Abstract
Thyroid hormone exerts effects across all organ systems. Hence, patients with thyroid dysfunction are at a risk of numerous complications. The stresses encountered during the perioperative period may exacerbate underlying thyroid disorders, potentially precipitating decompensation, and even death. Thus, it is of the utmost importance for the clinician to comprehend the mechanisms by which thyroid disease may complicate surgery and postoperative recovery and to optimize the status of thyrotoxic and hypothyroid patients. This article describes the adverse effects of thyroid dysfunction in patients undergoing nonthyroid surgery and recommends treatment approaches aimed at appropriate build-up to decrease perioperative risk.
Keywords: Hyperthyroid, hypothyroid, nonthyroid surgery, perioperative management, thyrotoxicosis
INTRODUCTION
Thyroid dysfunction is the second most common endocrine disorder after diabetes. The prevalence of hypothyroidism in India is reported to 10.95% and of subclinical hypothyroidism 8.02%.[1] Hyperthyroidism is prevalent in 0.67% and subclinical hyperthyroidism in 1.27% of the general population.[1] Due to the multisystem effects of thyroid hormone, the effects of thyroid dysfunction are manifold and may complicate surgical procedures and postoperative recovery. The ideal way to mitigate excess perioperative surgical risk would be by rendering the patient euthyroid. However, in situations where emergent surgery needs to be performed, achieving euthyroidism may not be possible. Build-up should begin as soon as surgery is planned and thyroid dysfunction is detected. Surgeons and anesthetists should be cognizant of the complications that may arise and choose the anesthetic agents and analgesics accordingly. This article will address the issues concerning the perioperative management of thyroid disease in patients with hypothyroidism and hyperthyroidism who are undergoing nonthyroid surgery.
PREOPERATIVE SCREENING
Routine preoperative thyroid function testing is not recommended for all patients undergoing surgery but may be performed if there is a history of symptoms or clinical signs suggesting underlying thyroid disease. In patients with known hypothyroidism or hypothyroidism who have been undergoing treatment, a thyroid function test should be included in the preoperative assessment to determine the adequacy of treatment and to ensure that thyroid therapy is optimized before surgery. In patients with well-compensated thyroid disease, if the patient is on a stable dose of medication and euthyroidism was documented within the past three to six months, additional testing before surgery is not necessary.[2] Thyroid function testing may also be useful in patients undergoing neurosurgery for sellar and even nonsellar masses due to a higher risk of central hypothyroidism. Central hypothyroidism may be more prevalent in patients with traumatic brain injury and cerebrovascular accidents and hence may be performed in this subset of patients irrespective of symptoms.[3]
COMPLICATIONS OF HYPOTHYROIDISM
The cardiovascular concerns are among the most relevant in perioperative situations. Hypothyroidism decreases cardiac output by 30% to 50%, with both slowing of the pulse and decreased contractility.[4] Loss of direct vasodilatory effect of T3 on vascular smooth muscles leads to an increase in peripheral vascular resistance, resulting in an increase in diastolic pressure, increased cardiac afterload, and a decreased pulse pressure.[5,6] Hypothyroid patients have a depressed adrenergic tone, despite increased catecholamine levels due to downregulation of b-adrenergic receptors.[7] A decreased cardiac output coupled with reduced baroreceptor responsiveness predisposes hypothyroid patients to develop hypotension under anesthesia. Perioperative major adverse cardiovascular events may occur, possibly due to increased cholesterol levels, prolonged half-life of multiple coagulation factors, and anemia.[8,9,10,11] Nonspecific ST wave in electrocardiogram (ECG) changes and low voltage on electrocardiogram are observed and, less commonly, “torsade de pointes” ventricular tachycardia have been reported.[12]
In addition to the cardiovascular concerns, hypothyroid patients face additional challenges due to the ventilatory dysfunction associated with this condition. The presence of an enlarged tongue, relaxed oropharyngeal tissues, large goiter, or obesity creates airway management challenges making intubation difficult. Decreased hypoxic and hypercarbic ventilatory drive can increase sensitivity to sedatives and delay weaning in the postoperative period.[13] Effusions can occur due to extravascular fluid shifts. Impaired respiratory muscle function and reduced surfactant production worsen atelectasis and predispose to lung collapse and pneumonia in the postoperative period.[14,15] Extracellular Glycosaminoglycans (GAG) deposition worsens the coexisting hypoalbuminemia-induced decreased oncotic pressure, with resultant extracellular fluid shifts. This decreases circulatory filling pressure and renal perfusion.[16] Furthermore, hypothyroidism-induced increased antidiuretic hormone (ADH), decreased atrial natriuretic factor (ANF), and decreased activity of the renin-angiotensin-aldosterone system (RAAS) lead to hyponatremia.[17] Hyponatremia will depress sensorium and increase the effect of sedatives. Impaired renal clearance will prolong the action of anesthetics, opioids, and other drugs.[18,19] Hypothyroidism-induced atony and hypomobility of the gastrointestinal tract will compound the surgery-induced paralytic ileus and delay initiation of enteral nutrition.[20] Bowel wall edema also impairs absorption of amino acids and sugars. At times, megacolon can occur, with increased mortality.
Hypothyroidism is associated with several hematologic effects. Most commonly described is a normochromic, normocytic anemia.[10] However, because of the increased prevalence of pernicious anemia among patients with hypothyroidism, concomitant pernicious anemia may cause macrocytosis.[21] Microcytic anemia secondary to iron deficiency can also be seen if menorrhagia develops secondary to hypothyroidism. Coagulation defects include a decrease in factor VIII activity, prolonged partial thromboplastin time, and acquired von Willebrand disease.[22,23] This increases the risk of surgical site bleeding. On the other hand, half-life of coagulation factors, such as factor II, VII, and X is prolonged.[24] This necessitates prolonged anticoagulation with heparin and a higher dose of warfarin for deep vein thrombosis prophylaxis in postsurgery. The most dreaded complication of surgery in hypothyroid patients is myxedema coma, associated with a high mortality up to 80%.[11] Myxedema coma is characterized by altered mental status, which may manifest as coma or seizure, and hypothermia, bradycardia, hyponatremia, heart failure, and hypopnea.
PERIOPERATIVE MANAGEMENT OF HYPOTHYROIDISM
In elective surgeries, postpone surgery until euthyroidism is achieved. A full replacement dose of 1.6 mg/kg/day can be initiated except in the elderly or those with known coronary artery disease where the initial dose is usually 25 mg daily, with a planned increase every 2 to 6 weeks until a euthyroid state is attained. For elderly, it is advisable to start at lower dose with gradual uptitration to target serum thyroid-stimulating hormone (TSH) to 4-6 mIU/L in persons aged more than 70-80 years. Pregnant women with overt hypothyroidism should receive levothyroxine replacement therapy with the dose titrated to achieve a thyrotropin concentration within the trimester-specific reference range. Pediatric dosage are age based, with newborns typically requiring 10 mg/kg/d, 1-year-old children 4-6 mg/kg/d, and adolescents 2-4 mg/kg/d, with transition to the average adult dose of 1.6 mg/kg/d. The treatment goals of hypothyroidism are the same for patients with psychosocial, behavioral, and mental health conditions, as for the general population. In patients with secondary hypothyroidism, treatment goal should be to maintain the serum-free thyroxine values in the upper half of the reference range.[25] Consensus on treatment of subclinical hypothyroidism is variable. The American Thyroid Association guidelines for hypothyroidism in adults recommend starting thyroid hormone treatment when serum TSH is more than 10 mIU/L and considering treatment in those with increased cardiovascular disease risk when the serum TSH is 4.5-10 mIU/L.[25] European thyroid association (ETA) 2013 recommends replacement therapy with L-thyroxine is recommended for younger patients (aged <65-70 years) with serum TSH >10 mU/L and a trial in younger Subclinical hypothyroidism (SCH) patients (serum TSH <10 mU/L) with symptoms suggestive of hypothyroidism. In old patients (aged >80-85 years) with elevated serum TSH ≤10 mU/L, a wait-and-see strategy is recommended and local age-specific reference range may be used.[26] The dose of L-thyroxine is usually lower in subclinical hypothyroidism.
In cases of urgent surgery, proceedings with surgery may be fraught with complications discussed above. In a retrospective study,[27] 40 hypothyroid surgical patients, most of whom had mild to moderate severe hypothyroidism, were compared with 80 euthyroid surgical patients. Among those undergoing noncardiac surgery, intraoperative hypotension occurred at a higher rate in the hypothyroid group. For those undergoing cardiac surgery, heart failure was more prevalent in the hypothyroid group. Also, hypothyroid patients had a higher rate of gastrointestinal and neuropsychiatric complications. Hypothyroid patients were less likely to be febrile, although the rate of postoperative infection was similar in both the groups. No differences were noted in perioperative blood loss, duration of hospitalization, rates of arrhythmia, hypothermia, hyponatremia, delayed recovery from anesthesia, tissue integrity, wound healing, pulmonary complications, or death. Another retrospective study[28] compared the outcome of anesthesia and surgery in 59 hypothyroid patients with 50 euthyroid patients. There were no differences in duration of surgery or anesthesia, lowest temperature and blood pressure recorded during surgery, need for vasopressors, time to extubation, fluid and electrolyte imbalances, incidence of arrhythmias, pulmonary or myocardial infarction, sepsis, need for postoperative respiratory assistance, bleeding complications, or time to hospital discharge. A subgroup analysis of patients based on their thyroxine levels (thyroxine level <1.0 mg/dL, 1.0 to <3.0 mg/dL, and ≥3.0 mg/dL) also revealed no differences in outcomes. The study concluded that in mild to moderate hypothyroidism, there is no evidence to justify deferring needed surgery, but in severe hypothyroidism, there is insufficient evidence to make a recommendation because there were only seven patients in the group with the lowest T4 concentration. In a prospective study[29] comparing postoperative outcomes in patients with subclinical hypothyroidism, undergoing coronary artery bypass grafting, there was an increase in the rate of postoperative atrial fibrillation in the subclinical hypothyroidism group. But no increase in major adverse cardiovascular events, wound problems, mediastinitis, leg infection, respiratory complications, delirium, or reoperation during the same hospitalization was noted.
To summarize, hypothyroid patients may be stratified on the basis of severity of hypothyroidism [Figure 1]. Mild hypothyroidism includes patients with subclinical hypothyroidism, defined biochemically as a normal serum-free T4 concentration in the presence of an elevated serum TSH concentration. Moderate hypothyroidism includes all patients with overt hypothyroidism (elevated TSH, low free T4) without the features of severe hypothyroidism. Severe hypothyroidism includes patients with severe clinical symptoms of chronic hypothyroidism such as altered mentation, pericardial effusion, or heart failure, those with very low levels of total thyroxine (T4) (less than 1.0 mcg/dL) or free T4 (less than 0.5 ng/dL) or myxedema coma. Mild and moderate hypothyroid patients may undergo urgent surgery without delay, with the knowledge that minor perioperative complications might develop. For severe hypothyroid, the patient should be treated as soon as the diagnosis is made. T4 is given in a loading dose of 200 to 300 mcg intravenously (IV) followed by 50 mcg daily IV.[30] If there is concern about existing or precipitating myxedema coma, preferably treat patients with both triiodothyronine (T3) and T4 to rapidly normalize thyroid function. T3 is given simultaneously in a dose of 5 to 20 mcg IV followed by 2.5 to 10 mcg every eight hours depending upon the patient’s age and coexistent cardiac risk factors. If the status of the pituitary adrenal axis is uncertain and deficiency is considered likely, patients should be given stress doses of corticosteroids until the integrity of the axis is ascertained.[31] If IV levothyroxine is not available, then oral LT4 regimen consisting of a loading dose of 300-500 mg, followed by taper over the next 3-5 days can be administered.[32] Patients who require cardiac revascularization are the only subset of patients who may not benefit from preoperative replacement of thyroid hormone. The risk of precipitating or worsening unstable coronary syndromes with thyroid hormones (by increasing myocardial oxygen demand) conflicts with the concern that untreated hypothyroidism might worsen heart failure or hypotension in the cardiac surgical patient. As retrospective and prospective studies of cardiac patients undergoing cardiac surgery or catheterization found no increase in the rate of adverse events in those patients whose hypothyroidism had not been treated, it is reasonable to proceed with the revascularization procedure before repleting thyroid hormone.[33,34]
Figure 1.
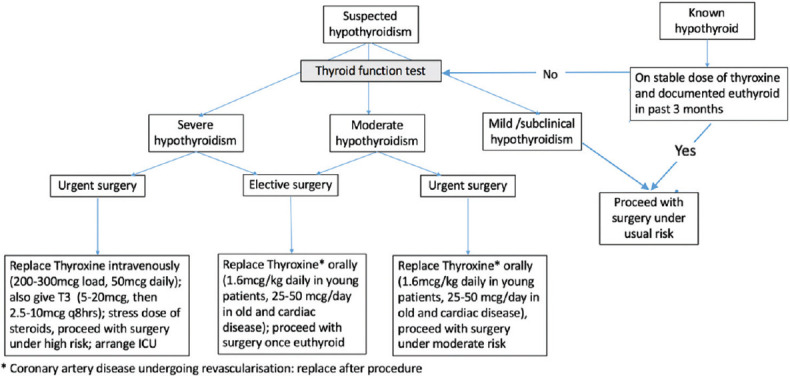
Approach to a patient with suspected or diagnosed hypothyroidism
During surgery, Ketamine may be the induction agent of choice because it increases sympathetic activity and is a positive inotrope [Table 1].[31] Thiopental is not an optimal choice because of its effects on systemic vascular resistance and its antithyroid properties.[35] Rapid-sequence induction with succinylcholine should be considered because of delayed gastric emptying. Rapid-acting nondepolarizing muscle relaxants may also be used, but because of the potential for decreased metabolism and baseline muscle weakness, there may be prolonged paralysis. Anesthesia may be maintained with nitrous oxide and short-acting opioid supplementation with or without volatile anesthetics.[31] During maintenance of anesthesia, particular vigilance must be paid to signs of cardiac depression, prolonged skeletal muscle paralysis, and hypothermia. Peripheral arterial catheterization and measurement of cardiac filling pressures are recommended for patients with severe hypothyroidism if surgery is expected to be of long duration or associated with significant blood loss. Non-narcotic analgesics may be preferred over opioids because of prolonged effects.
Table 1.
Preferred anaesthetic agents during general anaesthesia in thyroid dysfunction[31]
| Hypothyroidism | Hyperthyroidism | |
|---|---|---|
| Induction Agent | Ketamine | Sodium thiopental |
| Maintenance Agent | Nitrous oxide and short acting opioids/non-narcotic analgesics | Isoflurane |
| Intubation | Rapid-sequence induction with succinylcholine or rapid acting non-depolarizing muscle relaxants | Succinylcholine or a non-depolarizing muscle relaxant |
| Reversal | Glycopyrrolate preferred over atropine |
Postoperatively, if oral intake cannot be resumed in five to seven days, then T4 should be given IV or intramuscularly. The dose should be approximately 70% to 80% of the patient’s usual oral dose because that is approximately the fraction of oral T4 that is absorbed. Patients who were receiving chronic T4 therapy and are unable to eat for several days need not be given T4 parenterally.[36] Another challenge in the postoperative period is the development of nonthyroidal illness syndrome/low T3 syndrome, which is commonly seen following major surgeries such as coronary artery bypass grafting. Diagnosis should be considered in patients with low free T3 and/or free T4 with normal/low TSH. There is no conclusive evidence to treat nonthyroidal illness syndrome, unless prolonged and severe which may then be treated with Thyrotropin releasing hormone (TRH). The only exception to this is patients with head injury where low T3 may be indicative of central hypothyroidism.[37]
HYPERTHYROIDISM
Cardiovascular effects have been particularly well documented and include increases in resting heart rate, stroke volume, and cardiac output by 50%-300%.[38] Hyperthyroidism increases the risk of worsening high output heart failure. Surgical stress can compound pre-existing risk of atrial fibrillation, especially in older patients.[39] New onset arrhthymias together with an increased cardiac workload can worsen angina and even result in acute coronary event.[31,40] Metabolic derangements (hypokalemia) and the catabolic state associated with severe hyperthyroidism can cause weakness of respiratory muscles. This respiratory muscle weakness along with hypercarbia due to an increased basal oxygen consumption can result in prolonged mechanical ventilation. Increased calorigenesis, hyperthermia predisposes to dehydration. In addition, increased gut motility associated with hyperdefecation and malabsorption worsen the catabolic state during surgery.[31] Most sinistral complication is thyroid storm, characterized by cardiovascular, gastrointestinal, thermoregulatory, and central nervous system symptoms and a high mortality ranging from 10%-30%.[41]
PERIOPERATIVE MANAGEMENT OF HYPERTHYROIDISM
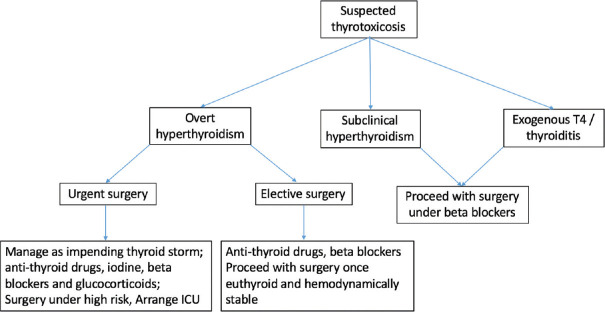
The severity of thyrotoxicosis may be described as mild (subclinical) hyperthyroidism defined as a low TSH with normal free T4 and T3 or overt, defined as a suppressed TSH with elevated free T4 and/or T3 concentrations [Figure 2]. Patients scheduled for elective surgery should be made euthyroid before surgery (this usually requires weeks) and cardiovascular stability should be ensured. In case of urgent surgery, administer a beta blocker (e.g., atenolol 25 to 50 mg daily) preoperatively to older patients (aged >50 years), or younger patients with cardiovascular disease, especially atrial arrhythmias, and taper after recovery. For overtly thyrotoxic patients in whom surgery cannot be postponed, optimized treatment plan directed against hormone synthesis and secretion by thyroid gland (thionamides, iodine), against peripheral sympathetic effects of thyroxine (beta blockers, corticosteroids) and against systemic decompensation (fluids, nutrition), should be administered.[42,43] If hyperthyroidism is due to Graves’ disease, toxic adenoma, or multinodular goiter, thionamides should be initiated with the aim of controlling hyperthyroidism in the postoperative period. Thionamides block de novo thyroid hormone synthesis but have no effect upon the release of preformed hormone from the thyroid gland and will therefore not have a significant effect on thyroid hormone levels over only a few preoperative days.[43] Methimazole is usually preferred to propylthiouracil, except during the first trimester of pregnancy, because of its longer duration of action (allowing for single-daily dosing) and a lesser degree of toxicity.[44] Dosing depends on the degree of hyperthyroidism (biochemical and clinical) and goiter size. Propylthiouracil (100 to 150 mg every six to eight hours) is preferred by some clinicians for the initial treatment of thyroid storm because it reduces T4-to-T3 conversion.[44] For patients who cannot take orally, rectal administration of thionamides, in the form of enema or suppository may be administered.[45,46,47] Iodine is the only medication that inhibits the release of thyroid hormones and is very useful in the urgent treatment of thyrotoxicosis. However, iodine should be given only after antithyroid drugs (at least one hour later) to avoid a thyroid hormone surge. In patients with Graves’ disease, however, exogenous iodine is unlikely to exacerbate hyperthyroidism by acting as substrate and therefore can be used alone with beta blockers in patients with thionamide intolerance. But in patients with toxic adenoma or multinodular goiter, iodine can exacerbate hyperthyroidism unless iodine organification has been fully blocked by pretreatment with a thionamide. Thus, thionamide therapy should therefore be started first and continued without interruption, preferably in divided dosing. In the absence of contraindications, beta blockers should be administered. The longer-acting beta blockers (e.g., atenolol) are preferred because an oral dose taken one hour before surgery will usually maintain adequate beta blockade until the patient is able to take oral medications postoperatively.[48] Start with atenolol 25 to 50 mg daily and increase the dose as needed to maintain the pulse rate less than 80 beats/minute; up to 200 mg daily may be needed for the symptomatic treatment of hyperthyroidism and control of tachycardia. IV propranolol (0.5 to 1 mg over 10 minutes followed by 1 to 2 mg over 10 minutes every few hours) can be used to control fever, hypertension, and tachycardia intraoperatively.[49] Beta blockers should be continued until the patient’s thyroid disease is under control. Patients with relative contraindications to beta blockade may better tolerate beta-1-selective agents, such as atenolol or metoprolol.[48] Calcium channel blockers can also be used for rate control in patients in whom beta blockers are contraindicated. Glucocorticoids may also be given because these agents are thought to decrease the release and peripheral conversion of thyroid hormone.[50] Moreover, in the setting of thyrotoxicosis, glucocorticoids may be necessary to treat relative adrenal insufficiency. Cholestyramine is an additional modality that may be used to rapidly lower thyroid hormone levels in thyrotoxic patients by binding thyroid hormone in the intestine and decreasing its reabsorption. In cases of thyrotoxicosis due to exogenous thyroid hormone or thyroiditis, no specific targeted therapy is indicated other than β-blockade or calcium channel blockade, as needed, to stabilize cardiovascular status, as the excess hormone will get metabolized with the passage of time.
Figure 2.
Approach to a patient with suspected or diagnosed thyrotoxicosis
During surgery, sodium thiopental may be a preferred induction agent because it has antithyroid activity [Table 1].[35] Drugs such as ketamine and ephedrine that increase sympathetic tone should be avoided. Succinylcholine or a nondepolarizing muscle relaxant devoid of having deleterious cardiovascular effects is acceptable for facilitation of intubation after induction. When reversing neuromuscular blockade, anticholinergics (atropine or glycopyrrolate) may lead to exaggerated sympathetic responses, particularly profound tachycardia. Glycopyrrolate, which has less chronotropic effect than atropine, may be the preferred choice.[35]
Postoperatively, patients who are taking a thionamide preoperatively (whether chronic or recently started), who will not be able to take oral medications for longer than a day or two postoperatively, can be treated with rectal preparations [Table 2]. The decision to use rectal methimazole postoperatively depends on the patient’s clinical status and the availability of rectal preparations.[46] Patients well controlled on long-term methimazole therapy (TSH is normal), there is usually a delay of at least 7 to 10 days before patients develop recurrent hyperthyroidism after omission of treatment. In patients on long-term treatment for more than 6 to 12 months, recurrence after stopping treatment may take weeks or months, especially if thyroid-stimulating immunoglobulins are no longer elevated. If antipyretics are needed, aspirin must be avoided, because this may cause an increase in thyroid hormone bioavailability by causing displacement of T4 from thyroid-binding globulin.[31] As thyroid storm can occur during surgery and postoperatively, its prevention, prompt identification, and management must be done to decrease mortality.
Table 2.
| Methimazole | Propylthiouracil |
|---|---|
| Suppository: Dissolve 1200 mg methimazole in 12 mL of water and add to 52 mL cocoa butter containing two drops of polysorbate (Span) 80. Stir mixture to form an emulsion and pour into 2.6 mL suppository molds to cool. |
Suppository: For Dissolve 200 mg of propylthiouracil in a polyethylene glycol base and put into suppository tablets. Retention enema: Dissolve eight 50 mg tablets of propylthiouracil in 60 mL of mineral oil enema (eg, Fleet mineral oil) or in 60 mL of sodium phosphates enema solution* (eg, Fleet enema phospho soda) |
CONCLUSION
The ubiquitous effects of thyroid hormone on multiple organ systems predispose patients with either hypothyroidism or hypothyroidism to specific perioperative complications, some of which can be severe or even fatal. Thus, the goal of therapy in the perioperative patient should be to achieve a euthyroid state and when that is not feasible, to use other measures that will increase hemodynamic stability and prevent decompensation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Unnikrishnan AG, Kalra S, Sahay RK, Bantwal G, John M, Tewari N. Prevalence of hypothyroidism in adults:An epidemiological study in eight cities of India. Indian J Endocrinol Metab. 2013;17:647–52. doi: 10.4103/2230-8210.113755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palace MR. Perioperative management of thyroid dysfunction. Health Serv Insights. 2017;10:1178632916689677. doi: 10.1177/1178632916689677. doi:10.1177/1178632916689677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Persani L, Becck-Peccoz P. 11th ed. J. B.Lippincott Co; Philadelphia: 2021. Central hypothyroidism. Werner &Ingbar's The Thyroid:A Fundamental and Clinical Text; pp. 566–74. [Google Scholar]
- 4.Anthonisen P, Holst E, Thomsen AA. Determination of cardiac output and other hemo- dynamic data in patients with hyper- and hypothyroidism, using dye dilution technique. Scand J Clin Lab Invest. 1960;12:472–80. doi: 10.3109/00365516009065412. [DOI] [PubMed] [Google Scholar]
- 5.Ojamaa K, Balkman C, Klein I. Acute effects of triiodothyronine on arterial smooth muscle cells. Ann Thorac Surg. 1993;56:S61–7. doi: 10.1016/0003-4975(93)90556-w. [DOI] [PubMed] [Google Scholar]
- 6.Park KW, Dai HB, Ojamaa K, Lowenstein E, Klein I, Sellke FW. The direct vasomotor effect of thyroid hormones on the skeletal hormones on rat skeletal muscle resistance arteries. Anesth Analg. 1997;85:734–8. doi: 10.1097/00000539-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Bilezikian JP, Loeb JN. The influence of hyperthyroidism and hypothyroidism on a and b-adrenergic receptor systems and adrenergic responsiveness. Endocr Rev. 1983;4:378–88. doi: 10.1210/edrv-4-4-378. [DOI] [PubMed] [Google Scholar]
- 8.Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women:The Rotterdam study. Ann Intern Med. 2000;132:270–8. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- 9.Diekman T, Lansberg PJ, Kastelein JJ, Wiersinga WM. Prevalence and correction of hypothyroidism in a large cohort of patients referred for dyslipidemia. Arch Intern Med. 1995;155:1490–5. [PubMed] [Google Scholar]
- 10.Loeliger EA, van der Esch B, Mattern MJ, Hemker HC. The biological disappearance rate of prothrombin, factors VII, IX, and X from plasma in hypothyroidism, hyperthyroidism and during fever. Thromb Diath Haemorrh. 1964;10:267–77. [PubMed] [Google Scholar]
- 11.Axelrod AR, Berman L. The bone marrow in hyperthyroidism and hypothyroidism. Blood. 1951;6:436–53. [PubMed] [Google Scholar]
- 12.Fredlund BO, Olsson SB. Long QT interval and ventricular tachycardia of a “torsade de pointe” type in hypothyroidism. Acta Med Scand. 1983;213:231–5. doi: 10.1111/j.0954-6820.1983.tb03724.x. [DOI] [PubMed] [Google Scholar]
- 13.Ingbar DH. The pulmonary system in hypothyroidism. In: Braverman LE, Utiger RD, editors. Werner & Ingbar's The Thyroid:A Fundamental and Clinical Text. 8th ed. J. B. Lippincott Co; Philadelphia: 2000. pp. 783–9. [Google Scholar]
- 14.Gosselin LE, Zhan W-Z, Sieck GC. Hypothyroid-mediated changes in adult rat diaphragm muscle contractile properties and MHC isoform expression. J Appl Physiol. 1996;80:1934–9. doi: 10.1152/jappl.1996.80.6.1934. [DOI] [PubMed] [Google Scholar]
- 15.Dulchavsky SA, Bailey J. Triiodothyronine treatment maintains surfactant synthesis during sepsis. Surgery. 1992;112:475–9. [PubMed] [Google Scholar]
- 16.DeRubertis FR, Jr, Michelis MF, Bloom ME, Bloom ME, Mintz DH, Field JB, et al. Impaired water excretion in myxedema. Am J Med. 1971;51:41–53. doi: 10.1016/0002-9343(71)90322-6. [DOI] [PubMed] [Google Scholar]
- 17.Park CW, Shin SY, Ahn JS, Kim SY, Choi EJ, Chang YS, et al. Thyroxine treatment induces upregulation of renin-angiotensin-aldosterone system due to decreasing effective plasma volume in patients with primary myxedema. Nephrol Dial Transplant. 2001;16:1799–806. doi: 10.1093/ndt/16.9.1799. [DOI] [PubMed] [Google Scholar]
- 18.Kim JM, Hackman L. Anesthesia for untreated hypothyroidism:Report of three cases. Anesth Analg. 1977;56:299–302. [PubMed] [Google Scholar]
- 19.Abbot TR. Anaesthesia in untreated myxedema. Br J Anaesth. 1967;39:510–4. doi: 10.1093/bja/39.6.510. [DOI] [PubMed] [Google Scholar]
- 20.Bastenie PA. Paralytic ileus in severe hypothyroidism. Lancet. 1946;1:413–416. doi: 10.1016/s0140-6736(46)90364-9. [DOI] [PubMed] [Google Scholar]
- 21.Hines JD, Halsted CH, Griggs RC, Harris JW. Megaloblastic anemia secondary to folate deficiency associate with hypothyroidism. Ann Intern Med. 1968;68:792–805. doi: 10.7326/0003-4819-68-4-792. [DOI] [PubMed] [Google Scholar]
- 22.Simone JV, Abildgaard CF, Schulman I. Blood coagulation in thyroid dysfunction. N Engl J Med. 1965;273:1057–61. doi: 10.1056/NEJM196511112732001. [DOI] [PubMed] [Google Scholar]
- 23.Dalton RG, Dewar MS, Savidge GF, Kernoff PB, Matthews KB, Greaves M, et al. Hypothyroidism as a cause of acquired von Willebrand's disease. Lancet. 1987;1:1007–9. doi: 10.1016/s0140-6736(87)92272-0. [DOI] [PubMed] [Google Scholar]
- 24.Mathew V, Misgar RA, Ghosh S, Mukhopadhyay P, Roychowdhury P, Pandit K, et al. Myxedema coma:A new look into an old crisis. J Thyroid Res. 2011;2011:493462. doi: 10.4061/2011/493462. doi:10.4061/2011/493462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism:Prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce SH, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S, et al. 2013 ETA Guideline:Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215–28. doi: 10.1159/000356507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ladenson PW, Levin AA, Ridgeway EC, Daniels GH. Complications of surgery in hypothyroid patients. Am J Med. 1984;77:261–6. doi: 10.1016/0002-9343(84)90701-0. [DOI] [PubMed] [Google Scholar]
- 28.Weinberg AD, Brennan MD, Gorman CA, Marsh HM, O’Fallon WM. Outcome of anesthesia and surgery in hypothyroid patients. Arch Intern Med. 1983;143:893–7. [PubMed] [Google Scholar]
- 29.Park YJ, Yoon JW, Kim KI, Lee YJ, Kim KW, Choi SH, et al. Subclinical hypothyroidism might increase the risk of transient atrial fibrillation after coronary artery bypass grafting. Ann Thorac Surg. 2009;87:1846–52. doi: 10.1016/j.athoracsur.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 30.Bennet-Guerrero E, Kramer DC, Schwinn DA. Effect of chronic and acute thyroid hormone reduction on perioperative outcome. Anesth Analg. 1997;85:30–6. doi: 10.1097/00000539-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Graham GW, Unger BP, Coursin DB. Perioperative management of selected endocrine disorders. Int Anesthesiol Clin. 2000;38:31–67. doi: 10.1097/00004311-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Rajendran A, Bhavani N, Nair V, Pavithran PV, Menon VU, Kumar H. oral levothyroxine is an effective option for myxedema coma:A single-centre experience. Eur Thyroid J. 2021;10:52–8. doi: 10.1159/000507855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drucker DJ, Burrow GN. Cardiovascular surgery in the hypothyroid patient. Arch Intern Med. 1985;145:1585–7. [PubMed] [Google Scholar]
- 34.Myerowitz PD, Kamienski RW, Swanson DK, Chopra PS, Berkoff HA, Kroncke GM, et al. Diagnosis and management of the hypothyroid patient with chest pain. J Thorac Cardiovasc Surg. 1983;86:57–60. [PubMed] [Google Scholar]
- 35.Stoelting RK, Dierdorf SF. Endocrine disease. In: Stoelting RK, Dierdorf SF, editors. Anesthesia and Co-Existing Disease. 3rd ed. Churchill Livingstone; New York: 1993. pp. 347–51. [Google Scholar]
- 36.Himes CP, Ganesh R, Wight EC, Simha V, Liebow M. Perioperative evaluation and management of endocrine disorders. Mayo Clin Proc. 2020;95:2760–74. doi: 10.1016/j.mayocp.2020.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Biondi B, Wartofsky L. Treatment with thyroid hormone. Endocr Rev. 2014;35:433–512. doi: 10.1210/er.2013-1083. [DOI] [PubMed] [Google Scholar]
- 38.Kohl BA, Schwartz S. Surgery in the patient with endocrine dysfunction. Med Clin North Am. 2009;93:1031–47. doi: 10.1016/j.mcna.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Sawin CT, Geller A, Wolf P, Belanger AJ, Baker E, Bacharach P, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–252. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 40.Parker JLW, Lawson DH. Death from thyrotoxicosis. Lancet. 1973;2:894–895. doi: 10.1016/s0140-6736(73)92019-9. [DOI] [PubMed] [Google Scholar]
- 41.Chiha M, Samarasinghe S, Kabaker AS. Thyroid storm:An updated review. J Intensive Care Med. 2015;30:131–40. doi: 10.1177/0885066613498053. [DOI] [PubMed] [Google Scholar]
- 42.Burman KD, Cooper DS. Evaluation and management of hyperthyroidism. In: Cooper DS, editor. Medical Management of Thyroid Disease. New York: Marcel Dekker Inc; 2001. pp. 33–92. [Google Scholar]
- 43.Baeza A, Aguayo J, Barria M, Pineda G. Rapid preoperative preparation in hyperthyroidism. Clin Endocrinol (Oxf) 1991;35:439–42. doi: 10.1111/j.1365-2265.1991.tb03562.x. [DOI] [PubMed] [Google Scholar]
- 44.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26:1343–421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 45.Yeung SC, Go R, Balasubramanyam A. Rectal administration of iodide and propylthiouracil in the treatment of thyroid storm. Thyroid. 1995;5:403–5. doi: 10.1089/thy.1995.5.403. [DOI] [PubMed] [Google Scholar]
- 46.Nabil N, Miner DJ, Amatruda JM. Methimazole:An alternative route of administration. J Clin Endocrinol Metab. 1982;54:180–1. doi: 10.1210/jcem-54-1-180. [DOI] [PubMed] [Google Scholar]
- 47.Walter RM, Bartle WR. Rectal administration of propylthiouracil in the treatment of Graves'disease. Am J Med. 1990;88:69–70. doi: 10.1016/0002-9343(90)90130-6. [DOI] [PubMed] [Google Scholar]
- 48.Gerst PH, Fildes J, Baylor P, Zonszein J. Long-acting beta-adrenergic antagonists as preparation for surgery in thyrotoxicosis. Arch Surg. 1986;121:838–40. doi: 10.1001/archsurg.1986.01400070108022. [DOI] [PubMed] [Google Scholar]
- 49.Shanks RG, Hadden DR, Lowe DC, McDevitt DG, Montgomery DA. Controlled trial of propranolol in thyrotoxicosis. Lancet. 1969;1:993–4. doi: 10.1016/s0140-6736(69)91797-8. [DOI] [PubMed] [Google Scholar]
- 50.Langley RW, Burch HB. Perioperative management of the thyrotoxic patient. Endocrinol Metab Clin North Am. 2003;32:519–34. doi: 10.1016/s0889-8529(03)00010-0. [DOI] [PubMed] [Google Scholar]