Summary
Pulmonary hypertension is a group of disorders characterized by elevated mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance. To test our hypothesis that combining two drugs useful in experimental pulmonary hypertension, statins and dehydroepiandrosterone sulfate (DHEA-S), is more effective than either agent alone, we induced pulmonary hypertension in adult male rats by exposing them to hypoxia (10 %O2) for 3 weeks. We treated them with simvastatin (60 mg/l) and DHEA-S (100 mg/l) in drinking water, either alone or in combination. Both simvastatin and DHEA-S reduced mPAP (froma mean±s.d. of 34.4±4.4 to 27.6±5.9 and 26.7±4.8 mmHg, respectively), yet their combination was not more effective (26.7±7.9 mmHg). Differences in the degree of oxidative stress (indicated by malondialdehydeplasma concentration), the rate of superoxide production (electron paramagnetic resonance), or blood nitric oxide levels (chemiluminescence) did not explain the lack of additivity of the effect of DHEA-S and simvastatin on pulmonary hypertension. We propose that the main mechanism of both drugs on pulmonary hypertension could be their inhibitory effect on 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, which could explain their lack of additivity.
Keywords: Statin, Dehydroepiandrosterone, Pulmonary hypertension, Rats
Introduction
Pulmonary hypertension is a diverse group of disorders characterized by elevated mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance. Both elevated pulmonary vascular smooth muscle tension and remodeling of the pulmonary vascular wall contribute, to a variable degree, to increased pulmonary vascular resistance. In the absolute majority of patients, pulmonary hypertension is a secondary consequence of some other disease, especially circulatory or respiratory [1]. The prognosis of primary disease is significantly worse if it is complicated by pulmonary hypertension. Although the possibilities of therapy have improved significantly in the last two decades, they still have limited effectiveness and there is a clear need for new therapeutic procedures, including off-label and combination approaches [2].
A good example of an off-label therapy for pulmonary hypertension is the use of statins. Although this group of competitive inhibitors of 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase is used primarily to reduce cardiovascular risks by lowering blood cholesterol levels [3], there is a large body of evidence that they prevent and can reduce pulmonary hypertension in experimental animals [4–7]. The mechanisms of this effect seem to include restoration of nitric oxide (NO) synthase expression and/or activity reduced in some (but not all [8]) forms of pulmonary hypertension [9,10], decreased plasma concentration of asymmetric dimethylarginine (an endogenous NO synthase inhibitor) [10], and antioxidant activity [11,12] (although a pro-oxidant effect of statins has also been reported [12]). The results with statins in human patients are less conclusive and may depend on the particular type of pulmonary hypertension [13–16]. Therefore, it is worth testing whether combining statins with other therapies could be more effective than statins alone. For example, the combination of statins with sildenafil was shown to be more effective against pulmonary hypertension than either therapy alone in some [17] but not all [18] studies.
Another promising approach to pulmonary hypertension therapy could be the use of dehydroepiandrosterone (DHEA). DHEA is a naturally occurring cholesterol-derived steroid hormone synthesized mainly in the adrenal cortex that serves as a precursor for both estrogens and androgens and has a variety of biological effects of its own [19]. DHEA circulates in the blood mostly in the form of its 3β-sulfate ester (DHEA-sulfate, DHEA-S), which alsomediates many of its effects [20]. DHEA/DHEA-S posess several properties that may be beneficial in pulmonary hypertension, including antioxidant activity [21], stimulation of NO synthesis [22], activation of Ca2+-gated K+ (KCa) channels with large conductance (BKCa) [23] that mediate vasodilation in the pulmonary circulation [24,25], and inhibition of L-type voltage-gated Ca2+ channels [26]. Furthermore, DHEA can block superoxide production by alveolar macrophages [27], which is also part of the hypoxic pulmonary hypertension mechanism [28,29]. Because of these properties, and because its exogenous administration is well tolerated in humans, DHEA (or DHEA-S) is attractive as a potential treatment of pulmonary hypertension, especially as plasma levels of DHEA/DHEA-S are reduced in patients with this disease [30]. It has been shown repeatedly that administration of DHEA or DHEA-Spartially prevents and reduces experimental pulmonary hypertension [23,31–33].
The present study was therefore designed to test the hypothesis that a combination therapy with oral statin (simvastatin) and oral DHEA-S will be more effective in preventing chronic hypoxic pulmonary hypertension in rats than either of the treatments alone. Since orally ingested DHEA is converted to DHEA-S when passing through the intestines and liver [20], we decided to use DHEA-S in drinking water.
Methods
The experiments were carried out according to EU regulations for the use of experimental animals and in accordance with the ARRIVE guidelines. They were approved by the Charles University Second Faculty of Medicine Animal Studies Committee.
All drugs and chemicals were purchased from Sigma-Aldrich (Prague, Czech Republic).
Experimental groups and drug administration
The study utilized adult Wistar rats (~350 g at the beginning of the experiment). For the sake of compatibility with previously published studies with statins and DHEA-S, only males were used. They were randomly assigned to one of five groups - one normoxic control (NC, n=16, kept in room air throughout the experiment) and 4 chronically hypoxic (normobaric 10 % O2 for 3 weeks). Of those, one group was treated with an inhibitor of HMG-CoA reductase, simvastatin, administered in drinking water at a dose of 60 mg/l, throughout the hypoxic exposure (group HS, n=9). Since simvastatin is not soluble in water, it was first dissolved in a small volume of ethanol and then added to the drinking water to yield a final ethanol concentration of 0.5 %. The dose of simvastatin was calculated using our earlier observation (now confirmed) that an adult male rat in 10 % O2 drinks about 30 ml/day and there ported effective dose by gavage [7]. Another group received, also in drinking water, DHEA-S at 100 mg/l [31] for the entire duration of the hypoxic exposure (group HD, n=10). The third hypoxic group was treated with a combination of DHEA-S (100 mg/l) and simvastatin (60 mg/l) in drinking water with 0.5 % ethanol (group HDS, n=9). The last group drinking plain water in hypoxia served as hypoxic controls (HC, n=9).
We consider the amount of ethanol consumed with simvastatin negligible, as doses ten times higher are used as a model of moderate alcohol use [34,35]. However, to verify that the differences between the groups were not due to the presence of ethanol in the drinking water of simvastatin-treated rats, parts of the remaining groups also received 0.5 % ethanol (8 of 16 rats in NC, 4 of 10 in HD, and 3 of 9 in HC). Unless otherwise stated, the results were the same with and without ethanol and they were thus pooled for statistical analysis.
Experimental protocol and measurements
After 3 weeks of hypoxia (or equivalent age in NC), rats were anesthetized with thiopental (30 mg/kg of body weight, i.p.). Pulmonary artery pressure was measured in intact chest rats spontaneously breathing room air by pulmonary artery catheterization as previously described [31,36]. The trachea was then accessed through a throat skin incision and used to intubate and ventilate the rat with air (50 breaths/min; peak inspiratory pressure 10 cm H2O; end-expiratory pressure 0 cm H2O). The chest was then opened by sternotomy and an ultrasound flow probe (Transonic Systems Inc, Ithaca, NY, USA) was placed on the ascending aorta to measure cardiac output [31]. In some rats, this procedure caused excessive bleeding, so the number of rats for which we have cardiac output values is somewhat lower than that for which we have other variables. Cardiac index was calculated as cardiac output/body weight and pulmonary vascular resistance index (PVRI) as mPAP/cardiac output/body weight. Subsequently, arterial blood samples were collected to measure hematocrit. To assess a possible role of NO alterations, the sum of plasma concentrations of NO and its oxidation products (nitrites and nitrates, NOx) was measured by chemiluminescence (NOA 280i, Sievers, Boulder, CO, USA) after hot acidic reduction as previously described [37].
Serum samples were also used to determine the concentration of malondialdehyde (MDA) with HPLC [38] as a measure of oxidative stress [39]. Briefly, 0.05 % butylhydroxytoluene, 0.44 M H3PO4 and 42 mM thiobarbituric acid were added to samples and standards (1,1,3,3-tetraethoxypropane) of different concentrations. The samples were vortexed and then heated at 100 °C for 1 hour. They were then cooled on ice for 5 minutes and the MDA-thiobarbituric acid complex was extracted into butanol. The tubes were centrifuged for 5 minutes at 10,000 g to form two separate phases. Aliquots were pipetted into vials and measured by HPLC (Jasco, Tokyo, Japan). The analysis was performed on the Agilent Zorbax Eclipse Plus C18 4,6x 250 mm, 5 μm column. The optimal flow rate was set at 1 ml/min, for a mobile phase with a composition of methanol/50 mM KH2PO4 (40:60, v/v). The fluorescence detector was set at 515/553 nm (excitation/emission). Evaluation was performed using ChromNAV software (Jasco).
Electron paramagnetic resonance (EPR) using 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine hydrochloride (CMH, 10 μM) as a superoxide (O2−) detecting spin probe was applied to measure the superoxide production rate in fresh blood [40]. Samples were prepared by adding 5 μM diethyldithiocarbamate, 25 μM desferroxamine, and 10 μM CMH (Noxygen, Elzach, Germany) and 5 μl of fresh blood in 50 μl of Krebs-Hepes buffer. The samples were placed in airtight glass capillaries and the spectra were recorded in an EPR spectrometer with a temperature-controlled resonator (Escan, Bruker Corp., Billerica, MA, USA). The EPR settings for the CMH spin label were center field 3455 G, sweep width 10 G, frequency 9.7690 GHz, microwave power 23.89 mW, and modulation amplitude 2.93 G. Spectra were recorded over 10 min.
After obtaining all samples, the heart was dissected and weighed in parts in the fresh state. The weight of the right ventricle relative to body weight and to the sum of left ventricle plus septum weight was used as a measure of right ventricular hypertrophy.
Statistical analysis
All differences between the groups were analyzed using 1-way ANOVA followed (if significant) by Fischer’s least significant difference post hoc test using the Prism 9 software (GraphPad Software, San Diego, CA, USA). p <0.05 was preselected to reject a null hypothesis of no difference in all cases. The results are reported as means ± s.d.
Results
All rats assigned to the experimental groups survived till the end of the study with no obvious problems. Compared to normoxic controls, all rats exposed to chronic hypoxia had reduced body weight, but there were no differences in body weight among the treatments, indicating similar water (and thus drug) intake in all hypoxic groups (Table 1). Hematocrit was increased similarly in all hypoxic groups (Table 2), indicating that the effects of therapy on pulmonary vascular resistance and mPAP were not caused by changes in blood viscosity.
Table 1.
Body and heart ventricles weights
| Group (n) | BW (g) | RV (mg) | LV+S (mg) | RV/BW (%) | LV+S/BW (%) | RV/LV+S |
|---|---|---|---|---|---|---|
| NC (16) | 446 ± 75 | 210 ± 21 | 838 ± 79 | 0.048 ± 0.007 | 0.19 ± 0.02 | 0.251 ± 0.015 |
| HC (9) | 327 ± 15* | 269 ± 41** | 695 ± 51* | 0.082 ± 0.011* | 0.21 ± 0.01 | 0.386 ± 0.042* |
| HD (10) | 304 ± 43* | 263 ± 56*** | 638 ± 49* | 0.086 ± 0.010* | 0.21 ± 0.02 | 0.409 ± 0.061* |
| HS (9) | 281 ± 23* | 239 ± 48 | 606 ± 50*† | 0.085 ± 0.014* | 0.22 ± 0.01 | 0.395 ± 0.073* |
| HDS (9) | 288 ± 33* | 241 ± 61 | 606 ± 6 3*† | 0.083 ± 0.015* | 0.21 ± 0.01 | 0.393 ± 0.071* |
NC, normoxic control group; HC, hypoxic control group (3 weeks in 10 % O2); HD, group treated with DHEA-S throughout the 3-weeks exposure to hypoxia; HS, group treated with simvastatin throughout the 3-weeks exposure to hypoxia; HDS, group treated with a combination of simvastatin + DHEA-S throughout the 3-weeks exposure to hypoxia; BW, body weight; RV, right ventrikle weight; LV+S, the sum of the weights of the left ventricle and septum. Data are means ± s.d. Differences among the groups not specifically marked are not statistically significant (p<0.05, 1-way ANOVA and Fischer’s least significant difference post hoc test).
P<0.0001 vs. NC,
P<0.005 vs. NC,
P<0.01 vs. NC
Table 2.
Cardiac output, cardiac index, and hematocrit
| Group | Cardiac output (ml/min) | Cardiac index (ml/min/kg BW) | Hematocrit (%) |
|---|---|---|---|
| NC | 36.9 ± 9.7 (n=14) | 84 ± 24 (n=14) | 51.4 ± 3.4 (n=16) |
| HC | 36.8 ± 21.0 (n=6) | 113 ± 65 (n=6) | 63.7 ± 3.2* (n=9) |
| HD | 27.3 ± 6.5 (n=7) | 91 ± 17 (n=7) | 59.6 ± 6.0 (n=8) |
| HS | 29.0 ± 6.7 (n=8) | 103 ± 17 (n=8) | 62.3 ± 3.8* (n=9) |
| HDS | 27.0 ± 8.0 (n=7) | 92 ± 22 (n=7) | 62.4 ± 4.3* (n=9) |
Group abbreviations as in Table 1. Data are means ± s.d. Differences among the groups not specifically marked are not statistically significant (p<0.05, 1-way ANOVA and Fischer’s least significant difference post hoc test).
P<0.0001 vs. NC
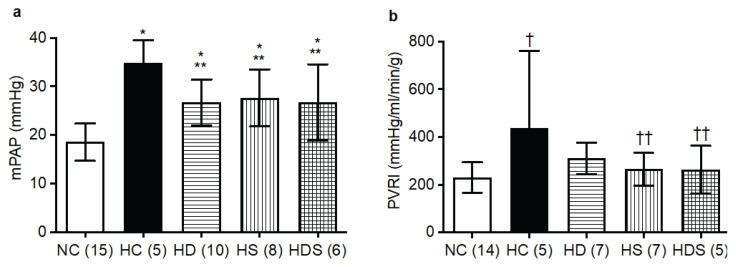
As expected, the increase in mPAP caused by chronic hypoxia was reduced (approximately by one half) by the treatment with DHEA-S alone. Similarly, simvastatin treatment alone also significantly reduced mPAP compared to hypoxic controls; the mPAP reducing effect of statin treatment was about the same as that of DHEA-S (Fig. 1a). Contrary to our hypothesis, simultaneous treatment with both drugs (DHEA-S + statin) did not result in any additional reduction in mPAP (Fig. 1a). Cardiac output and cardiac index did not differ among the groups (Table 2). However, PVRI, significantly elevated in hypoxic controls, did not significantly differ from normoxic controls in any of the treated groups (HD, HS and HDS) and was significantly lower in both groups treated with simvastatin (HS and HDS) than in hypoxic controls (Fig. 1b).
Fig. 1.
Mean pulmonary arterial pressure (mPAP, a) and pulmonary vascular resistance index (PVRI, b), elevated by chronic hypoxia, are reduced by treatment with DHEA-S, simvastatin, and their combination. NC, normoxic control group; HC, hypoxic control group (3 weeks in 10 % O2); HD, group treated with DHEA-S throughout the 3-weeks exposure to hypoxia; HS, group treated with simvastatin throughout the 3-weeks exposure to hypoxia; HDS, group treated with a combination of simvastatin + DHEA-S throughout the 3-weeks exposure to hypoxia. The columns and lines represent means and s.d., respectively; in parentheses are the ns. Differences among the groups not specifically marked are not statistically significant (p<0.05, 1-way ANOVA and Fischer’s least significant difference post hoc test). *p<0.002 vs. NC, †p=0.005 vs. NC, **p<0.013 vs. HC, ††p<0.045 vs. HC
The weight of the left ventricle plus the septum relative to body weight was similar in all groups (Table 1). The weight of the right ventricle relative to body weight, as well as relative to the left ventricle plus septum weight, was increased similarly in all groups exposed to chronic hypoxia (Table 1).
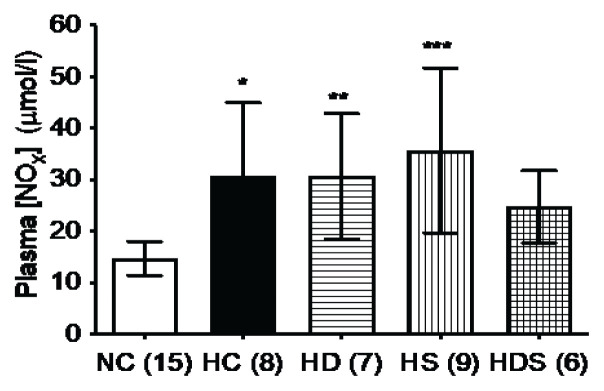
DHEA-S alone or simvastatin alone. In rats treated with the DHEA-S + simvastatin combination, the NOx values Plasma NOx concentration was significantly elevated by chronic hypoxia. The values were similar in hypoxic controls and in rats treated in hypoxia withdid not differ significantly (p=0.064) from those in normoxic controls, but they also did not differ significantly (p=0.313) from the hypoxic controls (Fig. 2).
Fig. 2.
Plasma concentration of nitric oxide and its oxidation products (NOx), elevated by chronic hypoxia, is not affected by DHEA-S or simvastatin treatment. When simvastatin and DHEA-S are combined, plasma NOx no longer differs from normoxic controls. Group abbreviations as in Fig. 1; in parentheses are the ns. The columns and lines represent means and s.d., respectively. Differences among the groups not specifically marked are not statistically significant (p<0.05, 1-way ANOVA and Fischer’s least significant difference post hoc test). *p=0.0017 vs. NC, **p=0.0027 vs. NC, ***p<0.0001 vs. NC
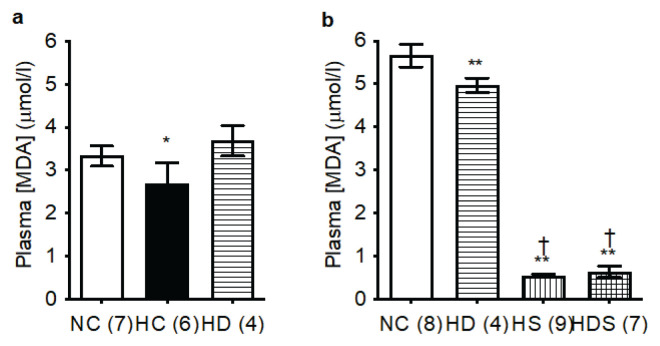
Plasma MDA concentration, a marker of oxidative stress, was one of the few variables in our study affected by the solvent that the rats drank (water vs. 0.5 % ethanol). MDA levels were significantly higher in animals drinking this weak ethanol solution compared to otherwise identically treated groups drinking water. This was so in normoxic controls and, to a lesser extent, in rats treated with DHEA-S in hypoxia. The same trend existed also in the hypoxic controls, where, however, we were able to measure only two rats drinking ethanol solution, so in this case the data are far from conclusive.
For this reason, we made separate statistical comparisons of MDA for rats drinking ethanol solution (with the exclusion of the too small hypoxia-only group) and water. In water-drinking rats, plasma MDA was slightly reduced by chronic hypoxia and restored by DHEA-S treatment (Fig. 3a). In rats drinking water with 0.5 % ethanol, plasma MDA was highly significantly reduced by DHEA-S treatment and even more so in both groups treated with simvastatin (alone or in combination with DHEA-S) compared to normoxic controls. The two simvastatin groups (HS and HDS) did not differ between each other (Fig. 3b).
Fig. 3.
Simvastatin markedly reduces plasma malondialdehyde (MDA) concentration. a) Rats drinking no alcohol. b) Rats drinking a weak ethanol solution (0.5 %). Group abbreviations as in Fig. 1; in parentheses are the ns. The columns and lines represent means and s.d., respectively. Differences among the groups not specifically marked are not statistically significant (p<0.05, 1-way ANOVA and Fischer’s least significant difference post hoc test). *p<0.01 vs. NC and HD, **p<0.0001 vs. NC, †p<0.0001 vs. HD
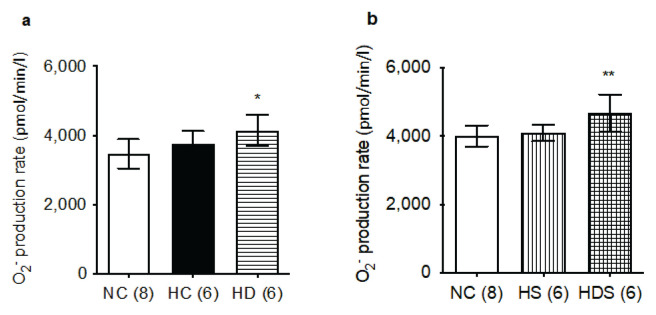
The rate of superoxide production also appeared to be affected by the solvent consumed by the rats, at least in normoxic controls, where we had sufficient numbers for direct comparison (3471 ± 423 pmol/min/l of blood in water-drinking rats and 4006 ± 304 pmol/min/l in ethanolsolution-drinking rats, p=0.0113). For this reason, we calculated the differences between groups separately for animals drinking each solvent.
In water-drinking rats we found an increased rate of superoxide production in the DHEA-S treated group compared to the normoxic controls group (Fig. 4a). In the rats drinking weak ethanol solution, the rate of superoxide production was higher in the HDS group compared to both the normoxic controls and rats treated in hypoxia with simvastatin alone (Fig. 4b). Taken together, these data seem to indicate that neither chronic hypoxia nor simvastatin treatment alters the rate of superoxide production. DHEA-S treatment, alone or in combination with statin, on the other hand, does elevate this variable.
Fig. 4.
DHEA-S, but not simvastatin or chronic hypoxia, increase the rate of superoxide (O2−) production. a) Rats drinking no alcohol. b) Rats drinking a weak ethanol solution (0.5 %). Group abbreviations as in Fig. 1; in parentheses are the ns. The columns and lines represent means and s.d., respectively. Differences among the groups not specifically marked are not statistically significant (p<0.05, 1-way ANOVA and Fischer’s least significant difference post hoc test). *p=0.008 vs. NC, **p<0.02 vs. NC and HS
Discussion
The main finding of the present study is that treatment of rats with a combination of DHEA-S and simvastatin is about as effective in reducing pulmonary hypertension in a hypoxic rat model as either treatment alone.
Statins are widely used for their cholesterol lowering effect. They competitively inhibit HMG-CoA reductase, the rate-controlling enzyme in the production of cholesterol [3]. In addition to this main effect, statins have a number of other, so-called pleiotropic effects that include antioxidant, antiproliferative, antithrombotic, and anti-inflammatory properties. Following the initial report that statin treatment can reduce experimentally induced pulmonary hypertension [4], a number of studies described the ability of various statins to partly prevent the development of pulmonary hypertension, or reduce an already established one, in several animal models [5–7], although there are also reports of minimal effectiveness of statins against established monocrotaline pulmonary hypertension [41,42].
In human patients, the studies are less conclusive. Three independent meta-analyzes did not find any beneficial effect of statin therapy on pulmonary hypertension from all causes [13–15]. This could be related to the paradox that statins reduce low-density lipoproteincholesterol [43], but low-density lipoprotein cholesterol is already low in patients with pulmonary arterial hypertension, and successful pulmonary hypertension therapy increases these low levels [44]. Nevertheless, analyses focused only on patients with chronic obstructive pulmonary disease (COPD) found reduced pulmonary hypertension in those treated with statins [16]. Our rat model of pulmonary hypertension induced by chronic hypoxia corresponds well with the situation of COPD patients, where the main cause for the development of pulmonary hypertension is their chronically hypoxemic status.
DHEA is an abundant steroid hormone that exists in the blood mainly in the form of its 3β-sulfate ester, DHEA-S, to which it is converted by sulfotransferases especially in the liver and adrenal cortex. Most of the effects of DHEA are mediated by DHEA-S[20]. We and others have shown that DHEA/DHEA-S treatment partly prevents and reverses experimental pulmonary hypertension [23,31–33]. Improved mPAP and pulmonary vascular resistance were then demonstrated in a small group of patients with COPD-related pulmonary hypertension [45]. The beneficial effect of DHEA supplementation corresponds to reduced plasma levels of DHEA-S in patients with pulmonary hypertension [30]. Our results fully confirm the beneficial effect of DHEA/DHEA-S supplementation on pulmonary hypertension.
Somewhat surprisingly, the positive effects of simvastatin, DHEA-S, and their combination on mPAP and PVRI were not reflected by reductions of right ventricle hypertrophy. While traditionally the enlargement of the right ventricle had been considered a simple mechanistic consequence of the afterload increased by the pulmonary hypertension, today it is evident that the relationship between right ventricle size and mPAP is more complex. For example, several long-term studies in humans found no positive effect of epoprostenol treatment on the right ventricular mass despite improvement of pulmonary hypertension [46]. Dissociation between a positive treatment effect on mPAP and no effect on right ventricle hypertrophy has also been reported in animal models [47].
Why are the effects of simvastatin and DHEA-S on pulmonary hypertension not additive? One possibility is that the majority of the beneficial effects on pulmonary hypertension is through the antioxidant activity. The effect of simvastatin on plasma MDA was really profound and DHEA-S could add only little to it, especially as its own effect on MDA was relatively modest. However, this possibility seems unlikely since the effect of simvastatin on pulmonary hypertension was very similar to that of DHEA-S, yet the magnitude of their influence on plasma MDA was quite disparate.
One difference in the mechanism of action between statins and DHEA is their effect on various K channels that control pulmonary arterial vascular smooth muscle cell membrane potential and thus their tension. DHEA activates KCa channels, specifically the charybdotoxin sensitive BKCa (KCa1.1) [23]. The possible activation of voltage-gated K+ (KV) channels by DHEA was variably confirmed [23] and excluded (with a possible exception of KV1.3) [48]. Statins, on the other hand, are known to stimulate the ATP-sensitive K+ channels (that play little role in the regulation of pulmonary arterial vascular smooth muscle) [49]. Statins were reported to activate KV channels in general [50] and to inhibit KV1.3 channels in cancerous T cells [51]. In vascular smooth muscle, KV1.3 upregulation is important for proliferation and migration [52]. Statins’ influence on the activity of the BKCa channels has not been reported. Thus, DHEA appears to possess a mechanism for pulmonary vasodilation (BKCa channel activation) that statins lack. This would be expected to result in an additive effect on pulmonary hypertension. The lack of additivity may mean that the participation of the opening of the BKCa channel in the mechanism of the effect of DHEA on pulmonary hypertension is not essential. Alternatively, statins might activate BKCa channels indirectly, through their promotion of NO activity, since NO causes pulmonary vasodilation by activating BKCa channels [25]. Nevertheless, in our experiment, plasma NOx concentration was not elevated by simvastatin in rats with PH (Fig. 2).
Another possible explanation for the lack of DHEA-S and statin additivity in their effect against pulmonary hypertension could be related to the fact that cholesterol is a precursor of DHEA synthesis. It is thus possible that simvastatin treatment reduced endogenous DHEA (and DHEA-S) production and adding exogenous DHEA-S to the statin therapy merely reconstituted normal levels of DHEA-S rather than increasing them above normal. In fact, a meta-analysis of studies in humans showed that statins decrease DHEA levels in women with polycystic ovary syndrome, although this effect was reported only after atorvastatin therapy, but not simvastatin [53]. However, if DHEA-S and statin affected pulmonary hypertension by different mechanisms, one would still expect the effect of simvastatin alone (presumably with lower DHEA-S levels) to be smaller than its effect with DHEA-S added.
Finally, DHEA can resemble statins in their capacity to inhibit HMG-CoA reductase [54]. This would mean that the reduction of pulmonary hypertension by statins does not belong among their pleiotropic effects unrelated to the inhibition of HMG-CoA reductase. In fact, that is what Girgis et al. [6] have concluded from their data showing that attenuation of pulmonary hypertension by simvastatin could be prevented by supplementation with the product of HMG-CoA reductase activity, mevalonate. The mechanisms whereby decreased HMG-CoA reductase activity can lead to less pulmonary hypertension are unknown and deserve further exploration.
One limitation of the present study is that it only used males, but not females. Sex differences in pulmonary vascular physiology and pathophysiology are well established [55]. Normal DHEA-S levels are higher in men than in women [56]. DHEA/DHEA-S levels are lower in men with pulmonary hypertension compared to men without the disease [30]. Cardiovascular benefits of statin therapy seem to be less in women than in men [57]. Therefore, there are grounds to believe that both simvastatin alone and DHEA-S alone could have somewhat different effects in females than in males. However, the focus of the study was the combined effect of the simultaneous administration of both agents, and here it is difficult to imagine argumentation favoring the presence of such a combo effect in females when it was not found in males. Nevertheless, further work on this issue seems warranted.
In conclusion, we confirmed the beneficial effect of statin and DHEA-S treatment on mPAP and PVRI in pulmonary hypertension. We found that combined treatment with both drugs together does not have an additive effect on pulmonary hypertension. One possible explanation is that each of the drugs reduces pulmonary hypertension primarily through their inhibitory effect on HMG-CoA reductase. Statins and DHEA-S can be combined in pulmonary hypertension if needed for other reasons.
Acknowledgements
The authors are grateful to the late Prof. Jan Herget, M.D. (+2019) for fruitful discussions of the study. Technical assistance of Mrs. Veronika Smolková is acknowledged. Supported by the Czech Republic Grant Agency grant #17-11223S.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Corris PA, Seeger W. Call it by the correct name - pulmonary hypertension not pulmonary arterial hypertension: growing recognition of the global health impact for a well-recognized condition and the role of the Pulmonary Vascular Research Institute. Am J Physiol Lung Cell Mol Physiol. 2020;318:L992–L4. doi: 10.1152/ajplung.00098.2020. [DOI] [PubMed] [Google Scholar]
- 3.Davies JT, Delfino SF, Feinberg CE, Johnson MF, Nappi VL, Olinger JT, Schwab AP, Swanson HI. Current and emerging uses of statins in clinical therapeutics: a review. Lipid Insights. 2016;9:13–29. doi: 10.4137/LPI.S37450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura T, Faul JL, Berry GJ, Vaszar LT, Qiu D, Pearl RG, Kao PN. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am J Respir Crit Care Med. 2002;166:1403–8. doi: 10.1164/rccm.200203-268OC. [DOI] [PubMed] [Google Scholar]
- 5.Katsiki N, Wierzbicki AS, Mikhailidis DP. Pulmonary arterial hypertension and statins: an update. Curr Opin Cardiol. 2011;26:322–6. doi: 10.1097/HCO.0b013e32834659bf. [DOI] [PubMed] [Google Scholar]
- 6.Girgis RE, Mozammel S, Champion HC, Li D, Peng X, Shimoda L, Tuder RM, Johns RA, Hassoun PM. Regression of chronic hypoxic pulmonary hypertension by simvastatin. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1105–L10. doi: 10.1152/ajplung.00411.2006. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura T, Vaszar LT, Faul JL, Zhao G, Berry GJ, Shi L, Qiu D, Benson G, Pearl RG, Kao PN. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation. 2003;108:1640–5. doi: 10.1161/01.CIR.0000087592.47401.37. [DOI] [PubMed] [Google Scholar]
- 8.Hampl V, Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol Rev. 2000;80:1337–72. doi: 10.1152/physrev.2000.80.4.1337. [DOI] [PubMed] [Google Scholar]
- 9.Murata T, Kinoshita K, Hori M, Kuwahara M, Tsubone H, Karaki H, Ozaki H. Statin protects endothelial nitric oxide synthase activity in hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2005;25:2335–42. doi: 10.1161/01.ATV.0000186184.33537.48. [DOI] [PubMed] [Google Scholar]
- 10.Pei Y, Ma P, Wang X, Zhang W, Zhang X, Zheng P, Yan L, Xu Q, Dai G. Rosuvastatin attenuates monocrotaline-induced pulmonary hypertension via regulation of Akt/eNOS signaling and asymmetric dimethylarginine metabolism. Eur J Pharmacol. 2011;666:165–72. doi: 10.1016/j.ejphar.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Davignon J, Jacob RF, Mason RP. The antioxidant effects of statins. Coron Artery Dis. 2004;15:251–8. doi: 10.1097/01.mca.0000131573.31966.34. [DOI] [PubMed] [Google Scholar]
- 12.Beltowski J. Statins and modulation of oxidative stress. Toxicol Mech Methods. 2005;15:61–92. doi: 10.1080/15376520590918766. [DOI] [PubMed] [Google Scholar]
- 13.Anand V, Garg S, Duval S, Thenappan T. A systematic review and meta-analysis of trials using statins in pulmonary arterial hypertension. Pulm Circ. 2016;6:295–301. doi: 10.1086/687304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rysz-Gorzynska M, Gluba-Brzozka A, Sahebkar A, Serban MC, Mikhailidis DP, Ursoniu S, Toth PP, Bittner V, Watts GF, Lip GY, Rysz J, Catapano AL, Banach M. Efficacy of statin therapy in pulmonary arterial hypertension: a systematic review and meta-analysis. Sci Rep. 2016;6:30060. doi: 10.1038/srep30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Qu M, Chen Y, Zhou Y, Wan Z. Statins have no additional benefit for pulmonary hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2016;11:e0168101. doi: 10.1371/journal.pone.0168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Y, Chang R, Yao J, Xu X, Teng Y, Cheng N. Effectiveness of long-term using statins in COPD - a network meta-analysis. Respir Res. 2019;20:17. doi: 10.1186/s12931-019-0984-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao L, Sebkhi A, Ali O, Wojciak-Stothard B, Mamanova L, Yang Q, Wharton J, Wilkins MR. Simvastatin and sildenafil combine to attenuate pulmonary hypertension. Eur Respir J. 2009;34:948–57. doi: 10.1183/09031936.00143508. [DOI] [PubMed] [Google Scholar]
- 18.Lee DS, Kim YK, Jung YW. Simvastatin, sildenafil and their combination in monocrotaline induced pulmonary arterial hypertension. Korean Circ J. 2010;40:659–64. doi: 10.4070/kcj.2010.40.12.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol. 2001;22:185–212. doi: 10.1006/frne.2001.0216. [DOI] [PubMed] [Google Scholar]
- 20.Celec P, Stárka L. Dehydroepiandrosterone - is the fountain of youth drying out? Physiol Res. 2003;52:397–407. [PubMed] [Google Scholar]
- 21.Schwartz AG, Pashko LL. Dehydroepiandrosterone, glucose-6-phosphate dehydrogenase, and longevity. Ageing Res Rev. 2004;3:171–87. doi: 10.1016/j.arr.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Liu D, Dillon JS. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Gαi2,3. J Biol Chem. 2002;277:21379–88. doi: 10.1074/jbc.M200491200. [DOI] [PubMed] [Google Scholar]
- 23.Bonnet S, Dumas-de-La-Roque E, Begueret H, Marthan R, Fayon M, Dos Santos P, Savineau J-P, Baulieu E-E. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA. 2003;100:9488–93. doi: 10.1073/pnas.1633724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan X-J. Voltage gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary artery myocytes. Circ Res. 1995;77:370–8. doi: 10.1161/01.RES.77.2.370. [DOI] [PubMed] [Google Scholar]
- 25.Hampl V, Huang JM, Weir EK, Archer SL. Activation of the cGMP-dependent protein kinase mimics the stimulatory effect of nitric oxide and cGMP on calcium-gated potassium channels. Physiol Res. 1995;44:39–44. [PubMed] [Google Scholar]
- 26.Ochi R, Chettimada S, Kizub I, Gupte SA. Dehydroepiandrosterone inhibits ICa,L and its window current in voltage-dependent and -independent mechanisms in arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2018;315:H1602–H13. doi: 10.1152/ajpheart.00291.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rom WN, Harkin T. Dehydroepiandrosterone inhibits the spontaneous release of superoxide radical by alveolar macrophages in vitro in asbestosis. Environ Res. 1991;55:145–56. doi: 10.1016/S0013-9351(05)80171-9. [DOI] [PubMed] [Google Scholar]
- 28.Žaloudíková M, Vytášek R, Rašková M, Vízek M, Uhlík J, Hampl V. The effect of exposure to hypoxia on superoxide formation by alveolar macrophages is indirect. Life Sci. 2019;236:116864. doi: 10.1016/j.lfs.2019.116864. [DOI] [PubMed] [Google Scholar]
- 29.Žaloudíková M, Vytášek R, Vajnerová O, Hniličková O, Vízek M, Hampl V, Herget J. Depletion of alveolar macrophages attenuates hypoxic pulmonary hypertension but not hypoxia-induced increase in serum concentration of MCP-1. Physiol Res. 2016;65:763–8. doi: 10.33549/physiolres.933187. [DOI] [PubMed] [Google Scholar]
- 30.Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, Klinger JR, Lima JA, Ouyang P, Palevsky HI, Palmisciano AJ, Krishnan I, Pinder D, Preston IR, Roberts KE, Kawut SM. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med. 2016;193:1168–75. doi: 10.1164/rccm.201509-1785OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hampl V, Bíbová J, Povýšilová V, Herget J. Dehydroepiandrosterone sulfate reduces experimental pulmonary hypertension in rats. Eur Respir J. 2003;21:862–5. doi: 10.1183/09031936.03.00084503. [DOI] [PubMed] [Google Scholar]
- 32.Oka M, Karoor V, Homma N, Nagaoka T, Sakao E, Golembeski SM, Limbird J, Imamura M, Gebb SA, Fagan KA, McMurtry IF. Dehydroepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc Res. 2007;74:377–87. doi: 10.1016/j.cardiores.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YT, Xue JJ, Wang Q, Cheng SY, Chen ZC, Li HY, Shan JJ, Cheng KL, Zeng WJ. Dehydroepiandrosterone attenuates pulmonary artery and right ventricular remodeling in a rat model of pulmonary hypertension due to left heart failure. Life Sci. 2019;219:82–9. doi: 10.1016/j.lfs.2018.12.056. [DOI] [PubMed] [Google Scholar]
- 34.Silva AF, Sousa-Nunes F, Faria-Costa G, Rodrigues I, Guimaraes JT, Leite-Moreira A, Henriques-Coelho T, Negrao R, Moreira-Goncalves D. Effects of chronic moderate alcohol consumption on right ventricle and pulmonary remodelling. Exp Physiol. 2021;106:1359–72. doi: 10.1113/EP088788. [DOI] [PubMed] [Google Scholar]
- 35.Jakoubek V, Hampl V. Alcohol and fetoplacental vasoconstrictor reactivity. Physiol Res. 2018;67:509–13. doi: 10.33549/physiolres.933609. [DOI] [PubMed] [Google Scholar]
- 36.Herget J, Paleček F. Pulmonary arterial blood pressure in closed chest rats. Changes after catecholamines, histamine and serotonin. Arch Int Pharmacodyn Ther. 1972;198:107–17. [PubMed] [Google Scholar]
- 37.Beitl E, Baňasová A, Miková D, Hampl V. Nitric oxide as an indicator for severity of injury in polytrauma. Bratisl Med J. 2016;117:217–20. doi: 10.4149/BLL_2016_041. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal R, Chase SD. Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J Chromatogr B. 2002;775:121–6. doi: 10.1016/S1570-0232(02)00273-8. [DOI] [PubMed] [Google Scholar]
- 39.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–28. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Chalupsky K, Kracun D, Kanchev I, Bertram K, Gorlach A. Folic acid promotes recycling of tetrahydrobiopterin and protects against hypoxia-induced pulmonary hypertension by recoupling endothelial nitric oxide synthase. Antioxid Redox Signal. 2015;23:1076–91. doi: 10.1089/ars.2015.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMurtry MS, Bonnet S, Michelakis ED, Bonnet S, Haromy A, Archer SL. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamcyin-atorvastatin-simvastatin study. Am J Physiol Lung Cell Mol Physiol. 2007;293:L933–L40. doi: 10.1152/ajplung.00310.2006. [DOI] [PubMed] [Google Scholar]
- 42.Sun X, Ku DD. Rosuvastatin provides pleiotropic protection against pulmonary hypertension, right ventricular hypertrophy, and coronary endothelial dysfunction in rats. Am J Physiol Heart Circ Physiol. 2008;294:H801–H9. doi: 10.1152/ajpheart.01112.2007. [DOI] [PubMed] [Google Scholar]
- 43.Stroes E. Statins and LDL-cholesterol lowering: an overview. Curr Med Res Opin. 2005;21:S9–S16. doi: 10.1185/030079905X59102. [DOI] [PubMed] [Google Scholar]
- 44.Kopec G, Waligora M, Tyrka A, Jonas K, Pencina MJ, Zdrojewski T, Moertl D, Stokwiszewski J, Zagozdzon P, Podolec P. Low-density lipoprotein cholesterol and survival in pulmonary arterial hypertension. Sci Rep. 2017;7:41650. doi: 10.1038/srep41650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dumas de La Roque E, Savineau JP, Metivier AC, Billes MA, Kraemer JP, Doutreleau S, Jougon J, Marthan R, Moore N, Fayon M, Baulieu EE, Dromer C. Dehydroepiandrosterone (DHEA) improves pulmonary hypertension in chronic obstructive pulmonary disease (COPD): a pilot study. Ann Endocrinol. 2012;73:20–5. doi: 10.1016/j.ando.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Vonk Noordegraaf A, Galie N. The role of the right ventricle in pulmonary arterial hypertension. Eur Respir Rev. 2011;20:243–53. doi: 10.1183/09059180.00006511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.all MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK, Steinhorn RH, Shah SJ, Schumacker PT. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am J Respir Crit Care Med. 2014;189:314–24. doi: 10.1164/rccm.201302-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng W, Hoidal JR, Farrukh IS. Role of a novel KCa opener in regulating K+ channels of hypoxic human pulmonary vascular cells. Am J Respir Cell Mol Biol. 1999;20:737–45. doi: 10.1165/ajrcmb.20.4.3390. [DOI] [PubMed] [Google Scholar]
- 49.Sehra D, Sehra S, Sehra ST. Cardiovascular pleiotropic effects of statins and new onset diabetes: is there a common link: do we need to evaluate the role of KATP channels? Expert Opin Drug Saf. 2017;16:823–31. doi: 10.1080/14740338.2017.1338269. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Zhang H, Liu H, Cao A. Mechanisms of simvastatin-induced vasodilatation of rat superior mesenteric arteries. Biomed Rep. 2016;5:491–6. doi: 10.3892/br.2016.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teisseyre A, Uryga A, Michalak K. Statins as inhibitors of voltage-gated potassium channels Kv1.3 in cancer cells. J Mol Struct. 2021;1230:129905. doi: 10.1016/j.molstruc.2021.129905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheong A, Li J, Sukumar P, Kumar B, Zeng F, Riches K, Munsch C, Wood IC, Porter KE, Beech DJ. Potent suppression of vascular smooth muscle cell migration and human neointimal hyperplasia by KV1.3 channel blockers. Cardiovasc Res. 2011;89:282–9. doi: 10.1093/cvr/cvq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang S, Gu YY, Jing F, Yu CX, Guan QB. The effect of statins on levels of dehydroepiandrosterone (DHEA) in women with polycystic ovary syndrome: a systematic review and meta-analysis. Med Sci Monit. 2019;25:590–7. doi: 10.12659/MSM.914128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pascale RM, Simile MM, De Miglio MR, Nufris A, Seddaiu MA, Muroni MR, Danni O, Rao KN, Feo F. Inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase activity and gene expression by dehydrocpiandrosterone in preneoplastic liver nodules. Carcinogenesis. 1995;16:1537–42. doi: 10.1093/carcin/16.7.1537. [DOI] [PubMed] [Google Scholar]
- 55.Hampl V, Bíbová J, Ošťádalová I, Povýšilová V, Herget J. Gender differences in the long-term effects of perinatal hypoxia on the pulmonary circulation in rats. Am J Physiol Lung Cell Mol Physiol. 2003;285:L386–L92. doi: 10.1152/ajplung.00389.2002. [DOI] [PubMed] [Google Scholar]
- 56.Goldman N, Glei DA. Sex differences in the relationship between DHEAS and health. Exp Gerontol. 2007;42:979–87. doi: 10.1016/j.exger.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karp I, Chen S-F, Pilote L. Sex differences in the effectiveness of statins after myocardial infarction. CMAJ. 2007;176:333–8. doi: 10.1503/cmaj.060627. [DOI] [PMC free article] [PubMed] [Google Scholar]