Abstract
Pseudomonas aeruginosa is a significant human pathogen, and no vaccine is commercially available. Passive antibody prophylaxis using monoclonal antibodies (MAb) against protective P. aeruginosa epitopes is an alternative strategy for preventing P. aeruginosa infection, but mouse MAb are not suitable for use in humans. Polyclonal human antibodies from multiple donors have variable antibody titers, and human MAb are difficult to make. We used immunoglobulin-inactivated transgenic mice reconstituted with megabase-size human immunoglobulin loci to generate a human MAb against the polysaccharide (PS) portion of the lipopolysaccharide O side chain of a common pathogenic serogroup of P. aeruginosa, 06ad. The anti-PS human immunoglobulin G2 MAb made from mice immunized with heat-killed P. aeruginosa was specific for serogroup 06ad pseudomonas. The MAb was highly opsonic for the uptake and killing of P. aeruginosa by human polymorphonuclear leukocytes in the presence of human complement. In addition, 25 μg of the MAb protected 100% of neutropenic mice from fatal P. aeruginosa sepsis. DNA sequence analysis of the genes encoding the MAb revealed VH3 and Vκ2/A2 variable-region genes, similar to variable-region genes in humans immunized with bacterial PS and associated with high-avidity anti-PS antibodies. We conclude that human MAb to P. aeruginosa made in these transgenic mice are highly protective and that these mice mimic the antibody response seen in humans immunized with T-cell-independent antigens such as bacterial PS.
Pseudomonas aeruginosa remains a serious pathogen in a variety of human patients, including patients with cancer and receiving chemotherapy, individuals with burns, patients in critical care units, and children with cystic fibrosis and AIDS (9, 28, 29, 30, 33, 34). Morbidity and mortality from infection with P. aeruginosa remain high despite the availability of antibiotics to which the organism is sensitive. In addition, there is no commercially available vaccine to prevent infection with the organism, and experimental vaccines against a variety of surface epitopes have been complicated by toxicity and/or poor or inconsistent immunogenicity in target populations (5, 7, 10, 11, 14, 25).
Passive administration of antibodies against protective pseudomonas epitopes is an attractive alternative to active vaccination of patients who are at risk for pseudomonas infections. Many patients susceptible to infection with pseudomonas are immunocompromised, do not respond well to active immunization, and may not make high-avidity, protective antibodies (17, 18, 25). Other patients may require rapid acquisition of high-titer antibodies to prevent colonization or infection after acute, intense exposure to pseudomonas in environments such as burn units or intensive care units, so that active immunization with the accompanying delay in the formation of antibodies may not be ideal.
Thus, passive administration of polyclonal antibodies or monoclonal antibodies (MAb) prior to infection with P. aeruginosa has the potential advantage of providing immediate high-titer antibodies to susceptible individuals. Passive administration of antibodies against O antigens of P. aeruginosa lipopolysaccharide (LPS) has been shown to be protective in numerous animal models of infection with P. aeruginosa (4, 16, 20, 24, 26, 44, 45). Human antibodies against these antigens would be ideal for use in at-risk patient groups. Polyclonal antibody preparations derived from healthy human donors, however, have been problematic due to variable titers of protective antibodies and the high cost of purifying antibodies from multiple donors (6, 42). MAb have the theoretical advantage of unlimited quantity, low cost, and custom-designed specificity. Mouse MAb against the polysaccharide (PS) portion of the pseudomonas LPS O side chain have been shown to be protective against sepsis and pneumonia and to facilitate clearance of the bacteria from the intestinal tract (4, 26, 36). Mouse MAb, however, are not suitable for repeated administration to humans, due to the induction of antibodies by foreign mouse proteins. Similarly, engineered mouse-human chimeric antibodies still contain some elements of mouse antibodies and are expensive to produce (23, 28). More recently, human MAb have been demonstrated to protect mice and guinea pigs from pseudomonas infection, and they have been well tolerated in human clinical trials (16, 33, 44, 45). However, human MAb are difficult to make, and most of the antibodies tested to date have been immunoglobulin (Ig) M (IgM), which penetrates poorly into pulmonary tissue and can be associated with immune complex formation and enhanced inflammation (16, 33).
Recently, another technique for manufacturing human MAb of an appropriate isotype and directed against selected antigens has become available. The use of Ig-inactivated mice that have been reconstituted with human Ig loci now allows the generation of entirely human MAb from immunized mice by standard hybridoma techniques (22). These XenoMouse (Abgenix) mice were constructed by introducing multiple megabase-size contiguous fragments of human Ig loci on yeast artificial chromosome transgenes into mice whose heavy- and light-chain Ig genes were inactivated by targeted deletion. These mice bear a majority of human heavy (66 VH) and kappa light (32 Vκ) variable genes in germ line configuration (12, 13, 22). Human MAb have been successfully made from XenoMouse animals against both protein and bacterial PS antigens, and antibody recombination, class switching, and affinity maturation appear to occur in a manner similar to that observed in humans (13, 22, 31).
In this report, we examine the functional capability of a human MAb generated in these transgenic mice and directed against the high-molecular-weight PS portion of the P. aeruginosa LPS O side chain. In particular, we address the hypothesis that a multivalent, human antipseudomonas MAb preparation could have practical use in the prevention of infection with P. aeruginosa in a variety of susceptible patients. Our data show that human anti-pseudomonas LPS antibodies made from these mice have high avidity, are opsonic for the uptake and killing of the bacteria by human polymorphonuclear leukocytes (PMN), and are highly efficacious in preventing mortality in the neutropenic mouse model of pseudomonas sepsis. We also show that anti-PS antibody variable (V)-region gene usage in these mice is similar to that observed in humans following immunization with PS vaccines.
MATERIALS AND METHODS
Bacterial strain.
P. aeruginosa serogroup 06ad was used for mouse immunizations, mouse protection experiments, and opsonic assays. Bacteria for mouse challenge experiments and the killing assay were freshly plated onto Pseudosel agar (Becton Dickinson, Sparks, Md.) and incubated at 37°C overnight. One CFU was picked from the plate, inoculated into Luria-Bertani (LB) broth, and incubated at 37°C in a shaking water bath to a concentration of 5 × 108 CFU/ml. Bacteria were centrifuged at 10,000 rpm for 10 min in a Sorvall RC5C centrifuge, resuspended, washed in chilled phosphate-buffered saline (PBS) three times, and diluted as needed. Bacteria for immunization experiments were grown as described above, heat killed at 60°C for 1 h, and stored at 4°C until use. Labeled bacteria used in the flow cytometry opsonic assays were grown and heat killed as described above. However, these bacteria were resuspended in alkaline conjugation buffer (a 1:3 solution of 0.5 M Na2CO3 and 0.5 M NaHCO3 [pH 9.5]) to give a concentration of 109/ml as described previously (31). An equal volume of alkaline conjugation buffer with 0.06% fluorescein isothiocyanate isomer I (FITC; Amresco, Solon, Ohio) was added and incubated for 20 h at room temperature with gentle shaking. Bacteria were washed in Veronal-buffered saline, resuspended in PBS at 109/ml, and stored at −80°C.
Antigens.
The high-molecular-weight PS (high MW PS) portions of the LPS O side chains from P. aeruginosa strains 06ad (International Typing System [IATS]), 011 (IATS), 016 (IATS 02a,b,e), 170003 (IATS 02a,b), and PAO1 Holloway (IATS 05) were made as previously described and were lyophilized for storage (14). These PSs were used to coat 96-well plates for enzyme-linked immunosorbent assays (ELISA) as described below. The 06ad high MW PS was also used in the blocking and avidity assays described below.
XenoMouse animal immunization and generation of hybridomas secreting anti-LPS antibodies.
Mice that were transgenic for human heavy and light Igs were bred and maintained by Abgenix, Inc., Fremont, Calif. The strain of Xeno-Mouse animals used was XMG2, which is an Ig-inactivated mouse strain reconstituted with a yeast artificial chromosome containing cointegrated human heavy- and light-chain Ig transgenes (22). This mouse strain has been reconstituted with only one human IgG constant region (IgG2). Mice were housed in microisolator cages in a pathogen-free facility after shipping, and food, bedding, and water were autoclaved prior to use. Mice were immunized with 107 heat-killed 06ad P. aeruginosa twice per week intraperitoneally (i.p.) (107 bacteria in PBS) (21) and/or in the footpad (107 bacteria and RIBI adjuvant; Sigma-Aldrich, St. Louis, Mo.). Their sera were screened for 06ad high MW PS O-side-chain antibodies by the ELISA described below.
Hybridomas were generated by fusing spleen and/or lymph node cells from immunized, seropositive XenoMouse animals with the nonsecreting sp2/0 myeloma cell line as described previously (22, 36). Supernatants from hybridomas were screened for the production of human MAb against the 06ad high MW PS using the ELISA procedure described below. Hybridomas found to be secreting IgG anti-PS antibodies were then cloned three times by limiting dilution. One IgG2-secreting clone, S20, was chosen for further experiments based on initial experiments measuring the strength of binding to solid-phase 06ad PS.
Detection of antibodies to LPS O side chains.
An ELISA was used to detect antibodies to the 06ad high MW PS in sera of immunized mice and in hybridoma supernatants as previously described (36). Briefly, each well of 96-well microtiter polystyrene plates (Nalge Nunc, Naperville, Ill.) was coated with 100 μl of 06ad high MW PS (2 μg/ml) overnight at 4°C. Plates were washed, and each well was blocked with 200 μl of 1% bovine serum albumin (Sigma-Aldrich) in PBS–0.05% Tween 20 (Amresco). Plates were washed and incubated overnight with serial dilutions of MAb or sera in 1% bovine serum albumin in PBS. Plates were washed, and bound antibodies were detected by adding isotype-specific alkaline phosphatase-conjugated mouse anti-human polyclonal antibodies (Southern Biotechnology Associates, Birmingham, Ala.). Each well of the plates was developed with 100 μl of p-nitrophenylphosphate (Sigma-Aldrich) chromogenic substrate in diethanol amine buffer. Optical densities were measured at 415 nm with a microplate reader (Bio-Rad, Hercules, Calif.).
Blocking assays to determine MAb specificity were performed in an identical fashion except that soluble 06ad high MW PS or control PS at different concentrations was added to the MAb prior to addition to PS-coated 96-well ELISA plates. Similarly, the cross-reactivity ELISA was run in an identical fashion except that PSs from P. aeruginosa 011, 016, 170003, and PA01 Holloway were used to coat plates in addition to 06ad, and 2.5 μg of human MAb/ml was then added. The relative avidities of the MAb were calculated with an assay similar to one that has been previously described (3, 35, 41). Antibodies were added to the wells of a high MW PS-coated ELISA plate, followed by serial dilutions of 06ad high MW PS or equal volumes of PBS (negative control). The concentration of PS required to inhibit 50% of the maximum absorbance was calculated, and the inverse of this value was used to represent relative avidity.
Characterization of V-region genes.
Dideoxy DNA sequencing was performed as previously described to determine the sequences of the V regions of the human MAb (32, 35). Total RNA was isolated from hybridoma cells from nine different clones using TRIZOL reagent (Gibco BRL) and converted into randomly primed cDNA for use as a template in PCR. Human heavy-chain and light-chain V regions were amplified using degenerate leader peptide primers and constant-region primers provided in the Human Ig-Primer Set (Novagen, Madison, Wis.). The PCR products were analyzed on a Tris-acetate-EDTA agarose gel. Samples positive in the PCRs were extracted with chloroform-isoamyl alcohol (24:1) and cloned into the EcoRI site of pT7Blue (Novagen) (39). The clones were sequenced by the dideoxy method with a Sequenase V2.0 DNA sequencing kit (U.S. Biochemical Corp., Cleveland, Ohio). Gene usage analysis was performed using the Vbase database (Medical Research Council Centre for Protein Engineering, Cambridge, United Kingdom; www.mrc-cpe.cam.ac.uk).
Antibody-mediated, complement-dependent opsonization.
The ability of the human MAb to opsonize P. aeruginosa 06ad for uptake by human PMN was measured by flow cytometry as previously described (35). P. aeruginosa 06ad was grown, heat killed, and FITC labeled as described above. Opsonization was carried out by incubating the labeled bacteria with MAb with or without 1% human serum from an agammaglobulinemic patient as the complement source. Bacteria were washed in PBS containing 6% dextran and 0.2% glucose and then were resuspended in Hanks balanced salt solution with 0.1% gelatin. PMN were isolated from peripheral human blood via venipuncture of healthy adult volunteers as previously described (35, 40) in accordance with the institutional review board guidelines of the University Hospitals of Cleveland. PMN were resuspended to achieve a concentration of 107 cells/ml and were activated for 30 min with 10 μl of a 10−6 dilution of N-formyl-Met-Leu-Phe (FMLP; Peninsula Laboratories, San Carlos, Calif.) per ml of cells. PMN were added to each opsonized bacterial sample, incubated at 37°C, separated from free bacteria by differential centrifugation, and resuspended in PBS. Single-color flow cytometric analysis of PMN was performed utilizing a FACScan and CellQuest software (Becton Dickinson, Mountain View, Calif.), and phagocytosis was expressed in relative units of mean fluorescence of 10,000 PMN for each sample. To demonstrate that the observed opsonophagocytosis was associated with bacterial killing, an alternative assay was used in which 25,000 CFU of live 06ad P. aeruginosa were mixed with agammaglobulinemic human serum, various concentrations of MAb, and 106 fresh human PMN obtained as described above in RPMI medium (400-μl final volume). Samples were obtained at the beginning and end of a 90-min 37°C incubation, after which bacteria were diluted and then plated for bacterial enumeration (37).
Protection of neutropenic mice from fatal sepsis.
The protective efficacy of the human MAb against invasive infection with P. aeruginosa was measured with the neutropenic mouse model as described previously (27, 36). Female 6-week-old BALB/c ByJ mice (Jackson Laboratories, Bar Harbor, Maine) were maintained in a pathogen-free, pseudomonas-free environment in which water, bedding, and food were autoclaved prior to use. Neutropenia was established by administering 3 mg of cyclophosphamide (Cytoxan; Bristol-Myers Squibb, Princeton, N.J.) i.p. to each mouse on days 1, 3, and 5. On day 5, the cyclophosphamide was administered at time zero; 2 h later, 25 μg of antibody was administered i.p.; and 103 CFU of live 06ad P. aeruginosa was administered 2 h later. Negative control mice received either PBS or an irrelevant MAb at the same dose (MOPC 21; Sigma-Alrich). Positive controls received mouse MAb D8, directed against the O-side-chain PS from the same pseudomonas serotype and previously reported to protect neutropenic mice from fatal sepsis (36). Mice were observed daily thereafter for 5 days, and cumulative mortality was the outcome measured.
Statistics.
Comparison of mortality between treatment groups in the neutropenic mouse experiments was performed using Fisher's exact test and the StatView statistical program for MacIntosh, version 4.5 (Abacus Concepts, Inc., Berkeley, Calif.).
RESULTS
V-region gene usage in transgenic mouse-derived anti-LPS antibodies.
Immunization of the transgenic mice with heat-killed 06ad P. aeruginosa resulted in the production of IgM and IgG2 human antibodies directed to the 06ad LPS O side chain, consistent with the constant-region reconstitution of these mice (data not shown). V-region genes from hybridomas obtained from the fusion of spleen cells from P. aeruginosa-immunized transgenic mice with the nonsecreting sp2/0 cell line were cloned and sequenced in order to determine V-region gene usage. The protective IgG2 anti-LPS MAb that was made in the human Ig transgenic mice and that was chosen for further study utilized genes from the VH3 gene family, similar to the restricted VH gene usage described after immunization of humans with a variety of bacterial PSs (GenBank accession number AF332470) (3, 8, 19, 38). Nine other MAb made in fusion experiments with spleen cells from pseudomonas-immunized transgenic mice yielded antibodies that also used the VH3 gene family for the heavy-chain gene elements, as determined by DNA sequencing (data not shown). More specifically, DNA sequence analysis showed that the VH3/V3–33 and JH4 genes were used in the protective anti-LPS MAb, similar to the gene usage in antipneumococcal MAb made in the same transgenic XenoMouse mice (31). Similarly, the light-chain gene segments used were Vκ2/A2 and Jκ1, also previously reported as being commonly used in humans after PS vaccine immunization (GenBank accession number AF332471) (3). In summary, gene usage for anti-LPS antibodies after P. aeruginosa immunization in these transgenic mice appeared to be remarkably similar to that observed in humans after PS immunization.
Human MAb binds to the LPS O side chain of P. aeruginosa strain 06ad.
The IgG2 human MAb produced in the transgenic mice bound to the O side chain of P. aeruginosa strain 06ad. Blocking assays revealed over a 90% reduction in the binding of the MAb to solid-phase 06ad LPS high MW PS after preincubation of the MAb with the same PS, compared to less than 10% inhibition with the control PS (purified type 6B pneumococcal capsular PS) (Fig. 1). The MAb also bound to Fisher Devlin immunotype 1 P. aeruginosa, currently considered part of the 06 serogroup (data not shown). The MAb, however, did not cross-react with LPSs from other P. aeruginosa serotypes, since no binding could be demonstrated to solid-phase LPS O-side-chain high MW PS from a variety of pseudomonas strains, including IATS 011, IATS 016, 170003, and PAO1 Holloway (Fig. 2). The concentration of high MW PS that inhibited 50% of the maximum absorbance of MAb binding to high MW PS was determined and the inverse of this value was used to calculate the relative avidity of the MAb. Human anti-PS antibodies are often of low relative avidity, particularly after immunization with pure PS vaccines. We found, unexpectedly, however, that the relative avidity of the human anti-LPS MAb (316 × 106 M−1) was similar to the previously reported increased relative avidity of human anti-PS antibodies obtained after immunization with a Haemophilus influenzae type b PS-protein conjugate vaccine in a similar assay (3).
FIG. 1.
Blocking of the binding of human anti-LPS MAb S20 to solid-phase P. aeruginosa strain 06ad LPS O-side-chain PS by preincubation with 06ad PS (▪) but not control PS from Streptococcus pneumoniae serotype 6B (♦) in an ELISA. The x axis shows micrograms of 06ad PS or control PS per milliliter added to each reaction tube. The y axis depicts the percent reduction of absorbance obtained with the PS inhibitor. Data represent means from three separate assays, each run in duplicate, and standard errors of the means.
FIG. 2.
Human MAb S20 binds only to P. aeruginosa 06ad LPS O-side-chain PS and not to LPS O-side-chain PSs from other strains. The x axis depicts the serotype of pseudomonas O-side-chain PS used to coat the ELISA plate, while the y axis indicates the absorbance (OD, optical density). Bars represent the mean absorbance from triplicate wells in one representative assay.
Human MAb against LPS is opsonic for uptake and killing of P. aeruginosa by human PMN.
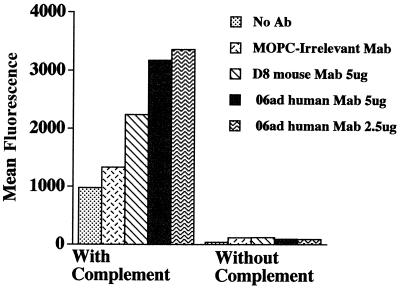
Although we showed that the IgG2 human MAb produced after P. aeruginosa immunization of mice was specific and avid for 06 P. aeruginosa LPS, functional activity was not yet demonstrated. Thus, we next showed that the S20 human MAb was highly opsonic for the uptake of labeled P. aeruginosa by fresh human PMN in a complement-dependent assay. In fact, the human MAb was more opsonic than a previously described protective mouse MAb, D8, against the same epitope (2.5 μg of human MAb yielded almost twice the mean fluorescence as 5 μg of mouse MAb) (Fig. 3). Antibody alone was a poor opsonin, confirming our previous data that Fcγ receptor stimulation without complement receptor stimulation is not optimal for the phagocytosis of P. aeruginosa by human PMN (2). Complement alone yielded some marginal increased uptake of labeled bacteria by PMN, but the phagocytosis was greatly enhanced with antibody and complement together, as predicted when both Fcγ and complement receptors are stimulated together in human PMN. Presumably antibody bound to the bacteria yielded polymeric IgG2 that then bound to the relatively low-affinity FcγR2 and FcγR3 PMN receptors, as well as facilitating the deposition of complement onto the bacterial surface. Finally, an ELISA showed C3 deposition on the surface of P. aeruginosa facilitated by the addition of the S20 MAb (data not shown), again suggesting that S20 is effective at fixing complement to the bacterial surface. Further studies are required to determine if the human MAb is capable of opsonizing pseudomonas for uptake by other phagocytes, such as alveolar macrophages, in which phagocytosis is primarily mediated by high-affinity FcγR1 receptors, not low-affinity receptors and complement receptors (2). Different IgG subclasses of anti-PS antibodies may also be required to bind to such receptors in order to initiate phagocytosis.
FIG. 3.
Opsonization of FITC-labeled P. aeruginosa 06ad by human MAb S20 for uptake by fresh human PMN in the presence of human complement (serum from an agammaglobulinemic patient). Opsonization and phagocytosis by PMN are shown as mean fluorescence obtained by flow cytometry gated for human PMN as described in Materials and Methods. The results shown are from one representative experiment out of four separate experiments, all with similar results. MOPC, MOPC 21.
Since the bacteria were heat killed prior to labeling with FITC, it seemed possible that increased susceptibility to antibody and complement-mediated opsonization could have occurred due to damage to the bacterial surface. Thus, we also measured opsonization, phagocytosis, and killing of 06ad P. aeruginosa by PMN in an assay in which live bacteria were opsonized with antibody and human complement and then colony counts were determined after exposure to fresh human PMN. The S20 MAb was also effective in this assay, in which more than a 90% reduction in P. aeruginosa CFU was obtained in the presence of complement (Fig. 4).
FIG. 4.
Killing of S20 MAb-opsonized P. aeruginosa 06ad in the presence of human PMN and complement. The y axis depicts percent killing of the inoculum of approximately 25,000 CFU of P. aeruginosa 06ad after 90 min of incubation with antibody, 106 human PMN, and human complement. Error bars denote standard errors of the means from two assays, each assay run in quadruplicate. MOPC 21 (mopc) was used as an irrelevant MAb and did not cause bacterial killing, and S20 antibody alone without complement did not yield bacterial killing (data not shown).
Human MAb from transgenic mice protects neutropenic mice from fatal P. aeruginosa sepsis.
In order to determine whether the in vitro avidity, specificity, and opsonic ability of the MAb translated to in vivo protective efficacy, we rendered BALB/c mice neutropenic by treatment with cyclophosphamide. Mice were then treated with 25 μg of human MAb, control antibody, or saline i.p. and challenged with 1,000 CFU of strain 06ad P. aeruginosa in a model designed to imitate the type of human host likely to develop severe sepsis and invasive disease with the organism. Mice receiving saline injection or irrelevant MAb and then challenged with P. aeruginosa sustained 100% mortality, most dying within 48 h after challenge, consistent with previous descriptions of mortality in nonimmune mice in this model (36). In contrast, mice receiving the human MAb derived from the XenoMouse animals were 100% protected from mortality (P, <0.0001, compared to saline controls), as were mice receiving a previously described, highly protective murine MAb at the same 25-μg dosage (P, <0.0003) (36) (Table 1).
TABLE 1.
Protective efficacy of XenoMouse-produced human MAb in the neutropenic mouse model of P. aeruginosa sepsisa
| Material administered | % Cumulative mortality | No. of mice |
|---|---|---|
| PBS | 100 | 6 |
| 25 μg of MOPC 21 (control) | 100 | 6 |
| 25 μg of MAb D8 (mouse) | 0b | 8 |
| 25 μg of MAb S20 (human) | 0c | 10 |
Control mice received either saline or the irrelevant MOPC 21 in the same volume as test MAb recipients. Results from two separate experiments with identical procedures and reagents were combined.
P value of <0.0003 compared to result for saline or MOPC 21 recipients.
P value of <0.0001 compared to result for saline or MOPC 21 recipients.
DISCUSSION
The current study demonstrates that immunization of the XenoMouse animals with killed P. aeruginosa yielded IgG2 anti-LPS MAb that utilized VH3 and Vκ2 genes, strikingly similar to the V-region gene usage in humans immunized with T-cell-independent bacterial PS vaccines. The role of the restricted V-region gene usage in the human antibody response to PS antigens remains unclear. However, recent data obtained from human immunodeficiency virus-positive adults suggested that a shift away from VH3 gene usage was associated with decreased responsiveness to immunization with bacterial PSs such as the pneumococcal vaccine, possibly due to depletion of certain B cells (1). Thus, the ability of these mice to appropriately utilize VH3 genes for antibodies directed against this PS suggests that further immunization with different PSs is likely to yield normal adult human-type antibody responses. Our data are similar to those obtained in a recent report, which showed isotype restriction as well as VH3 gene usage in these same transgenic mice after immunization with pneumococcal capsular PSs (31). This study also suggested that the B-cell repertoire in these mice strongly resembles that seen in normal adult humans. Finally, the light chain used by our human MAb, Vκ2/A2, is also commonly utilized by humans after immunization with bacterial PSs such as the capsule of H. influenzae type b. In fact, immunization with the H. influenzae type b conjugate vaccine (HbOC—an oligosaccharide conjugated to CRM197, a diphtheria toxin mutant) induces antibodies that preferentially utilize Vκ2/A2 genes. Anti-PS antibodies made after immunization with this vaccine have higher avidity than antibodies made after immunization with different conjugate vaccines that induce antibodies that use non-A2 genes, perhaps explaining the high relative avidity of our antibody (3, 41). Further experiments are required to determine if the XenoMouse animals utilize the same heavy- and light-chain V-region genes after immunization with these conjugate vaccines. An animal model that mimics both the isotype restriction and V-region gene usage seen in humans after effective vaccination may provide a unique mechanism for screening the immunogenicity of new bacterial PS-based vaccines as well as yield better methods to investigate human V-region gene usage after immunization.
Despite the existence of appropriate antibiotics, P. aeruginosa continues to be an aggressive and potentially lethal pathogen in many patient groups. Cystic fibrosis patients, for example, have a unique predilection for chronic pneumonia with P. aeruginosa that greatly increases morbidity and ultimately results in respiratory failure. Although these individuals appear to be initially infected with pseudomonas strains that express O-side-chain PSs, once chronic infection is established, these pseudomonas strains usually undergo phenotypic conversion, express mucoid exopolysaccharide, and become deficient in LPS O-side-chain PS. By the time chronic infection has occurred, it seems unlikely that any active or passive immunization intervention would be successful. However, an effective cocktail of antibodies to the commonly occurring LPS O side chains might be prophylactic against infection if given to young cystic fibrosis patients prior to colonization with O-side-chain-containing P. aeruginosa. Moreover, one might expect that the youngest cystic fibrosis patients needing antipseudomonas serotherapy would not be responsive to many of the components of a purified PS vaccine. Finally, active vaccination against LPS O-side-chain antigens may also be problematic in light of animal studies that have indicated antagonistic effects between different high MW PS antigens when combined into a multivalent vaccine (15). Similarly, burn, intensive care, and neutropenic cancer patients all have a strong risk of acquiring invasive infections with P. aeruginosa, and all these patient groups appear to develop colonization within just a few days of hospitalization. Thus, active vaccination may not be practical because of the need for a multivalent vaccine and the duration required to develop antibodies and because debilitated and immunocompromised hosts may respond poorly to immunization. On the other hand, administration of multivalent high-titer O-side-chain-specific opsonic antibodies prior to the acquisition of the initial O-side-chain-replete pathogen may be a potential prophylactic therapy that could prevent or significantly delay initial colonization from a variety of pseudomonas strains (43).
Use of human polyclonal antibodies as well as mouse or mouse-human MAb has proven problematic for a variety of reasons, including variability in antibody titer in polyclonal preparations and costliness of engineered MAb. It is clear that new technologies that provide cost-effective, specific human antibodies directed to protective surface epitopes of pseudomonas and other pathogens would provide reagents that are a strong addition to the clinical armamentarium (17).
The human MAb chosen for study was highly opsonic for the uptake of a homologous strain of P. aeruginosa by human PMN. The opsonic ability was complement dependent, since the addition of complement alone enhanced the uptake of the labeled bacteria and killing of the organism. Antibody alone, however, was a relatively poor opsonin. The addition of antibody to complement greatly enhanced the phagocytic uptake of the bacteria, presumably since optimal stimulation of both Fc and complement receptors on the PMN occurred. Most previous studies have found a strong link between the opsonic ability of antipseudomonas antibodies and in vivo protection; thus, it was likely that the antibody would also function well in vivo (25–28). Pretreatment of neutropenic mice with the human MAb provided strong protection against fatal pseudomonas sepsis. Thus, this MAb fulfilled the criteria necessary for full antipseudomonas function: the antibody was of relatively high avidity for antigen, it fixed complement to the bacterial surface, it opsonized bacteria for uptake by human phagocytes, and it protected animals from death from a homologous strain of bacteria. Further studies are required to determine if such antibodies can function in humans with equal efficiency.
The IgG2 antibody chosen for detailed investigation in our study was specific for the 06ad serogroup LPS O side chain and did not cross-react in an ELISA with LPS O side chains from several other P. aeruginosa serogroup strains. Although we did not challenge mice with other serogroups of P. aeruginosa, it seems very likely that any protection afforded by the antibody was serogroup specific. Since a variety of pseudomonas serogroups circulate in the community and can cause human disease, a multivalent preparation would be required to prevent colonization and/or infection in humans. There are approximately 10 serogroups of P. aeruginosa comprising a vast majority of isolates from human clinical sources (14), suggesting a minimum number of components in a multivalent preparation. Furthermore, the restricted IgG reconstitution of the mice (IgG2) currently limits the number of IgG subclasses that can be produced, although isotype switching or new mice reconstituted with other IgG constant regions will yield antibodies of a variety of IgG subclasses.
Despite these challenges, the transgenic mice provide an excellent platform to produce multiple antibodies against a variety of P. aeruginosa LPS O side chains. Thus, using routine, inexpensive mouse MAb technology with transgenic mice to yield functional human antibodies instead of mouse antibodies may make the production of a high-titer MAb preparation containing antibodies against a variety of P. aeruginosa serogroups feasible.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI-32596 (to J.R.S.) and AI-46667 (to J.R.S.) and by a pilot feasibility study from grant DK-27651 (P. Davis).
We thank Rhonda Kimmel and Sheryl Peterson for technical support.
REFERENCES
- 1.Abadi J, Friedman J, Mageed R A, Jefferis R, Rodriguez-Barradas M C, Pirofski L. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of VH3 gene segment usage. J Infect Dis. 1998;178:707–716. doi: 10.1086/515369. [DOI] [PubMed] [Google Scholar]
- 2.Berger M, Norvell T M, Tosi M F, Emancipator S N, Konstan M W, Schreiber J R. Tissue-specific Fcγ and complement receptor expression by alveolar macrophages determines relative importance of IgG and complement in promoting phagocytosis of Pseudomonas aeruginosa. Pediatr Res. 1994;35:68–77. doi: 10.1203/00006450-199401000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Chung G H, Kim K H, Daum R S, Insel R A, Siber G R, Sood S, Gupta R K, Marchant C, Nahm M H. The V-region repertoire of Haemophilus influenzae type b polysaccharide antibodies induced by immunization of infants. Infect Immun. 1995;63:4219–4223. doi: 10.1128/iai.63.11.4219-4223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cryz S J, Jr, Furer E, Germanier R. Passive protection against Pseudomonas aeruginosa infection in an experimental leukopenic mouse model. Infect Immun. 1983;40:659–664. doi: 10.1128/iai.40.2.659-664.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cryz S J, Jr, Furer E, Cross A S, Wegmann A, Germanier R, Sadoff J C. Safety and immunogenicity of a Pseudomonas aeruginosa O-polysaccharide toxin A conjugate vaccine in humans. J Clin Investig. 1987;80:51–56. doi: 10.1172/JCI113062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cryz S J, Jr, Furer E, Sadoff J C, Fredeking T, Que J U, Cross A S. Production and characterization of a human hyperimmune intravenous immunoglobulin against Pseudomonas aeruginosa and Klebsiella species. J Infect Dis. 1991;163:1055–1061. doi: 10.1093/infdis/163.5.1055. [DOI] [PubMed] [Google Scholar]
- 7.Cryz S J, Jr, Sadoff J C, Furer E, Germanier R. Pseudomonas aeruginosa polysaccharide-tetanus toxoid conjugate vaccine: safety and immunogenicity in humans. J Infect Dis. 1986;154:682–688. doi: 10.1093/infdis/154.4.682. [DOI] [PubMed] [Google Scholar]
- 8.Emara G M, Tout N L, Kaushik A, Lam J S. Diverse VH and Vκ genes encode antibodies to Pseudomonas aeruginosa LPS. J Immunol. 1995;155:3912–3921. [PubMed] [Google Scholar]
- 9.Fergie J E, Shema S J, Lott L, Crawford R, Patrick C C. Pseudomonas aeruginosa bacteremia in immunocompromised children: analysis of factors associated with a poor outcome. Clin Infect Dis. 1994;18:390–394. doi: 10.1093/clinids/18.3.390. [DOI] [PubMed] [Google Scholar]
- 10.Garner C V, DesJardins D, Pier G B. Immunogenic properties of Pseudomonas aeruginosa mucoid exopolysaccharide. Infect Immun. 1990;58:1835–1842. doi: 10.1128/iai.58.6.1835-1842.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilleland H E, Jr, Gilleland L B, Matthews-Greer J M. Outer membrane protein F preparation of Pseudomonas aeruginosa as a vaccine against chronic pulmonary infection with heterologous immunotype strains in a rat model. Infect Immun. 1988;56:1017–1022. doi: 10.1128/iai.56.5.1017-1022.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green L L. Antibody engineering via genetic engineering of the mouse: XenoMouse strains are a vehicle for the facile generation of therapeutic human monoclonal antibodies. J Immunol Methods. 1999;231:11–23. doi: 10.1016/s0022-1759(99)00137-4. [DOI] [PubMed] [Google Scholar]
- 13.Green L L, Jakobovits A. Regulation of B cell development by variable gene complexity in mice reconstituted with human immunoglobulin yeast artificial chromosomes. J Exp Med. 1998;188:483–495. doi: 10.1084/jem.188.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatano K, Boisot S, DesJardins D, Wright D G, Brisker J, Pier G B. Immunogenic and antigenic properties of a heptavalent high-molecular-weight O-polysaccharide vaccine derived from Pseudomonas aeruginosa. Infect Immun. 1994;62:3608–3616. doi: 10.1128/iai.62.9.3608-3616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatano K, Pier G B. Complex serology and immune response of mice to variant high-molecular-weight O polysaccharides isolated from Pseudomonas aeruginosa serogroup O2 strains. Infect Immun. 1998;66:3719–3726. doi: 10.1128/iai.66.8.3719-3726.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hector R F, Collins M S, Pennington J E. Treatment of experimental Pseudomonas aeruginosa pneumonia with a human IgM monoclonal antibody. J Infect Dis. 1989;160:483–489. doi: 10.1093/infdis/160.3.483. [DOI] [PubMed] [Google Scholar]
- 17.Krause R M, Dimmock N J, Morens D M. Summary of antibody workshop: the role of humoral immunity in the treatment and prevention of emerging and extant infectious diseases. J Infect Dis. 1997;176:549–559. doi: 10.1086/514074. [DOI] [PubMed] [Google Scholar]
- 18.Lang A B, Schaad U B, Rudeberg A, Wedgwood J, Que J U, Furer E, Cryz S J., Jr Effect of high-affinity anti-Pseudomonas aeruginosa lipopolysaccharide antibodies induced by immunization on the rate of Pseudomonas aeruginosa infection in patients with cystic fibrosis. J Pediatr. 1995;127:711–717. doi: 10.1016/s0022-3476(95)70158-3. [DOI] [PubMed] [Google Scholar]
- 19.Lucas A H, Larrick J W, Reason D C. Variable region sequences of a protective human monoclonal antibody specific for the Haemophilus influenzae type b capsular polysaccharide. Infect Immun. 1994;62:3873–3880. doi: 10.1128/iai.62.9.3873-3880.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacIntyre S, Lucken R, Owen P. Smooth lipopolysaccharide is the major protective antigen for mice in the surface extract from IATS serotype 6 contributing to the polyvalent Pseudomonas aeruginosa vaccine PEV. Infect Immun. 1986;52:76–84. doi: 10.1128/iai.52.1.76-84.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCool T L, Harding C V, Greenspan N S, Schreiber J R. B- and T-cell immune responses to pneumococcal conjugate vaccines: divergence between carrier- and polysaccharide-specific immunogenicity. Infect Immun. 1999;67:4862–4869. doi: 10.1128/iai.67.9.4862-4869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez M J, Green L L, Corvalan J R, Jia X C, Maynard-Currie C E, Yang X D, Gallo M L, Louie D M, Lee D V, Erikson K L, Luna J, Roy C M, Abderrahim H, Kirschenbaum F, Noguchi M, Smith D H, Fukushima A, Hales J F, Finer M H, Davis C G, Zsebo K M, Jakobovits A. Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice. Nat Genet. 1997;15:146–156. doi: 10.1038/ng0297-146. [DOI] [PubMed] [Google Scholar]
- 23.Morrison S L, Ol V T. Genetically engineered antibody molecules. Adv Immunol. 1989;44:65–92. doi: 10.1016/s0065-2776(08)60640-9. [DOI] [PubMed] [Google Scholar]
- 24.Pennington J E, Pier G B. Efficacy of cell wall Pseudomonas aeruginosa vaccines for protection against experimental pneumonia. Rev Infect Dis. 1983;5:S852–S857. doi: 10.1093/clinids/5.supplement_5.s852. [DOI] [PubMed] [Google Scholar]
- 25.Pier G B. Safety and immunogenicity of high molecular weight polysaccharide vaccine from immunotype 1 Pseudomonas aeruginosa. J Clin Investig. 1982;69:303–308. doi: 10.1172/JCI110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pier G B, Meluleni G, Goldberg J B. Clearance of Pseudomonas aeruginosa from the murine gastrointestinal tract is effectively mediated by O-antigen-specific circulating antibodies. Infect Immun. 1995;63:2818–2825. doi: 10.1128/iai.63.8.2818-2825.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pier G B, Thomas D, Small G, Siadak A, Zweerink H. In vitro and in vivo activity of polyclonal and monoclonal human immunoglobulins G, M, and A against Pseudomonas aeruginosa lipopolysaccharide. Infect Immun. 1989;57:174–179. doi: 10.1128/iai.57.1.174-179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preston J M, Gerceker A A, Reff M E, Pier G B. Production and characterization of a set of mouse-human chimeric immunoglobulin G (IgG) subclass and IgA monoclonal antibodies with identical variable regions specific for Pseudomonas aeruginosa serogroup O6 lipopolysaccharide. Infect Immun. 1998;66:4137–4142. doi: 10.1128/iai.66.9.4137-4142.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabin E R, Graber C D, Vogel E H, Finkelstein R A, Tumbusch W A. Fatal pseudomonas infection in burned patients. A clinical, bacteriologic and anatomic study. N Engl J Med. 1961;265:1225–1231. doi: 10.1056/NEJM196112212652501. [DOI] [PubMed] [Google Scholar]
- 30.Roilides E K, Butler K M, Husson R N, Mueller B U, Lewis L L, Pizzo P Z. Pseudomonas infections in children with human immunodeficiency virus infection. Pediatr Infect Dis J. 1992;11:547–553. doi: 10.1097/00006454-199207000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Russell N D, Corvalan J R F, Gallo M L, Davis C G, Pirofski L A. Production of protective human antipneumococcal antibodies by transgenic mice with human immunoglobulin loci. Infect Immun. 2000;68:1820–1826. doi: 10.1128/iai.68.4.1820-1826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanger F, Nicklen S, Coulson A A. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saravoltz L D, Markowitz N, Collins M S, Bogdanoff D, Pennington J E. Safety, pharmacokinetics, and functional activity of human anti-Pseudomonas aeruginosa monoclonal antibodies in septic and nonseptic patients. J Infect Dis. 1991;164:803–806. doi: 10.1093/infdis/164.4.803. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber J R, Goldmann D. Infections complicating cystic fibrosis. Curr Clin Top Infect Dis. 1986;7:51–81. [Google Scholar]
- 35.Schreiber J R, Cooper L J N, Diehn S, Dahlhauser P A, Tosi M F, Glass D D, Patawaran M, Greenspan N S. Variable region-identical monoclonal antibodies of different IgG subclass directed to Pseudomonas aeruginosa lipopolysaccharide O-specific side chain function differently. J Infect Dis. 1993;167:221–226. doi: 10.1093/infdis/167.1.221. [DOI] [PubMed] [Google Scholar]
- 36.Schreiber J R, Nixon K L, Tosi M F, Pier G B, Patawaran M B. Anti-idiotype-induced, lipopolysaccharide specific antibody response to Pseudomonas aeruginosa. II. Isotype and functional activity of the anti-idiotype-induced antibodies. J Immunol. 1991;146:188–193. [PubMed] [Google Scholar]
- 37.Schreiber J R, Pier G B, Grout M, Nixon K, Patawaran M. Induction of opsonic antibodies to Pseudomonas aeruginosa mucoid exopolysaccharide by an anti-idiotypic monoclonal antibody. J Infect Dis. 1991;164:507–514. doi: 10.1093/infdis/164.3.507. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y, Park M K, Kim J, Diamond B, Solomon A, Nahm M H. Repertoire of human antibodies against the polysaccharide capsule of Streptococcus pneumoniae serotype 6B. Infect Immun. 1999;67:1172–1179. doi: 10.1128/iai.67.3.1172-1179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabor S, Richardson C C. Selective inactivation of the exonuclease activity of bacteriophage T7 DNA polymerase by in vitro mutagenesis. J Biol Chem. 1989;264:6447–6458. [PubMed] [Google Scholar]
- 40.Tosi F M, Zakem H, Berger M. Neutrophil elastase cleaves C3bi on opsonized Pseudomonas as well as CRI on neutrophils to create a functionally important opsonin receptor mismatch. J Clin Investig. 1990;86:300–308. doi: 10.1172/JCI114699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Using W R, Lucas A H. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun. 1999;67:2366–2370. doi: 10.1128/iai.67.5.2366-2370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Wye J E, Collins M S, Baylor M, Pennington J E, Hsu Y, Sampanvejsopa V, Moss R B. Pseudomonas hyperimmune globulin passive immunotherapy for pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol. 1990;9:7–18. doi: 10.1002/ppul.1950090104. [DOI] [PubMed] [Google Scholar]
- 43.Zeitlin L, Cone R A, Whaley K J. Using monoclonal antibodies to prevent mucosal transmission of epidemic infectious diseases. Emerg Infect Dis. 1999;5:54–64. doi: 10.3201/eid0501.990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zweerink H J, Detolla L J, Gammon M C, Hutchison C F, Puckett J M, Sigal N H. A human monoclonal antibody that protects mice against Pseudomonas-induced pneumonia. J Infect Dis. 1990;162:254–257. doi: 10.1093/infdis/162.1.254. [DOI] [PubMed] [Google Scholar]
- 45.Zweerink H J, Gammon M C, Hutchison C F, Jackson J J, Lombardo D, Miner K M, Puckett J M, Sewell T J, Sigal N H. Human monoclonal antibodies that protect mice against challenge with Pseudomonas aeruginosa. Infect Immun. 1988;56:1873–1879. doi: 10.1128/iai.56.8.1873-1879.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]