Abstract
Background.
Corneal ulcers are a common cause of blindness in low- and middle-income countries, usually resulting from traumatic corneal abrasions during agricultural work. Antimicrobial prophylaxis of corneal abrasions may help prevent corneal ulcers, but delays in initiation of therapy are frequent.
Methods.
A cluster-randomized trial was performed in Nepal to determine the effectiveness of a community-based corneal ulcer prevention program (clinicaltrials.gov #NCT01969786). The randomization unit was the Village Development Committee (VDC). VDCs in the study area with <15,000 people were eligible for inclusion. In the intervention arm, pre-existing Female Community Health Volunteers (FCHVs) were trained to diagnose corneal abrasions and provide a three-day course of ophthalmic antimicrobials. The primary outcome was incident corneal ulceration, determined by masked assessment of corneal photographs.
Findings.
Between February 4, 2014 and October 20, 2017, 12 VDCs were randomized to receive the intervention and 12 control VDCs to receive no intervention. 213,697 individuals were included on the baseline census. FCHVs diagnosed and provided antimicrobials for 4,777 corneal abrasions. Medication allergy was self-reported in 0.2%. The census identified 289 incident corneal opacities among 246,893 person-years in the intervention arm and 262 opacities among 239,170 person-years in the control arm (incidence 1.21, 95%CI 0.85–1.74 per 1000 person-years in the intervention arm and 1.18, 95%CI 0.82–1.70 in the control arm); intention-to-treat incidence rate ratio (IRR) 1.03, 95%CI 0.63–1.67; P=0.93. Exploratory subgroup analyses suggested the effectiveness might depend on geography (e.g., IRR 0.50, 95%CI 0.19–1.30 in rural areas versus IRR 1.31, 95%CI 0.86–1.99 in peri-urban or urban areas; P=0.04 for subgroup interaction).
Interpretation.
This trial failed to detect a reduction in incident corneal ulceration during the first three years of a community-based corneal ulcer prevention program. Further study may be warranted in more rural areas where basic eye care facilities are not available.
Keywords: corneal injuries, corneal ulcer, community health workers, secondary prevention, anti-bacterial agents, antifungal agents
INTRODUCTION
Corneal opacity is an important cause of blindness globally.1 As traditional infectious causes of corneal opacity like trachoma and onchocerciasis have declined, the importance of corneal ulcer (i.e., microbial keratitis) has increased.1,2 In low- and middle-income countries, the most common cause of corneal ulcer is traumatic corneal abrasion, which is often experienced during agricultural work or other manual labor.3–9 In these settings, care-seeking for ocular trauma is often delayed, if pursued at all, increasing the likelihood of developing a corneal ulcer and a subsequent vision-threatening corneal opacity.3,8
Earlier initiation of antimicrobial prophylaxis for corneal abrasions may be an effective way to reduce the burden of corneal infections in resource-limited settings.10 For example, a previous study of patients with corneal abrasions in Nepal found that corneal ulcers were more likely to develop if antibiotic therapy was delayed by more than 18 hours after the inciting trauma.11 Several studies in Southeast Asia have attempted to reduce the time from trauma to treatment by instituting programs in which community health workers identify cases of ocular trauma or corneal abrasion and provide antimicrobial prophylaxis directly or through referral.11–15 These studies had promising results, with very few ulcers detected after program implementation. None of the studies included an untreated control group, so it was not possible to determine whether the paucity of ulcers was caused by the program or by other factors. To address this key limitation, a cluster-randomized trial was designed to test the effectiveness of a community-based corneal ulcer prevention program relative to an untreated control group. The cluster-randomized design was appropriate for the community-based nature of the intervention and reduced the risk of contamination compared to an individually-randomized trial. The trial tested the hypothesis that institution of a community health volunteer program for quick diagnosis and management of corneal abrasions would ultimately reduce the cluster-level incidence of corneal opacities.
METHODS
Study design and participants
The Village-Integrated Eye Worker (VIEW) trial was a cluster-randomized trial performed from February 4, 2014 to October 20, 2017 in the Chitwan and Nawalparasi districts of Nepal. Study clusters were government-defined administrative units known as Village Development Committees (VDCs), which were randomized to receive a corneal ulcer prevention program or to no intervention and followed for 3 years for photographic evidence of corneal opacity. The design and methods of the VIEW trial have been reported elsewhere.16 Ethical approval for the trial was obtained from the UCSF Committee on Human Research, Nepal Netra Jyoti Sangh, and the Nepal Health Research Council. A data and safety monitoring committee reviewed the protocol before study implementation and provided oversight during the trial. The trial adhered to the tenets of the Declaration of Helsinki.
The study was set in a low-lying, agrarian, relatively densely populated part of Nepal; approximately three-quarters of the population was literate in the 2011 national census.17 VDCs were administrative divisions in existence at the start of the study; each VDC consisted of 9 wards. Each ward had a government-supported Female Community Health Volunteer (FCHV) elected by the community to perform public health tasks (e.g., family planning, nutrition education for pregnant women). VIEW leveraged the FCHV program, adding eye care to their portfolio of services.
Because the intervention was thought to likely be most effective in rural areas, VDCs in the catchment area of Bharatpur Eye Hospital with a population of less than 15,000 on the 2001 Nepal census were deemed eligible. Twenty-four VDCs met the inclusion criteria and were included in the trial (appendix, p 5). Residents of all ages from all households in study communities were offered enrollment through an annual door-to-door census. Verbal consent was obtained for census activities before randomization was performed. Written consent was obtained for the actual FCHV intervention.
Randomization and masking
VDCs were stratified by district and randomized to receive the intervention or to no intervention. The study biostatistician (TCP) used R version 3 (R Project for Statistical Computing, Vienna, Austria) to generate the random allocation sequence after the baseline census was completed. Allocation was concealed by enrolling all study communities before randomization and by offering the prevention program to all community members in intervention communities simultaneously. Local study staff at the Bharatpur Eye Hospital implemented the randomization sequence.
Given the nature of this community-based intervention, study participants and the study personnel administering the intervention were not masked. However, census workers were not informed of the randomization allocation and all publicity materials (e.g., posters) were removed during census periods to avoid unmasking census workers. Outcome assessors (i.e., photo-graders) were masked to intervention allocation. Contamination was possible since people traveling from control communities to intervention communities could have seen publicity materials, although the large size of the randomization units limited the magnitude of contamination, and FCHVs offered services only to people living in the intervention communities.
Procedures
In intervention VDCs, existing FCHVs were trained to diagnose corneal abrasions using fluorescein strips, 2.5× magnifying loupes, and an ultraviolet flashlight. If a corneal abrasion was diagnosed, the FCHV provided 1% chloramphenicol ointment in single-dose applicaps (Chloromycetin Kaps, Pfizer India) and 1% itraconazole ointment (Itral, Jawa Pharmaceuticals), each of which was to be applied 3 times daily for 3 days. Pregnant women or those with a self-reported chloramphenicol allergy received 1% azithromycin ointment (Zaha, Ajanta Pharma Ltd) instead of chloramphenicol. Chloramphenicol was chosen based on its efficacy and safety in prior community-based studies of corneal ulcer prophylaxis;11–14 because its formulation as an ointment would provide a better barrier to super-infection relative to an eyedrop; because its packaging as single-dose applicaps aided in determining adherence; and because the high concentrations achievable with a topical preparation of this broad-spectrum antibiotic would likely be effective for most gram positive and many gram negative organisms. Itraconazole was chosen because of its availability as an ointment and its relatively low cost. Together, these two medications would cover the vast majority of pathogens that cause infectious keratitis in Nepal.18–23 The FCHV administered the first dose of the antimicrobial ointments. Participants were asked to return to the FCHV for a follow-up examination after 3 days, at which point the FCHV re-examined the affected eye with fluorescein as before, and asked an open-ended question about adverse events. FCHVs recorded participant information on paper forms. Any participant with a corneal ulcer, bilateral corneal abrasions, visual acuity worse than Counting Fingers in the non-affected eye, or another referable eye problem at the initial visit was referred to Bharatpur Eye Hospital or the nearest primary eye care center; participants with a corneal ulcer, non-healing corneal abrasion, or allergic reaction at the follow-up visit were also referred. FCHVs obtained written informed consent from all participants. Because the goal of the research was to assess the effectiveness of a community-based corneal ulcer prevention program versus no program, the control communities did not receive any study interventions.
A publicity campaign was conducted in intervention VDCs to encourage residents to present to FCHVs within 24 hours of experiencing ocular trauma (i.e., any injury to the eye or eyelid, typically accompanied by pain, tearing, and blurry vision). Publicity activities included orientation meetings with local leaders and community groups, door-to-door visits by FCHVs, and the distribution of posters, pamphlets, calendars, and pens.
An intervention awareness survey was conducted annually in intervention and control VDCs by masked survey workers. A random sample of households was selected from the prior census to participate in the survey and visited by trained survey workers, who asked an adult in each selected household a series of questions to determine their level of awareness of the intervention. The survey provided information on the effectiveness of the publicity campaign in intervention VDCs as well as the level of contamination to control VDCs.
A door-to-door census was started at months 0, 12, 24, and 36 in all study communities. Each census was conducted during the calendar year of the study (i.e., 2014 to 2017), with an initial pass of households taking several months followed by mop-up activities to increase photographic coverage for the remainder of the year. Trained census workers visited all households in the community and asked all household members a series of screening questions for eye trauma (e.g., eye trauma, sudden decreased vision, eye pain, and corneal infection) occurring since the prior census. Data were collected on mobile devices using a custom-designed mobile application (Conexus, Los Gatos, CA). Corneal photographs were taken using the Corneal CellScope (Development Impact Lab, Berkeley, CA, USA), a custom-made 3D-printed smartphone attachment with a +25 diopter lens and external illumination. Photographs were taken of both eyes of all residents at the baseline and final censuses, and of both eyes of residents answering positively to one of the screening questions during interim censuses.
Photographs were graded for the presence of incident corneal opacities by trained, masked graders. Photographs from individuals who answered any screening question positively at a follow-up census were presented to two trained graders in a random order, without identifying information. Photographs were presented for each eye and each census separately and graded for opacity on a 4-point scale (e.g., definitely yes, probably, possibly, definitely no). If the eye was graded as having a possible, probable, or definite opacity then all photographs from previous censuses were displayed in order to make a determination of whether the opacity had newly developed relative to a prior photograph. If photographs from a previous phase were of a different eye, this was noted. All eyes graded as possible, probable, or definite opacity were subsequently graded by one of three cornea specialists using the same procedures. An eye was classified as having an incident opacity if the cornea specialist judged the eye to have a definite or probable opacity at one phase and the same eye had definitely no opacity or a possible opacity at a prior phase.
Outcomes
The primary outcome of incident corneal ulceration was defined as the count of incident corneal opacities per randomization unit, as assessed during the census. An eye was classified as having an incident opacity if graders identified a new corneal opacity (i.e., active ulcer or inactive scar) on a follow-up census photograph that was not present on photographs from a previous census. Individuals were allowed to contribute multiple incident opacities to the overall community count but could contribute no more than one new incident ulcer per eye per census period. For the time-at-risk, individuals began contributing person-time at the first census in which they had photographs taken and continued to contribute time until their final census visit.
Participants were censored if documented as having died or moved on the census, or at the final month 36 census visit, whichever occurred first. Individuals with incident opacities continued to contribute person-time since they remained at risk for additional opacities. Pre-specified secondary outcomes included the prevalence of visual impairment caused by incident corneal opacities and the cost-effectiveness of the intervention (each to be reported in a separate publication) as well as the fidelity indicators of time from eye trauma to FCHV presentation (intervention arm only) and awareness of study intervention (both arms).
Statistical analysis
We estimated that including 12 VDCs per arm would provide greater than 80% power to detect a 30% reduction in the incidence of corneal ulcers at an alpha of 0.05. This calculation assumed 9,000 individuals per cluster (VDC) based on average VDC size in the study area, an annual incidence in the control arm of 100 per 100,000 person-years based on previous studies, an intra-class correlation coefficient (ICC) of 0.00015, and three years of follow-up.24
The primary, intention-to-treat analysis employed negative binomial regression on VDC-level data to model the count of incident corneal ulcers during the entire study period as a function of treatment arm, with log-person time at risk as an offset. The model’s dispersion parameter accounted for the clustered nature of the data. Significance testing was performed by Monte Carlo permutation (N=10,000 replications, accounting for stratified randomization), with an overall alpha of 0.05 for the interim and final analyses. The pre-planned interim analysis was performed after the first follow-up census; this analysis spent 0.001 alpha and did not meet pre-specified criteria for early stopping. Exploratory subgroup analyses were performed using similar regression models, but on ward-level data. Analyses were performed in R (version 4). The manual of procedures (https://osf.io/t5wp4/) and statistical analysis plan (https://osf.io/rmezw/) are available. The study was registered on ClinicalTrials.gov (NCT01969786) and overseen by a Data and Safety Monitoring Committee.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
RESULTS
The initial census started on February 4, 2014 and enumerated 213,697 people in the 24 study clusters (Figure 1). Randomization was performed on May 22, 2014. Baseline characteristics of the 12 intervention and 12 control clusters were well-balanced (Table 1).
Figure 1.
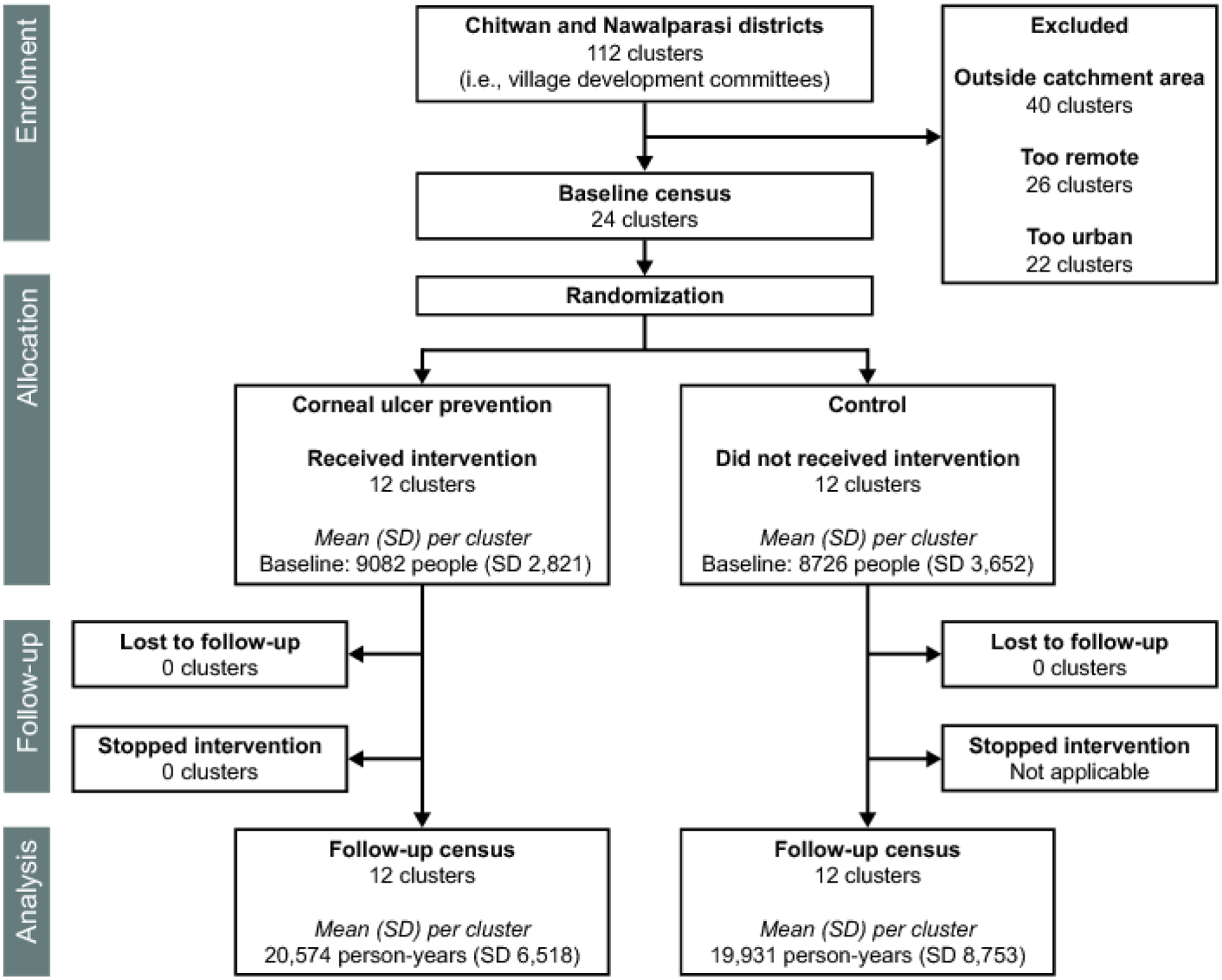
Trial flow
Table 1. Baseline characteristics of 24 Village Development Committees (VDCs) enrolled in the trial.
Values indicate means across the 12 VDCs per treatment arm, with 95% confidence intervals.
| Mean (95%CI) | ||
|---|---|---|
| Intervention N=12 VDCs | Control N=12 VDCs | |
| Wards, Na | ||
| Urban | 0.9 (0–2.3) | 0.6 (0–1.8) |
| Peri-urban | 6.8 (5.2–8.3) | 6.8 (4.8–8.7) |
| Rural | 1.3 (0.3–2.5) | 1.6 (0.2–3.4) |
| Population, N | 9082 (8267–9825) | 8726 (7695–9465) |
| Proportion female, % | 57% (56–58%) | 56% (56–57%) |
| Age distribution, % | ||
| 0–19 y | 41% (37–41%) | 41% (39–41%) |
| 20–39 y | 30% (30–31%) | 31% (30–31%) |
| 40–59 y | 19% (18–20%) | 19% (18–20%) |
| ≥60 y | 10% (10–12%) | 10% (9–10%) |
| Proportion photographed, % | 85% (77–88%) | 85% (76–86%) |
Values represent the mean per community among the 12 VDCs in each treatment arm.
Each VDC in the study area was comprised of 9 wards. Wards were classified as urban, peri-urban, or rural for internal study purposes by local study staff who visited communities as part of the study and were masked to study results. Classification was based on a synthesis of numerous factors, including the geography (plains vs hills); access to transport; distance from the nearest large city, government offices, hospital, and main highway; and government designation as rural or urban.
In June 2014, 116 FCHVs from the 12 intervention clusters were trained to diagnose corneal abrasions and provide antimicrobial ointments as prophylaxis. A group of 6 study supervisors performed periodic home visits and held monthly refresher trainings for the remainder of the study, focusing on review of examination procedures and assessment of ophthalmic knowledge through a set of photographs of corneal abrasions and ulcers. The average attendance at the monthly refresher trainings across all sessions of the study period was 91% (95%CI 88–95%). FCHV services were publicized through written materials posted throughout the community and by word of mouth.16 An annual survey performed on a random sample of 15 households per community found a marked increase in awareness of the FCHV program over the three years of the study (Figure 2). In the final study year, 25% (95%CI 13–37%) of respondents in the intervention arm said their FCHV was their provider of choice for ocular trauma and 40% (95%CI 27–57%) were aware that the FCHV provided services for ocular trauma, compared to 0.2% (95%CI 0–0.4%) and 0.6% (95%CI 0.1–1.4%) of the control arm, respectively.
Figure 2. Intervention awareness survey.
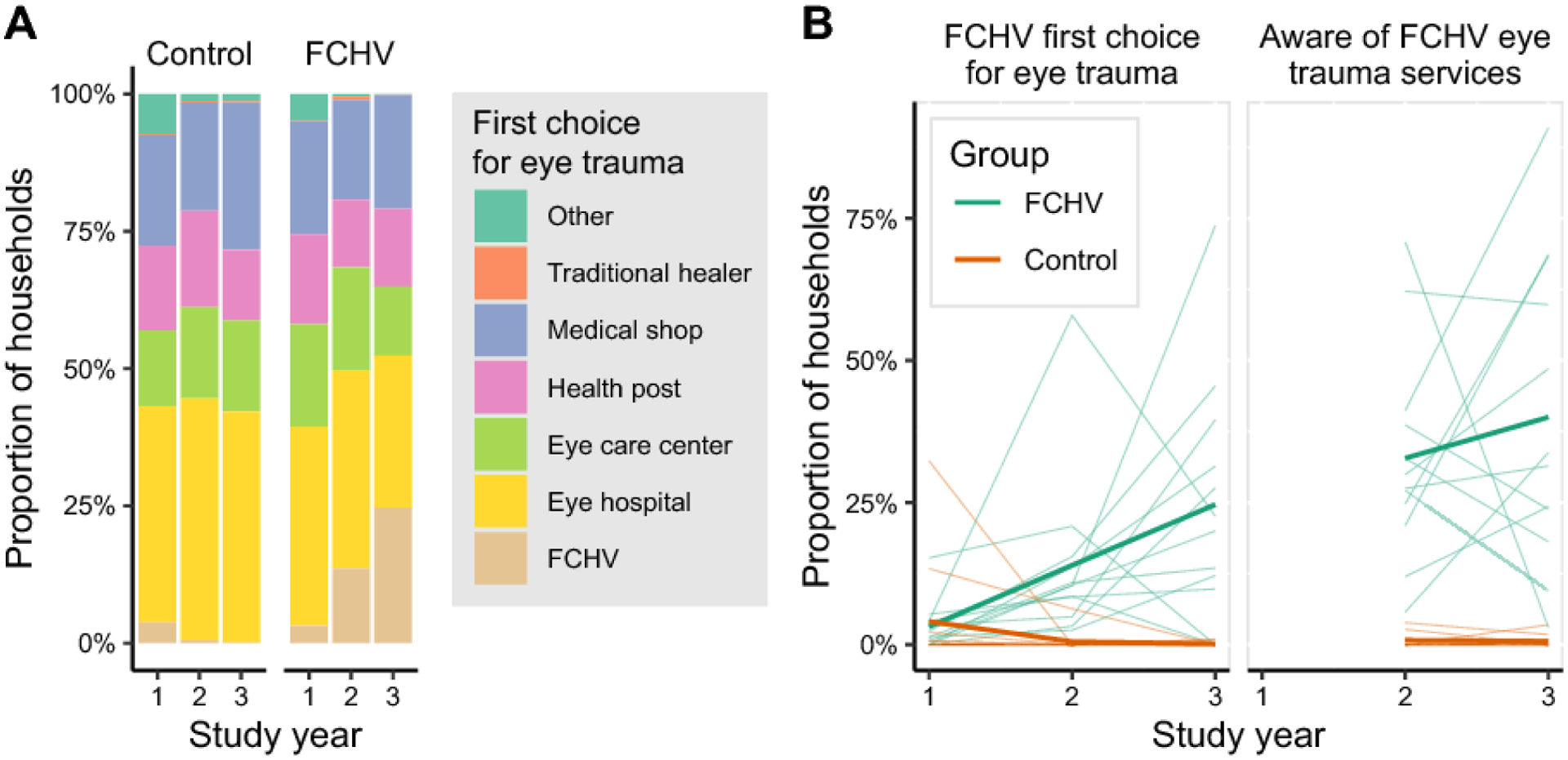
Starting three months after randomization, and then annually, a random sample of households in each community was surveyed. A member of the household was asked in an open-ended question where they would go if they experienced eye trauma, and their first response was recorded. In the second two annual surveys the respondent was asked about all providers available for eye trauma, regardless of whether it was their first choice. Panel A shows the distribution of responses for the first-choice provider of eye care across each annual survey, with all villages aggregated. In panel B, each thin line depicts a single village development committee over time. The thick line depicts the mean. The female community health volunteer (FCHV) intervention clusters are shown in green and control clusters in orange.
Over the 3-year study period, FCHVs completed 10,363 initial patient visits, of which 6,411 (62%) were due to ocular trauma and 4,777 (46%) were diagnosed with a corneal abrasion. Ophthalmic antimicrobials were provided for all cases of corneal abrasion. Self-reported adherence to prophylactic antimicrobial therapy was very high, and the vast majority of abrasions healed upon repeat examination by the FCHV (Table 2). The number of corneal abrasions diagnosed by the FCHV more than doubled each year over the three years of the study (appendix, p 6).
Table 2. Characteristics, adherence, and outcomes of individuals visiting Female Community Health Volunteers (FCHVs) for eye trauma.
Values indicate means across the 12 Village Development Committees, with 95% confidence intervals.
| Characteristic | Mean (95%CI) N=12 VDCs |
|---|---|
| FCHV visits due to eye trauma, N | 534 (447–644) |
| % Female | 60% (58–63%) |
| % <20 years | 22% (19–24%) |
| % 20–39 years | 35% (33–37%) |
| % 40–59 years | 33% (32–35%) |
| % ≥60 years | 10% (9–11%) |
| % Presenting within 18 hours | 59% (56–62%) |
| % Presenting within 24 hours | 72% (69–75%) |
| FCHV-diagnosed corneal abrasion,a N | 398 (307–511) |
| % Completing 4-day follow-up | 96% (94–98%) |
| % Completing antimicrobial prophylaxisb | 95% (94–97%) |
| % Self-reporting allergy to antimicrobial | 0.2% (0.1–0.3%) |
| % Abrasion healed at 4 daysa | 94% (92–96%) |
| % Abrasion not healed at 4 daysa | 2% (1–3%) |
| % Referred to eye hospitalc | 4% (3–5%) |
Corneal abrasions as observed by FCHV with fluorescein strips, ultraviolet flashlight, and loupes
Completion of all doses of a 3-day course of thrice daily antibiotic and antifungal, by self-report at 4-day follow-up
Indications for referral included bilateral corneal abrasions, suspicion for a corneal ulcer, visual acuity worse than Counting Fingers in the unaffected eye, a non-healed abrasion at the 4-day follow-up visit, or another abnormality the FCHV could not diagnose.
The number of residents from each community participating at each phase of the three follow-up censuses is summarized in the appendix (p 3). Of 130,579 unique individuals in the intervention arm and 121,960 unique individuals in the control arm enrolled at the month 0, 12, or 24 census, 114,569 (87.5%) and 109,102 (83.3%) were eligible for inclusion in the primary outcome (i.e., present and photographed at one census and present on at least one subsequent census). Of these, 17,287 people in the intervention arm and 21,020 in the control arm answered affirmatively to at least one corneal ulcer screening question at a follow-up census, of whom 16,961 (98.1%) and 20,540 (97.7%) had corneal photographs available from ≥2 censuses for grading, respectively.
A total of 85,237 distinct sets of eye photographs from one of the three follow-up censuses were screened by two independent graders during a first round of photo-grading, of which 13,925 were sent to a cornea specialist for review because of a possible corneal opacity. Of these, the vast majority were judged to be of good (N=12,381; 89%) or acceptable (N=1,375; 10%) quality, with only 169 (1%) sets of photographs deemed ungradable. A total of 289 incident corneal opacities were discovered in the intervention arm and 262 in the control arm during the three years of follow-up (representative examples shown in appendix, p 7). Annual incidence rates are shown for each cluster in the appendix (p 4). The estimated incidence was 1.21 (95%CI 0.85–1.74) per 1000 person-years in the intervention arm and 1.18 (95%CI 0.82–1.70) per 1000 person-years in the control arm (incidence rate ratio [IRR] 1.03, 95%CI 0.63–1.67; permutation P=0.93; pre-specified primary analysis). Intra-cluster correlation of incident corneal ulceration was estimated as an ICC of binary person-level data in the control arm (ANOVA-based ICC=0.001, 95%CI 0 to 0.003). In exploratory subgroup analyses, wards that were more rural and remote tended to experience fewer corneal opacities in the presence of the intervention (Table 3). For example, fewer incident opacities were observed in the intervention arm in wards classified by the hospital as rural (IRR 0.50, 95%CI 0.19–1.30), but not in wards classified as peri-urban or urban (IRR 1.31, 95%CI 0.86–1.99); P=0.04 for study arm by subgroup interaction (appendix, p 8).
Table 3. Subgroup analyses.
Study wards were stratified according to several criteria likely related to the rural-urban status of the community. The incidence of corneal opacity is shown for each treatment group across each stratum, along with the incidence rate ratio for the stratum (ward-level analyses).
| Incidence Rate (95%CI) | |||||
|---|---|---|---|---|---|
| Subgroup | Wards | Intervention | Control | Incidence Rate Ratio (95%CI) | Interaction P-valuea |
| Elevationb | 0.34 | ||||
| Low (< 300 m) | 168 | 1.16 (0.81–1.65) | 0.98 (0.65–1.48) | 1.17 (0.68–2.02) | |
| High (≥ 300 m) | 48 | 1.28 (0.95–1.73) | 1.79 (0.77–4.12) | 0.72 (0.30–1.75) | |
| Population densityc | 0.02 | ||||
| Above median | 108 | 1.13 (0.78–1.63) | 0.72 (0.51–1.01) | 1.57 (0.95–2.60) | |
| Below median | 108 | 1.23 (0.95–1.59) | 1.72 (1.01–2.92) | 0.72 (0.40–1.29) | |
| Distance from roadd | 0.04 | ||||
| Close (< 3 km) | 128 | 1.34 (0.90–1.98) | 0.94 (0.69–1.28) | 1.43 (0.87–2.36) | |
| Far (≥ 3 km) | 88 | 1.00 (0.70–1.42) | 1.85 (0.85–4.05) | 0.54 (0.23–1.28) | |
| Local classificatione | 0.04 | ||||
| Urban or peri-urban | 182 | 1.15 (0.85–1.57) | 0.88 (0.66–1.17) | 1.31 (0.86–1.99) | |
| Rural | 34 | 1.43 (0.94–2.18) | 2.88 (1.21–6.81) | 0.50 (0.19–1.30) | |
Interaction of study arm (intervention vs control) by subgroup in negative binomial regression of ward-level data with robust standard errors to account for village-level clustering
Extracted with R package elevatr for each household using baseline global positioning system (GPS) coordinates; summarized as a ward-level mean. Communities at higher elevation would be expected to be more rural.
Estimated for each household as the average distance to its 10 nearest neighbors, using GPS data from the baseline census; summarized as a ward-level mean. Communities with greater population density would be expected to be more rural.
Distance from the East-West Highway, a major urbanized thoroughfare in Nepal. Estimated for each household using baseline GPS data; wards in which all households were within 3km were classified as close, and the remaining as far. Communities farther from the road would be expected to be more rural.
Classified for internal study purposes based on the opinions of local study staff who visited communities as part of the study and were masked to study results.
The only reported adverse events were self-reported ocular allergic reactions, which occurred in 8 of the 4,777 participants who took the ophthalmic study medications (mean fraction per cluster: 0.2%, 95%CI 0.1–0.3%; Table 2).
DISCUSSION
Infectious keratitis frequently results in corneal scarring and subsequent vision loss, even when treated successfully with antimicrobial therapy.25 Thus, the most effective way to reduce corneal blindness may be to prevent the corneal ulcers from occurring in the first place.2 To that end, the present trial assessed whether the incidence of corneal ulcers would be reduced by a community-based program designed to promptly identify and provide antimicrobial prophylaxis for traumatic corneal abrasions. Despite evidence of intervention uptake and awareness, the communities randomized to the community-based intervention continued to experience similar levels of corneal opacity as control communities. Exploratory analyses found a substantial difference in intervention effectiveness when considering geography, with greater effectiveness in more rural and remote areas.
Corneal abrasions due to eye trauma have been shown to be the most important risk factor for corneal ulcers in low -and middle-income countries.6 Corneal trauma removes the protective corneal epithelium, providing a route for bacterial or fungal infection. Corneal epithelial defects typically take 24–72 hours to heal, and should be treated with topical antimicrobial prophylaxis until healing has occurred.26 Delayed initiation of antimicrobial prophylaxis in a patient with a corneal abrasion is thought to be an important risk factor for development of a corneal ulcer.11 In the present trial, 72% of people with eye trauma presented to an FCHV within 24 hours, suggesting that at least for those individuals who participated in the intervention, antimicrobial prophylaxis was started relatively promptly. It is difficult to know how many community members who developed a corneal abrasion did not present to the FCHV, and thus may have had a longer delay to antimicrobial prophylaxis or not received treatment at all.
Many requirements would need to be met in order for the intervention to be effective. Perhaps most importantly, both the FCHVs and community members would need to buy in to the program. Community members would need to be aware the intervention existed and need to have confidence in the diagnostic abilities of the FCHV and accept the antimicrobial therapy being offered. Community members would need to know where the FCHVs lived and be able to transport themselves for a visit. FCHVs would need to accept this new job responsibility—for which they were not paid—and demonstrate skill at diagnosis and organization in maintaining their inventory of supplies and medications. FCHVs would also need to maintain their examination skills over time, since they may go weeks without seeing a patient. FCHVs would need to maintain their enthusiasm and willingness to participate in the program over time. Aside from this, the medications themselves would need to be effective in preventing corneal infections when instilled in an eye with a corneal abrasion.
It is difficult to determine which factors were the most important for this trial’s null result. FCHVs were quite engaged with the program and received frequent visits from study supervisors in addition to the more formal annual training. Study staff helped FCHVs maintain their inventories of supplies and printed materials for publicity campaigns. However, it took a while to build awareness of the intervention, and awareness still had not reached saturation by the time of the third annual household survey. It is possible that the intervention was simply not instituted for a long enough period of time in order for community members to both be aware of the FCHV program and also trust the FCHV’s diagnostic abilities and management skills. Although FCHV visits for abrasions increased dramatically each year of the study, the intervention almost certainly continued to miss some community members, either because of a lack of awareness or because they preferred to seek care elsewhere. Furthermore, community members in the control communities may also have been receiving prompt treatment, either from a health care professional or from a medical shop. A survey of medical shops in the study area found that antibiotics were recommended for most eye conditions, so it is certainly possible that community members with abrasions in the control communities were also receiving appropriate prophylaxis.27
An intriguing finding of this study, albeit in an exploratory, hypothesis-generating analysis, was the suggestion that the intervention may be more effective in rural and remote areas than in urban and peri-urban areas. We can speculate about the reasons such an intervention could be more effective in rural areas. Rural communities are farther from health care providers and of lower economic means, and thus may have been more willing to accept the free services from the nearby FCHV. In contrast, someone living in an urban area may have found it more convenient to simply visit a medical shop or clinic instead of coordinating with the FCHV. Individuals from rural communities may also have been more likely to sustain eye trauma during agricultural work, and thus may have had more opportunities to visit the FCHV.
This study had several limitations. A large geographical area was chosen as the randomization unit in order to minimize contamination, but this limited the number of randomization units for analysis, reducing the statistical power of the trial. The corneal opacity outcome was based on photography in order to enable a masked assessment. However, some otherwise eligible participants did not contribute data from photographs—either because photographs were not captured, or because the photographs were not of sufficient quality. In addition, although previous work has demonstrated high sensitivity and specificity of smartphone photography for detecting corneal opacities and several rounds of grading increased the precision of the outcome, photographs nonetheless still provide less information than a standard ophthalmologic examination, and could have been misclassified.28 The study medications may have offered incomplete coverage, especially since Pseudomonas spp. have intrinsic resistance to chloramphenicol and intraconazole has poor efficacy for Fusarium spp.29 However, Pseudomonas and Fusarium account for a small fraction of ulcers in Nepal, and very few abrasions in the intervention arm did not heal after the three-day course of study medications, suggesting that the medications chosen for the trial were likely sufficient for prophylaxis purposes.18–23 Finally, the study was done in a particular location of Nepal, and it is not clear whether the results can be generalized to other settings. Given the results of the exploratory analyses, further study may be worthwhile specifically in areas that are rural or hard-to-reach.
In summary, this trial was unable to demonstrate that implementation of a community-based corneal ulcer prevention program in Nepal reduced the incidence of corneal opacities over three years, although exploratory subgroup analyses suggested the intervention could potentially be more effective in rural and remote communities. Awareness and participant responsiveness increased over the study period, suggesting that such community-based programs may need longer observation periods to determine effectiveness.
Data sharing:
A protocol has been published.16 The manual of procedures and statistical analysis plan are provided as supplemental files.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study.
We searched PubMed from inception to Dec 1, 2021, for studies published in English, using the search terms “cornea*[Title] AND (ulcer*[Title] OR opacit*[Title]) AND prevent*[Title]”. Observational studies of community health volunteer programs for corneal ulcer prevention performed in several settings in Southeast Asia found very low rates of corneal ulceration, and a randomized trial in India comparing antibiotic versus antifungal prophylaxis found very low rates of corneal ulceration in both groups. However, the low rate of corneal ulcers in these studies prevented an assessment of the effectiveness of the programs relative to the absence of a program.
Added value of this study.
The Village-Integrated Eye Worker (VIEW) trial was a cluster-randomized trial in which existing Female Community Health Volunteers in intervention communities were trained to diagnose corneal abrasions and provide prophylaxis with topical antibiotics and antifungals. Control communities received no intervention. A census with corneal photography was performed each year during the three-year trial, and incident corneal ulcers were determined by masked photo-graders. Ultimately the rate of corneal ulceration was similar in the two groups, although subgroup analyses found that the intervention might be more effective in areas that were more rural and remote. Strengths of the study include its large size, long duration, and masked outcome assessment, as well as its setting in an area with a relatively high incidence of corneal opacity.
Implications of all the available evidence.
Community health volunteer programs are a promising intervention for corneal ulcer prevention, but such programs may not be effective in all settings and the effectiveness may depend on socio-economic development and availability of health facilities. Further study of similar interventions should be targeted to rural and remote communities with poor access to basic health services.
Acknowledgements:
The authors would like to thank the District Public Health Offices (Chitwan and Nawalparasi, Nepal); the Nepal Health Research Council (Kathmandu, Nepal); Nepal Netra Jyoti Sangh (Krishna Raj Dharel, Tejendra Bdr Khadka, Chij Kumar Maskey, Sailesh Kumar Mishra, Tirtha Prasad Mishra, Jaya Ram Shrestha, Laximi Charan Shrestha, Unnat Shrestha); the DSMC members (William Barlow [chair], Patricia Buffler, Kavita Dhakhwa, Leslie Hyman, Art Reingold, Serge Resnikoff, Larry Schwab and Carrie Thiessen); and the study’s NIH Program officers Don Everett and Jimmy Le.
Funding:
This work was supported by grant U10EY022880 from the National Institutes of Health—National Eye Institute (Bethesda, MD, USA), That Man May See (San Francisco, CA, USA), the Peierls Foundation (Austin, TX, USA), the ALTA Foundation (San Francisco, CA), and Research to Prevent Blindness (New York, NY, USA).
Footnotes
Declaration of interests: We declare no competing interests.
REFERENCES
- 1.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health 2017; 5(12): e1221–e34. [DOI] [PubMed] [Google Scholar]
- 2.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ 2001; 79(3): 214–21. [PMC free article] [PubMed] [Google Scholar]
- 3.Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea 2002; 21(6): 555–9. [DOI] [PubMed] [Google Scholar]
- 4.Nirmalan PK, Katz J, Tielsch JM, et al. Ocular trauma in a rural south Indian population: the Aravind Comprehensive Eye Survey. Ophthalmology 2004; 111(9): 1778–81. [DOI] [PubMed] [Google Scholar]
- 5.Sheng XL, Li HP, Liu QX, et al. Prevalence and associated factors of corneal blindness in Ningxia in northwest China. Int J Ophthalmol 2014; 7(3): 557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, south India. Br J Ophthalmol 1997; 81(11): 965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan M, Mascarenhas J, Rajaraman R, et al. The steroids for corneal ulcers trial: study design and baseline characteristics. Arch Ophthalmol 2012; 130(2): 151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Upadhyay MP, Karmacharya PC, Koirala S, et al. Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. Am J Ophthalmol 1991; 111(1): 92–9. [DOI] [PubMed] [Google Scholar]
- 9.Khor WB, Prajna VN, Garg P, et al. The Asia Cornea Society Infectious Keratitis Study: A Prospective Multicenter Study of Infectious Keratitis in Asia. Am J Ophthalmol 2018; 195: 161–70. [DOI] [PubMed] [Google Scholar]
- 10.Upadhyay MP, Srinivasan M, Whitcher JP. Microbial keratitis in the developing world: does prevention work? Int Ophthalmol Clin 2007; 47(3): 17–25. [DOI] [PubMed] [Google Scholar]
- 11.Upadhyay MP, Karmacharya PC, Koirala S, et al. The Bhaktapur eye study: ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. Br J Ophthalmol 2001; 85(4): 388–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Getshen K, Srinivasan M, Upadhyay MP, Priyadarsini B, Mahalaksmi R, Whitcher JP. Corneal ulceration in South East Asia. I: a model for the prevention of bacterial ulcers at the village level in rural Bhutan. Br J Ophthalmol 2006; 90(3): 276–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maung N, Thant CC, Srinivasan M, et al. Corneal ulceration in South East Asia. II: a strategy for the prevention of fungal keratitis at the village level in Burma. Br J Ophthalmol 2006; 90(8): 968–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasan M, Upadhyay MP, Priyadarsini B, Mahalakshmi R, Whitcher JP. Corneal ulceration in south-east Asia III: prevention of fungal keratitis at the village level in south India using topical antibiotics. Br J Ophthalmol 2006; 90(12): 1472–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan M, Ravilla T, Vijayakumar V, et al. Community health workers for prevention of corneal ulcers in South India: a cluster-randomized trial. Am J Ophthalmol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien KS, Byanju R, Kandel RP, et al. Village-Integrated Eye Worker trial (VIEW): rationale and design of a cluster-randomised trial to prevent corneal ulcers in resource-limited settings. BMJ Open 2018; 8(8): e021556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Government of Nepal Central Bureau of Statistics. National Population and Housing Census 2011. https://unstats.un.org/unsd/demographic-social/census/documents/Nepal/Nepal-Census-2011-Vol1.pdf (accessed December 3, 2021.
- 18.Rai PG, Chaudhary M, Sharma AK, Gautam V. Direct microscopy in suppurative keratitis: a report from tertiary level hospital in Nepal. Nepal J Ophthalmol 2016; 8(16): 128–38. [DOI] [PubMed] [Google Scholar]
- 19.Suwal S, Bhandari D, Thapa P, Shrestha MK, Amatya J. Microbiological profile of corneal ulcer cases diagnosed in a tertiary care ophthalmological institute in Nepal. BMC Ophthalmol 2016; 16(1): 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amatya R, Shrestha S, Khanal B, et al. Etiological agents of corneal ulcer: five years prospective study in eastern Nepal. Nepal Med Coll J 2012; 14(3): 219–22. [PubMed] [Google Scholar]
- 21.Dhakhwa K, Sharma MK, Bajimaya S, Dwivedi AK, Rai S. Causative organisms in microbial keratitis, their sensitivity pattern and treatment outcome in western Nepal. Nepal J Ophthalmol 2012; 4(1): 119–27. [DOI] [PubMed] [Google Scholar]
- 22.Feilmeier MR, Sivaraman KR, Oliva M, Tabin GC, Gurung R. Etiologic diagnosis of corneal ulceration at a tertiary eye center in Kathmandu, Nepal. Cornea 2010; 29(12): 1380–5. [DOI] [PubMed] [Google Scholar]
- 23.Khanal B, Deb M, Panda A, Sethi HS. Laboratory diagnosis in ulcerative keratitis. Ophthalmic Res 2005; 37(3): 123–7. [DOI] [PubMed] [Google Scholar]
- 24.Gonzales CA, Srinivasan M, Whitcher JP, Smolin G. Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol 1996; 3(3): 159–66. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan M, Mascarenhas J, Rajaraman R, et al. The steroids for corneal ulcers trial (SCUT): secondary 12-month clinical outcomes of a randomized controlled trial. Am J Ophthalmol 2014; 157(2): 327–33 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim CH, Turner A, Lim BX. Patching for corneal abrasion. Cochrane Database Syst Rev 2016; 7: CD004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhandari S, Nesemann JM, Kandel RP, et al. Knowledge and Practices in the Diagnosis and Treatment of Corneal Infections by Nepalese Pharmaceutical Shop Workers. Am J Trop Med Hyg 2020; 103(4): 1694–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar A, Ali FS, Stevens VM, et al. Smartphone-based anterior segment imaging: a comparative diagnostic accuracy study of a potential tool for blindness prevalence surveys. Ophthalmic Epidemiol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalitha P, Shapiro BL, Srinivasan M, et al. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch Ophthalmol 2007; 125(6): 789–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


