Abstract
The Mxi-Spa type III secretion system of Shigella flexneri directs the host cell contact-induced secretion of a set of invasins, referred to as Ipas. In this study, we examined the role of Spa33 in Ipa secretion. A spa33-null mutant was both noninvasive and unable to translocate the Ipas from inner membrane to outer membrane (OM) positions of the Mxi-Spa transmembrane channel. Spa33 was found to be a Mxi-Spa substrate that is translocated to the bacterial cell surface upon the induction of Ipa secretion. This mobility may serve to drive Ipa translocation within Mxi-Spa toward OM positions. Consistent with a second distinct role in regulating Ipa traffic, the overexpression of Spa33 also blocked Ipa secretion and resulted in Ipa accumulation at the OM. Co-overexpression of Spa33 and another OM-associated element, Spa32, did not disrupt Ipa secretion, suggesting an interaction between the two proteins and an effect on the mechanism which serves to regulate Ipa release from the OM. These findings indicate that Spa33 is a mobile element within Mxi-Spa, which is required to control Ipa translocation into and out of OM positions of the secretory structure.
Shigella flexneri is the causative agent of bacillary dysentery, an invasive disease of the human colonic and rectal mucosa marked by an acute inflammatory response which leads to widespread necrosis and epithelial destruction. The invasive phenotype, a hallmark of Shigella pathogenesis, relies in part on a highly evolved mechanism for the subversion of epithelial cell function (36, 38). Bacterial contact with a target host cell triggers the secretion of a set of virulence plasmid-encoded proteins (Ipas) via a type III pathway (Mxi-Spa). Secreted Ipas form heteropolymeric complexes in the extracellular medium and/or at the bacterial cell surface which may be inserted into the host membrane, forming pores through which additional type III effector proteins are injected into the host cytosol (3, 23, 26). This delivery effects the nucleation and polymerization of actin filaments, which drive a macropinocytosis-like process and bacterial internalization within a loosely associated vacuole (4, 47). Vacuolar lysis occurs in an Ipa-dependent manner, releasing shigellae into the cytosol (1, 13, 37). The cytosolic environment supports both rapid bacterial growth and the elaboration of an actin-based process of intracellular motility (33, 37). When movement results in bacterial contact with the inner face of the host membrane, the force of actin polymerization generates protrusions that extend into neighboring uninfected cells. Protrusion phagocytosis occurs, followed by an Ipa-dependent process of secondary vacuole lysis and entry into a previously uninfected cytosol (2, 39, 44).
While the Ipa proteins and the survival strategy they support are specific to Shigella spp., the type III secretion system used for their delivery is broadly conserved across a diverse range of gram-negative plant and mammalian pathogens (15, 43). Of the 20 Mxi-Spa proteins believed to specify the Shigella type III transmembrane complex, 18 exhibit 16 to 68% identity with known or putative elements from other type III systems. These sets of homologous loci are usually encoded within large operons (displaying similar gene orders between the different systems) that map either to chromosomal pathogenicity islands or to large virulence plasmids. Eleven of the most highly conserved type III secretory proteins are also homologous to and are believed to have evolved from a group of inner membrane (IM)-associated elements from the flagellar subunit export pathways and outer membrane (OM)-associated elements from type II secretion, type IV pilus biogenesis, and filamentous phage extrusion pathways (15, 43). This group of proteins includes (i) a “secretin” that assembles in an OM pore through which protein traffic flows (9); (ii) a “secretin pilot,” a lipoprotein that directs secretin insertion into the OM (7, 12); (iii) a putative transmembrane protein, which may assemble a ring-like structure spanning the IM and OM; and (iv) a set of integral and peripheral IM proteins, predicted to form an IM pore and to energize the secretion process. Homologs of these proteins in type III systems are probably integrated to form much of the basic framework of a proteinaceous channel spanning the IM and OM. Such channels, elaborated by the type III systems of both Shigella and Salmonella spp., have been visualized by electron microscopy and appear as similar looking needle-like structures with bulbous bases embedded in the cell envelope (3, 17). These “secretons” probably represent the general structure of all type III systems, based on the genetic relatedness of their components. Conserved structures imply mechanistic similarities, an idea supported by functions that are common among many type III systems (i.e., host cell contact-induced secretion, effector protein injection, and the elaboration of pilus-like appendages) (15, 43).
The structural and functional similarities between type III systems are surprising considering that each system secretes a largely dissimilar set of specific effector proteins (15). Clearly, poorly conserved structural elements of each type III secreton must exist to mediate direct interactions with the effectors as they are translocating. Candidates for such adapter proteins were identified within a set of type III secretory proteins referred to as the YscO-YscP-YscQ family (15, 31, 32). Members of this family, including Spa13, Spa32, and Spa33 of Shigella spp. and SpaM, SpaN, and SpaO of Salmonella SPI-I, are encoded within colinear genes found in almost every type III system and are flanked by loci encoding the most highly conserved type III secreton elements (those displaying up to 60% identity). Despite this conserved gene organization, proteins within the YscO-YscP-YscQ family display only low-level sequence homology (e.g., Spa33 is only 24% identical to SpaO and 20% identical to YscQ). These findings are consistent with a common ancestry for this family and a subsequent divergence for the purpose of species-specific functions (i.e., interactions with substrate proteins). Additionally, SpaN and SpaO of Salmonella spp. (5, 6, 18), YscO and YscP of Yersina pestis (31, 32), and Spa32 of Shigella flexneri (49) have been demonstrated to be mobile secretory elements; that is, they are required for effector secretion and are themselves translocated substrates of their respective systems (i.e., exported to the cell surface or supernatant prior to or concurrently with the effector substrates). These findings have contributed to the hypothesis that at least some members of this family form a dynamic moving core within type III systems, with their mobility being coupled to or driving that of the secreted effectors (31).
Here, we sought to characterize the contribution of Spa33 to the virulent phenotype of Shigella spp., in particular, its role in Ipa secretion. Spa33 was of interest to us based on its unusual pattern of homology with other YscO-YscP-YscQ family members—its sequence similarities are restricted to a C-terminal domain (15). Such a protein could establish interactions with both the effector elements of each type III system and the more conserved structural elements. To initiate this study, we first demonstrated that Spa33 is a subunit of the Mxi-Spa type III system, required for Ipa secretion and target cell invasion. We showed that Spa33 is mobile within Mxi-Spa and is exported to the bacterial cell surface where it may interact with Spa32. By examining Ipa subcellular distribution patterns in wild-type, spa33-null mutant, and Spa33-overexpressing derivatives, we identified distinct Spa33-dependent steps in the process of Ipa secretion through the Mxi-Spa structure—transfer between IM and OM positions and release from the OM. Spa33 and perhaps the other mobile type III system elements previously identified may directly mediate translocation events specific to OM-associated segments of the secretory channel.
MATERIALS AND METHODS
Bacterial strains and growth media.
S. flexneri strains used in this study are described in Table 1. The Escherichia coli strains used were SM10 λpir (27) for delivery of pGP704 derivatives to S. flexneri and DH5α (Gibco BRL) for plasmid constructions. Bacteria were grown in tryptic soy broth (TSB) or Luria broth (LB) with aeration at 37°C unless otherwise stated. Analysis of Congo red binding was performed on TSB plates (1.5% agar) supplemented with 0.025% Congo red (Sigma Chemical Co.). The following concentrations of antibiotics were used: ampicillin, 100 μg ml−1; chloramphenicol, 10 μg ml−1; gentamicin, 50 μg ml−1; kanamycin, 50 μg ml−1; and streptomycin, 200 μg ml−1. Induction and repression of PBAD-directed gene expression from pBAD18 was performed using media supplemented with arabinose (0.2%) and glucose (0.2%), respectively.
TABLE 1.
Shigella strains and plasmids used in this study
| Strain or plasmid | Relevant information | Source or reference |
|---|---|---|
| Strains | ||
| 2457T | Wild-type S. flexneri serotype 2a | 8 |
| BS103 | Virulence plasmid-cured 2457T | 21 |
| BS228 | 2457T ϕ(ipaB-lacZ 17.6) | 14 |
| BS473 | 2457T streptomycin resistant | Lab stock |
| BS545 | 2457T spa33-1 (spa33::aphA-3) | This work |
| BS546 | BS545/pRRS18 (PBAD-spa33+) | This work |
| BS567 | M90T ipaB::aphA-3 | 24 |
| BS569 | M90T ipaD::aphA-3 | 24 |
| BS622 | 2457T/pRRS18 (PBAD-spa33+) | This work |
| BS623 | BS567/pRRS18 (PBAD-spa33+) | This work |
| BS624 | BS569/pRRS18 (PBAD-spa33+) | This work |
| BS625 | BS545/pRRS19 (PBAD-spa33+-his) | This work |
| BS626 | BS103/pRRS19 (PBAD-spa33+-his) | This work |
| BS627 | BS228/pRRS19 (PBAD-spa33+-his) | This work |
| BS628 | 2457T/pRRS20 (PBAD-spa32+) | This work |
| BS629 | 2457T/pRRS21 (PBAD-spa32+-spa33+) | This work |
| BS634 | 2457T/pRRS19 (PBAD-spa33+-his) | This work |
| Plasmids | ||
| pBAD18 | Arabinose-inducible PBAD expression vector, Ampr | 11 |
| pBluescript SK(+) | Cloning vector, Ampr | Stratagene |
| pGP704 | Suicide vector used to disrupt spa33, Ampr | 27 |
| pUC18K | Vector bearing the aphA-3 cassette, Ampr Kanr | 24 |
| pRRS15 | 1,952-bp PCR-generated fragment, extending from spa32 (position 3096 [GenBank accession number D13663]) to spa24 (position 5047), ligated with HindIII-BamHI-digested pBluescript SK(+) | This work |
| pRRS16 | 840-bp XmaI fragment (Klenow treated) of pUC18K, bearing aphA-3, ligated in the proper orientation with BspEI (Klenow treated)-EcoRV-digested pRRS15 (resulting in a 360-bp internal deletion in spa33) | This work |
| pRRS17 | 2,431-bp HindIII-SpeI-digested (Klenow-treated) fragment of pRRS16, bearing the spa33::aphA-3 allele, ligated with EcoRV-digested pGP704 | This work |
| pRRS18 | 921-bp PCR-generated fragment, extending from 20 bp upstream of the spa33 start codon to 19 bp downstream of the stop codon, ligated with EcoRI-HindIII-digested pBAD18 | This work |
| pRRS19 | 920-bp PCR-generated fragment, extending from 20 bp upstream of the spa33 start codon to its stop codon (including sequence encoding six histidine residues inserted immediately 5′ to the stop codon), ligated with NheI-HindIII-digested pBAD18 | This work |
| pRRS20 | 898-bp PCR-generated fragment, extending from 19 bp upstream of the spa32 start codon to 1 bp downstream of its stop codon, ligated with NheI-digested pBAD18 | This work |
| pRRS21 | 1,792-bp PCR generated fragment, extending from 19 bp upstream of the spa32 start codon to 21 bp downstream of the spa33 stop codon, was ligated with NheI-HindIII-digested pBAD18 | This work |
Plasmid and strain constructions.
The plasmids used in this study are described in Table 1. Analysis of DNA, plasmid manipulations, conjugation, P1 transduction, and transformation procedures were all based on standard protocols. PCR amplifications for cloning and plasmid-screening purposes used the Pfu (Stratagene) and Taq (Qiagen, Inc.) DNA polymerases, respectively, and were performed according to the manufacturers' protocols. To confirm the fidelity of PCRs, many of the PCR-generated plasmid inserts were sequenced. Templates for sequence analysis were prepared with the ABI Prism dye terminator cycle sequencing core kit and processed using an ABI Prism 377 DNA sequencer.
After its construction, plasmid pRRS17 was transferred into S. flexneri strain BS473 by conjugation. Transconjugants in which a double-crossover recombination event replaced the virulence plasmid-borne spa33 allele with the spa33::aphA-3 allele (referred to herein as spa33-1) were identified by ampicillin sensitivity and confirmed by PCR analysis. The aphA-3 cassette used in this study is designed for creating nonpolar mutations in the internal loci of large operons (24). After its construction, the spa33-1 allele was transferred by P1 transduction into strain 2457T, creating strain BS545. The structure of spa33-1 in BS545 was confirmed by PCR and Southern blot analysis.
Membrane preparations.
Total membrane preparations were isolated and fractionated as described by Osborn et al. (29), with some modifications. S. flexneri strains were grown in LB supplemented with arabinose and the appropriate antibiotics. At mid-late log phase, cell pellets (corresponding to equivalent numbers of bacteria for each strain used) and culture supernatants were separated by centrifugation at 4°C. Cell pellets were rapidly suspended in ice-cold 0.75 M sucrose–10 mM Tris-HCl (pH 7.8). After addition of lysozyme (final concentration, 100 μg ml−1) and a 2-min incubation on ice, the cells were diluted slowly with 2 volumes of ice-cold 1.5 mM EDTA (pH 7.5) added slowly over a period of 10 min. At this point, conversion to spheroplasts was confirmed by light microscopy. Spheroplasts were sonicated, and the resulting cellular debris was removed by centrifugation at 1,200 × g for 5 min at 4°C. Membranes were then separated from the soluble fraction by centrifugation at 100,000 × g for 1.5 h. The membrane-bearing pellet was then resuspended in 1.0 ml of 25% sucrose–5 mM EDTA (pH 7.5) and layered above a discontinuous sucrose density gradient consisting of 2.1 ml each of 50, 45, 40, 35, and 30% (wt/wt) sucrose (in 5 mM EDTA [pH 7.5]) over a 0.5-ml cushion of 55% sucrose. Gradients were centrifuged at 35,000 rpm for 20 h at 4°C in a Beckman SW40 rotor. Twelve 1.0-ml fractions were then collected stepwise from the top of each gradient. Samples of each fraction were analyzed to determine the refractive index and sucrose concentration (percent, by weight), protein content (using the Bio-Rad protein assay), and NADH oxidase activity (as described by Osborn et al. [29]). The Bio-Rad protein assay confirmed that equivalent total amounts of protein were present in each gradient. Protein in 500 μl of each fraction was precipitated with 10% (vol/vol) trichloroacetic acid for 2 h on ice and pelleted by centrifugation for 30 min at 14,000 × g. Samples were washed with 90% acetone, resuspended in electrophoresis buffer, neutralized with 1 M Tris-HCl (pH 8.0), and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
Supernatant protein preparations.
The indicated S. flexneri strains were grown in LB supplemented with arabinose and the appropriate antibiotics. At an optical density at 600 nm of ∼0.5, Congo red dye was added to the cultures (to a final concentration of 20 μM) to induce type III secretion. After 30 min at 37°C, the cultures were pelleted by centrifugation (3,000 × g for 5 min at 4°C). The culture supernatant was removed and filtered with a 0.45-μm-pore-size filter, and protein was precipitated with 10% (vol/vol) trichloroacetic acid for 2 h on ice and pelleted by centrifugation at 14,000 × g for 15 min at 4°C. Precipitated proteins were then washed with 90% acetone, resuspended in electrophoresis buffer, neutralized with 1 M Tris-HCl (pH 8.0), and analyzed by SDS-PAGE and immunoblotting. The bacterial cell pellet prepared as described above was washed with 1× phosphate-buffered saline, and an aliquot was removed and plated on LB plates to determine the bacterial titer. The remaining sample was pelleted, resuspended in electrophoresis buffer, and examined with the supernatant proteins by SDS-PAGE and immunoblotting.
A variation on this technique was also used (40), whereby the Congo red-induced cultures were passed four times each through a 28-gauge needle prior to the separation of supernatant and cell pellet fractions. All other manipulations were the same as that described above. This technique shears off cell surface-associated structures into the culture supernatant.
SDS-PAGE and immunoblot analysis.
Protein electrophoresis was performed in 12.5% SDS-polyacrylamide minigels. The separated proteins were transferred to polyvinylidene difluoride membranes (Schleicher & Schuell, Inc.) and treated with a blocking agent (1% casein hydrolysate in Tris-buffered saline). Immunodetection was performed using anti-penta-histidine (Qiagen, Inc.), anti-MxiM (42), anti-BlaM (5′-3′, Inc.), or a cocktail containing anti-IpaB and anti-IpaC (28) antibodies. The activity of an appropriate alkaline phosphatase-labeled secondary antibody was then visualized using the chemiluminescent substrate, CDP-Star (Boehringer Mannheim), as described by the manufacturer.
Protease sensitivity experiments.
Protease sensitivity experiments were based on a protocol described by Loferer et al. (19). S. flexneri strains were grown in LB supplemented with arabinose and the appropriate antibiotics. At an optical density at 600 nm of ∼0.8, standardized culture volumes were removed, washed once with proteinase K buffer (5 mM CaCl2–50 mM Tris-HCl [pH 7.5]), and resuspended in one-tenth of the original culture volume of proteinase K buffer supplemented with 10 μg of chloramphenicol per ml. Then, 0.3-ml aliquots were distributed into Eppendorf tubes and incubated in the presence or absence of Congo red (final concentration, 20 μM) and/or proteinase K (final concentration, 100 μg ml−1) and incubated for 2 h at 37°C. After one wash in proteinase K buffer, a 10-μl aliquot was diluted and plated for enumeration, while the remainder of the sample was pelleted, resuspended in electrophoresis buffer, and analyzed by SDS-PAGE and immunoblotting.
Invasion assay.
The ability of S. flexneri strains to invade semiconfluent L2 cell fibroblast monolayers was assessed using the gentamicin protection assay previously described (35). All of the strains examined were grown in the presence of 0.2% arabinose prior to infection.
RESULTS
Spa33 is a component of the Mxi-Spa type III secretory pathway.
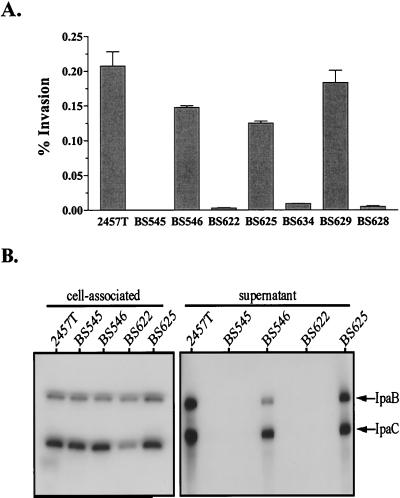
A 360-bp internal fragment of the virulence plasmid-encoded spa33 locus in S. flexneri strain 2457T was replaced with an 840-bp aphA-3 cassette (Fig. 1). The resulting mutant strain BS545 (spa33-1) was ∼3,000-fold less invasive than the virulent parental strain 2457T (Fig. 2A) and displayed a Congo red negative phenotype on TSB agar plates (data not shown). The binding of Congo red dye by Shigella colonies results in red coloration and is a phenotype of wild-type bacteria, like 2457T, which express a functional Mxi-Spa system (41). To confirm the deficiency in Mxi-Spa function, we examined the ability of spa33-1 to produce and secrete the IpaB and IpaC substrates. Immunoblot analyses demonstrated that Spa33 is required for secretion but not synthesis of the Ipas (Fig. 2B). The defects observed in BS545 were not attributable to polar effects exerted by the aphA-3 insertion, since arabinose-induced expression of wild-type spa33 in trans in strain BS546 restored the invasion, Ipa secretion, and Congo red-binding phenotypes (Fig. 2 and data not shown). Not surprisingly, growth of BS546 in the presence of glucose (thus repressing PBAD-directed expression) resulted in virulence defects that were nearly indistinguishable from those of the parental mutant strain, BS545 (data not shown). These findings confirm that Spa33 is a Mxi-Spa subunit required for Ipa secretion.
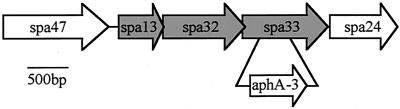
FIG. 1.
Genetic organization of the spa locus adjacent to spa33. Shaded loci encode known (spa32) and putative (spa13 and spa33) mobile secretory elements. Unshaded loci encode the highly conserved secretory proteins, Spa47 and Spa24, which are up to 58 and 60% identical to elements of other type III systems. The internal fragment of spa33 replaced with aphA-3 in strain BS545 (spa33-1) is shown.
FIG. 2.
Effect of spa33 mutation and overexpression on S. flexneri virulence phenotypes. Strains: 2457T, wild type; BS545, spa33-1; BS546, spa33-1/PBAD-spa33+; BS622, 2457T/PBAD-spa33+; BS625, spa33-1/PBAD-spa33+-his; BS634, 2457T/PBAD-spa33+-his; BS629, 2457T/PBAD-spa32+-spa33+; BS628, 2457T/PBAD-spa32+. Each culture was grown with arabinose. (A) Invasion of semiconfluent L2 cells by S. flexneri. Values represent the means and standard deviations for triplicate samples and represent the percentage of the bacterial inoculum which survived gentamicin treatment for 1.5 h. (B) Immunoblot analysis of IpaB and IpaC in the supernatants and cell-associated protein fractions of Congo red-induced S. flexneri strains. Protein isolated from 1 × 106 (for cell-associated samples) and 2.5 × 106 (for supernatant samples) bacteria was examined with IpaB and IpaC monoclonal antibodies. Bands corresponding to each of the Ipas are indicated by arrows.
Spa33 overexpression blocks Mxi-Spa function.
The YscP mobile secretory protein of Yersinia spp. (which is a homolog of Spa32) blocks Yop secretion when overexpressed, probably by preventing the installation of another secretory subunit into the type III structure (32). Since we hypothesized that Spa33 was a mobile secretory protein, we examined whether it exerted a similar overexpression phenotype. Toward this end, we constructed strain BS622 (2457T/PBAD-spa33+), in which spa33 is expressed from both its native promoter and the inducible PBAD promoter of pBAD18. The invasiveness of BS622 cultures grown in the presence of arabinose was reduced nearly 100-fold compared to the wild type (Fig. 2A), while Congo red binding and Ipa secretion were not detectable (data not shown and Fig. 2B, respectively). When grown in the presence of glucose (thus not overexpressing Spa33), BS622 reverted to a wild-type-like invasive phenotype (data not shown). These findings indicate that Spa33 expression levels must normally be tightly regulated, because overexpression disrupts Mxi-Spa function. This overexpression-induced inhibition of virulence is not a nonspecific result of similarly overexpressing any Mxi-Spa component, since excess expression of MxiI, MxiM, MxiE, MxiC, or MxiA does not disrupt virulence (data not shown).
Identification and localization of Spa33.
For insight into the function of Spa33, we determined its distribution in cellular and extracellular bacterial fractions. Since repeated attempts to generate Spa33 antisera were unsuccessful, these studies were performed using an easily detected C-terminal His-tagged version of Spa33. The tag did not alter Spa33 function, since (i) arabinose-induced spa33+-his expression in the spa33-1 mutant (BS625) restored invasion and Ipa secretion defects (Fig. 2) and (ii) arabinose-induced spa33+-his expression in strain 2457T (BS634) resulted in the overexpression-induced inhibition of invasion seen with wild-type Spa33 (Fig. 2A). His-tagged Spa33 is, therefore, a functional equivalent of wild-type Spa33. Expression of Spa33-His in most cases was provided by the PBAD promoter of pBAD18. Previous studies in our laboratory have demonstrated that PBAD-directed expression levels obtained in our in vitro analyses closely approximate the levels provided by the strongly expressed native promoters of the ipa, mxi, and spa operons and do not necessarily result in nonphysiological levels of protein (14, 44).
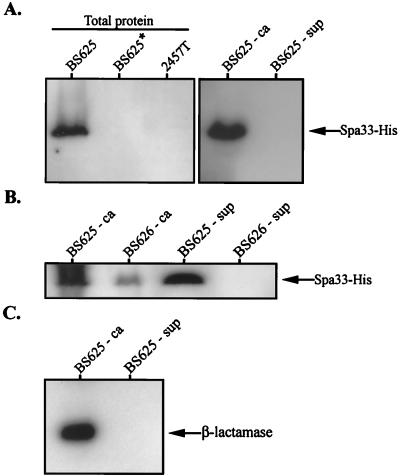
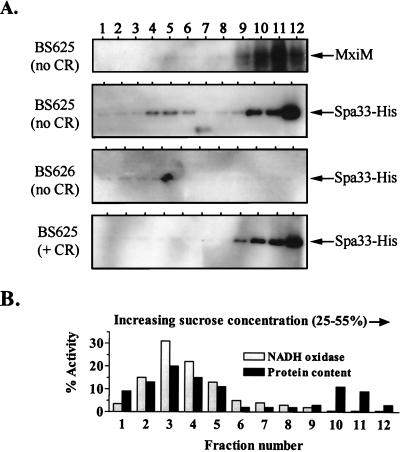
We detected a ∼35-kDa protein corresponding to Spa33-His in the total protein extract of strain BS625 (spa33-1/PBAD-spa33+-his) grown in the presence but not in the absence of arabinose (Fig. 3A). This band was not detected in 2457T, a strain lacking spa33+-his. Using the standard method for inducing Ipa secretion and separating cell-associated and soluble supernatant proteins, Spa33-His was detected only in the cell-associated fraction of BS625 (Fig. 3A). However, when a modified technique was used which shears off peripheral cell surface proteins prior to fractionation, Spa33-His (but not periplasmic β-lactamase) was detected in the supernatant of secretion-induced BS625 (Fig. 3B and C, respectively). Surface release of Spa33-His was observed to a lesser degree in BS625 cultures not induced for secretion (data not shown) and was absent in cultures of a Shigella derivative lacking Mxi-Spa (BS626) (Fig. 3B). Spa33-His is, therefore, translocated to the cell surface but not the supernatant in a Mxi-Spa-dependent manner.
FIG. 3.
Immunoblot analysis of Spa33-His localization in S. flexneri. All cultures were grown with arabinose unless indicated with an asterisk (indicating growth in the absence of arabinose). Fractionated samples were divided into cell-associated (ca) and supernatant (sup) pools. (A) Spa33-His synthesis and secretion. Total protein corresponding to 108 bacteria isolated from secretion-uninduced BS625 (spa33-1/PBAD-spa33+-his) and 2457T (wild type) were examined with an anti-His antibody. Cell-associated and supernatant protein samples corresponding to 1 × 108 and 2.5 × 109 bacteria, respectively, were isolated from secretion-induced BS625 and were similarly examined. (B) Shearing-induced release of Spa33-His into the supernatant. Cell-associated and supernatant protein samples corresponding to 108 and 109 bacteria, respectively, were isolated from secretion-induced BS625 and BS626 (BS103/PBAD-spa33+-his) and examined with an anti-His antibody. Prior to fractionation, each culture was sheared by passage through a 28-gauge needle. (C) Cell-associated and supernatant protein samples corresponding to 5 × 108 and 5 × 109 bacteria, respectively, isolated from secretion-induced BS625 and examined with an anti-β-lactamase antibody. Bands corresponding to Spa33-His or the cytoplasmic-periplasmic marker β-lactamase are indicated with arrows.
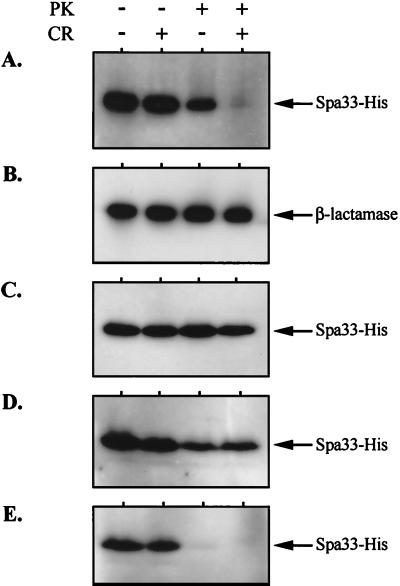
We also examined the susceptibility of Spa33-His to extracellular protease in the presence and absence of Congo red, an inducer of Ipa secretion (Fig. 4). Spa33-His was completely susceptible to proteinase K in secretion-induced BS625 (spa33-1/PBAD-spa33+-his), consistent with a surface-exposed position (Fig. 4A). This exposure was not due to membrane lysis, since the periplasmic β-lactamase marker in BS625 was stable regardless of treatment (Fig. 4B). Surface exposure and protease susceptibility did, however, require Mxi-Spa, because Spa33-His in the type III secretion-deficient strain, BS626, was also stable regardless of treatment (Fig. 4C). In the secretion-uninduced BS625 aliquot, Spa33-His was only partially susceptible to protease, indicating that a large portion of the Spa33 pool is protected within the cell envelope prior to secretion induction.
FIG. 4.
Spa33-His susceptibility to extracellular protease. Each strain was grown with arabinose. Equivalent culture aliquots were incubated in the presence or absence of proteinase K (PK, 100 μg ml−1) and/or Congo red (CR, 20 μM), as indicated. Total protein corresponding to 108 bacteria per sample was then analyzed by immunoblotting with either anti-His or anti-β-lactamase antibodies (indicated next to arrows). Strains: (A) BS625 (spa33-1/PBAD-spa33+-his); (B) BS625 (spa33-1/PBAD-spa33+-his); (C) BS626 (virulence plasmid-cured strain bearing a PBAD-spa33+-his fusion in trans); (D) BS634 (2457T/PBAD-spa33+-his); and (E) BS627 (ΔipaB/PBAD-spa33+-his).
By following the subcellular distribution of Spa33-His in the cell envelope of secretion-induced and -uninduced BS625, we examined whether Spa33 undergoes a change in localization (Fig. 5). For this study, total envelope proteins were fractionated using a technique that separates IM and OM proteins, because they differentially migrate into low- and high-density regions, respectively, of a sucrose gradient. NADH oxidase activity, which is largely restricted to the IM (29), peaked in our lower-density fractions, while MxiM, which is lipid anchored to the OM (42), peaked in the higher-density fractions. Spa33 was not associated with either membrane of a strain lacking a Mxi-Spa system (BS626). In the presence of a Mxi-Spa system (strain BS625) which was not induced with Congo red, the Spa33-His signal was primarily found in the OM fractions and to a lesser degree in the IM fractions. In fact, the OM signal represents the vast majority of the total cellular Spa33 pool (data not shown). Upon Congo red induction of BS625, the distribution of Spa33-His changed only slightly and was exclusively detected in the OM. These results indicate that Spa33 is primarily associated with the OM regardless of the secretion state. The protease resistance of Spa33 prior to secretion induction is, therefore, attributable to its protection within an OM-associated structure like Mxi-Spa. This finding is compatible with the model for Mxi-Spa secretion induction in which the secretory pore is blocked by a IpaB-IpaD complex in the OM (which is resistant to the action of extracellular protease) until a signal is received which drives opening and release of type III substrates to the cell surface or supernatant (25). Spa33 is likely contained within the OM by this IpaB-IpaD complex prior to its Congo red-induced release to the cell surface.
FIG. 5.
Spa33 distribution within the cellular envelope as determined by sucrose density gradient centrifugation. Strains were grown with arabinose prior to the isolation and fractionation of total cell envelope preparations. (A) Immunoblot analysis of samples isolated from strains BS625 (spa33-1/PBAD-spa33+-his) and BS626 (virulence plasmid-cured strain bearing the PBAD-spa33+-his fusion in trans) using anti-MxiM and anti-His antibodies. Equivalent aliquots of every fraction isolated from the Congo red (+ CR)-induced and -uninduced BS625 cultures were examined. For the BS626 (no CR) sample, five times more sample was analyzed per fraction. Exposure times for each Spa33-His blot are the same. The MxiM distribution patterns for strains BS625 (+ CR) and BS626 (no CR) were identical to that observed for BS625 (no CR) and for that reason are not shown. (B) NADH oxidase activity and total protein content in envelope fractions isolated from the BS625 (no CR) culture. The total percentage corresponds to the amount of NADH oxidase activity or protein per fraction divided by the total amount determined for each gradient. The NADH oxidase and protein distribution patterns for BS625 (+ CR) and BS626 (no CR) were identical and for that reason are not included.
Effect of Spa33 on the mechanism controlling Ipa secretion induction.
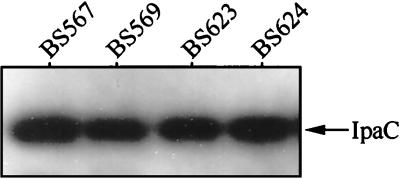
The Ipa secretion defect imposed by both a mutation in and overexpression of Spa33 suggested an interaction with the OM-associated IpaB-IpaD secretory plug. To examine this possibility, we determined the effect of Spa33 overexpression on a major phenotype associated with ipaB- or ipaD-null mutants that lack the OM-plug mechanism—constitutive secretion of Mxi-Spa substrates. For these studies, Spa33-overexpressing strains BS623 (ΔipaB/PBAD-spa33+) and BS624 (ΔipaD/PBAD-spa33+) were analyzed for the secretion of IpaC in the absence of Congo red. In each strain, the overexpression of Spa33 did not block the Congo red-independent secretion of IpaC (Fig. 6). If the secretion pore is constitutively open due to mutation in either ipaB or ipaD, then the ability of excess Spa33 to block Ipa secretion (Fig. 2B) is lost. This finding suggests that an effect of Spa33 overexpression is to inhibit activity of the IpaB-IpaD mechanism which controls the induction of Ipa secretion.
FIG. 6.
IpaC secretion in ΔipaB or ΔipaD backgrounds overexpressing Spa33. Strains: BS567 (ΔipaB); BS569 (ΔipaD); BS623 (ΔipaB/PBAD-spa33+); BS624 (ΔipaD/PBAD-spa33+). Supernatant proteins from arabinose-induced cultures were prepared as described, with the omission of Congo red dye. Secreted proteins corresponding to 5 × 105 bacteria were analyzed by immunoblotting with anti-IpaC antibodies. IpaC is indicated by the arrow.
We next examined how Spa33 overexpression affects its own surface exposure and how this is controlled by IpaB-IpaD. When Spa33-His was expressed in BS634 (a strain exhibiting the overexpression-induced inhibition of Ipa secretion [see Fig. 2A]), it was largely resistant to protease regardless of the presence of Congo red (Fig. 4D). This again suggests that excess Spa33 blocks loss of the IpaB-IpaD plug (and subsequent surface exposure of Spa33). When Spa33-His was similarly overexpressed in an ipaB mutant strain which lacks the IpaB-IpaD plug, it was susceptible to protease regardless of the presence of Congo red (Fig. 4E). The IpaB-IpaD plug controls the protease sensitivity of Spa33 and therefore controls its surface expression.
These results suggest a close interaction between Spa33 and the IpaB-IpaD secretory control mechanism. A hypothesis is suggested whereby Spa33 promotes IpaB-IpaD opening in response to induction signals, allowing Ipa release and full surface exposure for Spa33. When Spa33 is either absent or present in excess, opening cannot occur.
Effect of Spa33 on Ipa transmembrane traffic.
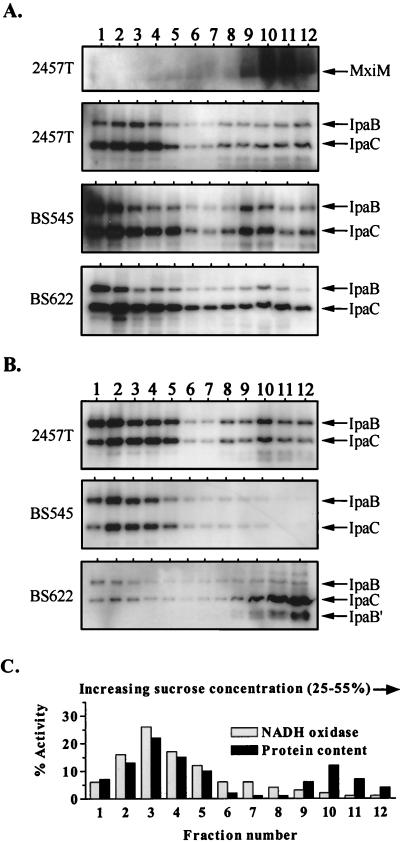
For detailed examination of the role for Spa33 in Ipa translocation, we determined how either a mutation in or overexpression of spa33 influences Ipa distribution in the cell envelope (Fig. 7). With a wild-type strain (2457T) grown in the absence of Congo red, both IpaB and IpaC were dispersed across the IM and OM protein-bearing fractions (Fig. 7A). We have previously observed this pattern and have taken it as an indication that Mxi-Spa exists in an Ipa-loaded state prior to the activation of secretory functions (42). Surprisingly, strains in which Spa33 was absent or overexpressed (BS545 and BS622, respectively) demonstrated a pattern of Ipa distribution that was nearly indistinguishable from that observed in 2457T (Fig. 7A).
FIG. 7.
Effect of Spa33 on IpaB and IpaC distribution within the cell envelope as determined by sucrose density gradient centrifugation. All cultures were grown with arabinose prior to the isolation and fractionation of envelope samples. For the immunoblot analyses, equivalent fraction aliquots were examined in each case, and the exposure times for all Ipa blots shown are identical. Arrows indicate the positions of corresponding proteins. (A) Immunoblot analysis of fractions from Congo red-uninduced 2457T (wild type), BS545 (spa33-1), and BS622 (2457T/PBAD-spa33+) using anti-MxiM, anti-IpaB, and anti-IpaC antibodies. The MxiM distribution patterns for strains BS545 and BS622 were identical to 2457T and for that reason are not shown. (B) Immunoblot analysis of fractions from Congo red-induced 2457T (wild type), BS545 (spa33-1), and BS622 (2457T/PBAD-spa33+) using anti-IpaB and anti-IpaC antibodies. IpaB′ is a specific degradation product of IpaB. MxiM distribution patterns for each strain were identical to those shown in panel A and for that reason are not shown. (C) NADH oxidase activity and total protein content in fractions isolated from Congo red-uninduced 2457T. The total percentage corresponds to the amount of NADH oxidase activity or protein per fraction divided by the total amount determined for each gradient. The NADH oxidase and protein distribution patterns for all other strains examined were identical and for that reason are not included.
The distribution of IpaB and IpaC in Congo red-induced 2457T was similar to that observed in the uninduced state (Fig. 7B), probably reflecting transient interactions which occur during Ipa translocation through Mxi-Spa. The absence of Spa33 (in BS545) deregulates this process, resulting in the restriction of IpaB and IpaC to IM positions (Fig. 7B). Ipas detected in the OM of BS545 prior to secretion induction either leaked out or were degraded and not replaced. An Ipa distribution pattern that was almost the exact opposite was observed with secretion-induced BS622 (the Spa33 overexpresser). In this strain, membrane-associated IpaC was restricted largely to the OM. The same was true for IpaB, although it was detectable only as a truncated derivative, probably due to instability. This IpaB instability in secretion-induced BS622 was not representative of the majority of the cell-associated (and presumably cytoplasmic) IpaB pool, which was shown to be stable (Fig. 2B). The finding that overexpression of Spa33 prevented Ipa release and led to accumulation in the OM fits with our model, in which excess Spa33 blocks opening of the IpaB-IpaD plug. Less clear are our findings that excess Spa33 also prevented Ipa accumulation in the IM and that the absence of Spa33 prevented IM-to-OM Ipa traffic. These results suggest a more complicated role for Spa33 in Ipa translocation than simply as a Mxi-Spa element which promotes opening of the OM IpaB-IpaD plug.
Interactions between Spa33 and other Mxi-Spa subunits.
In the flagellar biosynthetic pathway, severe overexpression phenotypes associated with either the FliN (a Spa33 homolog) or FliM (which has no Mxi-Spa homolog) subunits are reversed by their co-overexpression (45). This effect reflects a direct interaction between the two proteins, in that their proper function requires that each be present in roughly equivalent amounts. Based on these findings, we believed that co-overexpression studies could identify an interacting partner for Spa33, since it also exhibits an overexpression phenotype. Spa32 is the most likely choice for such a partnership since, like FliM and FliN of E. coli, Spa32 and Spa33 of Shigella spp. (i) are encoded in adjacent overlapping loci (suggesting translational coupling) and (ii) act at similar positions within the secretory structure (at the OM for Spa32 and Spa33) (reference 49 and this study). To test this hypothesis, we first constructed a Shigella strain that overexpressed Spa32 in an otherwise wild-type background (BS628) and examined it in the gentamicin protection invasion assay. This strain demonstrated an ∼40-fold reduction in invasiveness, compared to the wild-type strain, indicating that excess Spa32, like excess Spa33, inhibited the virulent phenotype (Fig. 2A). We then tested the effects of co-overexpressing both Spa32 and Spa33 using strain BS629 (2457T/PBAD-spa32+-spa33+) and found invasion to be at wild-type levels (Fig. 2A). Therefore, Spa32 and Spa33 must be maintained at similar levels to support Mxi-Spa function and may, like FliM and FliN in the flagellar system, interact within the secretory structure.
DISCUSSION
An important question in type III secretion concerns how highly conserved transmembrane structures are adapted for interactions with the distinct sets of effector proteins found in each type III system. Hueck (15) and Payne and Straley (31) suggested that this is mediated, at least in part, by a group of poorly conserved secretory proteins found in virtually all type III systems (referred to as the YscO-YscP-YscQ family). For reasons that include their mobility within type III structures, the sequence divergence within this family is believed to reflect pressures to interact with different groups of effectors. In this study, we identify Spa33 as one such mobile secretory protein in the Shigella type III system and show that it has a role in the regulation of protein traffic.
We first demonstrated that a nonpolar spa33 mutation rendered Shigella spp. deficient for both Ipa secretion and entry into the intracellular environment of L2 fibroblasts. Since these effects are similar to those described for mutants that lack components of the Mxi-Spa system (22, 42), Spa33 can be considered an essential subunit of the Shigella type III secretion system.
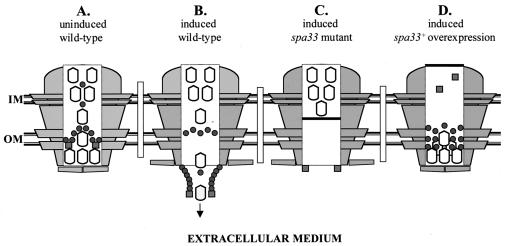
Prior to the induction of Ipa secretion, Spa33 was detected primarily at the OM in a form which was largely resistant to extracellular protease. Upon the induction of secretion, Spa33 was still primarily associated with the OM, however, in a form which was completely susceptible to extracellular protease. Taken with our findings that Spa33 could be sheared off the surface of secretion-induced cells (and to a much lesser extent from secretion-uninduced cells), these results indicate that Spa33 undergoes an alteration in its localization from within the OM to a more peripheral position at the cell surface (see Fig. 8A and B). This Ipa secretion-induced surface exposure indicated that Spa33 is a mobile secretory factor.
FIG. 8.
Models for Spa33 function within Mxi-Spa. Mxi-Spa transmembrane structures are depicted as lightly shaded structures flanking central open channels. Ipas are shown as open bullets, and Spa33 and Spa32 are shown as darkly shaded circles and squares, respectively. (A) Secretion-uninduced state in the wild-type strain. The Ipa proteins are arrayed across both membranes, while the IpaB-IpaD plug in the OM maintains the secretion “off” state. A Spa33-Spa32 complex at the OM will be needed for proper dissolution of the plug. (B) Secretion-induced state in the wild-type strain. The Ipa proteins are translocating through Mxi-Spa into the extracellular environment. Spa33 export to the cell surface may directly mediate Ipa translocation between the IM and OM. Spa32 at the cell surface is required for Ipa release. The arrow indicates the direction of Ipa traffic. (C) Secretion-induced state in the spa33-null mutant. The dark bar through Mxi-Spa indicates that IM-associated Ipas cannot be translocated into the OM without Spa33. (D) Secretion-induced state when Spa33 is overexpressed in an otherwise wild-type background. Envelope-associated Ipas accumulate at the OM. Excess Spa33 at the OM has displaced Spa32, thus preventing dissolution of the IpaB-IpaD plug. The dark bar indicates that Mxi-Spa cannot engage the cytoplasmic Ipa pool.
The mobilization of Spa33 to the OM and onto the cell surface requires the same Mxi-Spa channel used for Ipa export. Three lines of evidence support this contention. First, Spa33 lacks the primary sequence motifs which mediate transport and insertion into the OM (i.e., a signal sequence, hydrophobic domain, or β-sheet structure). Second, Spa33 is not associated with the cell envelope in a virulence plasmid-cured strain lacking Mxi-Spa. Third, the Congo red-induced release of Spa33 to the cell surface is controlled by the IpaB-IpaD secretory plug. Spa33 is, therefore, a Mxi-Spa substrate which, like the Spa32 mobile secretory factor (49; R. Schuch and A. T. Maurelli, unpublished data), is exported to the cell surface. These findings contrast with that of the mobile effectors from Salmonella (SpaM and SpaO) (6, 18) and Yersinia spp. (YscO and YscP) (31, 32) studied thus far, each of which is secreted into the supernatant upon the induction of type III secretion.
The overexpression of Spa33 had a profound impact on Ipa membrane distribution, which was surprisingly similar to the effect reported for a spa32-null mutant. In both backgrounds, Ipas accumulated at the OM and were not released into the supernatant (49) (Fig. 2B). In the case of Spa33 overexpression, we also found that the secretion inhibition was absent in a background lacking the IpaB-IpaD secretory plug mechanism. These phenotypes indicate that Spa32 and Spa33 may normally promote opening of the Mxi-Spa channel, perhaps by driving disengagement of the IpaB-IpaD plug in the OM. This function would require roughly equivalent amounts of Spa33 and Spa32 in Mxi-Spa, as indicated by our findings that Spa33 overexpression phenotypes are blocked by co-overexpression with Spa32. Excess Spa33 may displace or out-compete Spa32 for a position in the OM (Fig. 8D), while co-overexpression drives the equilibrium back to proper complex formation. This would explain why the spa32 mutant has the same phenotype as Spa33 overexpression. It is interesting that the overexpression of Spa32 alone has the same effect as Spa33 overexpression (that is, inhibition of Ipa secretion), indicating that excess Spa32 can also displace Spa33 from the secretory structure. Together, these findings suggest that Spa33 and Spa32 interact with the IpaB-IpaD mechanism to control the induction of Ipa secretion. The role for Spa33 here is probably indirect, mediated through displacement of Spa32 from its OM position.
The function of Spa33 was more precisely examined by looking at Ipa distribution in the cell envelopes of the spa33-null mutant and overexpressing strains. In secretion-uninduced conditions (i.e., envelopes isolated from cultures grown in the absence of Congo red), no overt phenotypes were observed. Spa33, therefore, does not grossly affect the Ipa-loading of Mxi-Spa prior to secretion. In secretion-induced conditions, however, the absence of Spa33 did noticeably manifest itself as a block in IM-to-OM Ipa traffic (see Fig. 8C), while excess Spa33 blocked Ipa traffic from the OM into the supernatant and prevented Ipa association with the IM (see Fig. 8D). These findings implicate Spa33 in processes specific to the regulation of Ipa traffic within Mxi-Spa. Such processes include the regulation of Ipa release, Ipa recruitment into the Mxi-Spa structure, and Ipa traffic within the Mxi-Spa channel.
Since Spa33 overexpression causes both Ipa accumulation at the OM and a block in Ipa recruitment into the IM, release of the Ipas from the OM may be a prerequisite for reloading Mxi-Spa at its base. Such a mechanism has been suggested for the type I pathway of HlyA secretion (46), where translocation may occur by continually alternating rounds of substrate recognition at the cytoplasmic face of a secretory structure and then release at the surface. The block in Ipa secretion imposed by Spa33 overexpression appears to freeze the Mxi-Spa system in the midst of such a process, at a point just prior to Ipa release and Ipa reloading.
Our results suggest several testable scenarios for mobile secretory protein function in Mxi-Spa. Spa33 and Spa32 may form part of a complex within the secretory channel which acts to receive the Ipas as they are pumped from IM positions and to promote their release from the surface. As part of this mechanism, Ipa translocation is probably coupled with Spa33 and Spa32 export to the cell surface. The mobility of Spa33 and Spa32 (i.e., Congo red-induced export from within the OM to the cell surface) also suggests that these proteins may assemble as part of the pilus-like structures synthesized by Mxi-Spa upon the induction of type III secretion (30). Similar structures are also made by the type III systems of Salmonella enterica serovar Typhimurium (10), Pseudomonas syringae (34), Ralstonia solanacearum (48), and enteropathogenic E. coli (16) and are believed to promote effector injection into the target host.
The primary sequences of Spa33 and other YscO-YscP-YscQ family members yield little structural information (i.e., coiled coil domains, membrane spanning segments, etc.). A notable feature of this family, however, concerns the pattern of sequence conservation found within it. Each member has an ∼70-residue C-terminal domain in common and a largely variable N-terminal extension, ranging from 47 residues for the flagellar homolog, FliN, to 150 to 200 residues for the type III secretion homologs (reference 15 and data not shown). It is of interest that FliN acts at the cytoplasmic face of the flagellar basal body structure, while the type III system homologs (at least Spa33 and SpaO) are exposed to the extracellular environment. Addition of the longer N-terminal extensions for the type III system homologs may have allowed adaptation of a FliN-like ancestor for interactions with type III pathway effector proteins (in the process freeing it from its IM position). While no Spa33-Ipa interactions have been identified yet, in a preliminary yeast two-hybrid screen, we did detect an interaction between the N-terminal variable region of Spa33 and the OM secretin protein, MxiD (data not shown). Such a finding supports the idea that the N-terminal extensions of the Spa33 family members promoted their release from IM-associated positions toward more axial positions at the OM.
In conclusion, we have shown that Spa33 is indispensable for Ipa secretion through the Mxi-Spa system of Shigella spp. Upon induction of secretory functions, Spa33 is mobilized from within the OM-associated segment of Mxi-Spa to the cell surface and, in the process, drives Ipa translocation from IM-associated positions toward the OM. In conjunction with another OM-associated mobile element (Spa32), Spa33 also acts at the level of controlling the induction of the secretory process. These findings indirectly implicate Spa33 as a Mxi-Spa component which mediates interactions with the Ipas and promotes their passage through the type III channel.
ACKNOWLEDGMENTS
This work was supported by grant AI24656 from the National Institute of Allergy and Infectious Diseases and grant RO7385 from the Uniformed Services University of the Health Sciences.
We thank Ed Oaks (Walter Reed Army Institute of Research) for the monoclonal IpaB and IpaC antibodies, Mike Flora (Biomedical Instrumentation Center, Uniformed Services University of the Health Sciences) for sequencing and primer synthesis, and William Day and Rachel Binet for critical reading of the manuscript.
REFERENCES
- 1.Adam T, Arpin M, Prévost M-C, Gounon P, Sansonetti P J. Cytoskeletal rearrangements and the functional role of T-plastin during entry of Shigella flexneri into HeLa cells. J Cell Biol. 1995;129:367–381. doi: 10.1083/jcb.129.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardini M L, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra-and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blocker A, Gounon P, Larquet E, Niebuhr K, Cabiaux V, Parsot C, Sansonetti P J. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J Cell Biol. 1999;147:683–693. doi: 10.1083/jcb.147.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clerc P, Sansonetti P J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987;55:2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collazo C M, Galán J E. Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect Immun. 1996;64:3524–3531. doi: 10.1128/iai.64.9.3524-3531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collazo C M, Zierler M K, Galán J E. Functional analysis of the Salmonella typhimurium invasion genes invI and invJ and identification of a target of the protein secretion apparatus encoded in the inv locus. Mol Microbiol. 1995;15:25–38. doi: 10.1111/j.1365-2958.1995.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 7.Daefler S, Guilvout I, Hardie K R, Pugsley A P, Russel M. The C-terminal domain of the secretin PulD contains the binding site for its cognate chaperone, PulS, and confers PulS dependence on pIVfl function. Mol Microbiol. 1997;24:465–475. doi: 10.1046/j.1365-2958.1997.3531727.x. [DOI] [PubMed] [Google Scholar]
- 8.Formal S B, Dammin G J, LaBrec E H, Schneider H. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J Bacteriol. 1958;75:604–610. doi: 10.1128/jb.75.5.604-610.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genin S, Boucher C A. A superfamily of proteins involved in different secretion pathways in gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol Gen Genet. 1994;243:112–118. doi: 10.1007/BF00283883. [DOI] [PubMed] [Google Scholar]
- 10.Ginocchio C C, Olmsted S B, Wells C L, Galán J E. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–724. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 11.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie K R, Seydel A, Guilvout I, Pugsley A P. The secretin-specific, chaperone-like protein of the general secretory pathway: separation of proteolytic protection and piloting functions. Mol Microbiol. 1996;22:967–976. doi: 10.1046/j.1365-2958.1996.01539.x. [DOI] [PubMed] [Google Scholar]
- 13.High N, Mounier J, Prévost M-C, Sansonetti P J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hromockyj A E, Maurelli A T. Identification of Shigella invasion genes by isolation of temperature-regulated inv::lacZ operon fusions. Infect Immun. 1989;57:2963–2970. doi: 10.1128/iai.57.10.2963-2970.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubori T, Matsushima Y, Nakamura D, Uralil J, Lara-Tejero M, Sukhan A, Galán J E, Aizawa S-I. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Ochman H, Groisman E A, Boyd E F, Solomon F, Nelson K, Selander R K. Relationship between evolutionary rate and cellular location among the Inv/Spa invasion proteins of Salmonella enterica. Proc Natl Acad Sci USA. 1995;92:7252–7256. doi: 10.1073/pnas.92.16.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loferer H, Hammar M, Normark S. Availability of the fibre subunit CsgA and the nucleator protein CsgB during assembly of fibronectin-binding curli is limited by the intracellular concentration of the novel lipoprotein CsgG. Mol Microbiol. 1997;26:11–23. doi: 10.1046/j.1365-2958.1997.5231883.x. [DOI] [PubMed] [Google Scholar]
- 20.Maurelli A T, Baudry B, d'Hauteville H, Hale T L, Sansonetti P J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985;49:164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurelli A T, Blackmon B, Curtiss R., III Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect Immun. 1984;43:397–401. doi: 10.1128/iai.43.1.397-401.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ménard R, Dehio C, Sansonetti P J. Bacterial entry into epithelial cells: the paradigm of Shigella. Trends Microbiol. 1996;4:220–225. doi: 10.1016/0966-842X(96)10039-1. [DOI] [PubMed] [Google Scholar]
- 23.Ménard R, Prévost M-C, Gounon P, Sansonetti P J, Dehio C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc Natl Acad Sci USA. 1996;93:1254–1258. doi: 10.1073/pnas.93.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ménard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ménard R, Sansonetti P J, Parsot C. The secretion of the Shigella flexneri Ipa invasins is induced by the epithelial cell and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ménard R, Sansonetti P J, Parsot C, Vasselon T. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell. 1994;79:515–525. doi: 10.1016/0092-8674(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 27.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills J A, Buysse J M, Oaks E V. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect Immun. 1988;56:2933–2941. doi: 10.1128/iai.56.11.2933-2941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osborn M J, Gander J E, Parisi E, Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. J Biol Chem. 1972;247:3962–3972. [PubMed] [Google Scholar]
- 30.Parsot C, Ménard R, Gounon P, Sansonetti P J. Enhanced secretion through the Shigella flexneri Mxi-Spa translocon leads to assembly of extracellular proteins into macromolecular structures. Mol Microbiol. 1995;16:291–300. doi: 10.1111/j.1365-2958.1995.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 31.Payne P L, Straley S C. YscO of Yersinia pestis is a mobile core component of the Yop secretion system. J Bacteriol. 1998;180:3882–3890. doi: 10.1128/jb.180.15.3882-3890.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Payne P L, Straley S C. YscP of Yersinia pestis is a secreted component of the Yop secretion system. J Bacteriol. 1999;181:2852–2862. doi: 10.1128/jb.181.9.2852-2862.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prévost M-C, Lesourd M, Arpin M, Vernel F, Mounier J, Hellio R, Sansonetti P J. Unipolar reorganization of F-actin layer at bacterial division and bundling of actin filaments by plastin correlate with movement of Shigella flexneri within HeLa cells. Infect Immun. 1992;60:4088–4099. doi: 10.1128/iai.60.10.4088-4099.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roine E, Wei W, Yuan J, Nurmiaho-Lassila E-L, Kalkkinen N, Romantschuk M, He S-Y. Hrp pilus: an hrp-dependent bacterial surface appendage produced by Pseudomonas syringae pv. tomato DC3000. Proc Natl Acad Sci USA. 1997;94:3459–3464. doi: 10.1073/pnas.94.7.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandlin R C, Goldberg M B, Maurelli A T. Effect of O side-chain length and composition on the virulence of Shigella flexneri 2a. Mol Microbiol. 1996;22:63–73. doi: 10.1111/j.1365-2958.1996.tb02656.x. [DOI] [PubMed] [Google Scholar]
- 36.Sansonetti P J. Molecular and cellular mechanisms of invasion of the intestinal barrier by enteric pathogens: the paradigm of Shigella. Folia Microbiol. 1998;43:239–246. doi: 10.1007/BF02818608. [DOI] [PubMed] [Google Scholar]
- 37.Sansonetti P J, Ryter A, Clerc P, Maurelli A T, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986;51:461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sansonetti P J, Tran Van Nhieu G, Egile C. Rupture of the intestinal epithelial barrier and mucosal invasion by Shigella flexneri. Clin Infect Dis. 1999;28:466–475. doi: 10.1086/515150. [DOI] [PubMed] [Google Scholar]
- 39.Sansonetti P J, Mounier J, Prévost M-C, Mège R M. Cadherin expression is required for the spread of Shigella flexneri between epithelial cells. Cell. 1994;76:829–839. doi: 10.1016/0092-8674(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 40.Sauvonnet N, Vignon G, Pugsley A P, Gounon P. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J. 2000;19:2221–2228. doi: 10.1093/emboj/19.10.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuch R, Maurelli A T. Virulence plasmid instability in Shigella flexneri 2a is induced by virulence gene expression. Infect Immun. 1997;65:3686–3692. doi: 10.1128/iai.65.9.3686-3692.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuch R, Maurelli A T. The Mxi-Spa type III secretory pathway of Shigella flexneri requires an outer membrane lipoprotein, MxiM, for invasion translocation. Infect Immun. 1999;67:1982–1991. doi: 10.1128/iai.67.4.1982-1991.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuch R, Maurelli A T. The type III secretion pathway: dictating the outcome of bacterial-host interactions. In: Brogden K A, et al., editors. Virulence mechanisms of bacterial pathogens. 3rd ed. Washington, D.C.: American Society for Microbiology; 2000. pp. 203–223. [Google Scholar]
- 44.Schuch R, Sandlin R C, Maurelli A T. A system for identifying post-invasion functions of invasion genes: requirements for the Mxi-Spa type III secretion pathway of Shigella flexneri in intercellular dissemination. Mol Microbiol. 1999;34:675–689. doi: 10.1046/j.1365-2958.1999.01627.x. [DOI] [PubMed] [Google Scholar]
- 45.Tang H, Billings S, Wang X, Sharp L, Blair D F. Regulated underexpression and overexpression of the FliN protein of Escherichia coli and evidence for an interaction between FliN and FliM in the flagellar motor. J Bacteriol. 1995;177:3496–3503. doi: 10.1128/jb.177.12.3496-3503.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thanabalu T, Koronakis E, Hughes C, Koronakis V. Substrate-induced assembly of a contiguous channel for protein export from E. coli: reversible bridging of an inner-membrane translocase to an outer membrane exit pore. EMBO J. 1998;17:6487–6496. doi: 10.1093/emboj/17.22.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran Van Nhieu G, Caron E, Hall A, Sansonetti P J. IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells. EMBO J. 1999;18:3249–3262. doi: 10.1093/emboj/18.12.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Gijsegem F, Vasse J, Camus J C, Marenda M, Boucher C. Ralstonia solanacearum produces hrp-dependent pili that are required for PopA secretion but not for attachment of bacteria to plant cells. Mol Microbiol. 2000;36:249–260. doi: 10.1046/j.1365-2958.2000.01851.x. [DOI] [PubMed] [Google Scholar]
- 49.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 1995;14:2461–2470. doi: 10.1002/j.1460-2075.1995.tb07243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]