Abstract
Cross-dehydrogenative coupling reactions provide a method to construct new chemical bonds by direct C–H activation without any pre-functionalization. Compared to functionalization of a C–H bond α- to ether oxygen, α- to carbonyl, or at a benzylic position, functionalization of unactivated hydrocarbons is difficult and often requires high temperatures, a transition-metal catalyst, or a superstoichiometric quantity of volatile, toxic, and explosive tert-butylhydroperoxide. Here, a cross-dehydrogenative C–O coupling reaction of N-hydroxyphthalimide with unactivated alkanes, nitriles, ethers, and thioethers has been realized by using iodobenzene diacetate as the radical initiator. The current protocol enables efficient functionalization of unactivated hydrocarbons and nitriles through inert C(sp3)–H bond activation under mild reaction conditions. O-substituted NHPI derivatives are generated in good yields under metal-free conditions.
Subject terms: Synthetic chemistry methodology, Synthetic chemistry methodology
Robust and selective C–H functionalisation of alkanes remains a challenge. Here, iodine(III) mediates the radical coupling of N-hydroxyphthalimide and unactivated C(sp3)–H bonds in a range of cyclic and acyclic alkanes.
Introduction
Cross-dehydrogenation coupling (CDC), a type of chemical bond construction by direct dehydrogenation, has emerged as an important approach in chemical synthesis because of its excellent efficiency, atomic economy, and wide substrate scope1–4. Catalysts based on transition metals such as copper, iron, ruthenium, and manganese are typically used in CDC reactions5–7. However, issues associated with these metallic catalysts, such as high cost, harsh reaction conditions, toxicity, and metal residues, have restricted their application in organic synthesis8,9. Therefore, the development of efficient, simple, environmentally friendly, and metal-free CDC reactions has become a major trend10.
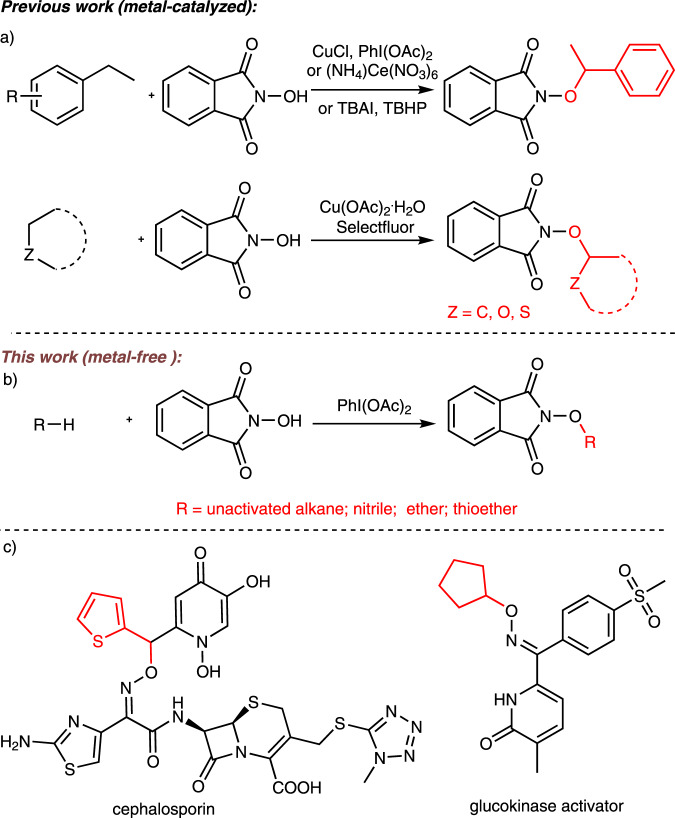
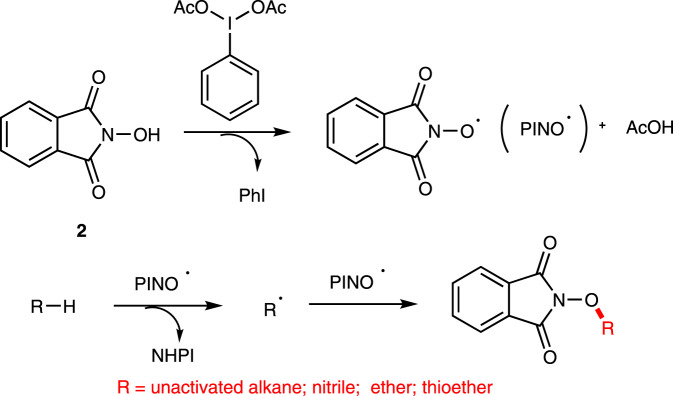
In 1985, the Masui group first applied N-hydroxyphthalimide (NHPI) as a metal-free oxidant to the oxidation of olefins via a free radical reaction11. The ‘non-persistent’ nitrogen-oxygen radical, i.e. phthalimide nitrogen-oxygen (PINO) radical12, is often used to activate inert hydrocarbon bonds due to its high activity13. Moreover, oxygen-substituted NHPI derivatives could be converted into alkoxyamines via hydrazinolysis14. Alkoxyamines can be further used to synthesize new cephalosporins, oxiconazoles, glucokinase activators, and other organic compounds with antibacterial activity15–17. Disadvantages of conventional synthetic methods for accessing these compounds include poor atom economy, tedious synthetic procedures, and unsatisfactory selectivity. Recent researches on the CDC reactions involving NHPI have focused mainly on activated C(sp3)–H bonds. Reaction sites are typically at benzylic positions or at the α position of carbonyl moieties or ethers18–39. The CDC reaction between NHPI and alkanes with inert C(sp3)–H bonds has rarely been studied, and only few examples have been reported so far (Fig. 1)40–43. For example, copper nitrate trihydrate-catalyzed functionalization of C(sp3)–H bonds adjacent to the oxygen atoms of ethers was realized with oxygen as the co-oxidant in 201737. Functionalization either α- to the oxygen atom of ethers or at the benzylic position of benzyl derivatives was accomplished with tetrabutylammonium iodide and tert-butylhydroperoxide under sonication31. It is noteworthy that the substrates in the previous reports did not involve acyclic alkanes or nitriles, and transition metal catalysts or superstoichiometric quantities of (volatile, toxic and explosive) tert-butylhydroperoxide were often required.
Fig. 1. Cross-dehydrogenative C–O coupling using NHPI.
a Approaches for the cross-coupling reactions under transition metal catalyst or tert-butylhydroperoxide. b Cross-dehydrogenative C–O coupling reaction of N-hydroxyphthalimide with unactivated C(sp3)–H by using iodobenzene diacetate. c Structures of cephalosporin and glucokinase activator.
Hypervalent iodine can be used as an initiator to trigger various chemical reactions, functioning like a transition metal catalyst, and this reagent has been widely applied to drug synthesis and total synthesis of natural products44–48. Considering the potential value of R–ONH2 compounds, we herein report an approach to constructing C–O bonds in unactivated alkanes, cyanides, ethers, and thioethers via a CDC reaction with NHPI in the presence of iodobenzene diacetate as a radical initiator (Fig. 1). The current method features a number of advantages, such as mild metal-free reaction conditions, high synthetic efficiency, environmentally friendliness, and applicability to a wide range of substrates.
Results and discussion
Reaction optimization
The project was commenced with the reaction of cyclohexane with NHPI at room temperature, in which the effect of oxidants [tert-butyl hydroperoxide (TBHP), PhI(OCOCF3)2, H2O2, and PhI(OAc)2] was investigated (Table 1, entries 1–5). The NHPI derivative 3 was formed in 40% yield only when PhI(OAc)2 was applied as the oxidant (entry 5). The solvents [chloroform, water, N,N-dimethylacetamide (DMA), 1,2-dichloroethane (DCE), acetonitrile (MeCN), ethyl acetate (EA), chlorobenzene (PhCl), dichloromethane (DCM), and benzene (PhH)] were next screened (entries 6–14). When PhI(OAc)2 was used as the oxidant, DCM (entry 13) and benzene (entry 14) gave the best results. The reaction with PhI(OCOCF3)2 as the oxidant in DCM and PhH was then investigated, and the desired product 3 was obtained in 42 and 49% yield, respectively (entries 15, 16). The influence of the temperature (0 oC and 60 oC) on the reaction was examined with DCM as the solvent and PhI(OAc)2 as the oxidant, and no better result was obtained (entries 17, 18). Notably, when DCM was used as the solvent, compound 4 was formed (15%) along with 3; the formation of 4 was obviously due to the reaction of the solvent molecule itself with NHPI (see Fig. 2 for the structure). The bond dissociation enthalpies (BDEs) for cyclohexane (cyclohexane-H) and DCM (DCM-H) were calculated by Gaussian 16 at B3LYP/6-31 G basis set level and found to be 402.3 and 416.5 kJ mol−1, respectively, which indicates that cyclohexane should be slightly more prone to undergo this kind of reaction.
Table 1.
Optimisation of the reaction conditionsa.
 | |||||
|---|---|---|---|---|---|
| Entry | 1:2:Oxidant | Oxidant | Solvent | T (°C) | Yieldb |
| 1c | 20:1:2 | TBHP | – | rt | N.D. |
| 2 | 2:1:2 | TBHP | H2O | rt | N.D. |
| 3c | 20:1:2 | PhI(OCOCF3)2 | – | rt | trace |
| 4c | 20:1:2 | H2O2 | – | rt | N.D. |
| 5c | 20:1:2 | PhI(OAc)2 | – | rt | 40 |
| 6 | 2:1:2 | PhI(OAc)2 | CHCl3 | rt | 39 |
| 7 | 2:1:2 | PhI(OAc)2 | H2O | rt | 16 |
| 8 | 2:1:2 | PhI(OAc)2 | DMA | rt | N.D. |
| 9 | 2:1:2 | PhI(OAc)2 | DCE | rt | 43 |
| 10 | 2:1:2 | PhI(OAc)2 | MeCN | rt | 46 |
| 11 | 2:1:2 | PhI(OAc)2 | EA | rt | 32 |
| 12 | 2:1:2 | PhI(OAc)2 | C6H5Cl | rt | 42 |
| 13 | 2:1:2 | PhI(OAc)2 | DCM | rt | 61 |
| 14 | 2:1:2 | PhI(OAc)2 | PhH | rt | 66 |
| 15 | 2:1:2 | PhI(OCOCF3)2 | DCM | rt | 42 |
| 16 | 2:1:2 | PhI(OCOCF3)2 | PhH | rt | 49 |
| 17 | 2:1:2 | PhI(OAc)2 | DCM | 0 | 25 |
| 18d | 2:1:2 | PhI(OAc)2 | DCM | 60 | 54 |
aGeneral procedure: the reaction was carried out with NHPI (1 mmol), cyclohexane (2 mmol), oxidant (2 mmol), and solvent (2 mL) at room temperature for 2 h.
bIsolated yield.
cCyclohexane (20 mmol) was used as the substrate and solvent.
dThe reaction was conducted in reflux.
Fig. 2. Reaction of DCM with NHPI.
DCM as both the solvent and the substrate.
When the reaction was performed with DCM as both the solvent and the substrate, compound 4 was obtained in 50% yield (Fig. 2). Although no additional coupling products were produced when benzene was adopted as the solvent, considering the toxicity of benzene, DCM was chosen as the solvent for most of the subsequent experiments.
Substrate scope
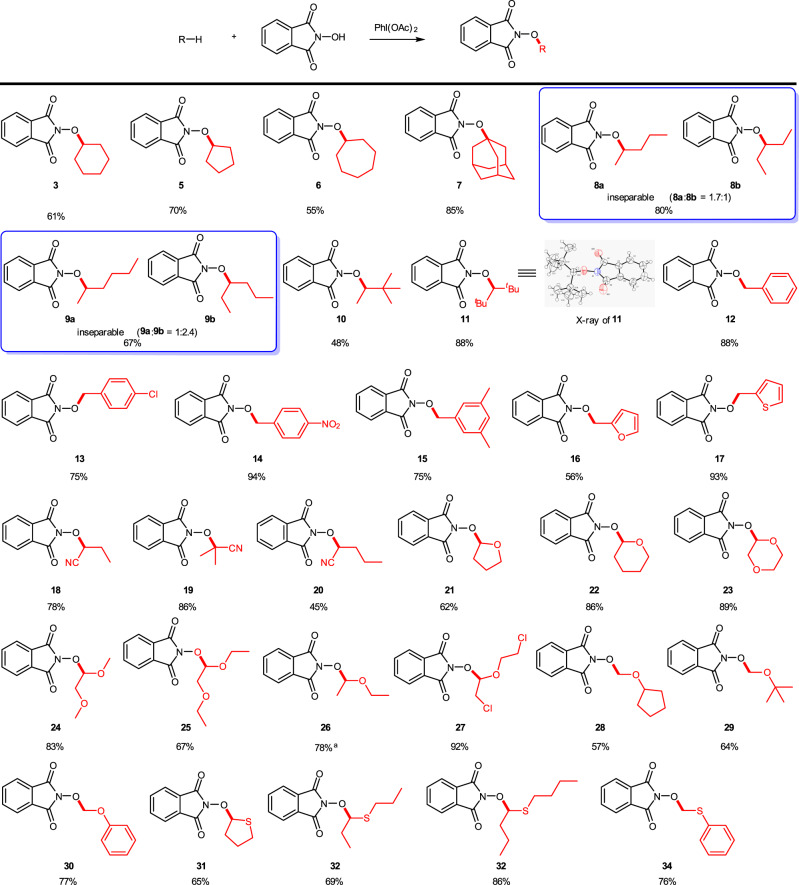
The substrate scope was then explored under the optimal reaction conditions obtained (see Table 1, entry 15), and the experimental results are summarized in Fig. 3. First, the reactions of the cycloalkanes with NHPI were investigated. The C–O CDC reactions of cyclopentane, cycloheptane, and amantadine with NHPI proceeded smoothly and afforded compounds (5–7) in the yields of 55–85%. It should be mentioned that no reports have been disclosed concerning this kind of C–H activation for acyclic alkanes so far. The reactions of acyclic alkanes (e.g., n-pentane and n-hexane) with NHPI were then tested and inseparable oxidation products 8a/8b (1.7/1) and 9a/9b (1/2.4) were obtained, respectively, while single products were delivered from the reaction of 2,2-dimethylbutane and 2,2,4,4-tetramethylpentane in 48% (10) and 88% (11) yields, respectively. In these cases, no activation of the primary C(sp3)–H bond occurred. Further studies revealed that toluene, trimethylbenzene, p-nitrotoluene, p-chlorotoluene, 2-methylfuran, and 2-methylthiophene were suitable substrates as well, and the corresponding oxidation products (12–17) were afforded in 56–94% yields. The benzyl C(sp3)–H bond was involved in the CDC reaction for these substrates, and the chloro substituent on the phenyl ring does not affect the desired transformation essentially.
Fig. 3. CDC coupling of various C–H reagents with NHPI.
General procedure: the reactions were carried out with NHPI (1 mmol), alkane (2 mmol), PhI(OAc)2 (2 mmol) in DCM (2 mL) at room temperature for 2 h. Isolated yield. aThe substrate (20 mmol) was used as the solvent.
Cyano compounds play an important role in drug synthesis49, and several research groups have realised direct oxidation of α–C(sp3)–H in alkyl nitrile with metal catalysts50,51. Hence, alkyl nitrile substrates were next studied under the current transition metal-free conditions. For butyronitrile, isobutyronitrile, and pentonitrile, the corresponding α–C(sp3)–H bond oxidation products (18–20) were generated in the yields of 45–86%. The CDC reaction of various ethers and sulfides was also investigated. Cyclic ethers (21–23), acyclic ethers (24–30), thioethers (31–34), and even haloether (27) were converted into the corresponding oxidation products smoothly.
Synthetic applications
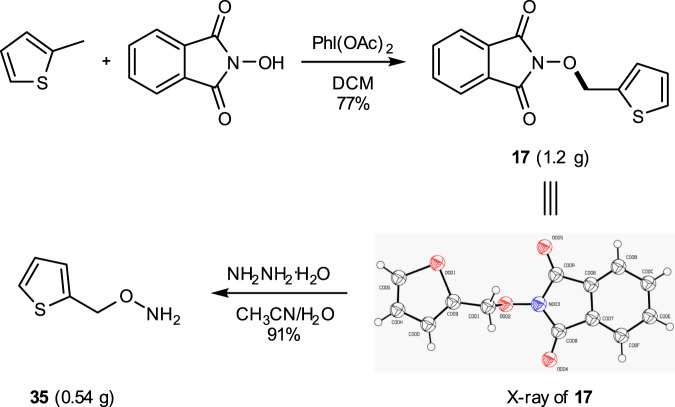
A gram-scale reaction was performed to demonstrate the application of the current synthetic method. O-(Thiophen-2-ylmethyl) hydroxylamine is a key intermediate for the synthesis of new cephalosporins. The CDC reaction was scaled up to gram-scale with 2-methylthiophene as the substrate and compound 17 was obtained in 77% yield (Fig. 4). Alkoxyamine 35 was then obtained via hydrazinolysis of 17.
Fig. 4. A gram-scale experiment.
Gram-scale with 2-methylthiophene and hydrazinolysis.
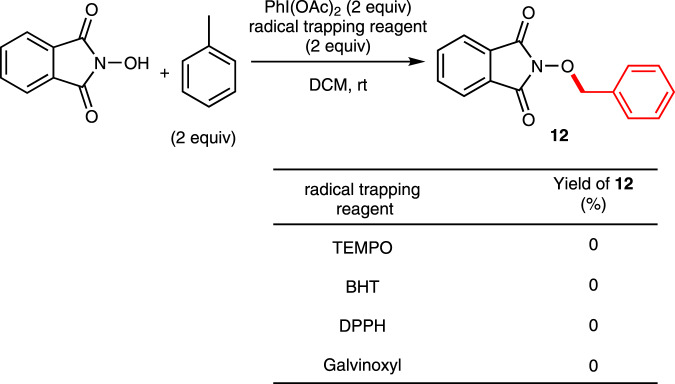
Relevant mechanistic studies were next performed (Fig. 5). The reaction of toluene (as the substrate) was found to be totally inhibited upon addition of 2,2,6,6-tetramethylpiperidinooxy (TEMPO), 2,6-di-tert-butyl-4-methylphenol (BHT), 2,2-diphenyl-1-picrylhydrazyl (DPPH), or Galvinoxyl. These results indicated that the current transformation might proceed via a radical reaction pathway.
Fig. 5. Effect of radical trapping reagents to the reaction mixture.
PhI(OAc)2 (0.54 mmol), radical trapping reagent (0.54 mmol) and NHPI (0.27 mmol) were added to a solution of toluene (0.54 mmol) in DCM (2 mL).
A plausible mechanism is proposed for the current reaction (Fig. 6). Oxidation of NHPI (2) with PhI(OAc)2 affords PINO radical35, which reacts with the substrate to form an alkyl radical via hydrogen abstraction. Finally, the alkyl radical couples with PINO radical to deliver the final product.
Fig. 6. Proposed reaction mechanism.
Oxidation of NHPI with PhI(OAc)2 affords PINO radical.
In conclusion, selective C–H functionalisation of alkanes remains a great challenge. PhI(OAc)2 has been applied to mediating radical coupling of N-hydroxyphthalimide and unactivated C(sp3)–H bonds in a range of cyclic and acyclic alkanes. The present reaction was conducted at room temperature which was compatible with various solvents. A variety of cycloalkanes, acyclic alkanes, cyanides, ethers, and thioethers reacted smoothly with NHPI avoiding volatile, toxic and explosive reagents, providing a direct and simple access to O-substituted NHPI derivatives.
Methods
General Information
All experiments were conducted under air, using commercially purchased analytical reagents and solvents which do not require further purification. 1HNMR and 13CNMR spectra were recorded on a Bruker spectrometer (at 400 and 100 MHz, respectively). TMS was used as reference for chemical shifts. High-resolution mass spectrometry (HRMS) was recorded on an Agilent Technologies LC-TOF instrument. X-ray crystallography of compounds 4, 11, and 17 were performed on a Bruker Smart Apex CCD area detector diffractometer using graphite-monochromated Mo Kα radiation. The regards to the suitability and safety warnings of PhH: avoid contact with the skin, the eyes; wear suitable protective clothing, gloves, eye/ face protection; avoid release to the environment.
General procedure for the synthesis of R-ONH2 compounds
PhI(OAc)2 (2 mmol) and NHPI (1 mmol) were added to a solution of a substrate (2 mmol) in DCM (2 mL), and the reaction mixture was stirred at room temperature for 2 h and filtered. The filtrate was concentrated under reduced pressure to give a crude product, which was purified by flash silica gel column chromatography (petroleum ether/EtOAc, 50:1 to 20:1) to give the product.
Procedure for compound 35
Water (36 mL) and N-hydrazine hydrate (1.8 g, 54 mmol) were added to a solution of 2-(thiophen-2-ylmethoxy)isoindoline-1,3-dione (1.2 g, 4.63 mmol) in acetonitrile (50 mL). The mixture was stirred at room temperature for 4 h. The crude product was purified by flash silica gel column chromatography (petroleum ether/EtOAc, 5:1) to give the product (0.54 g, 91%) as a brown solid.
Reaction of Toluene with NHPI in the presence of radical trapping reagent
PhI(OAc)2 (0.54 mmol), radical trapping reagent (0.54 mmol), and NHPI (0.27 mmol) were added to a solution of toluene (0.54 mmol) in DCM (2 mL), and the reaction mixture was stirred at room temperature for 2 h and detected by TLC.
Methods of the calculation of BDEs
The bond dissociation enthalpies (BDEs) were calculated by Gaussian 16 at B3LYP/6-31 G basis set level.
The BDEs for cyclohexane (cyclohexane-H) was calculated as:
[−234.992855 Hatree (Cyclohexane radical)] + [−0.497912 Hatree (H radical)] − [−235.644013 Hatree (Cyclohexane)] = 0.153246 Hatree = 402.3 kJ mol−1.
The BDEs for cyclohexane (DCM-H) was calculated as:
[−958.957469 Hatree (DCM radical)] + [−0.497912 Hatree (H radical)] − [−959.614026 Hatree (DCM)] = 0.158645 Hatree = 416.5 kJ mol−1.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by the State Key Basic Research Program of the PRC (2018YFC0310900), the National Natural Science Foundation of China (21602029, 21732001, 21871018), Key Projects of the Support Program for Outstanding Young Talents in Anhui Province Colleges and Universities (gxyqZD2020030), Horizontal Cooperation Project of Fuyang Municipal Government (XDHX201722), Shenzhen Science and Technology Innovation Committee (KQTD20190929174023858, JCYJ20180504165454447), Industry and Information Technology Bureau of Shenzhen Municipality (201806151622209330), Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions (2019SHIBS0004), Yifan Pharmaceutical Co., Ltd (HX2019033), Guangdong Science and Technology Program (2017B030314002), and the National Ten Thousand Talent Program (the Leading Talent Tier).
Author contributions
F.W., H.Z., and L.S. conceived the synthetic design and directed the project. X.H., X.L. and X.S. conducted the experimental work and data analysis., C.W., B.C., and J.Z. solved the X-ray structures and prepared the X-ray section of the Supplementary Intormation, Z.T. finished the calculation of bond dissociation enthalpies (BDEs).
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information file, and from the corresponding authors upon reasonable request. The characterization data are available in Supplementary Note 1 and NMR spectra are available in Supplementary Figs. 1~66. The supplementary crystallographic data (Supplementary Data 1) reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 2021450~2021452. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/. The calculated results are available in Supplementary Table 4. and XYZ co-ordinates for all optimized structures see Supplementary Data 2. The crystallographic informations of compounds 4, 11 and 17 are available in Supplementary Data 3–5.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fufang Wu, Email: fufang_wu@foxmail.com.
Liangquan Sheng, Email: shenglq@fync.edu.cn.
Hongbin Zhai, Email: zhaihb@pku.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-021-00480-8.
References
- 1.Girard SA, Knauber T, Li C-J. The cross-dehydrogenative coupling of C-H bonds: a versatile strategy for C-C bond formations. Angew. Chem. Int. Ed. 2014;53:74–100. doi: 10.1002/anie.201304268. [DOI] [PubMed] [Google Scholar]
- 2.Krylov IB, Vil’ VA, Terent’ev AO. Cheminform abstract: cross-dehydrogenative coupling for the intermolecular C-O bond formation. ChemInform. 2015;46:92–146. doi: 10.3762/bjoc.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, et al. Oxidative coupling between two hydrocarbons: an update of recent C–H functionalizations. Chem. Rev. 2015;115:12138–12204. doi: 10.1021/cr500431s. [DOI] [PubMed] [Google Scholar]
- 4.Yi H, et al. Recent advances in radical C–H activation/radical cross-coupling. Chem. Rev. 2017;117:9016–9085. doi: 10.1021/acs.chemrev.6b00620. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhofer A, Hioe J, Gschwind RM, König B. Photocatalytic phenol–arene C–C and C–O cross-dehydrogenative coupling. Eur. J. Org. Chem. 2017;2017:2194–2204. doi: 10.1002/ejoc.201700211. [DOI] [Google Scholar]
- 6.Dong J, et al. Photoredox-mediated direct cross-dehydrogenative coupling of heteroarenes and amines. Org. Lett. 2018;20:5661–5665. doi: 10.1021/acs.orglett.8b02389. [DOI] [PubMed] [Google Scholar]
- 7.Samanta S, Hajra A. Mn(II)-catalyzed C–H alkylation of imidazopyridines and N-heteroarenes via decarbonylative and cross-dehydrogenative coupling. J. Org. Chem. 2019;84:4363–4371. doi: 10.1021/acs.joc.9b00366. [DOI] [PubMed] [Google Scholar]
- 8.Prendergast AM, McGlacken GP. Transition metal mediated C–H activation of 2-pyrones, 2-pyridones, 2-coumarins and 2-quinolones. Eur. J. Org. Chem. 2018;2018:6068–6082. doi: 10.1002/ejoc.201800299. [DOI] [Google Scholar]
- 9.Faisca Phillips AM, Pombeiro AJL. Recent developments in transition metal-catalyzed cross-dehydrogenative coupling reactions of ethers and thioethers. ChemCatChem. 2018;10:3354–3383. doi: 10.1002/cctc.201800582. [DOI] [Google Scholar]
- 10.Parvatkar PT, Manetsch R, Banik BK. Metal-free cross-dehydrogenative coupling (CDC): molecular iodine as a versatile catalyst/reagent for CDC reactions. Chem. – Asian J. 2019;14:6–30. doi: 10.1002/asia.201801237. [DOI] [PubMed] [Google Scholar]
- 11.Masui M, Hosomi K, Tsuchida K, Ozaki S. Electrochemical oxidation of olefins using N-hydroxyphthalimide as a mediator. Chem. Pharm. Bull. 1985;33:4798–4802. doi: 10.1248/cpb.33.4798. [DOI] [Google Scholar]
- 12.Leifert D, Studer A. The persistent radical effect in organic synthesis. Angew. Chem. Int. Ed. 2020;59:74–108. doi: 10.1002/anie.201903726. [DOI] [PubMed] [Google Scholar]
- 13.Fu M-C, Shang R, Zhao B, Wang B, Fu Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science. 2019;363:1429–1434. doi: 10.1126/science.aav3200. [DOI] [PubMed] [Google Scholar]
- 14.Bag R, Sar D, Punniyamurthy T. Copper(II)-catalyzed direct dioxygenation of alkenes with air and N-hydroxyphthalimide: synthesis of β-keto-N-alkoxyphthalimides. Org. Lett. 2015;17:2010–2013. doi: 10.1021/acs.orglett.5b00770. [DOI] [PubMed] [Google Scholar]
- 15.Brown MF, et al. Pyridone-conjugated monobactam antibiotics with gram-negative activity. J. Med Chem. 2013;56:5541–5552. doi: 10.1021/jm400560z. [DOI] [PubMed] [Google Scholar]
- 16.Sharma GVM, et al. Self-assembling cyclic tetrapeptide from alternating C-linked carbo-β-amino acid [(S)-β-Caa] and α-aminoxy acid [(R)-Ama]: a selective chloride ion receptor. J. Org. Chem. 2010;75:1087–1094. doi: 10.1021/jo901923q. [DOI] [PubMed] [Google Scholar]
- 17.Yamawaki K, et al. A novel series of parenteral cephalosporins exhibiting potent activities against both Pseudomonas aeruginosa and other Gram-negative pathogens. Part 2: synthesis and structure–activity relationships. Bioorg. Med Chem. 2008;16:1632–1647. doi: 10.1016/j.bmc.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Iwahama T, Sakaguchi S, Ishii Y. Catalytic α-hydroxy carbon radical generation and addition. Synthesis of α-hydroxy-γ-lactones from alcohols, α,β-unsaturated esters and dioxygen. Chem. Commun. 2000;7:613–614. doi: 10.1039/b000707m. [DOI] [Google Scholar]
- 19.Zhou Q-F, et al. Phosphine-catalyzed [3+2] annulation of electron-deficient alkynes withN-hydroxyphthalimide: synthesis of 3a-hydroxyisoxazolo[3,2-a]isoindol-8(3aH)-ones. Adv. Synth. Catal. 2013;355:2787–2792. doi: 10.1002/adsc.201300426. [DOI] [Google Scholar]
- 20.Aruri H, et al. Cross-dehydrogenative coupling of azoles with alpha–C(sp3)–H of ethers and thioethers under metal-free conditions: functionalization of H-N azoles via C-H activation. J. Org. Chem. 2015;80:1929–1936. doi: 10.1021/jo502477r. [DOI] [PubMed] [Google Scholar]
- 21.Xia X-F, Zhu S-L, Zhang D. Copper-catalyzed C–O coupling of styrenes with N-hydroxyphthalimide through dihydroxylamination reactions. Tetrahedron. 2015;71:8517–8520. doi: 10.1016/j.tet.2015.09.040. [DOI] [Google Scholar]
- 22.Lerchen A, Knecht T, Daniliuc CG, Glorius F. Unnatural amino acid synthesis enabled by the regioselective cobalt(III)-catalyzed intermolecular carboamination of alkenes. Angew. Chem. Int. Ed. 2016;55:15166–15170. doi: 10.1002/anie.201608729. [DOI] [PubMed] [Google Scholar]
- 23.Feizpour F, Jafarpour M, Rezaeifard A. A photoinduced cross-dehydrogenative-coupling (CDC) reaction between aldehydes and N-hydroxyimides by a TiO2–Co ascorbic acid nanohybrid under visible light irradiation. N. J. Chem. 2018;42:807–811. doi: 10.1039/C7NJ03651E. [DOI] [Google Scholar]
- 24.Li J, et al. NHPI- and TBAI-co-catalyzed synthesis of allylic esters from toluene derivatives and alkenes. Synlett. 2018;29:840–844. doi: 10.1055/s-0036-1591748. [DOI] [Google Scholar]
- 25.Liu Z, Breit B. Rhodium-catalyzed regio- and enantioselective addition of N-hydroxyphthalimide to allenes: a strategy to synthesize chiral allylic alcohols. Org. Lett. 2018;20:300–303. doi: 10.1021/acs.orglett.7b03709. [DOI] [PubMed] [Google Scholar]
- 26.Mahmood S, et al. Cobalt/N-hydroxyphthalimide(NHPI)-catalyzed aerobic oxidation of hydrocarbons with ionic liquid additive. Mol. Catal. 2018;447:90–96. doi: 10.1016/j.mcat.2018.01.006. [DOI] [Google Scholar]
- 27.Mo Y, Jensen KF. Continuous N-hydroxyphthalimide (NHPI)-mediated electrochemical aerobic oxidation of benzylic C–H bonds. Chem. Eur. J. 2018;24:10260–10265. doi: 10.1002/chem.201802588. [DOI] [PubMed] [Google Scholar]
- 28.Su W, Jin C, Sun B, Yan Z. (Diacetoxyiodo)benzene-mediated transition-metal-free amination of C(sp3)–H bonds adjacent to heteroatoms with azoles: synthesis of N-alkylated azoles. Synlett. 2018;29:2432–2436. doi: 10.1055/s-0037-1610293. [DOI] [Google Scholar]
- 29.Tang S-Q, Wang A-P, Schmitt M, Bihel F. Dioxygenation of styrenes with molecular oxygen in water. Tetrahedron Lett. 2018;59:1465–1468. doi: 10.1016/j.tetlet.2018.03.009. [DOI] [Google Scholar]
- 30.Xu X, et al. Substrate-controlled regioselective iodooxygenation of olefins. Synlett. 2018;29:1634–1638. doi: 10.1055/s-0037-1609968. [DOI] [Google Scholar]
- 31.Jiang H, et al. Ultrasound accelerated synthesis of O-alkylated hydroximides under solvent- and metal-free conditions. Org. Biomol. Chem. 2019;17:10223–10227. doi: 10.1039/C9OB02245G. [DOI] [PubMed] [Google Scholar]
- 32.Krylov IB, Paveliev SA, Matveeva OK, Terent’ev AO. Cerium(IV) ammonium nitrate: reagent for the versatile oxidative functionalization of styrenes using N-hydroxyphthalimide. Tetrahedron. 2019;75:2529–2537. doi: 10.1016/j.tet.2019.03.030. [DOI] [Google Scholar]
- 33.Dian L, Wang S, Zhang-Negrerie D, Du Y, Zhao K. Organocatalytic amination of alkyl ethers via n-Bu4NI/t-BuOOH-mediated intermolecular oxidative C(sp(3))-N bond formation: novel synthesis of hemiaminal ethers. Chem. Commun. (Camb.) 2014;50:11738–11741. doi: 10.1039/C4CC05758A. [DOI] [PubMed] [Google Scholar]
- 34.Lee JM, Park EJ, Cho SH, Chang S, Cu-Facilitated C−O. Bond formation using N-hydroxyphthalimide: efficient and selective functionalization of benzyl and allylic C−H bonds. J. Am. Chem. Soc. 2008;130:7824–7825. doi: 10.1021/ja8031218. [DOI] [PubMed] [Google Scholar]
- 35.Qian P-C, et al. (Diacetoxyiodo)benzene-mediated oxygenation of benzylic C(sp3)-H bonds with N-hydroxyamides at room temperature. Eur. J. Org. Chem. 2015;2015:1680–1684. doi: 10.1002/ejoc.201403616. [DOI] [Google Scholar]
- 36.Krylov IB, Lopat’eva ER, Budnikov AS, Nikishin GI, Terent’ev AO, Metal-Free Cross-Dehydrogenative C-O. Coupling of carbonyl compounds with N-hydroxyimides: unexpected selective behavior of highly reactive free radicals at an elevated temperature. J. Org. Chem. 2020;85:1935–1947. doi: 10.1021/acs.joc.9b02656. [DOI] [PubMed] [Google Scholar]
- 37.Xu X, et al. Copper nitrate-catalyzed oxidative coupling of unactivated C(sp(3))-H bonds of ethers and alkanes with N-hydroxyphthalimide: synthesis of N-hydroxyimide esters. Org. Biomol. Chem. 2017;15:9875–9879. doi: 10.1039/C7OB02249B. [DOI] [PubMed] [Google Scholar]
- 38.Xu P, et al. Scalable photoelectrochemical dehydrogenative cross-coupling of heteroarenes with aliphatic C-H bonds. Angew. Chem. Int. Ed. Engl. 2020;59:14275–14280. doi: 10.1002/anie.202005724. [DOI] [PubMed] [Google Scholar]
- 39.Dian L, et al. Organocatalytic radical involved oxidative cross‐coupling of N‐hydroxyphthalimide with benzylic and allylic hydrocarbons. Adv. Synth. Catal. 2015;357:3836–3842. doi: 10.1002/adsc.201500623. [DOI] [Google Scholar]
- 40.Satoshi S, Susumu K, Takahiro I, Yasutaka I. An efficient aerobic oxidation of isobutane to t-butyl alcohol by n-hydroxyphthalimide combined with Co(II) species. Bull. Chem. Soc. Jpn. 1998;71:1237–1240. doi: 10.1246/bcsj.71.1237. [DOI] [Google Scholar]
- 41.Zhou J, Jin C, Li X, Su W. Copper-catalyzed oxidative esterification of unactivated C(sp3)–H bonds with carboxylic acids via cross dehydrogenative coupling. RSC Adv. 2015;5:7232–7236. doi: 10.1039/C4RA14586K. [DOI] [Google Scholar]
- 42.Guo Z, Jin C, Zhou J, Su W. Copper(ii)-catalyzed cross dehydrogenative coupling reaction of N-hydroxyphthalimide with alkanes and ethers via unactivated C(sp3)–H activation at room temperature. RSC Adv. 2016;6:79016–79019. doi: 10.1039/C6RA14697J. [DOI] [Google Scholar]
- 43.Deng Y, et al. Noel, C(sp3)-H functionalizations of light hydrocarbonsusing decatungstate photocatalysis in flow. Science. 2020;369:92–96. doi: 10.1126/science.abb4688. [DOI] [PubMed] [Google Scholar]
- 44.Liu GQ, Li YM. Regioselective (diacetoxyiodo)benzene-promoted halocyclization of unfunctionalized olefins. J. Org. Chem. 2014;79:10094–10109. doi: 10.1021/jo501739j. [DOI] [PubMed] [Google Scholar]
- 45.Moteki SA, Selvakumar S, Zhang T, Usui A, Maruoka K. A practical approach for the oxidation of unactivated Csp3-H bonds witho-nitro(diacetoxyiodo)benzene as an efficient hypervalent iodine(III)-based oxidizing agent. Asian J. Org. Chem. 2014;3:932–935. doi: 10.1002/ajoc.201402087. [DOI] [Google Scholar]
- 46.Xia X-F, et al. Metal-free three-component oxyazidation of alkenes with trimethylsilyl azide and N-hydroxyphthalimide. J. Org. Chem. 2015;80:290–295. doi: 10.1021/jo502327r. [DOI] [PubMed] [Google Scholar]
- 47.Doben N, Yan H, Kischkewitz M, Mao J, Studer A. Intermolecular acetoxyaminoalkylation of alpha-diazo amides with (diacetoxyiodo)benzene and amines. Org. Lett. 2018;20:7933–7936. doi: 10.1021/acs.orglett.8b03504. [DOI] [PubMed] [Google Scholar]
- 48.Peng H-C, et al. New, milder hypervalent iodine oxidizing agent: using μ-oxodi(phenyliodanyl) diacetate, a (Diacetoxyiodo)benzene derivative, in the synthesis of quinones. J. Chem. Educ. 2019;96:2622–2627. doi: 10.1021/acs.jchemed.8b00636. [DOI] [Google Scholar]
- 49.Velcicky J, et al. Palladium-catalyzed cyanomethylation of aryl halides through domino suzuki coupling-isoxazole fragmentation. J. Am. Chem. Soc. 2011;133:6948–6951. doi: 10.1021/ja201743j. [DOI] [PubMed] [Google Scholar]
- 50.Bunescu A, et al. Copper-catalyzed three-component carboazidation of alkenes with acetonitrile and sodium azide. Angew. Chem. Int. Ed. 2017;56:10555–10558. doi: 10.1002/anie.201705353. [DOI] [PubMed] [Google Scholar]
- 51.Wu T, et al. Palladium-catalyzed oxidative arylalkylation of activated alkenes: dual C-H bond cleavage of an arene and acetonitrile. Angew. Chem. Int. Ed. 2015;50:12578–12581. doi: 10.1002/anie.201104575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information file, and from the corresponding authors upon reasonable request. The characterization data are available in Supplementary Note 1 and NMR spectra are available in Supplementary Figs. 1~66. The supplementary crystallographic data (Supplementary Data 1) reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 2021450~2021452. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/. The calculated results are available in Supplementary Table 4. and XYZ co-ordinates for all optimized structures see Supplementary Data 2. The crystallographic informations of compounds 4, 11 and 17 are available in Supplementary Data 3–5.