Abstract
Lyme disease, caused by Borrelia burgdorferi, is characterized by the accumulation of lymphocytes and monocytes in the affected tissue. Endothelial cells line the blood vessel walls and control the trafficking of inflammatory leukocytes from the blood into the surrounding tissues. A model of the blood vessel wall, consisting of human umbilical vein endothelial cells (HUVEC) grown on amniotic connective tissue, was utilized to examine the effects of B. burgdorferi on the transendothelial migration of T lymphocytes. Maximal migration occurred when the HUVEC-amnion cultures were preincubated with B. burgdorferi for 24 h and T lymphocytes were added for an additional 4 h, yielding a two- to fourfold increase compared to migration across unstimulated cultures. The number of T lymphocytes that migrated was proportional to the number added. The anti-inflammatory cytokine interleukin 10 (IL-10), added during activation of the HUVEC, significantly diminished (by an average of 70% ± 21%) the migration of T lymphocytes across endothelium stimulated for 8 or 24 h with B. burgdorferi, but not IL-1. Compared to the initially added population of T lymphocytes, the population that migrated across untreated endothelium or HUVEC activated with B. burgdorferi or IL-1 contained a significantly smaller percentage of CD45RA+RO− (naïve) cells and a greater proportion of CD45RA+RO+ cells. The migratory population was also enriched for CD8+ T lymphocytes when the endothelium was incubated with either control medium or B. burgdorferi, but not IL-1. B. burgdorferi thus activates endothelium in a manner that promotes the transmigration of T lymphocytes, and IL-10 inhibits this activation. These data further suggest that endothelium plays an active role in promoting the recruitment of specific subpopulations of T lymphocytes.
Lyme disease is a chronic inflammatory illness that is caused by the spirochetal bacterium Borrelia burgdorferi. B. burgdorferi is introduced into the skin of its mammalian host by the bite of an infected Ixodes tick. The infection spreads locally, often resulting in an expanding rash called erythema migrans, which is characterized by infiltration of lymphocytes, plasma cells, and mast cells (12). The bacteria then hematogenously disseminate to secondary sites such as the nervous system, heart, muscles, joints, and distant cutaneous tissues. Histologically, these secondary sites exhibit an accumulation of inflammatory leukocytes, including lymphocytes, macrophages, plasma cells, and polymorphonuclear leukocytes (32). The majority of the T lymphocytes isolated from the synovial lesions of patients with Lyme arthritis are of the CD4+ phenotype (28, 33). However, CD8+ T lymphocytes are also present in these lesions (28).
Specific subsets of T lymphocytes may be important in controlling the outcome of infection with B. burgdorferi. In a murine model of Lyme disease, depletion of the CD4+ T lymphocytic subset in both disease-susceptible C3H/HeN mice and resistant BALB/c mice increases both severity of arthritis and spirochetal burden (18). In contrast, elimination of the CD8+ subset in the susceptible mice results in less severe arthritis and reduced numbers of spirochetes. The lymphokines secreted by specific subpopulations of T lymphocytes may also serve essential functions in controlling the pathogenesis of Lyme disease (5, 16, 17). For instance, the lymphokine interleukin 10 (IL-10) diminishes the production of gamma interferon (IFN-γ) by T lymphocytes that are isolated from patients with Lyme arthritis and stimulated with B. burgdorferi in vitro (37). Moreover, IL-10-deficient mice develop more severe arthritis than their wild-type counterparts when infected with B. burgdorferi (6).
The endothelial lining of the blood vessel wall is the first barrier encountered by circulating T lymphocytes in their journey toward infected or injured tissues. As such, it is likely to play a particularly important role in regulating the trafficking of these cells. In the context of Lyme disease, it has been shown that B. burgdorferi stimulates endothelial cells to secrete chemokines (7, 8) and to upregulate the expression of adhesion molecules for leukocytes (31). Consequently, exposure of endothelium to B. burgdorferi promotes the subsequent transmigration of neutrophils (8, 31), monocytes, and CD4+ T lymphocytes (7). Herein, we use a well-characterized in vitro model of the blood vessel wall to demonstrate that unfractionated, human peripheral blood T lymphocytes migrate in increased numbers across endothelial monolayers that have been activated with B. burgdorferi or the host proinflammatory cytokine IL-1. The anti-inflammatory cytokine IL-10 inhibits the migration of T lymphocytes across endothelium exposed to B. burgdorferi, but not IL-1. Phenotypic analysis of the T lymphocytes that migrate suggests that the endothelium may actively recruit specific subpopulations of T cells in a manner that partly depends on the inciting stimulus.
MATERIALS AND METHODS
Culture of spirochetes.
An isolate of B. burgdorferi derived from human blood (HBD1) (1) was cultured at 33°C in modified Barbour-Stoenner-Kelly medium containing low levels of lipopolysaccharide (31). Spirochetes (passages 42 to 60) were harvested in late log-phase growth by centrifugation and resuspended in medium 199 (M199; Life Technologies, Inc., Grand Island, N.Y.) containing 20% heat-inactivated fetal bovine serum (HyClone Laboratories, Logan, Utah). To make certain that exogenous lipopolysaccharide was not introduced during the course of experiments, sham preparations, which consisted of equal volumes of uninoculated spirochetal growth medium, were processed in parallel with the spirochetes.
Endothelial cell cultures.
Endothelial cells were isolated from human umbilical veins by collagenase perfusion and placed onto 1.5% gelatin-coated tissue culture plates (Corning Glass Works, Corning, N.Y.) (15). The human umbilical vein endothelial cells (HUVEC) were maintained in growth medium consisting of M199 with 20% fetal bovine serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (2 μg/ml) at 37°C in a 5% CO2 atmosphere. When confluent (within 3 to 5 days), cultures from several cords were trypsinized, pooled, and plated onto acellular amniotic connective tissue substrates fastened to Teflon rings (8). The amniotic membranes were obtained from human placentas shortly after delivery by separating the amnion from the chorion, fastening it to Teflon rings, and removing the epithelium with 0.25 N NH4OH and gentle scraping (31).
Isolation of T lymphocytes.
Blood was collected from healthy volunteers in syringes containing a final concentration of 0.12% EDTA and diluted with an equal volume of Dulbecco's phosphate-buffered saline (PBS) devoid of Ca2+ and Mg2+ (Life Technologies, Inc.). In a 50-ml polypropylene tube, 30 ml of the diluted blood was gently pipetted over 15 ml of Accu-Prep Lymphocytes gradient medium (Accurate Chemical & Scientific Corp., Westbury, N.Y.) and centrifuged at 675 × g for 20 min at room temperature. The peripheral blood mononuclear cells were collected from the interface, diluted with an equal volume of PBS lacking Ca2+ and Mg2+, centrifuged, and washed twice more. T lymphocytes were then isolated from the mononuclear cell fraction by negative selection using a MACS Pan T Cell Isolation kit (Miltenyi Biotec, Auburn, Calif.) according to the manufacturer's instructions. The purified T lymphocytes were consistently >98% pure and viable, as determined by flow cytometry for CD3 and trypan blue exclusion, respectively.
Transendothelial migration of T lymphocytes.
HUVEC (3 × 105) were plated onto pieces of amniotic tissue with a surface area of 2 cm2 and cultured for 7 to 10 days, a time that permits transendothelial electrical resistance to develop (15). HUVEC-amnion cultures were treated with either control medium, recombinant human IL-1β (1 U/ml; Collaborative Biomedical, Bedford, Mass.), 10 B. burgdorferi organisms per endothelial cell, or a sham preparation of spirochetes at 37°C for various times. Cultures were then washed, and purified T lymphocytes were added to the apical sides and allowed to incubate at 37°C for the indicated times. At the end of the assay, medium was removed, and the cultures were fixed overnight in 10% buffered formalin at 4°C. The tissues were stained with Wright stain and viewed en face by light microscopy. The total number of T lymphocytes associated with each culture was determined by counting nine fields (magnification, ×400) chosen at random.
To distinguish the T lymphocytes that were adherent to the apical surface of the HUVEC from those that had migrated across the endothelium and into the amniotic tissue, a portion of each culture was embedded in glycol methacrylate (Polysciences Inc., Warrington, Pa.), sectioned perpendicularly to the plane of the endothelial monolayer, and stained with toluidine blue (31). The percentage of T lymphocytes that migrated beneath the HUVEC was calculated by determining the positions, with respect to the endothelium, of at least 100 T cells for each sample. Correction for loss of adherent T lymphocytes during the embedding procedure was performed as described previously (27).
Harvesting of migrated T lymphocytes.
To analyze the phenotypes of migrated T cells, HUVEC-amnion cultures were left untreated or activated with IL-1β (1 U/ml) or 10 spirochetes per endothelial cell for 24 h at 37°C. Cultures were washed, and 2 × 106 to 4 × 106 T cells were added for 4 h at 37°C. T lymphocytes adherent to the apical surface of the endothelium were removed by washing the cultures vigorously three times in beakers containing Hanks' balanced salt solution (HBSS) (Life Technologies, Inc.) devoid of Mg2+ and Ca2+. T lymphocytes that had migrated across unstimulated or activated HUVEC were released from the amniotic tissue by incubating the cultures in collagenase D (0.5 mg/ml; Boehringer Mannheim, Indianapolis, Ind.) and hyaluronidase (10 μg/ml; Worthington Biochemical Corporation, Lakewood, N.J.) in HBSS at 37°C for 20 to 30 min. The digestion solution also contained soybean trypsin inhibitor (10 μg/ml), tosyl-l-phenylalanine chloromethyl ketone (37 μg/ml), and one Mini-Complete EDTA-free protease inhibitor cocktail tablet per 10 ml (all from Boehringer Mannheim). When incubated with purified T lymphocytes, in either the presence or absence of amniotic tissue, the digestion solution did not affect the viability of the cells or their expression of surface markers of interest, as determined by flow cytometry. The released cells were washed three times in HBSS and resuspended in PBS containing 0.5% bovine serum albumin (Sigma, St. Louis, Mo.). For each set of experimental conditions, T lymphocytes were harvested from three to four HUVEC-amnion cultures and pooled for subsequent phenotypic analysis. An additional two cultures were fixed and analyzed microscopically, as described in the preceding section, to verify that the treated endothelium was indeed activated and that adherent lymphocytes were removed by the washing procedure.
Flow cytometry.
The phenotypes of the initially added and migrated populations of T lymphocytes were compared by flow cytometry. An aliquot of the starting population of T cells, which had been held in culture medium at 37°C for the duration of the transmigration assay, was incubated with fluorescently labeled antibody (0.6 μg/ml) for 20 min at 0°C. A higher concentration (1.2 μg/ml) was needed to label the migratory population to saturation, perhaps due to nonspecific binding of the antibodies to debris or endothelial cells. Phycoerythrin (PE)-labeled monoclonal antibodies (MAb) to human CD4 (immunoglobulin G1 [IgG1]), CD8 (IgG1), and CD45RO (IgG2a) were purchased from Becton Dickinson, San Jose, Calif., as was fluorescein isothiocyanate (FITC)-labeled MAb to human CD45RA (IgG1) and CD69 (IgG1). FITC-labeled MAb to human CD3 (IgG2a) was from Biosource International (Camarillo, Calif.). In various experiments, T cells were stained with the following combination of MAb: (i) FITC-labeled anti-CD45RA and PE-labeled anti-CD45RO; (ii) FITC-labeled anti-CD69 and PE-labeled MAb to either CD4 or CD8; or (iii) FITC-labeled anti-CD3 and PE-labeled MAb to either CD4 or CD8. Cells incubated with fluorescently labeled, isotype-matched MAbs to irrelevant antigens (Caltag, Burlingame, Calif.) served as negative controls. After staining, the cells were washed three times, fixed in 1% buffered formalin, and stored at 4°C in the dark until analysis, usually within 24 h. Two-color flow cytometry was performed using a FACScan flow cytometer (Becton Dickinson). Lymphocytes were gated according to their forward scatter versus side scatter to exclude debris, clumps, and endothelial cells. A total of 10,000 cells was analyzed for each sample.
Statistics.
The data obtained for the transendothelial migration assays were subjected to an ordinary one-way analysis of variance with the Tukey-Kramer multiple-comparison test using the GraphPad Instat statistical software program (GraphPad Software Inc., San Diego, Calif.). The data obtained from phenotypic analyses were subjected to a two-way analysis of variance of a randomized block design with the Tukey-Kramer multiple-comparison test using the Jandel SigmaStat statistical software package (SPSS Science, Chicago, Ill.).
RESULTS
B. burgdorferi activates endothelium to promote the transmigration of T lymphocytes.
We previously reported that activation of endothelium by B. burgdorferi stimulates the subsequent transmigration of neutrophils (8, 31) and monocytes (7), using an in vitro model of the blood vessel wall. This model consists of HUVEC grown to confluence on acellular amniotic connective tissue. These endothelial cell cultures resemble endothelium in vivo with respect to both morphology and permeability properties (15). In addition, the CD4+ subset of T lymphocytes, isolated by positive selection with immunomagnetic beads, also exhibits enhanced migration across B. burgdorferi-stimulated endothelium (7). In the present study, experiments were performed to examine the migration of the entire T-lymphocytic population, rather than CD4+ T cells only. Moreover, a negative selection strategy, using immunomagnetic beads to remove monocytes, natural killer cells, B cells, and dendritic cells, was employed to minimize perturbation of the T lymphocytes during their isolation.
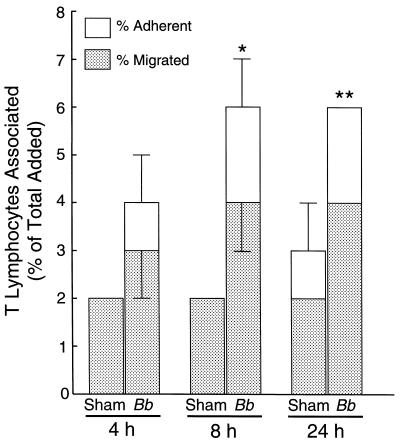
To determine whether exposure of endothelium to B. burgdorferi promotes the transmigration of these unfractionated T lymphocytes, HUVEC-amnion cultures were incubated with either spirochetes or a sham preparation of bacteria for 4, 8, or 24 h. The cultures were then washed to remove any unbound bacteria, and T lymphocytes were added for an additional 2 h. Incubation of the HUVEC-amnion cultures with B. burgdorferi for 8 to 24 h resulted in maximal migration of the T lymphocytes (a twofold increase compared to the sham control) (Fig. 1). Therefore, an incubation period of 24 h was chosen for stimulation of the HUVEC in subsequent studies.
FIG. 1.
Stimulation of HUVEC with B. burgdorferi for 8 to 24 h results in maximal migration of T lymphocytes. Unfractionated T lymphocytes (106 per culture) were incubated for 2 h with HUVEC-amnion cultures that had been treated with either a sham preparation of spirochetes or B. burgdorferi (Bb) at 10 bacteria/endothelial cell for 4, 8, or 24 h. The total height of each bar represents the number of T lymphocytes associated with each culture, expressed as a percentage of the total number of cells that was initially added. The lower portion of each bar depicts the percentage of cells that migrated beneath the endothelium. The upper portion of each bar denotes the percentage of T lymphocytes that were adherent to the apical surface of the endothelium. Bars represent the means ± standard deviations (error bars) of three to four replicate samples. Asterisks denote levels of migration across B. burgdorferi-stimulated endothelium that are significantly different from those across sham-treated controls: ∗, P < 0.01; ∗∗, P < 0.001.
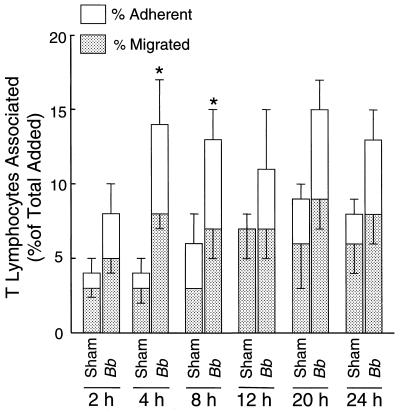
Next, the time for which T cells and endothelium must be coincubated to achieve maximum migration was determined. HUVEC-amnion cultures were preincubated with B. burgdorferi or a sham preparation of bacteria for 24 h and washed. T lymphocytes were then added for various times. Incubation for 4 h resulted in maximal migration of T lymphocytes (8% ± 1% of the total T cells added for spirochete-treated cultures, compared to 3% ± 1% for the sham control) (Fig. 2); consequently, this time point was used in all succeeding experiments. After 12 or more hours of incubation, the migration of T lymphocytes across sham-treated HUVEC monolayers increased to a level similar to that across monolayers that had been exposed to spirochetes.
FIG. 2.
Maximal numbers of T lymphocytes migrate across HUVEC stimulated with B. burgdorferi within 4 h. HUVEC were treated with a sham preparation of spirochetes or B. burgdorferi (Bb) at 10 bacteria/endothelial cell for 24 h, followed by incubation with T lymphocytes (106 per culture) for the indicated times. The total height of each bar represents the percentage of added T lymphocytes that became associated with each culture. The lower portion depicts the percentage of cells that migrated beneath the endothelium; the upper portion indicates the percentage that were adherent to the apical surface of the endothelium. Bars represent the means ± standard deviations (error bars) of three to four replicate samples. Data shown are representative of two separate experiments. The percentages of T cells migrating across B. burgdorferi-activated endothelium that are significantly different from those traversing sham-treated controls are shown by an asterisk (P < 0.05).
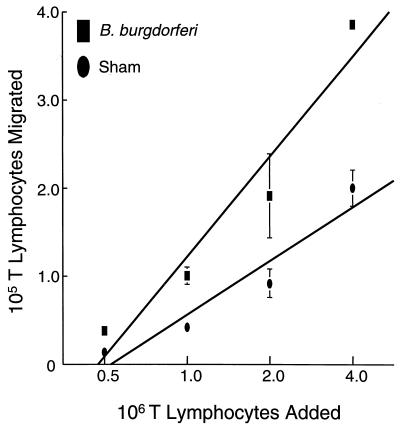
Pietschmann et al. (25) reported that when HUVEC are grown on collagen gels, increasing the number of T lymphocytes added augments the number of migrating T lymphocytes until a plateau is eventually reached. To see if the same phenomenon occurred in our in vitro system, HUVEC-amnion cultures were preincubated with B. burgdorferi or a sham preparation for 24 h, the cultures were washed, and various amounts of T lymphocytes (from 0.5 × 106 to 4 × 106 per culture) were added for an additional 4 h. Microscopic analysis of the cultures revealed that a linear relationship existed between the number of T lymphocytes added and the number of T lymphocytes that migrated, up to a point where it was no longer possible to count the number of cells that migrated (Fig. 3). Therefore, in our system, the endothelium does not impose a restriction on the number of T lymphocytes that can transmigrate.
FIG. 3.
The number of T lymphocytes that migrate is proportional to the number added. HUVEC were stimulated with either a sham preparation of spirochetes or B. burgdorferi (10 bacteria/endothelial cell) for 24 h. Increasing numbers of T lymphocytes were subsequently added and allowed to incubate for 4 h. Data points represent the means ± standard deviations (error bars) of three to four replicate samples. Data shown are representative of two separate experiments. The number of T lymphocytes that migrated across B. burgdorferi-activated endothelium is significantly greater than the number that migrated across sham-treated controls at all points tested (P < 0.05).
IL-10 inhibits the migration of T lymphocytes across endothelium stimulated by B. burgdorferi.
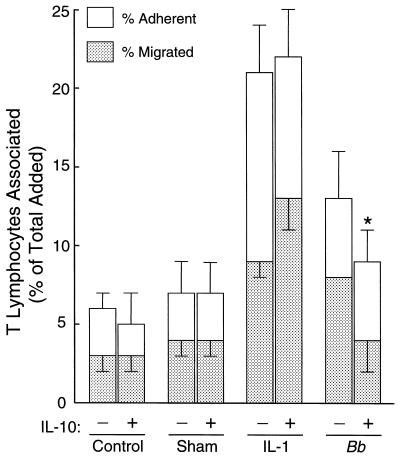
IL-10 is a lymphokine that has been implicated in the control of inflammation in murine models of Lyme disease (6). Addition of IL-10 during exposure of endothelium to B. burgdorferi reduces the transmigration of subsequently added monocytes by about half. In contrast, IL-10 has no effect on migration of monocytes across HUVEC stimulated with IL-1 (7). To determine whether IL-10 also diminishes transendothelial migration of T lymphocytes in a stimulus-specific manner, HUVEC-amnion cultures were incubated with medium only, a sham preparation of spirochetes, B. burgdorferi, or IL-1 for 8 h in the absence or presence of IL-10 (20 ng/ml). The cultures were then washed to remove the stimuli, and resting T lymphocytes were added, without IL-10, for an additional 4 h. In one experiment, IL-10 completely abolished (P < 0.01) the enhanced migration of T lymphocytes across B. burgdorferi-stimulated endothelium, reducing it to control levels (Fig. 4). In another experiment, using different donors for both the T lymphocytes and HUVEC, emigration was suppressed by 54% (P < 0.001) (data not shown). When coincubation of B. burgdorferi and HUVEC was extended to 24 h, IL-10 reduced the migration of T lymphocytes by 64% (P < 0.05) and 60% (P < 0.01) in two separate experiments (data not shown). In contrast, the migration of T lymphocytes across endothelium treated with either 0.01 or 5 U of IL-1 per ml was not reduced by IL-10 in any of these experiments.
FIG. 4.
IL-10 reduces the enhanced migration of T lymphocytes across B. burgdorferi-stimulated endothelium. HUVEC-amnion cultures were incubated with medium, a sham preparation of spirochetes, IL-1 (5 U/ml), or B. burgdorferi (Bb) at 10 bacteria/endothelial cell in the absence or presence of IL-10 (20 ng/ml) for 8 h. T lymphocytes (106 per culture) were then added for an additional 4 h. The total height of each bar represents the percentage of added T lymphocytes that became associated with each culture. The lower portion depicts the percentage of cells that migrated beneath the endothelium; the upper portion indicates the percentage that were adherent to the apical surface of the endothelium. Bars represent the means ± standard deviations (error bars) of three to four replicate samples. Significant reduction in migration by IL-10 is indicated by an asterisk (P < 0.01).
CD45RA+RO+ T lymphocytes preferentially traverse endothelium, whereas CD45RA+RO− cells migrate poorly.
To ascertain whether CD45RA−RO+ (memory), CD45RA+RO− (naïve), or CD45RA+RO+ T lymphocytes preferentially migrate across B. burgdorferi-stimulated endothelium, a T-lymphocyte transendothelial migration assay was conducted under the optimal conditions that were established as described above. Cultures were then washed vigorously in HBSS devoid of Ca2+ and Mg2+, which effectively removed T lymphocytes that were adherent to the apical surface of the endothelium (data not shown). The migrated T lymphocytes were liberated from the amniotic tissue with a solution containing collagenase, hyaluronidase, and a mixture of protease inhibitors. The harvested T lymphocytes were stained with fluorescently labeled MAb to CD45RA and CD45RO and analyzed by two-color flow cytometry. The phenotypes of the migrated T lymphocytes were then compared to those of an aliquot of the initial T-lymphocytic population, which had been incubated in culture medium at 37°C for the duration of the transmigration assay. As shown in Table 1, migratory populations contained a greater proportion of CD45RA+RO+ T cells and a smaller percentage of CD45RA+RO− cells than did the initial population, regardless of the state of activation of the endothelium. There was also a significant increase in the percentage of T cells that expressed an early marker of activation, CD69, in the populations of CD8+ lymphocytes that traversed either unstimulated or stimulated endothelium. Enrichment for CD4+ T cells that coexpressed CD69 was also noted. However, results were more variable than for the CD8+ population, and enrichment was of statistical significance only for CD4+ cells that migrated across IL-1-treated endothelium (Table 2). The number of migrated cells that expressed CD69 under any conditions never exceeded 7.5%. Therefore, the majority of the T lymphocytes that traversed the endothelium did not appear to have been activated recently.
TABLE 1.
CD45RA+RO+ T lymphocytes preferentially migrate across endothelium
| Expt. no.a | Markers | % of T lymphocytes with indicated phenotype
|
|||
|---|---|---|---|---|---|
| Initial population | Migrated across unstimulated HUVECb | Migrated across IL-1-treated HUVECbc | Migrated across B. burgdorferi-treated HUVECbc | ||
| 1 | CD45RA−RO+ | 39.3 | 43.2 | 36.4 | 45.7 |
| CD45RA+RO− | 36.2 | 5.1 | 4.5 | 5.5 | |
| CD45RA+RO+ | 24.0 | 51.5 | 59.0 | 48.6 | |
| 2 | CD45RA−RO+ | 38.9 | 33.0 | 30.2 | 25.8 |
| CD45RA+RO− | 36.2 | 5.5 | 3.3 | 2.8 | |
| CD45RA+RO+ | 22.2 | 60.8 | 66.3 | 71.3 | |
| 3 | CD45RA−RO+ | 23.9 | 25.6 | 31.9 | 31.4 |
| CD45RA+RO− | 49.7 | 9.7 | 7.7 | 9.5 | |
| CD45RA+RO+ | 17.5 | 62.4 | 59.6 | 58.4 | |
Each experiment was performed with T cells isolated from a different donor.
All migratory populations show a significant depletion of CD45RA+RO− T lymphocytes and enrichment for CD45RA+RO+ T lymphocytes compared to the initial population (P < 0.05).
On average, 2.0 ± 0.4-fold more T lymphocytes traversed stimulated HUVEC than unstimulated endothelium in these experiments.
TABLE 2.
The population of T lymphocytes that migrates across endothelium is enriched for CD69+ cells
| Expt. No.a | Markers | % of T lymphocytes expressing indicated markers
|
|||
|---|---|---|---|---|---|
| Initial population | Migrated across unstimulated HUVECb | Migrated across IL-1-treated HUVECbcd | Migrated across B. burgdorferi-treated HUVECbd | ||
| 1 | CD4 + CD69 | 0.5 | 1.2 | 6.8 | 5.0 |
| CD8 + CD69 | 0.3 | 5.0 | 5.2 | 5.0 | |
| 2 | CD4 + CD69 | 0.3 | 2.4 | 6.6 | 5.8 |
| CD8 + CD69 | 0.4 | 6.4 | 6.3 | 6.5 | |
| 3 | CD4 + CD69 | 0.4 | 1.0 | 2.5 | 1.0 |
| CD8 + CD69 | 0.7 | 7.5 | 6.7 | 6.0 | |
Each experiment was performed with T lymphocytes isolated from a different donor.
The populations that migrated across endothelium are significantly enriched for cells coexpressing CD8 and CD69 compared to the initial population (P < 0.05).
The population that traversed IL-1-treated endothelium shows significant enrichment for cells coexpressing CD4 and CD69 compared to the initial population (P < 0.05).
In these experiments, an average of 1.9 ± 0.1-fold more T lymphocytes migrated across activated endothelium than unstimulated HUVEC.
Endothelium stimulated by B. burgdorferi preferentially recruits CD8+ T lymphocytes.
Whether CD4+ or CD8+ T lymphocytes preferentially migrate across endothelium has been a matter of controversy (2, 13, 25). To address this issue, T lymphocytes were allowed to migrate across untreated HUVEC or HUVEC that had been stimulated with B. burgdorferi or IL-1. The migratory populations were harvested, stained for CD3 and either CD4 or CD8, and analyzed by two-color flow cytometry. Their phenotypes were then compared to those of the initial population of T lymphocytes. A statistically significant enrichment for CD8+ T lymphocytes in the migrated population occurred when the endothelium was incubated with either control medium or B. burgdorferi but not when the endothelium was stimulated with IL-1 (Table 3). Since the sum of the percentages of CD4+ and CD8+ T lymphocytes for each analysis was approximately 100%, an enrichment for the CD8+ subset corresponded to a depletion of CD4+ cells. Although the phenotypes of cells that migrated across untreated and B. burgdorferi-treated endothelium were similar, two- to fourfold more lymphocytes traversed the stimulated endothelium. These data suggest that the endothelium plays an active role in recruiting specific populations of T lymphocytes.
TABLE 3.
Unstimulated and B. burgdorferi-activated endothelium preferentially recruit CD8+ T lymphocytes
| Expt. no.b | CD4+/CD8+ T-lymphocyte ratioa
|
|||
|---|---|---|---|---|
| Initial population | Migrated across unstimulated HUVECc | Migrated across IL-1-treated HUVECd | Migrated across B.burgdorferi-treated HUVECcd | |
| 1 | 4.8 | 2.1 | 4.1 | 2.7 |
| 2 | 5.0 | 2.1 | 3.1 | 2.3 |
| 3 | 1.7 | 0.8 | 1.2 | 1.0 |
| 4 | 1.1 | 0.8 | 1.1 | 0.9 |
| 5 | 1.8 | 0.8 | 1.5 | 1.3 |
| 6 | 1.5 | 0.9 | 1.2 | 0.8 |
Data are expressed as the percentage of CD4+ T lymphocytes divided by the percentage of CD8+ T lymphocytes. In these analyses, the sum of the percentages of CD4+ and CD8+ T cells averaged 99% ± 3%.
Experiments 1 and 2 used T cells isolated from the same donor on different days; the remaining experiments all used cells from different donors.
Populations that migrated across unstimulated or B. burgdorferi-treated endothelium are significantly enriched for CD8+ T lymphocytes compared to the initial population (P < 0.05).
In these experiments, an average of 2.4 ± 0.6-fold more T lymphocytes migrated across stimulated endothelium than unstimulated endothelium.
DISCUSSION
Using a well-characterized in vitro model of the blood vessel wall, we demonstrated that B. burgdorferi activated endothelium in a manner that facilitated recruitment of specific subpopulations of T lymphocytes. Addition of the anti-inflammatory cytokine IL-10 significantly diminished this enhanced migration but had no effect on the migration of T lymphocytes across IL-1-treated HUVEC. Compared to the initially added population, the population of T lymphocytes that underwent transendothelial migration was both significantly enriched for CD45RA+RO+ cells and depleted of the CD45RA+RO− phenotype, regardless of the activation status of the endothelium. In addition, the migratory population was enriched for CD8+ T lymphocytes when the HUVEC were left untreated or exposed to B. burgdorferi but not when they were activated with IL-1.
In our in vitro model, approximately 2 to 4% of added T lymphocytes migrated across resting endothelium. In contrast, other investigators reported that 10 to 35% of T lymphocytes migrate across unstimulated endothelium grown on collagen (13, 25). In these studies, T cells were incubated overnight before executing the transmigration assay. This extended incubation may account for the increased level of migration compared to those found in our studies and those of Masuyama and colleagues (2, 23, 24), which employed freshly isolated lymphocytes. It may also explain why these investigators observed little (25) or no (13) increase in the number of T cells that migrated across cytokine-activated, as compared to resting, endothelium. In contrast, we observed on average a greater than twofold increase in the number of T lymphocytes that traversed HUVEC stimulated with either B. burgdorferi or IL-1.
Maximal numbers of T lymphocytes migrated across HUVEC exposed to B. burgdorferi within 4 h after addition of the leukocytes. At this time, comparatively few T cells migrated across HUVEC that had been treated with a sham preparation of spirochetes. When coincubation of endothelium and T lymphocytes was extended to 12 h or more, migration across the sham-treated cultures increased to levels comparable to those seen using spirochete-stimulated HUVEC (Fig. 2). This result suggests that, with increased incubation times, T lymphocytes and endothelium interact in a manner that facilitates transmigration. One possible scenario is that prolonged contact of T cells with endothelium results in activation of the leukocytes (30), which, in turn, may secrete cytokines that stimulate the endothelium to upregulate adhesion molecules and produce chemoattractants. These events would be expected to promote emigration of T lymphocytes.
The anti-inflammatory cytokine IL-10 significantly diminished the enhanced migration of T lymphocytes across B. burgdorferi-stimulated HUVEC but not across IL-1-treated endothelium (Fig. 4). Similarly, IL-10 inhibits migration of monocytes across endothelium activated by B. burgdorferi, but not by IL-1 (7). These observations suggest that B. burgdorferi and IL-1 activate endothelium via two distinct mechanisms, one that is sensitive to IL-10 and one that is not. The ability of IL-10 to diminish migration of lymphocytes and monocytes across spirochete-stimulated endothelium may be explained, at least in part, by its capacity to suppress endothelial production of monocyte chemoattractant protein-1 (7). In a murine model of Lyme disease, IL-10 plays an important role in the regulation of arthritis severity and host defense (6). Mice that are deficient in IL-10, when infected with B. burgdorferi, develop more severe arthritis than do wild-type mice, but have 10-fold fewer spirochetes in the affected tissue. Therefore, IL-10 may protect against the symptoms of Lyme disease by diminishing the recruitment of inflammatory leukocytes across endothelium. As a consequence, clearance of the bacteria is impeded.
Examination of the phenotypes of T lymphocytes that migrated across unstimulated HUVEC or HUVEC activated by B. burgdorferi or IL-1 revealed that CD45RA+RO+ T lymphocytes preferentially traversed the endothelium whereas CD45RA+RO− T lymphocytes were selectively depleted, regardless of the inciting stimulus (Table 1). This observation is consistent with several previous in vitro studies that demonstrate an enrichment for CD45RO-bearing T lymphocytes in the migratory population (2–4, 13, 29). Studies of sheep have led to the view that circulating memory (CD45RA−RO+) T lymphocytes preferentially enter nonlymphoid tissues, whereas naïve (CD45RA+RO−) T cells tend to migrate across the specialized high endothelial venules of lymphoid organs (21). Recently, however, the general validity of this conclusion has been questioned (36). Our data support the idea that naïve cells migrate poorly across nonlymphoid endothelium, but in our model CD45RA+RO+ cells traversed HUVEC monolayers more efficiently than did CD45RA−RO+ memory T cells. In vitro studies have suggested that CD45RA+RO+ cells are in transition from a naïve to a memory phenotype (10, 26), although the origin and functions of this subset in vivo are uncertain (35).
Expression of the CD45RO isoform occurs within 24 h after activation of CD45RA+RO− T lymphocytes in vitro (10). Therefore, we cannot rule out the possibility that the apparent enrichment for CD45RA+RO+ T cells in the migratory population stems from acquisition of CD45RO by previously negative cells during the 4 h assay. However, fewer than 7.5% of the migratory cells displayed the early activation marker CD69 (Table 2), which is expressed 1 to 2 h after stimulation (22, 34). This observation suggests that the majority of T lymphocytes that undergo transendothelial migration have not been recently activated. Consequently, it appears that enrichment for CD45RA+RO+ cells in the migratory population results not from a rapid phenotypic change but rather from preferential migration.
Greater than 95% of CD4+ T lymphocytes in the synovial fluids of patients with Lyme arthritis express CD45RO (28). Whether this enrichment for CD45RO+ T lymphocytes in lesions of Lyme disease is due to a greater capacity of CD45RO+ T cells to emigrate from the vasculature; local differentiation from naïve T cells; or enhanced survival, proliferation, or retention within the tissues is not certain. However, the results obtained in vitro support the notion that at least some enrichment occurs at the level of transendothelial migration. Such preferential recruitment might result from selective engagement of endothelial adhesion molecules; in vitro, CD45RA−RO+ T lymphocytes interact more effectively with E- and P-selectin and vascular cell adhesion molecule 1 than do naïve cells under conditions of flow (19). Chemokines, secreted by either endothelium or stromal cells, might lead to further selectivity by virtue of their ability to attract specific subsets of lymphocytes (20).
Analysis of expression of CD4 and CD8 by the migratory populations suggests that endothelium may be capable of recruiting dissimilar subsets of T lymphocytes under different conditions. The population that migrated across either unstimulated HUVEC or HUVEC exposed to B. burgdorferi was significantly enriched for CD8+ T cells compared to the population that was initially added (Table 3). Enrichment for CD8+ T cells in the population that migrates across resting endothelium has also been observed using HUVEC grown on collagen gels (13, 25), although others report that CD4+ T cells selectively accumulate in a similar model (2). In contrast, we saw no significant difference in the percentages of CD8+ T cells in the starting population and the population that migrated across IL-1-stimulated endothelium (Table 3). These results suggest that enrichment for CD8+ T lymphocytes is not due simply to an inherently greater capacity of these cells to transmigrate. Rather, it depends on the stimulus that was used to activate the HUVEC and thus, presumably, on the adhesion molecules and chemoattractants that were elaborated by the endothelium. However, some caution in making this conclusion is warranted, since the difference in the proportions of CD8+ T lymphocytes in the populations that migrated across endothelium stimulated by IL-1 versus B. burgdorferi does not reach statistical significance. Nonetheless, the implication that B. burgdorferi- and IL-1-stimulated endothelia are not identical is compatible with our observation that IL-10 diminishes activation of HUVEC by the spirochetes, but not by IL-1 (Fig. 4).
Two studies have assessed the ratios of CD4+ to CD8+ T lymphocytes in synovial tissues or fluids of patients with Lyme arthritis (28, 33). However, neither compared these ratios with those present in peripheral blood. The question of whether CD8+ T lymphocytes are enriched in lesions of Lyme disease thus remains open. Nevertheless, evidence suggests that these cells play a critical part in the progression of this illness. B. burgdorferi-specific CD8+ T lymphocytes are present in the synovial fluids of patients with Lyme arthritis (9). In a murine model of Lyme disease, abrogation of the CD8+ T-lymphocytic subset reduces both the severity of arthritis and the numbers of spirochetes in the joints and skin (18). Therefore, it appears that the CD8+ T cells promote the disease process by interfering with the generation of protective immunity. In mice, CD8+ T lymphocytes are activated early during the immune response to B. burgdorferi and are the major producers of IFN-γ (11). IFN-γ secreted by activated CD8+ T lymphocytes in Lyme disease may increase inflammation and arthritis by promoting macrophages to secrete proinflammatory mediators (14), thereby perpetuating the illness.
Collectively, our data suggest that subsets of T lymphocytes with similar phenotypes migrated across unstimulated endothelium and endothelium that had been exposed to B. burgdorferi. However, more than twice as many T cells traversed the spirochete-activated monolayers. One possible explanation for these observations is that B. burgdorferi simply induces endothelium to produce greater amounts of the same adhesion molecules and chemoattractants that it expresses under resting conditions. Alternatively, the populations of T lymphocytes that migrate across resting versus B. burgdorferi-activated endothelium may be distinct in ways that were not revealed by the phenotypic markers that we examined.
Accumulation of particular classes of T lymphocytes in lesions of patients with Lyme disease undoubtedly reflects a complex interplay among many different cell types and cytokines, and our in vitro model does not duplicate all of the factors that contribute to this process in vivo. Our results, however, raise the possibility that the composition of such T-lymphocytic infiltrates is determined, at least in part, at the level of emigration from the vasculature. Identification of the mechanisms responsible for the recruitment of specific subsets of T lymphocytes in Lyme disease will likely contribute to a better understanding of the progression of this illness and of chronic inflammatory disorders in general.
ACKNOWLEDGMENTS
This work was supported by research awards from the National Office and Long Island Chapter of the Arthritis Foundation.
We thank James Rohlf and Raymond Mugno for their expert help with statistics, Christopher Pullis and Corinne Leombruno for performing flow cytometry, Jennifer Raffanello for excellent technical assistance, and Jorge L. Benach for critical review of the manuscript.
REFERENCES
- 1.Benach J L, Bosler E M, Hanrahan J P, Coleman J L, Habicht G S, Bast T F, Cameron D J, Ziegler J L, Barbour A G, Burgdorfer W, Edelman R, Kaslow R A. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- 2.Berman J S, Mahoney K, Saukkonen J J, Masuyama J. Migration of distinct subsets of CD8+ blood T cells through endothelial cell monolayers in vitro. J Leukoc Biol. 1995;58:317–324. doi: 10.1002/jlb.58.3.317. [DOI] [PubMed] [Google Scholar]
- 3.Bird I N, Spragg J H, Ager A, Matthews N. Studies of lymphocyte transendothelial migration: analysis of migrated cell phenotypes with regard to CD31 (PECAM-1), CD45RA and CD45RO. Immunology. 1993;80:553–560. [PMC free article] [PubMed] [Google Scholar]
- 4.Brezinschek R I, Lipsky P E, Galéa P, Vita R, Oppenheimer-Marks N. Phenotypic characterization of CD4+ T cells that exhibit a transendothelial migratory capacity. J Immunol. 1995;154:3062–3077. [PubMed] [Google Scholar]
- 5.Brown C R, Reiner S L. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect Immun. 1999;67:3329–3333. doi: 10.1128/iai.67.7.3329-3333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown J P, Zachary J F, Teuscher C, Weis J J, Wooten R M. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns M J, Furie M B. Borrelia burgdorferi and interleukin-1 promote the transendothelial migration of monocytes in vitro by different mechanisms. Infect Immun. 1998;66:4875–4883. doi: 10.1128/iai.66.10.4875-4883.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns M J, Sellati T J, Teng E I, Furie M B. Production of interleukin-8 (IL-8) by cultured endothelial cells in response to Borrelia burgdorferi occurs independently of secreted IL-1 and tumor necrosis factor alpha and is required for subsequent transendothelial migration of neutrophils. Infect Immun. 1997;65:1217–1222. doi: 10.1128/iai.65.4.1217-1222.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busch D H, Jassoy C, Brinckmann U, Girschick H, Huppertz H I. Detection of Borrelia burgdorferi-specific CD8+ cytotoxic T cells in patients with Lyme arthritis. J Immunol. 1996;157:3534–3541. [PubMed] [Google Scholar]
- 10.Deans J P, Boyd A W, Pilarski L M. Transitions from high to low molecular weight isoforms of CD45 (T200) involve rapid activation of alternate mRNA splicing and slow turnover of surface CD45R. J Immunol. 1989;143:1233–1238. [PubMed] [Google Scholar]
- 11.Dong Z, Edelstein M D, Glickstein L J. CD8+ T cells are activated during the early Th1 and Th2 immune responses in a murine Lyme disease model. Infect Immun. 1997;65:5334–5337. doi: 10.1128/iai.65.12.5334-5337.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duray P H. The surgical pathology of human Lyme disease. An enlarging picture. Am J Surg Pathol. 1987;11(Suppl. 1):47–60. doi: 10.1097/00000478-198700111-00005. [DOI] [PubMed] [Google Scholar]
- 13.Galéa P, Brezinschek R, Lipsky P E, Oppenheimer-Marks N. Phenotypic characterization of CD4−/αβ TCR+ and γδ TCR+ T cells with a transendothelial migratory capacity. J Immunol. 1994;153:529–542. [PubMed] [Google Scholar]
- 14.Harty J T, Tvinnereim A R, White D W. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 15.Huang A J, Furie M B, Nicholson S C, Fischbarg J, Liebovitch L S, Silverstein S C. Effects of human neutrophil chemotaxis across human endothelial cell monolayers on the permeability of these monolayers to ions and macromolecules. J Cell Physiol. 1988;135:355–366. doi: 10.1002/jcp.1041350302. [DOI] [PubMed] [Google Scholar]
- 16.Kang I, Barthold S W, Persing D H, Bockenstedt L K. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keane-Myers A, Maliszewski C R, Finkelman F D, Nickell S P. Recombinant IL-4 treatment augments resistance to Borrelia burgdorferi infections in both normal susceptible and antibody-deficient susceptible mice. J Immunol. 1996;156:2488–2494. [PubMed] [Google Scholar]
- 18.Keane-Myers A, Nickell S P. T cell subset-dependent modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;154:1770–1776. [PubMed] [Google Scholar]
- 19.Lichtman A H, Ding H, Henault L, Vachino G, Camphausen R, Cumming D, Luscinskas F W. CD45RA−RO+ (memory) but not CD45RA+RO− (naive) T cells roll efficiently on E- and P-selectin and vascular cell adhesion molecule-1 under flow. J Immunol. 1997;158:3640–3650. [PubMed] [Google Scholar]
- 20.Luster A D. Chemokines—chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 21.Mackay C R, Marston W L, Dudler L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J Exp Med. 1990;171:801–817. doi: 10.1084/jem.171.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzio R, Mauël J, Betz-Corradin S. CD69 and regulation of the immune function. Immunopharmacol Immunotoxicol. 1999;21:565–582. doi: 10.3109/08923979909007126. [DOI] [PubMed] [Google Scholar]
- 23.Masuyama J, Berman J S, Cruikshank W W, Morimoto C, Center D M. Evidence for recent as well as long term activation of T cells migrating through endothelial cell monolayers in vitro. J Immunol. 1992;148:1367–1374. [PubMed] [Google Scholar]
- 24.Masuyama J, Yoshio T, Suzuki K, Kitagawa S, Iwamoto M, Kamimura T, Hirata D, Takeda A, Kano S, Minota S. Characterization of the 4C8 antigen involved in transendothelial migration of CD26hi T cells after tight adhesion to human umbilical vein endothelial cell monolayers. J Exp Med. 1999;189:979–990. doi: 10.1084/jem.189.6.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietschmann P, Cush J J, Lipsky P E, Oppenheimer-Marks N. Identification of subsets of human T cells capable of enhanced transendothelial migration. J Immunol. 1992;149:1170–1178. [PubMed] [Google Scholar]
- 26.Prince H E, York J, Jensen E R. Phenotypic comparison of the three populations of human lymphocytes defined by CD45RO and CD45RA expression. Cell Immunol. 1992;145:254–262. doi: 10.1016/0008-8749(92)90329-n. [DOI] [PubMed] [Google Scholar]
- 27.Randolph G J, Furie M B. A soluble gradient of endogenous monocyte chemoattractant protein-1 promotes the transendothelial migration of monocytes in vitro. J Immunol. 1995;155:3610–3618. [PubMed] [Google Scholar]
- 28.Roessner K, Trivedi H, Gaur L, Howard D, Aversa J, Cooper S M, Sigal L H, Budd R C. Biased T-cell antigen receptor repertoire in Lyme arthritis. Infect Immun. 1998;66:1092–1099. doi: 10.1128/iai.66.3.1092-1099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Röhnelt R K, Hoch G, Reiß Y, Engelhardt B. Immunosurveillance modelled in vitro: naive and memory T cells spontaneously migrate across unstimulated microvascular endothelium. Int Immunol. 1997;9:435–450. doi: 10.1093/intimm/9.3.435. [DOI] [PubMed] [Google Scholar]
- 30.Sancho D, Yáñez-Mo M, Tejedor R, Sánchez-Madrid F. Activation of peripheral blood T cells by interaction and migration through endothelium: role of lymphocyte function antigen-1/intercellular adhesion molecule-1 and interleukin-15. Blood. 1999;93:886–896. [PubMed] [Google Scholar]
- 31.Sellati T J, Burns M J, Ficazzola M A, Furie M B. Borrelia burgdorferi upregulates expression of adhesion molecules on endothelial cells and promotes transendothelial migration of neutrophils in vitro. Infect Immun. 1995;63:4439–4447. doi: 10.1128/iai.63.11.4439-4447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 33.Steere A C, Duray P H, Butcher E C. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis. Comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 1988;31:487–495. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- 34.Testi R, Phillips J H, Lanier L L. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering. Requirement for receptor cross-linking, prolonged elevation of intracellular [Ca++] and stimulation of protein kinase C. J Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- 35.van Lier R A, Baars P A. Assessing the replicative history of human T cells. Mutat Res. 1999;431:177–180. doi: 10.1016/s0027-5107(99)00158-x. [DOI] [PubMed] [Google Scholar]
- 36.Westermann J, Pabst R. How organ-specific is the migration of ‘naive’ and ‘memory’ T cells? Immunol Today. 1996;17:278–282. doi: 10.1016/0167-5699(96)80545-7. [DOI] [PubMed] [Google Scholar]
- 37.Yin Z, Braun J, Neure L, Wu P, Eggens U, Krause A, Kamradt T, Sieper J. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 1997;40:69–79. doi: 10.1002/art.1780400111. [DOI] [PubMed] [Google Scholar]