungs. Medicine. 2021;100(14):e25275. doi: 10.1097/MD.0000000000025275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Judd J, Zibelman M, Handorf E, O'Neill J, Ramamurthy C, Bentota S, Doyle J, Uzzo RG, Bauman J, Borghaei H, et al. Immune-Related Adverse Events as a Biomarker in Non-Melanoma Patients Treated with Programmed Cell Death 1 Inhibitors. Oncologist. 2017;22(10):1232–1237. doi: 10.1634/theoncologist.2017-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spigel DR, Vicente D, Ciuleanu TE, Gettinger S, Peters S, Horn L, Audigier-Valette C, Pardo Aranda N, Juan-Vidal O, Cheng Y, et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331(☆) Ann Oncol. 2021;32(5):631–641. doi: 10.1016/j.annonc.2021.01.071. [DOI] [PubMed] [Google Scholar]
- 72.Oh DY, Cham J, Zhang L, Fong G, Kwek SS, Klinger M, Faham M, Fong L. Immune Toxicities Elicted by CTLA-4 Blockade in Cancer Patients Are Associated with Early Diversification of the T-cell Repertoire. Can Res. 2017;77(6):1322–1330. doi: 10.1158/0008-5472.CAN-16-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2: Supplementary Figure S1. Proportion of irAE outcomes for seven ICIs reported by the FDA annually (January 1, 2015 to June 30, 2022). The irAE cases for each drug were divided into seven outcome groups, including died, disabled, hospitalized, life-threatening, non-serious, required intervention and other outcomes. Percentages of hospitalized and life-threatening outcomes are indicated for each drug every year. Data for ICIs targeting PD-1, PD-L1 and CTLA4 are shown in (A), (B) and (C), respectively. Supplementary Figure S2. Proportion of serious irAEs among total irAEs for various types of cancer. Data for ICIs targeting PD-1, PD-L1 and CTLA4 are shown in (A), (B) and (C), respectively. Supplementary Figure S3. Proportion of serious irAEs among total irAEs grouped by 18 tissue or organ disorders and associated with patient age. Data for ICIs targeting PD-1, PD-L1 and CTLA4 are shown in (A), (B) and (C), respectively. Supplementary Figure S4. Proportion of serious irAEs among total irAEs grouped by 18 tissue or organ disorders and associated with patient sex. Data for ICIs targeting PD-1, PD-L1 and CTLA4 are shown in (A), (B) and (C), respectively.
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information.