Abstract
Fibroblast growth factor receptor (FGFR) dysregulation is involved in a variety of tumorigenesis and development. Cholangiocarcinoma is closely related with FGFR aberrations, and pemigatinib is the first drug approved to target FGFR for the treatment of cholangiocarcinoma. Herein, we undertake biochemical and structural analysis on pemigatinib against FGFRs as well as gatekeeper mutations. The results show that pemigatinib is a potent and selective FGFR1–3 inhibitor. The extensive network of hydrogen bonds and van der Waals contacts found in the FGFR1-pemigatinib binding mode accounts for the high potency. Pemigatinib also has excellent potency against the Val-to-Ile gatekeeper mutation but less potency against the Val-to-Met/Phe gatekeeper mutation in FGFR. Taken together, the inhibitory and structural profiles exemplified by pemigatinib may help to thwart Val-to-Ile gatekeeper mutation-based resistance at earlier administration and to advance the further design and improvement for inhibitors toward FGFRs with gatekeeper mutations.
Subject terms: Kinases, X-ray crystallography, Kinases, X-ray crystallography
Pemigatinib is an FDA-approved drug for the treatment of cholangiocarcinoma, but while it is known to target fibroblast growth factor receptors, its mechanism of action is still not fully understood. Here, biochemical and structural analyses reveal that pemigatinib is a potent and selective FGFR1–3 inhibitor, and has excellent potency against the Val-to-Ile gatekeeper mutation.
Introduction
Fibroblast growth factor receptors (FGFRs) belong to the tyrosine kinase receptor family1. It can regulate cell migration, proliferation, and cell differentiation by activating downstream signaling pathways, including the RAS–MAPK–ERK, PI3K–AKT and Janus kinase–signal transducer and activator of transcription (JAK–STAT) signaling pathways1–4. Genetic alterations in FGFRs would lead to the aberrations of the activated FGFR signaling pathway and further the establishment and development of a wide variety of cancers5–8. FGFR2 aberrations are found in 10–15% of patients with intrahepatic cholangiocarcinoma9. Approximately 50% of bladder cancers are associated with FGFR3 mutations, and 30% of hepatocellular carcinoma patients have abnormally increased FGFR4 expression10,11. Therefore, targeting the FGFR signaling pathway represents an attractive therapeutic target. Many small-molecule inhibitors are being discovered and approved by the FDA for patients harboring FGFR alterations, such as pemigatinib (Fig. 1A), erdafitinib (Fig. 1B) and infigratinib (Fig. 1C).
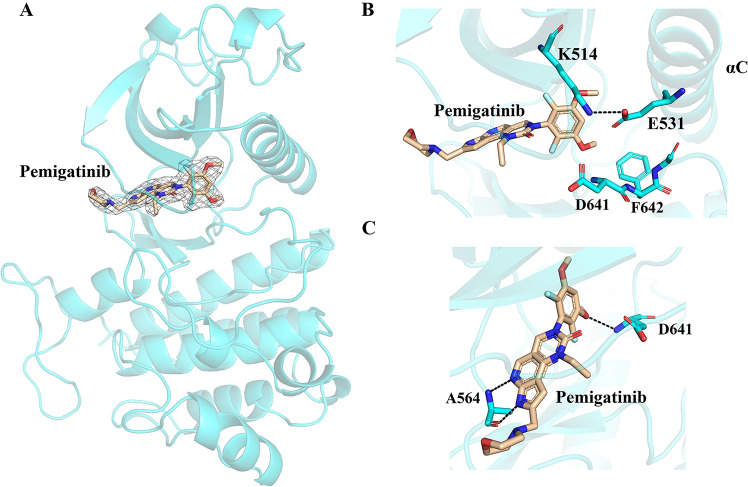
Fig. 1. Pemigatinib is a potent FGFR1-3 inhibitor.
Chemical structures of pemigatinib (INCB054828) (A), Erdafitinib (JNJ-42756493) (B) and Infigratinib (BGJ398) (C). Inhibitory effects of pemigatinib against wild type FGFR1-4 using kinase activity inhibition assays (D) and Ba/F3 cell models expressing FGFR1-4 (E). Error bars represent the standard deviation for at least three independent measurements.
Pemigatinib was the first targeted therapy approved by the FDA in April 2020 to treat patients with previously treated, unresectable, locally advanced, or metastatic cholangiocarcinoma and an FGFR2 fusion or other rearrangement12,13. In the Phase II FIGHT-202 trial (NCT02924376), pemigatinib yielded an objective response rate of 35.5% (2.8% complete responses) and a disease control rate of 82.0%14. These encouraging outcomes underlie the potential benefit of pemigatinib in clinical use. It is supposed that pemigatinib would become a first-line treatment for these patients. An ongoing clinical trial, FIGHT-302, will evaluate the efficacy and safety of pemigatinib therapy versus gemcitabine plus cisplatin combination therapy15. Additionally, pemigatinib is expected to treat other FGFR-driven malignancies, such as urothelial carcinoma, and relevant clinical trials are launched and undergoing in various countries worldwide12.
After an initial response to tyrosine kinase inhibitor therapies, acquired drug resistance gradually develops, correlated with therapy discontinuation and cancer advances, which can be mediated by the alternation in the protein-drug binding and/or the activation of bypass signaling16,17. Gatekeeper mutations in kinase domains represent a common theme, which maps at the beginning of the hinge region, curbs the accessibility of the inhibitor to the ATP pocket and contributes to the loss of effectiveness of the inhibitor, such as Abl T315I and EGFR T790M18–21. Ponatinib and osimertinib, third-generation tyrosine kinase inhibitors, were developed to overcome Abl T315I and EGFR T790M, respectively22,23. Therefore, overcoming resistance by gatekeeper mutations is a novel and subsequent direction of drug design and improvement for next-generation FGFR inhibitors. As the first-generation FGFR inhibitor, pemigatinib needs further development so deeper insights into the inhibitory and structural profiles of pemigatinib are necessary.
In this study, we aimed to explore the structural basis for the high potency of pemigatinib against FGFRs and predict the sensitivity to gatekeeper mutations. We performed biochemical and structural analysis on pemigatinib against FGFRs and gatekeeper mutations. The results show that pemigatinib is capable of inhibiting FGFR1-3 and retains excellent potency against FGFR2 V564I but lower potency against FGFR4 and other gatekeeper mutations. Our results may provide some structural basis and design directions for further optimization of FGFR inhibitors.
Results
Potent inhibition of FGFRs by pemigatinib
To confirm the inhibitory effect of pemigatinib on FGFR1-4, we performed kinase activity inhibition assays. As shown in Fig. 1, pemigatinib exhibits potent inhibitions against FGFR1-3 with half maximal inhibitory concentrations (IC50) of 0.2, 1.2, and 1.4 nM, respectively. Compared with FGFR1-3, the inhibition of FGFR4 is greatly reduced (~75-fold less potent compared with FGFR1), with an IC50 of 15 nM. We also carried out cellular proliferation assays in Ba/F3 cells to further verify the inhibitory effect of pemigatinib. Pemigatinib has no impact on the growth of parental Ba/F3 cells with IC50 values exceeding 5 μM. It has great potency against the proliferation of FGFR1-3-translocated Ba/F3 cells (IC50 of 1.2, 0.3 and 1.2 nM, respectively) but lower potency against the proliferation of FGFR4-translocated Ba/F3 cells (IC50 of 12 nM), consistent with the results of kinase assays. Taken together, these results substantiate pemigatinib as a potent FGFR1-3 inhibitor.
Structural basis of the pemigatinib-FGFR1 interaction
To gain structural insights into the mechanism of FGFR inhibition by pemigatinib, the crystal structure of the pemigatinib/FGFR1 kinase domain was determined at a high resolution of 2.5 Å. The detailed collection data are shown in Supplementary Table 1 and the validation report is available as Supplementary Data 1. The chemical structure of pemigatinib is described in Fig. 1A, and its electron density and structural characteristics are well represented in the crystal structure with the FGFR1 kinase domain (Fig. 2A). Pemigatinib occupies the ATP-binding pocket of FGFR1, where the activation loop adopts a DFG-in conformation (Fig. 2B), resembling other selective FGFR inhibitors. The pyrrolopyridine moiety forms two hydrogen bonds with the N-H group and the carbonyl group of Ala564 in the hinge region of FGFR1 (Fig. 2C). The difluoromethoxyphenyl ring is oriented perpendicular to the tricyclic scaffold and occupies the hydrophobic pocket containing the gatekeeper residue Val561. One of the methoxy oxygen atoms of the difluoromethoxyphenyl ring forms a hydrogen bond with the backbone nitrogen atom of Asp641 (Fig. 2C). The morpholine solubilizing group extends away from the hinge region toward the solvent exposed region and does not make any specific interactions with FGFR1. Apart from direct interactions, pemigatinib binds to FGFR1 indirectly via the water molecule that links the oxygen atom of the tricyclic scaffold to the amino side chain of Lys514 and the carboxylate side chain of Asp641 (Supplementary Fig. 1). More interactions, including van der Waals forces, are also described in Supplementary Fig. 1. These results demonstrate that pemigatinib firmly binds FGFR1 via an extensive interaction network.
Fig. 2. Crystal structure of pemigaitnib in complex with FGFR1.
A Overall structure of the pemigatinib/FGFR1 complex. B The activation loop of pemigaitnib kept in DFG-in conformation. C Hydrogen-bond interaction between pemigatinib and FGFR1.
Structural comparison of pemigatinib to erdafitinib/infigratinib in complex with FGFR1
To date, FGFR inhibitors approved by the FDA exclusively include pemigatinib, erdafitinib and infigratinib. To help fully understand the profiles of these three drugs, we performed a structural comparison of their binding to the FGFR1 kinase domain. The activation loops of these three drugs binding FGFR1 are all kept in the DFG-in conformation (Fig. 3A–C), indicating that they are classified as Type I inhibitors24,25. A 3,5-dimethoxyphenyl ring is observed in these three inhibitors, and one of the methoxy oxygen atoms is involved in a hydrogen bond with Asp641, which increases the selectivity for FGFRs8. With regard to the molecular scaffold, the quinoxaline in erdafitinib formed one hydrogen bond with Ala564, whereas the tricyclic urea scaffold in pemigatinib and the N-aryl-N’-pyrimidin-4-yl urea scaffold in infigratinib formed two hydrogen bonds with Ala564 (Fig. 3D–F). More hydrogen bonds formed could stabilize the complex conformations, which may explain why pemigatinib (IC50 of 0.2 nM) and infigratinib (IC50 of 0.9 nM) have more potency toward FGFRs than erdafitinib (IC50 of 1.2 nM)25,26. Moreover, they all possess a solubilizing group. As to pemigatinib, the solubilizing group is composed of a morpholine group, and to infigratinib and erdafitinib are ethyl piperazine and methyl pyrazole, respectively, which could increase their solubility and drug dissolution in the body8,25. Overall, these results indicate that the binding mode of pemigatinib in complex with FGFR1 is similar to that of erdafitinib/infigratinib.
Fig. 3. Structural comparison of pemigatinib to erdafitinib and infigratinib in complex with FGFR1.
A–C The DFG motif conformation of FGFR1. FGFR1 adopts a DFG-in activation loop conformation with pemigatinib, erdafitinib (PDB: 5EW8) and infigratinib (PDB: 3TT0). D–F Hydrogen-bond interactions between FGFR1 and pemigatinib/erdafitinib/infigratinib.
The effect of FGFR gatekeeper mutations on the potency of pemigatinib
The question of whether gatekeeper mutations in the FGFR kinase domain influence on the inhibitory efficacy of pemigatinib has not been addressed. Thus, kinase activity inhibition assays were carried out to assess the sensitivity of pemigatinib to typical and frequent gatekeeper mutations, involving FGFR1 V561M, FGFR2 V564I/F and FGFR3 V555M (Fig. 4). Unexpectedly, pemigatinib retains excellent potency against FGFR2 V564I with an IC50 of 7 nM in the kinase assay, which exhibits merely a 4.7-fold reduction in inhibitory efficacy compared to that against the wild type (Fig. 4A, B). In contrast, the efficacy of pemigatinib is evidently diminished by the introduction of methionine to replace valine. It is found that pemigatinib exhibits ~745-fold lower potency against FGFR1 V561M (IC50 of 149 nM), and ~76-fold lower potency against FGFR3 V555M (IC50 of 107 nM) (Fig. 4A, B). Moreover, pemigatinib is vulnerable to the V564F mutation in FGFR2. A great reduction in efficacy for pemigatinib is observed in the V564F mutation, with an IC50 of 263 nM (~219-fold lower potency than FGFR1) (Fig. 4A, B). Likewise, decreased potencies against V564F and V555M are also observed in the cellular proliferation assay in Ba/F3 cells (Supplementary Fig. 2). These results reveal that pemigatinib could exhibit significant inhibition against the Val-to-Ile mutation but attenuated potency against the Val-to-Met/Phe mutation in FGFRs.
Fig. 4. Pemigatinib remains the excellent inhibitory efficacy for Val-to-Ile gatekeeper mutation but lower potency against Val-to-Met/Phe gatekeeper mutation.
A Inhibitory effects of pemigatinib against FGFR1-3 gatekeeper mutants using kinase activity inhibition assays. B The collective half maximal inhibitory concentration (IC50) values for FGFR1-3 gatekeeper mutations summarized in detail. Error bars represent the standard deviation for at least three independent measurements. C–F Structural models of FGFR2 V564I/F, FGFR1 V561M and FGFR3 V555M in complex with pemigatinib. Pemigatinib colored cyan is from the binding models with the corresponding wild type where pemigatinib is docked into FGFR2 (PDB ID: 6LVL), and FGFR3 (PDB ID: 7DHL), and is aligned with the structural models of gatekeeper mutants. Pemigatinib colored orange is the predicted structure with gatekeeper mutants. The structural models of FGFR2 V564I/F, FGFR1 V561M and FGFR3 V555M in complex with pemigatinib are generated by substitution of gatekeeper residues on the basis of FGFR1 (PDB: 7WCL), FGFR2 (PDB: 6LVL) and FGFR3 (PDB: 7DHL) structures.
Structural basis of FGFR gatekeeper mutations in response to pemigatinib
To further understand the characteristics of pemigatinib, we evaluated the dynamic binding of pemigatinib with other FGFR types by flexible molecular docking using AutoDock Tools program27. Pemigatinib was redocked into FGFR1 to evaluate the reliability of this method. Then, pemigatinib was docked into FGFR2 (PDB ID: 6LVL)28, FGFR3 (PDB ID:7DHL)29 and FGFR4 (PDB ID: 7F3M)30, respectively. Structural models predict that FGFR1-4 in complex with pemigatinib exhibit similar binding modes and similar interaction patterns (Supplementary Fig. 3), with low predicted binding energies (Supplementary Table 2).
Furthermore, we also evaluated the dynamic binding of pemigatinib with FGFR gatekeeper mutations by flexible molecular docking. Structural superposition of FGFR2 V564I/pemigatinib and wild type FGFR2/pemigatinib indicates that pemigatinib binds with FGFR2 V564I in the similar position with wild type FGFR2 (Fig. 4C, Supplementary Fig. 4A and Supplementary Table 2). The less severe hindrance from the small isoleucine side chain makes the binding conformation nearly identical with the wild type, which underlies the sensitivity of pemigatinib toward FGFR2 V564I. When docked into FGFR1 V561M, FGFR2 V564F and FGFR3 V555M, pemigatinib moderately moves out of the binding pocket in order to evade the steric clash with the bulky methionine and phenylalanine residues compared with their wild type (Fig. 4D–F). The movement of pemigatinib in these mutants may result in impaired inhibitory potencies and lower predicted binding energies (−6.53 Kcal·mol−1 for FGFR1 V564M, −8.65 Kcal·mol−1 for FGFR2 V564F and -9.29 Kcal·mol−1 for FGFR3 V555M) (Fig. 4D–F, Supplementary Fig. 4B–D and Supplementary Table 2). Taken together, these structural models suggest that pemigatinib-FGFR binding is more susceptible to the disruption by the introduction of Met/Phe than Ile, due to the more severe steric clash caused by the bulky Met/Phe. Thus, the less severe hindrance could preserve excellent inhibitory potency against gatekeeper mutations.
Discussion
Pemigatinib is the first small molecule targeted drug to treat cholangiocarcinoma12. Despite slight loss of efficacy for FGFR4, pemigatinib has great potency against FGFR1-3, indicating that pemigatinib represents a potential therapy for other FGFR-driven malignancies. Related clinical trials have been undertaken to expand the application of pemigatinib12. Structural analysis shows that pemigatinib can be divided into three important chemical components according to their interactions with FGFR1. First, the 3,5-dimethoxylphenyl ring is essential for the selectivity for FGFR, which may be enhanced by the introduction of fluorine/chlorine8. Second, the tricyclic urea scaffold features hydrogen-bond interactions with the hinge region, which could serve as a template for further design and screening of drug structures of FGFR and/or other kinase receptor inhibitors. Third, the solubilizing morpholine group regulates the lipophilicity and contributes to excellent rat and cynomolgus monkey PK profiles as well as abrogation of time-dependent inhibition issue8. This extensive network of pemigatinib-FGFR1 contact reinforces the strength of drug binding and accounts for the high potency of pemigatinib, providing an exemplification for the further design and optimization of FGFR inhibitors with high selectivity and high potency.
Furthermore, the structural comparison of pemigatinib to erdafitinib and infigratinib in complex with FGFR1 highlights the importance of these three chemical components in drug design and optimization. The 3,5-dimethoxyphenyl ring, molecular scaffold and solubilizing group are all observed in these three drugs with extensive and indispensable interactions with FGFR1, which are essential for drug discovery and design. By overlaying the structures of these three compounds binding to FGFR1 and the modeled gatekeeper mutations, we found that the dimethoxyphenyl rings of erdafitinib and infigratinib were slightly away from gatekeeper residue Val561/Ile561, similar to that of pemigatinib, suggesting that erdafitinib and infigratinib might have excellent potency toward FGFR1 V561I as with pemigatinib (Supplementary Fig. 5A, B). For FGFR1 V561M/F (Supplementary Fig. 5C, D), these mutations may have a great impact on the response to pemigatinib, erdafitinib and infigratinib resulting from severe steric hindrance. These modeling data provide the structural basis for the fact that FGFR1 V561M/F and FGFR4 V550M confer resistance to erdafitinib, and FGFR1 V561M/F, FGFR2 V564F and FGFR3 V555M confer resistance to infigratinib31–35.
Pemigatinib is the first-generation FGFR inhibitor. According to previous experience with tyrosine kinase inhibitors35–38, resistance would occur due to the gatekeeper mutations, such as FGFR1 V561M, FGFR2 V564I/F, FGFR3 V555M and so on39. In our study, we found that pemigatinib can maintain excellent activity against the Val-to-Ile gatekeeper mutation, but exhibit significant attenuated potency toward the Val-to-Met/Phe gatekeeper mutation in FGFR, which may be attributed to the severe steric clash between the bulky Met/Phe and the 3,5-dimethoxyphenyl ring. Therefore, overcoming resistance may be achieved by substituting a smaller group for the 3,5-dimethoxyphenyl ring with a larger space to accommodate the bulky gatekeeper residues. Moreover, increasing the flexibility of molecular inhibitors may also take effect. In the discovery of pemigatinib, the prototype is cyclized and generates a tricyclic urea scaffold to rigidify the structure and restore the FGFR activity8, which reduces the flexibility of the compound and makes it vulnerable to Val-to-Met/Phe gatekeeper mutation. Hence, increased inhibitor flexibility could allow multiple inhibitor binding models to better accommodate gatekeeper mutation as well.
Except for the gatekeeper mutations, there are some other mutations conferring drug resistance to the FGFR inhibitors. For example, FGFR2 M535I, M537I, I547V, N549K/H/S/T, E565A/G, L617M/V, K641N/R and K659E/M/N have been reported40. Among of these mutations, six mutations have been observed in the treatment of pemigatinib, including N549K/H, E565A, K659M, L617V and K641R41. When mapped in our structure, these mutants are located outside of the binding pocket and are sorted into three categories (Supplementary Fig. 6). N549, E565 and K641 act as a “molecular brake” and keep the kinase in an autoinhibited state42. Thus, mutations of any residue of the triad might result in the disengagement of the “brake” and constitutive activation of the kinase42. Mutation of K659 would mimic the action of A-loop tyrosine phosphorylation, thereby constitutively activating the kinase42. L617M/V, M535I, M537I and I547V would strengthen the hydrophobic spine and stabilize the active kinase conformation, probably leading to less favorable pemigatinib-binding conditions32,33,40. In summary, these residues are all conserved in FGFR1-3, and any mutation of these residues might confer resistance to pemigatinib/erdafitinib/infigratinib by stabilizing the active kinase conformation either through disengaging the molecular brake or strengthening the hydrophobic spine or mimicking the action of A-loop tyrosine phosphorylation.
In this study, we performed kinase activity inhibition assays and cellular proliferation assays to demonstrate that pemigatinib is a potent and selective FGFR1–3 inhibitor. The structural analysis provides the features and nature of the pemigatinib-FGFR1 binding pattern, accounting for the high potency of pemigatinib. Pemigatinib could sustain the superior potency against the Val-to-Ile gatekeeper mutation than that against the Val-to-Met/Phe gatekeeper mutation, suggesting that the administration of pemigatinib at earlier stages of disease may evade Val-to-Ile mutation-based resistance. Collectively, these structural and inhibitory profiles exemplified by pemigatinib may be applied to the exploration of next-generation FGFR inhibitors to overcome gatekeeper mutation-based resistance.
Methods
Plasmid construction
Human FGFRs were prepared as previously described43–46. Briefly, the kinase domains of FGFR1 (residues 458–765), FGFR2 (residues 453–770) and FGFR3 (residues 450–758) were respectively cloned into a modified pET28a expression vector with an N-terminal 6×His tag followed by a PreScission cleavage site. The mutant plasmids, FGFR1 C584S, FGFR1 V561M, FGFR2 V564I, FGFR2 V564F and FGFR3 V555M, were constructed by PCR using primers with the desired mutations.
Protein expression and purification
Plasmids were expressed in E. coli BL21 Rosetta cells. For FGFR1 C584S, Rosetta cells were co-expressed with YOPH to obtain non-phosphorylated proteins. The colony was inoculated into the liquid LB culture with 50 μg/mL kanamycin at 37 °C and induced at 16 °C for 18 h by the addition of 0.5 mM IPTG between OD600nm of 0.7~0.8. The cells were harvested by 15-min centrifugation at 3000 rpm. The pellets were resuspended in lysis buffer (20 mM Tris-HCl, pH 8.0, 500 mM NaCl, 20 mM imidazole, 0.5 mM TCEP) and then lysed by a high-pressure homogenizer. The lysate supernatants were obtained after 35 min centrifugation at 18000 rpm and incubated with equilibrated Ni-NTA beads (GE Healthcare) for 1.5 h. Then, the beads were loaded into a gravity flow column and washed with lysis buffer containing 50 mM imidazole. The target proteins were eluted with lysis buffer containing 250 mM imidazole and digested with PreScission protease overnight to remove the N-terminal 6×His tag. Untagged FGFRs were further purified by anion exchange chromatography (GE Healthcare), and peak fractions collected were concentrated to 5–16 mg/mL. For crystallization, the pooled FGFR1 C584S was put through a Superdex 200 column (GE Healthcare) in storage buffer (20 mM Tris-HCl, pH 8.0, 20 mM NaCl, 0.5 mM TCEP).
Kinase inhibition assay
The kinase assays were performed using the ADP-Glo (Promega) methodology. Proteins (0.025–0.2 μM), pemigatinib (triple dilution method), ATP (10 μM) and poly (4:1 Glu, Tyr) peptides (Abcam, UK) (10 μM) were diluted with optimized kinase buffer (40 mM Tris-HCl pH 7.5, 20 mM MgCl2, 20 mM NaCl, 0.1 mg/mL BSA, 1 mM TCEP, and 4% DMSO). The specific steps were prepared as previously described47. In short, the kinase reactions were initiated by mixing protein, inhibitor, ATP, and poly (4:1 Glu, Tyr) peptides, reacting at room temperature for 30 min, and then terminated by adding ADP-Glo after 40 min of incubation. Kinase activities were detected on a plate reader (Perkin Elmer) after the addition of detection reagent. IC50 values were calculated with log[Inhibitor] versus kinase activity ratio using GraphPad Prism software.
Crystallization and structure determination
The hanging drop vapor diffusion method was used for crystallization as previously described48,49. FGFR1/pemigatinib crystals were produced by micro-seeding FGFR1 crystals. The FGFR1 crystals were grown at 4 °C by mixing 0.8 μL of protein solution with 0.8 μL of crystallization buffer comprising 18% (w/v) PEG 8000, 0.2 M LiSO4, and 0.1 M MES (pH 6.5). After 1 week, the crystals of FGFR1 were broken into microcrystals. FGFR1/pemigatinib crystals were produced similarly to the apo FGFR1 crystals but with the inhibitor molecule pre-incubated with FGFR1 in a 1:2 ratio overnight at 4 °C before micro-seeding and with identical crystallization conditions as apo FGFR1. The FGFR1/pemigatinib crystals were cryoprotected in the buffer supplemented with 20% glycerol and then flash-cooled in liquid nitrogen prior to data collection.
The X-ray diffraction data were collected in our lab, with HKL3000 employed for data integration and scaling50. The initial structures were solved by molecular replacement by Phaser from the PHENIX package with a search model from PDB entry 4RWJ51. Further structure refinement and model building were performed by Phenix. refine and Coot50. Full details are described in Supplementary Table 1. PyMOL and LigPlot+ were used for structural descriptions and protein–ligand interaction representations, respectively52,53. The coordinates and structure factors have been deposited in the PDB under accession numbers 7WCL.
Ba/F3 cell line proliferation assays
Cell proliferation and viability were evaluated in parental Ba/F3 cells and TEL-FGFR1-4/FGFR mutation-translocated Ba/F3 cell lines54. Cells were seeded in 96-well plates at 2 × 103 cells/well and incubated with various concentrations of pemigatinib in a final volume of 100 μL for 72 h at 37 °C. Subsequently, Cell Counting Kit-8 (Vazyme, China) was added, and after a 2-h incubation, the absorbance was measured at 450 nm using a multimode plate reader (Perkin Elmer). Each assay was performed in triplicate, and the IC50 values were calculated for the inhibitory potency of pemigatinib in vivo by GraphPad Prism 7.0.
Molecular docking
Computational docking was performed to predict the binding of pemigatinib to FGFR2-4 and FGFR gatekeeper mutations. Pemigatinib was docked into FGFR2 (PDB ID: 6LVL), FGFR3 (PDB ID:7DHL) and FGFR4 (PDB ID: 7F3M), respectively. The structures of FGFR gatekeeper mutations were prepared by protein mutagenesis based on their corresponding wild type FGFR. The waters were eliminated and the polar hydrogen was added by PyMOL53. Gasteiger charges and rotatable bonds to pemigatinib were assigned by AutoDock Tools program27. The docking simulations were conducted with a flexible protein and a flexible ligand. A docking grid with the dimensions of 40*40*40 points in the x-, y-, and z-axis directions was built, which encompassed the entire ligand-binding clefts.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The present study was financially supported by the Natural Science Foundation of China (No. 81570537, 81974074 and 82172654), Hunan Provincial Science and Technology Department (2018RS3026 and 2021RC4012) and National Science Foundation of Hunan Province (grants 2021JJ40961 and 2022JJ40763). We thank the staff from the Shanghai Synchrotron Radiation Facility (SSRF) beamline BL17U/BL19U/BL02U/BL10U/BL17B for assistance during diffraction data collection.
Author contributions
Q.L. and X.C. performed experiments; L.Q. and M.G. performed data collection and structure determination. H.W. and S.D. analyzed the data. Q.L., X.C., L.J. and Y.C. prepared the manuscript.
Peer review
Peer review information
Communications Chemistry thanks Yanmin Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The coordinates and structure factors are deposited in the Protein Data Bank under the accession codes 7WCL (FGFR1/pemigatinib complex). Validation report is available as Supplementary Data 1. All other relevant data supporting the key findings of this study are available within the article and its Supplementary Information files. A reporting summary is available as a Supplementary Information file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Qianmeng Lin, Xiaojuan Chen.
Contributor Information
Longying Jiang, Email: longyingj1024@163.com.
Yongheng Chen, Email: yonghenc@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-022-00718-z.
References
- 1.Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer. 2017;17:318–332. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 2.Kimelman D, Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987;51:869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- 3.Corson LB, Yamanaka Y, Lai KM, Rossant J. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development. 2003;130:4527–4537. doi: 10.1242/dev.00669. [DOI] [PubMed] [Google Scholar]
- 4.Brewer JR, Mazot P, Soriano P. Genetic insights into the mechanisms of Fgf signaling. Genes. Dev. 2016;30:751–771. doi: 10.1101/gad.277137.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grose R, Dickson C. Fibroblast growth factor signaling in tumorigenesis. Cytokine Growth Factor. Rev. 2005;16:179–186. doi: 10.1016/j.cytogfr.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug. Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knights V, Cook SJ. De-regulated FGF receptors as therapeutic targets in cancer. Pharmacol. Ther. 2010;125:105–117. doi: 10.1016/j.pharmthera.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Wu L, et al. Discovery of pemigatinib: a potent and selective fibroblast growth factor receptor (FGFR) inhibitor. J. Med. Chem. 2021;64:10666–10679. doi: 10.1021/acs.jmedchem.1c00713. [DOI] [PubMed] [Google Scholar]
- 9.Arai Y, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59:1427–1434. doi: 10.1002/hep.26890. [DOI] [PubMed] [Google Scholar]
- 10.Ho HK, et al. Fibroblast growth factor receptor 4 regulates proliferation, anti-apoptosis and alpha-fetoprotein secretion during hepatocellular carcinoma progression and represents a potential target for therapeutic intervention. J. Hepatol. 2009;50:118–127. doi: 10.1016/j.jhep.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Cappellen D, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat. Genet. 1999;23:18–20. doi: 10.1038/12615. [DOI] [PubMed] [Google Scholar]
- 12.Hoy SM. Pemigatinib: first approval. Drugs. 2020;80:923–929. doi: 10.1007/s40265-020-01330-y. [DOI] [PubMed] [Google Scholar]
- 13.Weng Q, Tan W, Yu RY, Xu RA, Chen Y. A novel bioanalytical method for the quantification of pemigatinib in rat plasma by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2021;202:114137. doi: 10.1016/j.jpba.2021.114137. [DOI] [PubMed] [Google Scholar]
- 14.Abou-Alfa GK, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekaii-Saab TS, et al. FIGHT-302: first-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020;16:2385–2399. doi: 10.2217/fon-2020-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krook MA, et al. Fibroblast growth factor receptors in cancer: genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br. J. Cancer. 2021;124:880–892. doi: 10.1038/s41416-020-01157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schram AM, Chang MT, Jonsson P, Drilon A. Fusions in solid tumours: diagnostic strategies, targeted therapy, and acquired resistance. Nat. Rev. Clin. Oncol. 2017;14:735–748. doi: 10.1038/nrclinonc.2017.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Shah K, Yang F, Witucki L, Shokat KM. A molecular gate which controls unnatural ATP analogue recognition by the tyrosine kinase v-Src. Bioorg. Med. Chem. 1998;6:1219–1226. doi: 10.1016/S0968-0896(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 19.O’Hare T, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews Wright NM, Goss GD. Third-generation epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer. Transl. Lung Cancer Res. 2019;8:S247–S264. doi: 10.21037/tlcr.2019.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah NP, et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 22.Soo, R. A. et al. A randomised phase II study of osimertinib and bevacizumab versus osimertinib alone as second-line targeted treatment in advanced NSCLC with confirmed EGFR and acquired T790M mutations: the European Thoracic Oncology Platform (ETOP 10-16) BOOSTER trial. Ann. Oncol.10.1016/j.annonc.2021.11.010 (2021). [DOI] [PubMed]
- 23.Parker WT, et al. The impact of multiple low-level BCR-ABL1 mutations on response to ponatinib. Blood. 2016;127:1870–1880. doi: 10.1182/blood-2015-09-666214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patani H, et al. Landscape of activating cancer mutations in FGFR kinases and their differential responses to inhibitors in clinical use. Oncotarget. 2016;7:24252–24268. doi: 10.18632/oncotarget.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guagnano V, et al. Discovery of 3-(2,6-dichloro-3,5-dimethoxy-phenyl)-1-{6-[4-(4-ethyl-piperazin-1-yl)-phenylamin o]-pyrimidin-4-yl}-1-methyl-urea (NVP-BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J. Med. Chem. 2011;54:7066–7083. doi: 10.1021/jm2006222. [DOI] [PubMed] [Google Scholar]
- 26.Perera TPS, et al. Discovery and pharmacological characterization of JNJ-42756493 (Erdafitinib), a functionally selective small-molecule FGFR family inhibitor. Mol. Cancer Ther. 2017;16:1010–1020. doi: 10.1158/1535-7163.MCT-16-0589. [DOI] [PubMed] [Google Scholar]
- 27.Morris GM, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuriwaki I, et al. Structure-based drug design of 1,3,5-triazine and pyrimidine derivatives as novel FGFR3 inhibitors with high selectivity over VEGFR2. Bioorg. Med. Chem. 2020;28:115453. doi: 10.1016/j.bmc.2020.115453. [DOI] [PubMed] [Google Scholar]
- 29.Kuriwaki I, et al. Synthesis and structure-activity relationships of pyrimidine derivatives as potent and orally active FGFR3 inhibitors with both increased systemic exposure and enhanced in vitro potency. Bioorg. Med. Chem. 2021;33:116019. doi: 10.1016/j.bmc.2021.116019. [DOI] [PubMed] [Google Scholar]
- 30.Qu L, et al. Structural insights into the potency and selectivity of covalent pan-FGFR inhibitors. Commun. Chem. 2022;5:5. doi: 10.1038/s42004-021-00623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang K, et al. GZD824 overcomes FGFR1-V561F/M mutant resistance in vitro and in vivo. Cancer Med. 2021;10:4874–4884. doi: 10.1002/cam4.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goyal L, et al. TAS-120 overcomes resistance to ATP-Competitive FGFR inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma. Cancer Discov. 2019;9:1064–1079. doi: 10.1158/2159-8290.CD-19-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goyal L, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2017;7:252–263. doi: 10.1158/2159-8290.CD-16-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Facchinetti F, et al. Facts and new hopes on selective FGFR inhibitors in solid tumors. Clin. Cancer Res. 2020;26:764–774. doi: 10.1158/1078-0432.CCR-19-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yue S, et al. FGFR-TKI resistance in cancer: current status and perspectives. J. Hematol. Oncol. 2021;14:23. doi: 10.1186/s13045-021-01040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hochhaus A, et al. Roots of clinical resistance to STI-571 cancer therapy. Science. 2001;293:2163. doi: 10.1126/science.293.5538.2163a. [DOI] [PubMed] [Google Scholar]
- 37.Choi YL, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N. Engl. J. Med. 2010;363:1734–1739. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi S, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 39.Nakanishi Y, et al. The fibroblast growth factor receptor genetic status as a potential predictor of the sensitivity to CH5183284/Debio 1347, a novel selective FGFR inhibitor. Mol. Cancer Ther. 2014;13:2547–2558. doi: 10.1158/1535-7163.MCT-14-0248. [DOI] [PubMed] [Google Scholar]
- 40.Byron SA, et al. The N550K/H mutations in FGFR2 confer differential resistance to PD173074, dovitinib, and ponatinib ATP-competitive inhibitors. Neoplasia. 2013;15:975–988. doi: 10.1593/neo.121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silverman IM, et al. Clinicogenomic analysis of FGFR2-rearranged cholangiocarcinoma identifies correlates of response and mechanisms of resistance to pemigatinib. Cancer Discov. 2021;11:326–339. doi: 10.1158/2159-8290.CD-20-0766. [DOI] [PubMed] [Google Scholar]
- 42.Chen H, et al. A molecular brake in the kinase hinge region regulates the activity of receptor tyrosine kinases. Mol. Cell. 2007;27:717–730. doi: 10.1016/j.molcel.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu D, et al. LY2874455 potently inhibits FGFR gatekeeper mutants and overcomes mutation-based resistance. Chem. Commun. 2018;54:12089–12092. doi: 10.1039/C8CC07546H. [DOI] [PubMed] [Google Scholar]
- 44.Wlodawer, A. et al. Crystal structure of the FGFR4/LY2874455 complex reveals insights into the Pan-FGFR selectivity of LY2874455. PLoS One11, 10.1371/journal.pone.0162491 (2016). [DOI] [PMC free article] [PubMed]
- 45.Ni F, et al. Remarkably stereospecific utilization of ATP α,β-Halomethylene analogues by protein kinases. J. Am. Chem. Soc. 2017;139:7701–7704. doi: 10.1021/jacs.7b03266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rezende Miranda R, et al. Development of a potent and specific FGFR4 inhibitor for the treatment of hepatocellular carcinoma. J. Med. Chem. 2020;63:11484–11497. doi: 10.1021/acs.jmedchem.0c00044. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Z, et al. Characterization of FGF401 as a reversible covalent inhibitor of fibroblast growth factor receptor 4. Chem. Commun. 2019;55:5890–5893. doi: 10.1039/C9CC02052G. [DOI] [PubMed] [Google Scholar]
- 48.Guo, M. et al. Characterization of ibrutinib as a non-covalent inhibitor of SRC-family kinases. Bioorg. Med. Chem. Lett. 34, 10.1016/j.bmcl.2020.127757 (2021). [DOI] [PubMed]
- 49.Deng W, et al. Investigation of covalent warheads in the design of 2-Aminopyrimidine-based FGFR4 inhibitors. ACS Med. Chem. Lett. 2021;12:647–652. doi: 10.1021/acsmedchemlett.1c00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams PD, et al. The Phenix software for automated determination of macromolecular structures. Methods. 2011;55:94–106. doi: 10.1016/j.ymeth.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sohl CD, Ryan MR, Luo B, Frey KM, Anderson KS. Illuminating the molecular mechanisms of tyrosine kinase inhibitor resistance for the FGFR1 gatekeeper mutation: the Achilles’ heel of targeted therapy. ACS Chem. Biol. 2015;10:1319–1329. doi: 10.1021/acschembio.5b00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Modeling. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- 53.Schrödinger, L. L. C. The PyMOL Molecular Graphics System, Version 1.8 (2015).
- 54.Duensing, A. et al. Targeting wild-type and mutationally activated FGFR4 in rhabdomyosarcoma with the inhibitor ponatinib (AP24534). PLoS One8, 10.1371/journal.pone.0076551 (2013). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The coordinates and structure factors are deposited in the Protein Data Bank under the accession codes 7WCL (FGFR1/pemigatinib complex). Validation report is available as Supplementary Data 1. All other relevant data supporting the key findings of this study are available within the article and its Supplementary Information files. A reporting summary is available as a Supplementary Information file.