Abstract
We present a case of a woman in her 20s, with a prior history of paediatric sacrococcygeal germ cell tumour, presenting with a 6-month history of perianal pain. An MRI pelvis revealed a heterogeneous soft tissue mass causing destruction of the sacrococcygeal bone. A staging CT demonstrated metastatic deposits in the lungs and hypodense foci in the liver suspicious of metastatic disease. Her alpha-fetoprotein levels were raised and a CT-guided biopsy was in keeping with recurrent germ cell tumour. She was referred to a national centre for the treatment of germ cell tumours in adults and was rechallenged with cisplatin-based multiagent chemotherapy with a curative intent. This case raises the important question of how long we should follow-up these patients and whether they can ever be safely discharged from oncology surveillance.
Keywords: Oncology, Cancer intervention
Background
Germ cell tumours (GCTs) are premalignant or cancerous neoplasms derived from germ cells. In children, GCTs arise from primordial germ cells, which migrate, during embryogenesis, from the yolk sac to the gonads and can be classified as gonadal or extragonadal. The majority of extragonadal GCTs arise in midline sites, such as the sacrococcyx, the mediastinum and the retroperitoneum, and can also occur in the brain. Childhood GCTs include germinomas, yolk-sac tumours, embryonal carcinomas, mature teratomas and immature teratomas. Childhood GCTs make up approximately 3% of malignancies in children aged 0–18 with their rate of incidence increasing at the onset of puberty.1 GCTs often secrete the tumour marker proteins: alpha-fetoprotein (AFP) and/or human chorionic gonadotrophin (hCG), which both can be measured in the blood. These markers can be important in the diagnosis and follow-up of GCTs.
As with adult GCTs, the mainstay of treatment for childhood GCTs is cisplatin-based multiagent chemotherapy.1
Since childhood GCTs are rare, there is minimal evidence available on the optimal post-treatment follow-up period. It is generally recommended that for paediatric extracranial GCTs, tumour markers are performed monthly for a period of 6 months and then every 3 months following, for a total of 2–3 years depending on tumour type.2
Case presentation
A woman in her 20s presented to her local hospital with a 6-month history of perianal pain radiating down the right thigh resulting in difficulty sitting. The onset of her pain coincided with an injury at the gym which she had initially attributed as the cause of her pain. On examination, there were no palpable masses or skin changes, however, a well-healed historic scar was noted over the coccyx. She was initially treated for a pilonidal sinus.
Of note, at the age of 14 months the patient was diagnosed with a stage IV yolk sac tumour arising from the coccyx with lung metastases. This had presented with a palpable mass and bowel obstruction and, subsequently, a temporary colostomy was performed. She was treated with neoadjuvant chemotherapy—six cycles of etoposide, bleomycin and carboplatin over a 4-month period with subsequent excision of the residual sacrococcygeal mass. Two months later, she suffered a biochemical relapse with a rising AFP tumour marker (AFP), and was treated with further chemotherapy (adriamycin, vincristine, actinomycin and cyclophosphamide) over the following year to a marker negative remission.
She remained under surveillance by the paediatric oncology team who facilitated yearly clinical review, 5 yearly echocardiograms and lung function tests due to the prior treatment with anthracycline and bleomycin, respectively.
The patient’s family history is significant for breast cancer in her mother (BRCA negative), maternal grandmother and both maternal great-grandmothers.
The patient lived with family, did not smoke and drank alcohol socially.
Investigations
Given her ongoing perianal pain, an MRI pelvis was performed. This revealed a 12.2 cm × 12.7 cm × 7.9 cm heterogeneous soft tissue mass causing destruction of the sacrococcygeal bone (figure 1 and figure 2). A staging CT revealed metastatic deposits in the lungs and hypodense foci in the liver suspicious of metastatic disease.
Figure 1.
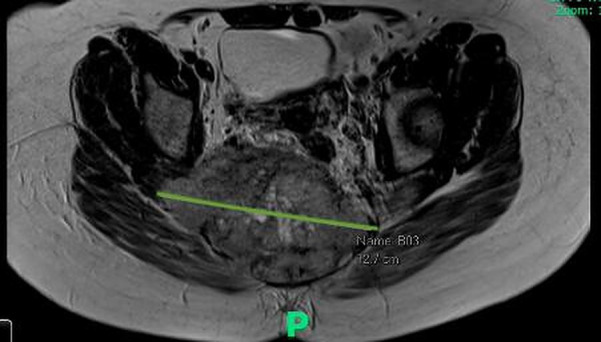
Transverse view of MRI pelvis that revealed a 12.2 cm × 12.7 cm × 7.9 cm heterogeneous soft tissue mass causing destruction of the sacrococcygeal bone.
Figure 2.

Sagittal view of MRI pelvis that revealed a 12.2 cm × 12.7 cm × 7.9 cm heterogeneous soft tissue mass causing destruction of the sacrococcygeal bone.
The patient was referred to an orthopaedic oncology specialist centre who carried out a CT-guided biopsy of the sacrum. The histology revealed abundant material of a highly cellular grade malignancy with solid or anastomosing trabecular or cobb webb-like growth pattern and was mitotically active, in keeping with a yolk sac tumour. Immunohistochemical analysis showed the tumour cells expressed cytokeratin AE1/AE3, cytokeratin Cam 5.2, glypican-3, AFP (focal), CD30 (very focal), spalt-like transcription factor 4 (very focal) and CD117 (very focal). They did not express octamer binding transcription factor 3/4 or epithelial membrane antigen, and HCG was equivocal. HepPar 1 and oestrogen receptor were negative and progesterone receptor had weak patchy expression. PD-L1 was negative.
Regarding tumour markers (blood), her AFP level was significantly elevated at 42, 537 ng/mL with a normal hCG.
Treatment
The patient was referred to the oncology team at a national centre for the treatment of GCTs in adults. She was rechallenged with etoposide and cisplatin chemotherapy with curative intent. Initially, she had a good biochemical response, however, after six cycles the AFP plateaued at around 20 000 ng/mL.
Treatment was changed to POMB/ACE chemotherapy (cisplatin, vincristine, methotrexate, bleomycin, actinomycin, cyclophosphamide, etoposide) with the aim of complete marker response. Unfortunately, following the second cycle she was unwell and was admitted to her local hospital for management of urosepsis, acute kidney injury, electrolyte derangement and was also found to be positive for COVID-19. She was pancytopenic and suffered a subdural haemorrhage requiring neurosurgical evacuation.
Given her bone marrow suppression with POMB/ACE chemotherapy, it was felt she was not a candidate for further high-dose treatment and was switched to salvage chemotherapy with TE/TCARBO (paclitaxel and etoposide alternating with paclitaxel and platinum with carboplatin). The patient was readmitted after the first cycle with pancytopenia and sepsis and underwent a cholecystectomy for source control. After a period of recovery, she continued the same chemotherapy regime with a dose reduction of carboplatin. Initially, her AFP fell to approximately 8000 ng/mL, however, after five cycles, her AFP began to rise and repeat imaging showed minor progression of the primary sacrococcygeal tumour but stable small volume liver and lung metastases. Additionally, her renal function continued to deteriorate with no clear cause.
The plan was to switch the patient to ifosfamide and doxorubicin chemotherapy, however, given the deterioration in renal function, this was not possible. She received one cycle of single agent doxorubicin and had a course of palliative radiotherapy for her primary tumour.
Outcome and follow-up
The patient had been clinically improving with down trending tumour markers (her most recent AFP was 5000 ng/mL). She was awaiting follow-up imaging and was due to recommence dose modified ifosfamide and doxorubicin.
Unfortunately, she was admitted to her local hospital with presumed sepsis where she suffered a cardiac arrest and died.
Discussion
A complete clinical remission is defined as normalisation of the tumour markers and the absence of residual lesions.3 Late relapses of malignant GCTs are rare events generally defined as recurrence at least 2 years after completion of successful primary treatment.4 They are associated with a poor prognosis, based on advanced-stage presentation, and resistance to chemotherapy.5
A large population-based series of 1949 patients with malignant GCTs identified 25 patients that developed late relapse, and of those, only 2 relapsed after 10 years; at 10 and 18 years, respectively.6 To the best of our knowledge, this is the first case reported in the literature of a recurrent extragonadal malignant GCT over 20 years later.
Diagnosis and proper treatment of patients with late‐relapsing GCTs is challenging and should be restricted to experienced centres only. Referral of late‐relapsing patients to high‐volume institutions ensures the best chances of cure.
This case raises the important question of how long we should follow-up these patients and whether they can ever be discharged from oncology surveillance. Monitoring tumour markers is relatively non-invasive, and tumour marker elevation is a highly sensitive method of relapse surveillance in GCTs.7 Tumour marker monitoring could aid in prompt recognition and treatment of relapse which would likely lead to improved outcomes especially in extragonadal GCTs.8
In our patient’s case, markers were secreted at relapse, emphasising how non-invasive surveillance could have led to earlier detection. Given that late relapses have poorer outcomes, prognosis was guarded from the outset, and whether earlier detection would have changed outcome is unknown.
It is also worth noting the importance of oncological surveillance for patients who have mature teratomas resected at the end of treatment. Although benign, these tumours can recur and undergo malignant transformation. Therefore, these patients should never be discharged from follow-up, ensuring any relapse can be detected early. If these patients are marker secreting, surveillance is straight forward. However, some patients do not secrete markers at relapse which raises further questions regarding the frequency of surveillance imaging.
Learning points.
In patients with a history of germ cell tumour, relapse should always be considered as a differential regardless of the time frame. This should be investigated by measuring tumour markers and considering further imaging.
Monitoring tumour markers is relatively non-invasive and tumour marker elevation is a highly sensitive method of relapse surveillance in germ cell tumours and could aid in prompt recognition and treatment leading to improved outcomes.
Prompt referral of late‐relapsing germ cell tumours to high‐volume institutions ensures the best chances of cure.
Footnotes
Contributors: H-CY and GK performed a background literature search and prepared the initial draft of the manuscript. MS contributed critical revisions of the manuscript. NS identified the case for reporting and contributed critical revisions of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
References
- 1.Pierce JL, Frazier AL, Amatruda JF. Pediatric germ cell tumors: a developmental perspective. Adv Urol 2018;2018:1–8. 10.1155/2018/9059382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.PDQ Pediatric Treatment Editorial Board . Childhood extracranial germ cell tumors treatment (PDQ®). Available: https://www.cancer.gov/types/extracranial-germ-cell/hp/germ-cell-treatment-pdq# [Accessed 12 Jan 2021].
- 3.Göbel U, Schneider DT, Calaminus G, et al. Germ-Cell tumors in childhood and adolescence. Annals of Oncology 2000;11:263–72. 10.1023/A:1008360523160 [DOI] [PubMed] [Google Scholar]
- 4.Oldenburg J, Alfsen GC, Wæhre H, et al. Late recurrences of germ cell malignancies: a population-based experience over three decades. Br J Cancer 2006;94:820–7. 10.1038/sj.bjc.6603014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronnen EA, Kondagunta GV, Bacik J, et al. Incidence of late-relapse germ cell tumor and outcome to salvage chemotherapy. J Clin Oncol 2005;23:6999–7004. 10.1200/JCO.2005.21.956 [DOI] [PubMed] [Google Scholar]
- 6.Oldenburg J, Alfsen GC, Waehre H, et al. Late recurrences of germ cell malignancies: a population-based experience over three decades. Br J Cancer 2006;94:820–7. 10.1038/sj.bjc.6603014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonseca A, Xia C, Lorenzo AJ, et al. Detection of relapse by tumor markers versus imaging in children and adolescents with Nongerminomatous malignant germ cell tumors: a report from the children's Oncology Group. J Clin Oncol 2019;37:396–402. 10.1200/JCO.18.00790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann JT, Nichols CR, Droz J-P, et al. Prognostic variables for response and outcome in patients with extragonadal germ-cell tumors. Ann Oncol 2002;13:1017–28. 10.1093/annonc/mdf176 [DOI] [PubMed] [Google Scholar]


