Abstract
Background:
Although hypertriglyceridemia (HTG) is a well-established cause of acute pancreatitis (AP), there are no definitive management guidelines. Studies comparing clinical severity and outcome of hypertriglyceridemia-induced acute pancreatitis (HTGAP) and non- HTGAP are scarce. Hence, the present study was undertaken.
Materials and Methods:
All consecutive patients admitted with AP from January 2017 to August 2021 at university teaching hospital were included in this study. Data with regards to patient demographics; clinical, laboratory, and radiologic parameters; management strategies; and outcome were collected and compared between HTGAP and non-HTGAP patients.
Results:
Overall, 550 patients with AP were admitted during the study period, of which 21 (3.8%) were HTG related. Mean age of HTGAP patients was 34.3 years (M: F = 14:7), and the mean serum triglyceride (TG) levels on admission were 3,718.9 mg/dL (range 1,094–11,991). Insulin infusion therapy was used in 18 patients with HTGAP and the target TG levels of ≤500 mg/dL was achieved in 4.2 days (mean). Compared to non-HTGAP patients, HTGAP patients had higher body mass index (29.2 vs. 25.6), higher clinical (BISAP 2.6 vs. 2.06) and radiologic severity scores (CT severity score 7.5 v/s 4.8), and required prolonged hospital stay (12.9 vs. 6.5 days).
Conclusion:
HTGAP occurred in young patients with high BMI and was associated with more severe disease, that required prolonged hospitalization than patients with non-HTGAP. Insulin infusion therapy was effective in reducing serum TG levels.
Keywords: Body mass index, hypertriglyceridemia, insulin, pancreatitis
INTRODUCTION
Hypertriglyceridemia (HTG) accounts for approximately 1 to 14% of all cases of acute pancreatitis (AP) and is the third most common etiology for AP after alcohol and gallstones.[1,2,3,4,5] Typically, triglyceride (TG) levels >1,000 mg/dL are thought to precipitate AP with a risk of around 5% that increases to 10–20% when TG levels exceed 2,000 mg/dL.[6] There is a regional variation in the occurrence of HTGAP and its prevalence seems to be increasing given the change in dietary habits, sedentary lifestyle, alcohol consumption, obesity, and diabetes mellitus.[3] There are no accepted guidelines for treatment of HTGAP, and the effectiveness of various interventions such as insulin and/or heparin infusion and plasmapheresis has been reported mostly in small case series and case reports.[6] Hence, this prospective study was undertaken at our tertiary referral center to assess the epidemiology, clinical features, and outcomes of HTGAP in comparison to non-HTGAP.
MATERIALS AND METHODS
In this prospective study, we enrolled consecutive patients diagnosed with acute pancreatitis (AP) who presented to the Emergency Department within 48 h of symptom onset. The study period extended from January 2017 to August 2021. The diagnostic criteria of acute pancreatitis (AP) were in accordance with the 2012 revised Atlanta classification, and hypertriglyceridemia (HTG) as a cause of pancreatitis when fasting serum TG level of ≥1,000 mg/dL.[7,8] Serum TG levels were measured as close to the onset of pain or hospital presentation as possible as serum TG levels can rapidly decrease with fasting. The clinical features and outcome of HTGAP were compared to patients with non-HTGAP. Patients who met at least two out of four criteria for systemic inflammatory response syndrome (SIRS) during their hospitalization were defined as SIRS positive.[9] CT severity index score (CTSI) and Bedside Index for Severity in Acute Pancreatitis (BISAP score which includes 1 point each for Blood urea nitrogen >25 mg/dL, Impaired mental status, SIRS, Age >60 years, and Pleural effusion) was calculated for all patients. The exclusion criteria was patients presenting after 48 h of pain onset.
All patients were treated according to standard guidelines for the management of AP which included intravenous rehydration therapy, pain control, correction of electrolyte disorders if any, and organ function support after admission.[10] In patients with “worrisome features” such as uncontrolled blood sugars with diabetic ketoacidosis, severe lactic acidosis, presence of two or more signs of SIRS, worsening organ failure, and in those patients with persistently high TG levels after 48 h of conservative management were treated with insulin infusion, at a rate of 0.1 U/kg/h until TG target level of ≤500 mg/dL was achieved, while monitoring for hypoglycemia. Plasmapheresis was utilized in whom insulin infusion could not be used due to frequent hypoglycemia. Clinical data during hospitalization were collected including demographic and laboratory data, disease severity, organ failure, length of hospital stay, and outcome following treatment. The primary therapeutic goal was reduction in the serum TGs levels to ≤500 mg/dL. The study was approved by the Institutional Ethical Committee.
Statistical analysis
The categorical variables were expressed as number with percentages and continuous variables as mean, median, and standard deviation. The categorical data were compared using Fisher’s exact test and the continuous variables were compared using the Student’s t-test and Mann–Whitney test. A P value of <0.05 was considered significant.
RESULTS
A total of 550 patients with AP due to various causes were admitted during the study period, of which HTGAP accounted for 21 patients (3.8%). The mean (±standard deviation) age of patients with HTGAP was 34.33 (±7.68) years with a male preponderance (M: F = 14:7). The baseline characteristics and laboratory findings of patients with HTGAP are as shown in Table 1. Diabetes mellitus was seen in 11 of 21 (52%) patients. The mean TG levels in patients with HTGAP was 3,718.9 mg/dL; however, there were 16 patients who had serum TG level between 500 to 1,000 mg/dL who had alcohol as a concurrent cause for pancreatitis. Overall, 14 patients with “worrisome clinical features” at admission and four patients who had no remarkable decline in their TG levels from admission levels after 48 h of conservative management were treated with insulin infusion therapy. Target level of serum TG ≤500 mg/dL was achieved with insulin infusion at a mean of 4.2 days [Figure 1]. Recurrent hypoglycemia was observed in three patients during insulin infusion therapy including one pregnant lady who underwent in plasmapheresis. In the other two patients with hypoglycemia, insulin infusion was reduced to 0.05 U/kg/h along with dextrose therapy.
Table 1.
Clinical characteristics of patients with hypertriglyceridemia-induced acute pancreatitis
| Clinical Parameter | Number |
|---|---|
| Total Number of patients | n=21 |
| Male to female ratio | 14:7 |
| Mean age±Standard deviation/range (years) | 34.33 (± 5.12), range 27 to 60 years |
| Comorbidities | |
| Diabetes mellitus | 11 |
| Hypertension | 4 |
| Hypothyroidism | 2 |
| Alcohol (social drinking only) | 6 |
| Body Mass Index | |
| Normal (18.5-24.9) | 3 |
| Overweight (25-29.9) | 11 |
| Obese (30.0 and above) | 7 |
| Mean (± SD) triglycerides level at admission | 3,718.9±2,710.83 (range 1,094 to 11,991) |
| Elevated amylase level to at least 3 times normal | 12 (57.14%) |
| Elevated lipase level to at least 3 times normal | 18 (85.7%) |
| Duration (in days) for triglycerides to fall <500 mg/dL (mean±SD) | 5.9±3.315 days (range 3 to 18 days) |
| Average duration of hospitalization (days) | 12.9 (range 3 to 60 days) |
| CT severity index score | |
| 0-3 | 0 |
| 4-6 | 6 |
| 7-10 | 15 |
| Complications | |
| Local | n=13 |
| Walled off pancreatic necrosis | 6 |
| Acute necrotic collection | 2 |
| Acute pancreatic fluid collection | 5 |
| Vascular | n=6 |
| Portal vein thrombosis | 2 |
| Splenic vein thrombosis Combined portal and splenic vein thrombosis Mesenteric vein thrombosis | 2 1 1 |
| BISAP score | |
| ≤2 | 5 |
| ≥3 | 16 |
| In-hospital mortality | Nil |
| Occurrence of recurrent pancreatitis | 2 (13.3%) |
Figure 1.
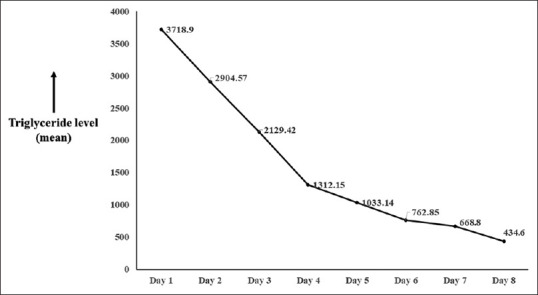
Graph showing the mean reduction in serum triglyceride levels after initiating insulin infusion
The baseline characteristics and outcome of patients with HTGAP in comparison to non- HTGAP are as shown Table 2. Patients with HTGAP were 12 years younger in comparison to non-HTGAP (34 vs. 46) and had a higher BMI (29 vs. 25). The clinical and radiologic severity index scores (BISAP and CTSI) of pancreatitis were significantly higher in patients with HTGAP and these patients required prolonged hospitalization (12 days vs. 6.5 days) in comparison to non-HTGAP. The comparison of patients with HTGAP and non-HTGAP according to different age groups [Table 3] revealed that patients with HTGAP had higher BMI, higher CTSI, and required prolonged hospitalization. All patients of HTGAP improved with therapy. Recurrent AP secondary to noncompliance with dietary restrictions and/or medications was observed in 2 patients, who presented with a serum TG >1,000 mg/dL during their subsequent admissions for recurrent AP.
Table 2.
Comparison of baseline characteristics and outcomes of patients with HTGAP and non-HTGAP
| Parameter | HTG-AP | Non-HTGAP | P |
|---|---|---|---|
| Total cases | 21 | 529 | |
| Age (Mean±Standard deviation) years | 34.3±5.12 | 46.27±19.68 | 0.005 |
| Sex | |||
| Male | 14 | 415 | NA* |
| Female | 7 | 114 | NA |
| Body mass index | 29.22±4.194 | 25.6927±5.22 | 0.004 |
| Comorbidities | |||
| Diabetes mellitus | 11 | 86 | NA |
| Hypertension | 4 | 61 | NA |
| Hypothyroidism | 2 | 11 | NA |
| Social habits | |||
| Smoking | 4 | 137 | NA |
| Alcohol | 6 (social drinking only) | 140 | NA |
| Pancreatic enzyme elevation (IU/L) | |||
| Amylase | 514±484.603 | 1,017.4788±1,433.7647 | 0.1372 |
| Lipase | 2,992.555±2,939.587 | 7,142.4840±7,982.0671 | 0.0279 |
| HDL cholesterol level (mg/dL) on admission | 15.0555±6.0631 | 34.223±18.554 | <0.0001 |
| Mean (±standard deviation) triglyceride levels on admission | 3,718.9±2,710.83 | 150.826±107.85 | <0.0001 |
| BISAP score | 2.647±0.861 | 2.067±1.199 | 0.0051 |
| CTSI score | 7.555±2.4307 | 4.8070±3.2707 | 0.0004 |
| Electrolyte levels (mean±standard deviation) | |||
| Sodium (mEq/L) | 123.611±6.408 | 126.122±7.803 | 0.1753 |
| Calcium (mg/dL) | 7.905±1.174 | 8.269±1.061 | 0.1505 |
| Vascular complications | 5 | 32 | NA |
| Duration of hospital stay (days) | 12.9±14.9 | 6.5±7.6 | 0.0006 |
| Mortality | 0 | 11.5% (61/529) |
*Not Applicable
Table 3.
Comparison between HTGAP and non-HTGsAP cohort across different age groups
| Variable | Age Group (years) | HTG AP | Non-HTP AP | P | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Number of patients | Mean (SD) | Number of patients | Mean (SD) | |||
| BMI | 18-30 | 4 | 30.67 (2.2) | 182 | 24.39 (5.2) | 0.0171 |
| 31-40 | 10 | 32.36 (3.66) | 160 | 25.22 (5.09) | 0.0001 | |
| 41-50 | 6 | 28.57 (2.42) | 82 | 24.92 (4.5) | 0.052 | |
| 51-60 | 1 | 27 | 91 | 27.13 | NA | |
| >60 | 0 | - | 14 | 27.63 | NA | |
| CTSI | 18-30 | 4 | 8 (2.4) | 182 | 4.38 (3.27) | 0.029 |
| 31-40 | 10 | 8 (1.97) | 160 | 5.27 (3.1) | 0.006 | |
| 41-50 | 6 | 8.29 (1.63) | 82 | 4.51 (3.3) | 0.006 | |
| 51-60 | 1 | 6 | 91 | 5.25 | NA | |
| >60 | 0 | - | 14 | 4.83 | NA | |
| Triglyceride level (mg/dL) | 18-30 | 4 | 2,808.75 (1,917.43) | 182 | 127.33 (102.66) | <0.0001 |
| 31-40 | 10 | 3,392.1 (3,180.80) | 160 | 215.10 (107.85) | <0.0001 | |
| 41-50 | 6 | 4,513 (2,961.23) | 82 | 423.57 (118.6) | <0.0001 | |
| 51-60 | 1 | 2,634 | 91 | 234.46 | NA | |
| >60 | 0 | - | 14 | 154.14 | NA | |
| Duration of Hospitalization (days) | 18-30 | 4 | 12.67 (1.6) | 182 | 5.48 (6.6) | 0.03 |
| 31-40 | 10 | 13 (11.54) | 160 | 6.42 (6.6) | 0.004 | |
| 41-50 | 6 | 15.29 (3.01) | 82 | 6.75 (5.8) | 0.0006 | |
| 51-60 | 1 | 10 | 91 | 6.48 | NA | |
| >60 | 0 | - | 14 | 6.27 | NA | |
DISCUSSION
Our prospective observational study demonstrates that HTGAP accounts for 3.8% (21/550) of all cases of acute pancreatitis. HTGAP is seen a decade earlier in comparison to patients with non-HTGAP and had higher body mass index (BMI). Two-thirds of patients with HTGAP had severe pancreatitis and required prolonged hospitalization (12 days vs. 6.5 days). Despite developing severe pancreatitis, there was no increased mortality among patients with HTGAP.
Although HTG has been regarded as the third most common cause of AP after gallstones and alcohol, the exact incidence of HTGAP is not known and various studies have estimated HTG as the causative factor in up to 1-14% of all cases of AP and may account for up to 56% of all cases of gestational pancreatitis with significant regional variations.[2,4,11] The wide variation in the prevalence of HTGAP is probably due to the use of lower cut-off TG levels for defining of HTGAP (>500 mg/dl) in some studies and also due to geographical variation of the metabolic risk factors.[12] The proposed mechanism of HTGAP is due to hydrolysis of TGs by pancreatic lipase with subsequent release of free fatty acids which provoke inflammatory changes and free radical injury in the pancreas. Elevated TG-rich chylomicrons increase the blood viscosity which impairs pancreatic capillary blood flow resulting in ischemia. The risk of developing AP increases with each incremental increase in levels of triglyceride above 1,000 mg/dl. In our study, mean TG level of 3,718 mg/dL was observed among cohort of patient with HTGAP. In our study, it was observed that HTGAP occurred in younger age group (mean, 34 years) as compared to the patients with non-HTGAP (mean, 46 years) pancreatitis. Similar finding was observed in prospective study by Nawaz H et al.,[13] in cohort of 400 consecutive patients with acute pancreatitis the mean age of patients with HTGAP was 44 years as compared to 52 years in patients with non- HTGAP. Patients with HTGAP were predominantly male, and had significantly higher BMI (mean, 29.22) as compared to patients with non-HTGAP (mean, 25.27) possibly due higher prevalence of hypertriglyceridemia in patients with higher BMI which concur with other studies.[14,15,16]
Although the initial presentation of HTGAP is similar to that of AP due to other causes, some studies have shown an association with severe pancreatitis than those with other causes of pancreatitis.[5,14,17,18] An association between the degree of TG elevation and the severity of AP has also been reported.[5,13,17,19] In our study, 82.35% (n = 14) showed a CTSI score of 7–10 with CT demonstrating acute pancreatic necrosis with peripancreatic fluid collections and pleural effusions. Six of these patients also had vascular thrombosis; while three had splenic vein thrombosis, two had main portal vein thrombosis, one had a combined splenic vein thrombosis and partial thrombosis of main portal vein, and one patient with mesenteric vein thrombosis. We did not find any association between the degree of TG elevation and the severity of AP in our study group.
Severe hypertriglyceridemia may cause the serum to appear milky [Figure 2]. Although this may be a useful diagnostic sign, it can interfere with routine laboratory measurements of sodium and amylase resulting in pseudo-hyponatremia and a falsely normal amylase level. Hence, elevation in serum amylase and lipase to >three times the upper limit of normal is seen in only 54% and 67% of HTGAP patients respectively.[2,20,21,22] In our study, the amylase level was elevated (>3 times upper limit of normal) in only 12 (57.14%) while 18 (85.7%) patients had elevated lipase level. Initial management of a patient with AP consists of supportive care with fluid resuscitation, pain control, restricting oral intake, and nutritional support with additional specific therapies tailored to lower serum TG levels. There are currently no established guidelines or randomized control trials for the treatment of HTGAP, although anecdotal evidence suggests that insulin infusion alone or in combination with heparin is an effective therapy for severe HTGAP. Apart from enhancing lipoprotein lipase activity, insulin also inhibits hormone-sensitive lipase in adipocytes, the key enzyme for breaking down adipocyte TG and releasing free fatty acids (FFA) into the circulation.[23,24] The use of plasmapheresis is limited to selective patients with severe HTGAP given concerns regarding its cost, availability, and efficacy.[25] A randomized control study has shown that plasmapheresis can lower TG levels more rapidly and efficiently than heparin plus insulin therapy; however, it was not found to be superior in terms of clinical outcomes and costs.[26] We initiated insulin infusion as early as possible in patients with “worrisome clinical features” such as severe lactic acidosis, uncontrolled sugars, two or more symptoms of SIRS, signs of worsening organ failure (n = 14). In the remainder of the patients (n = 7), without “worrisome features,” we opted for watchful waiting and conservative management. However, in four of these seven patients, there was no remarkable decline in their TG levels from admission levels, hence we initiated insulin infusion. In our study cohort, plasmapheresis was used in a pregnant lady who had recurrent hypoglycemia due to insulin infusion. The target levels of triglyceride levels of ≤500 mg/dL was achieved in mean 4.2 days which is in concordance with other studies.[27] Various case series and case reports have documented successful treatment of HTGAP using a combination of heparin and insulin infusion.[28,29] However, given the controversial role of heparin (transient reduction of TG levels, depletion of plasma stores resulting in lipoprotein lipase deficiency, potential lipotoxicity from FFA, and an increased risk of bleeding), we refrained from using heparin in our study.[30]
Figure 2.
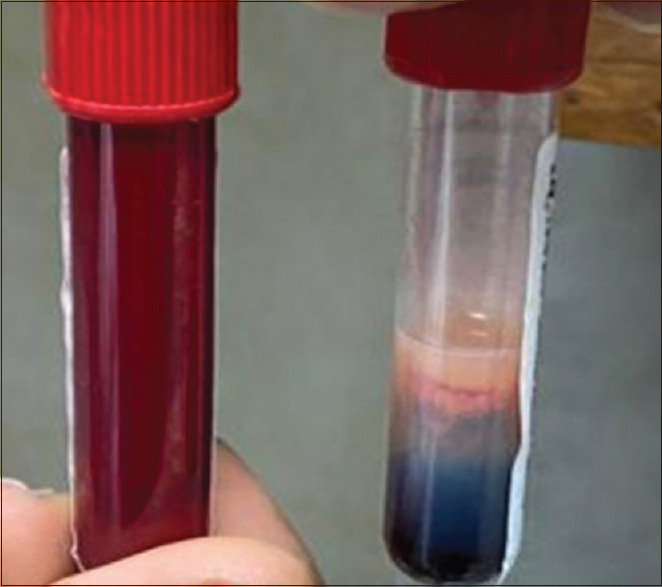
Lactescent serum (right test tube) characteristic of patients with triglycerides >1,000 mg/dL. The appearance of a lactescent (“creamy or milky”) layer floating on top of the serum is due to increased chylomicron particles present in the serum sample. The left test tube shows blood sample from a normal individual
Once triglyceride levels were <500 mg/dL, patients with HTGAP require long-term therapy to prevent recurrent pancreatitis and to prevent other complications of HTG. This consists of both pharmacologic therapy (e.g., oral fenofibrates) and dietary modification (e.g., fat- and simple sugar-restricted diet). Other non-pharmacologic interventions include weight loss in obese patients, aerobic exercises, avoidance of concentrated sugars and medications that raise serum TG levels, and strict glycemic control in diabetics. All our patients on discharge were initiated on oral fenofibrate and omega-3 fatty acids and advised on lifestyle and dietary changes; however, two patients who were noncompliant with the above instructions had recurrent AP.
Among the HTGAP cohort, despite 14 out of 21 (82.35%) patient developing severe pancreatitis, all patient survived with conservative supportive care and insulin therapy. Whereas, in the non-HTGAP cohort, 61 out of 529 patients (11.5%) died. Although the exact reason for this is not clear, it could be related to early identification and institution of TG lowering therapy in the form of insulin infusion. However, larger studies are required to ascertain the same.
Strengths of our study include a prospective enrollment of one of the largest series of 21 patients with HTGAP, uniformity of therapy with insulin, and a rigorous follow-up for recurrence of AP. Limitations of the study are the single center study design and noncomparison of other treatment modalities such as heparin and/or plasmapheresis.
CONCLUSION
HTGAP patients in comparison to those with non-HTGAP pancreatitis tend to be younger with higher BMI. Significantly a greater number of HTGAP patients develop severe pancreatitis requiring prolonged hospitalization as compared to non-HTGAP. HTGAP is the third most common cause of AP and is often associated with normal or minimally elevated serum amylase and lipase levels. Asymptomatic hyponatremia and hypocalcemia tend to be common in patients with HTGAP. Aside from supportive treatment, our experience suggests that insulin infusion alone is helpful in reducing TG levels in all these patients. Plasmapheresis may be useful in a small subset of patients who experience hypoglycemia or in whom rapid reduction in TG levels is required. Diet and lifestyle changes, weight reduction, strict control of diabetes along with lipid-lowering medications is critical in preventing recurrence of AP in these patients.
Ethics approval
Institute ethical committee clearance was obtained for this study, IEC Reference No. 166/2020.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis:Epidemiology, pathophysiology, and clinical management. United European Gastroenterol J. 2018;6:649–55. doi: 10.1177/2050640618755002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fortson MR, Freedman SN, Webster PD., 3rd Clinical assessment of hyperlipidemic pancreatitis. Am J Gastroenterol. 1995;90:2134–9. [PubMed] [Google Scholar]
- 3.Zhu Y, Pan X, Zeng H, He W, Xia L, Liu P, et al. A study on the etiology, severity, and mortality of 3260 patients with acute pancreatitis according to the revised Atlanta classification in Jiangxi, China over an 8-year period. Pancreas. 2017;46:504. doi: 10.1097/MPA.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 4.Chang CC, Hsieh YY, Tsai HD, Yang TC, Yeh LS, Hsu TY. Acute pancreatitis in pregnancy. Zhonghua Yi Xue Za Zhi (Taipei) 1998;61:85–92. [PubMed] [Google Scholar]
- 5.Koutroumpakis E, Slivka A, Furlan A, Dasyam AK, Dudekula A, Greer JB, et al. Management and outcomes of acute pancreatitis patients over the last decade:A US tertiary-center experience. Pancreatology. 2017;17:32–40. doi: 10.1016/j.pan.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Toskes PP. Hyperlipidemic pancreatitis. Gastroenterol Clin North Am. 1990;19:783–91. [PubMed] [Google Scholar]
- 7.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis--2012:Revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 8.Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, et al. Evaluation and treatment of hypertriglyceridemia:An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969–89. doi: 10.1210/jc.2011-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American College of chest physicians/Society of critical care medicine. Chest. 1992;101:1644e55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 10.Tenner S, Baillie J, DeWitt J, Vege SS American College of Gastroenterology. American College of Gastroenterology guideline:Management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–15. doi: 10.1038/ajg.2013.218. 1416. [DOI] [PubMed] [Google Scholar]
- 11.Anderson F, Thomson SR, Clarke DL, Buccimazza I. Dyslipidaemic pancreatitis clinical assessment and analysis of disease severity and outcomes. Pancreatology. 2009;9:252–7. doi: 10.1159/000212091. [DOI] [PubMed] [Google Scholar]
- 12.Deng LH, Xue P, Xia Q, Yang XN, Wan MH. Effect of admission hypertriglyceridemia on the episodes of severe acute pancreatitis. World J Gastroenterol. 2008;14:4558–61. doi: 10.3748/wjg.14.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nawaz H, Koutroumpakis E, Easler J, Slivka A, Whitcomb DC, Singh VP, et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol. 2015;110:1497–503. doi: 10.1038/ajg.2015.261. [DOI] [PubMed] [Google Scholar]
- 14.Lloret Linares C, Pelletier AL, Czernichow S, Vergnaud AC, Bonnefont-Rousselot D, Levy P, et al. Acute pancreatitis in a cohort of 129 patients referred for severe hypertriglyceridemia. Pancreas. 2008;37:13–2. doi: 10.1097/MPA.0b013e31816074a1. [DOI] [PubMed] [Google Scholar]
- 15.Bessembinders K, Wielders J, van de Wiel A. Severe hypertriglyceridemia influenced by alcohol (SHIBA) Alcohol Alcohol. 2011;46:113–6. doi: 10.1093/alcalc/agq088. [DOI] [PubMed] [Google Scholar]
- 16.Sandhu S, Al-Sarraf A, Taraboanta C, Frohlich J, Francis GA. Incidence of pancreatitis, secondary causes, and treatment of patients referred to a specialty lipid clinic with severe hypertriglyceridemia:A retrospective cohort study. Lipids Health Dis. 2011;10:157. doi: 10.1186/1476-511X-10-157. doi:10.1186/1476-511X-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan J, He W, Zhu Y, Zhu Y, Zeng H, Liu P, et al. Stratified analysis and clinical significance of elevated serum triglyceride levels in early acute pancreatitis:A retrospective study. Lipids Health Dis. 2017;16:124. doi: 10.1186/s12944-017-0517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vipperla K, Somerville C, Furlan A, Koutroumpakis E, Saul M, Chennat J, et al. Clinical profile and natural course in a large cohort of patients with hypertriglyceridemia and pancreatitis. J Clin Gastroenterol. 2017;51:77–85. doi: 10.1097/MCG.0000000000000579. [DOI] [PubMed] [Google Scholar]
- 19.Pascual I, Sanahuja A, García N, Vázquez P, Moreno O, Tosca J, et al. Association of elevated serum triglyceride levels with a more severe course of acute pancreatitis:Cohort analysis of 1457 patients. Pancreatology. 2019;19:623–9. doi: 10.1016/j.pan.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Howard JM, Reed J. Pseudohyponatremia in acute hyperlipemic pancreatitis. A potential pitfall in therapy. Arch Surg. 1985;120:1053–5. doi: 10.1001/archsurg.1985.01390330063013. [DOI] [PubMed] [Google Scholar]
- 21.Fallat RW, Vester JW, Glueck CJ. Suppression of amylase activity by hypertriglyceridemia. JAMA. 1973;225:1331–4. [PubMed] [Google Scholar]
- 22.Saligram S, Lo D, Saul M, Yadav D. Analyses of hospital administrative data that use diagnosis codes overestimate the cases of acute pancreatitis. Clin Gastroenterol Hepatol. 2012;10:805–11.e1. doi: 10.1016/j.cgh.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg IJ. Lipoprotein lipase and lipolysis:Central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- 24.Stefanutti C, Di Giacomo S, Labbadia G. Timing clinical events in the treatment of pancreatitis and hypertriglyceridemia with therapeutic plasmapheresis. Transfus Apher Sci. 2011;45:3–7. doi: 10.1016/j.transci.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 25.He WH, Yu M, Zhu Y, Xia L, Liu P, Zeng H, et al. Emergent triglyceride-lowering therapy with early high-volume hemofiltration against low-molecular-weight heparin combined with insulin in hypertriglyceridemic pancreatitis:A prospective randomized controlled trial. J Clin Gastroenterol. 2016;50:772–8. doi: 10.1097/MCG.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 26.Dhindsa S, Sharma A, Al-Khazaali A, Sitaula S, Nadella S, McKee A, et al. Intravenous insulin versus conservative management in hypertriglyceridemia-associated acute pancreatitis. J Endocrine Soc. 2019;4:bvz019. doi: 10.1210/jendso/bvz019. doi:10.1210/jendso/bvz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikhail N, Trivedi K, Page C, Wali S, Cope D. Treatment of severe hypertriglyceridemia in nondiabetic patients with insulin. Am J Emerg Med. 2005;23:415–7. doi: 10.1016/j.ajem.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 28.Berger Z, Quera R, Poniachik J, Oksenberg D, Guerrero J. [Heparin and insulin treatment of acute pancreatitis caused by hypertriglyceridemia. Experience of 5 cases] Rev Med Chil. 2001;129:1373–8. [PubMed] [Google Scholar]
- 29.Näsström B, Olivecrona G, Olivecrona T, Stegmayr BG. Lipoprotein lipase during continuous heparin infusion:Tissue stores become partially depleted. J Lab Clin Med. 2001;138:206–13. doi: 10.1067/mlc.2001.117666. [DOI] [PubMed] [Google Scholar]
- 30.Khatua B, El-Kurdi B, Singh VP. Obesity and pancreatitis. Curr Opin Gastroenterol. 2017;33:374–82. doi: 10.1097/MOG.0000000000000386. [DOI] [PMC free article] [PubMed] [Google Scholar]


