Abstract
Molecules that exhibit multiple resonance (MR) type thermally activated delayed fluorescence (TADF) are highly efficient electroluminescent materials with narrow emission spectra. Despite their importance in various applications, the emission mechanism is still controversial. Here, a comprehensive understanding of the mechanism for a representative MR-TADF molecule (5,9-diphenyl-5,9-diaza-13b-boranaphtho[3,2,1-de]anthracene, DABNA-1) is presented. Using the equation-of-motion coupled-cluster singles and doubles method and Fermi’s golden rule, we quantitatively reproduced all rate constants relevant to the emission mechanism; prompt and delayed fluorescence, internal conversion (IC), intersystem crossing, and reverse intersystem crossing (RISC). In addition, the photoluminescence quantum yield and its prompt and delayed contributions were quantified by calculating the population kinetics of excited states and the transient photoluminescence decay curve. The calculations also revealed that TADF occurred via a stepwise process of 1) thermally activated IC from the electronically excited lowest triplet state T1 to the second-lowest triplet state T2, 2) RISC from T2 to the lowest excited singlet state S1, and 3) fluorescence from S1.
Subject terms: Electronic devices, Electronic materials, Excited states, Quantum chemistry
Molecules that exhibit multiple resonance thermally activated delayed fluorescence (MR-TADF) are highly efficient electroluminescent materials, but their emission mechanism is still not fully understood. Here, the emission mechanism of a representative MR-TADF molecule is studied through quantum chemistry calculations, with two consecutive decay processes unveiled.
Introduction
Since highly efficient thermally activated delayed fluorescence (TADF) was used in organic light-emitting diodes (OLEDs)1, many TADF molecules containing various electron donor and acceptor groups have been developed2–9. An important factor for TADF efficiency is the energy difference between the lowest triplet state (T1) and the lowest excited singlet state (S1), ∆E(T1 → S1); a small ∆E(T1 → S1) (<200 meV) is required to induce T1 → S1 reverse intersystem crossing (RISC). Another important factor is a large transition-dipole moment between S1 and ground-state S0 to accelerate the rate of S1 → S0 fluorescence. Experimentally, efficient RISC and high photoluminescence quantum yields (PLQYs) have been simultaneously realized by combining suitable donor and acceptor units that control the spatial overlap between the highest-occupied molecular orbitals (HOMOs) and the lowest-unoccupied molecular orbitals (LUMOs)1–9. It is important to note that the fluorescence spectra were broadened because of the charge-transfer character of S1, which was a significant drawback for OLED applications in displays.
In 2016, Hatakeyama et al. developed a new class of TADF molecules10. Using a triphenylboron core possessing two nitrogen atoms (5,9-diphenyl-5,9-diaza-13b-boranaphtho[3,2,1-de]anthracene, DABNA-1, Fig. 1a), they observed a HOMO–LUMO separation without a conventional donor–acceptor structure, providing efficient TADF with a narrow emission spectrum. The HOMO–LUMO separation resulted from a multiple resonance (MR) effect, that is an opposite resonance effect induced by the boron and nitrogen atoms. MR–TADF molecules emitting blue-to-red fluorescence have been reported11–39.
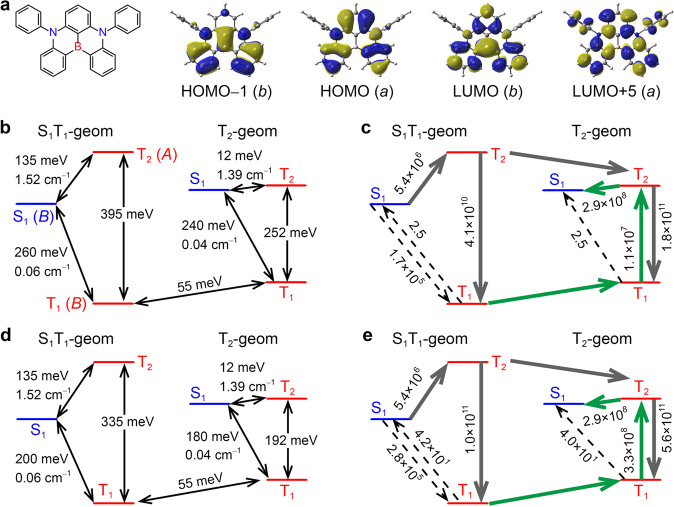
Fig. 1. Molecular orbitals and electronic transitions of DABNA-1.
a Structure of DABNA-1 and HOMO-1, HOMO, LUMO, and LUMO+5 distributions calculated at the RHF/6-31 G level (HOMO = highest-occupied molecular orbital, LUMO = lowest-unoccupied molecular orbital). The orbital symmetry is shown in parentheses. b Energy differences (meV) between S1, T1, and T2. S1–T1 and S1–T2 spin–orbit couplings (cm−1). The state symmetry is shown in parentheses. c The stepwise S1 → T2 → T1 and T1 → T2 → S1 processes of DABNA-1. The gray solid arrow depicts stepwise S1 → T2 → T1; the green solid arrow depicts stepwise T1 → T2 → S1; the dotted arrows show minor decay and upconversion processes. The values are calculated rate constants for one-step transitions. d The energy differences calculated using the corrected T1 energy. e Calculated rate constants for one-step transitions using the corrected T1 energy.
Several theoretical studies have attempted to reveal the TADF mechanism in MR molecules. Northey et al. investigated an intersystem-crossing (ISC) mechanism in DABNA-1 using quantum dynamics and time-dependent density-functional theory (TD-DFT) at the PBE0/6-31 G(d) level40. There was only a 0.02-eV energy difference between T2 and S1, ∆E(T2 → S1). However, ∆E(T1 → S1) and ∆E(T1 → T2) were large, 0.59 eV and 0.61 eV, respectively, which could not explain efficient RISC from T1 to S1. Gao et al. examined the density-functional dependence of ∆E(T1 → S1) within the framework of TD-DFT41. The MPWK1CIS functional reproduced the experimental ∆E(T1 → S1)41, as reviewed by Suresh et al.21. However, the RISC rate constant (kRISC) was not calculated and the TADF mechanism was unclear. Also, they considered TADF in terms of direct (one-step) T1 → S1 RISC, which differed from the work of Northey et al40. Pershin et al. reported that TD-DFT methods overestimated ∆E(T1 → S1) for MR molecules, and that the spin-component-scaling second-order approximate coupled-cluster (SCS-CC2) method outperformed TD-DFT for predicting ∆E(T1 → S1)42,43. The partial inclusion of double excitations within the SCS-CC2 method was responsible for the improved accuracy in predicting ∆E(T1 → S1). The SCS-CC2 calculation of DABNA-1-based molecules revealed the relationship between its molecular structure and electronic properties, ∆E(T1 → S1), and the fluorescence rate constant. However, the TADF mechanism was still unclear because the kRISC was not calculated. Lin et al. calculated rate constants for fluorescence (kF), ISC (kISC), kRISC, and internal conversion (IC) (kIC) for DABNA-144. However, the calculated kRISC was 6.7 × 102 s−1, which was much less than the experimental value of 1.0 × 104 s−110. The calculated kISC of 1.4 × 104 s−1 was two orders of magnitude less than the experimental value of 4.5 × 106 s−110. In addition, the calculated nonradiative decay, kIC(S1 → S0), was greater than the kF(S1 → S0), suggesting that the PLQY of DABNA-1 was less than 50%, in contrast with the 88% experimental value10. All these previous studies40–44 only partially explained the photophysical properties of MR-TADF mainly because crucial RISC was not elucidated.
Here, we report a comprehensive understanding of the TADF mechanism using the representative MR molecule, DABNA-1. So far, ∆E(T1 → S1) (and spin–orbit coupling (SOC) in some cases) and oscillator strength have been considered to understand the TADF mechanism and to design TADF molecules. However, these are not sufficient for the above aim; quantifications of all types of rate constants and those of energy levels, including higher-lying states relevant to the emission process, are required for the complete understanding of the emission mechanism. Recently, we reported rate-constant predictions enabled by a proposed cost-effective calculation method based on Fermi’s golden rule45. Using the method, we calculated kIC, kISC, kRISC, kF, and the rate constant for phosphorescence (kPhos), of benzophenone as an example. The calculated rate constants agreed well with experimental values. Here, we applied the same method to DABNA-1 and determined all relevant rate constants. We also determined the lifetime of the delayed component in transient PL (trPL), the rate constant of TADF (kTADF), and the PLQY, by calculating the population kinetics of the excited states and the trPL decay curve. The calculations also indicated that, after photoexcitation, T1 was first generated via S1 → T2 ISC and T2 → T1 IC. Then, triplet-to-singlet conversion occurred via thermally activated T1 → T2 IC and T2 → S1 RISC.
Results and discussion
Excited states of DABNA-1
S0, S1, T1, and T2 geometries of DABNA-1 were obtained at the TPSSh/6-31+G(d) level. Then, excited states were computed using the equation-of-motion coupled-cluster singles and doubles (EOM-CCSD) method (see “Methods” section). At the TD-TPSSh level, S1 and T1 local-energy minima were located at the S1- and T1-optimized geometries, respectively. However, as shown in Supplementary Table 10–12 and Supplementary Fig. 3, the lowest S1 and T1 energy levels calculated at the EOM-CCSD level were both located at the T1 geometry (3.37 and 3.12 eV, respectively). In addition, the S1 and T1 structures were nearly the same, including the dihedral angles (Supplementary Table 5), hence, the structure was denoted the S1T1 geometry. Because the Stokes shift was experimentally observed for DABNA-1 in solution and in solid films10, it was reasonably assumed that fluorescence occurs in the S1 structure at the adiabatic energy minimum. The S0, S1, T1, and T2 electronic states and the S1T1 and T2 equilibrium geometries were used to model TADF for DABNA-1 (eight electronic states in all). Because S2 states were 0.89 eV higher in energy than the S1 states, their contributions were neglected (Supplementary Fig. 3). The T3 states were only 0.2 eV higher than S1, but were neglected because the electronic-transition rate constants from S1 to T3 were far smaller than those of the competing processes (S1–T1 and S1–T2) and that from T2 to T3 was smaller than that from T2 to T1 (Supplementary Fig. 3). In the EOM-CCSD calculations, S1 and T1 involved predominantly HOMO → LUMO transitions, whereas T2 involved predominantly a linear combination of HOMO → LUMO + 5 and HOMO − 1 → LUMO transitions (Fig. 1a, Supplementary Fig. 2, and Supplementary Tables 6–9). Figure 1b shows a calculated energy-level diagram of DABNA-1. The adiabatic T1 → S1 energy difference calculated with the EOM-CCSD/6-31 G method (S1T1 geometry) was only 0.06 eV larger than the experimentally obtained 0.2 eV. Thus, EOM-CCSD significantly improved the overestimation of ∆E(T1 → S1), relative to those by previously reported TD-DFT methods40,42–44.
Calculation of rate constants and luminescence quantum efficiencies
We examined triplet formation from S1. Figure 1b shows calculated energy differences and SOCs, and Table 1 lists calculated and experimental values of kIC, kISC, kRISC, kF, and kPhos. The raw rate constants in Table 1 were calculated using excitation energies calculated via EOM-CCSD. Corrected values are discussed below. Equations for kIC, kISC, kRISC, kF, and kPhos are given in Supplementary Equations S1–S9 and Supplementary Fig. 4.
Table 1.
Calculated and experimental photophysical properties of DABNA-1.
| Calc. | Expt. | ||
|---|---|---|---|
| Raw* | Corrected** | ||
| ∆E(T1 → S1) | 240 | 180 | |
| ∆E(T2 → S1) | −12 | −12 | |
| ∆E(T1 → T2) | 252 | 192 | 196 |
| kF(S1 → S0) | 1.4 × 108 s−1 | 1.4 × 108 s−1 | 1.0 × 108 s−1 |
| kIC(S1 → S0) | 1.2 × 107 s−1 | 1.2 × 107 s−1 | 1.3 × 107 s−1 |
| kISC(S1 → T1) | 1.7 × 105 s−1 | 2.8 × 105 s−1 | |
| kISC(S1 → T2) | 5.4 × 106 s−1 | 5.4 × 106 s−1 | |
| kIC(T2 →T1) | 1.8 × 1011 s−1 | 5.6 × 1011 s−1 | |
| kRISC(T2 → S1) | 2.9 × 108 s−1 | 2.9 × 108 s−1 | |
| kPhos(T1 → S0) | 9.4 × 10−1 s−1 | 1.9 × 10−1 s−1 | |
| kIC(T1 → T2) | 1.1 × 107 s−1 | 3.3 × 108 s−1 | |
| kRISC(T1 → S1) | 2.5 s−1 | 4.2 × 101 s−1 | |
| Φ | 0.92 | 0.92 | 0.88 |
| Φp | 0.89 | 0.89 | 0.85 |
| ΦTADF | 2.9 × 10−2 | 3.2 × 10−2 | 3.5 × 10−2 |
| ΦISC | 3.5 × 10−2 | 3.5 × 10−2 | 4.0 × 10−2 |
| τTADF | 5.2 × 10−4 s | 5.1 × 10−5 s | 9.4 × 10−5 s |
| kTADF | 0.16 × 104 s−1 | 1.8 × 104 s−1 | 0.94 × 104 s−1 |
| k(T1 → T2 → S1) | 0.17 × 104 s−1 | 1.9 × 104 s−1 | 1.0 × 104 s−1 |
| k(S1 → T2 → T1) | 5.4 × 106 s−1 | 5.4 × 106 s−1 | 4.5 × 106 s−1 |
Calculated values of kF(S1 → S0) (1.4 × 108 s−1) and kIC(S1 → S0) (1.2 × 107 s−1) agreed quantitatively with experimental results (1.0 × 108 s−1 and 1.3 × 107 s−1, respectively) determined by Hatakeyama et al.10 from PL decay curves of a 1-wt% DABNA-1: 9,9′-biphenyl-3,3′-diylbis-9H-carbazole film at 300 K (Table 1). The experimentally obtained kISC value (4.5 × 106 s−1) agreed with kISC(S1 → T2) (5.4 × 106 s−1), rather than kISC(S1 → T1) (1.7 × 105 s−1), suggesting that the experimental kISC should be assigned to kISC(S1 → T2). The kISC(S1 → T2) was ten times greater than kISC(S1 → T1), because of the larger S1–T2 SOC (1.52 cm−1) relative to that of S1–T1 (0.06 cm−1). The large S1 → T2 SOC enhanced S1 → T2 ISC, despite the uphill transition from S1 to T2 (the Franck–Condon energy difference was 135 meV, Fig. 1b and Supplementary Table 13), compared with the downhill transition from S1 to T1 (260 meV). The small S1–T1 SOC resulted from very similar S1 and T1 orbital configurations (HOMO → LUMO transition). These results suggested that the ISC (S1 → T1 conversion) of DABNA-1 occurred via the stepwise S1 → T2 → T1 process, rather than by the one-step S1 → T1 ISC, irrespective of the geometry relaxation (gray arrows in Fig. 1c). Such T2-supported ISC is experimentally observed for a TADF system when ∆E(S1 → T2) is sufficiently small46. Because T2 → T1 IC was much faster than S1 → T2 ISC, the latter was the rate-determining step of the S1 → T2 → T1 process (Fig. 1c). Hence, the net rate constant k(S1 → T2 → T1) was approximately equal to kISC (S1 → T2) = 5.4 × 106 s−1 (Table 1).
For RISC from T1, the calculated kRISC(T1 → S1) (2.5 s−1) was considerably less than the experimental value (1 × 104 s−1)10, indicating that T1 → S1 conversion did not occur in one-step but via a more effective process. Because the S2 and T3 energy levels, as well as higher singlet and triplet states, could not contribute to the RISC from T1, as discussed above, and T2 was close to S1 (see Supplementary Fig. 3), stepwise T1 → T2 → S1 was the most promising mechanism (green arrows in Fig. 1c). To determine k(T1 → T2 → S1), we calculated the trPL decay curve (Fig. 2) via kinetic equations (Supplementary Equations S10–S17, Supplementary Table 15, and Supplementary Note 1). The total PLQY (Φ) and the TADF lifetime (τTADF) were then obtained from the trPL decay curve (Fig. 2). The prompt and delayed components of Φ (ΦP and ΦTADF, respectively) were calculated from
| 1 |
| 2 |
and the ISC quantum yield (ΦISC) was calculated from
| 3 |
Fig. 2. Calculated transient photoluminescence (trPL) decay curve.
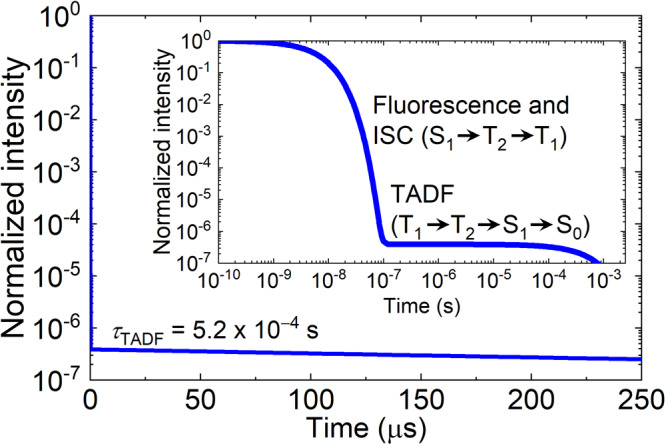
The inset is a log–log plot of the decay curve.
Then, kTADF was determined from10
| 4 |
Finally, k(T1 → T2 → S1) was calculated from
| 5 |
Table 1 also lists calculated Φ, ΦP, ΦTADF, ΦISC, τTADF, kTADF, k(T1 → T2 → S1), and k(S1 → T2 → T1) values. The Φ, ΦP, ΦTADF, and ΦISC values were quantitatively consistent with the experimental values because of the quantitative predictions for kF(S1 → S0), kIC(S1 → S0), kISC(S1 → T2), and kISC(S1 → T1). The calculated conversion rate constant k(S1 → T2 → T1) was consistent with the experimental , suggesting that the experimental ISC should be assigned to S1 → T2 → T1 ISC rather than direct S1 → T1 ISC. All the calculated single-step rate constants and k(S1 → T2 → T1) agreed well with the experimental values. However, the agreements of the calculated kTADF and k(T1 → T2 → S1) (0.16 × 104 s−1 and 0.17 × 104 s−1, respectively) and the experiments (0.94 × 104 s−1 and 1.0 × 104 s−1, respectively) were not as good as we expected, compared with the case of k(S1 → T2 → T1). Considering that they included the uphill-energy T1 → T2 IC, there was a possibility that the overestimation of ∆E(T1 → T2) leads to an underestimation of kIC(T1 → T2), kTADF, and k(T1 → T2 → S1).
To examine the effect of ∆E(T1 → T2) on kIC(T1 → T2), kTADF, and k(T1 → T2 → S1), we recalculated these rate constants using a corrected ∆E(T1 → T2). The rate-determining step for the T2-mediated RISC was T1 → T2 IC; hence, ∆E(T1 → T2) could be viewed as the activation energy for DABNA-1 TADF and RISC. As discussed above, the energy difference between the calculated and experimental T1 → S1 was 60 meV. Thus, 60 meV was subtracted from the EOM-CCSD-calculated ∆E(T1 → T2) value of 252 meV. The energy levels and rate constants calculated with the corrected ∆E(T1 → T2) are also listed in Table 1. The value of k(S1 → T2 → T1) was unchanged, suggesting that the T1 energy correction had little effect on the ISC. In contrast, kIC(T1 → T2) increased from 1.1 × 107 s−1 to 3.3 × 108 s−1. As a result, kTADF and k(T1 → T2 → S1) increased to 1.8 × 104 s−1 and 1.9 × 104 s−1, respectively, which were in much better agreements with the experiments. These results suggested that the ∆E(T1 → T2) overestimation was responsible for the underestimation of kTADF and k(T1 → T2 → S1). Thus, kTADF and k(T1 → T2 → S1) depended largely on kIC(T1 → T2), which was the sum of IC rate constants for individual molecular vibrations, kIC,α(T1 → T2), where α denoted the αth vibrational mode (Supplementary Equations S5–S7). A combined in-plane and out-of-plane bending mode (743 cm−1) had the largest kIC,α(T1 → T2) value (Supplementary Table 14 and Supplementary Fig. 5), suggesting that the T1 → T2 IC was predominantly accelerated by the bending mode.
Summary
In this work, we calculated the IC, ISC, RISC, fluorescence, phosphorescence, and TADF rate constants for DABNA-1, using EOM-CCSD wave functions and Fermi’s golden rule. The values quantitatively reproduced those from experiments, and thus validated the calculations. Various quantum yields, including PLQY, were also predicted and we revealed the DABNA-1 decay mechanism. The calculated population kinetics and the trPL decay curve indicated that TADF from DABNA-1 occurs via consecutive two processes, T1 → T2 IC, T2 → S1 RISC, and S1 → S0 radiative decay. Our proposed method here will be useful for accurate prediction of all rate constants and quantum yields relevant to OLED phenomena for various compounds with a wide range of structures.
Methods
Calculation of matrix elements between vibronic states
Geometry optimization and frequency analysis of S0 for DABNA-1 were performed using the TPSSh/6-31+G(d) method, whereas those of S1, T1, and T2 were performed using the TD-TPSSh/6-31+G(d) method with spin multiplicity = 1 (Supplementary Table 1–5 and Supplementary Fig. 1). The polarizable continuum model (PCM) of a CH2Cl2 solvent was used to consider the effects on vibronic states. We used the functional and the PCM conditions for ground- and excited-state geometry optimizations because Gao et al. reported that the TPSSh/6-31+G(d)-PCM model reproduced experimental DABNA-1 emission and absorption wavelengths in CH2Cl241. The geometry optimization and frequency analysis were performed with the Gaussian 16 program package47. For MR molecules, EOM-CCSD method and algebraic diagrammatic construction of the second order, have been shown to be suitable for calculating singlet–triplet energy differences42,48–50. Here, excited-state calculations were performed using the EOM-CCSD/6-31 G method implemented in the Q-Chem program package51. Excitation energies, SOCs, vibronic couplings, transition-dipole moments, and permanent dipole moments were calculated using EOM-CCSD wave functions. Examples of Q-Chem input files for running SOC calculations are shown in the Supplementary Methods.
Calculation of IC rate constant
Mathematical formulation of a method of calculating vibronic couplings and kIC is described elsewhere45,52 and reviewed in Supplementary Equation S5–S9. First, for each vibrational mode α and each transition, and were obtained from the EOM-CCSD calculations (Supplementary Equation S7) and was obtained from the frequency analyses performed using the TPSSh/6-31+G(d)-PCM model (Supplementary Equations S8 and S9). Then, was calculated (Supplementary Equation S6). Finally, was calculated by summing over α (Supplementary Equation S5). This protocol was also used for calculating the IC rate constants for transitions.
Supplementary information
Acknowledgements
The quantum chemical calculations using the Gaussian 16 and Q-Chem program packages were performed on the SuperComputer System, Institute for Chemical Research, Kyoto University. It was also supported by JSPS KAKENHI grant numbers: 19K05629 and JP20H05840 (Grant-in-Aid for Transformative Research Areas, “Dynamic Exciton”). We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this paper.
Author contributions
K.S. performed the theoretical calculations. H.K. planned and supervised the project. All authors contributed to the writing of this paper and have approved the final version.
Peer review
Peer-review information
Communications Chemistry thanks Jianzhong Fan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer-reviewer reports are available.
Data availability
All relevant data are available from the authors upon request.
Code availability
The code used to generate Fig. 2 is available from the authors upon request.
Competing interests
The authors have no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-022-00668-6.
References
- 1.Uoyama H, Goushi K, Shizu K, Nomura H, Adachi C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature. 2012;492:234–238. doi: 10.1038/nature11687. [DOI] [PubMed] [Google Scholar]
- 2.Adachi C. Third-generation organic electroluminescence materials. Jpn. J. Appl. Phys. 2014;53:060101. doi: 10.7567/JJAP.53.060101. [DOI] [Google Scholar]
- 3.Wong MY, Zysman-Colman E. Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Adv. Mater. 2017;29:1605444. doi: 10.1002/adma.201605444. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka H, Shizu K, Miyazaki H, Adachi C. Efficient green thermally activated delayed fluorescence (TADF) from a phenoxazine-triphenyltriazine (PXZ-TRZ) derivative. Chem. Commun. 2012;48:11392–11394. doi: 10.1039/c2cc36237f. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q, et al. Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes. J. Am. Chem. Soc. 2012;134:14706–14709. doi: 10.1021/ja306538w. [DOI] [PubMed] [Google Scholar]
- 6.Kaji H, et al. Purely organic electroluminescent material realizing 100% conversion from electricity to light. Nat. Commun. 2015;6:8476. doi: 10.1038/ncomms9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shizu K, et al. Strategy for designing electron donors for thermally activated delayed fluorescence emitters. J. Phys. Chem. C. 2015;119:1291–1297. doi: 10.1021/jp511061t. [DOI] [Google Scholar]
- 8.Shizu K, et al. Enhanced electroluminescence from a thermally activated delayed-fluorescence emitter by suppressing nonradiative decay. Phys. Rev. Appl. 2015;3:014001. doi: 10.1103/PhysRevApplied.3.014001. [DOI] [Google Scholar]
- 9.Wada Y, Nakagawa H, Matsumoto S, Wakisaka Y, Kaji H. Organic light emitters exhibiting very fast reverse intersystem crossing. Nat. Photon. 2020;14:643–649. doi: 10.1038/s41566-020-0667-0. [DOI] [Google Scholar]
- 10.Hatakeyama T, et al. Ultrapure blue thermally activated delayed fluorescence molecules: efficient HOMO–LUMO separation by the multiple resonance effect. Adv. Mater. 2016;28:2777–2781. doi: 10.1002/adma.201505491. [DOI] [PubMed] [Google Scholar]
- 11.Nakatsuka S, Gotoh H, Kinoshita K, Yasuda N, Hatakeyama T. Divergent synthesis of heteroatom-centered 4,8,12-triazatriangulenes. Angew. Chem. Int. Ed. 2017;56:5087–5090. doi: 10.1002/anie.201701246. [DOI] [PubMed] [Google Scholar]
- 12.Matsui K, et al. One-shot multiple borylation toward BN-doped nanographenes. J. Am. Chem. Soc. 2018;140:1195–1198. doi: 10.1021/jacs.7b10578. [DOI] [PubMed] [Google Scholar]
- 13.Liang X, et al. Peripheral amplification of multi-resonance induced thermally activated delayed fluorescence for highly efficient OLEDs. Angew. Chem. Int. Ed. 2018;57:11316–11320. doi: 10.1002/anie.201806323. [DOI] [PubMed] [Google Scholar]
- 14.Han SH, Jeong JH, Yoo JW, Lee JY. Ideal blue thermally activated delayed fluorescence emission assisted by a thermally activated delayed fluorescence assistant dopant through a fast reverse intersystem crossing mediated cascade energy transfer process. J. Mater. Chem. C. 2019;7:3082–3089. doi: 10.1039/C8TC06575F. [DOI] [Google Scholar]
- 15.Kondo Y, et al. Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter. Nat. Photon. 2019;13:678–682. doi: 10.1038/s41566-019-0476-5. [DOI] [Google Scholar]
- 16.Oda S, Kawakami B, Kawasumi R, Okita R, Hatakeyama T. Multiple resonance effect-induced sky-blue thermally activated delayed fluorescence with a narrow emission band. Org. Lett. 2019;21:9311–9314. doi: 10.1021/acs.orglett.9b03342. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, et al. Multi-resonance induced thermally activated delayed fluorophores for narrowband green OLEDs. Angew. Chem. Int. Ed. 2019;58:16912–16917. doi: 10.1002/anie.201911266. [DOI] [PubMed] [Google Scholar]
- 18.Suresh SM, et al. A deep blue B,N-doped heptacene emitter that shows both thermally activated delayed fluorescence and delayed fluorescence by triplet–triplet annihilation. J. Am. Chem. Soc. 2020;142:6588–6599. doi: 10.1021/jacs.9b13704. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, et al. Achieving pure green electroluminescence with CIEy of 0.69 and EQE of 28.2% from an Aza-fused multi-resonance emitter. Angew. Chem. Int. Ed. 2020;59:17499–17503. doi: 10.1002/anie.202008264. [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Park IS, Yasuda T. Full-color, narrowband, and high-efficiency electroluminescence from boron and carbazole embedded polycyclic heteroaromatics. J. Am. Chem. Soc. 2020;142:19468–19472. doi: 10.1021/jacs.0c10081. [DOI] [PubMed] [Google Scholar]
- 21.Madayanad Suresh S, Hall D, Beljonne D, Olivier Y, Zysman-Colman E. Multiresonant thermally activated delayed fluorescence emitters based on heteroatom-doped nanographenes: recent advances and prospects for organic light-emitting diodes. Adv. Funct. Mater. 2020;30:1908677. doi: 10.1002/adfm.201908677. [DOI] [Google Scholar]
- 22.Tanaka H, et al. Hypsochromic shift of multiple-resonance-induced thermally activated delayed fluorescence by oxygen atom incorporation. Angew. Chem. Int. Ed. 2021;60:17910–17914. doi: 10.1002/anie.202105032. [DOI] [PubMed] [Google Scholar]
- 23.Oda S, et al. Carbazole-based DABNA analogues as highly efficient thermally activated delayed fluorescence materials for narrowband organic light-emitting diodes. Angew. Chem. Int. Ed. 2021;60:2882–2886. doi: 10.1002/anie.202012891. [DOI] [PubMed] [Google Scholar]
- 24.Nagata M, et al. Fused-nonacyclic multi-resonance delayed fluorescence emitter based on ladder-thiaborin exhibiting narrowband sky-blue emission with accelerated reverse intersystem crossing. Angew. Chem. Int. Ed. 2021;60:20280–20285. doi: 10.1002/anie.202108283. [DOI] [PubMed] [Google Scholar]
- 25.Yang M, et al. Wide-range color tuning of narrowband emission in multi-resonance organoboron delayed fluorescence materials through rational imine/amine functionalization. Angew. Chem. Int. Ed. 2021;60:23142–23147. doi: 10.1002/anie.202109335. [DOI] [PubMed] [Google Scholar]
- 26.Yuan Y, et al. The design of fused amine/carbonyl system for efficient thermally activated delayed fluorescence: novel multiple resonance core and electron acceptor. Adv. Opt. Mater. 2019;7:1801536. doi: 10.1002/adom.201801536. [DOI] [Google Scholar]
- 27.Hall D, et al. Improving processability and efficiency of resonant TADF emitters: a design strategy. Adv. Opt. Mater. 2020;8:1901627. doi: 10.1002/adom.201901627. [DOI] [Google Scholar]
- 28.Naveen, K. R. et al. Achieving high efficiency and pure blue color in hyperfluorescence organic light emitting diodes using organo-boron based emitters. Adv. Funct. Mater. 32, 2110356 (2022).
- 29.Ikeda N, et al. Solution-processable pure green thermally activated delayed fluorescence emitter based on the multiple resonance effect. Adv. Mater. 2020;32:2004072. doi: 10.1002/adma.202004072. [DOI] [PubMed] [Google Scholar]
- 30.Sun D, et al. The design of an extended multiple resonance TADF emitter based on a polycyclic amine/carbonyl system. Mater. Chem. Front. 2020;4:2018–2022. doi: 10.1039/D0QM00190B. [DOI] [Google Scholar]
- 31.Liu G, et al. Facile synthesis of multi-resonance ultra-pure-green TADF emitters based on bridged diarylamine derivatives for efficient OLEDs with narrow emission. J. Mater. Chem. C. 2021;9:8308–8313. doi: 10.1039/D1TC01427G. [DOI] [Google Scholar]
- 32.Hua T, et al. Heavy-atom effect promotes multi-resonance thermally activated delayed fluorescence. Chem. Eng. J. 2021;426:131169. doi: 10.1016/j.cej.2021.131169. [DOI] [Google Scholar]
- 33.Jiang P, et al. Simple acridan-based multi-resonance structures enable highly efficient narrowband green TADF electroluminescence. Adv. Opt. Mater. 2021;9:2100825. doi: 10.1002/adom.202100825. [DOI] [Google Scholar]
- 34.Jiang P, et al. Quenching-resistant multiresonance TADF emitter realizes 40% external quantum efficiency in narrowband electroluminescence at high doping level. Adv. Mater. 2021;34:2106954. doi: 10.1002/adma.202106954. [DOI] [PubMed] [Google Scholar]
- 35.Wu X, et al. Fabrication of circularly polarized MR-TADF emitters with asymmetrical peripheral-lock enhancing helical B/N-doped nanographenes. Adv. Mater. 2022;34:2105080. doi: 10.1002/adma.202105080. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y, Wang Q, Cai X, Li C, Wang Y. Highly efficient electroluminescence from narrowband green circularly polarized multiple resonance thermally activated delayed fluorescence enantiomers. Adv. Mater. 2021;33:2100652. doi: 10.1002/adma.202100652. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, et al. Materials with high color purity based on strong acceptor attachment onto B–N-containing multiple resonance frameworks. CCS Chem. 2021;3:2077–2091. doi: 10.31635/ccschem.021.202101033. [DOI] [Google Scholar]
- 38.Liu Y, Xiao X, Ran Y, Bin Z, You J. Molecular design of thermally activated delayed fluorescent emitters for narrowband orange–red OLEDs boosted by a cyano-functionalization strategy. Chem. Sci. 2021;12:9408–9412. doi: 10.1039/D1SC02042K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, et al. Multi-resonance deep-red emitters with shallow potential-energy surfaces to surpass energy-gap law. Angew. Chem. Int. Ed. 2021;60:20498–20503. doi: 10.1002/anie.202107848. [DOI] [PubMed] [Google Scholar]
- 40.Northey T, Penfold TJ. The intersystem crossing mechanism of an ultrapure blue organoboron emitter. Org. Electron. 2018;59:45–48. doi: 10.1016/j.orgel.2018.04.038. [DOI] [Google Scholar]
- 41.Gao Y, et al. Realizing performance improvement of blue thermally activated delayed fluorescence molecule DABNA by introducing substituents on the para-position of boron atom. Chem. Phys. Lett. 2018;701:98–102. doi: 10.1016/j.cplett.2018.04.036. [DOI] [Google Scholar]
- 42.Pershin A, et al. Highly emissive excitons with reduced exchange energy in thermally activated delayed fluorescent molecules. Nat. Commun. 2019;10:597. doi: 10.1038/s41467-019-08495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall, D. et al. The modelling of multi-resonant thermally activated delayed fluorescence emitters – properly accounting for electron correlation is key! Preprint athttps://chemrxiv.org/engage/chemrxiv/article-details/6173bad70c048095df40e5f3 (2021).
- 44.Lin L, Fan J, Cai L, Wang C-K. Excited state dynamics of new-type thermally activated delayed fluorescence emitters: theoretical view of light-emitting mechanism. Mol. Phys. 2018;116:19–28. doi: 10.1080/00268976.2017.1362119. [DOI] [Google Scholar]
- 45.Shizu K, Kaji H. Theoretical determination of rate constants from excited states: application to benzophenone. J. Phys. Chem. A. 2021;125:9000–9010. doi: 10.1021/acs.jpca.1c06165. [DOI] [PubMed] [Google Scholar]
- 46.Han C, et al. Ladder-like energy-relaying exciplex enables 100% internal quantum efficiency of white TADF-based diodes in a single emissive layer. Nat. Commun. 2021;12:3640. doi: 10.1038/s41467-021-23941-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frisch, M. J. et al. Gaussian 16 Rev. C.01. (2016).
- 48.de Silva P. Inverted singlet–triplet gaps and their relevance to thermally activated delayed fluorescence. J. Phys. Chem. Lett. 2019;10:5674–5679. doi: 10.1021/acs.jpclett.9b02333. [DOI] [PubMed] [Google Scholar]
- 49.Pios S, Huang X, Sobolewski AL, Domcke W. Triangular boron carbon nitrides: an unexplored family of chromophores with unique properties for photocatalysis and optoelectronics. Phys. Chem. Chem. Phys. 2021;23:12968–12975. doi: 10.1039/D1CP02026A. [DOI] [PubMed] [Google Scholar]
- 50.Ehrmaier J, et al. Singlet–triplet inversion in heptazine and in polymeric carbon nitrides. J. Phys. Chem. A. 2019;123:8099–8108. doi: 10.1021/acs.jpca.9b06215. [DOI] [PubMed] [Google Scholar]
- 51.Shao Y, et al. Advances in molecular quantum chemistry contained in the Q-Chem 4 program package. Mol. Phys. 2015;113:184–215. doi: 10.1080/00268976.2014.952696. [DOI] [Google Scholar]
- 52.Shizu K, Adachi C, Kaji H. Effect of vibronic coupling on correlated triplet pair formation in the singlet fission process of linked tetracene dimers. J. Phys. Chem. A. 2020;124:3641–3651. doi: 10.1021/acs.jpca.0c03041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available from the authors upon request.
The code used to generate Fig. 2 is available from the authors upon request.