Abstract
Objective
Summarise longitudinal observational studies to determine whether diabetes (types 1 and 2) is a risk factor for frozen shoulder.
Design
Systematic review and meta-analysis.
Data sources
MEDLINE, Embase, AMED, PsycINFO, Web of Science Core Collection, CINAHL, Epistemonikos, Trip, PEDro, OpenGrey and The Grey Literature Report were searched on January 2019 and updated in June 2021. Reference screening and emailing professional contacts were also used.
Eligibility criteria
Longitudinal observational studies that estimated the association between diabetes and developing frozen shoulder.
Data extraction and synthesis
Data extraction was completed by one reviewer and independently checked by another using a predefined extraction sheet. Risk of bias was judged using the Quality In Prognosis Studies tool. For studies providing sufficient data, random-effects meta-analysis was used to derive summary estimates of the association between diabetes and the onset of frozen shoulder.
Results
A meta-analysis of six case–control studies including 5388 people estimated the odds of developing frozen shoulder for people with diabetes to be 3.69 (95% CI 2.99 to 4.56) times the odds for people without diabetes. Two cohort studies were identified, both suggesting diabetes was associated with frozen shoulder, with HRs of 1.32 (95% CI 1.22 to 1.42) and 1.67 (95% CI 1.46 to 1.91). Risk of bias was judged as high in seven studies and moderate in one study.
Conclusion
People with diabetes are more likely to develop frozen shoulder. Risk of unmeasured confounding was the main limitation of this systematic review. High-quality studies are needed to confirm the strength of, and understand reasons for, the association.
PROSPERO registration number
CRD42019122963.
Keywords: diabetes & endocrinology, epidemiology, primary care, rheumatology
Strengths and limitations of this study.
This systematic review is the first to summarise the results of studies estimating the longitudinal association between diabetes and the onset of frozen shoulder.
Robust meta-analytical methods were used to synthesise and analyse data.
Sensitivity to influential estimates and sensitivity to small study bias were assessed.
Risk of bias was judged to be high in seven studies and moderate in one study; this limits the certainty in evidence.
Only two cohort studies were identified, which meant that pooling of association estimates was not suitable.
Introduction
Frozen shoulder, also known as adhesive capsulitis, is a painful and severely debilitating condition. The inflammatory contracture of the glenohumeral joint capsule in frozen shoulder restricts both active and passive range of motion, with loss of external rotation being especially characteristic of this condition.1
Frozen shoulder generally presents between the ages of 50 years and 60 years and rarely presents before 40 years.2 Women (58%) are more likely to develop frozen shoulder than men (42%).3 The contralateral shoulder is also affected in 6%–17% of patients.4 Although the exact aetiology remains unclear, several factors have been found to be associated with frozen shoulder, including trauma,3 thyroid dysfunction,5–7 cardiovascular disease,2 8 metabolic factors7 9–11 and other musculoskeletal conditions such as Dupuytren’s contracture.12 13 The most common comorbidity in people with frozen shoulder is diabetes,2 both type 1 and type 2.6 The prevalence of frozen shoulder in the general population is around 0.75%,1 but the prevalence of frozen shoulder in people with diabetes is much higher. A meta-analysis of 13 cross-sectional studies estimated the prevalence of frozen shoulder in populations with diabetes to be 13.4% (95% CI 10.2% to 17.2%).14
Diabetes is a term used to describe a group of chronic diseases characterised by hyperglycaemia. The two most prevalent types of diabetes are type 1 and type 2, making up 8% and 90% of cases, respectively.15 It is well known that people with diabetes are at risk of complications such as cardiovascular disease, retinopathy, neuropathy and nephropathy,16 although the musculoskeletal complications of diabetes are not as well known.17 Musculoskeletal conditions, such as frozen shoulder, can significantly affect the quality of a patient’s life and should not be overlooked. Our previous systematic review and narrative synthesis of 28 studies has shown that patients with diabetes may experience worse outcomes from frozen shoulder than people without frozen shoulder.18
It has been suggested that diabetes may be a cause of frozen shoulder through glycation processes and/or inflammatory processes leading to capsular fibrosis and subsequent contracture.7 19 20 To understand whether diabetes could potentially be a cause of frozen shoulder, it is necessary (although not sufficient) to have evidence of the temporal relationship between diabetes and frozen shoulder.21 This systematic review aims to summarise evidence from longitudinal observational studies to understand the temporal relationship between diabetes and frozen shoulder.
Methods
Search strategy
The protocol for this systematic review was registered on PROSPERO (CRD42019122963), and the review was conducted and reported using Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.22 A systematic literature search of MEDLINE, Embase, AMED, PsycINFO, Web of Science Core Collection, CINAHL, Epistemonikos, Trip, PEDro, OpenGrey and The Grey Literature Report was carried out in January 2019 and updated in June 2021. Reference lists of eligible studies were screened. Additionally, a professional contact of one author (DvdW) was contacted to identify further studies. We retrieved all epidemiological studies containing index terms (eg, Medical Subject Headings) and free-text words related to diabetes and shoulder pain more generally (not limited to frozen shoulder) to reduce the risk of missing potentially relevant publications. The search strategy for MEDLINE, which was constructed with the support of a health information specialist, can be found in online supplemental appendix A.
bmjopen-2022-062377supp001.pdf (176.6KB, pdf)
Study selection
Reviewer BPD screened all titles and abstracts to check eligibility using the predefined inclusion and exclusion criteria, and reviewers MB and CB independently checked a 20% random sample. Reviewer BPD checked all full-texts for eligibility using the inclusion and exclusion criteria, and reviewers MB, CB and TR also independently checked eligibility. Disagreements regarding the inclusion of studies were resolved through discussion with DvdW.
Inclusion criteria
To be eligible for inclusion, studies were required to have a longitudinal, prospective or retrospective, observational study design. Cohort studies were required to have a study population consisting of people without frozen shoulder at inclusion and must have established whether diabetes was present at baseline (all types of diabetes were considered). Case–control studies were required to have a study population consisting of people with frozen shoulder and a control group without frozen shoulder, with diabetes defined as the exposure of interest. The paper must have presented an OR, risk ratio or HR, or they must have presented sufficient data to allow the associations to be estimated. There were no restrictions to setting; population-based as well as clinical cohorts were eligible. All non-English language papers were assessed by reviewers with appropriate language skills. Cross-sectional studies and case series were excluded. Studies were also excluded if a full text could not be obtained.
Data extraction and risk of bias
Data extraction was completed by reviewer BPD and was independently checked by reviewers MB and TR. Types of data extracted included details of study design, setting, sample characteristics, exposure/outcome/covariate measurement, inclusion and exclusion criteria, sample size, attrition, covariate conditioning, follow-up time, statistical analysis, association estimates (OR, risk ratio or HR) or raw data to estimate association sizes if they were not already presented. Risk of bias was independently assessed by pairs of reviewers (BPD, MB and TR). Risk of bias was judged using the Quality In Prognosis Studies (QUIPS) tool.23 The QUIPS tool covers six domains: (1) study participation, (2) study attrition, (3) prognostic/risk factor measurement, (4) outcome measurement, (5) study confounding and (6) statistical analysis and reporting. Each of the six domains is scored as being at a low, medium or high risk of bias.23 Domain scores were used to guide judgement of the overall risk of bias (scored as low, medium or high) for the study. Overall risk of bias was based on author judgement, and the use of a tallied or summated score was avoided. All disagreements regarding data extraction and assessment of risk of bias were resolved by discussion.
Data analysis
Case–control studies and cohort studies were analysed separately. Narrative synthesis was used where less than five studies were present and a random-effects meta-analysis model was used to calculate a summary estimate when five or more studies were present. Cohort study associations were measured using hazard ratios and case–control study associations were estimated using ORs. Where adjusted and crude estimates were both presented, the adjusted estimate was used. Where a zero-cell count was present within the results of a study, a continuity correction of 0.5 was added to all cells for that study. Restricted maximum likelihood estimation24 was used to estimate the between-study variance, τ2, and the Hartung-Knapp-Sidik-Jonkman variance correction method25 was used in the estimation of the pooled effect CI. Heterogeneity was assessed using Cochran’s Q statistic, complemented by the I2 index.26 Prediction intervals were not estimated since they are inaccurate when there is little heterogeneity (I2<0.3), or an imbalance in study sizes exists, both of which were found in the meta-analysis in this review (see Section 3).27 A forest plot was used to visualise results of individual results and of the pooled estimate. Evidence of small-study bias was assessed with a funnel plot of log ORs against their standard errors.28 A test for funnel plot asymmetry was not used since the meta-analysis included less than ten studies.29 The influence of each study on the overall pooled estimate was assessed by repeating the meta-analysis, each time leaving out a single study.30 Statistical analysis was carried out using Stata V.16.1.31
Patient and public involvement
No patient involved.
Results
The searches identified 1784 unique citations, 12 of which were selected for full-text screening, and 8 studies consisting of a total of 346 278 people fulfilled the inclusion criteria (figure 1). Table 1 summarises information on risk of bias, study design, setting, participants, sample size and methods used for diagnosing diabetes and frozen shoulder. Of the eight studies that met the criteria for inclusion, six32–37 had case–control designs and two38 39 had cohort designs. Three studies37–39 (including the two cohort studies) collected information from electronic health records (EHRs); four studies33–36 were hospital-based, and one study32 was based in a physical therapy clinic. Among the case–control studies, the percentage of female cases ranged from 52% to 75%, and the mean age for cases ranged from 52.8 years to 57.2 years.
Figure 1.
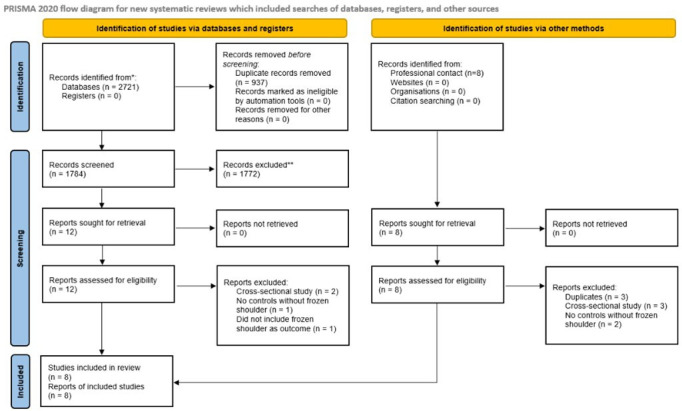
PRISMA flow diagram summarising record identification and study selection. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Characteristics of studies on diabetes as a risk factor for frozen shoulder
| Source | Risk of bias (QUIPS, overall assessment) | Design and setting | % Female | Mean age (years) | Sample size | Method to diagnose diabetes and frozen shoulder | Variables conditioned on |
| Case–control studies | |||||||
| Boyle-Walker et al32 |
High | Sex-matched case–control at physical therapy clinic in the USA |
Case group: 75%, control group: 68% |
Not reported | Cases: 32, controls: 31 |
Diabetes: self-reported questionnaire Frozen shoulder: clinically diagnosed |
Sex-matched |
| Li et al33 |
High | Hospital-based case–control matched on time of hospitalisation in China | Case group: 63%, control group: 55% |
Cases: 57.2, controls: 45.9 |
Cases: 182, controls: 196 |
Diabetes: face-to-face interview Frozen shoulder: clinically diagnosed |
Matched on time of hospitalisation, adjusted for history of minor shoulder trauma |
| Lee et al34 |
High | Hospital based age-matched and sex-matched case–control in South Korea | Case group: 55%, control group: not reported |
Cases: 52.8, controls: not reported |
Cases: 40, controls: 40 |
Diabetes: unclear Frozen shoulder: clinically diagnosed |
Age-matched and sex-matched |
| Milgrom et al35 | High | Hospital based age-matched case–control in Israel | Case group: 60%, control group: 65% |
Cases: 54.9, controls: 55.4 |
Cases: 126, controls: 98 |
Diabetes: If patient was receiving drug treatment for diabetes or whose serum glucose was higher than 200 mg/dL Frozen shoulder: clinically diagnosed |
Age-matched |
| Wang et al36 |
High | Hospital based age-matched and sex-matched case–control in Australia | Case group: 64%, control group: 58% |
Cases: 56, controls: 55.3 |
Cases: 87, controls: 176 |
Diabetes: self-reported Frozen shoulder: clinically diagnosed |
Age-matched and sex-matched |
| Kingston et al37 | High | Sex-matched case–control using EHRs in the USA | Case group: 58%, control group: 58% |
Cases: 56.4, controls: not reported |
Cases: 2190, controls: 2190 |
Diabetes: ICD-9 code Frozen shoulder: ICD-9 code |
Sex-matched |
| Cohort studies | |||||||
| Huang et al38 |
High | Age-matched and sex-matched cohort with 3-year follow-up using electronic health records in Taiwan |
Exposed group: 47%, non-exposed group: 47% |
Exposed group: 55.7, non-exposed group: 55.5 |
Exposed group: 78 827, non-exposed group: 236 481 |
Diabetes: ICD-9 code Frozen shoulder: ICD-9 code |
Age-matched and sex-matched, multivariable analysis adjusted for age, sex and dyslipidaemia |
| Lo et al39 |
Moderate | Cohort with 8-year follow-up using EHRs in Taiwan | Exposed group: 52%, non-exposed group: 51% |
Not reported | Exposed group: 5109, non-exposed group: 20 473 |
Diabetes: ICD-9 code Frozen shoulder: ICD-9 code |
Multivariable analysis adjusted for age, income, stroke, hypertension, hyperlipidaemia, obesity and chronic obstructive pulmonary disease |
EHR, electronic health record; ICD-9, International Classification of Diseases, Ninth Revision; QUIPS, Quality In Prognosis Studies.
Presence of diabetes was identified using ICD-9 codes (codes to classify diseases, symptoms, clinical findings and causes of disease and injury) from electronic health records in three studies,37–39 self-reported in three studies,32 33 36 identified with a glucose test or if the patient was receiving drug treatment for diabetes in one study35 and was unclear in one study.34 Frozen shoulder was identified using37–39 ICD-9 codes in three studies and was diagnosed clinically in five studies.32–36 Only one study39 reported the types of diabetes that the participants had. Lo et al39 stated that 296 (5.8%) of the 5109 people with diabetes in their study had type 1 diabetes. Two studies were conducted in Taiwan38 39; two were conducted in the USA32 37; and the remaining four were conducted in China,33 South Korea,34 Israel35 and Australia.36
Overall QUIPS risk of bias scores for each study can be found in table 1, and full QUIPS assessments can be found in table 2. Overall, there was a 75% agreement between reviewers across the individual bias domains, and reviewers agreed on four of the eight overall risk of bias scores. One of the cohort studies39 was scored as being at a moderate risk of bias for their overall study rating, and the other seven studies were rated as being at a high risk of bias overall. A bar graph of the scores for individual risk of bias domains can be found in online supplemental appendix figure B1. Risk of bias was generally high across most domains, but especially so for the risk of unaccounted confounding, which was scored as being at a high risk of bias in all eight studies. Five of the case–control studies32 34–37 only accounted for age, gender or a combination of the two. One study33 matched on the time of hospitalisation and adjusted for history of minor shoulder trauma. One cohort study38 adjusted for age, sex and dyslipidaemia; the other cohort study39 adjusted for age, income, stroke, hypertension, hyperlipidaemia, obesity and chronic obstructive pulmonary disease.
Table 2.
QUIPS domain scores for each primary study
| Source | Participation | Study attrition | Risk factor measurement | Outcome measurement | Confounding | Statistical analysis and presentation | Overall risk of bias |
| Case–control studies | |||||||
| Boyle-Walker et al32 | High | Moderate | High | Moderate | High | Moderate | High |
| Li et al33 | Moderate | Low | Moderate | High | High | High | High |
| Lee et al34 | Moderate | Low | Moderate | Moderate | High | Moderate | High |
| Milgrom et al35 | Moderate | Low | Low | Low | High | Low | High |
| Wang et al36 | Low | Low | Low | Low | High | Low | High |
| Kingston et al37 | Low | Moderate | Moderate | Low | High | Moderate | High |
| Cohort studies | |||||||
| Huang et al38 | Low | Moderate | Low | High | High | High | High |
| Lo et al39 | Low | Low | Low | Moderate | High | Low | Moderate |
QUIPS, Quality In Prognosis Studies.
Six case–control studies including a total of 5388 people were pooled in a random-effects meta-analysis, with a pooled OR of 3.69 (95% CI 2.99 to 4.56) (figure 2). The raw data extracted from each study that was used to calculate ORs can be found in online supplemental appendix table C1. The estimated between-study variance was small (τ2<0.01, 95% CI <0.01 to 0.23), and little heterogeneity was detected (Q=2.07, df=5, p=0.84; I2<0.01%, 95% CI <0.1% to 67.6%), but the estimate for I2 was imprecise as indicated by the wide 95% CI. The influence analysis showed that excluding the largest study,37 which contained 4380 of the 5388 participants, greatly reduced the precision of the pooled estimate but did not substantially affect the value of the pooled estimate (figure 3). Further, excluding any other single study did not substantially affect the value of the pooled estimate (figure 3). The two studies with the smallest SEs for their effect estimates had the largest ORs, making the funnel plot appear unsymmetrical. However, due to the small number of studies contributing to the funnel plot, the asymmetrical appearance could be due to chance (figure 4).
Figure 2.
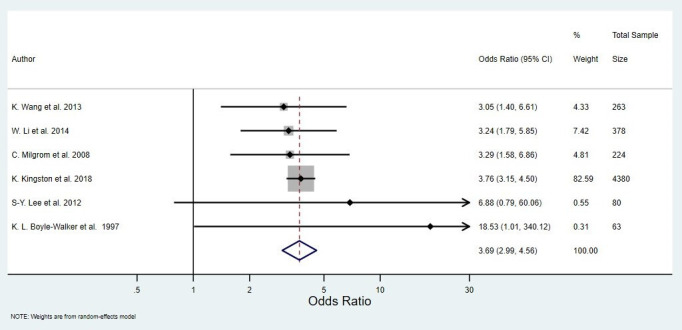
Random effects meta-analysis of the association between diabetes and the odds of developing frozen shoulder.
Figure 3.
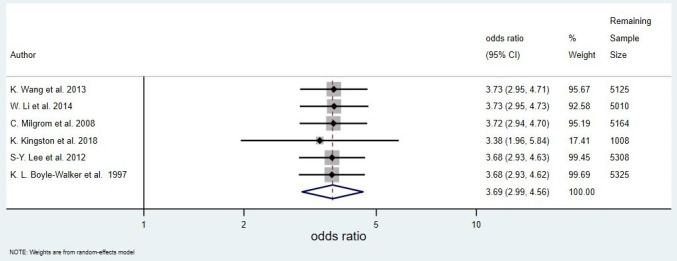
Influence plot showing the result of repeating the original meta-analysis (figure 2), each time with a different primary study removed.
Figure 4.
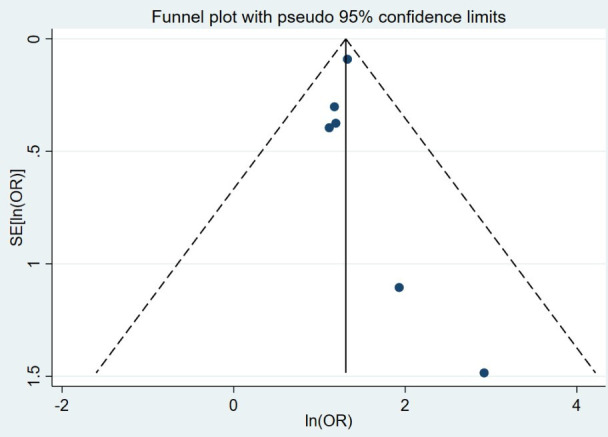
Funnel plot of log ORs for developing frozen shoulder in people with diabetes versus those without diabetes.
The two cohort studies that were identified used Cox proportional hazards models and obtained results suggesting that people with diabetes were more at risk of developing frozen shoulder. One cohort study38 using electronic health records from Taiwan, with a 3-year follow-up and consisting of 315 308 people reported an age-adjusted, sex-adjusted and dyslipidaemia-adjusted HR of 1.32 (95% CI 1.22 to 1.42). Another cohort study,39 with an 8-year follow-up, consisting of 25 582 people, also using electronic health records from Taiwan, estimated an age-adjusted, income-adjusted, stroke-adjusted, hypertension-adjusted, hyperlipidaemia-adjusted, obesity-adjusted and chronic obstructive pulmonary disease-adjusted HR of 1.67 (95% CI 1.46 to 1.91).
Discussion
This systematic review aimed to summarise evidence from longitudinal observational studies to determine whether diabetes (types 1 and 2) is a risk factor for frozen shoulder.
Eight studies met the eligibility criteria for the review; each individual study demonstrated evidence to suggest that diabetes is associated with the onset of frozen shoulder. Our meta-analysis of six case–control studies yielded a pooled OR of 3.69 (95% CI 2.99 to 4.56), and the value of the pooled estimate was robust to the omission of any individual study. The OR estimates of all but one study37 were imprecise with large CIs; this meant that the CIs overlapped well, resulting in a small I2 value. It is also important to note that Cochran’s Q statistic should be interpreted with caution since the number of studies included in the analysis was small.40
The funnel plot was unsymmetrical. However, given that a small number of studies were available, it was difficult to assess accurately whether any small-study bias was present or if the appearance was due to chance. Since our influence analysis has shown that the inclusion/exclusion of any individual study had very little impact on the pooled effect estimate, any potential small-study bias would be unlikely to substantially affect the results.
Two cohort studies were identified, both of which corroborate the evidence from the six case–control studies reported previously, that people with diabetes are more likely to develop frozen shoulder than those without diabetes. Of the two cohort studies, one was deemed to be at a high risk of bias and the other at a moderate risk of bias. The HRs in the two studies did differ, which could have partly been due to the differences in the covariates that were adjusted for and/or the differences in the duration of follow-up. Both studies were rated as being at a high of bias for the outcome-measurement domain as the length of follow-up (338 and 8 years39) was deemed too short to establish whether a patient would develop frozen shoulder in the future. Previous studies have suggested that the duration of diabetes may be associated with the risk of developing frozen shoulder,41 42 with one of the cohort studies in this review also stating that their study suggested that ‘the development of (frozen shoulder) is associated with the duration of diabetes’.38 Therefore, future studies should ensure that the follow-up period is long enough to observe participants from diabetes diagnosis through to the ages for which frozen shoulder is common. A cross-sectional study of 1373 patients presenting with frozen shoulder estimated that the mean age of onset for frozen shoulder was 55.4 years with an SD of 9.9 years.3
The following three paragraphs describe some limitations that may complicate the understanding of the association between diabetes and the onset of frozen shoulder.
The two cohort studies in the review were both conducted using EHRs. EHR datasets can provide large sample sizes with long follow-up periods and detailed patient medical record history.43 Misdiagnosis and miscoding in EHRs are common limitations and could potentially result in a risk of bias for frozen shoulder measurement.44 Research in the UK45 and in the Netherlands46 has shown that general practitioners often use non-specific shoulder pain codes instead of codes for specific shoulder conditions, for example, frozen shoulder. This would lead to an underdiagnosis of frozen shoulder. Further, this misclassification may be differential since clinicians may feel more confident in providing a specific frozen shoulder diagnosis in patients with diabetes due to the pre-existing knowledge of the association between the two conditions. Conversely, it has also been noted that frozen shoulder is sometimes used as a ‘waste-bin diagnosis’ for patients presenting with any stiff and painful shoulder.47 Thus, EHR data may include other shoulder conditions with similar clinical presentations being coded as frozen shoulder.
Another important limitation was the overall poor adjustment for confounding variables. All eight studies were rated as being at a high risk of unaccounted confounding. In each study, confounders were either ignored32 34–38 or inappropriate statistical methods, such as univariable prefiltering and stepwise selection, were used.33 38 39 These methods are especially poorly suited for aetiological models.48 Thus, these studies may have missed potentially important confounders33 38 39 or erroneously adjusted for mediators, such as stroke.39
The systematic review is also limited by there being only two cohort studies, meaning that pooling association estimates was not possible. Cohort studies are particularly useful for gaining a better understanding of temporal associations, as this review aimed to do. Further, both cohort studies were conducted in Taiwan using existing data from EHRs. Future studies with prospective designs will help to gauge whether the findings of these two cohort studies are reproducible and whether the results are consistent across different populations.
Previously, a meta-analysis of cross-sectional studies established that frozen shoulder was more prevalent in people with diabetes than among people without diabetes. This systematic review provides evidence of a temporal relationship between diabetes and frozen shoulder. Understanding the temporal relationship is key to explaining why diabetes and frozen shoulder are associated; however, further high-quality research with appropriate methods and study design is required to confirm the strength of the association and establish whether diabetes is indeed a cause of frozen shoulder.
While sound and reliable epidemiological evidence of a causal relationship between diabetes and frozen shoulder is currently unavailable, elsewhere in the literature, researchers have hypothesised about potential pathological mechanisms through which diabetes may lead to frozen shoulder. Current evidence, based on histological studies, suggests that a pathophysiological process consisting of chronic inflammation and capsular fibrosis leads to the contracture in frozen shoulder.49 50 It has been hypothesised that the accumulation of advanced glycation end products (AGEs), which lead to the cross-linking of collagen,51 52 may explain the fibrosis in the capsule of patients with frozen shoulder.33 Glycation is a process by which simple sugars bond to proteins, which is enhanced by persistent hyperglycaemia. Thus, the role of glycation and AGEs in the fibrosis of the shoulder capsule could potentially be a reason why diabetes is associated with frozen shoulder. Another potential reason why diabetes may be associated with frozen shoulder is that hyperglycaemia may induce proinflammatory cytokines53 which have been found to be elevated in the capsule and synovium of patients with frozen shoulder.54
The association between glycaemic control and the risk of developing frozen shoulder should also be a focus for future research. One study found evidence to suggest that poor long-term glycaemic control in people with diabetes is associated with an increased incidence of frozen shoulder,55 while another study found no association between HbA1c level in people with diabetes and the prevalence of frozen shoulder.56 Further research is required to investigate whether glycaemic control is associated with the development of frozen shoulder.
Conclusion
In summary, people with diabetes are more at risk of developing frozen shoulder than people without diabetes. However, existing research is limited by the high risk of unmeasured confounding. To better understand the nature of the relationship between diabetes and the onset of frozen shoulder, it is necessary to have high-quality cohort studies that use causal inference methods that are appropriate for aetiological modelling. Given the existing evidence that has been summarised in this review, clinicians should consider checking whether patients with diabetes are experiencing shoulder pain at their routine follow-up appointments. An early diagnosis will help the clinician to provide treatment for the pain and lack of function that result from frozen shoulder.
Supplementary Material
Footnotes
Contributors: Data extraction and risk of bias assessment were performed by BPD, MB and TR. BPD performed the meta-analysis and narrative synthesis of the results and drafted the initial manuscript. All authors contributed to the conception of the study and systematic review of the study selection, editing and approval of the final manuscript. BPD is the guarantor.
Funding: This study was supported by the Versus Arthritis PhD scholarship scheme (grant number 21899). The authors also thank the information specialists of evidence synthesis team at the Primary Care Centre Versus Arthritis for their advice regarding the search strategy and Elaine Willmore for taking time to check the results of our searches for this review. Further thanks are given to Dr Linda Chesterton, who was involved in the initial conceptualisation of this study. CB is funded by an National Institute for Health Research Clinical Lectureship.
Disclaimer: The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health and Social Care.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable. (Data have been included in table 1 and online supplemental appendix table C1).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Bunker T. (II) frozen shoulder. Orthop Trauma 2011;25:11–18. 10.1016/j.mporth.2011.01.007 [DOI] [Google Scholar]
- 2.Dias R, Cutts S, Massoud S. Frozen shoulder. BMJ 2005;331:1453–6. 10.1136/bmj.331.7530.1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho C-H, Koo TW, Cho N-S, et al. Demographic and clinical characteristics of primary frozen shoulder in a Korean population. Clin Shoulder Elbow 2015;18:133–7. [Google Scholar]
- 4.Rizk TE, Pinals RS. Frozen shoulder. Semin Arthritis Rheum 1982;11:440–52. 10.1016/0049-0172(82)90030-0 [DOI] [PubMed] [Google Scholar]
- 5.Cohen C, Tortato S, Silva OBS, et al. Association between frozen shoulder and thyroid diseases: strengthening the evidences. Rev Bras Ortop 2020;55:483–9. 10.1055/s-0039-3402476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang S-W, Lin J-W, Wang W-T, et al. Hyperthyroidism is a risk factor for developing adhesive capsulitis of the shoulder: a nationwide longitudinal population-based study. Sci Rep 2014;4:4183. 10.1038/srep04183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cucchi D, Marmotti A, De Giorgi S, et al. Risk factors for shoulder stiffness: current concepts. Joints 2017;5:217–23. 10.1055/s-0037-1608951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagy MT, Macfarlane RJ, Khan Y, et al. The frozen shoulder: myths and realities. Open Orthop J 2013;7:352–5. 10.2174/1874325001307010352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung C-M, Jung TS, Park HB. Are serum lipids involved in primary frozen shoulder? A case-control study. J Bone Joint Surg Am 2014;96:1828–33. 10.2106/JBJS.M.00936 [DOI] [PubMed] [Google Scholar]
- 10.Nayak SP, Panda CK. Is hyperlipidaemia a cause of primary frozen shoulder? A case-controlled study. Jebmh 2017;4:697–701. 10.18410/jebmh/2017/135 [DOI] [Google Scholar]
- 11.Austin DC, Gans I, Park MJ, et al. The association of metabolic syndrome markers with adhesive capsulitis. J Shoulder Elbow Surg 2014;23:1043–51. 10.1016/j.jse.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 12.Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren's disease. J Shoulder Elbow Surg 2001;10:149–51. 10.1067/mse.2001.112883 [DOI] [PubMed] [Google Scholar]
- 13.Bunker TD, Anthony PP. The pathology of frozen shoulder. A Dupuytren-like disease. J Bone Joint Surg Br 1995;77:677–83. 10.1302/0301-620X.77B5.7559688 [DOI] [PubMed] [Google Scholar]
- 14.Zreik NH, Malik RA, Charalambous CP. Adhesive capsulitis of the shoulder and diabetes: a meta-analysis of prevalence. Muscles Ligaments Tendons J 2016;6:26–34. 10.32098/mltj.01.2016.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NHS Digital . National Diabetes Audit 2017/18 - Report 1 Care Processes and Treatment Targets, 2020. Available: https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit/report-1-care-processes-and-treatment-targets-2017-18-short-report [Accessed 08 Sep 2022].
- 16.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 2008;26:77–82. 10.2337/diaclin.26.2.77 [DOI] [Google Scholar]
- 17.Sözen T, Başaran Nursel Çalık, Tınazlı M, et al. Musculoskeletal problems in diabetes mellitus. Eur J Rheumatol 2018;5:258–65. 10.5152/eurjrheum.2018.18044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyer BP, Burton C, Rathod-Mistry T, et al. Diabetes as a prognostic factor in frozen shoulder: a systematic review. Arch Rehabil Res Clin Transl 2021;3:100141. 10.1016/j.arrct.2021.100141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu C-L, Sheu WH-H. Diabetes and shoulder disorders. J Diabetes Investig 2016;7:649–51. 10.1111/jdi.12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietrzak M. Adhesive capsulitis: an age related symptom of metabolic syndrome and chronic low-grade inflammation? Med Hypotheses 2016;88:12–17. 10.1016/j.mehy.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 21.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Lippincott Williams & Wilkins, 2013. [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–6. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 24.Veroniki AA, Jackson D, Viechtbauer W, et al. Methods to estimate the between‐study variance and its uncertainty in meta‐analysis. Res Synth Methods 2016;7:55–79. 10.1002/jrsm.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014;14:25. 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partlett C, Riley RD. Random effects meta-analysis: Coverage performance of 95% confidence and prediction intervals following REML estimation. Stat Med 2017;36:301–17. 10.1002/sim.7140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterne JAC, Harbord RM. Funnel plots in meta-analysis. Stata J 2004;4:127–41. 10.1177/1536867X0400400204 [DOI] [Google Scholar]
- 29.Higgins JPT, Green S. Cochrane Handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2011. [Google Scholar]
- 30.Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods 2010;1:112–25. 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 31.StataCorp . Stata statistical software: release 16. College Station, TX: StataCorp LLC, 2019. [Google Scholar]
- 32.Boyle-Walker KL, Gabard DL, Bietsch E, et al. A profile of patients with adhesive capsulitis. J Hand Ther 1997;10:222–8. 10.1016/S0894-1130(97)80025-7 [DOI] [PubMed] [Google Scholar]
- 33.Li W, Lu N, Xu H, et al. Case control study of risk factors for frozen shoulder in China. Int J Rheum Dis 2015;18:508–13. 10.1111/1756-185X.12246 [DOI] [PubMed] [Google Scholar]
- 34.Lee S-Y, Park J, Song S-W. Correlation of Mr arthrographic findings and range of shoulder motions in patients with frozen shoulder. AJR Am J Roentgenol 2012;198:173–9. 10.2214/AJR.10.6173 [DOI] [PubMed] [Google Scholar]
- 35.Milgrom C, Novack V, Weil Y, et al. Risk factors for idiopathic frozen shoulder. Isr Med Assoc J 2008;10:361–4. [PubMed] [Google Scholar]
- 36.Wang K, Ho V, Hunter-Smith DJ, et al. Risk factors in idiopathic adhesive capsulitis: a case control study. J Shoulder Elbow Surg 2013;22:e24–9. 10.1016/j.jse.2012.10.049 [DOI] [PubMed] [Google Scholar]
- 37.Kingston K, Curry EJ, Galvin JW, et al. Shoulder adhesive capsulitis: epidemiology and predictors of surgery. J Shoulder Elbow Surg 2018;27:1437–43. 10.1016/j.jse.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 38.Huang Y-P, Fann C-Y, Chiu Y-H, et al. Association of diabetes mellitus with the risk of developing adhesive capsulitis of the shoulder: a longitudinal population-based followup study. Arthritis Care Res 2013;65:1197–202. 10.1002/acr.21938 [DOI] [PubMed] [Google Scholar]
- 39.Lo S-F, Chu S-W, Muo C-H, et al. Diabetes mellitus and accompanying hyperlipidemia are independent risk factors for adhesive capsulitis: a nationwide population-based cohort study (version 2). Rheumatol Int 2014;34:67–74. 10.1007/s00296-013-2847-4 [DOI] [PubMed] [Google Scholar]
- 40.Hardy RJ, Thompson SG. Detecting and describing heterogeneity in meta-analysis. Stat Med 1998;17:841–56. [DOI] [PubMed] [Google Scholar]
- 41.Thomas SJ, McDougall C, Brown IDM, et al. Prevalence of symptoms and signs of shoulder problems in people with diabetes mellitus. Journal of Shoulder and Elbow Surgery 2007;16:748–51. 10.1016/j.jse.2007.02.133 [DOI] [PubMed] [Google Scholar]
- 42.Bridgman JF. Periarthritis of the shoulder and diabetes mellitus. Ann Rheum Dis 1972;31:69–71. 10.1136/ard.31.1.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young JC, Conover MM, Funk MJ. Measurement error and misclassification in electronic medical records: methods to mitigate bias. Curr Epidemiol Rep 2018;5:343–56. 10.1007/s40471-018-0164-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Linsell L, Dawson J, Zondervan K, et al. Prevalence and incidence of adults consulting for shoulder conditions in UK primary care; patterns of diagnosis and referral. Rheumatology 2006;45:215–21. 10.1093/rheumatology/kei139 [DOI] [PubMed] [Google Scholar]
- 46.Dorrestijn O, Greving K, van der Veen WJ, et al. Patients with shoulder complaints in general practice: consumption of medical care. Rheumatology 2011;50:389–95. 10.1093/rheumatology/keq333 [DOI] [PubMed] [Google Scholar]
- 47.Bunker T. Time for a new name for frozen Shoulder—Contracture of the shoulder. Shoulder Elbow 2009;1:4–9. 10.1111/j.1758-5740.2009.00007.x [DOI] [Google Scholar]
- 48.Heinze G, Dunkler D. Five myths about variable selection. Transpl Int 2017;30:6–10. 10.1111/tri.12895 [DOI] [PubMed] [Google Scholar]
- 49.Wong PL, Tan HC. A review on frozen shoulder. Singapore Med J 2010;51:694–7. [PubMed] [Google Scholar]
- 50.Cho C-H, Song K-S, Kim B-S, et al. Biological aspect of pathophysiology for frozen shoulder. Biomed Res Int 2018;2018:1–8. 10.1155/2018/7274517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hwang KR, Murrell GAC, Millar NL, et al. Advanced glycation end products in idiopathic frozen shoulders. J Shoulder Elbow Surg 2016;25:981–8. 10.1016/j.jse.2015.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldin A, Beckman JA, Schmidt AM, et al. Advanced glycation end products. Circulation 2006;114:597–605. 10.1161/CIRCULATIONAHA.106.621854 [DOI] [PubMed] [Google Scholar]
- 53.Shanmugam N, Reddy MA, Guha M, et al. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 2003;52:1256–64. 10.2337/diabetes.52.5.1256 [DOI] [PubMed] [Google Scholar]
- 54.Lho Y-M, Ha E, Cho C-H, et al. Inflammatory cytokines are overexpressed in the subacromial bursa of frozen shoulder. J Shoulder Elbow Surg 2013;22:666–72. 10.1016/j.jse.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 55.Chan JH, Ho BS, Alvi HM, et al. The relationship between the incidence of adhesive capsulitis and hemoglobin A1c. J Shoulder Elbow Surg 2017;26:1834–7. 10.1016/j.jse.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 56.Yian EH, Contreras R, Sodl JF. Effects of glycemic control on prevalence of diabetic frozen shoulder. J Bone Joint Surg Am 2012;94:919–23. 10.2106/JBJS.J.01930 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-062377supp001.pdf (176.6KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information. Not applicable. (Data have been included in table 1 and online supplemental appendix table C1).


