Abstract
We report the structure and expression of the Coccidioides immitis BGL2 gene which encodes a previously characterized 120-kDa glycoprotein of this fungal respiratory pathogen. The glycoprotein is recognized by immunoglobulin M tube precipitin (TP) antibody present in sera of patients with coccidioidomycosis, a reaction which has been used for serodiagnosis of early coccidioidal infection. The deduced amino acid sequence of BGL2 shows 12 potential N glycosylation sites and numerous serine-threonine-rich regions which could function as sites for O glycosylation. In addition, the protein sequence includes a domain which is characteristic of family 3 glycosyl hydrolases. Earlier biochemical studies of the purified 120-kDa TP antigen revealed that it functions as a β-glucosidase (EC 3.2.1.21). Its amino acid sequence shows high homology to several other reported fungal β-glucosidases which are members of the family 3 glycosyl hydrolases. Results of previous studies have also suggested that the 120-kDa β-glucosidase participates in wall modification during differentiation of the parasitic cells (spherules) of C. immitis. In this study we showed that expression of the BGL2 gene is elevated during isotropic growth of spherules and the peak of wall-associated BGL2 enzyme activity correlates with this same phase of parasitic cell differentiation. These data support our hypothesis that the 120-kDa β-glucosidase plays a morphogenetic role in the parasitic cycle of C. immitis.
Coccidioidomycosis is a fungal respiratory disease of humans caused by Coccidioides immitis. It is recognized as a reemerging problem in regions of endemicity of the Southwestern United States (16). Diagnosis of early stages of C. immitis infection is aided by a serologic test which involves detection of patient immunoglobulin M (IgM) precipitin antibodies reactive with specific antigens of C. immitis in an immunodiffusion-tube precipitin (ID-TP) assay (28). We have previously described the isolation of a 120-kDa glycoprotein which is recognized by precipitin antibodies present in sera of patients with coccidioidomycosis (5, 22). The ability of this purified glycoprotein to bind patient IgM (TP) antibody was confirmed by both the classical TP reaction and an enzyme-linked immunosorbent assay (4, 22). We have also shown that the 120-kDa TP antigen is a β-glucosidase, and the active enzyme is present in the culture medium and within the walls of young parasitic cells (presegmented spherules) (23). We have demonstrated that the β-glucosidase can utilize isolated and boiled cell wall material of C. immitis spherules as a substrate. It was suggested that the wall-associated enzyme may cleave structural glucans of the spherule wall and thereby contribute to wall plasticity and isotropic growth of the parasitic cells (6, 23). Such in situ enzyme activity was supported by our observations that the active enzyme can be extracted from the wall of viable, presegmented spherules and that exposure of cultured parasitic cells to 1-deoxynojirimycin, a specific inhibitor of glucosidases, blocks diametric growth of the pathogen in vitro (23). Moreover, antibody raised against a conjugate of 1-deoxynojirimycin was used in an immunofluorescence study to show that the inhibitor was localized in the wall of the growth-arrested spherules.
Here we report the isolation of the BGL2 gene that encodes the 120-kDa β-glucosidase (TP) antigen, and present results of the analysis of BGL2 expression during the parasitic cycle of C. immitis.
MATERIALS AND METHODS
Fungal strain and growth conditions.
C. immitis strain C735 used in this study was originally isolated from a patient with disseminated coccidioidomycosis who resided in Southern California. The isolate is maintained in the Medical College of Ohio fungal culture collection. The saprobic phase was grown for 5 days in GYE liquid medium (1% glucose, 0.5% yeast extract) at 30°C, while the parasitic phase was grown in Converse medium for different periods of incubation as previously described (17).
Isolation and sequence analysis of the BGL2 genomic clone.
The strategy employed to isolate the gene that encodes the 120-kDa TP antigen was based on identification of two conserved amino acid sequences of selected fungal β-glucosidases which had been deposited in the GenBank database. An amino acid sequence alignment of these proteins was performed using the MacDNASIS Sequence Analysis Software (version 3.5; Hitachi, San Bruno, Calif.) to identify the conserved domains. The conserved sequences were used to design degenerate sense and antisense primers for use in a PCR with template genomic DNA of C. immitis to amplify a fragment of the putative BGL gene. The nucleotide sequence of the sense primer deduced from the conserved, upstream peptide sequence (GRNWEGF) was 5′-GGWMGDAAYTGGGARGGNTT-3′ (192-fold degeneracy) (where M is A or C; D is A, G, or T; N is A, C, G, or T; R is A or G; W is A or T; and Y is C or T). The nucleotide sequence of the antisense primer was designed on the basis of a conserved downstream peptide sequence (ELGFQGF) which had previously been identified as part of the signature motif that defines family 3 glycosyl hydrolases (18) (see Table 1). The nucleotide sequence of the antisense primer was 5′-GAAKCCYTGRAAKCCNARYTC-3′ (256-fold degeneracy) (where K is G or T).
TABLE 1.
Alignment of 18-aa signature sequence which defines fungal family 3 glycosyl hydrolases
| Fungus | Accession no.a | EC no.b | Amino acid sequencec | Sequence range (total aa)d |
|---|---|---|---|---|
| Coccidioides immitis BGL2 | AAF21242 | 3.2.1.21 | LLKGELGFQGFIMSDWQA | 268–283 (858) |
| Coccidioides immitis BGL1 | AAB67972 | 3.2.1.21 | ILKDELGFQGFVMTDWYA | 275–292 (870) |
| Histoplasma capsulatum H Antigen | AAA86880 | NDe | LLKAELGFQGFIMSDWQA | 267–284 (863) |
| Aspergillus niger BGL1 | CAB75696 | 3.2.1.21 | LLKAELGFQGFVMSDWAA | 266–283 (860) |
| Aspergillus aculeatus BGL | BAA10968 | 3.2.1.21 | LLKAELGFQGFVMSDWAA | 266–283 (860) |
| Saccharomycopsis fibuligera BGL1 | AAA34314 | 3.2.1.21 | LLKEELGFQGFVVSDWGA | 281–298 (876) |
| Pichia anomala BGL | CAA26662 | 3.2.1.21 | LLKEELGFQGFVMTDWGA | 285–302 (825) |
| Pichia capsulata BGLN | AAA91297 | ND | LLKSELGFQGFVVSDWGG | 269–286 (763) |
| Geaumannomyces graminis avenacinase I | AAB09777 | 3.2.1.21 | LLKTELGFQGFVVSDWAA | 265–282 (793) |
| Kluyveromyces marxianus BGL | CAA29353 | 3.2.1.21 | ILRDEWKWDGMLMSDWFG | 211–228 (845) |
Accession number for protein sequences in GenBank.
Enzyme nomenclature based on recommendations of the International Union of Biochemistry (18).
Conserved residues indicated in boldface type.
Positions of 18-aa sequence in total number of amino acids (in parentheses).
ND, not determined.
The PCR mixture (100 μl) contained 10 mM Tris-HCl (pH 8.3) plus 50 mM KCl, 1.5 mM MgCl2, a 0.2 mM concentration of each deoxynucleoside triphosphate (dNTP), a 5 μM concentration of each primer, 50 ng of C. immitis genomic DNA, and 2.5 U of Taq DNA polymerase (Promega, Madison, Wis.). Thirty-five cycles were conducted for amplification of the template genomic DNA. Initial denaturation was performed at 94°C for 3 min. Each subsequent cycle consisted of a melting step (94°C for 1 min), an annealing step (50°C for 1 min), and an extension step (72°C for 1 min). Three PCR products of different molecular size were observed by 3.0% agarose gel electrophoresis. The mixture of PCR amplicons was ligated into the pZErO 2.1 cloning vector (Invitrogen, Carlsbad, Calif.) by the TA cloning method (2). The clones were subsequently screened by PCR using primers derived from nucleotide sequences in the multiple cloning site of the vector (2). Selected clones were sequenced using the ThermoSequenase radiolabeled terminator cycle sequencing kit (Amersham, Cleveland, Ohio). A clone which contained a 423-bp insert was selected on the basis of homology of its translated sequence to the reported sequence of a secreted β-glucosidase of Histoplasma capsulatum (11, 13). This same glycosyl hydrolase of H. capsulatum has been identified as a seroreactive antigen (H antigen) and is used in the diagnosis of histoplasmosis (11). H. capsulatum has been shown to be a close relative of C. immitis (27). The 423-bp PCR product was purified, labeled with [α-32P]dCTP (>3,000 Ci/mmol; ICN, Costa Mesa, Calif.) using a Multiprimer DNA labeling system (Amersham), and used to screen a genomic library of C. immitis C735 which has been reported (32). Positive phages were selected and amplified, and DNA was extracted for restriction enzyme digestion and Southern hybridization using the 423-bp PCR product as previously described (33). This resulted in detection of a 4.9-kb XbaI genomic fragment of the BGL2 gene that was subcloned into pZErO 2.1 and sequenced as described above. The MacDNASIS software package was used for sequence analysis.
RACE and sequence analysis of BGL2 cDNA.
The rapid amplification of cDNA ends (RACE) procedure (17) was used to resolve the location of the nucleotide termination of the 5′ untranslated region (UTR) as well as the poly(A) addition sites of the BGL2 gene. In brief, total RNA was first isolated from mycelia of C. immitis as described (17). Reverse transcription (RT)-PCR was conducted as reported by Ausubel et al. (2). High fidelity Taq polymerase (SuperMix High Fidelity DNA Taq polymerase; Gibco BRL, Grand Island, N.Y.) was used for the PCR. The two gene-specific primers used for 5′ RACE were as follows: 5′-CCTTTTATCGTTTCAGCG-3′ (BG 2.4 [nucleotides {nt} 2300 to 2317] [see Fig. 2B]), and 5′-ACAAGCTTCTTGCATCCA-3′ (BG 2.10 [nt 1927 to 1944]). For 3′ RACE, one of the two primers used was the synthesized oligo-d(T)17-adapter construct described by Frohman (15). The amplified RT-PCR product was obtained using the BGL2 gene-specific primer, 5′-TGGTGTCAGCATCCTCAA-3′ (BG 2.15 [nt 3662 to 3679]) and the oligo(dT) construct. The PCR conditions were the same as those used for amplification of the genomic fragment described above. The RACE products were separated by 1.5% agarose gel electrophoresis, ligated into the pZErO 2.1 vector, and subjected to nucleotide sequence analysis as described above.
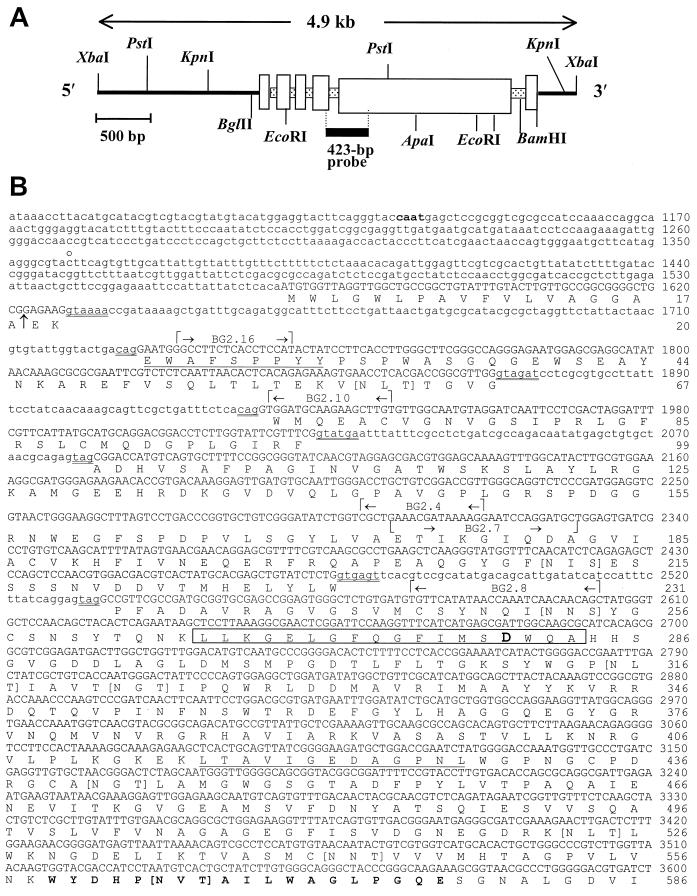
FIG. 2.
Restriction map of 4.9-kb λ phage insert digested with XbaI and subcloned into pZErO (A), and nucleotide sequence of C. immitis BGL2 gene and deduced amino acid sequence (B). The 423-bp probe in panel A is derived from PCR product (a) in Fig. 1. The two underlined amino acid sequences represent matched peptide sequence of the Lys-C-digested, native 120-kDa glycoprotein. The amino acid sequence in boldface type (aa 559 to 577) matched the peptide sequence of Lys-C-digested recombinant BGL2. The boxed sequence represents the 18-aa signature motif of family 3 glycosyl hydrolases. The aspartic acid residue within this sequence (boldface type) is the putative active site. Residues contained within square brackets are putative N glycosylation sites. The arrow between aa 18 and 19 indicates a putative cleavage site of the signal peptide. The double-underlined nucleotide sequences indicate conserved 5′-3′ sequences of introns. The gene-specific primers used for RACE and RT-PCR are indicated. The putative CAAT box (boldface type), 5′ end of the UTR (c̊), stop codon (asterisk), and putative poly(A) addition sites (•) are also indicated.
To amplify the remainder of the cDNA fragment of BGL2, two primers were synthesized on the basis of the sequences of the RACE products. The nucleotide sequences of the sense and antisense primers were 5′-GAAAGATCTGGCCTTCTCACCTCCATA (BG 2.16 [nt 1734 to 1751]) and 5′-ATGTCGACCCTACGAAGACGGGGCTAGAG (BG 2.18; nt 4485 to 4505), which contained engineered BglII and SalI restriction sites, respectively (nucleotides in boldface type). Thirty-five cycles were performed to amplify the RT-PCR product. The PCR conditions were the same as described above, except that the extension step was conducted at 72°C for 3 min. The 2.5-kb RT-PCR product was digested with BglII and SalI, separated by 1% agarose gel electrophoresis, isolated, and subcloned into the BamHI/SalI site of pET28b (Novagen, Madison, Wis.) to yield the pET28b-BGL2 plasmid construct (17). The plasmid insert was sequenced as described above.
The NCBI-BLAST and PSI-BLAST programs were used to search for protein sequences in the GenBank and SWISS-PROT databases with similarity to the translated sequence of the C. immitis BGL2 gene (1). The PROSITE database was used to identify motifs and signature sequences in BGL2 with homology to reported proteins (20), the PSORT database was used for for prediction of protein localization sites (24), and the CLUSTALW program was used to perform sequence alignments (19).
Southern hybridization.
Intact chromosomal DNA of C. immitis was prepared by the agarose-spheroplast procedure (25) and subjected to contour-clamped homogeneous electric field (CHEF) gel electrophoresis under the conditions previously described (34). The separated chromosomal DNA was transferred to a Zeta-probe GT Blotting Membrane (Bio-Rad, Hercules, Calif.) and hybridized with the radiolabeled, 423-bp PCR product which was described above. In addition, aliquots of equal amounts of total genomic DNA of C. immitis were separately digested with the restriction endonucleases XbaI, PstI, or KpnI and analyzed by Southern hybridization with the same 423-bp probe. Hybridization was conducted at low stringency as described by Yu et al. (33).
Purification of the 120-kDa TP antigen for amino acid sequence analysis.
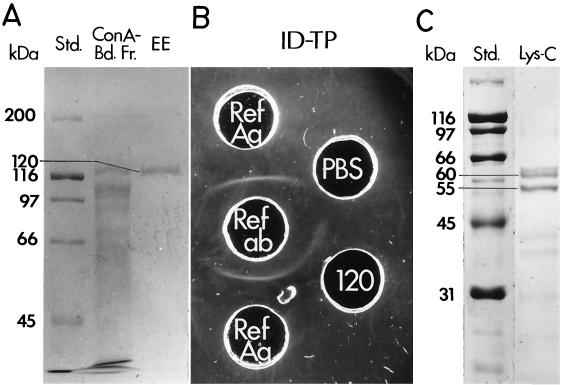
The 120-kDa glycoprotein was isolated from the mycelial filtrate-lysate (F-L) preparation of C. immitis as described previously (4). In brief, the F-L preparation was first subjected to concanavalin A (ConA) affinity chromatography. The ConA-bound fraction was eluted from the column and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions (22). The Coomassie brilliant blue (Sigma)-stained 120-kDa band was excised, and the glycoprotein was electroeluted from the gel, dialyzed against water, and concentrated by drying under vacuum. The purified antigen was reconstituted in phosphate-buffered saline (pH 7.2) to a concentration of 0.1 mg of protein/ml and tested for patient TP antibody reactivity in the ID-TP assay as previously reported (4).
Enzymatic digestion of the purified 120-kDa glycoprotein was conducted by use of the Protein Finger-printing System kit (Promega). The Lys-C digest was subjected to SDS-PAGE (10% polyacrylamide), and the separated fragments were electrotransferred to an Immobilon-P membrane (Millipore, Bedford, Mass.). Selected peptide bands visualized with Coomassie stain were excised and subjected to N-terminal sequence analysis in an Applied Biosystems model 477A gas phase sequencer by standard procedures (26).
Expression of BGL2 by Escherichia coli.
The 2.5-kb cDNA fragment of the BGL2 gene, which encodes a predicted 90.6-kDa protein (amino acids [aa] 23 to 858 [see Fig. 2B]), was amplified by PCR and subcloned into the pET28b vector as described above. The pET28b-BGL2 plasmid construct encodes a recombinant protein that contained a polyhistidine (His) tag at its N terminus derived from the vector. The stop condon in the plasmid construct was derived from the BGL2 gene insert. The pET28b-BGL2 construct was used to transform E. coli strain BL21(DE3) as described (17). Growth of the transformed cells, induction of expression, purification, and internal amino acid sequence analysis of the recombinant protein (rBGL2) were conducted as previously reported (17). C-terminal amino acid sequence analysis of the purified rBGL2 was conducted to confirm that a C-terminally truncated fragment of the recombinant protein was expressed by E. coli. The rBGL2 was isolated by nickel-affinity chromatography as previously described (33). C-terminal sequence analysis of the rBGL2 was determined with a Perkin-Elmer Applied Biosystems model 428 amino acid analyzer and conducted by the Macromolecular Structure Facility, Michigan State University, East Lansing, using a standard procedure (3).
Production of antiserum against rBGL2.
The chromatographically isolated rBGL2 was subjected to SDS-PAGE and electroeluted from the gel as previously described (26), and the purified recombinant protein was used to immunize BALB/c mice (6 weeks old) for production of specific antiserum as reported (21). The antiserum was used for examination of BGL2 protein production during in vitro growth of C. immitis by immunoblot analysis as described below. Preimmune mouse serum was used as a control.
RT-PCR evaluation of BGL2 gene expression during the parasitic cycle.
Semiquantitative analysis of BGL2 mRNA levels in different morphogenetic stages of the parasitic cycle was conducted essentially as described by Guevara-Olvera et al. (17). First-generation parasitic cells of C. immitis derived from arthroconidia were grown in Converse medium and harvested by centrifugation (1,500 × g for 10 min) after 16, 24, 36, 72, 84, 96, 120, and 132 h of incubation on a gyratory shaker under conditions previously reported (17). The first generation of parasitic cells isolated at these various times after inoculation with arthroconidia was fairly well synchronized in development (7). Mature, ruptured spherules which had released their endospores (i.e., 132 h postinoculation) were isolated, washed once with Converse medium, and used as the inoculum for second-generation cultures. The cells were grown in fresh Converse medium for 48 h and then harvested as described above. Cells harvested at each incubation time were separated into three aliquots; one was used for light-microscopic analysis of the degree of synchrony of cell development, one was used immediately for RNA extraction as described below, and the rest of the cells were quick-frozen in 1.5-ml microcentrifuge tubes and then stored at −70°C until processed for protein extraction. For light microscopy, the cell types isolated at each incubation time were scored to characterize their stage of development. At least 200 cells were examined in each aliquot. The cell types of the first generation were classified as follows: (i) swollen, cylindrical spherule initials that were <5 μm in diameter; (ii) swollen, cylindrical spherule initials that were ≥5 but <10 μm in diameter; (iii) spherules that were ≥10 μm but <20 μm in diameter; (iv) nonsegmented spherules that were >20 μm in diameter; (v) segmented spherules; (vi) early endosporulating (<50%) spherules; (vii) early endosporulating (>50%) spherules; and (viii) mature, ruptured spherules (≥90%) showing released endospores. The second-generation spherules were scored as nonsegmented parasitic cells that were 15 to 20 μm in diameter. To evaluate stages of segmentation, wall formation, and early endospore differentiation which occur within intact spherules, aliquots of parasitic cells from 72- to 120-h cultures were chemically fixed and sectioned as previously described (30). Thick sections (approximately 1 μm) were stained with wheat germ agglutinin (WGA) conjugated with fluorescein isothiocyanate (FITC) (Sigma) and examined by fluorescence microscopy. The WGA-FITC conjugate stained the chitin in the spherule and endospore wall.
Total RNA was isolated from each of the cell types described above and used for RT-PCR analysis of levels of BGL2 expression. Total RNA was also isolated from C. immitis mycelia grown in liquid GYE culture medium for 120 h at 30°C. Since the 120-kDa β-glucosidase was originally purified from 5-day mycelial cultures (23), the total RNA isolated from the saprobic phase served as a positive control for the RT-PCR. Total RNA was extracted from 100 mg of fresh parasitic cells or mycelial pellet using the Plant RNeasy mini kit (Qiagen, Chatsworth, Calif.) as previously described (17). The crude extract was digested with RQ1 RNase-free DNase (Promega), and the RNA was purified using the RNA clean-up protocol (Plant RNeasy mini kit; Qiagen). The purity and quantity of RNA were monitored by UV absorbance. A ratio of optical density at 260 nm to that at 280 nm that was >1.9 was obtained for each preparation.
Gene expression was examined by comparison of the level of mRNA which encodes BGL2 to that which encodes the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene of C. immitis (GenBank accession no. AF288134). The latter is expressed constitutively in C. immitis (J.-J. Yu, C.-Y. Hung, P. W. Thomas, and G. T. Cole, Abstr. 99th Gen. Meet. Am. Soc. Microbiol. 1999, abstr. F-52, p. 305, 1999). The RT-PCR protocol employed was essentially the same as previously reported (17). In brief, the cDNA was synthesized in a 50-μl solution containing 50 mM Tris-HCl (pH 8.3) plus 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol (DTT), 0.5 μM concentration of each dNTP, a 200 μM concentration of oligo PCR d(T)17-adapter primer, 5 μg of C. immitis total RNA, and 400 U of SuperScript II RNase H− reverse transcriptase (Gibco BRL). The reaction mixture was incubated at 42°C for 50 min and then shifted to 70°C for 10 min to denature the reverse transcriptase. The PCR mixture contained 1 μl of the cDNA, which was separated as aliquots diluted in Milli-Q (Millipore) water (1:1, 1:2, 1:4, 1:8, 1:16, 1:32, or 1:50). Each aliquot was mixed with 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM Mg Cl2, a 0.2 mM concentration of each dNTP, a 5 μM concentration of each primer, and 1 U of Taq DNA polymerase (Sigma) in a total volume of 25 μl. PCR primer pairs synthesized for C. immitis BGL2 and GAPDH were each designed to span at least one intron. Inclusion of the intron allowed us to distinguish cDNA from genomic DNA amplicons based upon their different sizes after separation by agarose gel electrophoresis. In order to further rule out contamination of the RNA preparations with genomic DNA, controls included samples of the C. immitis RNA preparation that had not been reverse transcribed but were subjected to PCR and examined by agarose gel electrophoresis as described above. For GAPDH, the sequences of the PCR primers were the same as reported by Guevara-Olvera et al. (17). For BGL2, the primer sequences were as follows: sense, 5′-GAAACGATAAAAGGAATCCAGGATGCT-3′ (BG2.7 [nt 2304 to 2330]), and antisense, 5′-GCTGTTGTTGATTTGGTTATATGAACA-3′ (BG2.8 [nt 2577 to 2603]). The primers yielded single RT-PCR products for GAPDH and BGL2 which were 246 and 243 bp, respectively. The PCR conditions were the same as described above, except that the annealing temperatures were 56°C for GAPDH and 60°C for BGL2. Thirty-five cycles were used to amplify the BGL2 and GAPDH genes. The PCR products were subjected to agarose gel electrophoresis (2.0%) and the amplified cDNA bands were visualized by staining with ethidium bromide (EtBr). The intensity of the EtBr stain for each band was determined by UV transillumination and densitometric analysis using the Bio-Rad Gel Documentation 1000 system and Multi-Analyst software program (Bio-Rad). The intensities of the bands, which represent amount of BGL2 and GAPDH amplicons for each dilution of cDNA template, were recorded. The relative amounts of BGL2 and GAPDH cDNA determined for each stage of parasitic cell development were calculated as the dilution factor that yielded the same band intensity as that of the mycelial BGL2 amplicon at a 1:50 dilution.
Evaluation of 120-kDa glycoprotein production by immunoblot analysis.
Detection of the 120-kDa glycoprotein in total homogenates of the 5-day mycelial mat and homogenates of cells from the same stages of parasitic cell development as described above were conducted by SDS-PAGE followed by immunoblot analysis using the murine antiserum raised against the rBGL2. Aliquots of each fungal cell isolate were mechanically disrupted with glass beads (50-μm diameter) in a Mini-Beadbeater (Biospec, Bartlesville, Okla.). Total protein of each cell preparation was extracted with 50 mM sodium acetate (NaAc) buffer (pH 5.5) containing octyl-β-d-glucopyranoside (1% [vol/vol]; Calbiochem, La Jolla, Calif.), 100 mM NaCl, 6 mM CaCl2, 1 mM phenylmethylsulfonyl fluoride, l-1-chloro-3-[4-tosylamido]-4-phenyl-2-butanone (50 μg/ml), leupeptin (1 μg/ml), and pepstatin (1 μg/ml); (Sigma). The protein concentration of each sample was determined using the Detergent Compatible Protein Assay kit (Bio-Rad), and adjusted so that equal amounts of protein were applied to each lane of the SDS-PAGE gel. The protein preparations (approximately 80 μg each) were separated by SDS-PAGE (10% polyacrylamide), and the reducing gels were stained with Coomassie brilliant blue. The immunoblot procedure was conducted as previously described (26), except that secondary antibody conjugated with horseradish peroxidase and a chemiluminescent substrate were used (ECL Western blot analysis system; Amersham, Arlington Heights, Ill.).
Evaluation of β-glucosidase activity.
The same set of equilibrated protein extracts as described above were also used to detect β-glycosyl-hydrolase activity by substrate gel electrophoresis (8). Approximately 40 μg of total protein of each cell homogenate were mixed with 6× sample buffer (0.35 M Tris-HCl [pH 6.8], 1% SDS, 10% glycerol, 0.002% bromophenol blue, and 0.6 M dithiothreitol), incubated at 37°C for 5 min, and separated by SDS-PAGE (10% polyacrylamide). The gel was washed three times with 50 mM NaAc buffer (pH 5.5) containing 0.1% Triton X-100 (37°C; 10 min each wash) to remove SDS and then incubated with 10 ml of a solution of 4-methyl-umbelliferyl-β-d-glucoside (Sigma) in the same NaAc buffer (0.1 mg/ml) for 30 min at 37°C. Fluorescent bands indicative of β-glucosidase activity were viewed under UV light, and their intensities were determined by image analysis as described above.
Nucleotide sequence accession number.
The GenBank accession number for the C. immitis BGL2 gene is AF022893, and that for the BGL2 protein is AAF21242.
RESULTS
Selection of putative BGL2 amplicon.
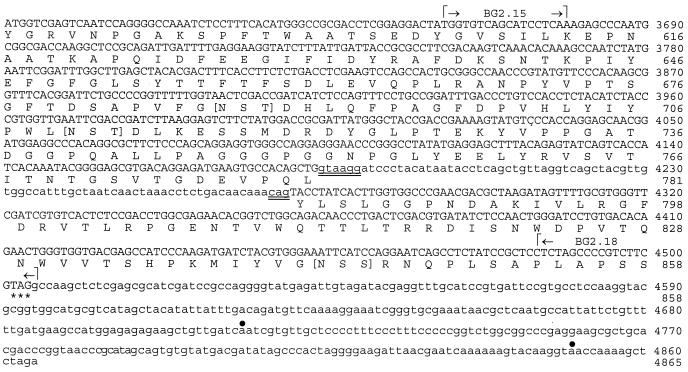
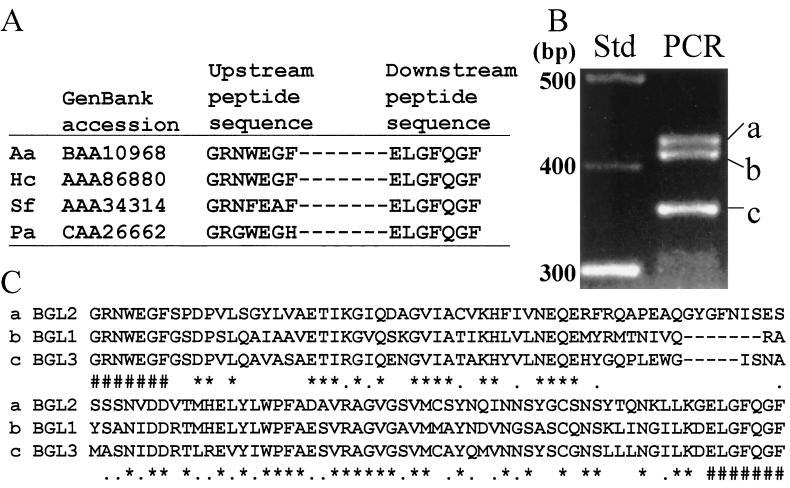
Four fungal β-glucosidase sequences obtained from the GenBank database were aligned, and two conserved regions were identified (Fig. 1A). The β-glucosidase sequences were selected on the basis that the native proteins were reported to have molecular sizes in the range of 80 to 144 kDa. Degenerate oligonucleotide primers designed on the basis of these sequences were used for PCR amplification with genomic template DNA. Three EtBr-stained amplicons visible in the agarose gel (Fig. 1B) were isolated, purified, cloned, and subjected to nucleotide sequence analysis. The sizes of the PCR products (amplicons a, b, and c), as determined by sequence analysis, were 423, 409, and 351 bp, respectively. Each amplicon was translated to yield the open reading frames shown in Fig. 1C. The translated PCR primer sequences are shown at the N and C termini. Alignment of the sequences, excluding the translated primer regions, was conducted using the CLUSTALW program, and the sequences showed 62 to 75% homology to each other and 45 to 58% homology to other nonfungal β-glucosidase sequences in the GenBank database. These results suggested that the three PCR products translate three distinct glycosyl hydrolases of C. immitis. In fact, the deduced amino acid sequence of amplicon b showed complete identity to BGL1, a cytosolic β-glucosidase of C. immitis which has been reported (J.-J. Yu and S. L. Smithson, Abstr. 96th Gen. Meet. Am. Soc. Microbiol. 1996, abstr. F-57, p. 83, 1996) and deposited in the GenBank database (accession no. AAB67972). BGL1 was not examined further in this study. The sequences of amplicons a and c were aligned with the sequence of a previously reported 144-kDa serodiagnostic antigen (H antigen) of H. capsulatum which was suggested to function as a β-glucosidase (11, 13). The homologies of sequences a and c to this putative β-glucosidase were 92 and 69%, respectively. Earlier studies in our laboratory have suggested a close phylogenetic relationship between C. immitis and H. capsulatum (27). For example, 72% amino acid sequence identity was revealed between the heat shock proteins (HSP60) of these two pathogens (31). On the basis of the above structural and functional homology data, we tentatively identified sequence a as a fragment of the gene which encodes the 120-kDa β-glucosidase of C. immitis, and we refer to this gene as BGL2. This 423-bp PCR product was used as a probe to screen the genomic library. The sequence of amplicon c was designated as a fragment of the BGL3 gene, which is further examined in temporal expression studies in this work.
FIG. 1.
(A) Alignment of amino acid sequences of two conserved regions of fungal β-glucosidase reported in GenBank for Aspergillus aculeatus (Aa), H. capsulatum (Hc), Saccharomycopsis fibuligera (Sf), and Pichia anomala (Pa). (B) PCR products (a, b, and c) amplified from C. immitis genomic DNA using degenerate primers designed on the basis of conserved amino acid sequences in panel A. (C) Translated sequences of PCR products shown in panel B. The translated primer sequences are indicated (#). An asterisk indicates amino acid identity; a dot indicates a conserved substitution (see Table 2).
Isolation and structure of the BGL2 gene.
The random hexamer primer-labeled 423-bp PCR product was used to screen a C. immitis genomic library constructed in λFIXII (32). A clone which hybridized with the probe was isolated, digested with XbaI to yield a 4.9-kb fragment, subcloned into pZErO 2.1, and subjected to DNA sequence analysis. The restriction map of the 4.9-kb fragment of the phage insert is shown in Fig. 2A. The deduced open reading frame of the cDNA sequence, which was obtained by 5′-3′ RACE as described above, matched the genomic sequence and confirmed that the gene contained five introns (Fig. 2B). A putative CAAT box was located 224 bp upstream of the 5′ end of the UTR (nt 1359). The latter was identified by 5′ RACE. No discernible TATA box was found. Two putative poly(A) tail addition sites were identified at nt 4712 and nt 4850.
The translated BGL2 gene contains 858 aa and, in the absence of any posttranslational modification, has a predicted molecular mass of 92.8 kDa and a pI of 5.0. Sequence analysis performed by PSORT (24) showed that 18 aa at the N terminus have the characteristic of a signal peptide with a putative cleavage site between A18 and E19. The predicted molecular size of the mature BGL2 protein is 90.9 kDa. The translated protein showed 12 potential N glycosylation sites, multiple S/T-rich regions which could function as O glycosylation sites, a glycosyl-hydrolase family 3 signature motif at aa 266 to 283 (18), and a predicted active-site residue at D280 (10). The 18-aa sequence of the signature motif of BGL2 is very similar to that reported for all other fungal family 3 glycosyl hydrolases currently deposited in the GenBank database, with the exception of Kluyveromyces marxianus (Table 1). The predicted full-length sequence of the C. immitis BGL2 protein was compared to reported amino acid sequences of fungal family 3 glycosyl hydrolases in the database. The highest identity (74.3%) was shown between BGL2 and the H antigen of H. capsulatum (Table 2).
TABLE 2.
Summary of calculated values for conserved amino acid similarities and identities between C. immitis BGL2 and β-glucosidase sequences of other fungi
| Sequence compareda | Similarity (%)b | Identity (%) |
|---|---|---|
| Histoplasma capsulatum H-antigen | 85.2 | 74.3 |
| Aspergillus aculeatus BGL1 | 79.3 | 66.6 |
| Aspergillus niger BGL1 | 79.2 | 66.2 |
| Saccharomycopsis fibuligera BGL1 | 58.8 | 42.9 |
| Coccidioides immitis BGL1 | 54.3 | 40.2 |
| Pichia capsulata BGLN | 52.4 | 37.8 |
| Pichia anomala BGL | 52.0 | 34.8 |
| Geaumannomyces graminis avenacinase I | 43.0 | 39.1 |
| Kluyveromyces marxianus BGL | 37.1 | 18.5 |
Southern hybridization.
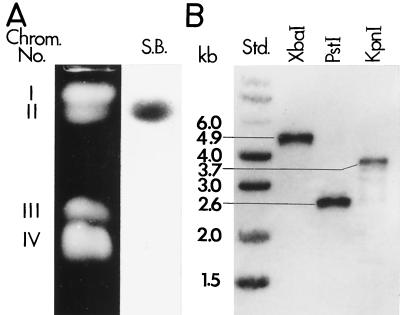
Southern hybridization of chromosomal DNA separated by CHEF gel electrophoresis was conducted using the same random hexamer primer-labeled 423-bp PCR amplicon as described above. The Southern blot showed that the BGL2 gene is located on chromosome II (Fig. 3A). Total genomic DNA preparations of C. immitis were separately digested with XbaI, PstI, and KpnI; subjected to agarose gel electrophoresis; and hybridized with the same labeled 423-bp probe (Fig. 3B). The sizes of the three single bands were predicted by the restriction map of the 4.9-kb BGL2 sequence (Fig. 2A). The Southern hybridization data indicate that BGL2 is a single-copy gene.
FIG. 3.
EtBr-stained CHEF electrophoresis gel of C. immitis strain C735 with Southern blot (S.B.) of separated chromosomal DNA (A), and Southern blot of restriction enzyme-digested genomic DNA (B). Abbreviations: Chrom., chromosome; Std., standard.
Purification and amino acid sequence analysis of 120-kDa TP antigen.
The dialyzed F-L preparation of the mycelial phase of C. immitis was bound to ConA, eluted, and separated by SDS-PAGE, and the 120-kDa glycoprotein was isolated from the gel by electroelution (Fig. 4A). Antigenic activity of the isolated glycoprotein was confirmed by the ID-TP assay (Fig. 4B). The purified TP antigen was subjected to Lys-C digestion, and two of the proteolytic fragments with molecular sizes of 55 and 60 kDa (Fig. 4C) were subjected to N-terminal amino acid sequence analysis. The sequence of the 60-kDa fragment (LTAVIGEDAGPNL) matched aa 416 to 428 of the translated BGL2 sequence (Fig. 2B), while the sequence of the 50-kDa fragment (EWAFSPPYY) was identical to aa 21 to 29.
FIG. 4.
(A) SDS-PAGE gel separation of ConA-bound fraction of mycelial filtrate plus lysate preparation (Con A-Bd. Fr.), and gel-electroeluted (EE) 120-kDa glycoprotein. Std., standard. (B) ID-TP assay of immunoreactivity of purified 120-kDa glycoprotein. (C) SDS-PAGE gel separation of Lys-C-digested 120-kDa glycoprotein.
Expression of rBGL2 and antibody production.
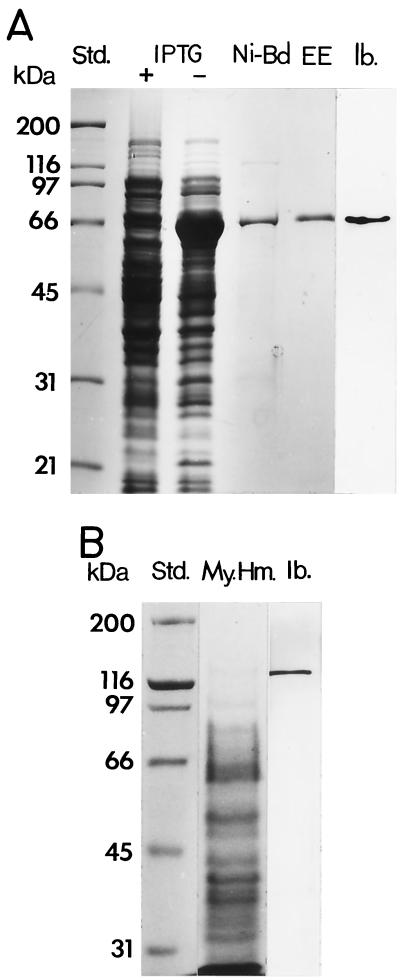
To express the BGL2 gene, the PCR-generated cDNA was subcloned into pET28b to yield the pET28b-BGL2 construct. The predicted molecular size of the recombinant protein was 94 kDa (including the vector-encoded peptide which contained the His tag at the N terminus). SDS-PAGE was used to analyze cell lysates obtained from E. coli strain BL21(DE3) which had been transformed with the plasmid construct and induced with IPTG (isopropyl-β-d-thiogalactopyranoside). An unpredicted 66-kDa band was detected in the lysate of IPTG-induced bacteria (Fig. 5A). The cDNA insert of the pET28b-BGL2 construct was sequenced and confirmed to contain an open reading frame that was identical to the sequence in Fig. 2B. The 66-kDa protein was isolated by nickel-affinity chromatography, separated by SDS-PAGE, and purified by electroelution (Fig. 5A). The protein was digested with Lys-C, separated by high-pressure liquid chromatography (17), and subjected to N-terminal amino acid sequence analysis, which yielded the following: WYDHPNVTAILWAGLPGQE. The sequence was identical to the predicted amino acid sequence of the BGL2 gene (aa 559 to 577) (Fig. 2B). C-terminal sequence analysis of the rBGL2, isolated by Ni-affinity chromatography, revealed that the last three residues were WAA. This sequence matches aa 600 to 602 of the translated sequence of the BGL2 gene (Fig. 2B). The predicted molecular size of rBGL2, taking this C terminus into account, is 66.1 kDa. This predicted size is the same as the SDS-PAGE estimate of the molecular size of the recombinant protein. It appears that the transformed bacteria produced a C-terminally truncated form of the rBGL2.
FIG. 5.
(A) SDS-PAGE and immunoblot analysis of E. coli-expressed rBGL2. Shown are standards (Std.), separations of lysates of transformed bacteria grown in the presence (+) or absence (−) of IPTG, nickel-affinity-isolated rBGL2 (Ni-Bd), purified rBGL2 obtained by gel electroelution (EE), and an immunoblot (Ib.) of the lysate of transformed E. coli using murine anti-rBGL2 antibody. (B) SDS-PAGE separation of detergent-extracted mycelial homogenate (My. Hm.) and corresponding immunoblot (Ib.) of native glycoprotein using anti-rBGL2 antibody.
The purified rBGL2 was used to immunize mice for production of polyclonal antibody. The antiserum recognized the 66-kDa recombinant protein in the bacterial lysate (Fig. 5A) as well as the native 120-kDa glycoprotein in the crude, detergent-extracted mycelial homogenate (Fig. 5B). The 120-kDa glycoprotein was isolated from the mycelial homogenate as reported (23) and confirmed to have β-glucosidase activity (data not shown). This same antiserum was subsequently used in the immunoblot assay of BGL2 in cell homogenates of C. immitis obtained from different stages of the parasitic cycle.
Isolation of C. immitis cell types for studies of BGL2 expression, BGL2 production, and enzyme activity.
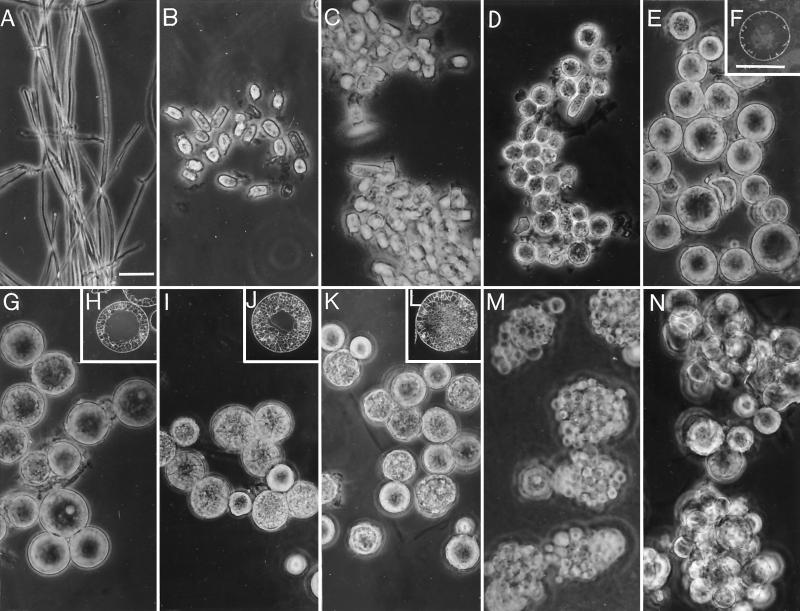
Figure 6A to N show light micrographs of the mycelia (Fig. 6A, 5-day culture), parasitic cell types isolated from first-generation cultures grown for 16 to 132 h (Fig. 6B to M), and a second-generation culture grown for 48 h (Fig. 6N). Inserts (Fig. 6F, H, J, and L) show thick sections of representative spherules isolated from 72-, 84-, 96-, and 120-h cultures which were stained with the WGA-FITC chitin-specific, lectin conjugate. The sectioned cells show stages of development of the segmentation wall complex (Fig. 6F and H) and early stages of endospore differentiation (Fig. 6J and L) within the intact spherules. The isotropic growth phase of the first-generation parasitic cells prior to segmentation is represented by Fig. 6B to E. Early differentiation of endospore initials (Fig. 6I and J) is signaled by isotropic growth of cells contained within the maternal spherule. Some swelling of the latter occurs at this stage as growth of the endospores occurs. Continued isotropic growth of the endospores (Fig. 6K and L) leads to rupture of the first generation spherules (Fig. 6M) and maturation of second-generation parasitic cells (Fig. 6N). The homogenate of each of these developmental stages was used to monitor BGL2 gene expression, 120-kDa glycoprotein production, and β-glucosidase activity. The similar morphology of the parasitic cells at each progressive stage of differentiation shown in Fig. 6B to N suggests the near-synchronous state of the first- and second-generation cultures grown in vitro.
FIG. 6.
Light micrographs of 5-day mycelia of C. immitis (A), and developmental stages of first-generation spherules (B to M) and second-generation spherules (N). Inserts (F, H, J, L) show WGA-FITC-stained sections of spherules at stages which correspond to parasitic cells shown in panels E, G, I, and K, respectively. Developmental stages (B, C, D, E, G, I, K, and M) are derived from parasitic-phase cultures inoculated with arthroconidia and incubated for 16, 24, 36, 72, 84, 96, 120, and 132 h, respectively. Second-generation spherules in panel N were derived from endosporulating spherules (M) which were incubated in fresh medium for 48 h. (A and F) Bars represent 20 μm.
RT-PCR analysis of BGL2 expression during the parasitic cycle.
A diagrammatic representation of first- and early second-generation parasitic cell development is shown in Fig. 7A. Each developmental stage is designated by the culture time (i.e., hours postinoculation), which was used to identify the cell homogenates examined in Fig. 7B to D and Fig. 8. The EtBr-stained bands in Fig. 7B represent PCR products of BGL2 cDNA amplification using diluted template cDNA (1:1 to 1:32). The cDNA templates were derived from RT of separate RNA preparations obtained from selected developmental stages of the parasitic cycle. The intensity of each gel band examined by densitometric analysis was compared to that of the mycelial BGL2 amplicon. The latter was generated by PCR amplification of mycelial cDNA template that was diluted by a factor of 1:50. The relative amounts of BGL2 and GAPDH cDNA at selected developmental stages of the parasitic cycle are shown in two separate analyses of gene expression (i.e., experiments 1 and 2 in Fig. 7C and D). The relative amounts of cDNA were calculated as described in Materials and Methods. As indicated, expression of the GAPDH gene was constitutive during the parasitic cycle. In contrast, expression of the BGL2 gene in first-generation cultures was elevated during the isotropic growth phase of spherules (16 to 36 h postinoculation) but decreased sharply once this diametric expansion was arrested and the cells began to undergo segmentation (72 h postinoculation in the first generation). As endospore differentiation was initiated (∼96 h postinoculation) and the cells began a second phase of isotropic growth, the expression level of BGL2 rose again. At 48 h after transfer of the released endospores to fresh culture medium, a sharp decrease in expression of BGL2 correlated with the near completion of isotropic growth of the second-generation spherules.
FIG. 7.
Diagrammatic representation of the parasitic cycle of C. immitis (A) and RT-PCR analysis of BGL2 and GAPDH expression (B to D). (A to D) Developmental stages of first- and second-generation parasitic cells are identified by incubation time after inoculation of the parasitic-phase cultures. (B) The EtBr-stained gels show BGL2 amplicons produced by RT-PCR as described in Materials and Methods. The dilution factors for the parasitic and mycelium phase-derived template cDNAs are indicated. The intensity of the gel bands was determined by densitometric analysis. (C and D) The relative amounts of BGL2 and GAPDH cDNAs are plotted as the dilution factor of the template cDNA that yielded the same EtBr-stained gel band intensity as that of the mycelial BGL2 amplicon using a cDNA template dilution of 1:50. The data presented in Experiment 1 (C) were derived from densitometric analysis of the gels shown in panel B.
FIG. 8.
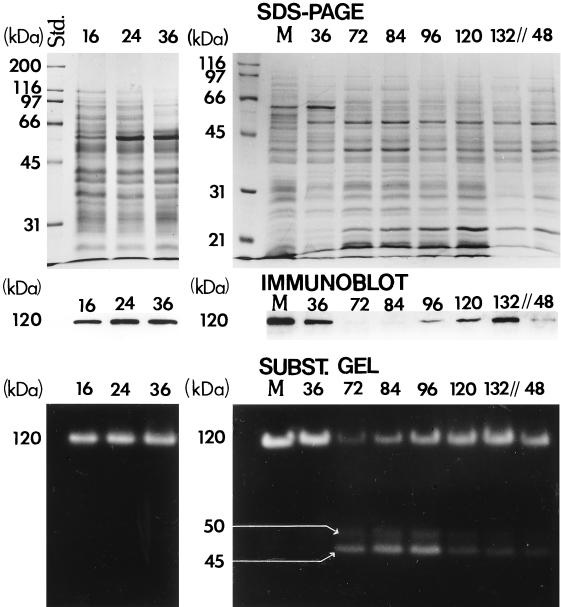
SDS-PAGE separation of Coomassie blue-stained detergent extracts of total parasitic cell and mycelial homogenates and results of both immunoblot analysis and substrate gel electrophoresis (SUBST. GEL) of these same total protein preparations. The developmental stages of the parasitic cycle are represented by incubation time (h) after inoculation of cultures (first generation, 16 to 132 h; second generation, 48 h). Lane M, homogenate of 5-day mycelial culture. Three bands are identified in the substrate gel with estimated molecular sizes of 120, 50, and 45 kDa.
BGL2 protein production.
The relative intensity of the Coomassie blue-stained protein bands of cell homogenates shown in Fig. 8 indicates that equal amounts of protein were applied to the respective lanes of the two SDS-PAGE gels. Detection of the BGL2 glycoprotein in each lane was accomplished by immunoblot analysis using the murine, polyclonal anti-rBGL2 antiserum. The results of this assay support the interpretation of the RT-PCR data. The 120-kDa glycoprotein was detected in cell homogenates during the isotropic growth phases of both the first-generation spherules (16, 24, and 36 h) and second-generation endospores (96, 120, and 132 h), but was not detectable once the parasitic cells ceased diametric growth and began to undergo segmentation. Detection of the 120-kDa glycoprotein in the immunoblot of the mycelial homogenate served as a positive control.
β-Glucosidase activity.
The results of substrate gel electrophoretic analysis of the same cell homogenates as described above are shown in Fig. 8. The parasitic cell homogenate preparations were first separated by SDS-PAGE under reducing conditions, and the gels were then washed to renature the proteins and remove the SDS. After incubation of the gel with 4-methyl-umbelliferyl-β-d-glucoside substrate, fluorescent bands were visible which corresponded to the 120-kDa β-glucosidase activity. The results suggest that enzyme activity remains high during the isotropic growth phase of both the first-generation spherules (16, 24, and 36 h) and endospores (96, 120, and 132 h) but decreases sharply once diametric growth is arrested (72 h). Results of the RT-PCR semiquantitative analysis of BGL2 expression at 72 and 84 h in the first generation showed the absence and then slight increase in BGL2 mRNA, respectively (Fig. 7B to D). The immunoblot assay demonstrated the presence of minute amounts of BGL2 protein at these same developmental stages. However, substrate gel analysis of BGL2 enzyme activity appears to be more sensitive than the immunoblot technique. We suggest that the absence of BGL2 mRNA at 72 h but detection of a low level of BGL2 enzyme activity at this same developmental stage (Fig. 8) is due to presence of a small amount of residual enzyme from the earlier developmental stage. On the other hand, the slightly elevated BGL2 enzyme activity at 84 h revealed by the substrate gel correlates with the slight increase in BGL2 mRNA at this same developmental stage (Fig. 7B to D). Similarly, the low level of BGL2 enzyme activity suggested by the fluorescent band, which represents the second-generation spherules at 48 h, correlates with the slightly elevated level of BGL2 mRNA (Fig. 7B to D) and BGL2 protein detected by immunoblot analysis of this same developmental stage (Fig. 8).
The second-generation cells in Fig. 6N (corresponds to lanes labeled 48 h in Fig. 7 and 8) had not initiated segmentation and, therefore, had not completed their isotropic growth phase. The substrate gel revealed two additional fluorescent bands with estimated molecular sizes of 45 and 50 kDa. In contrast to the 120-kDa β-glucosidase, the highest activity of these enzymes apparently correlated with phases of spherule segmentation (72 and 84 h) and early endosporulation (96 h) in the first generation of the parasitic cycle. To test whether the BGL3 gene fragment (Fig. 1C) possibly encodes either the 45- or 50-kDa glycosyl hydrolase, RT-PCR analysis of BGL3 expression was conducted using gene-specific primers. The results indicated that BGL3 is constitutively expressed during the parasitic cycle (data not shown). The anti-rBGL2 mouse serum, which was raised against approximately two-thirds of the mature BGL2 protein, including the putative active site, did not recognize the 45- or 50-kDa bands in the immunoblot. These data suggest that the 45- and 50-kDa proteins were not degradation products of BGL2 and may represent novel glycosyl hydrolases of C. immitis.
DISCUSSION
The secreted 120-kDa glycoprotein of C. immitis has been shown to be both a serodiagnostic antigen and a β-glucan-degrading enzyme (23). In this study, we have cloned and characterized the gene which encodes this TP antigen and wall-associated β-glucosidase. On the basis of analysis of the translated BGL2 gene sequence, the predicted molecular size of the mature protein is 90.9 kDa. It is argued, therefore, that the native 120-kDa glycoprotein is highly glycosylated and the carbohydrate moiety contributes approximately 25% of its molecular weight. In fact, the translated amino acid sequence of the BGL2 gene reveals several potential sites for both N and O glycosylation. The recombinant BGL2 protein expressed by E. coli was not recognized by the reference patient antibody which had been used to detect the purified, native TP antigen in the ID-TP assay. This result is consistent with our earlier finding that 3-O-methyl-d-mannose residues added to BGL2 by posttranslational modification are largely responsible for the reactivity of IgM precipitin antibodies with this TP antigen (5).
Our earlier studies had also confirmed that the purified 120-kDa glycoprotein is a β-glucosidase (23). Of the 70 recognized families of glycosyl hydrolases (http://afmb.cnrs-mrs.fr/∼pedro/CAZY/ghf_3.html) (9), the translated BGL2 sequence is closely related to family 3, which currently accommodates both prokayotic and eukaryotic enzymes with a broad range of substrate specifities (e.g., EC 3.2.1.21, -.37, -.52, -.55, -.57, -.58, and -.74). This family is characterized by an aspartate residue at the active site (10) and a specific peptide signature motif (9). Our sequence analysis showed that the location of the conserved 18-aa signature motif within the BGL2 sequence is very similar to that of all other reported fungal family 3 glycosyl hydrolases except for K. marxianus. However, the latter does contain the putative aspartate active site and four additional conserved residues within the signature motif of other members of this family.
The translated sequence of the C. immitis BGL2 gene showed 74% identity and 85% similarity to that of the serodiagnostic H antigen of H. capsulatum (11). Based on amino acid sequence homology of the H antigen to reported extracellular β-glucosidases of other fungi (11), and results of functional analysis of the recombinant protein (13), it has been reported that the H. capsulatum antigen is a secreted glycosyl hydrolase. We have shown that the 120-kDa β-glucosidase of C. immitis is both associated with the cell wall of presegmented spherules and secreted into the culture medium (4, 23). However, an important additional observation is that the concentration of the C. immitis macromolecule in the culture filtrate fluctuates during the parasitic cycle. The peaks of concentration of the secreted 120-kDa β-glucosidase in the medium corresponded to stages of endospore release, and the lowest concentrations corresponded to phases of isotropic growth (23). We previously showed that the active enzyme could be extracted from viable, intact, presegmented spherules by incubation of the cells with 1% octyl-β-d-thioglucoside (23). After isolation of the active β-glucosidase by this method the spherules remained viable, suggesting that the enzyme was derived from the spherule wall. Furthermore, exposure of spherule initials (Fig. 6C) to an inhibitor of the 120-kDa β-glucosidase resulted in the arrest of isotropic growth of the parasitic cells. These data suggested that the active enzyme is associated with the spherule wall during its isotropic growth phase and may perform a morphogenetic role during the parasitic cycle.
Our ability to further evaluate the function of the 120-kDa β-glucosidase was enhanced by the isolation of the BGL2 gene and our success in achieving near synchrony of parasitic cell development in liquid culture. The parasitic cycle can be separated into three fairly distinct morphogenetic phases: isotropic growth, segmentation, and endosporulation (17). An important developmental feature relevant to this study is that initiation of isotropic growth of endospores (i.e., cells which differentiate into new generations of spherules) occurs while the cells are still within the maternal spherule. The results of analyses of temporal expression of the BGL2 gene, production of the BGL2 protein, and activity of the BGL2 enzyme suggest that the peaks of 120-kDa β-glucosidase activity in detergent extracts of spherule homogenates correlate with the isotropic growth phases of first- and second-generation parasitic cells. The first morphological change that is observed after arthroconidia are incubated in Converse medium is cell swelling and most likely involves water uptake and concomitant increase in internal cell pressure, new wall biosynthesis, and wall loosening (12). The last of these events may be at least partly accomplished by BGL2. The 120-kDa β-glucosidase is capable of digesting boiled, β-mercaptoethanol-treated and washed, presegmented spherule wall material (23). Quantitative and qualitative analysis of the C. immitis β-glucosidase activity in the presence of laminarin (Km, 1.03 mM) indicated that the enzyme can utilize β-1,3-linked polyglucans as a substrate to release monomeric and polymeric fragments (23). The 120-kDa β-glucosidase is also capable of efficient digestion of the synthetic p-nitrophenol-β-d-glucopyranoside substrate, which is characteristic of β-1,3-exoglucosidases (14) rather than endoglucosidases as previously suggested (23). Fungal wall-associated exo- and endo-β-glucosidases have been proposed to play a role in morphogenesis (14). However, apparently not all such glycosyl hydrolases participate in cell wall modification. The ability of the C. immitis β-glucosidase to digest its own wall contrasts with activity of the exoG-II wall-associated hydrolase of Aspergillus fumigatus, which showed very limited activity in the presence of cell wall β-1,3-glucans (14). Although definitive proof of function of the C. immitis BGL2 is not yet available, the recent development of a transformation system for C. immitis (29) now permits us to evaluate the phenotype of a BGL2 knockout strain. Nevertheless, we have provided evidence for a morphogenetic role of BGL2 in isotropic growth of parasitic cells based on results of immunolocalization, biochemical analyses, in vitro inhibition studies, and temporal evaluations of gene, glycoprotein, and enzyme expression in near-synchronized parasitic-phase cultures of C. immitis.
ACKNOWLEDGMENTS
We are grateful to K. R. Seshan for technical assistance in culture preparation and morphological examinations.
This investigation was supported by Public Health Service grant AI 19149 and the National Institute of Allergy and Infections Diseases.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Wiley; 1989. [Google Scholar]
- 3.Boyd V L, Bozzini M, Zon G, Noble B L, Mattaliano R J. Sequencing of peptides and proteins from the carboxy terminus. Anal Biochem. 1992;206:344–352. doi: 10.1016/0003-2697(92)90376-i. [DOI] [PubMed] [Google Scholar]
- 4.Cole G T, Kruse D, Seshan K R. Antigen complex of Coccidioides immitiswhich elicits a precipitin antibody response in patients. Infect Immun. 1991;59:2434–2446. doi: 10.1128/iai.59.7.2434-2446.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole G T, Kruse D, Zhu S W, Seshan K R, Wheat R W. Composition, serologic reactivity, and immunolocalization of a 120-kilodalton tube precipitin antigen of Coccidioides immitis. Infect Immun. 1990;58:179–188. doi: 10.1128/iai.58.1.179-188.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole G T, Pishko E J, Seshan K R. Possible roles of wall hydrolases in the morphogenesis of Coccidioides immitis. Can J Bot. 1995;73(Suppl.):S1132–S1141. [Google Scholar]
- 7.Cole G T, Sun S H. Arthroconidium-spherule-endospore transformation in Coccidioides immitis. In: Szaniszlo P J, editor. Fungal dimorphism. New York, N.Y: Plenum Press; 1985. pp. 281–333. [Google Scholar]
- 8.Cole G T, Zhu S W, Pan S, Yuan L, Kruse D, Sun S H. Isolation of antigens with proteolytic activity from Coccidioides immitis. Infect Immun. 1989;57:1524–1534. doi: 10.1128/iai.57.5.1524-1534.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutinho P M, Henrissat B. The molecular structure of cellulases and other carbohydrate-active enzymes: an integrated database approach. In: Ohmiya K, Hayashi K, Sakka K, Kobayashi Y, Karita S, Kimura T, editors. Genetics, biochemistry and ecology of cellulose degradation. Uni Publishers Co., Tokyo, Japan. 1999. pp. 15–23. [Google Scholar]
- 10.Dan S, Marton I, Dekel M, Bravdo B A, He S, Withers S G, Shoseyov O. Cloning, expression, characterization, and nucleophile identification of family 3, Aspergillus nigerbeta-glucosidase. J Biol Chem. 2000;275:4973–4980. doi: 10.1074/jbc.275.7.4973. [DOI] [PubMed] [Google Scholar]
- 11.Deepe G S, Durose G G. Immunological activity of recombinant H antigen from Histoplasma capsulatum. Infect Immun. 1995;63:3151–3157. doi: 10.1128/iai.63.8.3151-3157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.d'Enfert C. Fungal spore germination: insights from the molecular genetics of Aspergillus nidulans and Neurospora crassa. Fung Genet Biol. 1997;21:163–172. [Google Scholar]
- 13.Fisher K L, Wood J P. Determination of beta-glucosidase enzymatic function of the Histoplasma capsulatumH antigen using a native expression system. Gene. 2000;247:191–197. doi: 10.1016/s0378-1119(00)00099-8. [DOI] [PubMed] [Google Scholar]
- 14.Fontaine T, Hartland R P, Diaquin M, Simenel C, Latgé J P. Differential patterns of activity displayed by two exo-beta-1,3-glucanases associated with the Aspergillus fumigatuscell wall. J Bacteriol. 1997;179:3154–3163. doi: 10.1128/jb.179.10.3154-3163.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frohman M A. RACE: rapid amplification of cDNA ends. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols: a guide to methods and applications. New York, N.Y: Academic Press; 1990. pp. 28–38. [Google Scholar]
- 16.Galgiani J N. Coccidioidomycosis: a regional disease of national importance. Rethinking approaches for control. Ann Intern Med. 1999;130:293–300. doi: 10.7326/0003-4819-130-4-199902160-00015. [DOI] [PubMed] [Google Scholar]
- 17.Guevara-Olvera L, Hung C-Y, Yu J-J, Cole G T. Sequence, expression and functional analysis of the Coccidioides immitisODC (ornithine decarboxylase) gene. Gene. 2000;242:437–448. doi: 10.1016/s0378-1119(99)00496-5. [DOI] [PubMed] [Google Scholar]
- 18.Henrissat B, Bairoch A. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J. 1993;293:781–788. doi: 10.1042/bj2930781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins D G, Sharp P M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann K, Bucher P, Falquet L, Bairoch A. The PROSITE database, its status in 1999. Nucleic Acids Res. 1999;27:215–219. doi: 10.1093/nar/27.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung C-Y, Ampel N M, Christian L, Seshan K R, Cole G T. A major cell surface antigen of Coccidioides immitiswhich elicits both humoral and cellular immune responses. Infect Immun. 2000;68:584–593. doi: 10.1128/iai.68.2.584-593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruse D, Cole G T. Isolation of tube precipitin antibody-reactive fractions of Coccidioides immitis. Infect Immun. 1990;58:169–178. doi: 10.1128/iai.58.1.169-178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruse D, Cole G T. A seroreactive 120-kilodalton β-1,3-glucanase of Coccidioides immitiswhich may participate in spherule morphogenesis. Infect Immun. 1992;60:4350–4363. doi: 10.1128/iai.60.10.4350-4363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakai K, Kanehisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics. 1992;14:897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan S, Cole G T. Electrophoretic karyotypes of clinical isolates of Coccidioides immitis. Infect Immun. 1992;60:4872–4880. doi: 10.1128/iai.60.11.4872-4880.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan S, Cole G T. Molecular and biochemical characterization of a Coccidioides immitis-specific antigen. Infect Immun. 1995;63:3994–4002. doi: 10.1128/iai.63.10.3994-4002.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan S, Sigler L, Cole G T. Evidence for a phylogenetic connection between Coccidioides immitis and Uncinocarpus reesii(Onygenaceae) Microbiology. 1994;140:1481–1494. doi: 10.1099/00221287-140-6-1481. [DOI] [PubMed] [Google Scholar]
- 28.Pappagianis D, Zimmer B L. Serology of coccidioidomycosis. Clin Microbiol Rev. 1990;3:247–268. doi: 10.1128/cmr.3.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reichard U, Hung C-Y, Thomas P W, Cole G T. Disruption of the gene which encodes a serodiagnostic antigen and chitinase of the human fungal pathogen Coccidioides immitis. Infect Immun. 2000;68:5830–5838. doi: 10.1128/iai.68.10.5830-5838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seshan K R, Cole G T. Structural studies in Coccidioides immitis. In: Maresca B, Kobayashi G S, editors. Molecular biology of pathogenic fungi. New York, N.Y: Telos Press; 1994. pp. 265–273. [Google Scholar]
- 31.Thomas P W, Wyckoff E E, Pishko E J, Yu J-J, Kirkland T N, Cole G T. The hsp60 gene of the human pathogenic fungus Coccidioides immitisencodes a T-cell reactive protein. Gene. 1997;199:83–91. doi: 10.1016/s0378-1119(97)00351-x. [DOI] [PubMed] [Google Scholar]
- 32.Wyckoff E E, Pishko E J, Kirkland T N, Cole G T. Cloning and expression of a gene encoding a T-cell reactive protein from Coccidioides immitis: homology to 4-hydroxyphenylpyruvate dioxygenase and the mammalian F antigen. Gene. 1995;161:107–111. doi: 10.1016/0378-1119(95)00250-a. [DOI] [PubMed] [Google Scholar]
- 33.Yu J-J, Smithson S L, Thomas P W, Kirkland T N, Cole G T. Isolation and characterization of the urease gene (URE) from the pathogenic fungus Coccidioides immitis. Gene. 1997;198:387–391. doi: 10.1016/s0378-1119(97)00342-9. [DOI] [PubMed] [Google Scholar]
- 34.Yu J-J, Zheng L, Thomas P W, Szaniszlo P J, Cole G T. Isolation and confirmation of function of the Coccidioides immitis URA5(orotate phosphoribosyl transferase) gene. Gene. 1999;226:233–242. doi: 10.1016/s0378-1119(98)00556-3. [DOI] [PubMed] [Google Scholar]