Abstract
Objective:
This study was done to analyse the demographic profile and presentation of diabetes in Central India.
Design:
Data was collected for this cross-sectional study from an electronic diabetes registry from 2014 to 2019. Demographic details, patient history and presence or absence of co-morbid conditions, duration of diabetes, age of onset of diabetes, drug history, personal history, presence of micro and/or macrovascular complications and investigations done were obtained.
Statistical Analysis:
The association between each factor and the outcome was studied in terms of prevalence ratio (PR) using the R-3.0.0 programming (R Foundation for Statistical Computing, Vienna, Austria) language. Statistical significance was evaluated at a 5% level.
Results:
Among 12,434 patients, 54.95% were below 50 years and 45.05% were above 50 years. 50.21% were females and 49.79% were males. The mean age was 47.49 ± 14.78 years and the mean body mass index (BMI) was 26.85 ± 5.19 kg/m2 with 62.29% of obese patients (>25 kg/m2). The mean overall duration of diabetes was 7.64 ± 7.63 years. Mean Glycosylated Haemoglobin (HbA1c) in patients <=50 years was 8.60 ± 2.63 and 8.90 ± 1.91 for over 50. 65.59% had uncontrolled blood sugars. 25.19% of patients had hypertension and 18.1% had dyslipidaemia. Coronary artery disease (CAD), nephropathy, neuropathy and retinopathy were observed in 21.49%, 9.60%, 33.65% and 14.65%, respectively. The adjusted PR of cardiovascular disease (CVD) was 5.374 times higher for patients over 50 (P < 0.0001); 3.775 times higher for males (P < 0.0001), 1.64 times higher for patients with BMI >25 kg/m2 (P < 0.0001) and 3.643 times higher in hypertensive cases (P < 0.0001). Similar associations were observed with nephropathy, neuropathy and retinopathy.
Conclusion:
From a large population study on diabetes, it was found a majority of the type 2 diabetes mellitus (T2DM) cases (65%) are sub-optimally controlled with HbA1c levels. Also, microvascular complications were related to the sub-optimal glycaemic control, but not the macro-vascular complications.
Keywords: CVD, diabetes, Hb1Ac, nephropathy, neuropathy, retinopathy
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disorder characterized by hyperglycaemia. It has dreadful complications and can significantly compromise the quality of life. In 2021, according to International Diabetes Federation Atlas, approximately 537 million adults (20–79 years) are living with diabetes. The total number of people living with diabetes is projected to rise to 643 million by 2030 and 783 million by 2045. The figures are expected to rise to 123 million in India itself by 2040.[1,2] World Health Organization (WHO) has projected that a maximum increase in people with diabetes would occur in India.[3] Diabetes is often associated with other co-morbid conditions like hypertension and dyslipidaemia and leads to increased morbidity and mortality.[4]
Multicentric, multistage, Indian Council of Medical Research-India Diabetes study (INDIAB) population-based study has revealed the overall prevalence of diabetes to be 7.3%, which varies widely from region to region.[4] Since the variation is wide and the Indian population is diverse in ethnicity, a regional demographic study of diabetic patients is important as it can pave the way for effective management and framing of preventive measures.[5] Considering the biodiversity, ethnic and geographical differences, the study of regional demographic profiles in India will help understand the common trends, nature of complications and gender differences while treating and screening for diabetes. In addition, diabetes registries are an important epidemiological tool. This helps monitor the prevalence of diabetes; they give a sound sampling base for epidemiologic and clinical studies, provide information to health service providers and planners on risk factors and complications, and are helpful in the overall monitoring of diabetes control programmes and form a base for framing guidelines as per local needs.[6]
Hence, a study was planned to know the demographic profile and presentation of diabetes in Central India.
MATERIALS AND METHODS
Study design
It is a cross-sectional study. The electronic diabetes registry was opened in 2014, and the data collection was done until 2019. The patient information, clinical examination findings and details of the investigations done were entered into the registry by primary care physicians and endocrinologists from Central India. A total of 15,892 individuals were enrolled in the study.
The data in the registry included demographic details (age, sex, height, weight, body mass index (BMI)), patient history of illness and presence or absence of co-morbid conditions (hypertension, dyslipidaemia, hypothyroidism or any other), duration of diabetes, age of onset of diabetes, drug history and personal history. The details of the clinical examination were recorded. The presence of micro and/or macrovascular complications and investigations done on the day of the visit or within a period of 3 months were entered by the consultants.
For the assessment of complications of diabetes (both microvascular as well as macrovascular), the records were studied in detail. Any documented history of ischaemic heart disease (IHD), peripheral vascular disease (PVD), cerebrovascular episode, retinopathy, neuropathy or nephropathy was recorded. Those patients in whom there was no history of a specific complication were evaluated for the same by the consultant. An electrocardiogram (ECG) and echocardiogram were done for cardiac evaluation. The ankle-brachial index was done to confirm PVD. A kidney function test and urine microalbumin were done to assess the renal status. Clinical examination with monofilament and tuning fork examination were done to assess neuropathy. Dilated fundus examination by an ophthalmologist was done for assessment of retinopathy.
Statistical analysis
The demographic, anthropometric and other co-morbidities data on diabetic patients were obtained through the survey and retrieved from the database. Continuous variables like age, BMI and glycated haemoglobin (HbA1c) were categorized, and other categorical variables like gender, presence of different co-morbidities and accordingly summary statistics were obtained in terms of frequencies and percentages. Since the study was cross-sectional, the association between each factor and the outcome was studied in terms of prevalence ratio (PR). The unadjusted PR defined by the ratio of prevalence of the outcome in the reference level relative to the prevalence in other levels was obtained for each factor. The adjusted estimates of PR were obtained using the log-binomial regression model, which is a generalized linear model in which the link function is the logarithm of proportion under study, and the error distribution is binomial. All the analyses were performed using R-3.0.0 programming language (R Core Team - 2015, Vienna, Austria); and the statistical significance was evaluated at a 5% level.
RESULTS
The survey involved 15,892 patients with diabetes from central India, out of which 91 (0.73%) gestational DM cases were excluded from the present analysis. The resulting 15,801 cases included 15,502 type II, 288 type I and 11 secondary DM cases, as shown in Figure 1. Further, data on some of the key parameters was missing for 3367 patients, resulting in a data set of 12,434 patients for downstream analysis. There were 54.95% of cases below 50 years of age, and 45.05% were above 50 years, as shown in Table 1. As regards sex distribution, there were 50.21% females and 49.79% males. The mean age of patients was 47.49 ± 14.78 years. There were 62.29% obese (>25 kg/m2) patients, and the mean BMI was 26.85 ± 5.19 kg/m2, and the mean overall duration of diabetes was 6.64 ± 7.63 years. For type I DM patients, it was 6.03 ± 7.08 years, while for type II patients, the mean was 7.69 ± 7.65 years. Overall, HbA1c was 8.78 ± 2.45. In the age <=50 years category, the mean HbA1C was 8.60 ± 2.63, and in above 50 years of age, it was 8.90 ± 1.91. Further, 65.59% had uncontrolled blood sugars with HbA1c values above 7.0%. There were 25.19% cases of hypertension, and 18.1% of patients had dyslipidaemia. The co-morbidities like coronary artery disease (CAD), nephropathy, neuropathy and retinopathy were observed in 21.49%, 9.60%, 33.65% and 14.65%, respectively.
Figure 1.
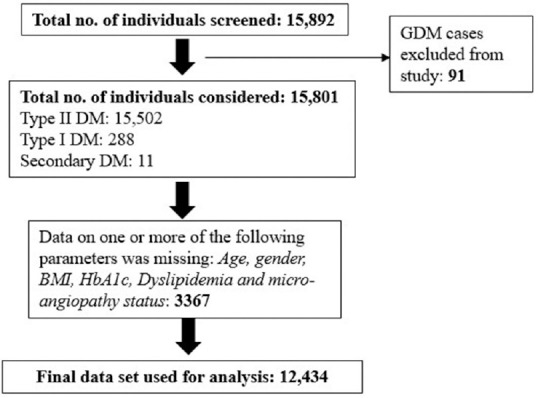
Flow diagram showing patient selection
Table 1.
Descriptive statistics for DM patients (n=12,434)
| Characteristics | Levels | Statistic |
|---|---|---|
| Age (years) [No (%)] | ≤50 | 6832 (54.95) |
| >50 | 5602 (45.05) | |
| Mean±SD | 47.49±14.78 | |
| Sex [No (%)] | Female | 6243 (50.21) |
| Male | 6191 (49.79) | |
| BMI (kg/m2) [No (%)] | ≤25 | 4689 (37.71) |
| >25 | 7745 (62.29) | |
| Mean±SD | 26.85±5.19 | |
| Duration of DM (years) [Mean±SD] | Overall | 6.64±7.63 |
| Type I | 6.03±7.08 | |
| Type II | 7.69±7.65 | |
| HbA1c [No (%)] | ≤7 | 4279 (34.41) |
| >7 | 8155 (65.59) | |
| Mean HbA1C±SD | 8.78±2.45 (≤ 50 years: 8.60±2.63 ≥50 years: 8.90±1.91) | |
| HTN [No (%)] | No | 9302 (74.81) |
| Yes | 3132 (25.19) | |
| Dyslipidaemia [No (%)] | No | 10184 (81.90) |
| Yes | 2250 (18.10) | |
| CVD [No (%)] | No | 9762 (79.09) |
| Yes | 2672 (21.49) | |
| Nephropathy [No (%)] | No | 11240 (91.06) |
| Yes | 1194 (9.60) | |
| Neuropathy [No (%)] | No | 8250 (66.84) |
| Yes | 4184 (33.65) | |
| Retinopathy [No (%)] | No | 10612 (85.98) |
| Yes | 1822 (14.65) |
The risk of these disorders associated with different factors was determined in terms of prevalence ratio. The key confounders considered were age, sex, BMI, duration of DM, HbA1c and dyslipidaemia. The unadjusted prevalence ratios were obtained for each factor. The adjusted prevalence ratios were obtained using the log-binomial regression model considering significant factors in the univariate (unadjusted) analysis. Table 2 provides the factors influencing the PR of cardiovascular disease (CVD). The adjusted PR of CVD associated with age >50 years was 5.374 [95% CI: 4.820-5.992] times higher than those with age ≤50 years, and the effect was statistically highly significant (P < 0.0001). Further, the adjusted PR of the disease in males was 3.775 [95% CI: 2.377–4.219] times higher than in females with P < 0.0001. The patients with BMI >25 kg/m2, had 1.64 [95% CI: 1.470–1.831] times the higher adjusted prevalence of CVD as compared to those with BMI <25 kg/m2, and the effect was statistically significant with P < 0.0001. Further, in hypertensive cases, the PR of the disease was 3.643 [95% CI: 3.260–4.071] times higher than in non-hypertensive cases with P < 0.0001. A sub-group analysis was performed in which females were categorized as <=50 years and >50 years. The CVD prevalence in females with age >50 years (3.03%) was compared with that of males (3.68%), which was statistically insignificant (P = 0.1581).
Table 2.
Risk factors associated with a prevalence ratio of CVD in the diabetic cohort
| Characteristics | Levels | CVD Present/Total (%) | Prevalence ratio [95% CI; P] | |
|---|---|---|---|---|
|
| ||||
| Unadjusted | Adjusted* | |||
| Age (years) | <=50 | 672/6832 (9.83) | Ref | Ref |
| >50 | 2000/5602 (35.7) | 5.090 [4.621-5.606; <0.0001] | 5.374 [4.820-5.992; <0.0001] | |
| Sex | Female | 734/6243 (11.75) | Ref | Ref |
| Male | 1938/6191 (31.30) | 3.420 [3.114-3.757; <0.0001] | 3.775 [21.377-4.219; <0.0001] | |
| BMI (kg/m2) | <=25 | 808/3997 (20.21) | Ref | Ref |
| >25 | 1610/6602 (24.38) | 1.273 [1.157-1.400; <0.0001] | 1.640 [1.470-1.831; <0.0001] | |
| Duration of DM (years) | <=5 | 1265/6123 (20.65) | Ref | |
| >5 | 1407/6311 (22.29) | 1.232 [1.011- 1.625; 0.0503] | ||
| HbA1c (%) | <=7 | 887/4279 (20.72) | Ref | |
| >7 | 1785/8155 (21.89) | 1.030 [0.942-1.215; 0.1410] | ||
| HTN | No | 1507/9302 (16.20) | Ref | Ref |
| Yes | 1165/3132 (37.19) | 3.064 [2.797-3.356; <0.0001] | 3.643 [3.260-4.071; <0.0001] | |
| Dyslipidaemia | No | 2082/10184 (20.44) | Ref | Ref |
| Yes | 590/2250 (26.22) | 1.383 [1.245-1.537; <0.0001] | 0.748 [0.655-0.854; 0.053] | |
*Factors with significant unadjusted ratio were included in multivariate model; Bold P values indicate statistical significance
On similar lines, the adjusted prevalence ratios of microangiopathy associated with these factors were determined. Table 3 provides the ratios for nephropathy. For diabetic patients with age >50 years, the adjusted PR of nephropathy was 3.630 [95% CI: 3.098–4.254] times higher as compared to those below 50 years, and the effect was statistically significant (P < 0.0001). Further, in males, the adjusted ratio was 3.068 [95% CI: 2.613–3.601] times higher than in females (P < 0.0001). The BMI >25 kg/m2 showed significantly higher PR of 1.311 [95% CI: 1.121–1.532] as compared to BMI <25 kg/m2 (P = 0.001). The presence of hypertension showed a significantly higher adjusted ratio of nephropathy 12.221 [95% CI: 10.458–14.282] as compared to non-hypertensive cases (P < 0.0001). However, a significant association was not noticed between HbA1C levels and nephropathy and the duration of diabetes.
Table 3.
Risk factors associated with a prevalence ratio of nephropathy in the diabetic cohort
| Characteristics | Levels | Nephropathy Present/Total (%) | Prevalence ratio [95% CI; P] | |
|---|---|---|---|---|
|
| ||||
| Unadjusted | Adjusted* | |||
| Age (years) | <=50 | 292/6832 (4.27) | Ref | Ref |
| >50 | 902/5602 (16.10) | 4.298 [3.747-4.930; <0.0001] | 3.630 [3.098-4.254; <0.0001] | |
| Sex | Female | 307/6243 (4.91) | Ref | Ref |
| Male | 887/6191 (14.32) | 3.234 [2.825-3.701; <0.0001] | 3.068 [2.613-3.601; <0.0001] | |
| BMI (kg/m2) | <=25 | 352/3997 (8.80) | Ref | Ref |
| >25 | 674/6602 (10.21) | 1.117 [1.028-1.348; 0.0197] | 1.311 [1.121-1.532; 0.001] | |
| Duration of DM (years) | <=5 | 561/6123 (9.61) | Ref | |
| >5 | 633/6311 (10.00) | 1.105 [0.981-1.245; 0.1000] | ||
| HbA1c (%) | <=7 | 392/4279 (9.17) | Ref | |
| >7 | 802/8155 (9.83) | 1.021 [0.904-1.166; 0.6935] | ||
| HTN | No | 293/9302 (3.15) | Ref | Ref |
| Yes | 901/3132 (28.76) | 12.418 [10.798-14.280; <0.0001] | 12.221 [10.458-14.282; <0.0001] | |
| Dyslipidaemia | No | 795/10184 (7.80) | Ref | Ref |
| Yes | 399/2250 (17.73) | 2.546 [2.235-2.900; <0.0001] | 1.539 [1.272-2.954; 0.077] | |
*Factors with significant unadjusted ratios were included in the multivariate model; Bold P values indicate statistical significance
The effect of these factors on neuropathy was studied, as shown in Table 4. The PR in males was 2.455 [95% CI: 2.165–2.761] times higher than that of females with P < 0.0001. The HbA1c value >7% showed significant effect on neuropathy with PR of 1.327 [95% CI: 1.154–1.524] as compared to patients with HbA1c <7% (P < 0.0001). In the hypertensive group, the adjusted ratio of neuropathy was 13.309 [95% CI: 11.461–15.456] times higher than the non-hypertensive group (P < 0.0001). The presence of dyslipidaemia also showed a significantly higher PR of 1.753 [95% CI: 1.319–2.922] (P = 0.048).
Table 4.
Risk factors associated with a prevalence ratio of neuropathy in the diabetic cohort
| Characteristics | Levels | Neuropathy Present/Total (%) | Prevalence ratio [95% CI; P] | |
|---|---|---|---|---|
|
| ||||
| Unadjusted | Adjusted* | |||
| Age (years) | <=50 | 1818/6832 (26.61) | Ref | Ref |
| >50 | 2366/5602 (42.23) | 2.016 [1.870-2.174; <0.0001] | 0.975 [0.869-1.094; 0.671] | |
| Sex | Female | 1415/6243 (22.66) | Ref | Ref |
| Male | 2769/6191 (44.72) | 2.716 [2.555-2.984; <0.0001] | 2.455 [2.165-2.761; <0.0001] | |
| BMI (kg/m2) | <=25 | 1324/3997 (33.12) | Ref | |
| >25 | 2302/6602 (34.86) | 1.081 [0.995-1.174; 0.0667] | ||
| Duration of DM (years) | <=5 | 2024/6123 (33.05) | Ref | |
| >5 | 2160/6311 (34.22) | 1.054 [0.978-1.135; 0.1673] | ||
| HbA1c (%) | <=7 | 1211/4279 (28.30) | Ref | Ref |
| > 7 | 2973/8155 (36.45) | 1.237 [1.099-1.392; <0.0001] | 1.327 [1.154-1.524; <0.0001] | |
| HTN | No | 1947/9302 (20.93) | Ref | Ref |
| Yes | 2237/3132 (71.42) | 9.422 [8.610-10.354; <0.0001] | 13.309 [11.461-15.456; <0.0001] | |
| Dyslipidaemia | No | 2998/10184 (29.43) | Ref | Ref |
| Yes | 1186/2250 (52.71) | 2.672 [2.434-2.932; <0.0001] | 1.753 [1.319-2.922; 0.0481] | |
*Factors with significant unadjusted ratio were included in the multivariate model; Bold P values indicate statistical significance
Table 5 shows the adjusted PR of retinopathy associated with different factors. An older age group (age >50 years) showed a 3.609 [95% CI: 3.085–4.225] times higher adjusted PR of retinopathy as compared to the younger group (Age ≤ 50 years) with P < 0.0001. Further, males had a significantly higher PR of 1.244 [95% CI: 1.069–1.449] than females (P = 0.005). The higher BMI also showed a significantly higher prevalence of retinopathy 1.278 [95% CI: 1.095–1.493] compared to normal cases (P = 0.002). The adjusted ratio of retinopathy in the hypertensive group was 7.254 [95% CI: 6.257–8.410] times higher than that of the non-hypertensive group, with P < 0.0001. Moreover, dyslipidaemia also showed 1.539 [95% CI: 1.471–2.056] times higher prevalence with a P value of 0.042.
Table 5.
Risk factors associated with a prevalence ratio of retinopathy in the diabetic cohort
| Characteristics | Levels | Retinopathy Present/Total (%) | Prevalence ratio [95% CI; P] | |
|---|---|---|---|---|
|
| ||||
| Unadjusted | Adjusted* | |||
| Age (years) | <=50 | 459/6832 (6.71) | Ref | Ref |
| >50 | 1363/5602 (24.33) | 4.464 [3.989-4.997; <0.0001] | 3.609 [3.085-4.225; <0.0001] | |
| Sex | Female | 722/6243 (11.56) | Ref | Ref |
| Male | 1100/6191 (17.76) | 1.652 [1.493-1.828; <0.0001] | 1.244 [1.069-1.449; 0.0051] | |
| BMI (kg/m2) | <=25 | 539/3997 (13.48) | Ref | Ref |
| >25 | 1074/6602 (16.26) | 1.246 [1.115-1.394; 0.0001] | 1.278 [1.095-1.493; 0.002] | |
| Duration of DM (years) | <=5 | 866/6123 (14.14) | Ref | |
| >5 | 956/6311 (15.15) | 1.108 [0.981-1.197; 0.1132] | ||
| HbA1c (%) | <=7 | 589/4279 (13.76) | Ref | Ref |
| >7 | 1233/8155 (15.12) | 1.171 [1.007-1.361; 0.0424] | 1.137 [0.952-1.359; 0.159] | |
| HTN | No | 678/9302 (7.28) | Ref | Ref |
| Yes | 1144/3132 (36.52) | 7.320 [6.578-8.144; <0.0001] | 7.254 [6.257-8.410; <0.0001] | |
| Dyslipidaemia | No | 1151/10184 (11.30) | Ref | Ref |
| Yes | 671/2250 (29.82) | 3.335 [2.990-3.720; <0.0001] | 1.539 [1.471-2.056; 0.042] | |
*Factors with significant unadjusted ratio were included in the multivariate model; Bold P values indicate statistical significance
DISCUSSION
The diabetes registry includes a collection of quality data and analysis for variations of various clinical and laboratory parameters. These data provide information specific to the population studied and can be compared with the available information and existing guidelines for patient care. At the patient level, this registry can provide an excellent tool to understand the nature of complications and the course of the disease and thus provide first-hand evidence-based local trends. This data can be useful in understanding the quality of care and gives useful information to healthcare providers and is important for assessing risk factors. Additionally, it can guide planning preventive strategies and provide useful information to health service providers and planners on risk factors and complications, and assess healthcare burden for better resource management facilitating better utilization of resources.[7]
This is the first registry from Central India that provides critical evidence about diabetes presentation, complications and patterns in this part of the country to the best of our knowledge.
The comprehensive picture of our study presents 54.95% of cases below the age of 50 years, which is a huge financial burden. The gender distribution was equal for type 2 diabetes mellitus (T2DM) (50.21% females and 49.79% males). A high percentage (65%) had uncontrolled DM as reflected by HbA1c levels of more than 7%. This is comparable to the international data, which indicates that even in developed countries, most diabetics have uncontrolled DM.[2] The average duration of diabetes was slightly above 7 years. There were 25.19% cases of hypertension, and 18.1% of patients had dyslipidaemia. The co-morbidities like CAD, nephropathy, neuropathy and retinopathy were observed in 21.49%, 9.60%, 33.65% and 14.65%, respectively.
Age above 50 years (P < 0.0001), male sex (P < 0.0001) and presence of hypertension (P < 0.0001) and BMI >25 kg/m2 (P < 0.0001) increased the risk of CVD; however, HbA1c did not influence CVD significantly (P > 0.05). This insignificant relationship could be a result of the cross-sectional nature of the study. As regards with sex, the difference became statistically insignificant when the age of females crossed 50. The same factors, such as age above 50 years (P < 0.0001), hypertension (P < 0.0001), male sex (P < 0.0001) and BMI >25 kg/m2 (P = 0.001) posed higher risk of nephropathy. In the case of neuropathy, hypertension, male sex and HbA1c >7% were the significant risk factors (P < 0.0001). As regards with retinopathy, male sex (p 0.005), older age group [>50 years] (P < 0.0001), dyslipidaemia (P = 0.042), BMI >25 kg/m2 [P = 0.002], HbA1c >7% (P < 0.0001) and presence of hypertension (P < 0.0001) were significant risk factors.
Of all the complications, only neuropathy and retinopathy were significantly associated with HbA1c >7%. It has been shown earlier that microvascular complications correlate more with glycaemic control than macrovascular complications.[8]
Our study has shown that 54% of the patients were below 50 years of age, in contrast to other studies from Gujrat.[5,8] The difference in sample size could explain this as our sample size is very large (n = 12,335) as compared to both of these studies. The results from INDIAB study have shown the mean age to be 41.3 years in most parts of India, whereas we report the mean age to be 47.49 ± 14.78 years. The sex distribution of cases matches with other studies from India reporting almost equal distribution in males and females.[1,3,4,5]
CAD prevalence in our cohort is 21.49% as against 21.4% in a study from South India and one more study, which is strikingly similar.[9,10] INDIA B study has shown this prevalence to be 8–10% in different states.[4] Another study from Central India has reported this prevalence to be 6.2%.[11] There could be ethnic and regional differences, and third, our sample size is very large, including a large number of newly detected diabetes.
The correlation of HbA1c with CAD has mixed results, with some reporting significant association while others showed contradictory results.[12] We have not found a significant association between HbA1C and CAD, whereas age, gender and hypertension are independent risk factors in our study. A few studies, including the Framingham cohort study, have also reported gender differences in this correlation wherein HbA1c is an independent risk factor in females whereas, in males, it is not.[13,14] Moreover, the cut-off for receiver operating characteristic (ROC) curve analysis for HbA1c, where risk increases, is found to be 5.1.[15] This could be why we did not get a significant correlation between HbA1C and CAD. But there are other studies, including a meta-analysis,[16] in which a significant association could not be established between CAD and HbA1c.[16,17,18]
The prevalence of microvascular complications has been reported by various studies in the range of 10–18% in the case of retinopathy, 2–4% in the case of nephropathy and 15–30% in neuropathy.[19] Our study reports nephropathy, neuropathy and retinopathy in 9.60%, 33.65% and 14.65%, respectively. The incidence of microvascular complications was 92.8, 106.2 and 130.2 per 1000 persons per year for retinopathy, neuropathy and nephropathy, respectively, in a study from Pakistan.[20] Only one study from India has reported a very high prevalence of these microvascular complications, 74%, 43% and 30% retinopathy, nephropathy and neuropathy.[21] The overall prevalence of microvascular complications in our study match with the reports from various other parts of India.[22] HbA1c had a significant effect only on neuropathy and retinopathy; for all other complications, the association was insignificant in our study.
Limitations of the study
The data entry was based mainly on self-reporting of the complications by the patients. The investigations reported are from different labs. However, with a very large sample size, the power of the study remains strong.
CONCLUSIONS
From this large database from central India, it can be concluded that the majority of the T2DM cases (65%) are sub-optimally controlled with HbA1c levels of more than 7%. There is equal distribution of cases amongst males and females. Microvascular complications like neuropathy and retinopathy are related to glycaemic control, but macrovascular complications like CAD are not related to glycaemic control. Control of other cardiac risk factors like obesity, hypertension and dyslipidaemia would help reduce the CAD risk in people with diabetes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work and have given final approval for the version to be published.
Medical writing and editorial support in the preparation of this article were provided by Dr. Punit Srivastava of Mediception Science Pvt Ltd (www.mediception.com).
REFERENCES
- 1.Mathur M, Mathur N, Singh O, Solanki J, Soni P, Sarva A, et al. Demographic characters and factors favouring emergence of diabetes mellitus type two. Int J Res Med Sci. 2018;6:950–4. [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetic Atlas. 10th Edition. [Last accessed on 2021 Dec 05]. Available from: http://www.idf.org/idf-diabete s-atlas-eighth-edition .
- 3.Gupta M, Prabhu K, Parijatham BO, Kalaiselvi VS, Rajendram SM, Rose J. Prevalence of diabetes mellitus in South India:A retrospective analysis. JIMSA. 2012;25:239–40. [Google Scholar]
- 4.Anjana RM, Deepa M, Pradeepa R, Mohanta J, Kanwar N, Das HK, et al. Prevalence of diabetes and prediabetes in 15 states of India:Results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–96. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 5.Rana HM, Chavda P, Rathod CC, Mavani M. Socio-demographic and anthropometric profile of diabetic patients attending diabetes clinic in tertiary care hospital of Central Gujarat. Natl J Community Med. 2015;6:554–7. [Google Scholar]
- 6.Russell KG, Rosenzweig J. Improving outcomes for patients with diabetes using Joslin Diabetes Center's Registry and risk stratification system. J Healthc Inf Manag. 2007;21:26–33. [PubMed] [Google Scholar]
- 7.Lakshminarayanan S, Kar SS, Gupta R, Xavier D, Bhaskar Reddy SV. Primary healthcare-based diabetes registry in Puducherry:Design and methods. Indian J Endocr Metab. 2017;21:373–7. doi: 10.4103/ijem.IJEM_296_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel M, Patel I, Patel Y, Rathi S. A hospital-based observational study of Type 2 diabetic subjects from Gujarat, India. J Health Popul Nutr. 2011;29:265–72. doi: 10.3329/jhpn.v29i3.7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohan V, Deepa R, Shanthi Rani S, Premalatha G. Prevalence of coronary artery disease and its relationship to lipids in a selected population in South India. J Am Coll Cardiol. 2001;38:682–7. doi: 10.1016/s0735-1097(01)01415-2. [DOI] [PubMed] [Google Scholar]
- 10.Hernández C, Candell-Riera J, Ciudin A, Francisco G, Aguadé-Bruix S, Hernández RS, et al. Prevalence and risk factors accounting for true silent myocardial ischemia:A pilot case-control study comparing type 2 diabetic with non-diabetic control subjects. Cardiovasc Diabetol. 2011;10:9. doi: 10.1186/1475-2840-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dash K, Tippisetty S, Kolukula V. Clinical and demographic profile of type 2 diabetes patients:An epidemiology report from Central India. J Endocr Soc. 2019;3:157. [Google Scholar]
- 12.Garg N, Moorthy N, Kapoor A, Tewari S, Kumar S, Sinha A, et al. Hemoglobin A (1c) in nondiabetic patients:An independent predictor of coronary artery disease and its severity. Mayo Clin Proc. 2014;89:908–16. doi: 10.1016/j.mayocp.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Singer DE, Nathan DM, Anderson KM, Wilson PW, Evans JC. Association of HbA1c with prevalent cardiovascular disease in the original cohort of the Framingham Heart Study. Diabetes. 1992;41:202–8. doi: 10.2337/diab.41.2.202. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda N, Hara H, Hiroi Y, Nakamura M. Gender specific difference of association between glycated hemoglobin and prevalence or complexity of coronary artery disease. J Am Coll Cardiol. 2014;63:A1540. [Google Scholar]
- 15.Jia EZ, An FH, Chen ZH, Li LH, Mao HW, Li ZY, et al. Hemoglobin A1c risk score for the prediction of coronary artery disease in subjects with angiographically diagnosed coronary atherosclerosis. Cell Physiol Biochem. 2014;34:672–80. doi: 10.1159/000363032. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Han Z, Hao G, Li Y, Dong X, Wang C. Hemoglobin A1c level is not related to the severity of atherosclerosis in patients with acute coronary syndrome. Dis Markers. 2015;2015:192108. doi: 10.1155/2015/192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Yang YM, Zhu J, Tan HQ, Liang Y, Li JD. Prognostic significance of hemoglobin A1c level in patients hospitalized with coronary artery disease. A systematic review and meta-analysis. Cardiovasc Diabetol. 2011;10:98. doi: 10.1186/1475-2840-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ertem AG, Bağbanc H, Kiliç H, Yeter E, Akdemir R. Relationship between HbA1c levels and coronary artery severity in nondiabetic acute coronary syndrome patients. Türk Kardiyol Dern Ars. 2013;41:389–95. doi: 10.5543/tkda.2013.95666. [DOI] [PubMed] [Google Scholar]
- 19.Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus:Distinct or continuum?Indian J Endocrinol Metab. 2016;20:546–51. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fawwad A, Mustafa N, Zafar AB, Khalid M. Incidence of microvascular complications of type 2 diabetes:A 12-year longitudinal study from Karachi-Pakistan. Pak J Med Sci. 2018;34:1058–63. doi: 10.12669/pjms.345.15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prakash B, Yadav LK. A study of micro vascular complications and associated risk factors in newly diagnosed patients of type 2 diabetes mellitus. Int J Community Med Public Health. 2018;5:2338–43. [Google Scholar]
- 22.Unnikrishnan R, Anjana R, Mohan V. Diabetes mellitus and its complications in India. Nat Rev Endocrinol. 2016;12:357–70. doi: 10.1038/nrendo.2016.53. [DOI] [PubMed] [Google Scholar]


