Abstract
In this study we have investigated the antibody and CD4 T-cell responses to the well-characterized malaria vaccine candidate MSP-1 during the course of a primary Plasmodium chabaudi chabaudi (AS) infection. Specific antibody responses can be detected within the first week of infection, and CD4 T cells can be detected after 3 weeks of infection. The magnitude of the CD4 T-cell response elicited during a primary infection depended upon the region of MSP-1. In general, the highest precursor frequencies were obtained when a recombinant MSP-1 fragment corresponding to amino acids 900 to 1507 was used as the antigen in vitro. By contrast, proliferative and cytokine responses against amino acids 1508 to 1766 containing the C-terminal 21-kDa region of the molecule were low. The characteristic interleukin 4 (IL-4) switch that occurs in the CD4 T-cell population after an acute blood stage P. c. chabaudi infection was only consistently observed in the response to the amino acid 900 to 1507 MSP1 fragment. A lower frequency of IL-4-producing cells was seen in response to other regions. Although the magnitudes of the immunoglobulin G antibody responses to the different regions of MSP-1 were similar, the isotype composition of each response was distinct, and there was no obvious relationship with the type of T helper cells generated. Interestingly, a relatively high antibody response to the C-terminal region of MSP-1 was observed, suggesting that T-cell epitopes outside of this region may provide the necessary cognate help for specific antibody production.
Merozoite surface protein 1 (MSP-1) of the malaria parasite is an important molecule involved in invasion of erythrocytes. In Plasmodium falciparum, MSP-1 is synthesized as a large precursor on the surfaces of merozoites (24). Proteolytic cleavage of MSP-1 leaves a C-terminal 19-kDa fragment (MSP-119) on the surface of the parasite, which is necessary for invasion of the erythrocyte (4, 5, 7). The remaining fragments are shed as a soluble complex (6). The C-terminal MSP-119 region is functionally conserved across species of the genus Plasmodium (33), and its tertiary structure is maintained by disulfide bridges (32). Immunization with MSP-119 of P. falciparum MSP-1, or its equivalent in rodent parasites, is able to generate protective immunity (16, 23, 29, 42), and development of MSP-1 as a potential vaccine has, therefore, concentrated on this region of the molecule.
During natural infection of humans, anti-MSP-1 antibodies are generated. However, their role in protective immunity is not known. Some studies show a correlation between antibodies to the C-terminal MSP-119 fragment and clinical immunity (18, 36). Others report no correlation with immunity or a correlation between the ability to clear infection and antibodies specific for N-terminal regions of the molecule (12, 17). Little is known about the repertoire of CD4 T cells responding to MSP-1 in a natural infection or the specificity of CD4 T cells required to provide help in a protective antibody response to MSP-1. We have previously observed that the precursor frequency of CD4 T cells from Plasmodium chabaudi chabaudi-immune mice responding to a region containing the C-terminal 21-kDa region of MSP-1 was lower than that of the response to a more N-terminal region (amino acids [aa] 900 to 1507) (35). This suggests that particular regions of MSP-1 may be immunodominant. Such a bias may influence the nature of MSP-1-specific T-cell help available and, thus, the antibody response. Epitopes recognized by human T cells have been found on P. falciparum MSP-1 (19, 34, 36, 38, 39, 43). There are several reports that suggests that epitopes may be clustered at the N terminus of the full-length molecule, and at the more N-terminal regions of the 42-kDa fragment of the molecule, rather than at the C-terminal 19-kDa fragment (14, 15, 22, 26, 36, 39, 43).
Since an important role for CD4 T cells in the response to MSP-1 is likely to be that of helper cells for the production of specific antibody, it would be of use to know whether effective MSP-1-specific helper T cells are generated and to which region of the molecule these cells respond. The most potent helper T cells are Th2 CD4 T cells, producing cytokines, which are necessary for B-cell growth and maturation (reviewed in reference 13). These cells are generally found at high frequency in P. chabaudi infection after the acute stage of a primary infection (27, 41). It is not known whether this population contains any cells recognizing peptides of MSP-1.
We have compared the precursor frequencies of CD4 T cells generated during a primary infection with P. c. chabaudi which were able to proliferate and produce cytokines characteristic of Th1 cells (gamma interferon [IFN-γ]) or Th2 cells (interleukin 4 [IL-4]) in response to four recombinant fragments making up the complete P. c. chabaudi MSP-1. In conjunction, the isotype of the antibody response to the same regions was measured. We show that the precursor frequency of CD4+ T cells generated in response to the C-terminal region of MSP-1 was low during the 12 weeks following a primary infection. Although the isotype composition of the immunoglobulin G (IgG) response to each fragment of MSP-1 was different, the overall magnitudes of the in vivo antibody responses to all regions were comparable. This suggests that T-cell help for an antibody response to the C terminus of MSP-1 may be provided by other regions of the molecule.
MATERIALS AND METHODS
Mice and parasites.
BALB/c mice were bred at the National Institute for Medical Research under specific-pathogen-free conditions. For experimental use, they were conventionally housed in sterilized cages with sterile bedding and food. P. c. chabaudi (AS) parasites were maintained as described previously (27). Female mice aged 6 to 12 weeks were infected with the blood stages of P. c. chabaudi (AS) by intraperitoneal injection of 105 infected erythrocytes. The course of infection was monitored by examination of Giemsa-stained (BDH, Poole, United Kingdom) blood films.
Preparation of recombinant MSP-1 proteins.
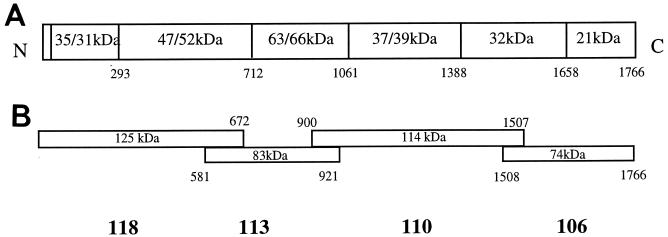
The P. c. chabaudi (AS) MSP-1 gene, cloned in four separate overlapping fragments into the pMalCR1 vector (New England Biolabs, Bishop Stortford, United Kingdom), was a kind gift of Paul McKean and Neil Brown (30, 31) (Division of Parasitology, National Institute for Medical Research, London, United Kingdom). Recombinant MSP-1 fragments (see Fig. 1B) were expressed as fusion proteins with maltose binding protein (MBP) in Escherichia coli as described previously (30, 35). The four MSP-1 fragments corresponded to the following amino acids in the native molecule: 118, aa 1 to 672; 113, aa 581 to 921; 110, aa 900 to 1507; and 106, aa 1508 to 1766. All of the protein fragments could be recognized by serum taken from mice repeatedly infected with P. c. chabaudi (hyperimmune serum [HIS] [see below]), suggesting that at least some of the structure of the native molecule was retained. The integrity of the structurally complex C-terminal fragment 106 was verified by its ability to bind to a monoclonal antibody (NIMP23 [10, 31]) which inhibits parasite growth in vivo (10) and recognizes a conformational epitope requiring disulfide bonding among the first four cysteines in the second epidermal growth factor-like domain (31). The binding of NIMP23 to fragment 106 is abolished by reduction and alkylation (31).
FIG. 1.
Schematic diagram showing the structure of native (A) and recombinant (B) fragments of MSP-1 of P. chabaudi chabaudi (AS) and indicating proteolytic cleavage sites and observed and expected molecular masses of the different polypeptides within the native molecule.
Limiting-dilution analysis of CD4 T-cell responses to MSP-1 antigens.
CD4 T cells were purified from the spleens of immune BALB/c mice at various times postinfection on a MACS column (Miltenyi Biotech, Bisley, Surrey, United Kingdom) as previously described (45). Biotinylated CD4+ T cells were labeled with streptavidin-phycoerythrin (Jackson Laboratories, Bar Harbor, Maine), and the purity of the CD4 T-cell population was routinely greater than 95% as assessed by flow cytometry. Limiting-dilution assays to estimate the precursor frequency of CD4+ T cells from immune mice that respond to malarial antigens were carried out as described previously (27, 44), using recombinant MSP-1 protein fragments as a source of antigen in the culture rather than infected erythrocytes. Recombinant MSP-1 fragments or MBP as a control antigen was added at a final concentration of 2 μM. Cultures without antigen were included as controls.
Microcultures were restimulated with irradiated (30 Gy) normal BALB/c mouse spleen cells (106 per ml) prepared as described previously (44) and MSP-1 fragments or MBP at 20 μM. After a further 48 h, 100 μl of supernatant was removed and used to measure IL-4 and IFN-γ by enzyme-linked immunosorbent assay (ELISA) (44). The microcultures were then pulsed overnight in 100 μl of Iscove's medium containing 1 mCi of [3H]thymidine (Amersham)/ml.
The response of individual microcultures was considered positive when proliferation or cytokine production exceeded the background response (antigen-presenting cells without T cells) by more than 3 standard deviations. Precursor frequencies were determined from the zero-order term of the Poisson distribution. The regression lines were fitted by the method of least squares, and the goodness of fit was determined by linear regression. All data shown have R2 values greater than 0.65.
Measurement of MSP-1-specific antibodies during the course of a P. c. chabaudi (AS) infection.
Mice were infected with 105 P. c. chabaudi (AS) parasites as described above and bled by intracardiac puncture under terminal anesthesia at various time points postinfection. The relative amounts of IgM, IgG, and the individual IgG isotypes specific for the different recombinant fragments of MSP-1 (Fig. 1B) were measured by ELISA. For this purpose, Polysorb ELISA plates (Nunclon, Copenhagen, Denmark) were coated overnight with the different recombinant MSP-1 antigens at 5 μg/ml in phosphate-buffered saline, and the ELISA was carried out as described previously for total P. c. chabaudi parasite lysate (28). MSP-1-specific antibodies were detected by using alkaline phosphatase-conjugated antisera specific for mouse IgM, IgG (all isotypes), IgG1, IgG2a, IgG2b, and IgG3 (Harlan Seralabs, Oxford, United Kingdom). A pool of plasma from BALB/c mice challenged at least six times with P. c. chabaudi (HIS) was used as a standard for each isotype. The amounts of each isotype are calculated in arbitrary units (AU) derived from this HIS standard, as described previously (28). The results are plotted as geometric means with standard errors of the mean number of AU (SEM) of four individual animals at each time point.
RESULTS
CD4+ T-cell proliferative responses to MSP-1 after 12 weeks of primary infection.
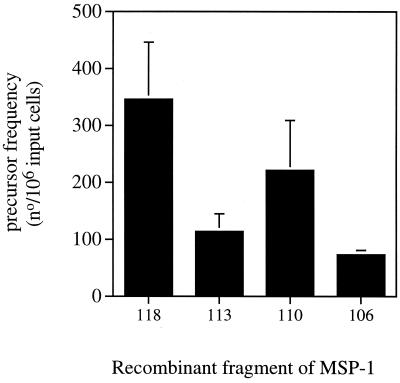
The precursor frequencies of CD4+ T cells of BALB/c mice after recovery from a primary P. c. chabaudi (AS) infection, which proliferated in response to different fragments of recombinant MSP-1 (Fig. 1B), were determined by limiting-dilution assays (Fig. 2). CD4 T cells proliferated in response to all fragments. However, the precursor frequencies measured against fragment 106 (aa 1508 to 1766) incorporating the C-terminal 21-kDa region of the native antigen were significantly lower than those in response to fragment 110 (aa 1508 to 1766) and fragment 118 (aa 1 to 672).
FIG. 2.
Precursor frequency of CD4+ T cells, isolated from mice at 12 weeks postinfection with P. chabaudi, proliferating in response to recombinant MSP-1 fragments. The bars represent the mean precursor frequencies with the SEM of three independent experiments, each using a pool of purified CD4 T cells obtained from two to three mice. The frequencies were calculated as described in Materials and Methods. Proliferation was measured by incorporation of [3H]thymidine. All R2 values were greater than 0.65.
Cytokine responses of CD4 T cells responding to MSP-1.
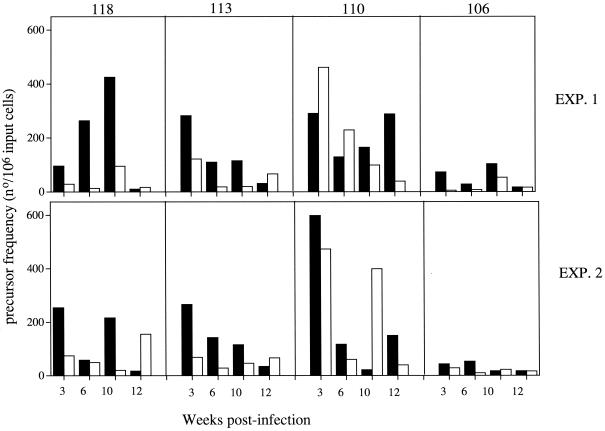
To determine whether the MSP-1-specific CD4 T-cell response switched to a Th2-like response (IL-4 production) after clearance of an acute infection, as has been described in P. c. chabaudi infections (27, 41), the precursor frequencies of IFN-γ- and IL-4-producing CD4 T cells responding to the different fragments of MSP-1 were determined. All regions of MSP-1 elicited IFN-γ and IL-4 responses (Fig. 3). The lowest frequencies were consistently observed in those CD4 T cells responding to fragment 106; at none of the times measured did the frequencies exceed 100 cells per 106 input cells. This is in general agreement with the proliferation data shown in Fig. 2.
FIG. 3.
Precursor frequency of CD4+ T cells producing IFN-γ (■) and IL-4 (□) in response to recombinant MSP-1 fragments and isolated from mice at different times postinfection with P. chabaudi. Two independent experiments (EXP.) consisting of pooled T cells from two to three mice at each time point are shown. The precursor frequencies were calculated as described in Materials and Methods. All R2 values were greater than 0.65.
Comparison of IFN-γ and IL-4 responses for each pool of CD4 T cells at each of the time points shows that only in the case of the response to fragment 110 were there comparable IL-4 responses. For this fragment, IL-4-producing cells were present at high frequency after 3 weeks of infection. By contrast, for fragments 118 and 113 the IFN-γ frequencies remained predominant for 10 weeks following infection but had decreased after 12 weeks of infection. This was not compensated for by a concomitant increase in the frequencies of IL-4-producing cells, with the exception of the response to fragment 118 at 12 weeks in experiment 2.
MSP-1-specific antibodies in plasma during a P. c. chabaudi infection.
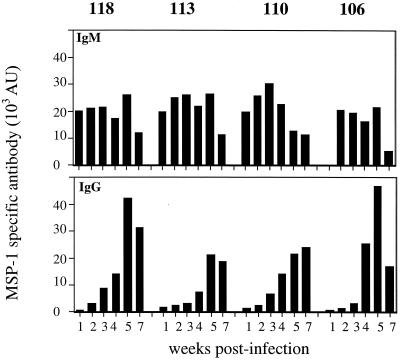
The antibody response to each of the different fragments of MSP-1 was measured in plasma taken at various times during a primary infection. All the fragments were recognized by IgM and IgG antibodies in the plasma of infected mice (Fig. 4). With the exception of fragment 106, an IgM response could be detected by week 1 of infection. After that time, the relative amounts of IgM antibodies specific for each fragment were similar, and they declined by week 7 in all cases. Little IgG was produced until after the peak of parasitemia (after 2 weeks), and production was greatest in response to fragments 118 and 106 (at week 5).
FIG. 4.
Serum antibodies specific for different recombinant MSP-1 antigens at different time points during a P. chabaudi chabaudi (AS) infection of female BALB/c mice. IgM (A) and IgG (B) antibodies were measured by ELISA. The bars represent the geometric means of the amounts of antibodies in the plasma of four to five individual animals expressed as AU. The AU were calculated from a standard HIS as described in Materials and Methods. The SEM were less than 10% of the means and are not shown.
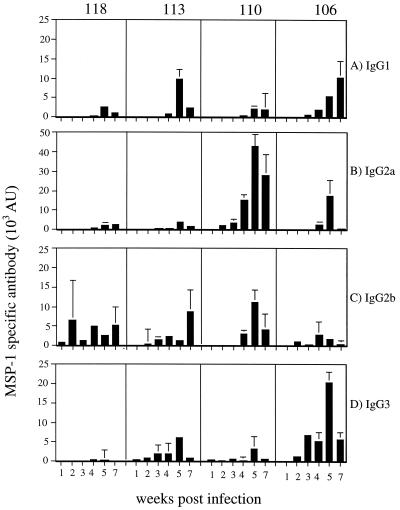
The isotype distribution of the IgG response, however, was different for each fragment (Fig. 5). The largest relative IgG3 and IgG1 responses were made to fragment 106, with negligible responses to fragments 118 and 110. IgG2a antibodies reacted predominantly with fragment 110, and IgG2b antibodies reacted with all fragments, the lowest response being to fragment 106. Therefore, it appears that the response to fragment 118 is largely restricted to antibodies of the IgG2b isotype, whereas the response to fragment 110 is characterized by a relatively large IgG2a response and the response to fragment 106 is characterized by relatively large IgG3 and IgG1 responses.
FIG. 5.
Isotype composition of the antibody response to different recombinant MSP-1 antigens at different times during a P. chabaudi chabaudi (AS) infection of female BALB/c mice. The AU of the different isotypes of IgG1 (A), IgG2a (B), IgG2b (C), and IgG3 (D) were measured as described in the legend to Fig. 4.
DISCUSSION
This study has shown that MSP-1 of P. c. chabaudi elicits an immune response during primary infection in mice. IgM antibodies were present within 1 week, IgG antibodies were present within 2 weeks, and specific CD4 T-cell responses were detectable in the spleen within 3 weeks of infection. Although antibodies specific for all of the conformational epitopes of MSP-1 may not be detected by using these recombinant fragments, recognition of this molecule early in the course of an infection suggests that MSP-1 is a very accessible target of the immune response. As the kinetics of antibody and CD4 T-cell responses following a single infection of P. falciparum have not been reported, direct comparison of these data with human responses is not possible. However, the rapid appearance of IgG antibodies to different parts of MSP-1 after the start of the transmission season, and their presence in sera of young children or adults after limited exposure to P. falciparum (11) or Plasmodium vivax (40), indicate that MSP-1 is also readily recognized by the human immune system. Studies of CD4 T-cell responses of exposed humans to MSP-1 demonstrate the presence of MSP-1-reactive T cells in a proportion of exposed children and adults (18, 19, 36, 43).
All regions of MSP-1 are recognized by CD4 T cells taken from infected BALB/c mice at various times during infection. However, there were clear differences among the responses to the different parts. Throughout the infection, there was a lower precursor frequency of CD4 T cells proliferating in response to fragment 113 and particularly to fragment 106 (corresponding to aa 581 to 921 and 1508 to 1766, respectively). By contrast, the response to fragment 110 (aa 900 to 1507) was generally higher. These differences were also observed in the cytokine responses of CD4 T cells. These data extend our previous observation showing that the CD4 T-cell response to fragment 110 is greater than the response to fragment 106 after 10 weeks of infection (35). The precursor frequencies of IL-4- and IFN-γ-producing cells responding to fragment 106 were also both low compared with that of the response to fragment 110. The IL-4 response to fragment 113 was low, and both cytokine responses to the N-terminal fragment 118 were intermediate and variable. A similar comparison of the frequencies of human T cells responding to different regions of the MSP-1 molecule of P. falciparum or P. vivax MSP-1 has not been made. Some studies using bulk culture assays indicate a lower prevalence of responders among exposed humans to the C-terminal 19-kDa region of P. falciparum (14, 15, 18, 19, 26, 36, 38, 43). Other studies, however, using synthetic peptides or protein fragments of P. vivax do not indicate any obviously lower responses to the 19-kDa region (40). In light of the data reported here, it would be interesting to compare the precursor frequencies of the human CD4 T-cell responses to the different parts of MSP-1.
The reasons for the difference among the responses to the four regions in our studies could be several. It might be that there are few peptides within the C-terminal fragment that can bind to the class II major histocompatibility molecules, I-Ad or I-Ed, thus inducing only a limited T-cell response. There are examples of malaria proteins that do not contain peptides able to bind particular major histocompatibility complex (MHC) molecules (2, 21). However, immunization of mice with recombinant fragments 110 and 106 can induce CD4 T-cell responses in BALB/c mice (S. Quin, unpublished data). This suggests that peptides that bind to I-Ad and I-Ed are processed from both parts of MSP-1. The complex cysteine-bonded structure of the C-terminal part of MSP-1, contained within fragment 106, may temporally hinder the processing of peptides for presentation on MHC class II. Therefore, the amount of peptide from fragment 106 available for stimulating T cells may be less than that from the region of MSP-1 represented by fragment 110. In support of this possibility is the observation by Egan et al. (19) showing that human T-cell responses to MSP-119 in vitro are greater when the disulfide bonds have been reduced, as well as our studies showing that the C-terminal region of P. chabaudi MSP-1 is presented more slowly than fragment 110 (35) and is processed only in the classical de novo MHC class II pathway compared with fragment 110, which is rapidly processed within the recycling class II pathway. Reduction of the disulfide bonds allows processing of fragment 106 in the recycling pathway and speeds up processing and presentation (35). Whether these differences in processing observed in vitro are directly responsible for the lower CD4 T-cell-responses seen in vivo is currently under investigation.
CD4 responses to the individual MSP-1 fragments did not follow the kinetics of the overall malaria-specific CD4 T-cell response previously reported for P. c chabaudi (AS) infections, in which there is switch from a predominantly Th1 response during the first 3 weeks of infection to a more pronounced Th2 response after that time (27, 45). Only in the response to fragment 110 was there a high frequency of IL-4-producing cells, which was generally equivalent to, or greater than, the frequency of IFN-γ-producing cells at the times measured. The frequency of cytokine-producing cells was either low overall (fragment 106) or strongly biased towards an IFN-γ response (fragments 118 and 113). These data suggest that the signals for CD4 T-cell differentiation may be different for the responses to different regions of MSP-1. Since polarization towards a Th1 or a Th2 response can depend to a large extent on the nature of the antigen-presenting cell, antigen dose, duration of stimulus, cell cycle, and cytokine environment (3, 13, 20, 25), a detailed analysis of antigen presentation and the amounts of MSP-1 peptides presented from the shed complex and the membrane-bound C-terminal region may give some clue. In this regard, the more rapid processing and presentation of fragment 110 by dendritic cells (35) may result in an increase both in the amount of peptide presented and in the relative duration of the stimulation, thus favoring the development of Th2 cells (25).
Despite a relatively poor T-cell response to the C terminus of MSP-1 in vivo, both IgM and IgG antibodies specific for this region which were similar in magnitude to the antibody response to other regions of the molecule were observed. The reason for the later appearance of IgM antibodies is unknown but may be due to the lower frequency of 106-reactive CD4 T cells able to provide cognate T-cell help. However, the subsequent switch giving rise to equivalent IgG antibody titers suggests either that the low MSP-121-specific T-cell response is eventually sufficient to provide B-cell help or that T cells specific for regions outside of the C terminus, or specific for other parasite proteins, are able to provide the necessary help for antibody production in vivo.
Although the amounts of the total specific IgG were comparable during the infection, there were differences in the relative amounts of the IgG isotypes specific for the different regions. Antibodies of all isotypes recognized fragment 106, with the levels of IgG1 and IgG3 relatively high compared with the responses to the other fragments. The response to fragment 110 was characterized by a predominant IgG2a response, and the response to fragment 118 was restricted to IgG2b antibodies. Immunization of mice with different Plasmodium yoelii MSP-1 fusion proteins in the presence of adjuvant has shown that all regions of the molecule can elicit similar patterns of IgG isotypes (1, 42). This response can be modulated using known T-cell epitopes from other proteins linked to recombinant MSP-119 (1). Together, these data lend support to the view that the nature of T-cell help and the environment in which T-cell help and/or B-cell differentiation and isotype switching is taking place are important, and these may not be the same for each region of MSP-1. However, there was no obvious correlation between the isotype in the plasma and the IFN-γ or IL-4 responses of specific CD4 T cells. In fact, the CD4 T-cell response to fragment 110 was composed of both IL-4 and IFN-γ-producing cells, while the antibody response was characterized by a large IgG2a component.
It is not yet clear whether isotypes such as those promoting phagocytosis or antibody-dependent cellular cytotoxicty or inhibition (8, 9) are necessary for protective immunity induced by MSP-1 in humans. Mouse studies with P. yoelii would suggest they are not (37, 46). Nevertheless, knowledge of the regulation of the antibody response to MSP-1 and the specificity of CD4 helper T cells involved would be useful. If there are similar restrictions in the CD4 T-cell response to P. falciparum MSP-1, it may be worthwhile to consider the use of regions outside of the C terminus in a vaccine in order to provide more effective T-cell help.
ACKNOWLEDGMENTS
This study was funded by the Medical Research Council and the Wellcome Trust (S.J.Q. is the recipient of a Wellcome Trust Prize Studentship).
We thank Pearline Benjamin for her skilled technical assistance and Tony Holder, Elsa Seixas, Ching Li, and Meike Hensmann for their critical review of this paper.
REFERENCES
- 1.Ahlborg N, Ling I T, Holder A A, Riley E M. Linkage of exogenous T-cell epitopes to the 19-kilodalton region Plasmodium yoelii merozoite surface protein-1 (MSP119) can enhance protective immunity against malaria and modulate the immunoglobulin subclass response to MSP119. Infect Immun. 2000;68:2102–2109. doi: 10.1128/iai.68.4.2102-2109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berzofsky J A, Cease J L, Cornette J L, Spouge J L, Margalit H, Berkower I, Good M F, Miller L H, DeLisi C. Protein antigenic structures recognized by T cells: potential application to vaccine design. Immunol Rev. 1987;98:9–52. doi: 10.1111/j.1600-065x.1987.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 3.Bird J, Brown D R, Mullen A, Moskowitz N H, Mahowald M, Sider J, Gajewski T F, Wang C R, Reiner S L. Helper T cell differentiation is controlled by cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 4.Blackman M, Heidrich H G, Donachie S, Mc.Bride J, Holder A. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990;172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackman M, Ling I, Nicholls S, Holder A. Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol Biochem Parasitol. 1991;49:29–34. doi: 10.1016/0166-6851(91)90127-r. [DOI] [PubMed] [Google Scholar]
- 6.Blackman M, Scott-Finnigan T, Shai S, Holder A. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med. 1994;180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackman M, Whittle H, Holder A. Processing of the Plasmodium falciparum major merozoite surface protein 1: identification of a 33-kilodalton secondary processing product which is shed prior to erythrocyte invasion. Mol Biochem Parasitol. 1991;49:35–44. doi: 10.1016/0166-6851(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 8.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongcuphajaisiddi T, Druilhe P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouharoun-Tayoun H, Oeuvray C, Druilhe P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–418. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle D B, Newbold C I, Smith C C, Brown K N. Monoclonal antibodies that protect in vivo against Plasmodium chabaudi recognize a 250,000-dalton parasite polypeptide. Infect Immun. 1982;38:94–102. doi: 10.1128/iai.38.1.94-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavanagh D, Elhassan I, Roper C, Robinson J, Giha H, Holder A, Hviid L, Theander L, Ar D, McBride J. A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J Immunol. 1998;161:347–359. [PubMed] [Google Scholar]
- 12.Chizzolini C, Dupont A, Akue J, Kaufman H, Verdini A, Pessi A, Del Giudice G. Natural antibodies against three distinct and defined antigens of P. falciparum in residents of a mesoendemic area of Gabon. Am J Trop Med Hyg. 1988;39:150–156. doi: 10.4269/ajtmh.1988.39.150. [DOI] [PubMed] [Google Scholar]
- 13.Constant S L, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 14.Crisanti A, Fruh K, Muller H, Bujard H. The T cell reactivity against the major merozoite protein of Plasmodium falciparum. Immunol Lett. 1990;25:143–148. doi: 10.1016/0165-2478(90)90106-z. [DOI] [PubMed] [Google Scholar]
- 15.Crisanti A, Muller H, Hilbich C, Sinigaglia F, Matile H, McKay M, Scaife J, Beyreuther K, Bujard H. Epitopes recognised by human T cells map within the conserved part of the GP190 of P. falciparum. Science. 1988;240:1324–1326. doi: 10.1126/science.2453924. [DOI] [PubMed] [Google Scholar]
- 16.Daly T, Long C. A recombinant 15-kilodalton carboxyl terminal fragment of Plasmodium yoelii yoelii 17XL merozoite surface protein-1 induces a protective response in mice. Infect Immun. 1993;61:2462–2467. doi: 10.1128/iai.61.6.2462-2467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodoo D, Theander T, Kurtzals J, Koram K, Riley E, Akanmori B D, Nkrumah F, Hviid L. Levels of antibody to conserved parts of Plasmodium falciparum merozoite surface protein-1 in Ghanaian children not associated with protection from clinical malaria. Infect Immun. 1999;67:2131–2137. doi: 10.1128/iai.67.5.2131-2137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egan A, Morris J, Barnish G, Allen S, Greenwood B, Kaslow D, Holder A, Riley E. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the Merozoite Surface Antigen, PfMSP-1. J Infect Dis. 1996;173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 19.Egan A, Waterfall M, Pinder M, Holder A, Riley E. Characterisation of human T- and B-cell epitopes in the C terminus of Plasmodium falciparum merozoite surface protein 1: evidence for poor T cell recognition of polypeptides with numerous disulfide bonds. Infect Immun. 1997;65:3024–3031. doi: 10.1128/iai.65.8.3024-3031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gett A V, Hodgkin P D. Cell division regulates T cell cytokine repertoire revealing a mechanism underlying immune class regulation. Proc Natl Acad Sci USA. 1998;95:9488–9493. doi: 10.1073/pnas.95.16.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good M F, Berzofsky J A, Maloy W L, Hayashi I, Fujii N, Hockmeyer W T, Miller L H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoide vaccine: widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986;164:655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guttinger M, Romagnoli P, Vandel L, Meloen R, Takacs B, Pink J R L, Sinigaglia F. HLA polymorphism and recognition of a conserved region of P170, a malaria vaccine candidate. Int Immunol. 1991;3:899–906. doi: 10.1093/intimm/3.9.899. [DOI] [PubMed] [Google Scholar]
- 23.Hirunpetcharat C, Tian J, Kaslow D, Rooijen N, Kumar S, Berzofsky J, Miller L, Good M. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of Merozoite Surface Protein-1 of Plasmodium yoelii expressed in Saccharomyces cerevisiae. J Immunol. 1997;159:3400–3411. [PubMed] [Google Scholar]
- 24.Holder A, Freeman R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J Exp Med. 1982;156:1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iezzi G, Scotet E, Scheidegger D, Lanzavecchia A. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur J Immunol. 1999;29:4092–4101. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Kabilan L, Sharma V, Kaur P, Ghosh K, Yadav R, Chauhan V. Cellular and humoral immune responses to well-defined blood stage antigens (major merozoite surface antigen) of Plasmodium falciparum in adults from an Indian zone where malaria is endemic. Infect Immun. 1994;62:685–691. doi: 10.1128/iai.62.2.685-691.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langhorne J, Gillard S, Simon B, Slade S, Eichmann K. Frequencies of CD4 T cells reactive with Plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int Immunol. 1989;1:416–424. doi: 10.1093/intimm/1.4.416. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Langhorne J. Tumor necrosis factor alpha p55 receptor is important for development of memory responses to blood stage malaria infection. Infect Immun. 2000;68:5724–5730. doi: 10.1128/iai.68.10.5724-5730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling I, Ogun S, Holder A. Immunisation against malaria with a recombinant protein. Parasite Immunol. 1994;16:63–67. doi: 10.1111/j.1365-3024.1994.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 30.McKean P, O'Dea K, Brown K. Nucleotide sequence analysis and epitope mapping of the merozoite surface protein 1 from Plasmodium chabaudi AS. Mol Biochem Parasitol. 1993;62:199–210. doi: 10.1016/0166-6851(93)90109-b. [DOI] [PubMed] [Google Scholar]
- 31.McKean P, O'Dea K, Brown K. A single amino acid determines the specificity of a monoclonal antibody which inhibits Plasmodium chabaudi AS in vivo. Mol Biochem Parasitol. 1993;62:211–222. doi: 10.1016/0166-6851(93)90110-j. [DOI] [PubMed] [Google Scholar]
- 32.Morgan W, Birdsall B, Frenkiel T, Gradwell M, Burghaus P, Syed S, Uthaipibull C, Holder A, Feeney J. Solution structure of an EGF module pair from the Plasmodium falciparum merozoite surface protein-1. J Mol Biol. 1999;289:113–122. doi: 10.1006/jmbi.1999.2753. [DOI] [PubMed] [Google Scholar]
- 33.O'Donnell R A, Saul A, Cowman A, Crabb B S. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nat Med. 2000;6:91–95. doi: 10.1038/71595. [DOI] [PubMed] [Google Scholar]
- 34.Quakyi I A, Currier J, Fell A, Taylor D W, Houghton R A, England R D, Bersofsky J A, Miller L H, Good M F. Analysis of human T cell clones specific for conserved peptide sequences within malaria proteins. Paucity of clones responsive to intact parasites. J Immunol. 1994;153:2082–2092. [PubMed] [Google Scholar]
- 35.Quin S, Seixas E, Cross C A, Berg M, Lindo V, Langhorne J. Low CD4+ T cell responses to the C-terminal region of the malaria merozoite surface protein 1 may be attributed to processing within distinct MHC Class II pathways. Eur J Immunol. 2001;31:72–81. doi: 10.1002/1521-4141(200101)31:1<72::aid-immu72>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 36.Riley E, Allen S, Wheeler J, Blackman M, Bennett S, Takacs B, Schonfeld H, Holder A, Greenwood B. Naturally acquired cellular and humoral immune responses to the major merozoite surface antigen (PfMSP-1) of Plasmodium falciparum are associated with reduced malaria morbidity. Parasite Immunol. 1992;14:321–337. doi: 10.1111/j.1365-3024.1992.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 37.Rotman H, Daly T, Long C. Plasmodium: immunisation with carboxyl-terminal regions of MSP-1 protects against homologous but not heterologous blood-stage parasite challenge. Exp Parasitol. 1999;91:78–85. doi: 10.1006/expr.1999.4357. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y P, Sayed U, Shoukat S H, Roberts J M, Udhayakumar V, Oloo A J, Hawley W A, Kaslow D C, Nahlen B L, Lal A A. Natural immune reponses to the C-terminal 19-kilodalton domain of Plasmodium falciparum merozoite surface protein-1. Infect Immun. 1996;64:2716–2723. doi: 10.1128/iai.64.7.2716-2723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinigaglia F, Takacs B, Jacot H, Matile H, Pink J, Crisanti A, Bujard H. Nonpolymorphic regions of p190, a protein of the Plasmodium falciparum erythrocytic stage, contains both T and B cell epitopes. J Immunol. 1988;140:3568–3572. [PubMed] [Google Scholar]
- 40.Soares I S, Leitus G, Souza J M, Del Portillo H A, Rodrigues M. Acquired immunity to the N- and C-terminal regions of Plasmodium vivax merozoite surface protein 1 in individuals exposed to malaria. Infect Immun. 1997;65:1606–1614. doi: 10.1128/iai.65.5.1606-1614.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevenson M M, Tam M F. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin Exp Immunol. 1993;91:77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian J, Kumar S, Kaslow D, Miller L. Comparison of protection induced by immunization with recombinant proteins from different regions of merozoite surface protein 1 of Plasmodium yoelii. Infect Immun. 1997;65:3032–3036. doi: 10.1128/iai.65.8.3032-3036.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Udhayakumar V, Anyona D, Kariuki S, Shi Y, Bloland P, Branch O, Weiss W, Nahlen B, Kaslow D, Lal A. Identification of T and B cell epitopes recognized by humans in the C-terminal 42kDa domain of the Plasmodium falciparum merozoite surface protein 1. J Immunol. 1995;154:6022–6030. [PubMed] [Google Scholar]
- 44.von der Weid T, Kopf M, Kohler G, Langhorne J. The immune response to Plasmodium chabaudi malaria in interleukin-4 deficient mice. Eur J Immunol. 1994;24:2285–2293. doi: 10.1002/eji.1830241004. [DOI] [PubMed] [Google Scholar]
- 45.von der Weid T, Langhorne J. Altered response of CD4+ T cell subsets to Plasmodium chabaudi chabaudi in B cell-deficient mice. Int Immunol. 1993;5:1343–1348. doi: 10.1093/intimm/5.10.1343. [DOI] [PubMed] [Google Scholar]
- 46.Vukovic P, Hogarth M, Barnes N, Kaslow D C, Good M. Immunoglobulin G3 antibodies specific for the 19-kilodalton carboxyl-terminal fragment of Plasmodium yoelii merozoite surface protein 1 transfer protection to mice deficient in Fc-γR1 receptors. Infect Immun. 2000;68:3019–3022. doi: 10.1128/iai.68.5.3019-3022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]