Abstract
Background
Percutaneous thermal ablation is a curative-intent locoregional therapy (LRT) for selected patients with unresectable colorectal liver metastasis (CLM). Several factors have been identified that contribute to local tumour control after ablation. However, factors contributing to disease progression outside the ablation zone after ablation are poorly understood.
Methods
In this retrospective study, using next-generation sequencing, we identified genetic biomarkers associated with different patterns of progression following thermal ablation of CLM.
Results
A total of 191 ablation naïve patients between January 2011 and March 2020 were included in the analysis, and 101 had genomic profiling available. Alterations in the TGFβ pathway were associated with increased risk of development of new intrahepatic tumours (hazard ratio [HR], 2.75, 95% confidence interval [95% CI] 1.39–5.45, P = 0.004); and alterations in the Wnt pathway were associated with increased probability of receiving salvage LRT for any intrahepatic progression (HR, 5.8, 95% CI 1.94–19.5, P = 0.003).
Conclusions
Our findings indicate that genomic alterations in cancer-related signalling pathways can predict different progression patterns and the likelihood of receiving salvage LRT following percutaneous thermal ablation of CLM.
Subject terms: Colorectal cancer, Prognostic markers, Metastasis
Background
Approximately two-thirds of all deaths related to colorectal cancer are attributed to colorectal liver metastasis (CLM) [1]. The gold standard local treatment of CLM is surgical resection, which is associated with a 5-year overall survival (OS) rate of 16–74% [2]. However, only about 25% of patients with CLM are eligible for surgical resection [3]. Thermal ablation (e.g., radiofrequency ablation or microwave ablation) is an alternative curative-intent local treatment option for selected patients with unresectable CLM and is associated with a 5-year OS rate of 24–58% [4]. Retrospective studies have shown that resection provides better local tumour control, especially for lesions >3 cm, but thermal ablation has the advantage of being minimally invasive and is associated with low morbidity, and is easily repeatable [5, 6].
Knowledge of the likelihood of various outcomes after thermal ablation of CLM would help oncologists decide which patients are best treated with this approach and which patients might benefit more from other forms of therapy. The Cancer Genome Atlas project has identified molecular subtypes of colorectal cancer, which may foster the discovery of new therapeutic targets [7]. However, these findings have not yet been incorporated into research on thermal ablation for CLM. To date, the only molecular features that have been studied for prognostic value among patients undergoing thermal ablation of CLM are single mutations—specifically, RAS mutations and their influence on the progression of ablated tumours [8–10]. Molecular tumour profiling using next-generation sequencing will soon become more widely available and might offer useful information for the prognostication of patients with CLM eligible for thermal ablation. Since thermal ablation is a local therapy, the molecular tumour profile could be especially useful for predicting patterns of progression beyond the ablated tumour.
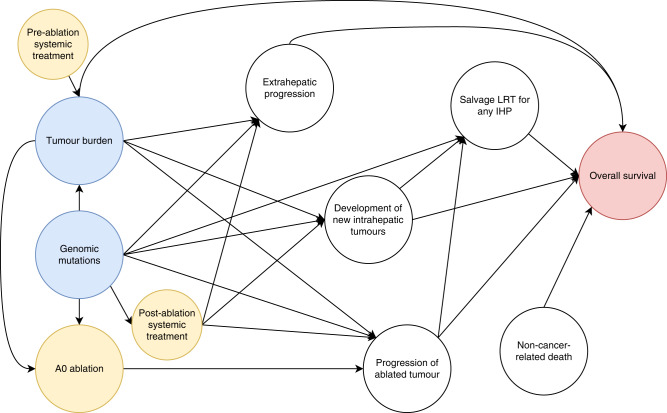
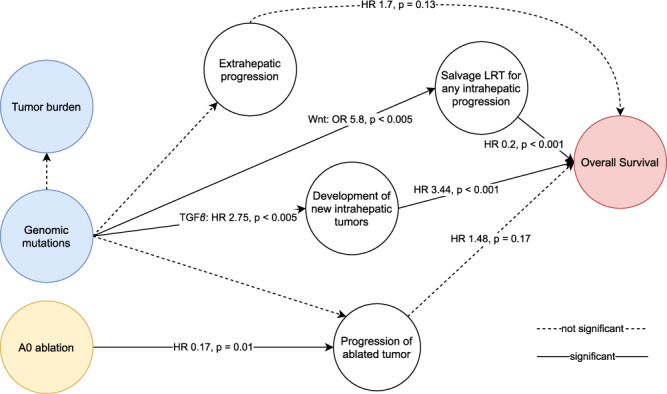
We hypothesised that genetic alterations in signalling pathways indirectly influence OS after thermal ablation of CLM via distinct progression patterns (Fig. 1). The aims of this study were to analyse the influence of disease progression patterns on OS and analyse the influence of genetic alterations in signalling pathways on these disease progression patterns.
Fig. 1. Directed acyclic graph of postulated causal relationships with OS after thermal ablation of CLM.
Treatments are depicted in yellow, risk factors are depicted in blue, and outcome is depicted in red. A0 ablation, defined as ablation with a >5 mm margin, has been reported multiple times to be the most important predictor of intrahepatic progression at the ablation zone following thermal ablation [9, 29–31]. In addition, factors reflecting tumour burden, like maximum tumour size, number of CLMs, extrahepatic metastasis (e.g., positive lymph nodes), and KRAS mutations, have been reported to further contribute to the progression of ablated tumours [9, 10]. Development of new intrahepatic tumours (IHP), extrahepatic progression (EHP), and salvage locoregional therapy (LRT) for any intrahepatic progression are drivers of OS [27]. In addition, non-cancer-related death are competing risks towards OS.
Methods
Study cohort
Patients who underwent percutaneous CT-guided thermal ablation (e.g., microwave ablation or radiofrequency ablation) for CLM between January 2011 and March 2020 at The University of Texas MD Anderson Cancer Center were identified from a prospectively managed institutional review board–approved registry. This retrospective study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. The requirement for written informed consent was waived for this retrospective analysis. Patients who received ablation combined with other LRT (e.g., resection, transarterial chemoembolization, radioembolization with Yttrium 90) in the same procedure, patients with less than 1 year of imaging follow-up, and patients who underwent non-thermal ablation were excluded from the study. From the patients included in the progression pattern analysis, patients with missing genetic sequencing, fewer than 46 genes sequenced, or missing APC mutation status (most frequently altered gene in the Wnt pathway) were excluded from the genomic mutation analysis. In patients who received repeated thermal ablation treatment, only the initial ablation treatment was included. This study was conducted according to the STROBE guidelines [11].
Data collection
Baseline characteristics per patient were collected from the electronic medical records. Procedural characteristics were retrieved from a custom structured report stored on the picture archiving and communication system for each CT-guided ablation intervention. Outcomes of patients treated before October 2015 were collected from the dataset of a previously published study [10]. Outcomes of the progression of ablated tumours were updated according to new criteria (at or within the ablation zone). Imaging follow-up of patients who were still alive or without disease progression at the conclusion of this study were updated. Follow-up information was collected until January 2022. All data were imported into the study database and validated independently by two investigators (IP and YML).
Somatic gene mutation profiling
Tumour DNA was extracted from formalin-fixed, paraffin-embedded samples from primary colorectal cancer. The Ion Torrent Personal Genome Machine (Life Technologies, Carlsbad, USA) was used to screen for mutations in cancer-related genes using an IT AmpliSeq cancer panel genomic library preparation protocol (Life Technologies) [12]. The reports of the mutational profiles were extracted from the institutional data warehouse and imported into the study database. The tested genes, exons, and codons of each panel are available in the supplementary materials (46-gene panel: Supplementary Table 6, 50-gene panel: Supplementary Table 7, 134-gene panel: Supplementary Table 8). One panel that was in use in 2018 had 143 tested genes but was missing the status of APC mutations – the predominantly mutated gene in the Wnt pathway. KRAS, NRAS, and BRAF mutations were grouped into a single category, RAS/BRAF mutation. This is supported by the fact that these mutations are mostly mutually exclusive and overlap functionally [13, 14].
Cancer-related signalling pathways
Genetic mutations in the AmpliSeq multigene panel were categorised according to the list by Sanchez-Vega et al. of ten canonical cancer-related signalling pathways [14]. Data were available for genes in seven of these pathways: p53, Wnt, RTK-RAS, TGFβ, PI3K, Notch and cell cycle. Data for genes in the Hippo, Myc and oxidative stress response/Nrf2 pathways was unavailable.
Image-guided thermal ablation procedure
Our institutional CT-guided percutaneous thermal ablation protocol has been reported in detail previously [10, 15]. In general, patients were eligible for percutaneous ablation if they presented with ≤5 CLMs and each was ≤5 cm in diameter. All procedures were performed under general anaesthesia and under CT guidance with additional ultrasound guidance if deemed necessary. Post-ablation contrast-enhanced CT was acquired for ablation confirmation if the renal function allowed the use of a contrast agent. Image fusion available on the CT console was used at the interventional radiologist’s discretion but no ablation confirmation software was used during the study period. Ablations were performed with radiofrequency ablation (Cool-tip Ablation System, Covidien, Boulder, USA) or microwave ablation (Certus Probe, Certus 140 2.4-GHz Ablation System, Neuwave, Madison, USA). Patients underwent initial post-ablation cross-sectional follow-up imaging within 2–8 weeks after ablation. After the initial post-ablation follow-up imaging, further follow-up imaging was performed at 2- to 6-month intervals until patient death or loss to follow-up. Cross-sectional follow-up imaging was performed with contrast-enhanced CT, positron emission tomography/CT, or magnetic resonance imaging.
Definitions
A0 ablation was defined as a tumour-free margin ≥5 mm, either estimated by visual inspection [10] or estimated using a software-based approach [16, 17]. To describe ablation endpoints, standardised terminology and reporting criteria were employed [18, 19]. All disease progression patterns are based on imaging follow-up findings. The start time for all time-to-event analyses was the date of ablation. In the survival analysis of OS survival after IHP, OS was measured from the time of diagnosis of IHP to death or last imaging follow-up. A residual tumour was defined as viable tumour within or at the edge of the ablation zone at initial follow-up imaging. Local tumour progression was defined as viable tumour within or at the edge of the ablation zone after at least one cross-sectional imaging study had demonstrated complete ablation. Time to progression of the ablated tumours was defined as the time from ablation to the first image evidence of residual tumour or local tumour progression. Thus, in patients treated for multiple CLMs, the time progression of the ablated tumours was measured on a patient level as the time to the residual tumour or local tumour progression at any of the ablation zones. The time to development of new intrahepatic tumours was defined as the time from ablation to the appearance of viable tumour within the liver outside the ablation zone. Intrahepatic progression of any kind is defined as either progression of the ablated tumours, the development of new intrahepatic tumours, or both. Extrahepatic progression was defined as the progression or new appearance of metastasis outside the liver. No disease progression was defined as no disease progression neither intrahepatic, nor extrahepatic after thermal ablation of CLM. Salvage LRT was defined as any LRT (e.g., ablation, resection, transarterial chemoembolization) for any intrahepatic progression. Patients ineligible for salvage LRT would undergo palliative treatment, including palliative chemotherapy, as per standard practice.
Statistical analysis
The Cox proportional hazards model was used to estimate the effects of progression patterns on OS and the effects of genetic mutations on the time to progression of the ablated tumour and the time to development of new intrahepatic tumours (time-to-event outcomes). Patients were right censored at the last imaging follow-up or death. Logistic regression was used to assess the effects of genetic mutations on salvage LRT after intrahepatic progression (binary outcome). All analyses were performed with multivariable models. The log-rank test was used to compare survival and cumulative incidence curves. A value of P < 0.05 was used for statistical significance, and two-sided 95% CIs were reported. A complete case analysis was performed. Estimates with infinite 95% CIs (e.g., estimates associated with rare mutations) were excluded from the model. Statistical analysis was performed using R (version 4.0.5) and RStudio (version 2021.09.2) [20]. Ggplot2 and ggpubr were used for graphics [21, 22], and tidyverse was used for data processing [23].
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Results
Study population
Of 226 patients who underwent initial percutaneous thermal ablation for CLM during the study period, 191 met the criteria for inclusion in the progression pattern analysis, and 101 (52.8%) met the criteria for inclusion in the pathway-centric genomic mutation sub-analysis (Fig. 2). The reasons for exclusion from the pathway-centric analysis were missing mutation profile (n = 41), <46 tested genes (n = 4), and profile with missing APC status (n = 45). The demographic and clinicopathologic characteristics of the study population are summarised in Table 1. The median age was 56.7 years (interquartile range [IQR] 48.6–66.6). Eighty-two patients (42.9%) died during the median follow-up period of 31.5 months (IQR 18.46–44.26). A total of 117 patients (61.3%) had previously undergone hepatic resection for CLM. One hundred thirty-five patients (70.7%) had intrahepatic progression of any kind. Among those, 43 patients (22.5%) had local progression of an ablated tumour, defined as the appearance of viable tumour at or within the ablation zone; and 121 patients (63.4%) had the development of new intrahepatic tumours; 143 patients (74.9%) had extrahepatic progression; 115 patients (60.2%) had multiple sites of progression; finally, 26 patients (13.6%) had no disease progression, of whom 20 were still alive at the time of data collection.
Fig. 2. Patient selection for progression pattern analysis and sub-analyses of genomic mutations.
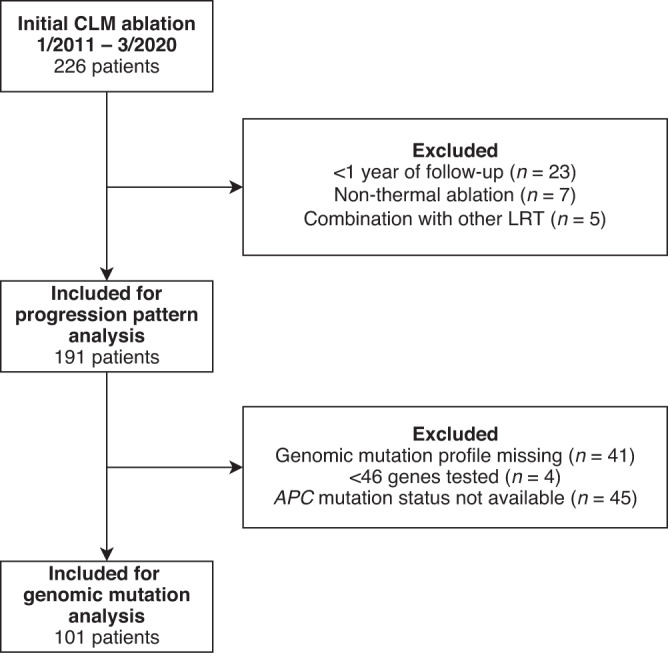
LRT locoregional therapy.
Table 1.
Baseline demographic and clinical characteristics of 191 patients who underwent initial thermal ablation for colorectal liver metastasis (CLM) and were included in this study per initial session.
| Characteristic | Value |
|---|---|
| Age, median (IQR), yr | 56.7 (48.6–66.6) |
| Male sex, no. (%) | 121 (63.4) |
| Rectal primary tumour, no. (%) | 39 (20.4) |
| Right-sided primary tumour, no. (%) | 45.0 (23.6%) |
| Node-positive primary tumour, no. (%) | 114 (59.7) |
| Liver metastasis, no. (%) | |
| Metachronous | 86 (45.0) |
| Synchronous | 102 (53.4) |
| N/A | 3 (1.6) |
| Prior hepatic resection for CLM, no. (%) | 117 (61.3%) |
| Extrahepatic metastasis, no. (%) | 111 (58.1) |
| Pre-ablation chemotherapy, no (%) | 142.0 (74.3%) |
| Fluorouracil-based regimen | 127 (66.5%) |
| Oxaliplatin | 74 (38.7%) |
| Irinotecan | 70 (36.6%) |
| Use of Bevacizumab | 90 (47.1%) |
| Lines of pre-ablation chemotherapy | |
| 0 | 49 (25.7%) |
| 1 | 83 (43.5%) |
| 2 | 54 (28.3%) |
| 3 | 5 (2.6%) |
| Number of ablated CLMs, median (IQR) | 1 (1–2) |
| Diameter of largest ablated CLM, median (IQR), cm | 1.4 (1.0–2.0) |
N/A not available, IQR interquartile range.
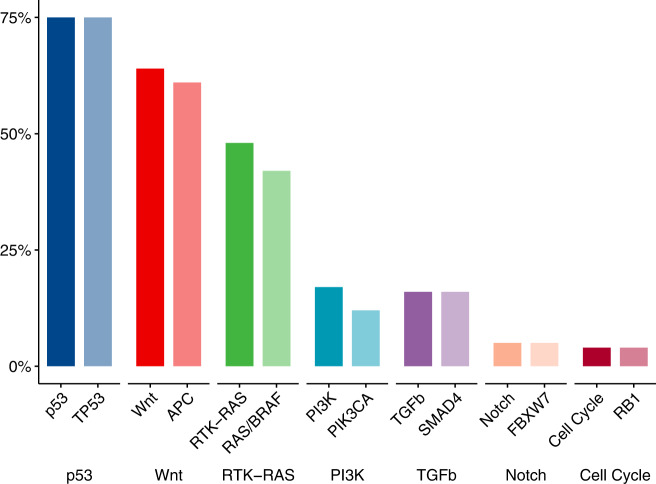
The most prevalent mutations were mutations in the p53 (74.3%), Wnt (63.4%) and RTK-RAS (47.5%) pathways (Fig. 3). Within these pathways, the vast majority of mutations were in the predominant genes TP53 (74.3%), APC (60.4%), and KRAS, NRAS, or BRAF (hereafter, referred to collectively as “RAS/BRAF”) (41.6%). The frequency of mutations by race and pathway is presented in Supplementary Fig. 1.
Fig. 3. Frequency of alterations in seven cancer-related signalling pathways. Alterations in the signalling pathways and mutations in the predominant member gene in each of these pathways among the 101 patients included in the mutation analysis.
Pathways are represented by solid bars and the member genes as light bars.
Effect of progression patterns on OS
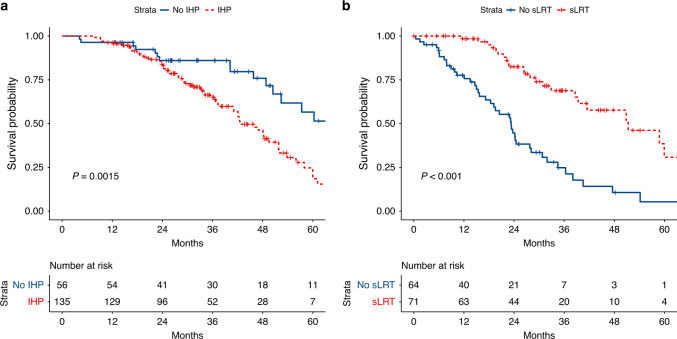
Supplementary Table 1 shows the effect of progression patterns on OS after thermal ablation of CLM after adjustment for clinicopathologic factors (age, prior hepatic resection, and tumour burden [diameter of largest CLM, number of CLMs, extrahepatic metastasis, and right-sided primary tumour]). Development of new intrahepatic tumours following ablation was associated with worse OS (HR = 3.44, 95% confidence interval [95% CI] 1.9–6.24, P < 0.001), whereas the progression of ablated tumours and extrahepatic progression were not. Salvage LRT for any intrahepatic progression was associated with better OS (HR 0.2, 95% CI 0.1–0.4, P < 0.001). These effects are also reflected in the Kaplan–Meier curves in Fig. 4, showing that patients with intrahepatic progression had significantly shorter OS (median OS 42.7 months, 95% CI 40.9–51.8) than patients without intrahepatic progression (median OS 70.3 months, 95% CI 52.4 to not reached) (P = 0.0015). Among the patients who had intrahepatic progression, those who received salvage LRT had significantly longer OS (median OS 51.3 months, 95% CI 39.3 to not reached) than patients who did not receive salvage LRT (median OS 23.3 months, 95% CI 18.4–28.1) (P < 0.001).
Fig. 4. Overall survival by intrahepatic progression and salvage LRT.
Left: OS according strativied by intrahepatic progression (IHP); Right: OS after IHP stratified by use of salvage LRT (sLRT). Startpoint for OS after IHP is the timepoint when IHP was first diagnosed.
Factors affecting intrahepatic progression patterns
Factors affecting intrahepatic progression of any kind
Factors affecting the intrahepatic progression of any kind after initial thermal ablation for CLM are reported in Supplementary Table 2. Alterations in the TGFβ pathway were associated with significantly worse intrahepatic progression-free survival, with a HR of 2.74 (95% CI 1.42–5.27, P = 0.003). Alterations in the Wnt pathway were associated with significantly better intrahepatic progression-free survival, with a HR of 0.56 (95% CI 0.33–0.94, P = 0.03). However, the cumulative incidence function does not show a significant difference (Supplementary Fig. 2). Further, A0 ablation was associated with significantly improved intrahepatic progression-free survival with a HR of 0.42 (95% CI 0.22–0.82, P = 0.01).
Factors associated with the development of new intrahepatic tumours
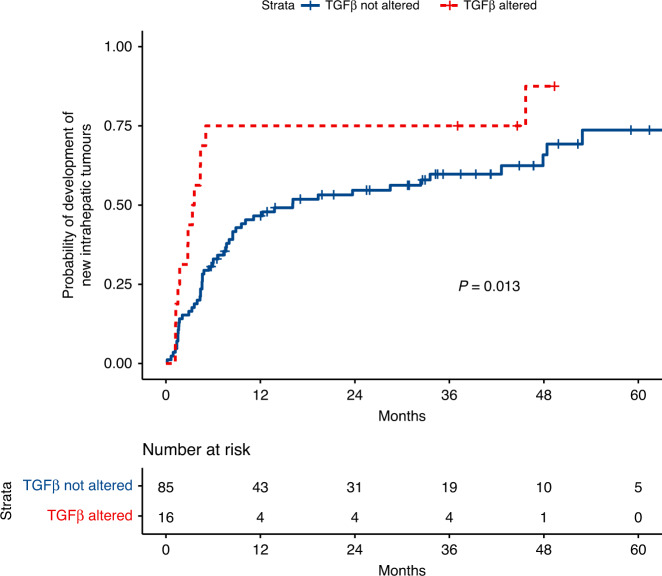
Factors associated with the development of new intrahepatic tumours (not including tumours at the edge of the ablation zone) after initial thermal ablation for CLM are reported in Supplementary Table 3. Alterations in the TGFβ pathway were associated with significantly shorter time to development of new intrahepatic tumours, with a HR of 2.75 (95% CI 1.39–5.45, P = 0.004). However, mutations in this pathway were relatively rare in this dataset (n = 16, all SMAD4 mutations). In addition, the overall number of ablated CLM at the initial session was associated with a significantly shorter time to development of new intrahepatic tumours (HR 1.77, 95% CI 1.2–2.63 P = 0.004). The median time to development of new intrahepatic tumours in patients with TGFβ alteration was 3.49 months (95% CI 1.75 to not reached) vs. 16.1 months (95% CI 8.5–47.9) (P = 0.013) (Fig. 5).
Fig. 5. Cumulative incidence of development of new tumours following initial ablation of CLM stratified by TGFβ alteration.
The cumulative incidence shows significantly faster time to development of new intrahepatic tumors for patients with alteration in the TGFβ pathway.
Factors associated with the progression of ablated tumours
Factors associated with the progression of ablated tumours after initial thermal ablation for CLM are reported in Supplementary Table 4. The main factors associated with progression of ablated tumours were A0 ablation (HR 0.17, 95% CI 0.05–0.66, P = 0.01), prior resection (HR 0.36, 95% CI 0.16–0.83, P = 0.016), and diameter of the largest ablated CLM (HR 1.59, 95% CI 1.04–2.42, P = 0.032). None of the altered pathways exhibited a significant effect on the progression of ablated tumours.
Factors associated with the use of salvage LRT
Factor associated with salvage LRT use following initial ablation are reported in Supplementary Table 5 and shows the ORs for receiving salvage LRT for intrahepatic progression among patients who developed any intrahepatic progression (n = 75). Alterations in the Wnt signalling pathway significantly increased the odds of receiving salvage LRT at the time of intrahepatic progression by 5.8-fold (95% CI 1.94–19.5, P = 0.003). Other altered pathways did not exhibit a significant influence on the odds of receiving salvage LRT.
Discussion
In this study, we have shown that the most important risk factors associated with worse OS following CLM ablation were the development of new intrahepatic tumours (HR 3.44, P < 0.001) and lack of salvage local therapy at the time of intrahepatic progression (HR = 0.2, P < 0.001). Alteration in the TGFβ pathway had a 2.75-fold increase in risk of the development of new intrahepatic tumours (HR 2.75, P = 0.004) and alteration in the Wnt pathway was associated with a reduced risk of any intrahepatic progression (HR 0.56, P = 0.03). Interestingly, patients with alterations in the Wnt pathway were 5.8 times more likely to receive salvage LRT if intrahepatic progression occurred (P = 0.003). However, with the present data, we could not identify a mediator via which mutations in Wnt would affect use of salvage LRT. None of the altered pathways examined exhibited a significant influence on the progression of the ablated tumour.
This study showed that different progression patterns had different effects on OS. Further, it showed that information about the genomic mutation status of CLMs provided valuable prognostic biomarkers of disease progression and the eligibility for salvage LRT. This kind of information per LRT (e.g., resection, ablation, transarterial chemoembolization) could be used to select optimal treatment modalities for patients with CLMs, depending on their individual risk for different progression patterns and thus the expected need for salvage LRT. In our study population, 86.4% of patients had progressive disease following ablation. More importantly, 70.7% of the patients had intrahepatic progression of any kind, which emphasises the role for salvage LRT, which was associated with significant OS improvement (median OS 51.3 months vs. 23.3 months, P < 0.001). However, only over half of the patients with intrahepatic progression received salvage LRT. The most common reason for ineligibility for salvage LRT was extrahepatic progression (>50%).
Several of our findings are in keeping with those previously reported in other studies of surgical and ablation series. Previous studies showed that patients receiving salvage LRT for intrahepatic progression following surgical resection had improved OS [24, 25], which is consistent with our finding of use of salvage LRT being a protective factor. Also, alterations in Wnt, and TGFβ have been found as protective and risk factors for OS, respectively, in a similar pathway-centric study of a cohort undergoing resection for CLM [26]. Salvage LRT for intrahepatic progression following thermal ablation of CLM has been reported to have a similar OS to those of patients without intrahepatic progression [27, 28], which is consistent with our finding of salvage LRT is associated with a reduction on the risk of death by 80%. A0 ablation (ablation with a margin of ≥5 mm) showed as the best predictor of lack of progression following ablation of CLM and was associated with an 83% risk reduction (HR = 0.17, P = 0.01), which has been reported in several previous studies on [9, 29–31]. We also confirmed in this expanded patient series our previous findings of a lower risk of intrahepatic progression at the ablation zone for patients with prior resection [15].
Current literature on the influence of RAS mutation on progression of ablated tumours has shown diverging results [9, 10, 29] and did not show a significant effect in our study cohort. We believe that the lack of RAS influence on the progression of ablated tumours in our present study might be attributed to several factors. First, we applied a stricter definition of the progression of ablated tumours than the one used in other published studies (any tumour directly in contact with or within the ablation zone vs. within 1 cm of the ablation zone), in agreement with current recommendations for standardised terminology [18, 32]. Second, the inclusion in our present analysis of additional tumour biomarkers not previously reported might have lessened the individual influence of RAS mutation among patients with multiple co-mutations. Third, there might be a selection bias since interventional radiologists may have performed more aggressive ablations in patients with RAS mutation with the aim to obtain larger ablation zones given our understanding of the recent literature [9, 10]. Finally, the inclusion in the multivariable analysis of margin assessment by using accurate ablation confirmation software might have further reduced the impact of RAS on the progression of ablated tumours, as demonstrated by the criticality of achieving A0 ablation.
This study has limitations due to its retrospective nature. Even though the study population is from a high-volume centre, genomic profiling with ≥46 genes and APC mutation status for first-time ablation treatments was available in only over half of the patients undergoing thermal ablation for CLM. One of the main reasons is that a large number of patients were referred to our institution from other institutions and underwent resection of their primary tumour at another institution. The resulting small sample size led to relatively low statistical power, and therefore it is likely that some true effects were not discovered, especially effects related to rarely altered genes. Due to the small sample size, we could also not translate the found effects of mutations on progression patterns directly onto OS. Our findings might also reflect the intrinsic practice patterns of our institution regarding the management of CLM, and therefore the findings require external validation. Due to the heterogeneous patient population referred to liver ablation at our centre, we did not analyse the effect of mutations on initial tumour burden. Further, there were major advances in percutaneous liver ablation procedures during the study period. Another limitation is very heterogenous pre- and post-ablation systemic treatment, which might have contributed to the progression patterns and affected the overall survival. These variations are likely due to the low level of evidence about the benefit of (neo-)adjuvant chemotherapy combined with thermal ablation of CLM [6].
In conclusion, our findings indicate that genomic alterations can predict different progression patterns following the thermal ablation of CLM. Alternation in the TGFβ pathway was associated with an increased risk of development of new tumours, and Wnt pathway alteration showed an increased likelihood of receiving salvage LRT following post-ablation intrahepatic recurrence, with both having direct implications on survival outcomes.
Supplementary information
Acknowledgements
We thank Stephanie Deming, Research Medical Library, MD Anderson Cancer Center, for editing the manuscript.
Author contributions
IP, BCO and JNV: study design; IP, YML and BCO: writing and editing; IP, YML, BCO, YK and HM: data collection and data review; IP: statistical analysis; IP, YML, YK, HM, AKJ, MC, SK, TN, KKB and BCO: critical review and editing; BCO: supervision of the project. All authors have read and agreed to this version of the manuscript.
Funding
IP is funded by a PostDoc Mobility Fellowship from the Swiss National Science Foundation under project number P2BEP3_195444. Research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under award numbers R01CA235564 and P30CA016672 and by the Image Guided Cancer Therapy Research Program at The University of Texas MD Anderson Cancer Center.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available because of human subjects' privacy concerns and institutional policies. Anonymized data are available from the corresponding author upon reasonable request.
Code availability
The complete R code for the statistical analysis is available in the supplementary materials.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This retrospective study was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board (Nr: 2021-0340) and conducted in accordance with the Declaration of Helsinki. The requirement for written informed consent was waived.
Consent to publish
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-022-02030-y.
References
- 1.Valderrama-Trevino AI, Barrera-Mera B, Ceballos-Villalva JC, Montalvo-Jave EE. Hepatic metastasis from colorectal cancer. Euroasian J Hepatogastroenterol. 2017;7:166–75. doi: 10.5005/jp-journals-10018-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. doi: 10.1186/1471-2407-14-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin YM, Paolucci I, Brock KK, Odisio BC. Image-guided ablation for colorectal liver metastasis: principles, current evidence, and the path forward. Cancers. 2021;13:3926. doi: 10.3390/cancers13163926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kron P, Linecker M, Jones RP, Toogood GJ, Clavien PA, Lodge JPA. Ablation or resection for colorectal liver metastases? A systematic review of the literature. Front Oncol. 2019;9:1052. doi: 10.3389/fonc.2019.01052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillams A, Goldberg N, Ahmed M, Bale R, Breen D, Callstrom M, et al. Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, The Interventional Oncology Sans Frontieres meeting 2013. Eur Radiol. 2015;25:3438–54. doi: 10.1007/s00330-015-3779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang BB, Yan K, Zhang ZY, Yang W, Wu W, Yin SS, et al. The value of KRAS gene status in predicting local tumor progression of colorectal liver metastases following radiofrequency ablation. Int J Hyperthermia. 2019;36:211–9. doi: 10.1080/02656736.2018.1556818. [DOI] [PubMed] [Google Scholar]
- 9.Calandri M, Yamashita S, Gazzera C, Fonio P, Veltri A, Bustreo S, et al. Ablation of colorectal liver metastasis: interaction of ablation margins and RAS mutation profiling on local tumour progression-free survival. Eur Radiol. 2018;28:2727–34. doi: 10.1007/s00330-017-5273-2. [DOI] [PubMed] [Google Scholar]
- 10.Odisio BC, Yamashita S, Huang SY, Harmoush S, Kopetz SE, Ahrar K, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg. 2017;104:760–8. doi: 10.1002/bjs.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–24. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Singh RR, Patel KP, Routbort MJ, Reddy NG, Barkoh BA, Handal B, et al. Clinical validation of a next-generation sequencing screen for mutational hotspots in 46 cancer-related genes. J Mol Diagn. 2013;15:607–22. doi: 10.1016/j.jmoldx.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell. 2018;173:321–37. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odisio BC, Yamashita S, Huang SY, Kopetz SE, Ahrar K, Mizuno T, et al. Impact of prior hepatectomy history on local tumor progression after percutaneous ablation of colorectal liver metastases. J Vasc Interv Radiol. 2018;29:395–403. doi: 10.1016/j.jvir.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson BM, Lin YM, Lin EY, Cazoulat G, Gupta S, Kyle Jones A, et al. A novel use of biomechanical model-based deformable image registration (DIR) for assessing colorectal liver metastases ablation outcomes. Med Phys. 2021;48:6226–36. doi: 10.1002/mp.15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson BM, Lin EY, Cardenas CE, Gress DA, Erwin WD, Odisio BC, et al. Automated contouring of contrast and noncontrast computed tomography liver images with fully convolutional networks. Adv Radiat Oncol. 2021;6:100464. doi: 10.1016/j.adro.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria−a 10-year update. J Vasc Interv Radiol. 2014;25:1691–705. doi: 10.1016/j.jvir.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puijk RS, Ahmed M, Adam A, Arai Y, Arellano R, de Baere T, et al. Consensus guidelines for the definition of time-to-event end points in image-guided tumor ablation: results of the SIO and DATECAN initiative. Radiology. 2021;301:533–40. doi: 10.1148/radiol.2021203715. [DOI] [PubMed] [Google Scholar]
- 20.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019.
- 21.Alboukadel Kassambara. ggpubr: ’ggplot2’ Based Publication Ready Plots. R package version 0.4.0. 2020. https://CRAN.R-project.org/package=ggpubr.
- 22.Wickham H. ggplot2: elegant graphics for data analysis. 2nd edn. New York: Springer-Verlag; 2016.
- 23.Wickham H, Averick M, Bryan J, Chang W, McGowan L, Françoi R, et al. Welcome to the Tidyverse. J Open Source Softw. 2019;4:1686.
- 24.Andreou A, Knitter S, Schmelzle M, Kradolfer D, Maurer MH, Auer TA, et al. Recurrence at surgical margin following hepatectomy for colorectal liver metastases is not associated with R1 resection and does not impact survival. Surgery. 2021;169:1061–8. doi: 10.1016/j.surg.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C. Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg. 2016;263:146–52. doi: 10.1097/SLA.0000000000001194. [DOI] [PubMed] [Google Scholar]
- 26.Kawaguchi Y, Kopetz S, Kwong L, Xiao L, Morris JS, Tran Cao HS, et al. Genomic sequencing and insight into clinical heterogeneity and prognostic pathway genes in patients with metastatic colorectal cancer. J Am Coll Surg. 2021;233:272–84. doi: 10.1016/j.jamcollsurg.2021.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology. 2016;278:601–11. doi: 10.1148/radiol.2015142489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–68. doi: 10.1148/radiol.12111851. [DOI] [PubMed] [Google Scholar]
- 29.Ruiter SJS, Tinguely P, Paolucci I, Engstrand J, Candinas D, Weber S, et al. 3D quantitative ablation margins for prediction of ablation site recurrence after stereotactic image-guided microwave ablation of colorectal liver metastases: a multicenter study. Front Oncol. 2021;11. [DOI] [PMC free article] [PubMed]
- 30.Shady W, Petre EN, Do KG, Gonen M, Yarmohammadi H, Brown KT, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29:268–75. doi: 10.1016/j.jvir.2017.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laimer G, Schullian P, Jaschke N, Putzer D, Eberle G, Alzaga A, et al. Minimal ablative margin (MAM) assessment with image fusion: an independent predictor for local tumor progression in hepatocellular carcinoma after stereotactic radiofrequency ablation. Eur Radiol. 2020;30:2463–72. doi: 10.1007/s00330-019-06609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puijk RS, Ahmed M, Adam A, Arai Y, Arellano R, de Baere T, et al. Consensus guidelines for the definition of time-to-event end points in image-guided tumor ablation: results of the SIO and DATECAN initiative. Radiology. 2021;203715; 10.1148/radiol.2021203715. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available because of human subjects' privacy concerns and institutional policies. Anonymized data are available from the corresponding author upon reasonable request.
The complete R code for the statistical analysis is available in the supplementary materials.