Abstract
Introduction:
Human Galectin-3 is a 32- to 35-kDa size lectin, mainly comprises a C-terminal carbohydrate recognition binding domain (CRD) and N-terminal domain. It acts as a powerful pro-inflammatory signalling factor, which plays an important role in the activation, chemotaxis, and cytokine release of inflammatory cells. Galectin-3 has also been studied in relation to development of insulin resistance. The levels of galectin-3 have been observed to be associated with both diabetes prevalence and incidence, independent of traditional diabetes risk factors. It is also associated with development of microvascular complications of diabetes mellitus like retinopathy, nephropathy and neuropathy.
Methods:
Tertiary care hospital-based cross-sectional prospective study. 150 patients selected by simple random sampling and were divided into 3 groups., Group A – Patients of Type 2 Diabetes mellitus without microvascular complications (n=50), Group B – patients of Type 2 diabetes mellitus with microvascular complications (n=50) and Group C - Healthy control (n=50)
Statistical Analysis:
Descriptive statistics was performed by calculating mean and standard deviation for the continuous variables. chi-square goodness-to-fit test, Student T test (unpaired) and Analysis of variance (ANOVA) and multivariate analysis were used to compare means. The p-value was taken significant when less than 0.05 (P<0.05) and a confidence interval of 95%.
Results:
In group A, B and C majority of patients were between 56-60 years with 34%, 40% and 36% cases, respectively. The mean BMI shows that the Patients with complications had significantly higher BMI than those without complications and controls had significantly lower BMI than patients having diabetes. The data shows statistical significance with deranged biochemical profile in patients with DM with complications as compared to patients without complications and control group. In both groups A and B patients with HbA1c between 9.1-12 had mean serum galectin level (20.2 in group A, 25.9 in group B) significantly higher than patients with HbA1c between 6.5-9 (18.5 in group A and 20.4 in group B). patients with deranged lipid profile had significantly higher serum galectin level in all 3 groups, with cases from group B having higher values than group A. While controls had the lowest value of serum galectin (P value<0.001). There was a highly significant correlation between high serum galectin levels and the incidence of both non-progressive and progressive retinopathy (P value=0.0001). The mean galectin of patients with neuropathy was 28.3 ± 3.1 ng/ml, which was significantly higher than patients from group B without neuropathy (24.5 ± 2.6 ng/ml). The mean serum galectin level of patients with macroalbuminuria was 30.1± 1.3 ng/ml which was significantly higher than those with microalbuminuria having mean galectin level of 22.8 ±4.8 ng/ml. There was a highly significant correlation between high serum galectin levels and the incidence of both micro and macroalbuminuria (P value=0.0001).
Conclusion:
This study concludes that elevated serum Galectin-3 levels are associated with diabetes-related chronic inflammatory processing pathway, and closely relates to the severity of diabetes in T2DM both with and without complications. Therefore, Galectin-3 may be helpful in the diagnosis and prognosis of microvascular and macrovascular complications in T2DM patients.
Keywords: Diabetes mellitus, galectin-3, microvascular complications
INTRODUCTION
Diabetes mellitus (DM) is a group of metabolic disorders which is characterised by hyperglycemia in the absence of treatment. Overall, the prevalence of pre-diabetes in India is 10.3%.[1] The prevalence of diabetes in Indian adults is as high as 20% in urban areas and 10% in rural areas.[1,2] Obesity and type 2 diabetes mellitus (T2DM) have reached epidemic proportions in the Western world with a soaring uprise in the Indian peninsula, nonetheless.[3,4] There is, resultantly, a parallel uprise in the incidence and prevalence of both conditions, which supports the concept of overweight and obesity being powerful risk factors resulting in the development of T2DM. Various proinflammatory and prooxidant signals have also been strongly implicated in obesity pathogenesis including the advanced accumulation of glycation end products (AGES)/advanced lipoxidation end products (ALEs) through the receptor for AGEs (RAGE) receptor.[3,5,6] The various long-term specific complications of diabetes include retinopathy, nephropathy, and neuropathy as the three most severe and cardinal sequelae in uncontrolled cases. People with diabetes also have an increased risk of other diseases including heart, peripheral arterial, and cerebrovascular disease, obesity, cataracts, erectile dysfunction, and nonalcoholic fatty liver disease.[7]
A handful of human studies have provided evidence suggesting that galectin-3 (Gal-3) levels are searingly upregulated in subjects suffering from obesity and T2DM.[8,9] Gal-3 which is encoded by the LGALS3 gene, is a member of the b-galactoside-binding lectin family subtype of galectin, formerly known as carbohydrate-binding protein-35 (CBP-35).[10,11] Human Gal-3 is a 32- to 35-kDa size lectin, which mainly comprises a C-terminal carbohydrate recognition binding domain (CRD) and an N-terminal domain.[12,13,14] Gal-3 is distributed in the human body tissues, such as haematopoietic tissues, thymus, lymph nodes, and spleen, and is mainly produced by immune cells such as macrophages, mast cells, eosinophils, and it can be secreted extracellularly.[15,16] It acts as a powerful proinflammatory signalling factor, which plays an important role in the activation, chemotaxis, and cytokine release of inflammatory cells.
Recently, Gal-3 has been implicated in the development of T2DM and obesity. Studies in humans have also shown that Gal-3 concentrations are significantly higher in obese and diabetic individuals and that it increases in conjunction with unbridled glucose homeostasis. On the other hand, Gal-3 levels correlated positively with insulin sensitivity and negatively with levels in patients with T2DM.
Gal-3 has also been studied in relation to the development of insulin resistance. Due to the presence of a collagenous domain in its structure, galectin can interact with a wide range of extracellular matrix proteins such as tenascin, fibronectin, and laminin. Gal-3 is produced by numerous cells including neutrophils, macrophages, labrocytes, fibroblasts, and osteoclasts. Gal-3 is found in the lungs, stomach, intestine, and uterus. It was determined that Gal-3 is involved in the inflammatory process, apoptosis, and angiogenesis and is associated with the development of insulin resistance.[17]
Previous animal and human studies targeted at exploring the relationship between Gal-3, and metabolic disorders have made conclusive observations of its pathogenesis in DM, its complications like retinopathy and nephropathy, as well as its role in the propagation of abnormal lipid profiles.[9,18] The fibrovascular tissues from proliferative diabetic retinopathy patients have the presence of increased levels of advanced glycation products, galectin, TRL4, and IL-1b in the macrophages, along with high IL-1b receptor-positive glial cells which express galectin-1. Therefore, diabetes-induced retinal galectin upregulation has been suggested to activate the IL-1b-related inflammatory cues in macrophages. This suggests that advanced glycation products’ triggered inflammation happens in DR in the presence of galectin.[8,9] Microalbuminuria can serve as an early indicator of glomerular disease and its progression; in many cases, the urinary albumin excretion may stay within the normal limits during early-stage renal damage. presence of clinically diagnosed DM. Several authors, including Kathryn et al.,[1] have observed that raised serum Gal-3 level is independently associated with the progression of nephropathy. Gal-3 has been found to exert an opposite role, depending on the stage of renal damage and the degree of tissue inflammation. The pharmacological inhibition of Gal-3 using small-molecule competitive inhibitors can prevent nephropathy hypertension in diabetics. Targeting the Gal-3 molecule has also been recently proposed as one of the latest therapeutic approaches against renal fibrosis.
Considering these findings and prior mechanistic studies, the association between Gal-3 and diabetes is likely mediated by islet-cell inflammation, tissue fibrosis, and ultimately via b-cell dysfunction. We, therefore, conducted this study to establish an association between galectin levels and the severity of diabetes. We also attempted to establish the link between the levels of galectin and the various microvascular complications of DM in this case-controlled study.
MATERIALS AND METHODS
STUDY DESIGN: It was a tertiary-care hospital-based cross-sectional prospective study. The study was conducted after taking proper approval from the institutional research and ethical committee.
STUDY POPULATION: Patients suffering from T2DM presenting to our institute for a period of 1 year were selected.
-
SAMPLE SIZE: A total of 150 patients were selected by simple random sampling and were divided into three groups.
- Group A – Healthy control (n = 50)
- Group B – Patients of T2DM without vascular complications (n = 50)
- Group C – Patients of T2DM with microvascular complications (n = 50)
Inclusion Criteria (for cases)
Patients of T2DM with and without microvascular complications
Age group of 40–60 years
Inclusion Criteria (for controls)
Age and sex-matched healthy individuals were recruited as controls
Exclusion Criteria (for cases and controls)
Patients with malignancy, chronic liver dysfunction, renal dysfunction, chronic alcoholics, coronary artery disease, active infections, gastroparesis, and skin changes
Patients with macrovascular complications of T2DM (acute cerebrovascular accident, myocardial infarction, acute kidney injury, peripheral vascular disease, and erectile dysfunction)
Pregnant females
Patients with T2DM
Patients who did not give consent to be a part of the study
T2DM was diagnosed according to the guidelines of the American Diabetes Association. The demographic and clinical parameters of all the participants were recorded. The patients were then investigated for complete blood count, serum Gal-3, blood urea and serum creatinine (Scr), fasting plasma glucose (FPG), urine routine and microscopic study, urine albumin creatinine ratio, lipid profile, and glycosylated haemoglobin (HbA1c).
The haemogram test was performed using a 5-part haematology analyser. Biochemical tests and the lipid profile were measured using a Merck SELECTRA PRO M Automated Analyzer, and serum Gal-3 levels were measured by the enzyme-linked immunosorbent assay (ELISA) method using specific kits, according to the manufacturer’s instructions (R&D Systems, Catalogue #SGAL30). The serum Gal-3 reference range was taken to be 2.47–15.7 ng/ml.
Statistical analysis
Descriptive statistics were performed by calculating the mean and standard deviation for the continuous variables. Categorical variables are presented as absolute numbers and percentages. Nominal categorical data between the groups were compared using the Chi-square goodness-to-fit test. The Student-t test (unpaired) was used to compare the mean of quantitative variables <30, and the Z test was used to compare the mean of quantitative variables >30. Other tests used were analysis of variance (ANOVA) and multivariate analysis. The P value was taken as significant when less than 0.05 (P < 0.05) and a confidence interval of 95% was taken. Appropriate statistical analysis was applied as and when required using SPSS software version 17.0. A P value < 0.05 was considered as significant
RESULTS
Our study included 50 cases of diabetes without complications (group A), 50 cases with complications (group B) and 50 controls (group C). In groups A, B and C, the majority of patients were between 56 and 60 years with 34%, 40% and 36% cases, respectively. All three groups were compared based on age (P-value = 0.15). The male-to-female ratio was 1.38:1 in group A, 1.27:1 in group B, and 0.38:1 in group C. The three groups were gender-matched (P-value = 0.06). The mean body mass index (BMI) of the study groups as shown in Table 1 shows that the patients with complications had a significantly higher BMI than those without complications. Additionally, controls had a significantly lower BMI than patients having diabetes, which makes the correlation statistically highly significant.
Table 1.
Showing demographic and clinical data of patients of all study groups
| Group A (DM without complications) | Group B (DM with complications) | Group C (controls) | P | |
|---|---|---|---|---|
| Mean Age | 52.98±4.69 | 54.12±3.65 | 49.92±3.78 | P=0.15 |
| Gender | ||||
| Male | 29 (58%) | 28 (56%) | 19 (38%) | P=0.06 |
| Female | 21 (42%) | 22 (44%) | 31 (62%) | |
| Demographics | ||||
| Rural | 20 (40%) | 21 (42%) | 21 (42%) | P=0.58 |
| Urban | 30 (60%) | 29 (58%) | 29 (58%) | |
| BMI | 26.1±2.6 | 28.7±2.5 | 20.2±2.1 | P=0.0001 |
There was a significant difference in the disease duration of DM between groups A and B. Whereas the mean duration of patients without complications was 2.15 ± 1.65 years, that of patients with complications was 7.95 ± 4.14 years (P-value < 0.0001) [Figure 1].
Figure 1.

Number of cases according to the duration of DM in two groups
The biochemical parameters in the three groups were compared. As shown in Table 2, patients with complications (group B) had significant deranged levels of kidney function tests, lipid profile, and HbA1c levels than those without complications (group A). Serum Gal-3 levels also show a significant increase in patients of group B and group A as compared to controls (group C) [Table 2]. The galectin levels in all three groups of patients are shown in Figure 2. Additionally, controls had significantly lower levels than those with diabetes. The data show statistical significance with deranged biochemical profiles in patients with DM with complications as compared to patients without complications and the control group.
Table 2.
Distribution of different biochemical parameters in different groups
| Parameter | Group A (Diabetics without Complications) | Group B (Diabetics with Complications) | Control Group | P | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Urea | 36.1 | 23.2 | 57.1 | 28.3 | 34.5 | 16.2 | 0.001 |
| Creatinine | 1.1 | 1.1 | 1.8 | 0.9 | 0.9 | 0.3 | 0.01 |
| HbA1c | 8.2 | 1.4 | 9.8 | 1.9 | 4.8 | 0.32 | 0.0001 |
| Galectin | 18.7 | 3.8 | 23.6 | 4.5 | 7.6 | 2.4 | 0.001 |
| Cholesterol | 263.8 | 34.8 | 281.8 | 39.5 | 175.2 | 23.46 | 0.0001 |
| Triglyceride | 279.8 | 45.3 | 361.6 | 58.9 | 133.1 | 20.1 | 0.02 |
| HDL | 39.1 | 3.9 | 36.5 | 4.3 | 67.8 | 7.5 | 0.0002 |
| LDL | 154.2 | 27.2 | 165.1 | 38.4 | 115.9 | 29.2 | 0.0001 |
Figure 2.

Distribution of patients according to serum galectin levels
In both groups A and B, patients with HbA1c between 9.1 and 12 had mean serum galectin levels (20.2 in group A and 25.9 in group B) significantly higher than in patients with HbA1c between 6.5 and 9 (18.5 in group A and 20.4 in group B). This difference was statistically significant in both groups (P-value = 0.04 in group A and 0.001 in group B) as shown in Table 3.
Table 3.
Mean serum galectin level of patients according to HbA1c level
| HbA1c | Diabetics without complications | Diabetics with complications | Control group | P | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Mean | SD | Mean | SD | Mean | SD | ||
| <6.5 | 0 | 0 | 0 | 0 | 7.6 | 2.4 | 0.0001 |
| 6.5-9.0 | 18.5 | 5.4 | 20.4 | 6.1 | 0 | 0 | 0.0001 |
| 9.1-12 | 20.2 | 5.9 | 25.9 | 9.3 | 0 | 0 | 0.0001 |
| >12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P | 0.04 | 0.001 | |||||
As shown in Table 4 and Figure 3, patients with deranged lipid profiles had significantly higher serum galectin levels in all three groups, with cases from group B having higher values than group A, whereas controls had the lowest value of serum galectin (P-value < 0.001).
Table 4.
Mean galectin level versus lipid profile
| Group A | Group B | Group C | P | |
|---|---|---|---|---|
| LDL level | ||||
| 100-159 | 16.1±1.2 | 19.2±1.6 | 6.9±1.3 | 0.0001 |
| ≥160 | 20.1±2.5 | 25.7±2.9 | 7.7±1.8 | 0.001 |
| CH level | ||||
| Optimum (<200) | 15.4±1.3 | - | 7.6±2.4 | 0.0001 |
| Intermediate (200-239) | 16.3±1.4 | 18.3±0.9 | - | 0.001 |
| Triglyceride level | ||||
| <200 mg/dl | 16.2±1.3 | 19.3±1.6 | 6.7±1.2 | 0.0001 |
| ≥200 mg/dl | 19.8±2.3 | 25.5±2.8 | 7.8±1.7 | 0.001 |
Figure 3.
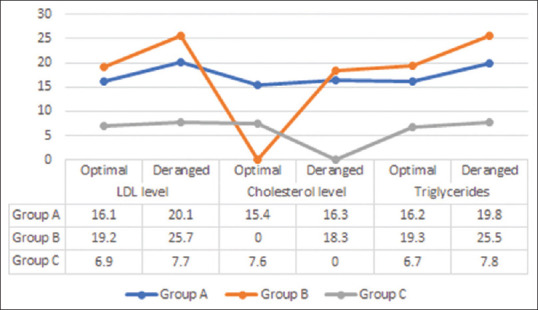
Distribution of patients according to lipid profile and serum galectin levels
There was a highly significant correlation between high serum galectin levels and the incidence of both non-progressive and progressive retinopathy (P-value = 0.0001). [Table 5] The ANOVA also revealed that patients with progressive retinopathy (from group B) had significantly higher serum galectin levels than patients without progressive retinopathy (from group B), and patients without complications (group A), as well as controls (group A). [Figure 4]
Table 5.
Distribution of cases according to the presence of microvascular complications
| Microvascular Complication | No. of Patients | Percent |
|---|---|---|
| Retinopathy | ||
| Absent | 18 | 36% |
| Non-progressive diabetic retinopathy (NPDR) | 28 | 56% |
| Progressive diabetic retinopathy (PDR) | 4 | 8% |
| Neuropathy | ||
| Absent | 35 | 70% |
| Present | 15 | 30% |
| Nephropathy | ||
| Absent | 27 | 54% |
| Microalbuminuria | 18 | 36% |
| Macroalbuminuria | 5 | 10% |
Figure 4.

Serum Gal-3 levels vs retinopathy
The mean galectin of patients with neuropathy was 28.3 ± 3.1 ng/ml, which was significantly higher than patients from group B without neuropathy (24.5 ± 2.6 ng/ml). There was a highly significant correlation between high serum galectin levels and the incidence of diabetic neuropathy (P-value = 0.0001). [Figure 5]
Figure 5.

Serum galectin levels vs diabetic neuropathy
The mean serum galectin level of patients with macroalbuminuria was 30.1 ± 1.3 ng/ml, which was significantly higher than those with microalbuminuria having a mean galectin level of 22.8 ± 4.8 ng/ml. There was a significant difference in the mean serum galectin level as the level of urine albumin/creatinine ratio (ACR)increased; that is, in patients having high albuminuria, a high serum galectin level was found (P-value = 0.0001). There was a highly significant correlation between high serum galectin levels and the incidence of both micro- and macroalbuminuria (P-value = 0.0001). The ANOVA also revealed that patients with macroalbuminuria had significantly higher serum galectin levels than patients with microalbuminuria. [Figure 6] [Table 6]
Figure 6.

Serum galectin levels vs diabetic nephropathy
Table 6.
Correlation of serum galectin with microvascular complications in DM
| Group C (Healthy controls) | Group A (DM with no complications) | Group B (DM with complications) | P | ||
|---|---|---|---|---|---|
| Diabetic Retinopathy | 7.6±3.6 | 18.7±2.6 | No Retinopathy | 20.7±2.7 | F ratio=159.8 |
| Non-progressive diabetic retinopathy | 24.4±4.4 | P<0.0001 | |||
| Progressive Retinopathy | 30.1±1.4 | ||||
| Diabetic Neuropathy | 7.6±3.6 | 18.7±2.6 | No neuropathy | 24.5±3.9 | F ratio=181.4 |
| Neuropathy | 28.3±3.1 | P<0.0001 | |||
| Diabetic Nephropathy | 7.6±3.6 | 18.7±2.6 | Microalbuminuria | 22.8±3.8 | F ratio=144.22 |
| Macroalbuminuria | 30.1±1.3 | P<0.0001 | |||
DISCUSSION
Gal-3 is a carbohydrate-binding protein that holds important regulatory roles in a multitude of inflammations, immunity, and malignancies.[19] Various findings suggest that Gal-3 accelerates the pathogenesis of metabolic diseases, by enhancing inflammatory infiltrates.[15,16] Inhibition of Gal-3 can also prevent acute diabetic retinopathy.[17]
In this case-control study, the study subjects were divided into three groups. Group A included T2DM cases without complications, group B consisted of T2DM cases with complications, whereas in group C, healthy individuals were recruited. There was a significant difference in the disease duration between groups A and B. Whereas the mean duration of diabetes in patients without complications was 2.15 ± 1.65 years, that of patients with complications was 7.95 ± 4.14 years (P-value < 0.0001). This finding was similar to the study by Zoungas et al.[20] In their study, patients with complications had a significantly longer mean duration of diabetes (7.9 ± 6.4 years) than those without complications (2.3 ± 1.9 years). Similar observations have been made by Krolewski et al.[21] and Jerneld et al.[22]
There was a male preponderance in our study in both groups. Similar observations were made by Alnour and Abdalla et al.[23] who found a male-to-female ratio of 1.5 in T2DM. Metan et al.[24] found a male-to-female ratio of 1:1 in their study.
In the present study, we used Asian guidelines for BMI estimation. In the diabetic-patients-without-complications group, a BMI range of 23–27.5 had most patients with 74%. The mean BMI of patients was 26.1 ± 2.6 kg/m2. In diabetics with complications, however, a majority (54%) of patients had a significantly higher BMI range between 27.5 and 33.6, and the mean BMI was 28.7 ± 2.5 kg/m2. In the control group, the BMI ranged between 18.5 and 22.9 and the mean BMI was 20.2 ± 2.1 kg/m2. There was a highly significant difference between BMI in all three groups (P-value = 0.0001). Topiwala et al.[25] found that 87 (28.0%), 89 (28.7%) and 134 (43.2%) patients had a BMI <23.0, 23.0 to <25.0 and ≥25.0 kg/m2, respectively, in diabetic patients. Kumbhalkar and Daware[26] found that the maximum number of patients (60%) of both groups had grade II obesity (BMI >30 to 40). The above studies support our observation. In our study, a maximum number of patients with diabetes in both complicated and non-complicated groups were overweight according to Asian guidelines for BMI, and the group with complications had a significantly greater BMI as compared to the group without complications; however, there was no significant difference in the BMI with sex. Recent literature has identified conclusive links between obesity and T2DM involving proinflammatory cytokines (tumour necrosis factor and IL-6), insulin resistance, deranged fatty acid metabolism, and cellular processes involving mitochondrial dysfunction and oxidative stress in the endoplasmic reticulum.[27]
In the present study, in group A (diabetes without complications), 86% of cases were in the category 6.5–9.0% of HbA1c, and the remaining 14% had HbA1c between 9.1 and 12%. In group B, a significantly higher proportion of patients (58%) had HbA1c between 9.1 and 12% with the remaining 42% cases having HbA1c between 6.5 and 9% (P-value = 0.01). Whereas comparing the serum galectin levels in patients stratified based on HbA1c, we observed that there was a significant difference in serum galectin as the level of HbA1c increased. Also, patients with complications had a higher level of galectin than those without complications for the same level of HbA1c. There was a significant difference between serum galectin levels in all three groups as well. (P-value = 0.01). Tan et al.[1] found that diabetic patients with complications who had a significantly poorer glycaemic control measured with HbA1c of 9.45 ± 2.31% as a reference had a significantly higher level of serum galectin compared to diabetic patients without complication (P < 0.001). Studies by Weigert et al.,[18] Jin Qi-hui et al.,[28] and Hodeib et al.[29] also found a positive association between plasma levels of galectin with HbA1c levels.
In our study, amongst patients from group B, 46% of patients had diabetic nephropathy. Of these 36% of patients had microalbuminuria, defined as 30–300 mg/g urine ACR or 30–300 mg albumin in urine/day, whereas the remaining 8% had macroalbuminuria (ACR >300 mg/g or >300 mg albumin in urine/day). The mean serum galectin level of patients with macroalbuminuria was 30.1 ± 1.37 ng/ml, which was significantly higher than those with microalbuminuria having a mean galectin level of 22.85 ± 4.83 ng/ml. There was a significant difference in the mean serum galectin level as the level of urine ACR increased; that is, in patients having high albuminuria, a high serum galectin level was found (P-value = 0.0001). A similar observation was made by Tan et al.[1], Jin Qi-hui et al.[28], Hodeib et al.,[29] and Allyan et al.,[30] and Hodeib et al.[29] found that the serum galectin level increased positively with an increase in urine ACR (correlation coefficient: 0.66, P value < 0.0001). Tan et al.[1] also observed that serum Gal-3 levels were significantly increased in type 2 diabetic individuals with microalbuminuria or macroalbuminuria when compared with an age- and sex-matched nondiabetic control group (7.58 ± 2.29 ng/ml vs 6.10 ± 1.91 ng/ml, respectively, P < 0.01). They also observed that after a mean follow-up of 9 years, Gal-3 was independently associated with a doubling of serum creatinine and incident macroalbuminuria. In their study, individuals with Gal-3 levels in the highest quartile had a fourfold risk of renal function loss and a threefold risk of incident macroalbuminuria. Our results could be extrapolated to their observations if our patients were followed up for a longer duration.
In our study, the mean serum galectin for females was higher than for males in all three groups. In group A, the mean level for females was 18.9 ± 4.9 ng/ml, whereas that for male patients was 18.5 ± 5.2 ng/ml (P-value = 0.45), and for group B, the average level in female patients was 23.9 ± 7.1 ng/ml, compared to 23.3 ± 6.5 ng/ml in males (P-value = 0.93). However, the difference was statistically insignificant in all three groups. In agreement with our results, Tan et al.,[1] Jin Qi-hui et al.,[28] and Hodeib et al.[29] independently observed no statistically significant difference between the mean plasma galectin level in female and male diabetic patients.
In our study, there was a highly significant association between high serum galectin and the incidence of both diabetic neuropathy and retinopathy. To date, there is barely any mention of the role of galectin in the development and progression of diabetic retinopathy and nephropathy. Most of the concerned literature only covers animal models.[31,32,33] Ours is one of the very few studies exploring this under-reported facet of diabetes. The role of galectin in diabetic retinopathy has been highlighted by Abu El-Asrar et al.[34] They observed that galectin-1 and vascular endothelial growth factor (VEGF) levels were significantly higher in vitreous samples from progressive diabetic retinopathy patients than in those from nondiabetic subjects (P-value < 0.001). They also found a significant positive correlation between the levels of galectin-1 and VEGF (r = 0.354; P = 0.022). However, Hodeib et al.[29] and Weigert et al.[18] found no correlation between serum galectin levels and the presence of these complications.
The levels of lipid profile parameters, namely, LDL, triglycerides (TG), and total cholesterol (TC) were found to be significantly increased in both groups A and B, with a significantly higher level in group B than in group A. Moreover, the value of HDL levels was significantly decreased in groups A and B, with a significantly higher fall in group B. Allyan et al.[30] also observed that there was a significant correlation between the deranged lipid profile and high galectin level. Galectin levels positively correlated with cholesterol (correlation coefficient: 0.333, P value < 0.0001) and TG (correlation coefficient: 0.335, P value < 0.0001) and negatively with HDL (correlation coefficient: 0.397, P value < 1.000). Similarly, our results were also in close agreement with those of Vora et al.[3] They observed that the serum galectin level of patients with normal lipid levels ranged between 5.9 and 11.8 ng/ml, whereas in patients with hyperlipidemia, the serum galectin was significantly higher and ranged between 17.1 and 99.2 ng/ml (P < 0.001).
The main strength of our study was the fact that it was the first study that described the association between serum Gal-3 and various complications and biochemical parameters among T2DM patients in India. Our results clearly demonstrated a significant difference in serum Gal-3 levels in T2DM with the complication group compared to T2DM without complication and control groups (P-value < 0.01). Furthermore, positive significant correlations were also found between serum Gal-3 and BMI, HbA1c, cholesterol, triglyceride, urinary albumin creatinine ratio, and increasing age.
CONCLUSION
This study concludes that elevated serum Gal-3 levels are associated with diabetes-related chronic inflammatory processing pathways and closely related to the severity of diabetes in T2DM both with and without complications. Therefore, Gal-3 may be helpful in the diagnosis and prognosis of microvascular and macrovascular complications in T2DM patients. However, whether serum Gal-3 is simply a biomarker of disease or also a mediator for the development and progression of diabetes still warrants further investigation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tan KCB, Cheung CL, Lee ACH, Lam JKY, Wong Y, Shiu SWM. Galectin-3 is independently associated with progression of nephropathy in type 2 diabetes mellitus. Diabetologia. 2018;61:1212–9. doi: 10.1007/s00125-018-4552-z. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Huang X, Ma XW, Mo W, Wang WJ, Song HY. Enzymatic vitreolysis with recombinant microplasminogen and tissue plasminogen activator. Eye (Lond) 2008;22:300–7. doi: 10.1038/sj.eye.6702931. [DOI] [PubMed] [Google Scholar]
- 3.Vora A, de Lemos JA, Ayers C, Grodin JL, Lingvay I. Association of Galectin-3 with diabetes mellitus in the Dallas heart study. J Clin Endocrinol Metab. 2019;104:4449–58. doi: 10.1210/jc.2019-00398. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D’Agostino RB., Sr Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s:The Framingham Heart Study. Circulation. 2006;113:2914–8. doi: 10.1161/CIRCULATIONAHA.106.613828. [DOI] [PubMed] [Google Scholar]
- 5.Friedrichs J, Manninen A, Muller DJ, Helenius J. Galectin-3 regulates integrin alpha2beta1-mediated adhesion to collagen-I and -IV. J Biol Chem. 2008;283:32264–72. doi: 10.1074/jbc.M803634200. [DOI] [PubMed] [Google Scholar]
- 6.Nio J, Takahashi-Iwanaga H, Morimatsu M, Kon Y, Iwanaga T. Immunohistochemical and in situ hybridization analysis of galectin-3, a beta-galactoside binding lectin, in the urinary system of adult mice. Histochem Cell Biol. 2006;126:45–56. doi: 10.1007/s00418-005-0142-5. [DOI] [PubMed] [Google Scholar]
- 7.Park K. Park's Textbook of Preventive and Social Medicine. 20th ed. Vol. 6. Banarsidas Bhanot; Jabalpur, India: 2009. Epidemiology of chronic non-communicable diseases and condition; p. 341. [Google Scholar]
- 8.Yang RY, Hsu DK, Liu FT. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc Natl Acad Sci USA. 1996;93:6737–42. doi: 10.1073/pnas.93.13.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mensah-Brown EP, Al Rabesi Z, Shahin A, Al Shamsi M, Arsenijevic N, Girdhar S, et al. An epidemiological study of overweight and obesity among women in an urban area of North India. Indian J Community Med. 2016;41:154–7. doi: 10.4103/0970-0218.173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yilmaz H, Cakmak M, Inan O, Darcin T, Akcay A. Increased levels of galectin-3 were associated with prediabetes and diabetes:New risk factor? J Endocrinol Invest. 2015;38:527–33. doi: 10.1007/s40618-014-0222-2. [DOI] [PubMed] [Google Scholar]
- 11.Newlaczyl AU, Yu LG. Galectin-3—a jack-of-all-trades in cancer. Cancer Lett. 2011;313:123–8. doi: 10.1016/j.canlet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Pugliese G, Iacobini C, Pesce CM, Menini S. Galectin-3. An emerging all-out player in metabolic disorders and their complications. Glycobiology. 2015;25:136–50. doi: 10.1093/glycob/cwu111. [DOI] [PubMed] [Google Scholar]
- 13.Pugliese G, Iacobini C, Ricci C, Fantauzzi CB, Menini S. Galectin-3 in diabetic patients. Clin Chem Lab Med. 2014;52:1413–23. doi: 10.1515/cclm-2014-0187. [DOI] [PubMed] [Google Scholar]
- 14.Iacobini C, Amadio L, Oddi G. Role of galectin-3 in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S264–70. doi: 10.1097/01.asn.0000077402.95720.b4. [DOI] [PubMed] [Google Scholar]
- 15.Pugliese G. Do advanced glycation end products contribute to the development of long-term diabetic complications? Nutr Metab Cardiovasc Dis. 2008;18:457–60. doi: 10.1016/j.numecd.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Gonz?alez GE, Cassaglia P, Truant SN. Galectin-3 is essential for early wound healing and ventricular remodelling after myocardial infarction in mice. Int J Cardiol. 2014;176:1423–5. doi: 10.1016/j.ijcard.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 17.MacKinnon AC, Gibbons MA, Farnworth SL. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am J Respir Crit Care Med. 2012;185:537–46. doi: 10.1164/rccm.201106-0965OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weigert J, Neumeier M, Wanninger J, Bauer S, Farkas S, Scherer MN, et al. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J Clin Endocrinol Metab. 2010;95:1404–11. doi: 10.1210/jc.2009-1619. [DOI] [PubMed] [Google Scholar]
- 19.Dumic J, Dabelic S, Flögel M. Galectin-3:An open ended story. Biochim Biophys Acta. 2006;1760:616–35. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57:2465–74. doi: 10.1007/s00125-014-3369-7. [DOI] [PubMed] [Google Scholar]
- 21.Krolewski AS, Warram JH, Rand LI, Kahn CR. Epidemiologic approach to the etiology of type I diabetes mellitus and its complications. N Engl J Med. 1987;317:1390–8. doi: 10.1056/NEJM198711263172206. [DOI] [PubMed] [Google Scholar]
- 22.Jerneld B, Algvere P. Relationship of duration and onset of diabetes to prevalence of diabetic retinopathy. Am J Ophthalmol. 1986;102:431–7. doi: 10.1016/0002-9394(86)90069-3. [DOI] [PubMed] [Google Scholar]
- 23.Alnour MSM, Abdalla MH. A study of fibrinogen level and c-reactive protein in type 1 and type 2 diabetes mellitus and their relation to glycemic control. Am J Med Sci. 2015;5:201–3. [Google Scholar]
- 24.Metan SB. Evaluation of plasma fibrinogen levels in type 2 diabetes mellitus and it co relation with HbA1c levels. GJRA Glob J Research Anal. 2017;6:7–9. [Google Scholar]
- 25.Topiwala M, Bahulikar A, Beke N, Khadke V, Phalgune D. Association between glycemic control and serum fibrinogen levels in type 2 diabetes mellitus patients. Int J Adv Med. 2019;6:469–74. [Google Scholar]
- 26.Kumbhalkar SD, Daware MA. Estimation of plasma fibrinogen in type II diabetes mellitus and it's correlation with glycemic control and urine albumin excretion rate. Vidarbha J Intern Med. 2018;24:12–7. [Google Scholar]
- 27.Eckel RH, Kahn SE, Ferrannini E, Goldfine AB, Nathan DM, Schwartz MW, et al. Obesity and type 2 diabetes:What can be unified and what needs to be individualized?J Clin Endocrinol Metab. 2011;96:1654–60. doi: 10.1210/jc.2011-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin QH, Lou YF, Li TL, Chen HH, Liu Q, He XJ. Serum galectin-3:A risk factor for vascular complications in type 2 diabetes mellitus. Chin Med J (Engl) 2013;11:2109–15. [PubMed] [Google Scholar]
- 29.Hodeib H, Hagras MM, Abdelhai D, Watany MM, Selim A, Tawfik MA, et al. Galectin-3 as a prognostic biomarker for diabetic nephropathy. Diabetes Metab Syndr Obes. 2019;12:325–31. doi: 10.2147/DMSO.S194410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allyan F, El Bilbeisi AH, Adris M, Zabut B, El Afifi A, Abu Mustafa A. The association between serum galectin-3 and biochemical parameters with cardiovascular diseases among type 2 diabetes patients in Gaza Strip, Palestine:A case-control study. Austin J Nutri Food Sci. 2020;8:1140. [Google Scholar]
- 31.Cardoso AL, Fernandes A, Aguilar-Pimentel JA, De Angelis MH, Guedes JR, Brito MA, et al. Towards frailty biomarkers:Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214–77. doi: 10.1016/j.arr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Abreu CA, De Lima SV, Mendonca HR, Goulart CO, Martinez AM. Absence of galectin-3 promotes neuroprotection in retinal ganglion cells after optic nerve injury. Histol Histopathol. 2017;32:253–62. doi: 10.14670/HH-11-788. [DOI] [PubMed] [Google Scholar]
- 33.Mendonca HR, Carvalho JNA, Abreu CA, Mariano de Souza Aguiar Dos Santos D, Carvalho JR, Marques SA, et al. Lack of Galectin-3 attenuates neuroinflammation and protects the retina and optic nerve of diabetic mice. Brain Res. 2018;1700:126–37. doi: 10.1016/j.brainres.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Abu El-Asrar AM, Ahmad A, Allegaert E, Siddiquei MM, Alam K, Gikandi PW, et al. Galectin-1 studies in proliferative diabetic retinopathy. Acta Ophthalmol. 2020;98:e1–12. doi: 10.1111/aos.14191. [DOI] [PubMed] [Google Scholar]


