Abstract
Dental caries is a biofilm-mediated, diet-modulated, multifactorial and dynamic disease that affects more than 90% of adults in Western countries. The current treatment for decayed tissue is based on using materials to replace the lost enamel or dentin. More than 500 million dental restorations are placed annually worldwide, and materials used for these purposes either directly or indirectly interact with dentin and pulp tissues. The development and understanding of the effects of restorative dental materials are based on different in-vitro and in-vivo tests, which have been evolving with time. In this review, we first discuss the characteristics of the tooth and the dentin-pulp interface that are unique for materials testing. Subsequently, we discuss frequently used in-vitro tests to evaluate the biocompatibility of dental materials commonly used for restorative procedures. Finally, we present our perspective on the future directions for biological research on dental materials using tissue engineering and organs on-a-chip approaches.
Keywords: Dental materials, Cell culture, Tissue chips, Biocompatibility
1. Introduction
Dental tissues comprise a combination of mineralized and non-mineralized tissues that present long-term durability under cyclic loading, temperature fluctuations, pH changes and microbiological challenges over time [1]. Dentin and pulp form a complex where mineralized and soft tissues communicate through an interface formed between dentinal tubules and odontoblastic processes, which protrude into the tubules such that the function of these tissues is interdependent [2]. The most common dental disease is dental caries. The standard strategy to treat caries is based on necrotic tissue removal and subsequent replacement with different restorative materials [3]. More than 500 million direct dental restorations are placed globally every year [4], resulting in a dental materials market valued at 1.5 billion USD with projections to reach 2 billion USD by 2026 [5]. Those restorative materials are used for various applications, which entail direct or indirect contact with the dental pulp and oral mucosa for up to several years [5]. Therefore, biocompatibility assessment of such dental materials is mandatory to assure patient’s safety. To that end, a stepwise process of in-vitro, in-vivo, and clinical testing is utilized to assess those materials’ clinical and biological safety [6,7].
Different approaches attempting to mimic the interactions in the material-dentin-pulp interface have been designed to address the complexity of dentin-pulp interface and the variety of materials used in different treatments. However, despite the remarkable progress achieved over the last decades, pre-clinical tests are still evolving to allow for a more reproducible and accurate estimate of the material behavior under the challenging conditions of the oral and dental environment [6,8] (Fig. 1). Therefore, the goals of the current review are to (i) examine the most critical aspects related to the biocompatibility of dental materials that are applied at the dental-pulp interface, (ii) critically discuss current in-vitro tests commonly used to evaluate a material’s biocompatibility in dental pulp research, and finally, (iii) present our perspective on the future directions for research and development of biocompatible restorative materials.
Fig. 1.
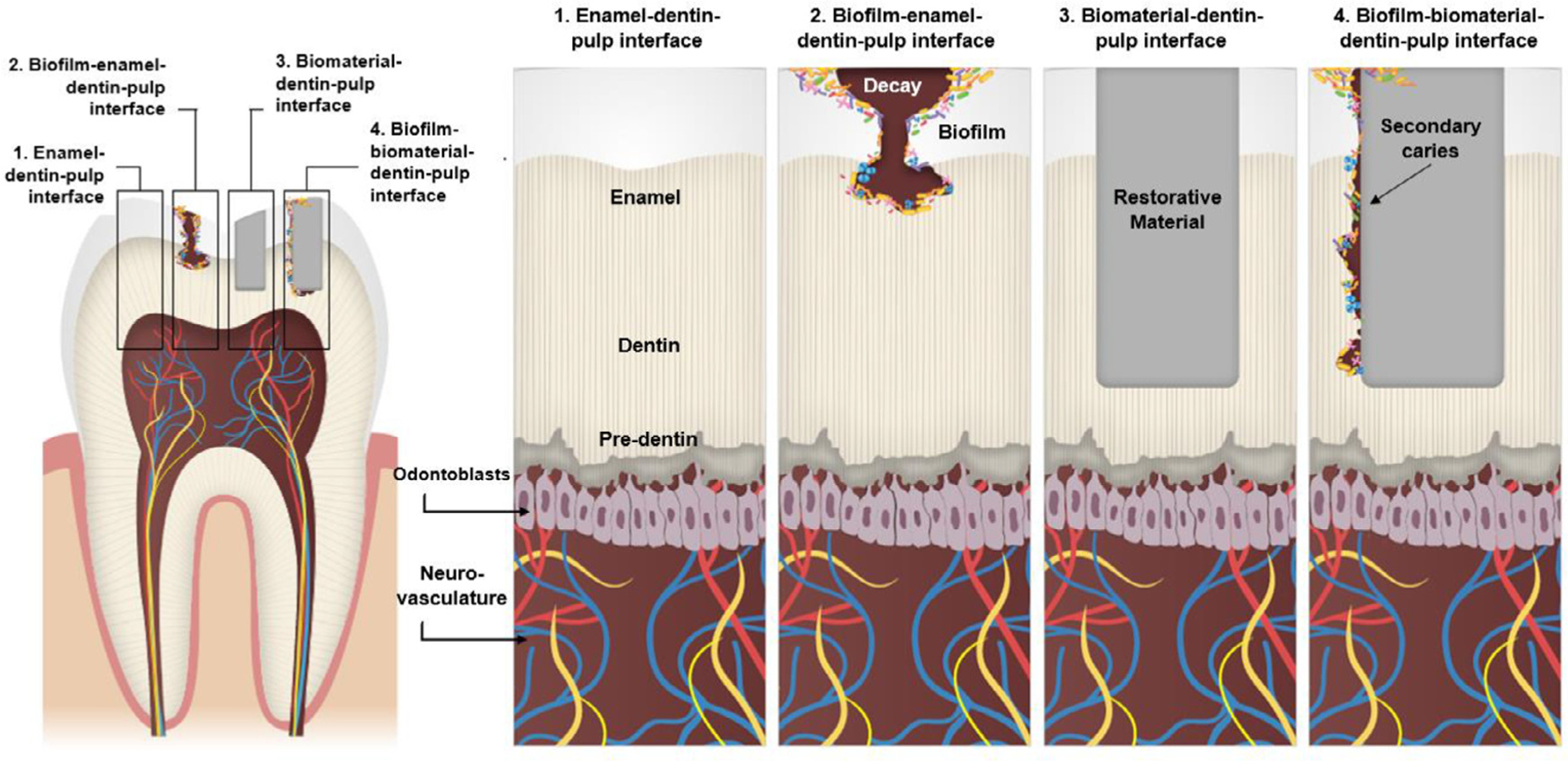
Key biological interfaces present in the dentin-pulp complex in physiologic and disease states to be considered in different study models.
2. Biocompatibility of restorative dental materials and biological interfaces
The essential characteristic that distinguishes a biomaterial from any other material is its ability to subsist in contact with tissues of the human body without causing an unacceptable degree of injury to that host [9]. The initial definition of biocompatibility as a lack of adverse reactions and material with inert properties, such as nontoxic, non-immunogenic, non-mutagenic, etc., has been gradually substituted by the understanding that biocompatibility is the ability of a material to perform with an appropriate host response in a specific situation [6,9]. The core principles of biocompatibility assessment were thoroughly discussed elsewhere Williams (2008) [9], Wataha (2012) [6], Schmalz & Galler (2017) [7], and Cieplik (2022) [10]. Briefly,(i) interactions at the material-tissue interface are dynamic and occur for both – the material elicits a response from the body and vice-versa, (ii) all materials will be changed at some level by their introduction into a biological environment, and as the material and biological tissue are modified by each other, the changes themselves may trigger other changes, (iii) the reactions at the material-tissue interface are a function of the tissue where the interface is created, i.e., implantation of material next to dentin creates a unique interface, distinct from the one created in bone or connective tissue, and (iv) it is possible to customize the interactions at the material-tissue interface by modifying the biological properties of the material [6].
The currently accepted paradigm of biocompatibility comprises the evaluation of distinct and interdependent responses of two or more interfaces within the biomaterial-tissue complex and an understanding of the biological phenomena that occur in short, medium, and long term [9]. To that end, the oral cavity can be divided into complex microenvironments and interfaces: enamel-dentin-pulp interface, biofilm-enamel-dentin-pulp interface, biomaterial-dentin-pulp interface, biofilm-dental material-dentin-pulp interface, etc. (Fig. 1). Different from most other organs and tissues, a unique aspect of dental interfaces is that most of them combine mineralized and soft tissues, such as dentin (mineralized), enamel (mineralized), and the dental pulp (soft), and periodontal ligament (soft). This combination poses challenges for microscopic visualization of the system and difficulties in simultaneously analyzing the outcomes for both soft and hard tissue. Furthermore, many restorative materials, especially uncured monomers from polymeric materials [11] and corrosion products from alloys [12], are known to promote pulp tissue response with short- and long-term effects. In addition, postoperative sensitivity is related to biocompatibility issues in the dentin-pulp complex [11]. When dentin damage is more pronounced, pulp capping strategies may be used, and materials are placed over a thin layer of dentin (0.5 mm-1 mm) in indirect strategies or directly placed over the pulp in direct pulp capping treatments. However, microleakage of bacteria, food debris, or saliva into the material-dentin-pulp interface may promote material degradation and the release of bacterial byproducts, causing or increasing injury to the dental pulp [13–15]. Thus, overall tests are designed to study either the mineralized tissue, or to determine soft tissue responses, unable to predict materials’ behavior on enamel, dentin and pulp concomitantly [7, 16].
3. 2D models for dental pulp studies
In vitro models to study the dental pulp have evolved from 2D cell cultures to complex organs on-a-chip. Each model has been designed to answer specific research questions (Fig. 2). Therefore, a head-to-head comparison of the pros and cons is perhaps less relevant than an understanding of the evolution of these methods over time, since it is unfair to assert disadvantages to methods and materials developed in the late 1960s versus methods developed in the late 2000s when biomedical technologies have evolved considerably.
Fig. 2.
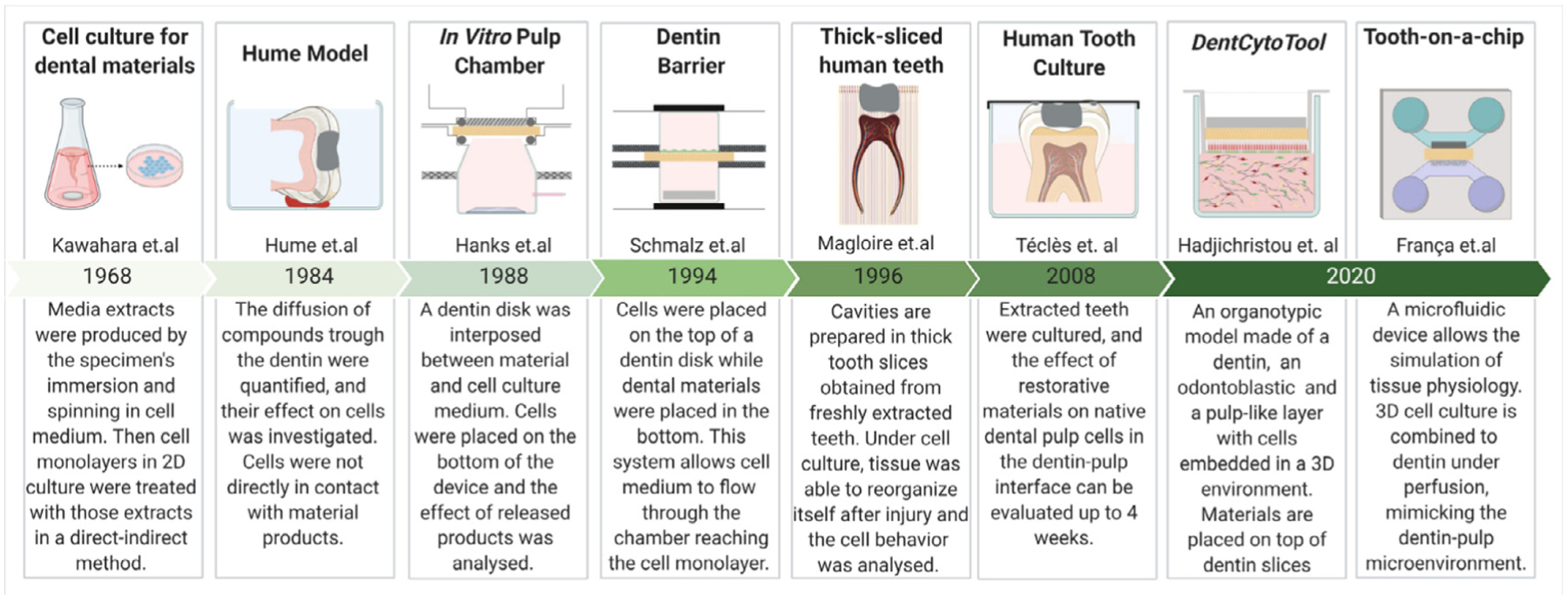
Timeline showing the evolution of in-vitro models used for dental-pulp-related studies.
Two-dimensional cell cultures have been used since the late 1960s to test the cytotoxicity of dental materials [17]. They represent the most used methods to test biocompatibility, and a general guide for in-vitro cytotoxicity testing is presented in ISO 10,993–1 [18] and ISO 7405 [19]. In addition, a separate standard (ISO 3990) is being established to test the antibacterial properties of restorative dental materials. For further details, this topic was recently revised by Cieplik et al. [10] and Kreth et al. [14].
Cell culture models in 2D are diverse, being generally divided into direct and indirect models. Briefly, in the direct model, cells are seeded directly in contact with the material, while in the indirect model, barriers are used between materials and cells (Fig. 3). The main advantages of 2D in vitro tests are the capability to isolate variables, such as control of the environment (cell medium composition, growth factors concentration, cell type, etc.). Also, compared to other methods, 2D cultures allow a detailed, straight-forward and precise measurement of cellular and sub-cellular responses at a low cost. For instance, dose-response curves can be determined within days in an easy and relatively inexpensive approach for highly reproducible quantitative results [6,20]. On the other hand, limitations inherent to 2D models are the lack of most environmental cues present in native tissues, such as the absence of three-dimensionality (3D) of the natural tissues [21] or multitype cell-cell interactions [22,23], lack of a circulatory system and immune response [24,25], all of which eventually result in compromising the clinical relevance of the findings [26]. In addition, direct contact of materials or leachates with 2D cultures is known to overestimate cell reactions to material products, leading to higher levels of cytotoxicity [20,27].
Fig. 3.
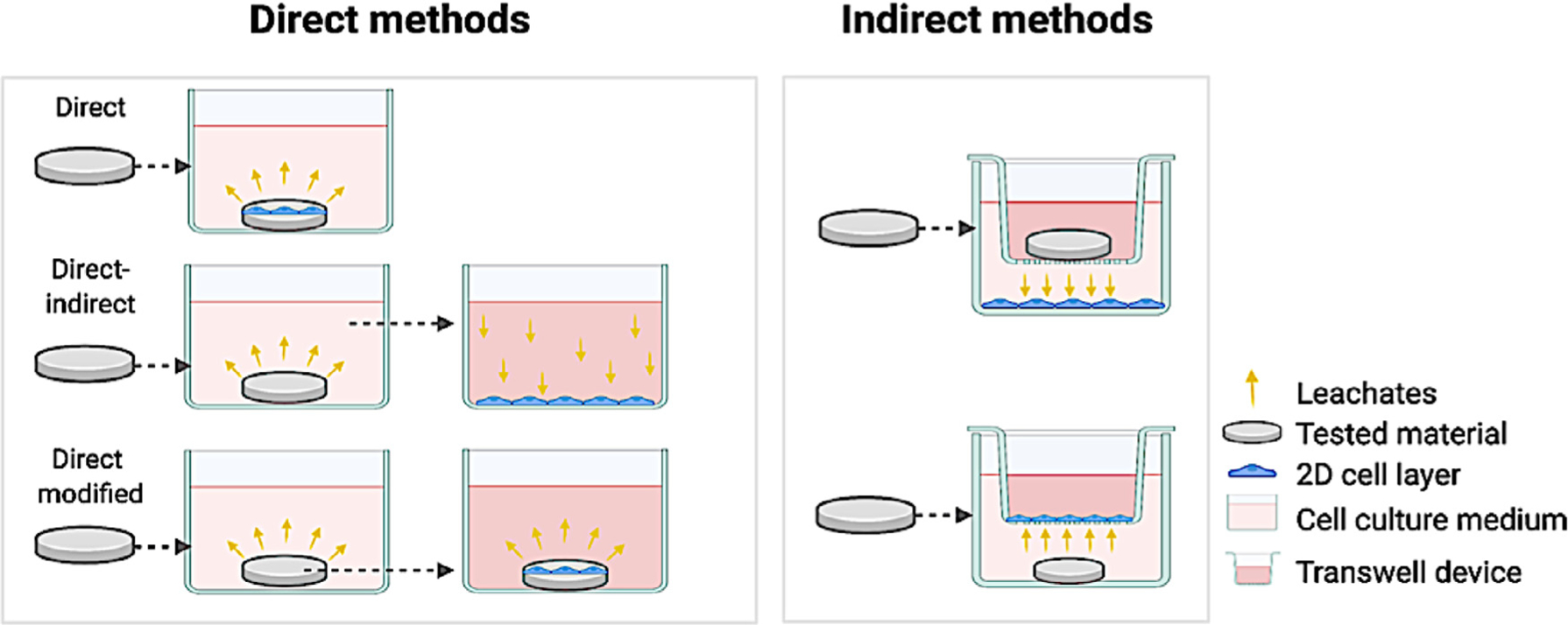
Direct and indirect cell culture models to test dental materials.
4. Barrier models for dental pulp studies
Extensive research has demonstrated that dentin can act as a protective barrier against the cytotoxic effects of dental materials [6,7,28]. Dentin is the largest structure in the tooth and is composed of hydroxyapatite mineral crystallites, collagen fibrils (primarily type I), and non-collagenous molecules [1, 29]. Besides the presence of dentin tubules, which give essential topographical cues for cell differentiation [1, 30], dentin has in its composition non-collagenous proteins, proteoglycans, and growth factors, known as dentin matrix molecules (DMM), which are fossilized within the mineralized matrix [31, 32]. Once released, these DMM coordinate a sequence of events that modulate pulp responses to injuries. During the carious process, fossilized biomolecules within the dentin matrix are solubilized upon demineralization or matrix degradation of dentin, allowing them to diffuse down the dentinal tubules to the pulp tissue and recruit stem cells [33]. In addition, many dental materials, such as calcium hydroxide and calcium silicate-based materials, have sought to leverage the ability of the dental pulp to ‘protect itself’ by inducing the solubilization of these molecules into the pulp [34–36].
Since 2D models cannot best reproduce the processes mentioned above that occur in-vivo, and the direct or indirect (through the tubules) contact of dental materials with cells may lead to erroneous indications of enhanced toxicity, different strategies have been proposed to separate the cells and the material, as typically happens in the restored tooth. Millipore® filter barriers or Transwell® assays are proposed to mimic a clinical setting in which most materials are placed onto the dentin and not directly onto the dental pulp (Fig. 3) [19,37,38]. While direct contact is avoided, a synthetic membrane fails to mimic the biological composition, thickness, and complexity of the dentin’s organic-inorganic tubular structure, resulting in oversimplified modeling of the dentin-pulp interface.
The concept of a ‘dentin barrier test’ was introduced in the early 1970s to provide more organotypic models for mimicking the dentin-pulp complex [39]. Approaches ranged from the use of dentin slices [40] to the development of ‘pulp chambers’ as proposed by Hume [41] and Hanks [42] (Fig. 2), successfully showing that the release of products from the material structure is modified by the presence of dentin barriers allowing a more realistic approach for the biocompatibility analysis. The incorporation of cell monolayers further improved these devices to simulate a biological interface for biomaterial screening [43,44]. Similar to the ‘in-vitro pulp chamber model’, a device for dentin barrier tests was developed by Schmalz et al. [45] in the 1990s. In this setup, fibroblasts were seeded directly on bovine dentin slices supported by plastic holders. The chamber was then sealed with an O-ring, which significantly improved the standardization of cytotoxicity tests. Subsequently, several studies used the dentin barrier device to test the effect of different materials on cell behavior [28,46–49]. The system evolved to allow the simulation of pulp pressure [47]. ISO 7405 standards recommend this method for testing dental materials (Fig. 2) [19,50]. The dentin barrier cytotoxicity test is applied to evaluate different concentrations of material components or extracts of polymerized or set materials when placed on one side of dentin and allowed to diffuse to the opposite side. Perfusion chambers are recommended with the dentin barrier device allowing an influx of culture media during the test. The perfusion of material products may be measured by colorimetry, spectrophotometry, and chromatography. Also, cell activity can be measured by scores of cell damage and by another cytotoxicity, immunogenicity, mutagenicity, and molecular tests. Positive and negative controls are recommended in the standard. While the negative control should not affect cell viability, components such as camphoroquinone and hydroxyethyl methacrylate (HEMA) are proposed as positive controls that should reduce cell viability by approximately 50% after 24 h exposure. The pulp-related response is also considered on ISO 7405 through the pulp and dentin usage tests, pulp capping tests, and endodontic usage tests via animal models. Although animal models were recommended, the standardization guidelines highlight that it is essential to consider them only after a full and careful review of the evidence indicating that a similar outcome cannot be achieved by other tests [19,50].
5. 3D models - ex-vivo, engineering tissue constructs, organoids, and organs on-a-chip
Despite all the possibilities of 2D and barrier models, these systems have limited ability to answer important questions related to the effect of biomaterials on both the dentin and pulp cells, as well as the interplay among biofilm, dentin, biomaterials, and pulp cells in near-physiologic conditions. To overcome this issue, an ex-vivo model was developed to simulate clinical conditions in extracted teeth [51,52].
Migration and reorganization of pulp cells have been investigated using the thick-sliced human teeth model [51] and human tooth culture [52] (Fig. 2). The thick-sliced human teeth model successfully showed that an injury in the dentin-pulp interface elicited migration and proliferation of cells nearby the injury site. Moreover, there was evidence of cell differentiation and pulp tissue revascularization near the thick-sliced dentin after 30 days in cell culture [51]. In the early 2000s Téclès et al. developed an entire tooth culture model to study the migration of stem cells from the dental pulp to the injured site (Fig. 2) [52,53]. In this method, immature third molars are cultured in well plates with cell medium for up to 4 weeks. The system allows the preparation of a tooth cavity and evaluation of some pulp cell responses, including early steps of dentin regeneration. The teeth can be fixed, stained, and imaged at pre-determined time points. This method showed the effect of pulp capping materials on dental pulp healing after an injury. Furthermore, this model enabled the understanding of some mechanisms for perivascular progenitor/stem cells proliferation, migration, differentiation and mineral secretion in response to odontoblast injury [52,53]. The static conditions of tooth culture regarding to pH and O2 and CO2 exchange are limitations in the current model as these conditions have great influence on the tissue response [52].
To mimic the complexity of the tooth interfaces, it is necessary to associate dental mineralized tissue with cells cultured in a 3D environment akin to the dental pulp. The advances in tissue engineering techniques enabled a deeper understanding and optimization of the conditions necessary to emulate the dental pulp environment. The most used hydrogels for 3D dental pulp studies are Matrigel [54,55], GelMA [56], decellularized pulp extracellular matrix (ECM) [57], Puramatrix [58], fibrin [59], collagen [60], pegylated fibrinogen [61] and polyethylene glycol [62]. These cell-loaded 3D matrices have been used as improved alternatives for the 2D cell monolayers [63]. However, the primary use of those matrices is targeted at dental pulp regeneration, with fewer studies focused on platforms for dental materials biocompatibility testing [63].
The current knowledge of the conditions necessary to emulate a dental pulp environment has culminated in the development of a tissue-engineered dentin/pulp analog, the DentCytoTool [63]. This system was developed to recapitulate the main components of the dentin-pulp complex and incorporate 3D microenvironmental cues. It is comprised of two compartments: the upper chamber, representing the dentin component with a layer of dental pulp stem cells cultured onto a membrane of a cell culture insert (Millicell) and covered by a disk-shaped human dentin matrix previously treated with an EDTA solution. The lower chamber emulates the pulp component consisting of a co-culture of endothelial cells and stem cells encapsulated in a collagen I/fibrin hydrogel (Fig. 4A–F) [63,64]. The authors tested the effect of lipopolysaccharide and resin monomers simultaneously in odontogenic and endothelial cells, in a 3D environment representative of the dentin-pulp complex. Their results showed that the in vitro model could emulate the tissue organization observed in clinical situations where bacteria products and resin monomers affect the dentin/pulp interface (Fig. 4 I,J). This tissue-engineered platform provides an important evolution in how dental materials are tested because it enables the evaluation of the mechanistic aspects of interactions between the dentin/pulp complex and dental materials, allowing for a systematic assessment of the interplay of vasculature, odontoblast-like cells, dentin, and dental materials.
Fig. 4.
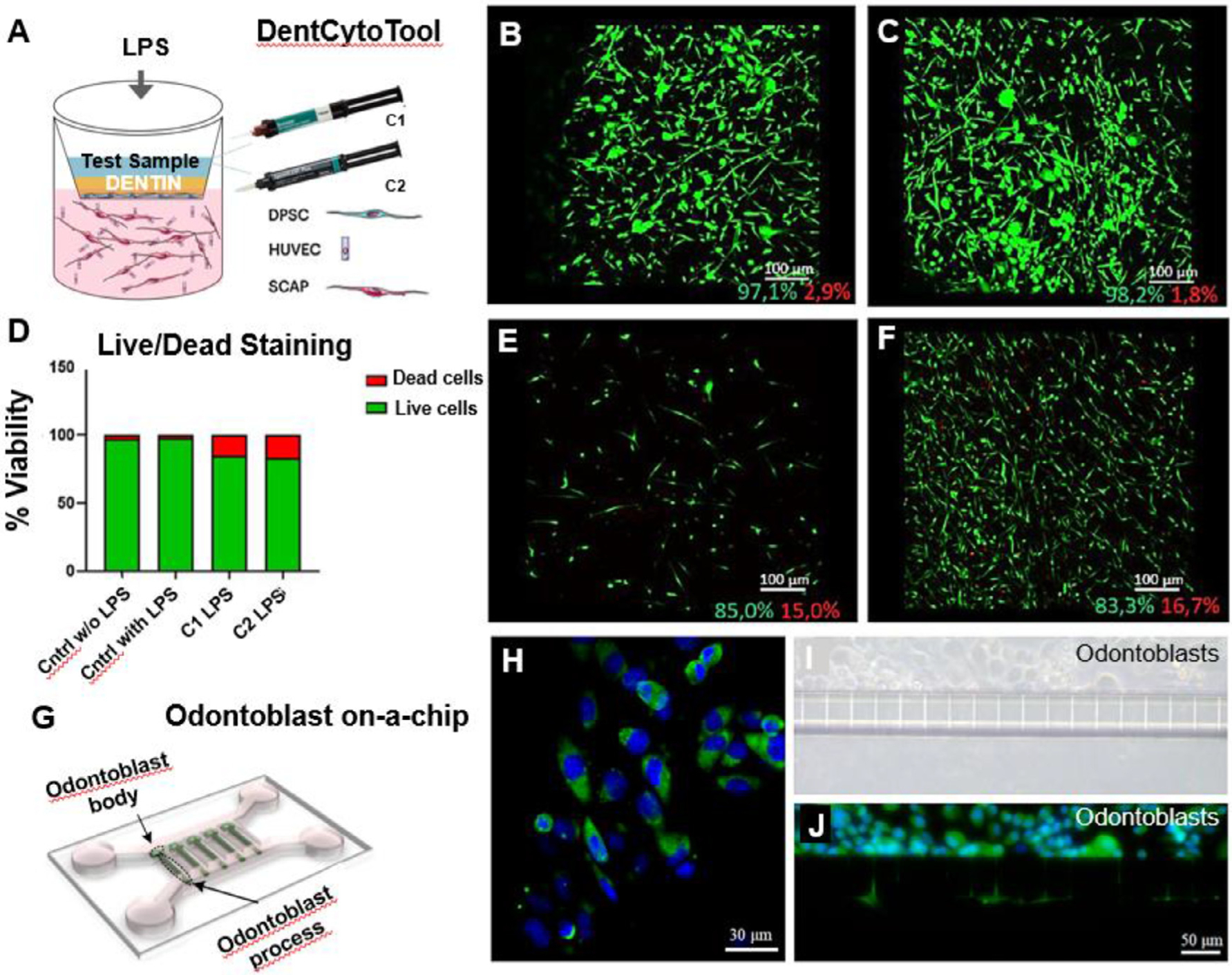
(A) DentCytotool is a 3D dentin/pulp tissue analogue comprised of upper and lower compartments, representing the dentin and pulp components respectively. The upper compartment consists of a layer odontoblast-like cells derived from dental pulp stem cells and a disk of human treated dentin matrix (hTDM), while the lower compartment consists of endothelial cells and stem cells from apical papillae (SCAP) encapsulated in hydrogel. (B-F) Live/dead fluorescent staining with Calcein AM and EthD-1, depicting cytotoxic effects of control-LPS (B), control + LPS (C), C1 + LPS (E) and C2 + LPS (F), on the of the HUVEC/SCAP co-cultures of the lower compartment (D). The green and red percentages below the scale bar of each image represent the live and dead cells respectively. Reproduced with permission [64]. (G) Schematic microfluidic chip for odontoblastic process growth. (H-J) Characterization of the odontoblast marker protein aquaporin 4 (AQP4). (H) Immunofluorescence image of odontoblasts on a Petri dish after staining with AQP4 (green) and DAPI (blue). (I) Brightfield microscope image of odontoblasts layer on the upper chamber of the chip. (J) Odontoblast-like cells on-chip stained with AQP4 and DAPI showing cytoplasmic extensions across the microchannels. Reproduced with permission [65].
Human 3D dental pulp organoid models were developed to screen dental materials’ biocompatibility and drugs on dental pulp cells [66]. The authors co-cultured human dental pulp cells with endothelial cells with and without human pulp-derived extracellular matrix, so organoids were assembled within two days. The organoids responded similarly to the pulp tissue by increasing the levels of interleukin 6 after exposure to LPS and showing calcium deposit formation in response to a calcium silicate cement (iRoot BP plus) [66]. Although this model does not emulate biophysical cues of the dentin-pulp interface, it is a promising platform for drug screening and developing restorative materials.
A promising approach to studying the various interfaces of the tooth with unprecedented access to real-time information is using tissue chips. Organs on-a-chip are microfluidic cell culture devices fabricated with microchip manufacturing methods that contain continuously perfused chambers inhabited by living cells arranged to simulate tissue- and organ-level physiology [67]. These devices model multicellular tissue architecture, tissue-tissue interfaces, physicochemical microenvironments, and liquid perfusion, replicating different levels of organ functionality that have not been possible with conventional 2D or 3D culture systems so far. Microfluidic devices also enable high-resolution, real-time imaging and in vitro analysis of living cells’ biochemical, genetic, and metabolic activities in a functional tissue and organ context, making them a handy platform to test drugs and other materials. The first organ-on-a-chip was a lung mimicking device published in 2010 [68]. Over the past decade, these devices have evolved to emulate several other organs, becoming a potentially transformational scientific paradigm. The impact of organs on-a-chip on drug discovery and development offers new perspectives for understanding and modeling different diseases and predicting the toxicity and efficacy of new compounds [69]. Also, there is a large body of evidence that this technology may provide more accurate, rapid, and cost-effective knowledge of tissue behavior [70–74], which was not previously possible with other in vitro models [75].
As for the development of on-chip platforms to test dental materials, Niu et al., 2019 developed a device to model the growth of odontoblast processes from a cell body in vitro using a microfluidic chip via soft lithography [76]. The device was made of poly-dimethylsiloxane (PDMS) and had 2-μm microchannels to mimic dentin tubules. Furthermore, the authors optimized the proper size of the microchannels and hydrostatic pressure to provide the optimal environment for the growth of odontoblastic processes[76]. This is a valuable platform for investigating odontoblast biology, dentin repair, and dental diseases (Fig. 4 G–J).
The first organ-on-a-chip designed to emulate the dentin-pulp interface was also referred to as the ‘Tooth-on-a-chip’ [77]. Briefly, this device is roughly the size of a USB stick. It is made of a transparent biocompatible material, molded to have parallel channels and chambers separated by a dentin fragment allowing the formation of a layer of odontoblast-like cells on one side of the dentin. In addition, exogenous components, such as dental materials, bacteria, and saliva, can be tested simultaneously on the other side of the dentin (Fig. 5 A–F). This spatial design mimics an actual tooth cavity, where the dental pulp interfaces directly with the dentin and indirectly with the oral cavity via dentin tubules. Besides the possibility to visualize cell responses in real-time, with inline assays and conventional microscope, the Tooth-on-a-chip enables the quantification of immediate responses of pulp cells to exogenous components, such as dental materials’ leachates, DMM extraction, and bacterial products [77–79].
Fig. 5.
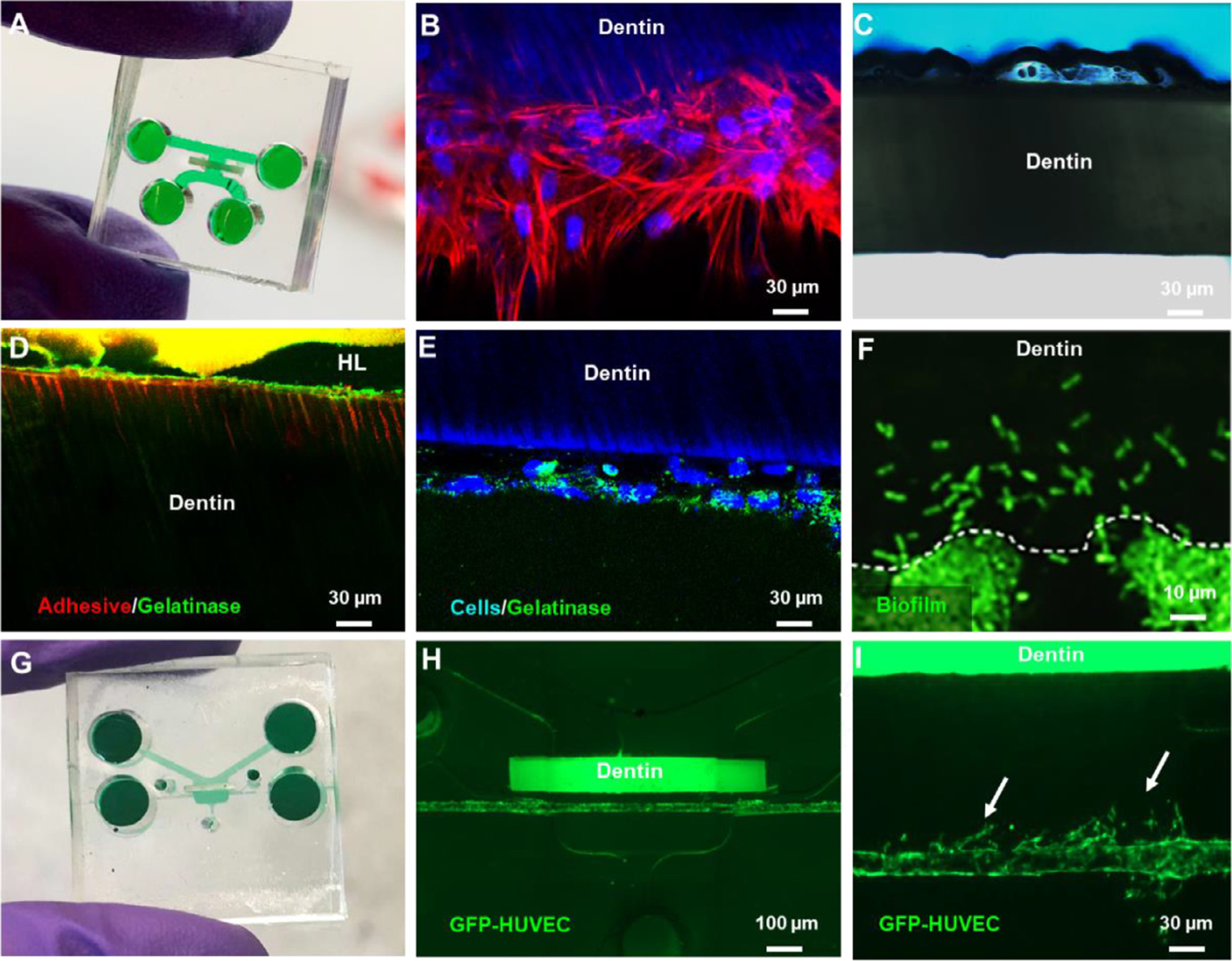
Applications of the Tooth on-a-chip to test dental materials. (A) Microfluidic device comprised of two parallel chambers separated by a fragment of native dentin. (B) Stem cell from apical papilla (SCAPs) seeded on-chip after 7 days protrude cytoplasmic processes into the dentin tubules akin to odontoblasts. (C) Interaction of phosphoric acid and native dentin recorded in real-time. (D) Tooth-on-a-chip devices were seeded with cells, and after 48 h, fluorescein-conjugated gelatin showed gelatinolytic activity in the hybrid layer and dentin tubules. (E) The gelatinolytic activity was co-localized with cell cytoplasm suggesting a role for cells in the enzymatic degradation of the hybrid layer. Reproduced with permission [77] (F). Streptococcus mutans seeded on-chip to evaluate real-time biofilm formation and interactions with pulp cells. (G) Tooth-on-a-chip for vasculature studies. (H) The device has a chamber on the ‘pulp side’ filled with collagen, seeded with mesenchymal stem cells and GFP-HUVECs. After 24 h, a pericyte-supported blood vessel is engineered with a controllable distance from the dentin. (I) Angiogenic sprouts toward the dentin on-chip.
This device advances the possibilities for testing dental materials since it allows for precise experimental control over multifactorial questions that are too challenging to study in vivo systematically (79). For example, the Tooth-on-a-chip was used to test the hypothesis that cells participated in the enzymatic degradation of the hybrid layer (Fig. 5 B–E) [77]. To that end, stem cells of odontogenic origin were seeded on-chip (Fig. 5 B), and devices without cells were used as controls. Next, the dentin was acid-etched (Fig. 5 C), dried, and had a multipurpose adhesive inserted to form a hybrid layer on the dentin (Fig. 5 D). After 24 h, all devices were tested for gelatinase activity. The results showed that chips with cells presented more gelatinolytic activity within the dentin tubules, at the hybrid layer and in the layer of odontoblast-like cells adjacent to the dentin (Fig. 5 D, E) compared to controls. This suggests that cell-derived proteases may have a greater impact on the degradation of the hybrid layer than dentin endogenous proteases. The real-time evaluation of both hard and soft tissues was not previously achievable with other platforms.
The dental pulp is highly vascularized, and most of its biological responses are related to some degree of modification in vascular biology. Thus, mature blood vessels were engineered in the Tooth-on-a-chip to determine the angiogenic activity of the diffusible dentin matrix molecules. To that end, a collagen/fibrin hydrogel laden with mesenchymal stem cells (MSCs) and GFP-expressing human umbilical vein endothelial cells (GFP-HUVECs) was placed in the device to engineer pericyte-supported blood vessels at controlled distances from the dentin. Dentin was then acid-etched with EDTA for 45 s to extract DMM, and subsequently, angiogenic sprouting was evaluated as a function of the distance from the dentin (Fig. 5 G–I). Enhanced angiogenesis was observed when vessels were distant around 900 μm from the dentin, but not when cells were closer to it (Fig. 5 H,I). These findings pave the way to test not only the odontoblast responses to dental materials but also the vasculature, innervation, and immune responses emulating more closely an actual tooth cavity.
Since restorative dental materials can eventually leach to adjacent tissues, organotypic models such as organ-on-a-chip platforms have evolved from hamster-based oral mucosal irritation tests. Currently, oral mucosa on a chip platforms [80–83] and periodontal epithelium on a chip [84] have been designed to test some dental monomers potentially harmful to tissues. For example, an oral mucosa was engineered on a chip to investigate the effect of HEMA [82,83] and S. mutans [83] on oral epithelial and submucosa integrity. Each chip contained sets of three microchannels separated by PDMS posts designed to intercalate square pores. Culture chambers consisted of a main channel and two parallel side channels. Oral keratinocytes were seeded in the pores between the posts, and the middle chamber was seeded with human gingival fibroblasts encapsulated in type I collagen. After 24 h of exposure to 25 mM of HEMA, significant cell death was observed in both keratinocytes and fibroblasts. Moreover, infiltration of the keratinocyte layer by S. mutans resulted in a 64% decrease in transepithelial electrical resistance (TEER) [83].
Similarly, full-thickness gingival equivalents were engineered on a chip to test the effect of silver diamine fluoride (SDF) on the oral mucosa [80]. This study showed that exposing oral keratinocytes to SDF for only 3 min is enough to induce detachment of the corneal layer, loss of intercellular cohesion, and a 50% decrease in cell viability, suggesting corrosive properties of the SDF [80]. The main advantage of both systems over conventional cell culture systems is the ability to simultaneously perform inline experiments tracking multitypic cell responses in the epithelial layer and submucosal fibroblast in real-time using super-resolution microscopy as needed [81].
6. Perspectives
How dental materials are being developed and tested has drastically changed recently [69]. Understanding a material’s interaction with tissues and organisms at the cellular and molecular levels and within different size scales is critical for advancing the development of a new generation of materials and enhancing existing biomaterials [85]. In addition, the demand for more ethical ways to test materials that spare animal lives and minimize the costs of in vivo pre-clinical studies [86] has pushed forward the development of microfluidic devices and organs on-a-chip. The dental field is aligned with this trend but faces challenges due to the complexity of dental tissues.
An important consideration for studies with organs on-a-chip is ‘how simple is complex enough?’, for example, to determine the effect of dental materials on dental pulp cells, a Tooth-on-a-chip model with a cell layer and dentin is enough. However, if the purpose is to investigate the effect of biomaterials or biofilm on inflammatory responses of the dental pulp, then it would be beneficial to use a model with vasculature incorporated into the system. The same principle is valid for studies evaluating materials’ effect on pulp innervation and immune responses. Therefore, organ on-a-chip applications for dental pulp research still present tremendous possibilities for growth. Particularly the combination of microfluidic devices with other biofabrication technologies is needed to allow the precise level of complexity necessary to answer unaddressed questions related to biofilm-dental materials and dentin-pulp complex interactions. To that end, coupling organs on-a-chip with other technologies, such as bioprinting and gene editing, offers a powerful way to deepen our understanding of pulp responses to biofilm and biomaterials, increasing the predictive power of tissue chips for assessing the biocompatibility of restorative materials.
Besides questions related to the development and testing of new materials, which are mostly restricted to academia and industry, we envision possibilities for personalized approaches in the choice of materials that will be placed in the patient as currently happens for drug testing in other fields [87,88]. Such personalized approaches are being implemented in other areas [89,90], and we envision that it is only a matter of time to have them adapted to the dental practice. For example, there proof of evidence for a personalized airway-on-a-chip to evaluate human lung inflammation and drug responses [72], blood vessel-on-chip models indicate the best drug combination for vascular tumors and thrombosis [91], and gut-on-a-chip predict personalized responses to dietary, prebiotic and probiotic interventions [92]. In addition, this assessment could help choose restorative material according to the patient’s specific saliva and biofilm [93].
As a caveat, although organs on-a-chip for lung, gut, heart, and skin continue along a path towards widespread commercialization, a challenge with models related to dental research is the relatively small market compared to medical devices. This poses a critical economic barrier to scaling up the manufacture and commercialization of dental-related devices to an industrial pace. To address this issue, funding agencies, such as the National Institutes of Health and the organs on-a-chip community, are coordinating global efforts to help this technology become widespread and ultimately help transform science and how drugs and materials are tested, signaling this is the way forward.
7. Conclusion
Tissue engineering models and organs on-a-chip present innovative ways to effectively model the dentin-pulp interface in physiological and diseased states, which is critical for a deeper understanding of the interplay of biomaterials and biofilm in the context of the dental pulp. Miniaturization of devices providing more capabilities within less space is currently possible, enabling inline tests to track cell-dentin-material-biofilm responses in real-time.
Statement of significance.
Dental caries is still the most prevalent infectious disease globally, requiring more than 500 million restorations to be placed every year. Regrettably, the failure rates of such restorations are still high. Those rates are partially based on the fact that current platforms to test dental materials are somewhat inaccurate in reproducing critical components of the complex oral microenvironment. Thus, there is a collective effort to develop new materials while evolving the platforms to test them. In this context, the present review critically discusses in-vitro models used to evaluate the biocompatibility of restorative dental materials and brings a perspective on future directions for tissue-engineered and organs-on-a-chip platforms for testing new dental materials.
Acknowledgments
This project was supported by funding from the National Institute of Dental and Craniofacial Research (R01DE026170 and 3R01DE026170-03S1 to LEB, K01DE03484-01A1 to CMF), the Oregon Clinical & Translational Research Institute (OCTRI) - Biomedical Innovation Program (BIP), the Innovation in Oral Care Awards sponsored by GlaxoSmithKline (GSK), International Association for Dental Research (IADR). This work was partially conducted during a scholarship supported by the Institutional Program for Internationalization PrInt- CAPES -Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Bertassoni LE, Dentin on the nanoscale: hierarchical organization, mechanical behavior and bioinspired engineering, Dent. Mater 33 (6) (2017) 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bjorndal L, Darvann T, Thylstrup A, A quantitative light microscopic study of the odontoblast and subodontoblastic reactions to active and arrested enamel caries without cavitation, Caries Res. 32 (1) (1998) 59–69. [DOI] [PubMed] [Google Scholar]
- [3].Schwendicke F, Frencken JE, Bjørndal L, Maltz M, Manton DJ, Ricketts D, Van Landuyt K, Banerjee A, Campus G, Doméjean S, Fontana M, Leal S, Lo E, Machiulskiene V, Schulte A, Splieth C, Zandona AF, Innes NP, Managing Carious Lesions: consensus Recommendations on Carious Tissue Removal, Adv. Dent. Res 28 (2) (2016) 58–67. [DOI] [PubMed] [Google Scholar]
- [4].Heintze SD, Rousson V, Clinical effectiveness of direct class II restorations - a meta-analysis, J. Adhes. Dent 14 (5) (2012) 407–431. [DOI] [PubMed] [Google Scholar]
- [5].Wood L, United States Dental Materials Market (2019 to 2026) - Analysis, Size & Trends - Researchandmarkets.Com, Ed., B.-A.B.H. company, 2020. [Google Scholar]
- [6].Wataha JC, Predicting clinical biological responses to dental materials, Dent. Mater 28 (1) (2012) 23–40. [DOI] [PubMed] [Google Scholar]
- [7].Schmalz G, Galler KM, Biocompatibility of biomaterials - Lessons learned and considerations for the design of novel materials, Dent. Mater 33 (4) (2017) 382–393. [DOI] [PubMed] [Google Scholar]
- [8].de Souza Costa CA, Hebling J, Scheffel DL, Soares DG, Basso FG, Ribeiro AP, Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques, Dent. Mater 30 (7) (2014) 769–784. [DOI] [PubMed] [Google Scholar]
- [9].Williams DF, On the mechanisms of biocompatibility, Biomaterials 29 (20) (2008) 2941–2953. [DOI] [PubMed] [Google Scholar]
- [10].Cieplik F, Aparicio C, Kreth J, Schmalz G, Development of standard protocols for biofilm-biomaterial interface testing, JADA Found. Sci 1 (2022) 100008. [Google Scholar]
- [11].Sahin N, Saygili S, Akcay M, Clinical, radiographic, and histological evaluation of three different pulp-capping materials in indirect pulp treatment of primary teeth: a randomized clinical trial, Clin. Oral Investig 25 (6) (2021) 3945–3955. [DOI] [PubMed] [Google Scholar]
- [12].Kitagawa M, Murakami S, Akashi Y, Oka H, Shintani T, Ogawa I, Inoue T, Kurihara H, Current status of dental metal allergy in Japan, J. Prosthodont. Res 63 (3) (2019) 309–312. [DOI] [PubMed] [Google Scholar]
- [13].Li Y, Carrera C, Chen R, Li J, Lenton P, Rudney JD, Jones RS, Aparicio C, Fok A, Degradation in the dentin-composite interface subjected to multi-species biofilm challenges, Acta Biomater. 10 (1) (2014) 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kreth J, Merritt J, Pfeifer CS, Khajotia S, Ferracane JL, Interaction between the Oral Microbiome and Dental Composite Biomaterials: where We Are and Where We Should Go, J. Dent. Res 99 (10) (2020) 1140–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cehreli SB, Tirali RE, Yalcinkaya Z, Cehreli ZC, Microleakage of newly developed glass carbomer cement in primary teeth, Eur. J. Dent 7 (1) (2013) 15–21. [PMC free article] [PubMed] [Google Scholar]
- [16].Van Landuyt KL, Nawrot T, Geebelen B, De Munck J, Snauwaert J, Yoshihara K, Scheers H, Godderis L, Hoet P, Van Meerbeek B, How much do resin-based dental materials release? a meta-analytical approach, Dent. Mater 27 (8) (2011) 723–747. [DOI] [PubMed] [Google Scholar]
- [17].Kawahara H, Yamagami A, Nakamura M, Biological testing of dental materials by means of tissue culture, Int. Dent. J 18 (2) (1968) 443–467. [PubMed] [Google Scholar]
- [18].I.O.f. Standardization, ISO-10993Biological Evaluation of Medical Devices, ISO, 2018. [Google Scholar]
- [19].O.f. Standardization, in: ISO 7405:2018 Dentistry — Evaluation of Biocompatibility of Medical Devices Used in Dentistry, Organization for Standardization, 2018, p. 43. [Google Scholar]
- [20].Hanks CT, Wataha JC, Sun Z, In vitro models of biocompatibility: a review, Dent. Mater 12 (3) (1996) 186–193. [DOI] [PubMed] [Google Scholar]
- [21].Brancato V, Oliveira JM, Correlo VM, Reis RL, Kundu SC, Could 3D models of cancer enhance drug screening? Biomaterials 232 (2020) 119744. [DOI] [PubMed] [Google Scholar]
- [22].Álvarez-García YR, Ramos-Cruz KP, Agostini-Infanzón RJ, Stallcop LE, Beebe DJ, Warrick JW, Domenech M, Open multi-culture platform for simple and flexible study of multi-cell type interactions, Lab Chip 18 (20) (2018) 3184–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Court M, Malier M, Millet A, 3D type I collagen environment leads up to a reassessment of the classification of human macrophage polarizations, Biomaterials 208 (2019) 98–109. [DOI] [PubMed] [Google Scholar]
- [24].Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ, Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement, Proc. Natl. Acad. Sci. U. S. A 106 (2) (2009) 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, Kamm RD, Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation, Proc. Natl. Acad. Sci. U. S. A 112 (1) (2015) 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li Y, Kilian KA, Bridging the Gap: from 2D Cell Culture to 3D Microengineered Extracellular Matrices, Adv. Healthc. Mater 4 (18) (2015) 2780–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pagano S, Lombardo G, Balloni S, Bodo M, Cianetti S, Barbati A, Montaseri A, Marinucci L, Cytotoxicity of universal dental adhesive systems: assessment in vitro assays on human gingival fibroblasts, Toxicol. in Vitro 60 (2019) 252–260. [DOI] [PubMed] [Google Scholar]
- [28].Schmalz G, Schweikl H, Esch J, Hiller KA, Evaluation of a dentin barrier test by cyctotoxicity testing of various dental cements, J. Endod 22 (3) (1996) 112–115. [DOI] [PubMed] [Google Scholar]
- [29].Bertassoni LE, Stankoska K, Swain MV, Insights into the structure and composition of the peritubular dentin organic matrix and the lamina limitans, Micron 43 (2–3) (2012) 229–236. [DOI] [PubMed] [Google Scholar]
- [30].Miyashita S, Ahmed NE, Murakami M, Iohara K, Yamamoto T, Horibe H, Kurita K, Takano-Yamamoto T, Nakashima M, Mechanical forces induce odontoblastic differentiation of mesenchymal stem cells on three-dimensional biomimetic scaffolds, J. Tissue Eng. Regen. Med 11 (2) (2017) 434–446. [DOI] [PubMed] [Google Scholar]
- [31].Bertassoni LE, Orgel JP, Antipova O, Swain MV, The dentin organic matrix - limitations of restorative dentistry hidden on the nanometer scale, Acta Biomater. 8 (7) (2012) 2419–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Horsophonphong S, Sercia A, França CM, Tahayeri A, Reddy AP, Wilmarth PA, Surarit R, Smith AJ, Ferracane JL, Bertassoni LE, Equivalence of human and bovine dentin matrix molecules for dental pulp regeneration: proteomic analysis and biological function, Arch. Oral. Biol 119 (2020) 104888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Salehi S, Cooper P, Smith A, Ferracane J, Dentin matrix components extracted with phosphoric acid enhance cell proliferation and mineralization, Dent. Mater 32 (3) (2016) 334–342. [DOI] [PubMed] [Google Scholar]
- [34].Graham L, Cooper PR, Cassidy N, Nor JE, Sloan AJ, Smith AJ, The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components, Biomaterials 27 (14) (2006) 2865–2873. [DOI] [PubMed] [Google Scholar]
- [35].Tomson PL, Lumley PJ, Smith AJ, Cooper PR, Growth factor release from dentine matrix by pulp-capping agents promotes pulp tissue repair-associated events, Int. Endod. J 50 (3) (2017) 281–292. [DOI] [PubMed] [Google Scholar]
- [36].Ferracane JL, Cooper PR, Smith AJ, Dentin matrix component solubilization by solutions of pH relevant to self-etching dental adhesives, J. Adhes. Dent 15 (5) (2013) 407–412. [DOI] [PubMed] [Google Scholar]
- [37].Lin CP, Chen YJ, Lee YL, Wang JS, Chang MC, Lan WH, Chang HH, Chao WM, Tai TF, Lee MY, Lin BR, Jeng JH, Effects of root-end filling materials and eugenol on mitochondrial dehydrogenase activity and cytotoxicity to human periodontal ligament fibroblasts, J. Biomed. Mater. Res. B Appl. Biomater 71 (2) (2004) 429–440. [DOI] [PubMed] [Google Scholar]
- [38].Paschalidis T, Bakopoulou A, Papa P, Leyhausen G, Geurtsen W, Koidis P, Dental pulp stem cells’ secretome enhances pulp repair processes and compensates TEGDMA-induced cytotoxicity, Dent. Mater 30 (12) (2014) e405–e418. [DOI] [PubMed] [Google Scholar]
- [39].Outhwaite WC, McKenzie DM, Pashley DH, A versatile split-chamber device for studying dentin permeability, J. Dent. Res 53 (6) (1974) 1503. [DOI] [PubMed] [Google Scholar]
- [40].Meryon SD, Jakeman KJ, An in vitro study of the role of dentine in moderating the cytotoxicity of zinc oxide eugenol cement, Biomaterials 7 (6) (1986) 459–462. [DOI] [PubMed] [Google Scholar]
- [41].Hume WR, An analysis of the release and the diffusion through dentin of eugenol from zinc oxide-eugenol mixtures, J. Dent. Res 63 (6) (1984) 881–884. [DOI] [PubMed] [Google Scholar]
- [42].Hanks CT, Craig RG, Diehl ML, Pashley DH, Cytotoxicity of dental composites and other materials in a new in vitro device, J. Oral Pathol 17 (8) (1988) 396–403. [DOI] [PubMed] [Google Scholar]
- [43].Scheffel DL, Soares DG, Basso FG, de Souza Costa CA, Pashley D, Hebling J, Transdentinal cytotoxicity of glutaraldehyde on odontoblast-like cells, J. Dent 43 (8) (2015) 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Leite MLAE, Costa CAS, Duarte RM, Andrade AKM, Soares DG, Bond Strength and Cytotoxicity of a Universal Adhesive According to the Hybridization Strategies to Dentin, Braz. Dent. J 29 (1) (2018) 68–75. [DOI] [PubMed] [Google Scholar]
- [45].Schmalz G, Schweikl H, Characterization of an in vitro dentin barrier test using a standard toxicant, J. Endod 20 (12) (1994) 592–594. [DOI] [PubMed] [Google Scholar]
- [46].Jiang RD, Lin H, Zheng G, Zhang XM, Du Q, Yang M, In vitro dentin barrier cytotoxicity testing of some dental restorative materials, J. Dent 58 (2017) 28–33. [DOI] [PubMed] [Google Scholar]
- [47].Schmalz G, Schuster U, Koch A, Schweikl H, Cytotoxicity of low pH dentin-bonding agents in a dentin barrier test in vitro, J. Endod 28 (3) (2002) 188–192. [DOI] [PubMed] [Google Scholar]
- [48].Schuster U, Schmalz G, Thonemann B, Mendel N, Metzl C, Cytotoxicity testing with three-dimensional cultures of transfected pulp-derived cells, J. Endod 27 (4) (2001) 259–265. [DOI] [PubMed] [Google Scholar]
- [49].da Silva JM, Rodrigues JR, Camargo CH, Fernandes VV, Hiller KA, Schweikl H, Schmalz G, Effectiveness and biological compatibility of different generations of dentin adhesives, Clin. Oral Investig 18 (2) (2014) 607–613. [DOI] [PubMed] [Google Scholar]
- [50].I.I.O.o. Standards, in: Dentistry - Evaluation of Biocompatibility of Medical Devices Used in Dentistry, ISO 7405, ISO, Geneva, Switzerland, 2018, p. 50. [Google Scholar]
- [51].Magloire H, Joffre A, Bleicher F, An in vitro model of human dental pulp repair, J. Dent. Res 75 (12) (1996) 1971–1978. [DOI] [PubMed] [Google Scholar]
- [52].Téclès O, Laurent P, Aubut V, About I, Human tooth culture: a study model for reparative dentinogenesis and direct pulp capping materials biocompatibility, J. Biomed. Mater. Res. B Appl. Biomater 85 (1) (2008) 180–187. [DOI] [PubMed] [Google Scholar]
- [53].Téclès O, Laurent P, Zygouritsas S, Burger AS, Camps J, Dejou J, About I, Activation of human dental pulp progenitor/stem cells in response to odontoblast injury, Arch. Oral. Biol 50 (2) (2005) 103–108. [DOI] [PubMed] [Google Scholar]
- [54].Xiang L, Yin Y, Zheng Y, Ma Y, Li Y, Zhao Z, Guo J, Ai Z, Niu Y, Duan K, He J, Ren S, Wu D, Bai Y, Shang Z, Dai X, Ji W, Li T, A developmental landscape of 3D-cultured human pre-gastrulation embryos, Nature 577 (7791) (2020) 537–542. [DOI] [PubMed] [Google Scholar]
- [55].Kaufman G, Skrtic D, Structural and recovery mechanisms of 3D dental pulp cell microtissues challenged with Streptococcusmutans in extracellular matrix environment, J. Med. Microbiol 65 (11) (2016) 1332–1340. [DOI] [PubMed] [Google Scholar]
- [56].Khayat A, Monteiro N, Smith EE, Pagni S, Zhang W, Khademhosseini A, Yelick PC, GelMA-Encapsulated hDPSCs and HUVECs for Dental Pulp Regeneration, J. Dent. Res 96 (2) (2017) 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen G, Chen J, Yang B, Li L, Luo X, Zhang X, Feng L, Jiang Z, Yu M, Guo W, Tian W, Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration, Biomaterials 52 (2015) 56–70. [DOI] [PubMed] [Google Scholar]
- [58].Cavalcanti BN, Zeitlin BD, Nör JE, A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells, Dent. Mater 29 (1) (2013) 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gorin C, Rochefort GY, Bascetin R, Ying H, Lesieur J, Sadoine J, Beckouche N, Berndt S, Novais A, Lesage M, Hosten B, Vercellino L, Merlet P, Le-Denmat D, Marchiol C, Letourneur D, Nicoletti A, Vital SO, Poliard A, Salmon B, Muller L, Chaussain C, Germain S, Priming Dental Pulp Stem Cells With Fibroblast Growth Factor-2 Increases Angiogenesis of Implanted Tissue-Engineered Constructs Through Hepatocyte Growth Factor and Vascular Endothelial Growth Factor Secretion, Stem Cells Transl. Med 5 (3) (2016) 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Iohara K, Murakami M, Takeuchi N, Osako Y, Ito M, Ishizaka R, Utunomiya S, Nakamura H, Matsushita K, Nakashima M, A Novel Combinatorial Therapy With Pulp Stem Cells and Granulocyte Colony-Stimulating Factor for Total Pulp Regeneration, Stem Cells Transl. Med 2 (10) (2013) 818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lu Q, Pandya M, Rufaihah AJ, Rosa V, Tong HJ, Seliktar D, Toh WS, Modulation of dental pulp stem cell odontogenesis in a tunable PEG-fibrinogen hydrogel system, Stem Cells Int (2015) 525367 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Galler KM, Brandl FP, Kirchhof S, Widbiller M, Eidt A, Buchalla W, Göpferich A, Schmalz G, Suitability of Different Natural and Synthetic Biomaterials for Dental Pulp Tissue Engineering, Tissue Eng. Part A 24 (3–4) (2018) 234–244. [DOI] [PubMed] [Google Scholar]
- [63].Hadjichristou C, Papachristou E, Bonovolias I, Bakopoulou A, Three-dimensional tissue engineering-based Dentin/Pulp tissue analogue as advanced biocompatibility evaluation tool of dental restorative materials, Dent. Mater 36 (2) (2020) 229–248. [DOI] [PubMed] [Google Scholar]
- [64].Hadjichristou C, Papachristou E, Vereroudakis E, Chatzinikolaidou M, About I, Koidis P, Bakopoulou A, Biocompatibility assessment of resin-based cements on vascularized dentin/pulp tissue-engineered analogues, Dent. Mater (2021). [DOI] [PubMed] [Google Scholar]
- [65].L N, H Z, Y L, Y W, A L, R L, R Z, Q Y, Microfluidic Chip for Odontoblasts in vitro, ACS Biomater. Sci. Eng 5 (2019). [DOI] [PubMed] [Google Scholar]
- [66].Xu X, Li Z, Ai X, Tang Y, Yang D, Dou L, Human three-dimensional dental pulp organoid model for toxicity screening of dental materials on dental pulp cells and tissue, Int. Endod. J 55 (1) (2022) 79–88. [DOI] [PubMed] [Google Scholar]
- [67].Bhatia SN, Ingber DE, Microfluidic organs-on-chips, Nat. Biotechnol 32 (8) (2014) 760–772. [DOI] [PubMed] [Google Scholar]
- [68].Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE, Reconstituting organ-level lung functions on a chip, Science 328 (5986) (2010) 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ingber DE, Is it time for reviewer 3 to request human organ chip experiments instead of animal validation studies? Adv. Sci. (Weinh) 7 (22) (2020) 2002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Barrile R, van der Meer AD, Park H, Fraser JP, Simic D, Teng F, Conegliano D, Nguyen J, Jain A, Zhou M, Karalis K, Ingber DE, Hamilton GA, Otieno MA, Organ-on-Chip Recapitulates Thrombosis Induced by an anti-CD154 Monoclonal Antibody: translational Potential of Advanced Microengineered Systems, Clin. Pharmacol. Ther (2018). [DOI] [PubMed] [Google Scholar]
- [71].Jain A, Barrile R, van der Meer AD, Mammoto A, Mammoto T, De Ceunynck K, Aisiku O, Otieno MA, Louden CS, Hamilton GA, Flaumenhaft R, Ingber DE, Primary Human Lung Alveolus-on-a-chip Model of Intravascular Thrombosis for Assessment of Therapeutics, Clin. Pharmacol. Ther 103 (2) (2018) 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Benam KH, Villenave R, Lucchesi C, Varone A, Hubeau C, Lee HH, Alves SE, Salmon M, Ferrante TC, Weaver JC, Bahinski A, Hamilton GA, Ingber DE, Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro, Nat. Methods 13 (2) (2016) 151–157. [DOI] [PubMed] [Google Scholar]
- [73].Musah S, Dimitrakakis N, Camacho DM, Church GM, Ingber DE, Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a Glomerulus Chip, Nat. Protoc 13 (7) (2018) 1662–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, Fatanat-Didar T, Koshy S, Weaver JC, Church GM, Ingber DE, Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip, Nat. Biomed. Eng 1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Low LA, Mummery C, Berridge BR, Austin CP, Tagle DA, Organs-on-chips: into the next decade, Nat. Rev. Drug Discov (2020). [DOI] [PubMed] [Google Scholar]
- [76].Niu L, Zhang H, Liu Y, Wang Y, Li A, Liu R, Zou R, Yang Q, Microfluidic Chip for Odontoblasts, ACS Biomater. Sci. Eng 5 (9) (2019) 4844–4851. [DOI] [PubMed] [Google Scholar]
- [77].França CM, Tahayeri A, Rodrigues NS, Ferdosian S, Puppin Rontani RM, Sereda G, Ferracane JL, Bertassoni LE, The tooth on-a-chip: a microphysiologic model system mimicking the biologic interface of the tooth with biomaterials, Lab Chip 20 (2) (2020) 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Rodrigues NS, França CM, Tahayeri A, Ren Z, Saboia VPA, Smith AJ, Ferracane JL, Koo H, Bertassoni LE, Biomaterial and Biofilm Interactions with the Pulp-Dentin Complex-on-a-Chip, J. Dent. Res 100 (10) (2021) 1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bertassoni LE, Progress and Challenges in Microengineering the Dental Pulp Vascular Microenvironment, J. Endod 46 (9S) (2020) S90–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hu S, Muniraj G, Mishra A, Hong K, Lum JL, Hong CHL, Rosa V, Sriram G, Characterization of silver diamine fluoride cytotoxicity using microfluidic tooth-on-a-chip and gingival equivalents, Dent. Mater (2022). [DOI] [PubMed] [Google Scholar]
- [81].Sriram G, Sudhaharan T, Wright GD, Multiphoton Microscopy for Noninvasive and Label-Free Imaging of Human Skin and Oral Mucosa Equivalents, Methods Mol. Biol 2150 (2020) 195–212. [DOI] [PubMed] [Google Scholar]
- [82].Ly KL, Rooholghodos SA, Rahimi C, Rahimi B, Bienek DR, Kaufman G, Raub CB, Luo X, An Oral-mucosa-on-a-chip sensitively evaluates cell responses to dental monomers, Biomed. Microdevices 23 (1) (2021) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rahimi C, Rahimi B, Padova D, Rooholghodos SA, Bienek DR, Luo X, Kaufman G, Raub CB, Oral mucosa-on-a-chip to assess layer-specific responses to bacteria and dental materials, Biomicrofluidics 12 (5) (2018) 054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Jin L, Kou N, An F, Gao Z, Tian T, Hui J, Chen C, Ma G, Mao H, Liu H, Analyzing Human Periodontal Soft Tissue Inflammation and Drug Responses In Vitro Using Epithelium-Capillary Interface On-a-Chip, Biosensors (Basel) 12 (5) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ferracane JL, Bertassoni LE, Interface between Materials and Oral Biology, J. Dent. Res 100 (10) (2021) 1009–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Fontana F, Figueiredo P, Martins JP, Santos HA, Requirements for Animal Experiments: problems and Challenges, Small 17 (15) (2021) e2004182. [DOI] [PubMed] [Google Scholar]
- [87].Prince E, Kheiri S, Wang Y, Xu F, Cruickshank J, Topolskaia V, Tao H, Young EWK, McGuigan AP, Cescon DW, Kumacheva E, Microfluidic Arrays of Breast Tumor Spheroids for Drug Screening and Personalized Cancer Therapies, Adv Healthc Mater, 2021. [DOI] [PubMed] [Google Scholar]
- [88].Chen Z, Kheiri S, Gevorkian A, Young EWK, Andre V, Deisenroth T, Kumacheva E, Microfluidic arrays of dermal spheroids: a screening platform for active ingredients of skincare products, Lab Chip 21 (20) (2021) 3952–3962. [DOI] [PubMed] [Google Scholar]
- [89].van den Berg A, Mummery CL, Passier R, van der Meer AD, Personalised organs-on-chips: functional testing for precision medicine, Lab Chip 19 (2) (2019) 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Jodat YA, Kang MG, Kiaee K, Kim GJ, Martinez AFH, Rosenkranz A, Bae H, Shin SR, Human-derived organ-on-a-chip for personalized drug development, Curr. Pharm. Des 24 (45) (2018) 5471–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Llenas M, Paoli R, Feiner-Gracia N, Albertazzi L, Samitier J, Caballero D, Versatile vessel-on-a-chip platform for studying key features of blood vascular tumors, Bioengineering (Basel) 8 (6) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gibbons SM, Gurry T, Lampe JW, Chakrabarti A, Dam V, Everard A, Goas A, Gabriele G, Kleerebez M, Lane J, Maukonen J, Penna ALB, Pot B, Valdes AM, Walton G, Weiss A, Zanzer YC, Venlet NV, Miani M, Perspective: Leveraging the Gut Microbiota to Predict Personalized Responses to Dietary, Prebiotic, and Probiotic Interventions, Adv Nutr, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Fugolin APP, Pfeifer CS, New Resins for Dental Composites, J. Dent. Res 96 (10) (2017) 1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]


