Summary
Hypoxia can cause basement membrane (BM) degradation in tissues. Matrix metalloproteinase 9 (MMP-9) is involved in various human cancers as well as BM degradation by downregulating type IV collagen (COL4). This study investigated the role of MMP-9 in hypoxia-mediated BM degradation in rat bone marrow based on its regulation of collagen type IV alpha 1 chain (COL4A1). Eighty male rats were randomly divided into four groups based on exposure to hypoxic conditions at a simulated altitude of 7,000 m, control (normoxia) and 3, 7, and 10 days of hypoxia exposure. BM degradation in bone marrow was determined by transmission electron microscopy. MMP-9 levels were assessed by western blot and real-time PCR, and COL4A1 levels were assessed by western blot and immunohistochemistry. Microvessels BMs in bone marrow exposed to acute hypoxia were observed by electron microscopy. MMP-9 expression increased, COL4A1 protein expression decreased, and BM degradation occurred in the 10-, 7-, and 3-day hypoxia groups compared with that in the control group (all P<0.05). Hypoxia increased MMP-9 levels, which in turn downregulated COL4A1, thereby increasing BM degradation. MMP-9 upregulation significantly promoted BM degradation and COL4A1 downregulation. Our results suggest that MMP-9 is related to acute hypoxia-induced BM degradation in bone marrow by regulating COL4A1.
Keywords: Bone marrow, Basement membrane, MMP-9, Hypoxia
Introduction
Hypoxia is a state of low oxygen content and reduced pressure in tissues [1–3]. Depending upon the tissue type, the metabolic demands, and the adaptability of the tissue to hypoxia, the response to hypoxia can have effects ranging from substantial adaptation to tissue damage [4, 5]. Tissue hypoxia can be caused by one of three general abnormalities: hypoxemia, impaired oxygen delivery to tissues, and impaired tissue oxygen extraction/utilization [6]. In particular, acute hypoxia is characterized by hypoxemia leading to exacerbated injury of multiple organs such as heart, lung, pancreas, including bone marrow[7–10], and these changes can lead to basement membrane (BM) degradation in bone marrow [11].
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases with more than 20 different members [12, 13]. In particular, MMP-9 plays a crucial role in regulating angiogenesis and BM degradation under hypoxic conditions [14, 15]. MMP-9 upregulation is often observed in different malignant tumors and has been shown to promote metastasis and invasion by inducing angiogenesis and BM degradation [16–18]. Further, MMP-9 functions in the degradation of COL4 [16,19–22]. Therefore, we hypothesized that MMP-9 upregulation is associated with acute hypoxia-induced BM degradation. To adapt to a hypoxic environment, the body can induce hypoxia-regulated genes, such as MMP-9 and vascular endothelial growth factor, which cause microvascular changes in the body, including degradation of the vascular BM [23].
The BM is composed of multiple proteins, and the most abundant is COL4, which accounts for approximately 50 % of the basal part of the membrane and thus has an important biological function. COL4 forms a stable super molecular structure with laminin and other components, thereby acting as a stent to ensure the stability of the BM, and its function is mediated by the interaction between the BM and cells [24,25]. In addition, COL4 includes many subunits, and its core function is mainly attributed to COL4A1, which is also the most studied gene in COL4 [26,27]. Recently, worldwide research on COL4A1 has focused on its role in vascular diseases.
Gould et al. [28] found that the loss of the COL4A1 gene could negatively affect the composition and expression of COL4, resulting in the abnormal development and structure of small blood vessels, which lead to the degradation of vascular BMs. Interestingly, an experimental study on a rat model of subarachnoid hemorrhage showed bleeding at different time points in the lateral cortex and altered distributions and contents of MMPs, and in the experimental group, the BM was damaged within 24~72 h after subarachnoid hemorrhage and showed increased blood-brain barrier (BBB) permeability, decreased COL4, and increased MMP-9 expression, which eventually led to neurogenic oedema and death [29].
A comparative study on rat bone [30] showed that simulating the low oxygen levels that occur in plateau areas resulted in increased MMP-9 expression in rat bone marrow, increased vascular BM degradation,. Further validation of chronic hypoxia-induced MMP-9 levels showed degradation of vascular basilemma and confirmed that low oxygen conditions and increased MMP-9 are closely related to the degradation of BM. Electron microscopy experiments revealed that under hypoxic conditions, significant differences occurred in the degradation of the microvascular BM of rat bone marrow compared with that under normoxic conditions, and these changes included a decreased thickness of the vascular BM and lack of uniformity.
Degradation of the vascular BM is related to COL4A1 destruction. As the main component of COL4, COL4A1 is closely related to the biological function of MMP-9. Therefore, we hypothesized that MMP-9 can degrade the vascular BM by regulating COL4A1 under different durations of hypoxic exposure.
To further define how oxygen deprivation over different durations is associated with the degradation mechanism of vascular basilemma, we exposed rats to simulated plateau conditions of 7,000 meters above sea level for 3, 7, and 10 days. We then examined the bone marrow at the three different times and determined how different durations of hypoxia affect MMP-9 and COL4A1 levels and BM degradation in bone marrow.
Methods
Animals
Specific pathogen-free (SPF) male Sprague Dawley (SD) rats weighing 200 ± 20 g were purchased from the Animal Centre of Xi’an Jiaotong University, China (Grant No. SCXK (Shan) 2018-005). This experimental protocol (P-SL-202102) was approved by the Institutional Animal Care and Use Committee of Affiliated Hospital of Qinghai University, and it complied with the animal management rules of the Chinese Ministry of Health. All rats were housed at an ambient temperature of 18 ± 2 °C and relative humidity of 40–60 % throughout the experiment, and they were fed a standard pellet diet and provided water ad libitum.
Reagents and instrumentation
The anti-COL4A1 antibody (1:200, PB9099) was purchased from Boster Bio, China. Anti-MMP-9 (# ab38898) and anti-β-actin (# ab8229) antibodies were obtained from Abcam (Cambridge, MA, USA). The forward and reverse primers for MMP-9 and GAPDH were designed using Primer 3 and synthesized by Jinsirui Co., Ltd. (Nanjing, China). The miRNeasy Mini Kit was purchased from Qiagen (Hilden, Germany). The PrimeScript RT reagent kit (catalogue no. #RR036A) and TB Green Premix Ex Taq (catalogue no. #RR820A) were purchased from TaKaRa Bio (Shiga, Japan). ProLong Gold antifade reagent (P36931) was obtained from Invitrogen (Carlsbad, CA, USA). The acute hypoxia rat model was established in an automatically adjusted low-pressure hypobaric chamber (DYC-300; Guizhou Fenglei Oxygen Chamber Co., Ltd., Guizhou, China).
Establishment of the animal model
In total, eighty rats were randomly divided into four groups (n = 20 rats per group), namely, a control group and three treatment groups based on the duration of exposure to hypoxic conditions: 3, 7, and 10 days. The rats in the control group were kept under normoxic conditions for 28 days. All rats except those in the control group were maintained continuously in a hypobaric chamber for the indicated time periods [31–33] under the same pressure and oxygen concentration as that at an altitude of 7,000 m. All rats were housed at an ambient temperature of 18 ± 2 °C and relative humidity of 40–60 % throughout the experiment, and they were fed a standard pellet diet and provided water ad libitum [1].
Collection of blood samples
The rats were anaesthetized using urethane (1.0 g/kg) and sacrificed by bleeding the abdominal aorta. Blood samples were collected for routine tests using a blood cell analyzer obtained from Mindray Biomedical Electronics Co., Ltd. (BC-5000Vet, Shenzhen, China), and the red blood cell (RBC), hemoglobin (Hb), hematocrit (HCT), and erythrocyte counts were recorded.
Collection of bone marrow samples
The thigh bones of the rats were extracted, homogenized, and centrifuged with 15 ml of 0.9 % normal saline at 3,00 × g for 5 min, and then the extracts were filtered to collect the bone marrow. A portion of each bone marrow sample was flash-frozen in liquid nitrogen and stored at −80 °C for RNA and protein extraction. The remaining samples were fixed in 4 % paraformaldehyde and 2.5 % glutaraldehyde for immunohistochemistry staining and transmission electron microscopy (TEM).
Immunohistochemistry staining for COL4A1
Paraffin sections were prepared for immunohistochemical analysis using the SP-HRP kit (SP-900; ZSGB Biotechnology Co. Ltd., Beijing, China). Antigen site retrieval was accomplished by a microwave heat-mediated method and incubation with 10 mmol/l citrate buffer (pH 6) for 10 min. The subsequent procedure was performed according to the manufacturer’s instructions as follows: sections were incubated in 3 % hydrogen peroxide for 10 min, washed three times (3 min each) with 0.01 mmol/l PBS (pH 7.4), and blocked with goat serum. Then, the sections were incubated for 14 h at 4 °C with rabbit anti-COL4A1 (1:200, PB9099; Boster Bio) primary antibody in 0.3 % Triton PBS (0.01 mmol/l). Next, the sections were washed with PBS three times (3 min each) and incubated with a biotinylated goat anti-rabbit secondary antibody for 15 min at 37 °C. After rinsing for 9 min in PBS, the sections were incubated with horseradish peroxidase-conjugated streptavidin for 15 min at 37 °C, and then they were washed again with PBS for 9 min. The reaction product was visualized using diaminobenzidine for 10 min at room temperature, and then the sections were stained with hematoxylin for 20 s. Images were acquired at 200× magnification, and the integrated optical density and area of protein expression were measured with Image Pro Plus software (Media Cybernetics, Rockville, MD, USA) and used to calculate the mean optical density value.
Transmission electron microscopy
BM degradation in bone marrow was examined by TEM. Tissues were fixed with 3 % buffered glutaraldehyde and stored in a refrigerator overnight (4°C). Thereafter, they were rinsed in 0.1 M phosphate buffer and post-fixed for 2 h with 1 % osmium tetroxide in 0.125 M sodium cacodylate buffer, dehydrated in increasing concentrations of ethanol (30–100 %), rinsed in acetone, and embedded in Araldite. Ultrathin sections (500-nm thickness) were stained with uranyl acetate and lead citrate and examined using a Tecnai Spirit Bio TWIN electron microscope (FEI Company, Hillsboro, OR, USA).
Real-time quantitative PCR
Total RNA was extracted from frozen bone marrow samples using the miRNeasy Mini Kit and quantified using a NanoDrop. cDNA was synthesized using the TaKaRa PrimeScript RT reagent kit. The mRNA expression of MMP-9 was determined using TB Green Premix Ex Taq (TaKaRa) on an ABI 7500 Real-time PCR system (Bio-Rad, Hercules, CA, USA). The primers used were as follows: MMP-9 forward: 5′-GCATCTGTATGGTCGTGGCT-3′, reverse: 5′-TGCAGTGGGACACATAGTGG-3′; GAPDH for-ward: 5′-AGTGCCAGCCTCGTCTCATA-3′, reverse: 5′-GAACTTGCCGTGGGTAGAGT-3′. Relative gene expression was calculated using the 2−ΔΔCt method, and all values were normalized to the housekeeping gene GAPDH. The PCR was programmed as follows: 95 °C for 10 min; 40 cycles of 95 °C for 10 s; 60 °C for 30 s; 72 °C for 30 s; and 72 °C for 5 min. All samples were examined in triplicate. The primers used to amplify the expression of MMP-9 are presented in Table 1.
Table 1.
MMP-9 and primers
| Primer | Sequence (5′-3′) | Length |
|---|---|---|
| MMP-9 F | 5′-GCATCTGTATGGTCGTGGCT-3′ | 112 bp |
| MMP-9 R | 5′-TGCAGTGGGACACATAGTGG-3′ | 112 bp |
| GAPDH-F | 5′-AGTGCCAGCCTCGTCTCATA-3′ | 201bp |
| GAPDH-R | 5′-GAACTTGCCGTGGGTAGAGT-3′ | 201bp |
Western blotting
The protein expression of COL4A1 and MMP-9 in bone marrow was determined by western blotting. Proteins were isolated from frozen bone marrow tissues by homogenization in RIPA buffer containing 1 mmol/l PMSF, and then centrifugation at 11,000 × g for 10 min at 4 °C was performed to collect the supernatant. The protein concentration was measured using the bicinchoninic acid assay, with bovine serum albumin as a standard sample. Proteins were resolved using 10 % SDS-PAGE and transferred to polyvinylidene difluoride membranes, and then the membranes were blocked with 5 % non-fat milk for 1 h and then incubated with anti-COL4A1 (1:1000) and anti-β-actin (1:300) antibodies at 4 °C overnight. Next, the membranes were incubated with goat anti-mouse/anti-rabbit IgG secondary antibodies (1:20,000) for 1 h at ambient temperature and detected with an enhanced chemiluminescence kit (ECL, Biyuntian Biotech Institute, Shanghai, China).
Statistical analysis
The results were analyzed using SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA) and expressed as the mean ± SD for normally distributed data. Differences between groups were analyzed by one-way analysis of variance (ANOVA), followed by the Student–Newman–Keuls test and Dunnett’s multiple comparison test. A value of P<0.05 was considered statistically significant.
Results
Characteristics of the acute hypoxia rat model
An acute hypoxia rat model was established in the 3-, 7-, and 10-day groups. Rats with acute hypoxia showed typical symptoms, including cyanosis in the mucous membrane of the lips, tongue, ears, palms, and soles of the feet compared to the rats in the control group. In addition, on day 3, the RBCs, Hb, and HCT were increased compared to that in the control group (P<0.05; Table 1). On day 7, the RBCs, Hb, and HCT were increased compared to that in the 3-day group (P<0.05, Table 1). On day 10, the RBCs, Hb, and HCT were increased compared to that in the 7-day group (P<0.05, Table 2).
Table 2.
Characteristics of the acute hypoxia rat model
| Index | Control (n = 10) | 3 days (n = 10) | 7 days (n = 10) | 10 days (n = 10) |
|---|---|---|---|---|
| RBC (× 10 12 /L) | 7.90 ± 0.68 | 8.63 ± 0.42a | 9.18 ± 0.43b | 9.71 ± 0.31c |
| Hb (g/L) | 170.00 ± 12.17 | 183.12 ± 14.21a | 201.23 ± 14.89b | 217.77 ± 12.10c |
| HCT (%) | 39.78 ± 4.19 | 44.83 ± 4.90a | 49.75 ± 3.00b | 55.91 ± 4.97c |
MMP-9 was upregulated in the hypoxic rat bone marrow
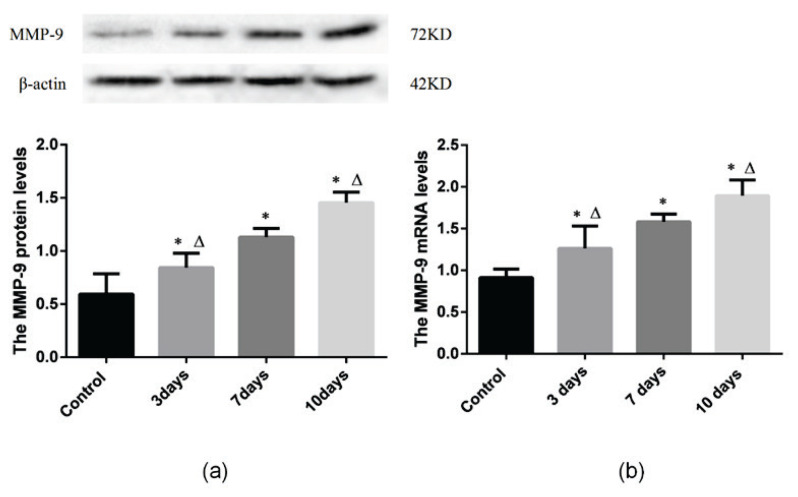
Western blot analysis showed that the MMP-9 levels in the hypoxia groups were significantly higher than those in the control group (P<0.05, Fig. 1). MMP-9 expression is 0.84±0.13 in the 3-day group which was significantly higher than 0.59±0.19 in the control group. Furthermore, MMP-9 expression is 1.13±0.83 in the 7-day group which was significantly higher than that in the 3-day group, and MMP-9 expression is 1.46±0.10 which was significantly increased in the 10-day group compared to that in 7-day group. (P<0.05, Fig. 1A).
Fig. 1.
MMP-9 protein (a) and mRNA (b) expression were increased in the bone marrow of rats with acute hypoxia. Control: control group; 3 days: acute hypoxia for 3 days; 7 days: Acute hypoxia for 7 days; 10 days: acute hypoxia for 10 days. Results are presented as the mean ± SEM (n = 6 rats per group). *P<0.05 vs. Control, ΔP<0.05 vs. 7 days.
In addition, RT-PCR showed that the mRNA expression of MMP-9 was significantly increased in the hypoxia groups compared to that in the control group (P<0.05, Fig. 1B). MMP-9 gene expression is 1.26±0.27 in the 3-day group which was significantly higher than 0.91±0.10 in the control group. Moreover, MMP-9 gene expression is 1.58±0.09 in the 7-day group which was significantly higher than that in the 3-day group, and MMP-9 gene expression is 1.89±0.19 which was significantly increased in the 10-day group compared to that in 7-day group. (P<0.05, Fig. 1B).
COL4A1 was decreased in the bone marrow of acute hypoxia rats
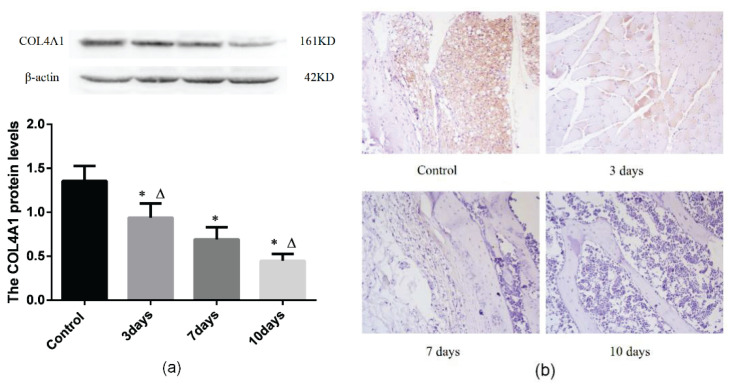
Western blot analysis showed that the COL4A1 levels in the bone marrow samples from the hypoxia groups were lower than those from the control group (P<0.05, Fig. 2A). The expression of COL4A1 is 0.94±0.16 in the 3-day group which was lower than 1.36±0.17 in the control group, and the expression of COL4A1 is 0.69±0.14 in the 7-day group which was lower than that in the 3-day group, while the expression of COL4A1 is 0.45±0.08 in the 10-day group which was lower than that in the 7-day group (P<0.05, Fig. 2A).
Fig. 2.
Expression of MMP-9 at different hypoxia time. (a) Immunohistochemical staining of COL4A1 in bone marrow (magnification: 400×). Red arrows indicate COL4A1-positive staining. (b) Western blot showing the protein expression of COL4A1. Control: control group; 3 days: acute hypoxia for 3 days; 7 days: acute hypoxia for 7 days; 10 days: acute hypoxia for 10 days. Results are presented as mean ± SEM (n = 6 rats per group). *P<0.05 vs. Control, ΔP<0.05 vs. 7 days.
The morphology of the BM was analyzed by immunohistochemical staining, which showed an even and continuous BM and increased COL4A1 expression in the control group compared to that in the hypoxia groups. In contrast, in the hypoxia groups, immunohistochemistry showed a thinner and more uneven BM, with the extent of BM damage progressively increasing in the 3-, 7-, and 10-day groups. Image Pro-Plus 6.0 software was used for quantitative analysis. The average optical density (AOD) of 5 high magnification scopes was calculated. The expression of COL4A1 is 0.14±0.01 in the 3-day group which was lower than 0.17±0.00 in the control group, and the expression of COL4A1 is 0.12±0.01 in the 7-day group which was lower than that in the 3-day group, while the expression of COL4A1 is 0.09±0.01 in the 10-day group which was lower than that in the 7-day group (P<0.05, Fig. 2B).
BM degradation occurred in the bone marrow of acute hypoxia rats
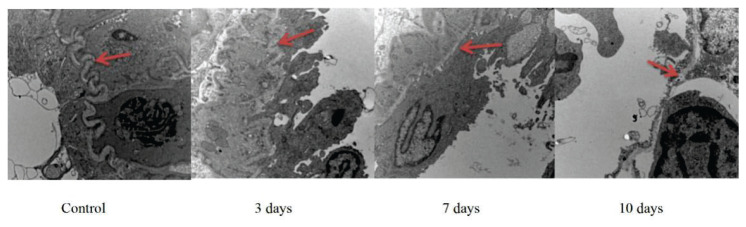
The BM of microvessels in the bone marrow were observed by TEM (Fig. 3). The control group showed a thick and continuous BM, whereas the hypoxia groups showed an uneven and thin BM with increased degradation. The BM thickness in the 3-day group was significantly higher than that in the other hypoxia groups, whereas the BM thickness in the 10-day group was significantly lower than that in the other groups.
Fig. 3.
Ultrastructural analyses of the basement membrane (BM). Representative photomicrographs of BM from one randomly selected slide per group. Scale bar = 500 nm. BM degradation was higher in the acute hypoxia groups than in the control group Control: control group; 3 days: acute hypoxia for 3 days; 7 days: acute hypoxia for 7 days; 10 days: acute hypoxia for 10 days. Red arrows indicate the BM of micro-vessels.
Discussion
Our study revealed five major findings: (1) rats developed erythropoiesis under hypoxic conditions; (2) the BM showed significant pathological changes (BM degradation) in the bone marrow microvessels of under acute hypoxia after 3, 7, and 10 days; (3) COL4A1, which is a major component of the BM, was downregulated in the hypoxia groups, and the level of downregulation was consistent with the extent of BM degradation; (4) acute hypoxia induced the upregulation of MMP-9 in bone marrow; and (5) the MMP-9 and COL4A1 levels in the bone marrow of acute hypoxia rats were positively correlated to the extent of BM degradation.
Hypoxia exposure can cause a variety of vascular pathological changes that lead to BM degradation, such as increased blood viscosity, which is consistent with the results of previous studies [36, 37]. In our previous study, we found that chronic hypoxia induced degradation of rat bone marrow microvascular BM and was closely related to high MMP-9 expression [30]. In this study, the degradation of vascular BM in the bone marrow of rats was increased and that the thickness of the vascular BM was decreased after exposure to hypoxic conditions for different durations compared with that in the normoxic group.
The COL4A1 gene is located on chromosome 13q34, and it gene encodes the collagen type IV alpha protein 1, an essential component of the vascular BM [38]. Previous studies [13, 28, 39] have shown that the mechanism underlying microvessels BM degradation in certain diseases, such as cancer and stroke, involves COL4A1, which regulates the progression of tumor metastasis. However, the role of COL4A1 in acute hypoxia-mediated microvessels BM degradation has not been previously reported and the mechanism underlying acute hypoxia-mediated microvessels BM degradation has not been studied. Our results indicated that MMP-9 expression was upregulated in the anoxic group; moreover, degree of the rat bone marrow microvascular BM degradation, as observed by electron microscopy, was found to be consistent with the level of MMP-9, which is consistent with previous studies [40,41]. BM destruction is an essential step in tumor progression and supports tumor invasion and metastasis by promoting angiogenesis [42].
Our study found that under different anoxic conditions, the BM of bone marrow microvessels in rats was degraded, COL4A1 expression was decreased, and the BM degradation level was consistent with the level of COL4A1. The role of COL4A1 in the degradation of hypoxia-mediated microvascular BM has not been previously reported and the mechanism of hypoxia-mediated microvascular BM degradation has not been previously studied.
Our results showed that by regulating the expression of COL4A1, MMP-9 was related to the degradation of BM in the bone marrow of hypoxic rats. The regulatory effect of MMP-9 on COL4A1 in the process of hypoxia-mediated microvascular BM degradation was analyzed here for the first time. We also found that in the hypoxic treatment groups, rat bone marrow BM degradation was the most serious in the 10-day group, in which the content of COL4A1 was the lowest and the expression of MMP-9 was the highest, BM degradation was relatively slight in the 3-day group, in which the content of COL4A1 was high and the expression of MMP-9 was the lowest.
Our results showed that MMP-9 expression was enhanced in the acute hypoxia groups. Consistent with previous studies [35, 43, 44]. In our study, we observed an increase in MMP-9 in the bone marrow samples of rats exposed to hypoxia, which resulted in increased BM degradation [16,21,22].
Further research is needed to clarify the role of MMP-9 in acute hypoxia-mediated BM changes.
Conclusions
In summary, we found that MMP-9 induced BM degradation under acute hypoxia, identifying the role of MMP-9 in acute hypoxia-induced BM degradation via the regulation of COL4A1 in bone marrow provides a foundation for further studies and shows the potential for the development of novel therapeutic strategies.
Acknowledgements
The study was conducted according to the standard operating procedures approved by the Affiliated Hospital of Qinghai University (P-SL-202102).
This research was funded by Natural Science Foundation of science and technology department of Qinghai Province (No. 2021-ZJ-966Q), Young and middle-aged Scientific Research Foundation project of Qinghai University (No. 2019-QYY-5) and Basic Research for Application of science and technology department of Qinghai Province (No. 2019-ZJ-7081) are gratefully acknowledged.
Abbreviations
- MMP-9
matrix metalloproteinase 9
- MMPs
matrix metalloproteinases
- BM
basement membrane
- COL4A1
collagen type IV alpha 1 chain
- COL4
type IV collagen
- BBB
blood-brain barrier
- SPF
specific pathogen-free
- SD
Sprague Dawley
- ANOVA
one-way analysis of variance
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.MacIntyre NR. Tissue hypoxia: implications for the respiratory clinician. Respir Care. 2014;59(10):1590–1596. doi: 10.4187/respcare.03357. [DOI] [PubMed] [Google Scholar]
- 2.Su J, Li Z, Cui S, Ji L, Geng H, Chai K, Ma X, Bai Z, Yang Y, Wuren T. The Local HIF-2α/EPO Pathway in the Bone Marrow is Associated with Excessive Erythrocytosis and the Increase in Bone Marrow Microvessel Density in Chronic Mountain Sickness. High Alt Med Biol. 2015;16(4):318–330. doi: 10.1089/ham.2015.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SH, Manandhar S, Lee YM. Roles of RUNX in Hypoxia-Induced Responses and Angiogenesis. Adv Exp Med Biol. 2017;962:449–469. doi: 10.1007/978-981-10-3233-2_27. [DOI] [PubMed] [Google Scholar]
- 4.Leach RM, Treacher DF. Oxygen transport-2. Tissue hypoxia. Bmj. 1998;317(7169):1370–1373. doi: 10.1136/bmj.317.7169.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin DS, Khosravi M, Grocott MP, Mythen MG. Concepts in hypoxia reborn. Crit Care. 2010;14(4):315. doi: 10.1186/cc9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger MM, Grocott MPW. Facing acute hypoxia: from the mountains to critical care medicine. Br J Anaesth. 2017;118(3):283–286. doi: 10.1093/bja/aew407. [DOI] [PubMed] [Google Scholar]
- 7.Prokudina ES, Naryzhnaya NV, Mukhomedzyanov AV, Gorbunov AS, Zhang Y, Yaggi AS, Tsibulnikov SY, Nesterov EA, Lishmanov YB, Suleiman MS. Effect of Chronic Continuous Normobaric Hypoxia on Functional State of Cardiac Mitochondria and Tolerance of Isolated Rat Heart to Ischemia and Reperfusion: Role of μ and delta2 Opioid Receptors. Physiol Res. 2019;68(6):909–920. doi: 10.33549/physiolres.933945. [DOI] [PubMed] [Google Scholar]
- 8.Honda J, Kimura T, Sakai S, Maruyama H, Tajiri K, Murakoshi N, Homma S, Miyauchi T, Aonuma K. The glucagon-like peptide-1 receptor agonist liraglutide improves hypoxia-induced pulmonary hypertension in mice partly via normalization of reduced ET(B) receptor expression. Physiol Res. 2018;67(Suppl 1):S175–s184. doi: 10.33549/physiolres.933822. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Ai L, Hai B, Cao Y, Li R, Li H, Li Y. Tempol alleviates chronic intermittent hypoxia-induced pancreatic injury through repressing inflammation and apoptosis. Physiol Res. 2019;68(3):445–455. doi: 10.33549/physiolres.934010. [DOI] [PubMed] [Google Scholar]
- 10.Kolesnikov SI, Popova AS, Krupitskaya LI, Sinitskii AI, Kolesnikova LI. Activity of Heme Synthesis Enzymes in the Bone Marrow and Liver of August and Wistar Rats During the Neonatal Period and After Acute Postnatal Hypoxia. Bull Exp Biol Med. 2015;160(2):193–195. doi: 10.1007/s10517-015-3125-0. [DOI] [PubMed] [Google Scholar]
- 11.Andreeva E, Matveeva D. Multipotent mesenchymal stromal cells and extracellular matrix: regulation under hypoxia. Human Physiology. 2018;44(6):696–705. doi: 10.1134/S0362119718060038. [DOI] [Google Scholar]
- 12.Misko A, Ferguson T, Notterpek L. Matrix metalloproteinase mediated degradation of basement membrane proteins in Trembler J neuropathy nerves. J Neurochem. 2002;83(4):885–894. doi: 10.1046/j.1471-4159.2002.01200.x. [DOI] [PubMed] [Google Scholar]
- 13.Hou H, Zhang G, Wang H, Gong H, Wang C, Zhang X. High matrix metalloproteinase-9 expression induces angiogenesis and basement membrane degradation in stroke-prone spontaneously hypertensive rats after cerebral infarction. Neural Regen Res. 2014;9(11):1154–1162. doi: 10.4103/1673-5374.135318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors (Basel) 2018;18(10) doi: 10.3390/s18103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lochter A, Sternlicht MD, Werb Z, Bissell MJ. The significance of matrix metalloproteinases during early stages of tumor progression. Ann N Y Acad Sci. 1998;857:180–193. doi: 10.1111/j.1749-6632.1998.tb10116.x. [DOI] [PubMed] [Google Scholar]
- 16.Hlobilkova A, Ehrmann J, Knizetova P, Krejci V, Kalita O, Kolar Z. Analysis of VEGF, Flt-1, Flk-1, nestin and MMP-9 in relation to astrocytoma pathogenesis and progression. Neoplasma. 2009;56(4):284–290. doi: 10.4149/neo_2009_04_284. [DOI] [PubMed] [Google Scholar]
- 17.Radenkovic S, Konjevic G, Jurisic V, Karadzic K, Nikitovic M, Gopcevic K. Values of MMP-2 and MMP-9 in tumor tissue of basal-like breast cancer patients. Cell Biochem Biophys. 2014;68(1):143–152. doi: 10.1007/s12013-013-9701-x. [DOI] [PubMed] [Google Scholar]
- 18.Yang JS, Lin CW, Hsieh YS, Cheng HL, Lue KH, Yang SF, Lu KH. Selaginella tamariscina (Beauv.) possesses antimetastatic effects on human osteosarcoma cells by decreasing MMP-2 and MMP-9 secretions via p38 and Akt signaling pathways. Food Chem Toxicol. 2013;59:801–807. doi: 10.1016/j.fct.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Wang S, Liu Z, Yang L, Liu J, Xiu M. Increased Six1 expression in macrophages promotes hepatocellular carcinoma growth and invasion by regulating MMP-9. J Cell Mol Med. 2019;23(7):4523–4533. doi: 10.1111/jcmm.14342. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Yuan J, Xu XJ, Lin Y, Chen QY, Sun WJ, Tang L, Liang QX. LncRNA MALAT1 expression inhibition suppresses tongue squamous cell carcinoma proliferation, migration and invasion by inactivating PI3K/Akt pathway and downregulating MMP-9 expression. Eur Rev Med Pharmacol Sci. 2019;23(1):198–206. doi: 10.26355/eurrev_201901_16765. [DOI] [PubMed] [Google Scholar]
- 21.Dong H, Diao H, Zhao Y, Xu H, Pei S, Gao J, Wang J, Hussain T, Zhao D, Zhou X. Overexpression of matrix metalloproteinase-9 in breast cancer cell lines remarkably increases the cell malignancy largely via activation of transforming growth factor beta/SMAD signalling. Cell Prolif. 2019;52(5):e12633. doi: 10.1111/cpr.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang HL, Thiyagarajan V, Shen PC, Mathew DC, Lin KY, Liao JW, Hseu YC. Anti-EMT properties of CoQ0 attributed to PI3K/AKT/NFKB/MMP-9 signaling pathway through ROS-mediated apoptosis. J Exp Clin Cancer Res. 2019;38(1):186. doi: 10.1186/s13046-019-1196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, He Z, Wang H, Xu J. A Discussion of the Necessity of Constructing Tourism Service Standards to Cope with Altitude Sickness in the High-Altitude Cold Area. 2021 International Conference on Culture-oriented Science & Technology (ICCST); 2021; IEEE; pp. 580–584. [DOI] [Google Scholar]
- 24.Xing Q, Parvizi M, Higuita ML, Griffiths LG. Basement membrane proteins modulate cell migration on bovine pericardium extracellular matrix scaffold. Scientific reports. 2021;11(1):1–10. doi: 10.1038/s41598-021-84161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy S, Kim D. Retinal capillary basement membrane thickening: Role in the pathogenesis of diabetic retinopathy. Progress Retinal Eye Res. 2021;82:100903. doi: 10.1016/j.preteyeres.2020.100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinlan C, Rheault MN. Genetic basis of type IV collagen disorders of the kidney. Clinical Journal of the American Society of Nephrology. 2021 doi: 10.2215/CJN.19171220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donner I, Sipilä LJ, Plaketti R-M, Kuosmanen A, Forsström L, Katainen R, Kuismin O, Aavikko M, Romsi P, Kariniemi J. Next-generation sequencing in a large pedigree segregating visceral artery aneurysms suggests potential role of COL4A1/COL4A2 in disease etiology. Vascular. 2021:17085381211033157. doi: 10.1177/17085381211033157. [DOI] [PubMed] [Google Scholar]
- 28.Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, Bousser MG, Heutink P, Miner JH, Tournier-Lasserve E. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354(14):1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- 29.Schöller K, Trinkl A, Klopotowski M, Thal SC, Plesnila N, Trabold R, Hamann GF, Schmid-Elsaesser R, Zausinger S. Characterization of microvascular basal lamina damage and blood-brain barrier dysfunction following subarachnoid hemorrhage in rats. Brain Res. 2007;1142:237–246. doi: 10.1016/j.brainres.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 30.Zhu M, Yang M, Yang Q, Liu W, Geng H, Pan L, Wang L, Ge R, Ji L, Cui S. Chronic Hypoxia-Induced Microvessel Proliferation and Basal Membrane Degradation in the Bone Marrow of Rats Regulated through the IL-6/JAK2/STAT3/MMP-9 Pathway. Biomed Res Int. 2020;2020:9204708. doi: 10.1155/2020/9204708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumari P, Roy K, Wadhwa M, Chauhan G, Alam S, Kishore K, Ray K, Panjwani U. Fear memory is impaired in hypobaric hypoxia: Role of synaptic plasticity and neuro-modulators in limbic region. Life Sci. 2020;254:117555. doi: 10.1016/j.lfs.2020.117555. [DOI] [PubMed] [Google Scholar]
- 32.Shaw S, Kumar U, Bhaumik G, Reddy MPK, Kumar B, Ghosh D. Alterations of estrous cycle, 3β hydroxysteroid dehydrogenase activity and progesterone synthesis in female rats after exposure to hypobaric hypoxia. Sci Rep. 2020;10(1):3458. doi: 10.1038/s41598-020-60201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khanna K, Mishra KP, Chanda S, Eslavath MR, Ganju L, Kumar B, Singh SB. Effects of Acute Exposure to Hypobaric Hypoxia on Mucosal Barrier Injury and the Gastrointestinal Immune Axis in Rats. High Alt Med Biol. 2019;20(1):35–44. doi: 10.1089/ham.2018.0031. [DOI] [PubMed] [Google Scholar]
- 34.Zimna A, Kurpisz M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed Res Int. 2015;2015:549412. doi: 10.1155/2015/549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fidler AL, Vanacore RM, Chetyrkin SV, Pedchenko VK, Bhave G, Yin VP, Stothers CL, Rose KL, McDonald WH, Clark TA. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc Natl Acad Sci U S A. 2014;111(1):331–336. doi: 10.1073/pnas.1318499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah A, Matsumura N, Quon A, Morton JS, Dyck JRB, Davidge ST. Cardiovascular susceptibility to in vivo ischemic myocardial injury in male and female rat offspring exposed to prenatal hypoxia. Clin Sci (Lond) 2017;131(17):2303–2317. doi: 10.1042/CS20171122. [DOI] [PubMed] [Google Scholar]
- 37.Konradi J, Mollenhauer M, Baldus S, Klinke A. Redox-sensitive mechanisms underlying vascular dysfunction in heart failure. Free Radic Res. 2015;49(6):721–742. doi: 10.3109/10715762.2015.1027200. [DOI] [PubMed] [Google Scholar]
- 38.Steffensen LB, Stubbe J, Lindholt J, Beck H, Overgaard M, Bloksgaard M, Genovese F, Nielsen SH, Tha M, Bang-Moeller S. Basement membrane collagen IV deficiency promotes abdominal aortic aneurysm formation. Sci Rep. 2021;11(1):1–13. doi: 10.1038/s41598-021-92303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niland S, Riscanevo AX, Eble JA. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int J Mol Sci. 2022;23(1):146. doi: 10.3390/ijms23010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mondal S, Adhikari N, Banerjee S, Amin SA, Jha T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur J Med Chem. 2020;194:112260. doi: 10.1016/j.ejmech.2020.112260. [DOI] [PubMed] [Google Scholar]
- 41.Sugiyama A, Okada M, Otani K, Yamawaki H. [Development of basic research toward clinical application of cleaved fragment of type IV collagen]. Nihon Yakurigaku Zasshi. 2021;156(5):282–287. doi: 10.1254/fpj.21016. [DOI] [PubMed] [Google Scholar]
- 42.Gomatou G, Syrigos N, Vathiotis IA, Kotteas EA. Tumor Dormancy: Implications for Invasion and Metastasis. Int J Mol Sci. 2021;22(9):4862. doi: 10.3390/ijms22094862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saleh M, Khalil M, Abdellateif MS, Ebeid E, Madney Y, Kandeel EZ. Role of matrix metalloproteinase MMP-2, MMP-9 and tissue inhibitor of metalloproteinase (TIMP-1) in the clinical progression of pediatric acute lymphoblastic leukemia. Hematology. 2021;26(1):758–768. doi: 10.1080/16078454.2021.1978763. [DOI] [PubMed] [Google Scholar]
- 44.Verma D, Zanetti C, Godavarthy PS, Kumar R, Minciacchi VR, Pfeiffer J, Metzler M, Lefort S, Maguer-Satta V, Nicolini FE. Bone marrow niche-derived extracellular matrix-degrading enzymes influence the progression of B-cell acute lymphoblastic leukemia. Leukemia. 2020;34(6):1540–1552. doi: 10.1038/s41375-019-0674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]