Abstract
Controlling the sequence of the three consecutive reactive carbon centres of Cu-allenylidene remains a challenge. One of the impressive achievements in this area is the Cu-catalyzed annulation of 4-ethynyl benzoxazinanones, which are transformed into zwitterionic Cu-stabilized allenylidenes that are trapped by interceptors to provide the annulation products. In principle, the reaction proceeds via a preferential γ-attack, while annulation reactions via an α- or β-attack are infrequent. Herein, we describe a method for controlling the annulation mode, by the manipulation of a CF3 or CH3 substituent, to make it proceed via either a γ-attack or an α- or β-attack. The annulation of CF3-substituted substrates with sulfamate-imines furnished densely functionalized N-heterocycles with excellent enantioselectivity via a cascade of an internal β-attack and an external α-attack. CH3-variants were transformed into different heterocycles that possess a spiral skeleton, via a cascade of an internal β-attack and a hydride α-migration followed by a Diels−Alder reaction.
Subject terms: Asymmetric catalysis, Asymmetric synthesis
Copper-catalysed annulation of allenylidenes provides an opportunity to target the alpha, beta, or gamma positions of the allenylidene, but achieving this control is challenging. Here substrate control allows selectivity between these three positions in the enantioselective cyclisation of ethynyl benzoxazinanones and sulfamate-derived cyclic imines.
Introduction
Due to their widespread occurrence in nature, indoles and indolines are considered important structural motifs in biologically active molecules, and they are often associated with impressive bioactivity1–7. In particular, polycyclic indole/indoline scaffolds that bear multiple stereocenters have received substantial attention from the pharmaceutical industry due to the intriguing drug-like space they present. This is exemplified in particular by the complex molecular structures of alkaloids (Fig. 1a)8–12. Conversely, non-natural/artificial organic compounds that possess a trifluoromethyl (CF3) group at a stereogenic carbon centre, such as efavirenz and DPC 083 (anti-HIV drugs), lotilaner (a veterinary drug), and esaxerenone (a nonsteroidal antimineralocorticoid) have been very successful on the pharmaceutical and agricultural markets (Fig. 1b)13–16. Efficient methods for the construction of alkaloid-like polycyclic indole scaffolds that contain a CF3 group at a stereogenic carbon centre would thus be a great advantage for the production of chemically novel drugs17–22.
Fig. 1. Examples of biologically active N-heterocycles.
a Representative biologically active alkaloids with polycyclic indole/indoline scaffolds. b Representative synthetic drugs (pharmaceuticals and agrochemicals) with a CF3 group at a stereogenic carbon centre.
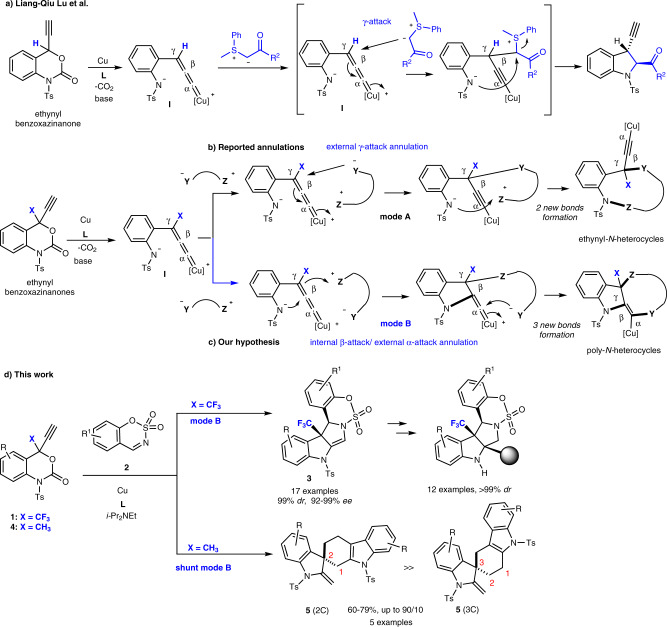
To this end, we are interested in cascade annulation reactions using 4-ethynyl benzoxazinanone23–41. Xiao, Lu, and co-workers synthesized ethynyl benzoxazinanone in 2016. Using Cu-catalysis, ethynyl benzoxazinanone was converted into the reactive zwitterionic Cu-allenylidene intermediate I via a decarboxylation. I was smoothly intercepted with various sulfur ylides to provide 3-ethynyl-indolines via a [4 + 1] cycloaddition reaction (Fig. 2a)23. Ethynyl benzoxazinanone soon became a popular tool for Cu-catalyzed ethynylative annulations. In the presence of suitable interceptors, various ethynyl-N-heterocycles can be constructed from 4-ethynyl benzoxazinanones in a similar fashion (Fig. 2b)23–41. That is, the intermolecular annulations start with an attack of the interceptor (Y−―Z+) at the γ-position of Cu-allenylidene intermediate I followed by the formation of the nitrogen-Z (N-Z) bond and the regeneration of the ethynyl moiety.
Fig. 2. Cu-catalyzed decarboxylative annulation of ethynyl benzoxazinanone.
a The seminal work demonstrating the Cu-catalyzed decarboxylative [4 + 1] annulation of ethynyl benzoxazinanone with sulfur ylides. b A representative annulation mechanism of ethynyl benzoxazinanones with an interceptor (Y−―Z+) using Cu-catalysis (mode A). c Our hypothesis for a different annulation mode (mode B). d This work: annulation reactions of ethynyl benzoxazinanones (1: X = CF3; 4: X = CH3) that provide pentacyclic-fused CF3-indolines 3 via mode B, and non-fluorinated spiral pentacyclic carbazole/indoline molecules 5 via shunt mode B.
Cu-allenylidene intermediates such as I contain three consecutive reactive carbon centres that are labelled, starting from the Cu atom, as the α, β, and γ-positions42–45. In principle, the reaction proceeds via mode A and involves a preferential attack of the interceptor at the γ-position of I to furnish the ethynyl-N-heterocycles having two new-bonds (mode A, Fig. 2b)23–33. Despite the rich reactivity of Cu-allenylidene intermediates such as I, annulation reactions that proceed via an α- or β-attack rather than a γ-attack have remained scarce39. We have hypothesized that annulation mode B involving preferential α- and β-attacks could be realized by controlling the steric and electric factors of suitable substituents X (X ≠ H). Thus, annulation mode B should arise from the pairwise combination of a successive internal β-attack and an external α-attack of I by the interceptor (Y−―Z+). Subsequent formation of a Cγ-Z bond would provide the non-ethynyl, poly-N-heterocycles with three new-bonds formation (Fig. 2c). During our research into the development of efficient synthetic methods for the synthesis of fluorine-containing heterocyclic compounds for drug discovery46–57, we noticed that the use of a CF3 substituent as the X group can direct the reaction pathway from mode A to B55. Herein, we realize the idea of a cascade of inter- and intramolecular annulations (mode B), which involves both α- and β-attacks by employing 4-ethynyl-4-CF3-benzoxazinanones 1 (X = CF3) and cyclic sulfamate-imines 2 (Fig. 2d; mode B). A wide variety of densely functionalized indoline heterocycles 3 that contain a CF3 group can be obtained in high yield with excellent diastereoselectivity and enantioselectivity (up to 99% dr and 99% ee). Examples of reactions that generate all-carbon CF3 quaternary stereocenters at the angular position are extremely rare58,59, and therefore the obtained results should accelerate corresponding areas of research, especially drug-discovery. The copper-catalyzed asymmetric synthesis via a Cu-allenylidene intermediate attracts much attention60–62. Substantial transformations of 3 into more complex molecules are also demonstrated.
The concept of altering the annulation mode by controlling the reactivity of the Cu-stabilized allenylidene intermediate I can also be applied to non-fluorinated substrates. Ethynyl benzoxazinanones 4 that contain a methyl (CH3) group instead of a CF3 group are transformed into very different poly-N-heterocycles with a spiral carbazole/indoline skeleton (5) in good yield with high regioselectivity (Fig. 2d; shunt mode B). The unusual formation of 5 occurs via a shunt pathway of mode B that involves the decarboxylative generation of I, followed by a cascade process that involves a cyclization, hydride-α-migration, and a Diels−Alder reaction. The series of spiral poly-N-heterocycles 5 generated here are also alkaloid-like indole-rich- molecules. Thus, this method can be expected to serve as a powerful tool for the generation of a drug-like space in a single step.
Results and discussion
Annulation reaction using 4-ethynyl CF3-benzoxazinanone 1
We commenced our investigation with an annulation reaction of 4-ethynyl CF3-benzoxazinanone 1a and sulfamate-derived cyclic imine 2a at room temperature in the presence of CuOTf·1/2 C6H6 (10 mol%), a methyl-substituted Pybox ligand (L1, 20 mol%), and i-Pr2NEt (2.4 equiv) in toluene (Table 1, entry 1). To our delight, the reaction proceeded smoothly and delivered the polycyclic indoline (3aa) that bears a CF3 group at the all-carbon quaternary centre. However, the yield of 3aa was only moderate and the enantioselectivity was poor (47% and 15% ee). Encouraged by this initial attempt, we systematically evaluated several chiral ligands (entries 2−4) and found that the phenyl-substituted Pybox ligand L2 stood out, producing the desired product 3aa in 70% yield with excellent enantioselectivity 96% ee (entry 2). Further results of the other ligands screened, such as L5 and (R)-BINAP are shown in Supplementary Table 1 in the Supporting Information. An investigation into the effect of the solvent on the reaction (Supplementary Table 2) revealed that toluene provides better reaction efficiency than other solvents. Gratifyingly, an evaluation of different Cu salts (entries 5−7 and Supplementary Table 3) revealed that Cu(OTf)2 (5 mol%) and L2 (10 mol%) resulted in an improved reaction efficiency with a slightly lower yield (68%) and enhanced enantioselectivity (98% ee). A slightly improved yield was observed when the reaction was performed with 1.1 equiv of 2a (entry 8, 71% yield, 98% ee; for more details, see Supplementary Table 4). An evaluation of different bases (Supplementary Table 5) showed that i-Pr2NEt was superior to other bases and the reaction efficiency was further improved by employing 0.5 equiv of the base (entry 9, 88% yield, 98% ee). The most favourable outcome was observed when the reaction was performed at 10 °C (entry 10, 94% yield, >99% dr, >99% ee). Further experiments revealed that the presence of the base was necessary for this transformation to proceed (entry 11). The absolute configuration of 3aa, induced by L2, was determined by a single-crystal X-ray diffraction analysis (CCDC2026703).
Table 1.
Optimization of the reaction conditions for the Cu-catalyzed annulation of 1a with 2aa.
 | |||||
|---|---|---|---|---|---|
| Entry | Ligand | Cu | drb | Yield (%)b | ee (%)c |
| 1 | L1 | CuOTf·1/2C6H6 | >95:5 | 47 | 15 |
| 2 | L2 | CuOTf·1/2C6H6 | >99:1 | 70 | 96 |
| 3 | L3 | CuOTf·1/2C6H6 | - | 28 | - |
| 4 | L4 | CuOTf·1/2C6H6 | >95:5 | 32 | 75 |
| 5 | L2 | Cu(OTf)2 | >99:1 | 71 | 99 |
| 6 | L2 | CuI | - | 21 | - |
| 7d | L2 | Cu(OTf)2 | >99:1 | 68 | 98 |
| 8d, e | L2 | Cu(OTf)2 | >99:1 | 71 | 98 |
| 9d, e, f | L2 | Cu(OTf)2 | >99:1 | 88 | 98 |
| 10d, e, f, g | L2 | Cu(OTf)2 | >99:1 | 94 | >99 |
| 11d, e, h | L2 | Cu(OTf)2 | - | NR | - |
aReaction conditions: 1a (0.1 mmol), 2a (0.1 mmol), [Cu] cat. (10 mol%), ligand (20 mol%), i-Pr2NEt (2.4 equiv) in dry toluene at rt.
bDetermined by 19F NMR analysis of the crude reaction mixture using PhCF3 as the internal standard.
cDetermined by chiral HPLC analysis.
dUsing Cu(OTf)2 (5 mol%) and L2 (10 mol%).
eUsing 2a (0.11 mmol).
fUsing i-Pr2NEt (0.5 equiv).
gReaction at 10 °C for 16 h.
hWithout base.
Substrate scope
With the optimal catalyst identified and the standard conditions in hand, we studied the scope of the sulfamate-derived cyclic imines 2 for this enantioselective decarboxylative annulation reaction. The results are summarized in Fig. 3. Cyclic sulfamate imines (2a-2i) that bear a variety of substituents at different positions of the benzene ring, regardless of whether they are electron-donating or electron-withdrawing groups, were tolerated and delivered the annulated products (3aa-3ai) in good to excellent isolated yield (52−91%) with excellent enantioselectivity (>92% ee). The variation of the substituent pattern has thus merely a marginal impact on the selectivity. For instance, substrates that bear halogen substituents such as 6-F (2d), 6-Br (2e), or 7-Cl (2i) reacted smoothly and delivered the desired products in decent yield (71−91%) with respectable enantioselectivity. We observed that the selectivity was slightly decreased from 7-halo (3ai; 98% ee) substitution to 6-halo (3ad, 3ae; 95% ee) substitution. Although 6-NO2 substituted cyclic imine 2 f led to a slightly lower yield, a high enantioselectivity was still achieved in this reaction (3af; 52%, 92% ee). Moreover, the naphthalene fused cyclic imine 2j reacted smoothly to produce the desired product in excellent yield and enantioselectivity (3aj; 87%, 99% ee).
Fig. 3. Substrate scope for the mode B annulation of 1 with 2.
Reaction conditions: 1 (0.1 mmol), 2 (0.11 mmol), Cu(OTf)2 (5 mol%), L2 (10 mol%), and i-Pr2NEt (0.05 mmol) in 1.0 mL of toluene. The yield values refer to the isolated yield. In all cases, a > 99:1 diastereomeric product (3) ratio was obtained. The ee values were determined by chiral HPLC analysis.
Further experiments were performed in order to evaluate the generality of the reaction. Substituents were introduced at different positions on the benzoxazinanone moiety to create excellent reaction partners and resulted in the desired products with good yield and enantioselectivity. Substrates bearing electron-withdrawing groups, such as 7-CF3 (1b), 6-F (1d), and 6-Cl (1e) reacted efficiently with different sulfamate-derived cyclic imines (2) and produced the desired products in good yield (>81%) with excellent enantioselectivity (up to 99% ee). Notably, the introduction of an ester group at the 7-position of the benzoxazinanone (1c), led to a similar product (3ca) in good yield (72%) with excellent diastereoselectivity (>99% dr) and enantioselectivity (96% ee). Nevertheless, this result should be noted due to the survival of the ester moiety under the applied reaction conditions. The reaction with an electron-donating substituent (CH3) at the 6-position of the benzoxazinanone moiety gave the desired product in a decent yield with optimum enantioselectivity (3fa; 77%, 98% ee). The stereochemistry of these products was assigned in analogy with 3aa. In all cases, the diastereoselectivity of 3 was found to be absolute.
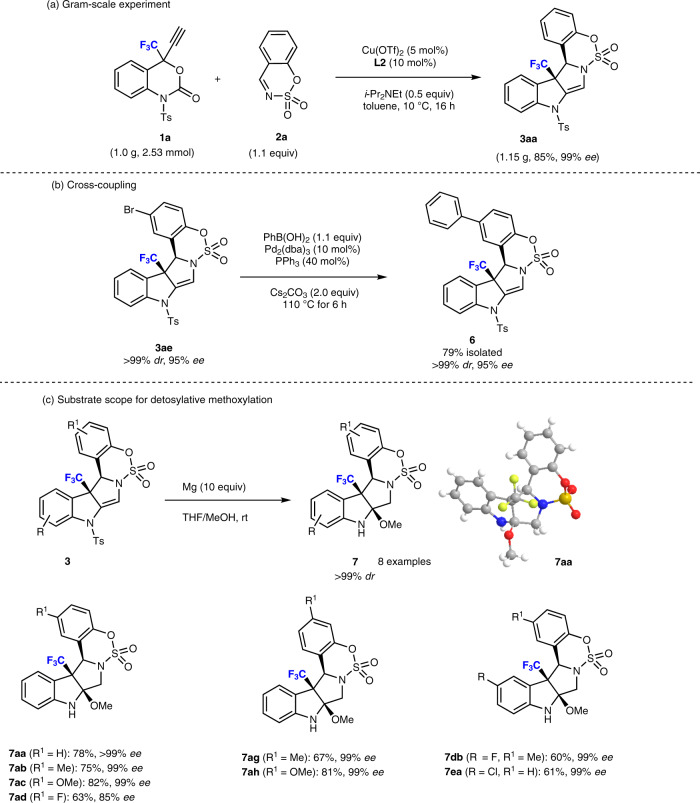
Synthetic utility I
To further showcase the synthetic potential of this Cu-catalyzed decarboxylative annulation reaction, a gram-scale synthesis of 3aa was carried out, which achieved an 85% isolated yield without deterioration of the optical purity (Fig. 4a). Gratifyingly, the Pd-catalyzed Suzuki coupling of bromo-substituted-indoline 3ae with phenylboronic acid afforded biphenyl product 6 in good yield under retention of the enantiopurity (Fig. 4b). As the removal of a p-toluenesulfonyl (tosyl) group from an amide usually requires relatively harsh reaction conditions63, we were concerned prior to attempting the detosylation of 3 due to its high-density functional structure. Interestingly, treatment of 3aa with Mg/MeOH under sonication generated another stereocenter, in which successive detosylation/methoxylation reactions occur in a single step and result in the angular methoxylated product 7aa in 78% yield with outstanding stereoselectivity (>99% dr). The absolute configuration of 7aa was determined by single-crystal X-ray diffraction analysis (CCDC2026704, Fig. 4c). The scope of the detosylative methoxylation was extended to different substrates, and the results are summarized in Fig. 4c. Various substituents on the benzene ring with electronically different properties were well tolerated and gave the corresponding product 7 in moderate to good yield (>63%) with excellent stereoselectivity (up to 99% ee). Moreover, halo-substituted indolines (3db, 3ea) afforded the desired products 7db (60%) and 7ea (61%) in moderate yield with very good selectivity (99% ee). In all cases, the enantiopurity was retained at 99% ee except the case of 7ad, and the sulfamate moiety remained intact under the detosylation conditions.
Fig. 4. Synthetic utility I.
a Gram-scale reaction of 1 with 2. b Cross-coupling reaction using 3. c Detosylative methoxylation of 3 to 7.
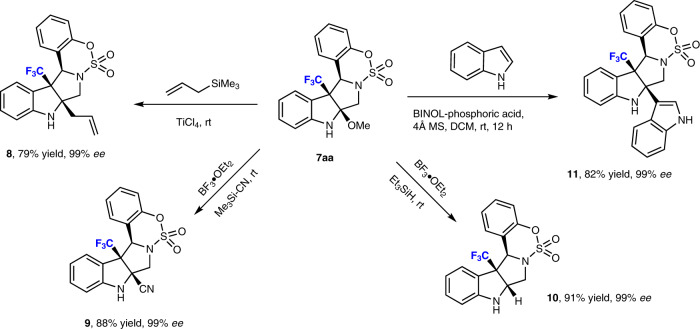
Synthetic utility II
To further explore the synthetic utility of this transformation, we decided to screen a number of acid-mediated substitution reactions of masked methoxy indole 7aa with the goal of introducing a substituent at the 2-position (Fig. 5). The Lewis-acid-mediated reaction of 7aa with allyltrimethylsilane afforded the desired allyl-substituted product 8 in 79% yield with 99% ee. Treatment of 7aa with trimethylsilyl cyanide (Me3Si-CN) and triethylsilane (Et3SiH) delivered the corresponding 2-cyano-product 9 and the reductive product 10 in 88 and 91% yield, respectively, without compromising the enantiopurity. The phosphoric-acid-catalyzed reaction of 7aa with indole furnished the sterically complex poly-N-heterocycle 11 in 82% yield under retention of the enantiopurity. These results suggest that methoxy poly-N-heterocycle 7 is a versatile compound that can be easily converted into synthetically challenging di-angular-substituted products in promising yields with optimum enantiopurity.
Fig. 5. Synthetic utility II.
Examples of Lewis-acid-mediated functionalization reactions of 7.
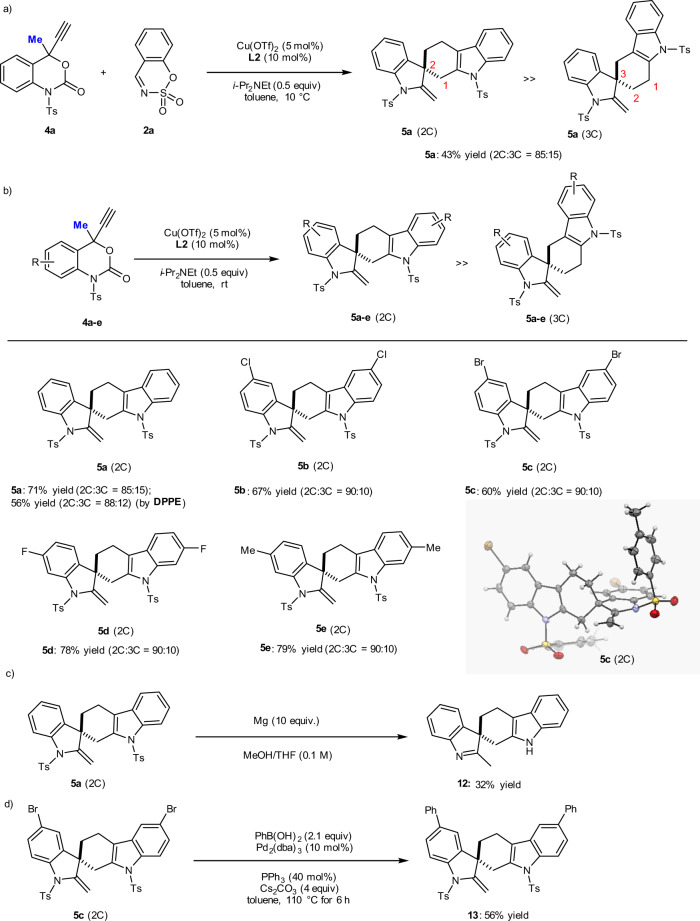
Annulation reactions using non-fluorinated, ethynyl CH3-benzoxazinanones
Next, we attempted the decarboxylative annulation of non-fluorinated, 4-ethynyl-4-CH3-benzoxazinanones 4 with 2 under the reaction conditions optimized for 1 (Fig. 6a). To our great surprise, a mixture of regioisomers of poly-N-heterocycles with a spiral carbazole/indoline skeleton 5a was obtained in 43% yield with high regioselectivity (2C/3C = 85:15). The expected cycloaddition product bearing the 2a sulfamate moiety was not formed in detectable quantities. The unique, alkaloid-like structure of 5a led us to investigate the scope of this regioselective transformation of 4 to 5. We thus treated 4a under the same catalytic conditions but without the addition of 2a. As expected, this transformation is generally applicable, and a variety of analogues of 4 were promptly converted into the corresponding spiro-carbazole/indoline molecules (Fig. 6b) in good to high yield (60−79%) with high 2C-regioselectivity (85:15-90:10). The poly-N-heterocyclic structure of 5 (2C) was determined unambiguously by single-crystal X-ray diffraction analysis of the brominated spiro-N-heterocycle 5c (2C). The X-ray crystal structure of 5c (2C) (CCDC2026705) and the HPLC analysis of 5a conclusively show that 5 is a racemate, which is useful information for the discussion of the reaction mechanism (vide infra). The transformation of 4a also proceeded smoothly with the non-chiral ligand 1,2-bis(diphenylphosphino)ethane (DPPE) to give the same spiro-carbazole/indoline 5a in similar yield (56%) and regioisomeric ratio (2C:3C = 88:12). The results using other ligands were also attempted and similar results were obtained (Supplementary Table 7). Removal of the tosyl group of 5a (2C) was achieved with Mg in MeOH/THF to yield spiro carbazole/indole derivative 12 with a 2-CH3-3H-indole skeleton (Fig. 6c). Suzuki−Miyaura coupling reaction of 5c (2C) with phenyl boronic acid (PhB(OH)2) under Pd-catalysis gave the bis-coupling product 13 in 56% yield (Fig. 6d).
Fig. 6. Cu-catalyzed decarboxylative annulation of CH3-benzoxazinanones 4.
a Reaction of 4a with 2a under Cu-catalysis. b Substrate scope for the Cu-catalyzed decarboxylative formation of spiral carbazole/indoline derivatives 5 from 4. c Detosylation of 5a. d Suzuki−Miyaura coupling reaction of 5c (2C) with PhB(OH)2.
Proposed reaction mechanisms
Based on the experimental results and our own hypotheses, we propose a feasible reaction mechanism to rationalize the formation of polycyclic merged indolines 3 from the reaction of 4-ethynyl 4-CF3-benzoxazinanones 1 with cyclic sulfamate imines 2 (Fig. 7a). Initially, a Cu catalyst (stabilized by its ligands) reacts with 1a in the presence of a base (i-Pr2NEt) to generate Cu acetylide A. Subsequently, the decarboxylation reaction of A generates zwitterionic Cu-allenylidene intermediate I. Due to the presence of the sterically demanding CF3 group at the γ-allenyl position, the cyclic sulfamate imine 2a does not smoothly approach the expected γ-position of I for the annulation mode A. A double-helical, pseudo-C2-symmetrical architecture of Cu-complex I optimized by DFT calculations is displayed with their selected atomic charge distributions (Fig. 7b, see more details in Supplementary Fig. 1). Ligand L1 was used for computations. The computed optimized conformation supports the fact that the CF3 group highly blocks the Cγ-position of I. Thus, a conventional γ-attack would be unfavourable. Besides, the nitrogen atom is close to the β-carbon of Cu-allenylidene intermediate I (N---Cβ: 2.59744 Å). It should be interesting to note that the β-carbon is more positive electronic density than others (Cα: −0.746; Cβ: 0.187; Cγ: −0.149). Annulation mode B should then arise from the pairwise combination of a successive internal β-attack by the anionic amide moiety in zwitterionic Cu-allenylidene intermediate I to zwitterionic Cu-indoline intermediate II, and a [2 + 3] cyclization of II and 2a, respectively. The [2 + 3] cyclization step of II and 2a consists of the formation of a C−C bond between the γ-carbon (Cu-indoline II) and the α-carbon (imine 2a), an external α-attack on II by the sulfamate imine 2a providing the [2 + 3] annulation product B as a Cu-salt. Finally, 3aa is obtained from protonation of intermediate B mediated by i-Pr2NEt, followed by the regeneration of the Cu catalyst in the final step. As illustrated in I and II, it should be noted that the β-cation of Cu-allenylidene zwitterionic intermediate I and γ-anion of Cu-indoline zwitterionic intermediate II are likely to be stabilized by negative hyperconjugation induced by a neighbouring CF3-substituent64–67. A newly generated (S)-CF3 at an angular position can be explained by the Si-face approach of the sulfamate imine 2a, based on the optimized conformation of Cu-indoline zwitterionic intermediate II (with L1) generated by DFT calculations (Fig. 7c).
Fig. 7. A proposed reaction mechanism.
a A Cu-catalyzed catalytic cycle for the decarboxylative annulation of 1a with 2a (mode B). b Optimized geometry of I (with L1) and calculated atomic charges. c Optimized geometry of II (with L1) and stereochemical model rationalizing the observed stereoselectivity.
The unexpected transformation of the non-fluorinated ethynyl Me-benzoxazinanones 4 into spiro-carbazole/indolines 5 can also be explained based on the proposed mechanism (Fig. 8). The first half of the process for the generation of Cu indoline zwitterionic intermediate II’ from 4a via zwitterionic Cu-allenylidene intermediate I′ is the same as the process for the formation of Cu indoline zwitterionic intermediate II in Fig. 7. Namely, the mode A is unfavourable due to the steric Me group based on the DFT calculation of I′ (with L1). The nitrogen atom is close to the β-carbon of Cu-allenylidene intermediate I′ (N---Cβ: 2.59749 Å), resulting in the intramolecular cyclization to Cu-indoline II. While the γ-anion is stabilized in the CF3-containing intermediates I and II by negative hyperconjugation (Fig. 7), that in the non-fluorinated Cu zwitterionic intermediates I′ and II′ (II′′) is unstable due to the positive inductive effect by Me group. The atomic charge distributions are shown in Fig. 8b, c, which indicates that both γ-carbons are relatively positive (Cγ in I′: 0.201; Cγ in II′ (II′′): 0.166). Thus the intermediate II′ promptly converts, even in the presence of interceptor 2a, into dimethylene-Cu-indoline C via a cascade process that involves an isomerization to II” and an intramolecular hydride migration of Me proton to the α-position (shunt-mode B). Cu-dimethylene-indoline C is then protonated to give indole-2,3-quinodimethane intermediate D68–73 under concomitant release of Cu/L/i-Pr2NEt. D spontaneously dimerizes via a Diels−Alder cyclization pathway to furnish the structurally complex spiro-carbazole-indoline 5a. The lack of asymmetric induction observed in the synthesis of 5a supports a pathway where D spontaneously dimerizes independently from the Cu-catalyzed catalytic cycle (Fig. 8a).
Fig. 8. Proposed reaction mechanisms.
a A Cu-catalyzed catalytic cycle for the decarboxylative annulation of 4a (with 2a) (shunt-mode B). b Optimized geometry of I′ (with L1) and calculated atomic charges. c Optimized geometry of II' (II′′) (with L1) and calculated atomic charges. d Regioselective formation of spiro-carbazole-indoline 5a (2C) rather than 5a (3C) via an endo-preferential Diels−Alder reaction. e HOMO (diene)-LUMO (dieneophile)-controlled Diels−Alder dimerization.
The high regioselectivity of 5a-I over 5a-II can be explained by the Alder endo rule74–76, which involves a favourable interaction between the π systems of the dienophile and the diene (Fig. 8d). While there are two possible endo-modes I and II, endo-mode I is much more suitable in a general HOMO (diene)-LUMO (dienophile)-controlled Diels−Alder process (Fig. 8e)77–79. The analyses were also in good agreement with reported concepts of o-quinodimethane chemistry80–83. Several methods for the generation of indole-2,3-quinodimethane intermediates have been reported;68–73,84–87, however, they require multistep synthesis and high reaction temperature. Our method proceeds under mild conditions, and the reaction mechanism is much different from others. This new method for the indole-2,3-quinodimethane generation could be extended for broad applications.
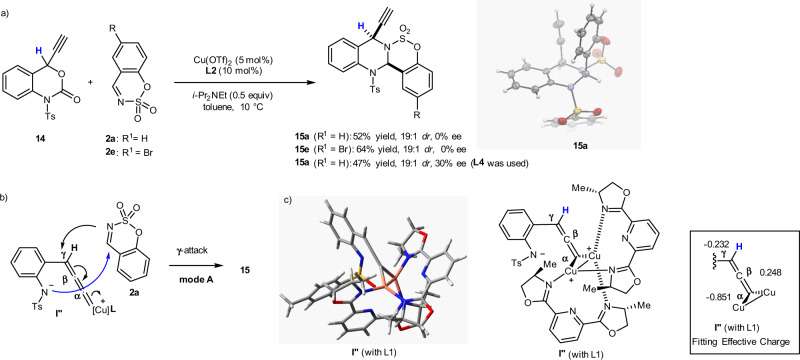
To shed further light on the possibility of controlling the annulation mode by altering the conformation of Cu-allenylidene zwitterionic intermediate I, we conducted the reaction of the non-substituted ethynyl benzoxazinanone 14 with two cyclic sulfamate imines (2a and 2e) under the same Cu-catalysis conditions. Remarkably, ethynyl substrate 14 could be transformed, via a Cu-catalyzed decarboxylative [4 + 2] annulation, into two tetracyclic 4-ethynyl-quinazoline derivatives (15a and 15e) in good yield (>52%) with excellent diastereoselectivity (19:1 dr) (Fig. 9a). An X-ray diffraction analysis of single crystals of 15a confirmed a tetracyclic quinazoline skeleton with a cis-stereochemical assignment (CCDC2026702). This result is consistent with our proposed mechanism, i.e., that the annulation reaction of the unsubstituted, non-steric, ethynyl 12 with 2 proceeds via mode A, which involves a Cu-allenylidene intermediate I′′ and a preferential γ-attack of interceptor 2 on I′′, to furnish the ethynyl-N-heterocycles 15 (Fig. 9b). Mode B, which involves the intramolecular β-attack, was not observed when non-substituted ethynyl benzoxazinanones 14 are used. The optimized conformation of the Cu-allenylidene intermediate I′′ with the atomic charge distributions (selected) by DFT calculations are shown (Fig. 9c, Supplementary Fig. 2). The γ-position is obviously opened to the nucleophile while the β-carbon is rather sterically shielded, which is in good agreement with the experimental observation mentioned above. Interestingly, however, the β-carbon is the most positive (Cα: −0.851; Cβ: 0.248; Cγ: −0.232). This fact suggests the balance of electronic and steric factor at Cu-allenylidene intermediate is crucial for the reactivity and reaction mode of the annulation reactions.
Fig. 9. Cu-catalyzed decarboxylative annulation of non-substituted 4-ethynyl benzoxazinanone 14.
a Cu-catalyzed reaction of 14 with 2a, e. b The reaction mechanism involves mode A, not mode B, due to the favourable conformation of I′′′. c Optimized geometry of I′′) (with L1) and calculated atomic charges.
Conclusion
In summary, we have demonstrated a strategy for controlling the annulation mode of ethynyl benzoxazinanones based on the reactivity of the Cu-allenylidene zwitterionic intermediate I that is formed during the reaction. Via a Cu-catalyzed decarboxylative annulation reaction of 4-ethynyl 4-CF3-benzoxazinanones 1 with cyclic sulfamate imines 2, densely functionalized indoline scaffolds 3, which bear a trifluoromethylated all-carbon quaternary centre, were constructed in excellent yield and enantioselectivity (up to 99% ee). The key step in the transformation is the unique generation of a Cu-indoline zwitterionic intermediate II from a Cu-allenylidene zwitterionic intermediate I by intramolecular β-attack. The obtained poly-N-heterocycles contain a chiral C-CF3 bond at an angular position; the synthesis of these types of molecules is usually very challenging. Notably, the chemical transformation of the CF3-poly-N-heterocycles 3 resulted in various types of derivatives with excellent selectivity. Significantly, this protocol represents the first example of the construction of optically pure trifluoromethylated merged indoline frameworks. These molecules will most likely become prospective drug candidates. The concept was extended to non-fluorinated, 4-ethynyl 4-CH3-benzoxazinanones 4. Under the same Cu-catalyzed decarboxylation conditions, CH3-substituted Cu-indoline zwitterionic intermediates II′ (II′′) were generated. The II′′ was promptly converted regioselectively into the alkaloid-like, spiro-carbazole-indoline derivatives 5 via a cascade process of internal β-attack and hydride α-migration (shunt-mode B) followed by a spontaneous Diels−Alder cyclization. This method is generally applicable to a variety of substrates, all of which are attractive for drug-discovery research. Although significant effort has already been devoted to control the reactivity of the α, β, and γ-positions of the Cu-allenylidene, the hitherto reported results are fragmented and no clear strategy has emerged. Our concept, which is based on exploiting the reactivity of the Cu-allenylidene zwitterionic intermediate I, may be substantially expanded to synthesize complex polycyclic molecules. The number of permutations and combinations of potential reactants and interceptors that can be used in this cascade annulation will lead to a rich variety of heterocyclic skeletons. Moreover, the concept could be extended to not only use Cu-catalysis but to also use Pd-catalysis and 4-vinyl-benzoxazinanones87,88. Further studies on the extension of this concept are currently in progress in our laboratory.
Methods
Computational methods
The ligand framework of zwitterionic Cu-allenylidene intermediates I (X = CF3), I′ (X = Me) and I′′′ (X = H) adopted a double-helical, pseudo-C2-symmetrical architecture according to the reported works of X-ray structure of [Cu2Cl(pybox)2]−[CuCl2]− 89–91, and computed structure of Cu-allenylidene complexed with L192. Ligand L1 was used instead of L2 for computation to simplify their calculations. The geometry optimizations and energy calculations were carried out at the B3LYP/6-311 G** level with Grimme’s dispersion correction methods of the D3. Atomic charge distributions were calculated from the B3LYP/6-311 G** level wave functions by electrostatic potential fitting using the Merz−Singh−Kollman scheme. The optimized geometries and their relative energies are displayed in Supplementary Figs. 1−3. Calculated atomic charges for optimized geometries are shown in Supplementary Fig. 2. The Gaussian 16 programme was used for the DFT calculations in Figs. 7b, c, 8b, c, 9b, c. The references are shown in Supporting Information.
General procedure for preparation of CF3-poly-N-heterocycles 3
In an argon filled glove box, a flame-dried 10 mL Schlenk tube was charged with copper(II) trifluoromethanesulfonate (1.81 mg, 0.005 mmol, 5 mol%), 2,6-bis[(4 R)-phenyl-2-oxazolin-2-yl]-pyridine L2 (3.69 mg, 0.01 mmol, 10 mol%) and anhydrous Toluene (1 mL). The resulting solution was stirred for 1 h at 80 °C. In an argon filled glove box, ethynyl benzoxazinanones 1 (0.1 mmol), benzoxathiazine 2 (0.11 mmol), and DIPEA (8.7 μL, 0.05 mmol, 0.5 equiv) were added. The resulting solution was stirred at 10 °C until the complete conversion of ethynyl benzoxazinanones (monitored by TLC). The reaction was quenched by saturated NH4Cl aqueous solution (2 mL). The resulting solution was extracted with ethyl acetate (5 mL × 3). The combined organic layers were dried over Na2SO4, filtered, and concentrated under vacuum. The diastereomeric ratio and crude yield were determined by 19F NMR analysis of the crude reaction mixture. The residue was purified by flash silica gel chromatography (Toluene) to afford the title compound 3. Full experimental details can be found in the Supplementary Methods.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Hiroto Uno for X-ray crystallographic analyses, and Chika Tanaka for preparing some starting materials. This work was supported by JSPS KAKENHI grants JP 21H01933 (KIBAN B, NS).
Author contributions
N.S. conceived the concept of this study. M.R.G. optimized the reaction conditions and surveyed the substrate scope. M.R.G., T.I., and K.I. prepared the starting materials. S.T. examined the DFT calculation. N.S. directed the project. N.S. and M.R.G. prepared the manuscript. The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.
Data availability
All characterization data including 1H, 13C, and 19F NMR spectral data and sample spectra, [α]D, HRMS, and HPLC chromatograms used to determine enantiomeric purity are included in the Supplementary Information (Supplementary Figs. 4–147). The X-ray crystallographic coordinates for structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2026703, CCDC 2026704, CCDC 2026705, and CCDC 2026702. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The CIF files of CCDC 2026703, CCDC 2026704, CCDC 2026705, and CCDC 2026702 are also included as Supplementary Data 1−4. The electronic structure calculations of I (with L1), I′ (with L1), II (with L1), II′, II′′ (with L1), and I′′ (with L1), are available as Supplementary Data 5.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Communications Chemistry thanks William Unsworth and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-021-00596-x.
References
- 1.Sundberg, R. J. Indoles (Academic Press, 1996).
- 2.Kochanowska-Karamyan AJ, Hamann MT. Marine indole alkaloids: potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010;110:4489–4497. doi: 10.1021/cr900211p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patil SA, Patil R, Miller DD. Indole molecules as inhibitors of tubulin polymerization: potential new anticancer agents. Future Med. Chem. 2012;4:2085–2115. doi: 10.4155/fmc.12.141. [DOI] [PubMed] [Google Scholar]
- 4.Kaushik NK, et al. Biomedical importance of indoles. Molecules. 2013;18:6620–6662. doi: 10.3390/molecules18066620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patil R, Patil SA, Beaman KD, Patil SA. Indole molecules as inhibitors of tubulin polymerization: potential new anticancer agents, an update (2013−2015) Future Med. Chem. 2016;8:1291–1316. doi: 10.4155/fmc-2016-0047. [DOI] [PubMed] [Google Scholar]
- 6.Chadha N, Silakari O. Indoles as therapeutics of interest in medicinal chemistry: Bird’s eye view. Eur. J. Med Chem. 2017;134:159–184. doi: 10.1016/j.ejmech.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Kumari A, Singh RK. Medicinal chemistry of indole derivatives: current to future therapeutic prospectives. Bioorg. Chem. 2019;89:103021. doi: 10.1016/j.bioorg.2019.103021. [DOI] [PubMed] [Google Scholar]
- 8.Tadeusz A. Alkaloids—Secrets of Life (Elsevier, 2007).
- 9.Roberts, M. F. Alkaloids: Biochemistry, Ecology, and Medicinal Applications (Springer, 2013).
- 10.Qiu S, et al. Natural alkaloids: basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014;12:401–406. doi: 10.1016/S1875-5364(14)60063-7. [DOI] [PubMed] [Google Scholar]
- 11.Aniszewski, T. Alkaloids: Chemistry, Biology, Ecology, and Applications (Elsevier Science, 2015).
- 12.Rasouli H, Yarani R, Pociot F, Popović-Djordjević J. Anti-diabetic potential of plant alkaloids: Revisiting current findings and future perspectives. Pharmacol. Res. 2020;155:104723. doi: 10.1016/j.phrs.2020.104723. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, et al. Fluorine in pharmaceutical industry: Fluorine-containing drugs introduced to the market in the last decade (2001–2011) Chem. Rev. 2014;114:2432–2506. doi: 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]
- 14.Swallow S. Fluorine in medicinal chemistry. Progress in medicinal chemistry. Prog. Med. Chem. 2015;54:65–133. doi: 10.1016/bs.pmch.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Inoue M, Sumii Y, Shibata N. Contribution of organofluorine compounds to pharmaceuticals. ACS Omega. 2020;5:10633–10640. doi: 10.1021/acsomega.0c00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa Y, Tokunaga E, Kobayashi O, Hirai K, Shibata N. Current contributions of organofluorine compounds to the agrochemical industry. iScience. 2020;23:101467. doi: 10.1016/j.isci.2020.101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nenajdenko, V. Fluorine in Heterocyclic Chemistry Vol. 1 (Springer, 2014).
- 18.Nenajdenko, V. Fluorine in Heterocyclic Chemistry Vol. 2 (Springer, 2014).
- 19.Kawai H, Shibata N. Asymmetric synthesis of agrochemically attractive trifluoromethylated dihydroazoles and related compounds under organocatalysis. Chem. Rec. 2014;14:1024–1040. doi: 10.1002/tcr.201402023. [DOI] [PubMed] [Google Scholar]
- 20.Huang YY, Yang X, Chen Z, Verpoort F, Shibata N. Catalytic asymmetric synthesis of enantioenriched heterocycles bearing a C-CF3 stereogenic center. Chem. Eur. J. 2015;21:8664–8684. doi: 10.1002/chem.201500361. [DOI] [PubMed] [Google Scholar]
- 21.He XH, Ji YL, Peng C, Han B. Organocatalytic asymmetric synthesis of cyclic compounds bearing a trifluoromethylated stereogenic center: recent developments. Adv. Synth. Catal. 2019;361:1923–1957. [Google Scholar]
- 22.Gui HZ, Wei Y, Shi M. Recent advances in the construction of trifluoromethyl-containing spirooxindoles through cycloaddition reactions. Chem. Asian J. 2020;15:1225–1233. doi: 10.1002/asia.202000054. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, et al. Catalytic asymmetric [4 + 1] annulation of sulfur ylides with copper–allenylidene intermediates. J. Am. Chem. Soc. 2016;138:8360–8363. doi: 10.1021/jacs.6b04414. [DOI] [PubMed] [Google Scholar]
- 24.Song J, Zhang ZJ, Gong LZ. Asymmetric [4 + 2] annulation of C1 ammonium enolates with copper-allenylidenes. Angew. Chem. Int. Ed. 2017;56:5212–5216. doi: 10.1002/anie.201700105. [DOI] [PubMed] [Google Scholar]
- 25.Lu XH, et al. Enantioselective cascade reaction for synthesis of quinolinones through synergistic catalysis using Cu-pybox and chiral benzotetramisole as catalysts. Chem. Eur. J. 2017;23:7689–7693. doi: 10.1002/chem.201701741. [DOI] [PubMed] [Google Scholar]
- 26.Shao W, You SL. Highly diastereo- and enantioselective synthesis of tetrahydro-5h-indolo[2,3-b]quinolines through copper-catalyzed propargylic dearomatization of indoles. Chem. Eur. J. 2017;23:12489–12493. doi: 10.1002/chem.201703443. [DOI] [PubMed] [Google Scholar]
- 27.Li TR, Lu LQ, Wang YN, Wang BC, Xiao WJ. Divergent synthesis of polycyclic indolines: copper-catalyzed cascade reactions of propargylic carbamates and indoles. Org. Lett. 2017;19:4098–4101. doi: 10.1021/acs.orglett.7b01903. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, et al. Asymmetric catalytic [4+2] cycloaddition via Cu–allenylidene intermediate: stereoselective synthesis of tetrahydroquinolines fused with a γ-lactone moiety. Org. Lett. 2018;20:1760–1763. doi: 10.1021/acs.orglett.8b00253. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, et al. Catalytic asymmetric [4+3] annulation of C,N-cyclic azomethine imines with copper allenylidenes. Org. Lett. 2018;20:6506–6509. doi: 10.1021/acs.orglett.8b02828. [DOI] [PubMed] [Google Scholar]
- 30.Ji D, Wang C, Sun J. Asymmetric [4 + 2]-cycloaddition of copper-allenylidenes with hexahydro-1,3,5-triazines: access to chiral tetrahydroquinazolines. Org. Lett. 2018;20:3710–3713. doi: 10.1021/acs.orglett.8b01584. [DOI] [PubMed] [Google Scholar]
- 31.Zhang ZJ, et al. N-Heterocyclic carbene/copper cooperative catalysis for the asymmetric synthesis of spirooxindoles. Angew. Chem. Int. Ed. 2019;58:12190–12194. doi: 10.1002/anie.201907188. [DOI] [PubMed] [Google Scholar]
- 32.Simlandy AK, Ghosh B, Mukherjee S. Enantioselective [4 + 2]-annulation of azlactones with copper-allenylidenes under cooperative catalysis: synthesis of α-quaternary α-acylaminoamides. Org. Lett. 2019;21:3361–3366. doi: 10.1021/acs.orglett.9b01103. [DOI] [PubMed] [Google Scholar]
- 33.Sun BB, et al. Asymmetric [4+2] cycloaddition of azlactones with dipolar copper–allenylidene intermediates for chiral 3,4-dhydroquinolin-2-one derivatives. Tetrahedron Lett. 2019;60:1967–1970. [Google Scholar]
- 34.Li TR, et al. A copper-catalyzed decarboxylative amination/hydroamination sequence: switchable synthesis of functionalized indoles. Angew. Chem. Int. Ed. 2016;55:12422–12426. doi: 10.1002/anie.201605900. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Liu M, Chen X, Wang H, Zhai H. Copper-catalyzed decarboxylative propargylation/hydroamination reactions: access to C3 β-ketoester-functionalized indoles. Chem. Commun. 2018;54:8375–8378. doi: 10.1039/c8cc04499f. [DOI] [PubMed] [Google Scholar]
- 36.Li TR, Zhang MM, Wang BC, Lu LQ, Xiao WJ. Synthesis of 3,3′-biindoles through a copper-catalyzed friedel–crafts propargylation/hydroamination/aromatization sequence. Org. Lett. 2018;20:3237–3240. doi: 10.1021/acs.orglett.8b01100. [DOI] [PubMed] [Google Scholar]
- 37.Jiang F, et al. Asymmetric [3 + 3] annulation of copper–allenylidenes with pyrazolones: synthesis of chiral 1,4-dihydropyrano[2,3-c]pyrazoles. Org. Lett. 2018;20:5278–5281. doi: 10.1021/acs.orglett.8b02214. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YC, Zhang ZJ, Fan LF, Song J. Enantioselective decarboxylative propargylation/hydroamination enabled by organo/metal cooperative catalysis. Org. Lett. 2018;20:2792–2795. doi: 10.1021/acs.orglett.8b01101. [DOI] [PubMed] [Google Scholar]
- 39.Wang BC, Wang YN, Zhang MM, Xiao WJ, Lu LQ. Copper-catalyzed decarboxylative cyclization via tandem C–P and C–N bond formation: access to 2-phosphorylmethyl indoles. Chem. Commun. 2018;54:3154–3157. doi: 10.1039/c8cc00739j. [DOI] [PubMed] [Google Scholar]
- 40.Lu S, Ong JY, Poh SB, Tsang T, Zhao Y. Transition-metal-free decarboxylative propargylic substitution/cyclization with either azolium enolates or acyl anions. Angew. Chem. Int. Ed. 2018;57:5714–5719. doi: 10.1002/anie.201801340. [DOI] [PubMed] [Google Scholar]
- 41.Lu Q, et al. Manganese(I)-catalyzed C-H (2-indolyl)methylation: expedient access to diheteroarylmethanes. Angew. Chem. Int. Ed. 2018;57:1399–1403. doi: 10.1002/anie.201710060. [DOI] [PubMed] [Google Scholar]
- 42.Hu X-H, Liu Z-T, Shao H, Hu X-P. Recent advances in catalytic stereocontrolled cycloaddition with terminal propargylic compounds. Synthesis. 2015;47:913–923. [Google Scholar]
- 43.Zhang D-Y, Hu X-P. Recent advances in copper-catalyzed propargylic substitution. Tetrahedron Lett. 2015;56:283–295. [Google Scholar]
- 44.Roh SW, Choi K, Lee C. Transition metal vinylidene- and allenylidene-mediated catalysis in organic synthesis. Chem. Rev. 2019;119:4293–4356. doi: 10.1021/acs.chemrev.8b00568. [DOI] [PubMed] [Google Scholar]
- 45.Li R, Liu D, Niu D. Asymmetric O-propargylation of secondary aliphatic alcohols. Nat. Catal. 2020;3:672–680. [Google Scholar]
- 46.Ogawa S, Iida N, Tokunaga E, Shiro M, Shibata N. Cinchona alkaloid/Ti(IV)-catalyzed enantioselective enamine-trifluoropyruvate condensation-cyclization reaction and its application to drug-like heterocycles. Chem. Eur. J. 2010;16:7090–7095. doi: 10.1002/chem.201000911. [DOI] [PubMed] [Google Scholar]
- 47.Matoba K, et al. Enantioselective synthesis of trifluoromethyl-substituted 2-isoxazolines: asymmetric hydroxylamine/enone cascade reaction. Angew. Chem. Int. Ed. 2010;49:5762–5766. doi: 10.1002/anie.201002065. [DOI] [PubMed] [Google Scholar]
- 48.Kawai H, et al. Organocatalytic asymmetric synthesis of trifluoromethyl-substituted diarylpyrrolines: enantioselective conjugate cyanation of β-aryl-β-trifluoromethyl-disubstituted enones. Angew. Chem. Int. Ed. 2012;51:4959–4962. doi: 10.1002/anie.201201278. [DOI] [PubMed] [Google Scholar]
- 49.Kawai H, et al. Diastereoselective additive trifluoromethylation/halogenation of isoxazole triflones: synthesis of all-carbon-functionalized trifluoromethyl isoxazoline triflones. ChemistryOpen. 2014;3:14–18. doi: 10.1002/open.201300044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Punna N, Das P, Gouverneur V, Shibata N. Highly diastereoselective synthesis of trifluoromethyl indolines by interceptive benzylic decarboxylative cycloaddition of nonvinyl, trifluoromethyl benzoxazinanones with sulfur ylides under palladium catalysis. Org. Lett. 2018;20:1526–1529. doi: 10.1021/acs.orglett.8b00237. [DOI] [PubMed] [Google Scholar]
- 51.Das P, et al. Access to benzo-fused nine-membered heterocyclic alkenes with a trifluoromethyl carbinol moiety via a double decarboxylative formal ring-expansion process under palladium catalysis. Chem. Sci. 2018;9:3276–3281. doi: 10.1039/c7sc05447e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Punna N, Harada K, Zhou J, Shibata N. Pd-catalyzed decarboxylative cyclization of trifluoromethyl vinyl benzoxazinanones with sulfur ylides: access to trifluoromethyl dihydroquinolines. Org. Lett. 2019;21:1515–1520. doi: 10.1021/acs.orglett.9b00330. [DOI] [PubMed] [Google Scholar]
- 53.Uno H, Imai T, Harada K, Shibata N. Synthesis of highly functionalized 12-membered trifluoromethyl heterocycles via a nondecarboxylative Pd-catalyzed [6 + 6] annulation. ACS Catal. 2020;10:1454–1459. [Google Scholar]
- 54.Uno H, Punna N, Tokunaga E, Shiro M, Shibata N. Synthesis of both mirror images of 9‐membered CF3‐heterocycles using a single antipode of chiral ligand under Pd‐catalyzed decarboxylative ring‐expansion with kinetic resolution. Angew. Chem. Int. Ed. 2020;59:8187–8194. doi: 10.1002/anie.201915021. [DOI] [PubMed] [Google Scholar]
- 55.Gannarapu MR, Zhou J, Jiang B, Shibata N. Two catalytic annulation modes via Cu-allenylidenes with sulfur ylides that are dominated by the presence or absence of trifluoromethyl substituents. iScience. 2020;23:100994. doi: 10.1016/j.isci.2020.100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uno H, Kawai K, Shiro M, Shibata N. Modular synthesis of medium-sized fluorinated and nonfluorinated heterocyclic lactones by sequential CN-bond-cleaving ring expansion under Pd catalysis. ACS Catal. 2020;10:14117–14126. [Google Scholar]
- 57.Kawai, K., Uno, H., Fujimoto, D. & Shibata, N. Transition-metal free catalytic synthesis of trifluoromethyl indolines by [4+1] cycloaddition of trifluoromethyl benzoxazinones with sulfur ylides. Helv. Chim. Acta. 10.1002/hlca.202000217 (2020).
- 58.Zhang ZZ, Zhang Y, Duan HX, Deng ZF, Wang YQ. Enantioselective (3+2) cycloaddition via N-heterocyclic carbene-catalyzed addition of homoenolates to cyclic N-sulfonyl trifluoromethylated ketimines: synthesis of fused N-heterocycle γ-lactams. Chem. Commun. 2020;56:1553–1556. doi: 10.1039/c9cc09269b. [DOI] [PubMed] [Google Scholar]
- 59.Troelsen NS, et al. The 3F library: fluorinated Fsp3 -rich fragments for expeditious 19 F NMR based screening. Angew. Chem. Int. Ed. 2020;59:2204–2210. doi: 10.1002/anie.201913125. [DOI] [PubMed] [Google Scholar]
- 60.Nakajima K, Shibata M, Nishibayashi Y. Copper-catalyzed enantioselective propargylic etherification of propargylic esters with alcohols. J. Am. Chem. Soc. 2015;137:2472–2475. doi: 10.1021/jacs.5b00004. [DOI] [PubMed] [Google Scholar]
- 61.Díez J, Gamasa MP, Panera M. Tetra-, di-, and mononuclear copper(I) complexes containing (S,S)-iPr-pybox and (R,R)-Ph-pybox ligands. Inorg. Chem. 2006;45:10043–10045. doi: 10.1021/ic061453t. [DOI] [PubMed] [Google Scholar]
- 62.Gao X, Cheng R, Xiao Y-L, Wan X-L, Zhang X. Copper-catalyzed highly enantioselective difluoroalkylation of secondary propargyl sulfonates with difluoroenoxysilanes. Chem. 2019;5:2987–2999. [Google Scholar]
- 63.Wuts, P. G. M. Greene’s Protective Groups in Organic Synthesis 5th edn. (John Wiley & Sons, 2014).
- 64.Uneyama K. in Organofluorine Chemistry 55−59 (Blackwell Publishing, 2006).
- 65.Saunders WH. Negative ion hyperconjugation in fluorocarbanions and the nature of the borderline between E1cB and E2 mechanisms. An ab initio study. J. Org. Chem. 1999;64:861–865. doi: 10.1021/jo981769l. [DOI] [PubMed] [Google Scholar]
- 66.David H. Critical examination of fluorine hyperconjugation in aromatic systems. Chem. Rev. 1971;71:139–145. [Google Scholar]
- 67.Exner O, Böhm S. Negative hyperconjugation of some fluorine containing groups. N. J. Chem. 2008;32:1449–1453. [Google Scholar]
- 68.Marinelli ER. Generation and cycloaddition reactions of indole-2,3-quinodimethanes. Tetrahedron Lett. 1982;23:2745–2748. [Google Scholar]
- 69.Fuwa H, Tako T, Ebine M, Sasaki M. A new method for the generation of indole-2,3-quinodimethanes from allenamides. Chem. Lett. 2008;37:904–905. [Google Scholar]
- 70.Laronze M, Sapi J. 3-Cyanomethyl-2-vinylindoles as thermal indole-2,3-quinodimethane equivalents: synthesis of functionalized 1,2,3,4-tetrahydrocarbazoles. Tetrahedron Lett. 2002;43:7925–7928. [Google Scholar]
- 71.Terzidis M, Tsoleridis CA, Stephanidou-Stephanatou J. Chromone-3-carboxaldehydes in Diels–Alder reactions with indole-o-quinodimethane. Synthesis of tetrahydrochromeno[2,3-b]carbazoles. Tetrahedron Lett. 2005;46:7239–7242. [Google Scholar]
- 72.Kuroda N, Takahashi Y, Yoshinaga K, Mukai C. A novel generation of indole-2,3-quinodimethanes. Org. Lett. 2006;8:1843–1845. doi: 10.1021/ol060418n. [DOI] [PubMed] [Google Scholar]
- 73.Fuwa H, Sasaki M. A new method for the generation of indole-2,3-quinodimethanes and 2-(N-alkoxycarbonylamino)-1,3-dienes. Intramolecular Heck/Diels–Alder cycloaddition cascade starting from acyclic α-phosphono enecarbamates. Chem. Commun. 2007;43:2876–2878. doi: 10.1039/b704374k. [DOI] [PubMed] [Google Scholar]
- 74.Alder K, Stein G. Untersuchungen über den Verlauf der Diensynthese. Angew. Chem. 1937;50:510–519. [Google Scholar]
- 75.Sauer J, Sustmann R. Mechanistic aspects of Diels−Alder reactions: a critical survey. Angew. Chem. Int. Ed. Engl. 1980;92:773–801. [Google Scholar]
- 76.Leal RC, Pereira DH, Custodio R. An energetic analysis of the Diels−Alder endo:exo selectivity reaction by using composite methods. Comput. Theor. Chem. 2018;1123:161–168. [Google Scholar]
- 77.Dewar MJS, Thiel W. Ground states of molecules. 38. The MNDO method. Approximations and parameters. J. Am. Chem. Soc. 1977;99:4899–4907. [Google Scholar]
- 78.Woodward, R. B. & Hoffmann, R. The Conservation of Orbital Symmetry (Academic Press, 1970).
- 79.Hoffmann R, Woodward RB. Orbital symmetries and endo-exo relationships in concerted cycloaddition reactions. J. Am. Chem. Soc. 1965;87:4388–4389. [Google Scholar]
- 80.Oppolzer, W. Intramolecular cycloaddition reactions of ortho-quinodimethanes in organic synthesis. Synthesis. 1978, 793−802 (1978).
- 81.Klundt I. Benzocyclobutene and its derivatives. Chem. Rev. 1970;70:471–487. [Google Scholar]
- 82.Charlton JL, Alauddin MM. Orthoquinodimethanes. Tetrahedron. 1987;43:2873–2889. [Google Scholar]
- 83.Funk RL, Vollhardt KPC. Thermal, photochemical, and transition-metal mediated routes to steroids by intramolecular Diels–Alder reactions of o-xylylenes (o-quinodimethanes) Chem. Soc. Rev. 1980;9:41–61. [Google Scholar]
- 84.Magnus P, Gallagher T, Brown P, Pappalardo P. The indole 2-3-quinodimethane strategy for the synthesis of indole alkaloids. Acc. Chem. Res. 1984;17:35–41. [Google Scholar]
- 85.Pindur U, Erfanian-Abdoust H. Indolo-2,3-quinodimethanes and stable cyclic analogs for regio- and stereocontrolled syntheses of [b]-annelated indoles. Chem. Rev. 1989;89:1681–1689. [Google Scholar]
- 86.Segura JL, Martin N. o-Quinodimethanes: efficient intermediates in organic synthesis. Chem. Rev. 1999;99:3199–3246. doi: 10.1021/cr990011e. [DOI] [PubMed] [Google Scholar]
- 87.Li TR, Wang YN, Xiao WJ, Lu LQ. Transition-metal-catalyzed cyclization reactions using vinyl and ethynyl benzoxazinones as dipole precursors. Tetrahedron Lett. 2018;59:1521–1530. [Google Scholar]
- 88.Singha S, Serrano E, Mondal S, Daniliuc CG, Glorius F. Diastereodivergent synthesis of enantioenriched α, β-disubstituted γ-butyrolactones via cooperative N-heterocyclic carbene and Ir catalysis. Nat. Catal. 2020;3:48–54. [Google Scholar]
- 89.Diez J, Gamasa MP, Panera M. Tetra-, di-, and mononuclear copper (I) complexes containing (S,S)-iPr-pybox and (R,R)-Ph-pybox ligands. Inorg. Chem. 2006;45:10043–10045. doi: 10.1021/ic061453t. [DOI] [PubMed] [Google Scholar]
- 90.Panera M, Diez J, Merino I, Rubio E, Gamasa MP. Synthesis of copper(I) complexes containing enantiopure pybox ligands. First assays on enantioselective synthesis of propargylamines catalyzed by isolated copper(I) complexes. Inorg. Chem. 2009;48:11147–11160. doi: 10.1021/ic901527x. [DOI] [PubMed] [Google Scholar]
- 91.Tsuchida K, Senda Y, Nakajima K, Nishibayashi Y. Construction of chiral tri- and tetra-arylmethanes bearing quaternary carbon centers: copper-catalyzed enantioselective propargylation of indoles with propargylic esters. Angew. Chem. Int. Ed. 2016;55:9728–9732. doi: 10.1002/anie.201604182. [DOI] [PubMed] [Google Scholar]
- 92.Li R-Z, et al. Enantioselective propargylation of polyols and desymmetrization of meso 1,2-Diols by copper/borinic acid dual catalysis. Angew. Chem. Int. Ed. 2017;56:7213–7217. doi: 10.1002/anie.201703029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All characterization data including 1H, 13C, and 19F NMR spectral data and sample spectra, [α]D, HRMS, and HPLC chromatograms used to determine enantiomeric purity are included in the Supplementary Information (Supplementary Figs. 4–147). The X-ray crystallographic coordinates for structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2026703, CCDC 2026704, CCDC 2026705, and CCDC 2026702. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The CIF files of CCDC 2026703, CCDC 2026704, CCDC 2026705, and CCDC 2026702 are also included as Supplementary Data 1−4. The electronic structure calculations of I (with L1), I′ (with L1), II (with L1), II′, II′′ (with L1), and I′′ (with L1), are available as Supplementary Data 5.