Summary
The pro-inflammatory status of adipose tissue (AT) has been found to be related to reverse cholesterol transport (RCT) from peritoneal macrophages. However, this finding was made in experimental models using induced peritonitis and isolated peritoneal macrophages of animals. This experimental relationship is in agreement with RCT changes in man in two extreme situations, sepsis or cardiovascular complications. Given the above, we sought to test RTC in relationship to macrophage polarization in the visceral AT (VAT) of living kidney donors (LKDs) and the effect of conditioned media obtained from their AT. The influence of ATCM on CE capacity was first assessed in an experiment where standard plasma was used as cholesterol acceptor from [14C] cholesterol labeled THP-1 cells. Conditioned media as a product of LKDs’ incubated AT showed no effect on CE. Likewise, we did not find any effect of individual plasma of LKDs on CE when individual plasma of LKDs were used as acceptors. On the other hand, we documented an effect of LKDs’ adipose cell size on CE. Our results indicate that the pro-inflammatory status of human AT is not likely induced by disrupted RCT but might be influenced by the metabolic status of LKDs’ adipose tissue.
Keywords: Cholesterol efflux, Pro-inflammatory macrophages, Conditioned media, Visceral adipose tissue, Adipocyte size
Introduction
Cardiovascular disease represents the most common cause of mortality in developed countries [1]. Consumption of high-fat, high-cholesterol diets – known as Western-type diets (WTDs) – can lead to hypercholesterolemia as well as clinical complications of atherosclerosis. Atherosclerosis is a chronic disease of the vascular wall characterized by atherosclerotic plaque formation with its main feature being a combination of high concentrations of low-density lipoprotein (LDL) and a systemic pro-inflammatory status. Pro-inflammatory mechanisms have been shown to play a key role in atheroma formation [2]. A high plasma LDL concentration leads to an increase in LDL influx into the arterial wall and further to intracellular cholesterol accumulation, pro-inflammatory changes in local macrophages and release of cytokines stimulating further macrophage accumulation in the arterial wall as the initial step in atherosclerosis development. High-density lipoproteins (HDL) probably oppose this process of cholesterol accumulation and are thought to reduce inflammation [3]. High-density lipoprotein subfractions feature a wide variety of anti-atherosclerotic properties (anti-inflammatory, protection against LDL oxidation, anti-apoptotic, anti-thrombotic, etc.) [4,5]. One of the most crucial atheroprotective roles of HDL particles is their function as cholesterol acceptor in reverse cholesterol transport (RCT) from extrahepatic cells [6,7].
Reverse cholesterol transport is a process that results in cholesterol efflux (CE) from peripheral tissue back to the liver via HDL particles [8]. The first step in the process is the transport of non-esterified cholesterol to the plasma membrane and, subsequently, to the extracellular space. The process is mediated by the transporters adenosine triphosphate-binding cassette subfamily A member 1 (ABCA1), adenosine triphosphate-binding cassette transporter G1 (ABCG1), scavenger receptor class B type 1 (SR-BI) and pre-β-HDL mature spherical HDL as cholesterol acceptors [9]. In the second step, lecithin-cholesterol acyltransferase (LCAT) in a complex with HDL, very low-density lipoprotein (VLDL) or LDL esterifies free cholesterol, which is more efficiently carried by lipoproteins transporting it to the liver [10].
It has been shown that a systemic pro-inflammatory status of the body is associated with changes in the RCT pathway [11]. Furthermore, according to a study examining the effect of murine model of obesity on RCT changes, obesity impairs RCT due to reduced plasma cholesterol uptake by hepatocytes and adipocytes and cholesterol efflux from these cells [12].
During the acute phase of response to infection, plasma HDL and apolipoprotein (Apo) AI levels are reduced in humans [13.] Many of the transport proteins and enzymes that play important roles in HDL metabolism are altered during the acute phase response (LCAT, cholesteryl ester transfer protein, and hepatic lipase decrease, while secretory phospholipase A2 [sPLA2] and endothelial cell lipase increase) [13]. In patients developing acute sepsis, CE from cultured macrophages to plasma or HDL is significantly decreased [14]. While these changes could be beneficial as part of the immune response to infection, this prolonged intracellular cholesterol accumulation inducing the immune response may cause chronic infections and autoimmune diseases and, importantly, is associated with chronic metabolic inflammation including the development of atherosclerosis [15,16].
The principle underlying the relationship of RCT to infection was investigated in detail [17] and tested in peritoneal macrophages of animal models by Tall [2]. Tall’s group used peritoneal macrophages obtained by inducing peritoneal infection. ABCA1- and ABCG1-deficient mice tended to accumulate free and esterified cholesterol in peritoneal macrophages and this defect of CE has been suggested as the cause of growing inflammation. As macrophage deficiency of ABCA1 and ABCG1 is proatherogenic and increases inflammation, CE from macrophages is assumed to have anti-inflammatory activity within the atherosclerotic plaque in mice [18].
Although the risk of coronary heart disease (CHD) in man has been related to HDL concentration in many studies, data reported recently by Cahill clearly demonstrated that the CHD risk did not relate simply to HDL cholesterol concentration but directly to CE capacity [19]. That was why we decided to test the possibility of a relationship between inflammation in human adipose tissue (AT) and RCT.
In our earlier study, we demonstrated a link between AT macrophage polarization and cardiovascular risk factors [20] and the term AT immunometabolism is currently commonly used in this area of research [21–23]. Under this concept, a role of interaction between adipocytes and immune cells has been acknowledged [24]. As AT involvement in the process of RCT is also widely accepted [25,26], we tested a hypothesis that the pro-inflammatory microenvironment within AT may be related to the ability of macrophages to be involved in RCT. Adipocyte size affects lipoprotein metabolism and large adipocytes are combined with increasing of triglycerides and decreasing of HDL cholesterol concentration [27,28]. Although fatty acid turnover is small and large adipocytes does not differ, an effect of cholesterol metabolism might be considered [29].
Our primary focus was to assess the effect of pre-treatment of THP-1 macrophages with AT-conditioned media (ATCM) on CE as it is probably the only way how to analyse the reverse cholesterol transport in human AT.
Methods
Subjects
Our study designed to analyze the structure of adipose tissue plasma membrane and including a total of 42 living kidney donors (LKDs) was conducted between March 2017 and October 2019. Part of the data obtained in this study have been presented elsewhere [30]. The project was approved by the joint Ethics Committee of the Institute for Clinical and Experimental Medicine and Thomayer University Hospital (G-16-06-22, 963/13) in compliance with the principles of the Declaration of Helsinki of 1975, as revised in 2000, and the study was conducted in accordance with the approved protocol. All participants signed informed consent and completed a standard questionnaire with a healthcare professional.
Adipose tissue samples
All donors underwent hand-assisted retroperitoneoscopic nephrectomy. Visceral adipose tissue (VAT) outside Gerota’s fascia was obtained in the operating theatre during cleansing of the kidney before transplantation. Tissue samples were immediately transported in Dulbeco’ s Phosphate Buffered Saline (PBS, Biosera, Nuaille, France) to the laboratory where they were divided into three parts. The first sample used for stromal vascular fraction (SVF) isolation and analysis of AT macrophages by flow cytometry. The second part was used to prepare ATCM and the third one for analysis of fat cell size measurement.
Biochemistry
Immediately before anesthesia induction, fasting blood samples were obtained by venipuncture. Plasma total cholesterol, LDL cholesterol, HDL cholesterol and triglyceride concentrations were determined enzymatically on a Cobas Mira Plus Autoanalyzer (Roche, Basel, Switzerland) using commercially available kits (Roche Diagnostics, Basel, Switzerland).
Flow cytometry
All visible blood vessels, connective tissue and residual blood were removed and AT was cut into small pieces and digested in a collagenase II solution (2 mg/ml, 3.4 ml per g of tissue, Sigma-Aldrich, St. Louis, MO, USA) for 20 minutes at 37 °C in a shaking water bath. Next, the resultant cell suspension was cooled, repeatedly filtered on ice (150 μm and 50 μm filters, Sysmex, Kobe, Japan) and purified (10 minutes, 200 rcf, 4 °C) to obtain the SVF. A sample of SVF was incubated with a mixture of conjugated antibodies for 20 minutes at room temperature (CD14 - PC7, clone RM052; CD16 - ECD, clone 3G8; CD36 - FITC, clone FA6.152, all Beckman Coulter, Brea, CA, USA, Fixable Viability Dye (FVD) - eFluor 780, Thermo Fisher Scientific, Waltham, MA, USA) to be subsequently analyzed by flow cytometry (CytoFLEX, Beckman Coulter, Brea, CA, USA). The absence of FVD staining indicated the number of live cells; only singlets were included.
The threshold for CD16 positivity was set on a blood sample from the same patient. The data were processed with FlowJo software (Becton, Dickinson & Company, Franklin Lakes, NJ, USA).
Based on previous results [30, 31, 32, 33], we defined CD14+CD16+ positive macrophages with high CD36 expression (CD14+CD16+CD36high) as pro-inflammatory (referred to as such hereinafter). Their proportion to total macrophages (CD14 positive cells) was expressed in %.
A detailed description of the technique of flow cytometry including the gating strategy was published elsewhere [32].
Immunohistochemistry of macrophages of adipose tissue
Cryosections were fixed in an ice-cold acetone-methanol mixture for 10 minutes. Heat-induced epitope retrieval was performed with an antigen unmasking solution (Tris-Based, 25 minutes, 95 °C). After washing, sections were blocked for 2 hours in PBS with goat serum (5 %), bovine serum albumin (1 %) and Triton X (0.3 %) and incubated with primary antibody (CD68, clone PG-M1; Agilent Technologies, 1:100) or respective isotype controls (BioLegend) overnight. After 1-hour incubation with secondary antibodies (goat anti-mouse antibody DyLight 488, Invitrogen), sections were mounted with mounting medium with DAPI (Abcam). Four representative photographs of each section were taken at 10× magnification using the Eclipse Ni-E upright microscope (Nikon Corporation). The number of CD68 positive cells was expressed as a percentage of all nuclei present.
Adipose tissue-conditioned media
A method of isolation of pieces of AT similar to that used for SVF preparation was employed to prepare ATCM. Adipose tissue-conditioned media were obtained by incubation of cleansed and minced VAT in culture media (1 ml per 1 g of AT) (EBM-2, Lonza, Basel, Switzerland) with 0.2 % fatty acid-free bovine serum albumin solution (BSA) (Sigma Aldrich, St. Louis, MO, USA with penicillin-streptomycin, Sigma-Aldrich, St. Louis, MO, USA in a thermostat for 24 hours (5 % CO2, 37 °C). Next, the ACTM were separated from pieces of tissue using a 150-μm strainer, then a 0.22-μm syringe filter and stored in a −80 °C freezer until used.
We have published earlier that the concentrations of pro-inflammatory cytokines (TNF-α, IL-1β) in conditioned media are related to the behavior of macrophages to the endothelium and, generally, to their pro-inflammatory phenotype [34]. We sought to assess any potential effect of ATCM on CE.
Cholesterol efflux analysis
Cholesterol efflux (CE) was measured using methods described earlier but with small modifications [35]. In Experiment 1, a macrophage culture had been pre-treated by individual ATCM before CE measurement using identical plasma for all media as the acceptor. On the contrary, in Experiment 2, CE from standard (unaffected) macrophages was analyzed using the plasma of individual LKDs as cholesterol acceptor.
THP-1 cells (human monocyte leukemia cells; ECACC 88,081,201, Salisbury, UK) were cultured in RPMI-1640 with 10 % fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), 2 mM L-glutamine (Sigma Aldrich, St. Louis, MO, USA) and penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C, 5 % CO2 to be subsequently seeded into 24-well plates at a density of approximately 0,4 × 106 cells/ml and differentiated into macrophages with phorbol 12-myristate 13-acetate (PMA, 100 ng/mL, Sigma Aldrich, St. Louis, MO, USA) for 72 hours. Before each of the following steps, the medium was aspirated and the cells washed with PBS containing 0.1 % fatty acid-free BSA (Sigma Aldrich, St. Louis, MO, USA).
Next, THP-1 cells were labeled in a medium containing [14C] cholesterol (ARC, St. Louis, MO, USA, specific activity 0.1 μCi/mL) for 48 hours. The culture medium with 10 % ATCM was subsequently added for 48 hours. Cholesterol efflux was measured by incubation of the labeled tissue culture in RPMI medium containing 5 % standard plasma. Labeled free cholesterol molecules only absorbed on the cell surface were subtracted as radioactivity after 15 min of incubation. In Experiment 1, standard identical plasma from all samples was used.
Aliquots of efflux media were first collected and measured at 15 min of incubation to assess labeled cholesterol on the surface of THP-1 cells. The next incubation period was at 240 minutes of CE measurement when the efflux phase ended. The media were subsequently centrifuged at 400 relative centrifugal force (rcf) at 4 °C for 5 minutes to remove any floating cells. Next, the supernatants were mixed with a scintillation liquid (Rotiszint eco plus, Roth, Germany) for liquid scintillation counting followed by double washing of medium-free cell monolayers with ice-cold PBS containing 0.1 % BSA. The cells were frozen for one hour, defrosted and then lysed in isopropanol-hexane (2:3) for one hour. Aliquots of the lysates were centrifuged at 400 rcf and evaporated in scintillation vials. Next, the scintillation liquid was added to aliquots for liquid scintillation counting. Cholesterol efflux (%) was expressed as the radioactivity of the efflux media divided by total radioactivity of the sample (media plus lysed cells). All the samples were analyzed in triplicate using a liquid scintillation analyzer (Tri-Carb 2900TR; PerkinElmer, Waltham, United States) and presented data are means of triplicates.
The above procedure was also used for CE measurement in Experiment 2, with acceptors being the plasma samples of individual LKDs.
Histological analysis of AT cell size
The third AT sample was used for fat cell size measurement. A small piece (approximately 100–150 mg) of adipose tissue was separated from the sample and prepared for long-term storage. The tissue was subsequently fixed overnight at room temperature in neutral buffered formalin. After washing with PBS, the tissue was incubated overnight in 30 % sucroce in PBS at 4 °C. Samples were then gently dried with cellulose wadding paper, mounted in OCT (Tissue-Tek O. C. T.™ Compound, Sakura Finetek Europe BV, Alphen aan den Rijn, the Netherlands) and flash frozen in an isopentane bath cooled by liquid nitrogen and stored at −80 °C until further use.
Fresh prepared cryosections (8 μm) were incubated in 96 % ethanol for 5 minutes. After a short wash in distilled water, the sections were stained with fresh Giemsa working solution (10 minutes), washed thoroughly in tap water and dried at room temperature. Dry sections were cleared briefly in xylene and immediately mounted (Pertex®, Histolab).
Four representative photographs of each section were taken at 10× magnification using an Eclipse Ni-E upright microscope (Nikon Corporation). Adipocyte area was measured using the Fiji Adiposoft plugin (semi-automatic approach; Fiji software [NIH-Fiji, Bethesda, MD, USA] and Adiposoft plugin [University of Navarra, Pamplona, Spain]). Damaged adipocytes and adipocytes on image margins were excluded from the analysis. A minimum of 400 cells per subject was analyzed.
Statistical analysis
Data were analyzed using the paired t-test. All results are expressed as mean ± SD. Probability values of less than 0.05 were considered significant.
Results
The main characteristics of the whole group of LKDs are presented in the Table 1. The study include a total of 42 LKDs (28 women and 14 men) whose baseline physiological and clinical characteristics were compared with data of age- and sex-matched individuals selected from a population study analyzing a 1 % representative Czech population sample aged 25–64 years [36]. Our group of LKDs was clearly slightly healthier than the representative Czech population sample as their BMI was significantly lower (p<0.05); however, there were no significant differences in non-HDL cholesterol and total HDL cholesterol concentrations. Slightly lower concentration of HDL cholesterol in LKDs corresponds to slightly lower concentrations of non-HDL cholesterol. Quite surprisingly, triglyceride concentrations were (non-significantly) higher in our LKDs.
Table.
Characteristics of the group of LKDs and age- and sex-matched controls from the 1 % representative Czech population sample
| LKDs | Czech population sample | p value | |
|---|---|---|---|
| Number (men/women) | 42 (28/14) | 42 (28/14) | ns. |
| Age (years) | 51.96±10.36 | 51.57±9.77 | ns. |
| BMI (kg/m 2 ) | 26.73±3.10 | 29.24±6.11 | p<0.05 |
| Non-HDL-C (mmol/l) | 3.37±0.96 | 3.95±1.12 | ns. |
| HDL-C (mmol/l) | 1.35±0.43 | 1.57±0.41 | ns. |
| Triglycerides (mmol/l) | 1.72±0.96 | 1.28±0.58 | ns. |
BMI: body mass index; non-HDL-C: non-high-density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol, ns: non-significant. Characteristics of the whole LKDs group compared with the Czech population sample. The number, age, BMI, non-HDL-C, triglyceride and HDL-C concentrations of the group of LKDs were compared with those of a representative sample of the Czech population whose age- and sex-matched individuals were selected from a population study analyzing a 1 % representative Czech population sample aged 25–64 years [34]. The total numbers of women and men were 28 and 14, respectively. Values are expressed as mean ± SD.
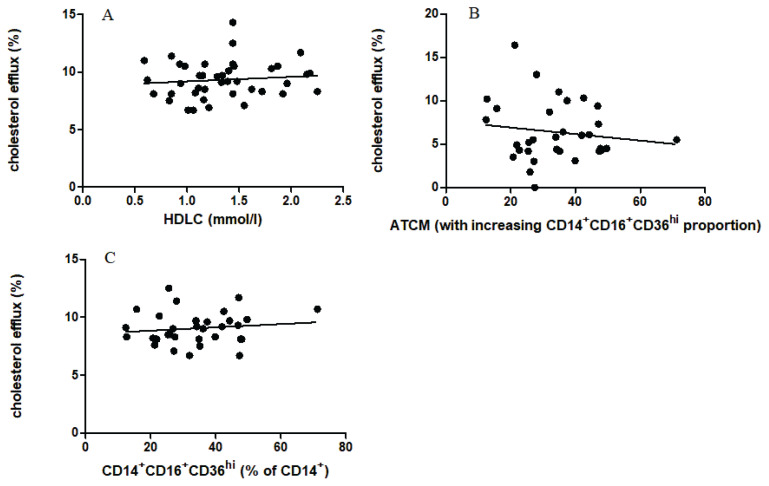
When comparing the relationship between CE and concentration of HDL cholesterol, no statistically significant correlation was found (Fig. 1A).
Fig. 1.
The relation of cholesterol efflux to total HDL cholesterol A (n=42). The relation of cholesterol efflux to the proportion of pro-inflammatory CD14+CD16+CD36hi macrophages influencing by the presence of ATCM B (n=32). The relation of cholesterol efflux to the proportion of pro-inflammatory CD14+CD16+CD36hi macrophages C (n=32, no cytometric analysis was available for 10 samples due to technical difficulties).
Experiment 1 was intended to detect a possible effect of ATCM on release radiolabeled cholesterol. As CE was not affected by the presence of ATCM in our experiment, we were unable to demonstrate any effect of ATCM obtained from a sample of incubated adipose tissue (Fig. 1B). The implication is that no molecule released from incubated VAT of LKDs could have affected CE from pre-labeled macrophages when using identical plasma samples as the acceptor of labeled cholesterol from cultured macrophages.
Experiment 2 was conducted to measure CE from pre-labeled macrophages to plasma samples of individual acceptors, i.e., various plasma samples from LKDs. Similarly, there was no statistically significant correlation between CE and the percentage of pro-inflammatory CD14+CD16+CD36hi cells as a measure of adipose tissue inflammation (Fig. 1C). However, the proportion of pro-inflammatory macrophages in visceral AT of LKDs varied between 15 and almost 60 % of total adipose tissue macrophages, there is no significant trend with analyzed CE.
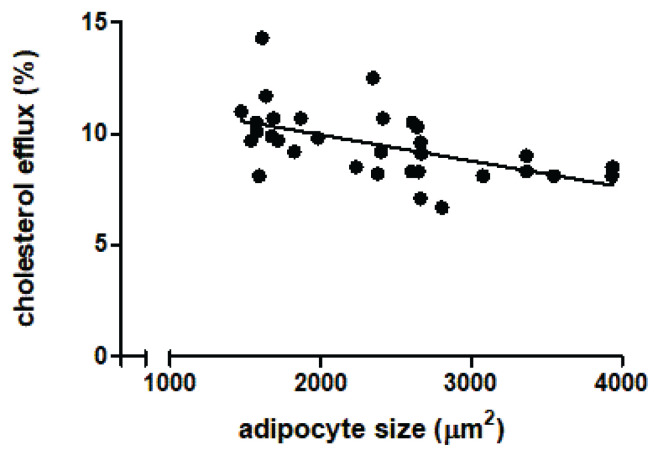
The same adipose tissue (used to prepare ATCM) was used to measure adipocyte size of LKDs (n=32) by immunohistochemical analysis. The pattern of distribution of CD68 positive macrophages is documented in Fig. 3 showing that it is rather homogeneous as it is documented in Fig. 2 a statistically significant decrease of CE to increasing adipocyte size of LKDs was documented (n=32; p<0.002; R=0.3).
Fig. 3.
Immunohistochemical analysis of adipose tissue CD68 positive macrophages (marked in green) of living kidney donors.
Fig. 2.
The relation of cholesterol efflux (measured with individual LKDs’ plasma) to adipocyte size (n=32; p<0.002; R=0.3). A total of 10 immunohistology samples were not taken at all because of very small amounts of tissue available.
Discussion
Experimental data of Tall’s laboratory [37, 2] together with decreases in RCT and CE in sepsis [14] and coronary artery disease [38] in man suggest the important role played by CE in inflammation. As adipose tissue inflammation is a most significant factor in cardiometabolic risk, our aim was to investigate the relationship between CE and the proportion of pro-inflammatory macrophages in adipose tissue. As both clinical cases documenting the relationship of inflammation and RCT are extreme ones, we sought to test CE to the plasma of a set of healthy individuals.
Our study was designed to analyze the adipose tissue of LKDs obtained intraoperatively during donated kidney isolation [32]. We focused primarily on macrophage polarization as a marker of pro-inflammatory changes in AT because of the widely recognized pivotal impact of these immune cells on AT dysfunction. To study the local effect of human AT, ATCM were prepared from the adipose tissue of LKDs. Adipose tissue-conditioned media were supposed to influence CE from macrophages during their incubation prior to CE measurement. Our model of RCT estimate based on measurement of CE from radiolabeled macrophages is widely employed (using the whole plasma [39,40] or different lipoprotein particles [41,42,43]).
Experiment 1 analyzing a possible effect of ATCM on CE was principally based on our data [34] suggesting that AT in vitro is able to produce the same pro-inflammatory stimuli. The negative results only document (using identical cells as well as an identical cholesterol acceptor) that conditioned media were not able to influence any effect on reverse cholesterol transport.
Similar to data derived from experimental models [2], we expected reduced RCT in individuals with higher proportions of pro-inflammatory macrophages in AT. Cholesterol efflux was measured using the plasma of individual LKDs and various proportions of pro-inflammatory macrophages in their visceral AT were compared with RCT.
Surprisingly, we were unable to document effect of different concentrations of HDL acceptors or the proportion of pro-inflammatory macrophages to CE. One could assume that, in healthy individuals, RCT is probably not related to inflammation expressed as a proportion of pro-inflammatory macrophages and to HDL concentration.
Despite our inability to confirm Tall’s hypothesis, we found a strong negative correlation between CE and adipocyte surface. The size of adipocytes is a significant predictor of the cardiometabolic alterations related to obesity. Adipocyte hypertrophy, especially in visceral AT, may be associated with cardiometabolic alterations [44]. This theory is consistent with our finding of reduced CE from adipocytes with a larger surface area as a characteristic feature suggesting that cholesterol accumulation in macrophages is associated with the development of cardiovascular disease. Adipocyte size has been shown to be an important determinant of adipokine secretion [45]. Furthermore, large adipocytes are presumably able to influence the activity of intracellular and extracellular lipases [46]. Although fatty acid turnover in small vs. large adipocytes is not different, large cells are, by definition, able to store greater amounts of fatty acids. The advantage to store high energy substrate in long history starvation of men was probably very important advantage in history. On the contrary in recent high prevalence of obesity and frequent supply high-energy meals it might be an important disadvantage. Similarly to the situation of higher risk of hypertension and genetically determination hypercholesterolemia [47].
In conclusion, changes in RTC are not related to macrophage polarization and pro-inflammatory status of human AT. On the contrary, it has been documented that larger adipocytes correlate positively with the concentration of triglycerides and negatively with HDL [48], and are also behind decreasing RTC.
Acknowledgements
This research was funded by the Ministry of Health of the Czech Republic, grant No. NU 20-01-0022.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Jokinen E. Obesity and cardiovascular disease. Minerva pediatr. 2015;67:25–32. [PubMed] [Google Scholar]
- 2.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Eckardstein A, Nofer JR, Assmann G. High density lipoproteins and arteriosclerosis role of cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2001;21:13–27. doi: 10.1161/01.ATV.21.1.13. [DOI] [PubMed] [Google Scholar]
- 5.Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SPA, Remaley AT, Sviridov D, Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 6.Sviridov D, Nestel P. Dynamics of reverse cholesterol transport: Protection against atherosclerosis. Atherosclerosis. 2002;161:245–254. doi: 10.1016/S0021-9150(01)00677-3. [DOI] [PubMed] [Google Scholar]
- 7.Catalano G, Duchene E, Julia Z, Le Goff W, Bruckert E, Chapman MJ, Guerin M. Cellular SR-BI and ABCA1-mediated cholesterol efflux are gender-specific in healthy subjects. J Lipid Res. 2008;49:635–643. doi: 10.1194/jlr.M700510-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Aiello RJ, Brees D, Bourassa PA, Royer L, Lindsey S, Coskran T, Haghpassand M, Francone OL. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler Thromb Vasc Biol. 2002;22:630–637. doi: 10.1161/01.ATV.0000014804.35824.DA. [DOI] [PubMed] [Google Scholar]
- 9.Jessup W, Gelissen IC, Gaus K, Kritharides L. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr Opin Lipidol. 2006;17:247–257. doi: 10.1097/01.mol.0000226116.35555.eb. [DOI] [PubMed] [Google Scholar]
- 10.Glomset JA. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968;9:155–167. doi: 10.1016/S0022-2275(20)43114-1. [DOI] [PubMed] [Google Scholar]
- 11.Feingold KR, Grunfeld C. The acute phase response inhibits reverse cholesterol transport. J Lipid Res. 2010;51:682–684. doi: 10.1194/jlr.E005454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duong MN, Uno K, Nankivell V, Bursill C, Nicholls SJ. Induction of obesity impairs reverse cholesterol transport in ob/ob mice. PLoS One. 2018;13:e0202102. doi: 10.1371/journal.pone.0202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Annema W, Nijstad N, Tölle M, Freark de Boer J, Buijs RVC, Heeringa P, van der Giet M, Tietge UJF. Myeloperoxidase and serum amyloid A contribute to impaired in vivo reverse cholesterol transport during the acute phase response but not group IIA secretory phospholipase A2. J Lipid Res. 2010;51:743–754. doi: 10.1194/jlr.M000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherer Y, Shoenfeld Y. Mechanisms of disease: Atherosclerosis in autoimmune diseases. Nat Clin Pract Rheumatol. 2006;2:99–106. doi: 10.1038/ncprheum0092. [DOI] [PubMed] [Google Scholar]
- 16.Post WS, Budoff M, Kingsley L, Palella FJ, Jr, Witt MD, Li X, George RT, Brown TT, Jacobson LP. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–467. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao GJ, Tang SL, Lv YC, Ouyang XP, He PP, Yao F, Chen WJ, Lu Q, Tang YY, Zhang M, Fu Y, Zhang DW, Yin K, Tang CK. Antagonism of Betulinic Acid on LPS-Mediated Inhibition of ABCA1 and Cholesterol Efflux through Inhibiting Nuclear Factor-kappaB Signaling Pathway and miR-33 Expression. PLoS One. 2013;8:e74782. doi: 10.1371/journal.pone.0074782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch C, Fisher EA, Wang N, Yvan-Charvet L, Tall AR. Deficiency of ATP-binding cassette transporters a1 and g1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112:1456–1465. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cahill LE, Sacks FM, Rimm EB, Jensen MK. Cholesterol efflux capacity, HDL cholesterol, and risk of coronary heart disease: A nested case-control study in men. J Lipid Res. 2019;60:1457–1464. doi: 10.1194/jlr.P093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kralova Lesna I, Cejkova S, Kralova A, Fronek J, Petras M, Sekerkova A, Thieme F, Janousek L, Poledne R. Human adipose tissue accumulation is associated with pro-inflammatory changes in subcutaneous rather than visceral adipose tissue. Nutr Diabetes. 2017;7:e264. doi: 10.1038/nutd.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteiro L, Da S, Pereira JA, Palhinha L, Moraes-Vieira PMM. Leptin in the regulation of the immunometabolism of adipose tissue-macrophages. J Leukoc Biol. 2019;106:703–716. doi: 10.1002/JLB.MR1218-478R. [DOI] [PubMed] [Google Scholar]
- 22.Kohlgruber AC, Lamarche NM, Lynch L. Adipose tissue at the nexus of systemic and cellularimmunometabolism. Semin Immunol. 2016;28:431–440. doi: 10.1016/j.smim.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Schipper HS, Prakken B, Kalkhoven E, Boes M. Adipose tissue-resident immune cells: Key players in immunometabolism. Trends Endocrinol Metab. 2012;23:407–415. doi: 10.1016/j.tem.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Engin AB. Adipocyte-macrophage cross-talk in obesity. Adv Exp Med Biol. 2017;960:327–343. doi: 10.1007/978-3-319-48382-5_14. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, McGillicuddy FC, Hinkle CC, O’Neill S, Glick JM, Rothblat GH, eilly MP. Adipocyte modulation of high-density lipoprotein cholesterol. Circulation. 2010;121:1347–1355. doi: 10.1161/CIRCULATIONAHA.109.897330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao JH, Zeng MY, Yu XH, Zeng GF, He LH, Zheng XL, Zhang DW, Ouyang XP, Tang CK. Visceral adipose tissue-derived serine protease inhibitor accelerates cholesterol efflux by up-regulating ABCA1 expression via the NF-κB/miR-33a pathway in THP-1 macropahge-derived foam cells. Biochem Biophys Res Commun. 2018;500:318–324. doi: 10.1016/j.bbrc.2018.04.066. [DOI] [PubMed] [Google Scholar]
- 27.Laforest S, Michaud A, Paris G, Pelletier M, Vidal H, Géloën A, Tchernof A. Comparative Analysis of Three Human Adipocyte Size Measurement Methods and Their Relevance for Cardiometabolic Risk. Obesity. 2017;25:122–131. doi: 10.1002/oby.21697. [DOI] [PubMed] [Google Scholar]
- 28.Honecker J, Weidlich D, Heisz S, Lindgren CM, Karampinos DC, Claussnitzer M, Hauner H. A distribution-centered approach for analyzing human adipocyte size estimates and their association with obesity-related traits and mitochondrial function. International Journal of Obesity. 2021;45(9):2108–2117. doi: 10.1038/s41366-021-00883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobsson Bo, Smith Ulf. Effect of cell size on lipolysis and antilipolytic action of insulin in human fat cells. Journal of Lipid Research. 1972;13(5):651–656. doi: 10.1016/S0022-2275(20)39370-6. [DOI] [PubMed] [Google Scholar]
- 30.Poledne R, Malinska H, Kubatova H, Fronek J, Thieme F, Kauerova S, Kralova Lesna I. Polarization of macrophages in human adipose tissue is related to the fatty acid spectrum in membrane phospholipids. Nutrients. 2020;12:8. doi: 10.3390/nu12010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poledne R, Kralova Lesna I. Adipose tissue macrophages and atherogenesis - a synergy with cholesterolaemia. Physiol Res. 2021;70(Suppl4):S535–S549. doi: 10.33549//physiolres.934745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kralova Lesna I, Kralova A, Cejkova S, Fronek J, Petras M, Sekerkova A, Thieme F, Janousek L, Poledne R. Characterisation and comparison of adipose tissue macrophages from human subcutaneous, visceral and perivascular adipose tissue. J Transl Med. 2016;14:208. doi: 10.1186/s12967-016-0962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cejkova S, Kubatova H, Thieme F, Janousek L, Fronek J, Poledne R, Kralova Lesna I. The effect of cytokines produced by human adipose tissue on monocyte adhesion to the endothelium. Cell Adhes Migr. 2019;13:293–302. doi: 10.1080/19336918.2019.1644856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kralova Lesna I, Suchanek P, Kovar J, Stavek P, Poledne R. Replacement of dietary saturated FAs by PUFAs in diet and reverse cholesterol transport. J Lipid Res. 2008;49:2414–2418. doi: 10.1194/jlr.M800271-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Cifkova R, Skodova Z, Bruthans J, Adamkova V, Jozifova M, Galovcova M, Wohlfahrt P, Krajcoviechova A, Poledne R, Stavek P, Lanská V. Longitudinal trends in major cardiovascular risk factors in the Czech population between 1985 and 2007/8. Czech MONICA and Czech post-MONICA. Atherosclerosis. 2010;211:676–681. doi: 10.1016/j.atherosclerosis.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao B, Tang Ch, Sinha A, Mayer PS, Davenport GD, Brot N, Oda MN, Zhao XQ, Heinecke JW. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res. 2014;114(11):1733–1742. doi: 10.1161/CIRCRESAHA.114.303454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mweva S, Paul JL, Cambillau M, Goudouneche D, Beaune P, Simon A, Fournier N. Comparison of different cellular models measuring in vitro the whole human serum cholesterol efflux capacity. Eur J Clin Invest. 2006;36:552–559. doi: 10.1111/j.1365-2362.2006.01673.x. [DOI] [PubMed] [Google Scholar]
- 40.Kralova Lesna IK, Suchanek P, Stavek P, Poledne R. May alcohol-induced increase of HDL be considered as atheroprotective? Physiol Res. 2010;59:407–413. doi: 10.33549/physiolres.931769. [DOI] [PubMed] [Google Scholar]
- 41.Marshall JD, Courage ER, Elliott RF, Fitzpatrick MN, Kim AD, Lopez-Clavijo AF, Woolfrey BA, Ouimet M, Wakelam MJO, Brown RJ. THP-1 macrophage cholesterol efflux is impaired by palmitoleate through Akt activation. PLoS One. 2020;15:e0233180. doi: 10.1371/journal.pone.0233180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motte A, Gall J, Salem JE, Dasque E, Lebot M, Frisdal E, Galier S, Villard EF, Bouaziz-Amar E, Lacorte JM, Charbit B, Goff WL, Lesnik P, Guerin M. Reduced reverse cholesterol transport efficacy in healthy men with undesirable postprandial triglyceride response. Biomolecules. 2020;10:810. doi: 10.3390/biom10050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohatgi A. High-Density Lipoprotein Function Measurement in Human Studies: Focus on Cholesterol Efflux Capacity. Prog Cardiovasc Dis. 2015;58:32–40. doi: 10.1016/j.pcad.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laforest S, Labrecque J, Michaud A, Cianflone K, Tchernof A. Adipocyte size as a determinant of metabolic disease and adipose tissue dysfunction. Crit Rev Clin Lab Sci. 2015;52(6):301–313. doi: 10.3109/10408363.2015.1041582. [DOI] [PubMed] [Google Scholar]
- 45.Skurk T, Alberti-Huber C, Herder Ch, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92(3):1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 46.De Naeyer H, Ouwens DM, Van Nieuwenhove Y, Pattyn P, Hart LM, Kaufman JM, Sell H, Eckel J, Cuvelier C, Taes YE, Ruige JB. Combined Gene and Protein Expression of Hormone-Sensitive Lipase and Adipose Triglyceride Lipase, Mitochondrial Content, and Adipocyte Size in Subcutaneous and Visceral Adipose Tissue of Morbidly Obese Men. Obes Facts. 2011;4:407–416. doi: 10.1159/000333445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poledne R, Zicha J. Human genome evolution and development of cardiovascular risk factors through natural selection. Physiol Res. 2018;67(2):155–163. doi: 10.33549/physiolres.933885. [DOI] [PubMed] [Google Scholar]
- 48.Veilleux A, Caron-Jobin M, Noël S, Laberge PY, Tchernof A. Visceral adipocyte hypertrophy is associated with dyslipidemia independent of body composition and fat distribution in women. Diabetes. 2011;60(5):1504–1511. doi: 10.2337/db10-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]