Abstract
It was previously demonstrated that Salmonella enterica serovar Typhimurium induces cell death with features of apoptosis in murine macrophages. Mice infected with Salmonella serovar Typhimurium develop systemic disease without diarrhea, whereas the infection in cattle and in humans is localized and characterized by diarrhea. Considering these clinical disease expression differences between mice and cattle, we investigated whether serovar Typhimurium is cytotoxic for bovine macrophages. Macrophages infected with serovar Typhimurium grown in the logarithmic phase quickly underwent cell death. Macrophages infected with stationary-phase cultures or with a mutant lacking sipB underwent no immediate cell death but did develop delayed cytotoxicity, undergoing cell death between 12 and 18 h postinfection. Both pathways were temporarily blocked by the general caspase inhibitor Z-VAD-Fmk and by the caspase 1 inhibitor Z-YVAD-Fmk. Comparisons of macrophages from cattle naturally resistant or susceptible to intracellular pathogens indicated no differences between these two genetic backgrounds in terms of susceptibility to serovar Typhimurium-induced cell death. We conclude that Salmonella serovar Typhimurium induces cell death in bovine macrophages by two distinct mechanisms, early sipB-mediated and delayed sipB-independent mechanisms.
Salmonellosis is one of the most important human enteric diseases worldwide. It is the most prevalent food-borne infection in the United States, where the number of infections has been estimated to range from 800,000 to 3,700,000 annually (4). Salmonella infections display a broad range of clinical manifestations that are dependent on both the host species and the serotype causing the infections (8). Murine infection by Salmonella enterica serovar Typhimurium has been used extensively as a model for human salmonellosis. However, the clinical disease caused by Salmonella serovar Typhimurium in mice is more similar to the nondiarrheal human systemic typhoid fever caused by S. enterica serovar Typhi than to the diarrheal syndrome in humans infected with serovar Typhimurium (32). In contrast, in cattle, serovar Typhimurium causes an enteric disease, characterized by diarrhea and dehydration, which infrequently progresses toward a systemic infection (8, 13, 35, 42). The pathogenesis of salmonellosis in mice has been linked to the ability of the organism to invade intestinal epithelial cells, preferentially M cells, and the ability to survive inside phagocytic cells (11, 12, 14, 19). Although it has also been demonstrated that serovar Typhimurium invades the intestinal epithelium in cattle, initially through M cells, and then undergoes phagocytosis by macrophages (13), the role of intracellular survival in the pathogenesis of diarrhea is not clear. On the other hand, a functional Salmonella pathogenicity island (SPI) 1 (SPI-1) is required for virulence and diarrhea in cattle (35).
A large number of the virulence genes of Salmonella are located in restricted regions of the genome called SPIs. Five SPIs have been identified so far (3, 15, 25, 40, 41). SPI-1, located at 63 min on the Salmonella serovar Typhimurium chromosome map, is a 40-kb segment that encodes a type III secretion system. Proteins secreted by SPI-1 are involved in cell invasion and in the induction of apoptosis in murine macrophages (reviewed in reference 7). SPI-2 at 31 min on the chromosome map is 40 kb long and encodes a type III secretion system that plays a role in intracellular survival (6, 25).
In vitro infection with virulent Salmonella serovar Typhimurium induces apoptosis in mouse macrophages and macrophage cell lines, such as J774 and RAW264.7 (5, 21, 23). The cytotoxicity of serovar Typhimurium observed at 2 h postinfection is related to the capacity of this organism to invade, but not with intracellular replication (23). Mutants lacking invasion proteins encoded by SPI-1 failed to induce apoptosis in murine macrophages at 2 h postinfection (5, 23). This cytotoxic phenotype is dependent on the stage of bacterial growth, since cultures in the logarithmic phase of growth are cytotoxic, whereas stationary-phase cultures are not (22). The ability of logarithmically growing Salmonella to induce apoptosis correlates with the expression of invasion proteins encoded by SPI-1, such as the secreted protein, SipB, the regulator of SPI-1 expression, HilA, and a structural protein of the type III secretion apparatus, PrgH. On the other hand, cultures in the stationary phase of growth do not express these proteins (22). Furthermore, the cytotoxicity observed at 2 h after infection of macrophages is dependent specifically on SipB which, after translocation to the macrophage cytoplasm, binds to and activates caspase 1, triggering apoptotic cell death. Activated caspase 1 cleaves the interleukin-1β precursor to give rise to the active proinflammatory cytokine, which may be released after cell death. This proposed mechanism of pathogenicity may be important in vivo for the induction of an inflammatory response (16). A similar mechanism had been previously proposed for Shigella-induced apoptosis. Here, IpaB, which is orthologous to the Salmonella invasion protein SipB, also binds to caspase 1, thereby triggering the release of inflammatory cytokines (17).
In addition to the cell death induced by the SPI-1 gene products, which occurs soon after infection, another pathway of cell death has been described for a mouse macrophage cell line. In this pathway, the cytotoxicity is delayed compared to that induced by SPI-1 and is not dependent on the expression of invasion genes. However, mutants lacking ompR do not have the late cytotoxic phenotype (21). The ompR gene is a regulator for the expression of the type III secretion system encoded by SPI-2 (20).
Considering the differences in clinical manifestations between Salmonella serovar Typhimurium infection in mice, a typhoid fever model, and the diarrheal disease caused in cattle, it is important to determine whether or not bovine macrophages are susceptible to the cytotoxic mechanisms of serovar Typhimurium. The variability in the susceptibility of host cells to bacterial infection is illustrated by Shigella infection, in which apoptosis induced in mouse macrophages is mediated by IpaB but in which cell death in human macrophages is induced by a nonapoptotic pathway (10).
Since SPI-1 invasion genes are required for enteropathogenicity in cattle (2, 35, 36, 38) and SipB, an SPI-1-encoded protein, can induce in murine macrophages apoptosis that is followed by the release of inflammatory mediators, it is possible that the induction of cell death in bovine macrophages by Salmonella serovar Typhimurium infection is involved in the pathogenesis of diarrhea. Thus, as a first step in addressing this question, this study was aimed at determining whether bovine monocyte-derived macrophages undergo cell death after serovar Typhimurium infection and whether SipB and caspases are involved in such a mechanism.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Salmonella serovar Typhimurium strain IR715 (31), a spontaneous nalidixic acid-resistant derivative of strain ATCC 14028, was used in this study. A derivative of ATCC 14028 carrying a nonpolar sipB deletion has been described by Tsolis et al. (34).
Bacteria were grown in 5 ml of Luria-Bertani (LB) broth for 20 h at 37°C under agitation (230 rpm). Then, 50 μl of the bacterial suspension was reinoculated into 5 ml of fresh LB broth and incubated under the same conditions as those described above for 5 h to obtain a logarithmic-phase inoculum and for 20 h to obtain a stationary-phase inoculum.
Animals.
Six crossbred cattle (one bull and five cows) ranging in age from 6 to 15 years were used. They were kept in U.S. Department of Agriculture-approved facilities and received hay, 10 lb of commercial food daily, mineral and vitamin supplements, and water ad libitum. The cattle were divided into two groups—naturally resistant (n = 3) and susceptible (n = 3) to intracellular pathogens—according to criteria previously reported (9, 26, 27). Except for the comparison between resistant and susceptible animals, all of the experiments were conducted using cells from a resistant cow.
Peripheral blood monocyte-derived macrophage isolation, culturing, and infection.
The protocol used for monocyte isolation was described previously (27). Briefly, venous blood was collected into anticoagulant (acid-citrate-dextrose), diluted 1:2 in phosphate-buffered saline (PBS)–citrate (pH 7.4), layered over a Percoll (Amersham Pharmacia Biotech AB, Uppsala, Sweden) solution with a specific density of 1.0770 (mixture of the following solutions: 10:1 Percoll and 1.5 M NaCl in 1.2% NaH2PO4; 130 mM trisodium citrate; 5% bovine serum albumin; and PBS [adjusted for a final refractive index of 1.3460]), and centrifuged at 1,000 × g for 30 min. The coat containing white blood cells was collected, washed in PBS-citrate, resuspended in supplemented RPMI medium (Gibco BRL, Life Technologies, Inc., Grand Island, N.Y.) with 4% autologous serum, and incubated at 37°C with 5% CO2 overnight in Teflon flasks. Then, the medium containing the nonadherent cells was removed and replaced with supplemented RPMI medium with 12.5% autologous serum. The medium was changed every 3 days. The monocytes differentiated into macrophages after 7 to 10 days in culture. All the experiments were conducted with cells kept in cultures for 10 to 11 days.
For inoculation, the bacterial suspension was diluted in supplemented RPMI medium. A multiplicity of infection (MOI) of 50:1 was used for all experiments, since preliminary experiments showed that with MOIs of 10:1 and 100:1, high percentages of cells (mean and standard deviation, 83.65% ± 3.23% and 97.02% ± 3.24%, respectively) were infected in our system. The inoculation was followed by centrifugation (500 × g, 5 min) and incubation at 37°C in 5% CO2 for 30 min. Subsequently, gentamicin (Gibco BRL) was added to the medium to a final concentration of 25 μg/ml in order to kill extracellular bacteria.
Cytotoxic assay.
Macrophages were harvested from Teflon flasks by placing the flasks on ice and then were resuspended in supplemented RPMI medium with 12.5% heat-inactivated autologous serum to make a suspension of 5 × 105 cells/ml. The macrophages were seeded in 96-well plates (50,000 cells/well), centrifuged (500 × g, 5 min), and incubated overnight at 37°C in 5% CO2. At 1, 6, 12, or 18 h after inoculation, the cells were fixed with 1.85% formaldehyde in PBS for 15 min, stained with 0.13% crystal violet for 2.5 h, and washed extensively. Absorption was measured by use of a microplate reader with a 630-nm filter (Dynatech Laboratories, Inc., Chantilly, Va.). The readings obtained for the uninfected wells were considered to represent 100% survival, and the survival of the infected cells was calculated based on the reading for the uninfected control [(A630 for infected cells/A630 for uninfected control) × 100]. For some experiments, cells were incubated with either a general caspase inhibitor, Z-VAD-Fmk, or the caspase 1 inhibitor Z-YVAD-Fmk or Z-WEHD-Fmk (Enzyme System Products, Dublin, Calif.) (33) for 1 h prior to inoculation.
TUNEL analysis of DNA.
For terminal deoxyribonucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays, macrophages were inoculated with Salmonella serovar Typhimurium in Teflon flasks (2 × 106 cells/flask). TUNEL analysis was performed using a commercial kit (Pharmingen, San Diego, Calif.) in accordance with the manufacturer's instructions, except for an additional incubation with purified mouse immunoglobulin G (Sigma, St. Louis, Mo.). The macrophages were harvested by placement on ice at 0, 20, 60, or 180 min after inoculation and incubation for 30 min as described above. The cells were fixed in 1% paraformaldehyde in PBS for 15 min on ice, washed, and stored in 70% ethanol at −20°C for 2 to 4 days. The cells were incubated with a labeling solution containing terminal deoxyribonucleotidyltransferase and bromo-dUTP, followed by washes and incubation with purified mouse immunoglobulin G, fluorescein-labeled antibody to bromo-dUTP, and finally propidium iodide. The cells were then analyzed by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, Calif.). Flow cytometric data were analyzed with Flow Jo (Tree Star, Inc., Palo Alto, Calif.).
Assessment of bacterial uptake and intracellular survival.
Bacterial uptake and intracellular survival were assessed following a protocol previously described but with modifications (27). Macrophages were seeded in 96-well plates (40,000 cells/well) and incubated overnight (37°C, 5% CO2). After inoculation, centrifugation, and incubation for 30 min (37°C, 5% CO2), gentamicin was added to the medium to a final concentration of 25 μg/ml. The cells were incubated for 1 h and washed four times with 100 μl of fresh medium per well. At 1 and 6 h after inoculation, the macrophages were lysed by the addition of 0.5% Tween 20 (Sigma). After the wells were washed three times, samples were diluted and plated on LB agar plates to enumerate CFU. As a control, the inoculum was grown in the absence of macrophages under the same conditions, except for the addition of gentamicin, to ensure that the bacteria survived and grew. Each inoculum was also incubated with medium containing gentamicin for 1 h to confirm the activity of the antibiotic.
Statistical analysis.
The quantitative data were submitted to analysis of variance, and the averages were compared by using the Duncan test. Percentage data underwent angular transformation before statistical analysis. Differences were considered significant when P was <0.05 (30).
RESULTS
Salmonella serovar Typhimurium induces DNA fragmentation (TUNEL) in bovine macrophages infected with both logarithmic- and stationary-phase cultures.
Logarithmic-phase cultures of Salmonella serovar Typhimurium express SPI-1 genes (22), which have previously been shown to be necessary for diarrhea in calves (34, 35). In murine macrophages, stationary-phase cultures of Salmonella serovar Typhimurium induce a late form of cell death in a sipB-independent manner, while bacteria grown logarithmically cause cell death by an early, sipB-dependent pathway (37). Therefore, we addressed the questions of whether Salmonella serovar Typhimurium is cytotoxic for bovine monocyte-derived macrophages and whether the cell death in this system is dependent on the stage of bacterial growth.
Macrophages were infected with wild-type Salmonella serovar Typhimurium grown to logarithmic or stationary phase and processed for TUNEL and flow cytometric analysis. Macrophages were harvested at 0, 20, 60, and 180 min after infection. A relative increase in the numbers of TUNEL-positive cells was observed in infected samples compared to the low background observed in uninfected controls. Although both logarithmic- and stationary-phase bacteria induced DNA fragmentation (TUNEL), the percentages of labeled cells were higher in the samples infected with logarithmically growing bacteria at all times studied. This difference was statistically significant at 20 and 60 min after infection. Table 1 summarizes the results (means and standard deviations) for five independent experiments.
TABLE 1.
Percentages of TUNEL-positive bovine macrophages (apoptotic cells) infected with logarithmic- or stationary-phase Salmonella serovar Typhimurium at 0, 20, 60, and 180 min after infection
| Time (min) | % of TUNEL-positive macrophages in the following samplesa:
|
||
|---|---|---|---|
| Uninfected control | Logarithmic phase | Stationary phase | |
| 0b | 14.47 ± 15.42 x | 47.75 ± 34.45 Ay | 38.51 ± 39.49 xy |
| 20 | 5.40 ± 6.20 x | 75.54 ± 28.34 ABy | 42.51 ± 28.88 z |
| 60 | 6.65 ± 5.36 x | 81.32 ± 26.97 By | 35.57 ± 31.92 x |
| 180 | 2.78 ± 1.88 x | 93.41 ± 5.53 By | 71.22 ± 13.39 y |
Values are means and standard deviations from five independent experiments. Different uppercase letters in the same column indicate significant difference (P < 0.05). Different lowercase letters in the same row indicate significant difference (P < 0.05).
Time zero corresponds to macrophages incubated for 30 min at 37°C after challenge with Salmonella serovar Typhimurium (see Materials and Methods).
A mutation in sipB reduces early Salmonella serovar Typhimurium-induced DNA fragmentation in bovine macrophages.
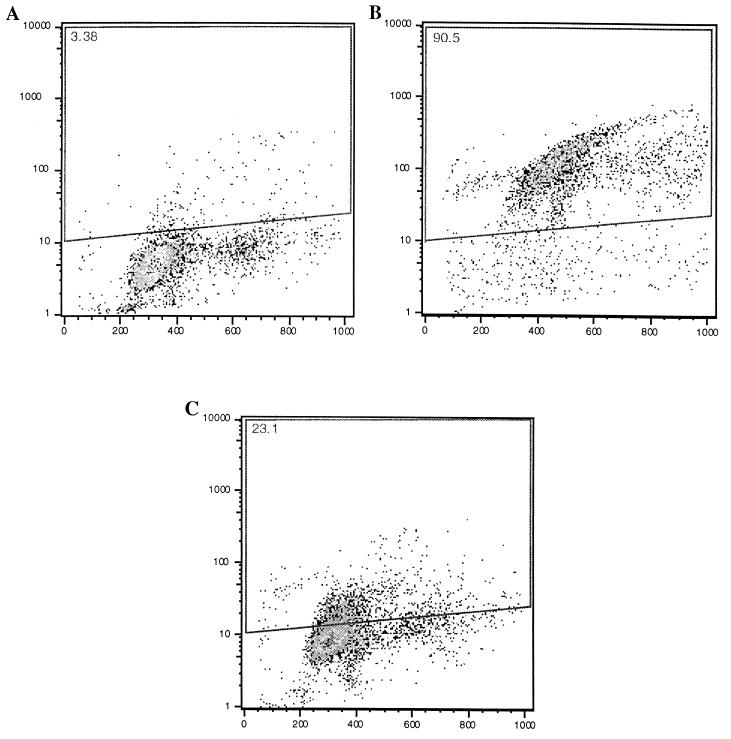
In the mouse model, it has been demonstrated that SipB is directly responsible for the induction of apoptosis by binding to caspase 1 (16). We therefore determined whether sipB is involved in the DNA fragmentation observed after infection of bovine macrophages with logarithmic-phase Salmonella serovar Typhimurium. Bovine macrophages were infected with either wild-type Salmonella serovar Typhimurium or a nonpolar mutant lacking sipB, both grown to logarithmic phase. One hour after infection, the cells were fixed, processed for TUNEL, and analyzed by flow cytometry. Significant differences in the percentages of cells showing TUNEL were observed between cultures infected with the wild type and those infected with the sipB mutant (P < 0.05). Higher percentages (means and standard deviations) of wild-type-infected macrophages (92.24% ± 2.71%; n = 3) than of macrophages infected with the sipB mutant (36.69% ± 20.58%; n = 3) were TUNEL positive. However, the sipB mutant was still able to induce DNA fragmentation in a fraction of cells that was significantly larger (P < 0.05) than the fraction of TUNEL-positive cells in uninfected controls (2.74% ± 0.48%; n = 3). These data indicate that early DNA fragmentation is at least partially associated with the secretion of SipB, although a low level of DNA fragmentation still can be observed in a mutant lacking sipB (Fig. 1).
FIG. 1.
Flow cytometric analysis of bovine macrophages infected with wild-type Salmonella serovar Typhimurium or a sipB mutant. All of the strains were grown to the logarithmic phase. Macrophages were infected in Teflon flasks with an MOI of approximately 50:1, harvested 1 h after infection, processed for TUNEL staining (see Materials and Methods), and analyzed by flow cytometry. In each dot plot, the x axis corresponds to propidium iodide staining and the y axis corresponds to bromo-dUTP incorporation. Apoptotic cells are within the area indicated by the quadrilateral, and the percentage of apoptotic cells is indicated at the top left corner of each panel. These data are from a representative experiment showing uninfected macrophages (A) with a low background of TUNEL-positive cells, macrophages infected with the wild type (B) and containing a high percentage of apoptotic cells, and macrophages infected with a mutant lacking sipB (C) and having a rate of apoptosis that was low but higher than that of the control.
The early cytotoxic effect of Salmonella serovar Typhimurium is sipB dependent, whereas delayed cytotoxicity is sipB independent, and both pathways require caspase activity.
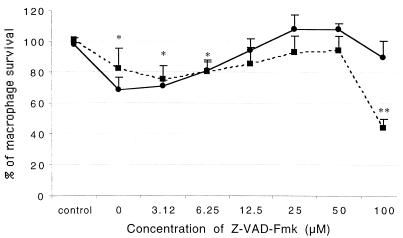
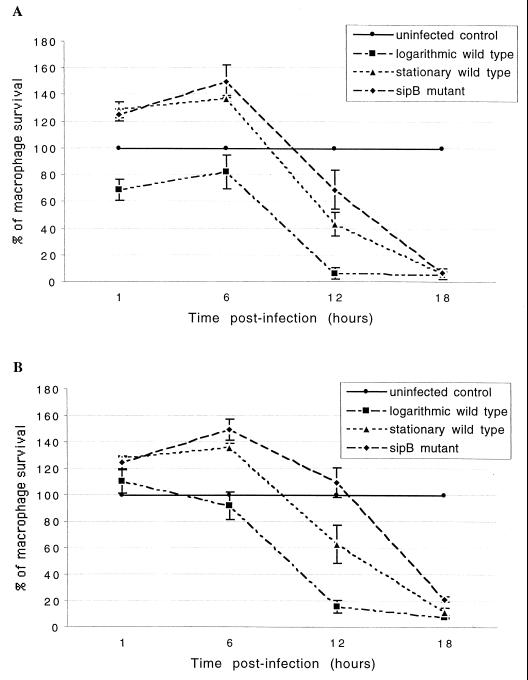
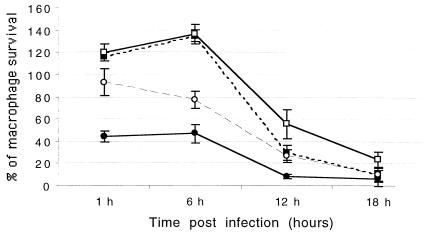
To determine whether sipB-mediated cell death involves caspase activity, the ability of a general caspase inhibitor (Z-VAD-Fmk) and two specific caspase 1 inhibitors (Z-YVAD-Fmk and Z-WEHD-Fmk) to block cell death measured by crystal violet staining was tested. The dose-response curve of Z-VAD-Fmk is shown in Fig. 2. The optimal concentration of the inhibitor was between 25 and 50 μM. Paradoxically, high concentrations of the inhibitor (100 μM) had no inhibitory effect at 1 h postinfection, and potentiation of the cytotoxic effect was observed at 6 h postinfection. A similar effect has been reported for cells undergoing tumor necrosis factor alpha (TNF-α)-induced apoptosis: Z-VAD-Fmk inhibited cell death at moderate concentrations but had the opposite effect at high concentrations (29). By 1 h after infection, 31.18% of untreated macrophages infected with the wild type grown logarithmically had died, and this cytotoxic effect was completely blocked by prior incubation of the cells with medium containing 25 μM general caspase inhibitor Z-VAD-Fmk. In contrast, macrophages infected with stationary-phase wild type or sipB mutant exhibited no cytotoxicity at 1 h after infection, and these cells even had an increase in the A630 reading. At 6 h after infection, the pattern of cytotoxicity was still the same; i.e., macrophages infected with stationary-phase wild type or sipB mutant showed no cytotoxicity (Fig. 3 and 4). Incubation of macrophages with a filter-sterilized supernatant from infected cells also caused an increase in the A630 reading (data not shown). In contrast, at 12 h after infection, all of the strains produced a cytotoxic effect (Fig. 4A) which could be inhibited partially by Z-VAD-Fmk (Fig. 4B). At 18 h after infection, most of the macrophages were dead regardless of the strain used (Fig. 4A), and the addition of Z-VAD-Fmk did not markedly inhibit cytotoxicity (Fig. 4B).
FIG. 2.
Inhibition of Salmonella serovar Typhimurium-induced cytotoxicity by the general caspase inhibitor Z-VAD-Fmk. Macrophages were incubated with concentrations of Z-VAD-Fmk ranging from 0 to 100 μM, infected in 96-well plates with wild-type Salmonella serovar Typhimurium grown to the logarithmic phase, fixed at 1 h (solid line) and 6 h (broken line) after infection, stained with crystal violet, and analyzed by use of a microplate reader with a 630-nm filter; 100% survival corresponds to the average A630 reading of the uninfected control without the inhibitor. The values corresponding to the control value indicate the survival of uninfected macrophages treated with Z-VAD-Fmk (100 μM). Values are means and standard deviations (n = 4). Single asterisks indicate that the values at both 1 and 6 h postinfection were significantly lower than those of the control (P < 0.05). Double asterisks indicate that the value was significantly lower than all of the other values (P < 0.05).
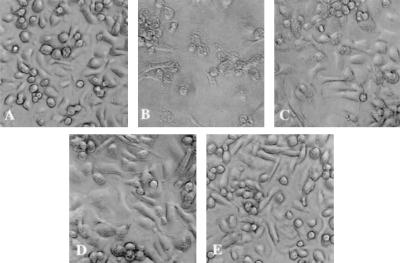
FIG. 3.
Salmonella serovar Typhimurium-induced cytotoxicity on monocyte-derived bovine macrophages at 6 h postinfection. A cytotoxic effect was observed in logarithmic-phase Salmonella serovar Typhimurium-infected macrophages, whereas macrophages inoculated with stationary-phase wild type, sipB mutant, and heat-inactivated Salmonella serovar Typhimurium remained intact. (A) Uninfected control. (B to E) Macrophages inoculated with wild-type Salmonella serovar Typhimurium grown to logarithmic phase (B), wild-type Salmonella serovar Typhimurium grown to stationary phase (C), sipB mutant (D), and heat-inactivated Salmonella serovar Typhimurium (E).
FIG. 4.
Time course of bovine macrophage survival. (A) Survival after infection with wild-type Salmonella serovar Typhimurium grown to the logarithmic or stationary phase and a sipB mutant grown to the logarithmic phase. (B) Survival in the presence of a caspase inhibitor. Macrophages were incubated with the general caspase inhibitor Z-VAD-Fmk (25 μM) for 1 h prior to infection. To determine survival, macrophages were infected in 96-well plates, fixed at different times, stained with crystal violet, and analyzed by use of a microplate reader with a 630-nm filter. Values are means and standard deviations (n = 4).
Approximately 92% of the cells were TUNEL positive at 1 h after infection with logarithmically growing Salmonella serovar Typhimurium (Fig. 1), while at this time the percentage of dead cells measured by crystal violet staining was considerably lower (Fig. 4). Thus, DNA fragmentation detected by TUNEL staining apparently preceded cell death detected by crystal violet staining during Salmonella serovar Typhimurium-induced cytotoxicity in bovine macrophages. However, significant differences were observed between stationary-phase wild type and the sipB mutant on the one hand and logarithmic-phase wild type on the other hand at 1 and 6 h postinfection (Fig. 4A), suggesting that crystal violet staining measured the sipB-dependent mechanism of cell death. Macrophages infected with the sipB mutant showed large numbers of TUNEL-positive cells at 6, 12, and 18 h postinfection (data not shown) which were similar to the numbers of TUNEL-positive cells infected with logarithmic-phase wild type at 60 and 180 min postinfection (Table 1). This result indicates a correlation between the cell death measured by these two methods.
The caspase 1 inhibitor Z-YVAD-Fmk was effective at 25 and 50 μM, blocking sipB-dependent cell death at 1 h after challenge and partially inhibiting the sipB-independent mechanism (Fig. 5). In contrast, when macrophages were preincubated with the specific caspase 1 inhibitor (Z-WEHD-Fmk) at concentrations ranging from 3.12 to 100 μM prior to infection with logarithmic-phase Salmonella serovar Typhimurium, no significant difference in the rate of survival compared to that for macrophages without inhibitor or with vehicle only was observed (P > 0.05; data not shown). Preincubation with 25 μM Z-WEHD-Fmk also had no effect on the delayed mechanism of cell death (data not shown).
FIG. 5.
Time course of bovine macrophage survival after infection with wild-type or sipB mutant Salmonella serovar Typhimurium grown to logarithmic phase in the presence or absence of a caspase 1 inhibitor (Z-YVAD-Fmk). Macrophages were incubated with Z-YVAD-Fmk (50 μM) for 1 h prior to infection. To determine survival, macrophages were infected in 96-well plates, fixed at different times, stained with crystal violet, and analyzed by use of a microplate reader with a 630-nm filter. Values are means and standard deviations (n = 4). Symbols: ●, wild type, 0 μM; ▪, sipB mutant, 0 μm; ○, wild type, 50 μM; □, sipB mutant, 50 ìM.
To test the contribution of soluble factors to cell death, we collected medium from macrophages infected with both logarithmic- and stationary-phase wild-type Salmonella serovar Typhimurium from 0 min up to 12 h after infection. The medium was centrifuged, and the supernatant was filtered (0.2-μm pore size) and stored at −70°C. Macrophages were incubated with the filter-sterilized supernatant for various times, and cell death was evaluated by crystal violet staining. No cytotoxicity was observed when the macrophages were incubated with the supernatant for up to 18 h. Indeed, there was an increase in the A630 reading in the wells incubated with the supernatant; this increase was associated with morphological changes characterized by cells spreading out on the bottom of the well and rough cellular boundaries in comparison to the appearance of the control (data not shown). Macrophages inoculated with heat-inactivated organisms (65°C for 20 min) were not killed at 1, 6, and 12 h after challenge. Surprisingly, there was a slight but significant decrease in survival after 18 h of incubation when the macrophages were challenged with heat-inactivated Salmonella serovar Typhimurium; these cells showed 78.44% survival compared to the control (P < 0.05; n = 4).
The cytotoxic effects of Salmonella serovar Typhimurium are similar in macrophages from cattle with naturally resistant and susceptible genetic backgrounds.
The experiments described above were performed with macrophages obtained from a single cow. To address whether this cow was representative of the general population, we compared cytotoxic responses produced by Salmonella serovar Typhimurium in macrophages isolated from different animals. In addition, the naturally resistant genetic background is important for in vitro resistance against intracellular infectious agents such as Brucella abortus, Mycobacterium bovis, and S. enterica serovar Dublin but not for resistance against Salmonella serovar Typhimurium (27). These experiments were designed to address the question of whether or not there is any difference in the cytotoxic effects of Salmonella serovar Typhimurium between resistant and susceptible individuals. No statistically significant difference was observed at 1 and 6 h postinfection in the A630 readings between macrophages from resistant and susceptible cattle infected with wild-type or sipB mutant Salmonella serovar Typhimurium (Table 2). The profile of cytotoxicity observed with these cattle was similar to that observed in the previous experiments, suggesting that the resistant animal used in the previous experiments was representative of the response in this host species.
TABLE 2.
Cytotoxicity of Salmonella serovar Typhimurium grown to logarithmic or stationary phase and a sipB mutant for macrophages from cattle naturally resistant or susceptible to B. abortus at 1 and 6 h after infection
| Strain | % Macrophage survival at the following hour postinfection for the indicated cattlea:
|
|||
|---|---|---|---|---|
| 1
|
6
|
|||
| Resistant | Susceptible | Resistant | Susceptible | |
| Logarithmic phase | 81.17 ± 6.74 | 88.71 ± 37.92 | 61.35 ± 12.38 | 65.01 ± 33.32 |
| Stationary phase | 130.61 ± 8.14 | 134.79 ± 42.31 | 132.44 ± 7.37 | 140.92 ± 37.95 |
| sipB mutant | 125.56 ± 10.67 | 132.22 ± 46.05 | 153.09 ± 2.06 | 153.46 ± 50.49 |
Values are means and standard deviations for the percentage of macrophage survival in comparison to that in the uninfected control (see Materials and Methods). The experiments were performed in quadruplicate with three animals in each group. There was no significant difference between resistant and susceptible cattle (P > 0.05).
Bacterial uptake and intracellular survival.
To ensure that the differences in cytotoxicity were not due to more efficient uptake or intracellular survival of the logarithmic-phase wild type than of bacteria grown to stationary phase or sipB mutant bacteria, the numbers of intracellular bacteria were quantified at 1 and 6 h after infection. The percentages (means and standard deviations) of organisms phagocytosed [(number of intracellular organisms/number of organisms in the inoculum) × 100] were 36.81 ± 0.71 and 21.7 ± 1.44 for stationary-phase wild type and sipB mutant, respectively. Both strains survived intracellularly for up to 6 h postinfection (Table 3). The rapid killing of macrophages inoculated with logarithmic-phase IR715 made it impossible to quantify intracellular bacteria with this method since, due to the early cytotoxicity, only 39.72% of the macrophages remained attached after washing. Since cytotoxicity resulted in the detachment of macrophages infected with the logarithmically growing wild type, the bacterial numbers recovered from these wells could not be directly compared to the bacterial numbers recovered from wells infected with noncytotoxic mutants. These results suggest that although there are slight differences in uptake and intracellular survival, all of the strains were able to survive in macrophages.
TABLE 3.
Uptake by bovine macrophages and intracellular survival of Salmonella serovar Typhimurium (wild type grown to the stationary phase and a sipB mutant) at 1 and 6 h postinfection
| Strain | CFU (106)/well at the following hour postinfectiona:
|
Ratio of 1-h to 6-h values (%b) | |
|---|---|---|---|
| 1 | 6 | ||
| Wild type | 1.583 ± 0.03 | 2.403 ± 0.582 | 1.518 (151.8) |
| sipB mutant | 1.063 ± 0.070 | 1.061 ± 0.238 | 0.998 (99.81) |
Values are means and standard deviations (n = 3).
Rate of intracellular survival from 1 h to 6 h postinfection.
DISCUSSION
Since the disease caused by Salmonella serovar Typhimurium in mice is systemic while that in cattle and humans is localized, bovine infection is a useful model for studying the pathogenesis of diarrhea. SPI-1 genes, including sipB, are required for the development of diarrhea in calves (34, 35). Recent reports indicated that SipB directly triggers apoptosis in murine macrophages. After SPI-1-dependent translocation of SipB into murine macrophage cytoplasm, the effector protein binds to caspase 1, which cleaves and activates interleukin-1β. This mechanism has been proposed to be a link between apoptosis and inflammation (16). Furthermore, caspase 1 knockout mice have an oral Salmonella serovar Typhimurium 50% lethal dose 1,000-fold higher than that for the wild type (24). In order to understand the role of SipB-mediated cytotoxicity during diarrheal disease in cattle, we investigated its contribution to eliciting cell death in bovine macrophages in vitro.
While this work was in progress, Watson et al. reported that Salmonella serovar Typhimurium kills bovine alveolar macrophages by a sipB-dependent pathway (39). Here, we demonstrated that in vitro infection of bovine monocyte-derived macrophages with Salmonella serovar Typhimurium induced cell death. There were two distinct mechanisms of cell death: the first, early cell death, which occurred very rapidly after infection and which depended on the presence of sipB, and the second, a delayed type of cell death, which occurred within 12 h after infection and which was sipB independent. DNA fragmentation occurred very early after infection, but DNA labeling was more intense when macrophages were infected with the logarithmically growing wild-type organism. Both mechanisms of cell death induced by Salmonella serovar Typhimurium were temporarily blocked by incubation with a general caspase inhibitor (Z-VAD-Fmk) or a caspase 1 inhibitor (Z-YVAD-Vmk) prior to infection. Surprisingly, Z-WEHD-Fmk, which is the optimal target sequence for caspase 1 (33), did not significantly affect the rate of cell death induced by Salmonella serovar Typhimurium in bovine macrophages. The sipB mutant was fully able to infect and survive intracellularly within macrophages, a result which indicated that the differences in cytotoxicity between the wild type and the sipB mutant were not due to differences in uptake or survival.
After infection with logarithmic-phase Salmonella serovar Typhimurium, there was a rapid increase in the percentage of apoptotic cells, as detected by TUNEL, during the first hour of infection (Table 1). The DNA fragmentation observed by TUNEL preceded the cytolysis observed by crystal violet staining, indicating that cytolysis was delayed in relation to DNA fragmentation. Based on the mouse model, the extremely fast mechanism of cell death triggered by logarithmic-phase organisms can be explained by the direct action of SipB binding to and activating caspase 1 (16). A longer period required for cell death in sipB-independent cytotoxicity might suggest an indirect mechanism. It has been demonstrated with the mouse model that stationary-phase Salmonella serovar Typhimurium lacking the expression of the type III secretion system encoded by SPI-2 (20, 21) is not cytotoxic for J774 cells (37). Why these SPI-2 genes are required for delayed cytotoxicity is not clear. Recently, a SipB-mediated, caspase 1-independent mechanism of cell death, which involves caspase 2 activation, was reported for murine macrophages (18).
Macrophages infected with stationary-phase wild type or sipB mutant showed increases in A630 readings at 1 and 6 h after infection (Fig. 4) that may have been related to the staining of infecting bacteria, as previously reported (22). However, macrophages incubated with supernatant from infected cells also showed an increase in the A630 reading that may have been due to activation of the macrophages.
A homologue of the natural resistance-associated macrophage protein 1 (NRAMP1), initially identified in mice, has been described for bovine species (9). This protein has been implicated as a putative mediator of natural resistance for intracellular pathogens (1). A previous report on the cytotoxicity of Salmonella serovar Typhimurium for bovine alveolar macrophages did not specify whether cells were derived from resistant or susceptible animals (39). To investigate whether NRAMP1 may affect the ability of Salmonella serovar Typhimurium to kill bovine macrophages, we compared its cytotoxicities for macrophages from genetically susceptible and resistant animals. No differences in the rates of apoptosis were observed for Salmonella serovar Typhimurium-infected macrophages from resistant and susceptible animals. These results were consistent with previous reports indicating that macrophages from cattle naturally resistant to intracellular pathogens are more efficient at killing or preventing the growth of B. abortus, M. bovis, and Salmonella serovar Dublin but not Salmonella serovar Typhimurium (27). Our results suggest that the cytotoxicity of Salmonella serovar Typhimurium may not be related to NRAMP1 in bovine species.
According to our results, both early and delayed mechanisms of cell death involve caspase activity, since the cytotoxic effect was either blocked or decreased when the macrophages were previously incubated with the general caspase inhibitor Z-VAD-Fmk (Fig. 4). The dose-response curve demonstrated maximal inhibition at concentrations of between 25 and 50 μM. The supernatant from infected bovine macrophages did not have a cytotoxic effect, suggesting that probably TNF-α and other soluble factors did not play an important role in the cell death induced by Salmonella serovar Typhimurium infection. In contrast, murine macrophages infected with Mycobacterium tuberculosis undergo apoptosis, and TNF-α plays a major role in this system (28). As previously proposed for Salmonella-induced murine macrophage apoptosis (16) and bovine alveolar macrophages (39), our results suggested that caspase 1 plays a role in the sipB-dependent mechanism of cell death in monocyte-derived bovine macrophages and apparently may have some function in the sipB-independent mechanism as well. The lack of activity of Z-WEHD-Fmk was not addressed in this study. These results suggest that Salmonella-mediated macrophage cell death is a proinflammatory mechanism that plays a significant role in the pathogenesis of enteritis and diarrhea in cattle. In vivo ligated ileal loop experiments with calves are the obvious next step to validate the implications of Salmonella-induced cell death in the pathogenesis of enteritis and diarrhea.
ACKNOWLEDGMENTS
This work was supported by grant DHHS/PHS/NIH-1 RO1 A144170–01A1 from the National Institutes of Health. R.L.S. was supported by CAPES, Brasília, Brazil, and Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
We thank José Angel Gutiérrez Pabello, Betty Rosenbaum, Roberta Pugh, and Doris Hunter for technical assistance; Colin Tanksley for care of animals; Arturo Zychlinsky for scientific advice; and Ando van der Velden for sharing data prior to publication.
REFERENCES
- 1.Adams L G, Templeton J W. Genetic resistance to bacterial diseases of animals. Rev Sci Tech. 1998;17:200–219. doi: 10.20506/rst.17.1.1085. [DOI] [PubMed] [Google Scholar]
- 2.Ahmer B M, van Reeuwijk J, Watson P R, Wallis T S, Heffron F. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol Microbiol. 1999;31:971–982. doi: 10.1046/j.1365-2958.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- 3.Blanc-Potard A B, Solomon F, Kayser J, Groisman E A. The SPI-3 pathogenicity island of Salmonella enterica. J Bacteriol. 1999;181:998–1004. doi: 10.1128/jb.181.3.998-1004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalker R B, Blaser M J A. A review of human salmonellosis. III. Magnitude of Salmonella infection in the United States. Rev Infect Dis. 1988;10:111–124. doi: 10.1093/clinids/10.1.111. [DOI] [PubMed] [Google Scholar]
- 5.Chen L M, Kaniga K, Galán J E. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 6.Cirillo D M, Valdivia R H, Monack D M, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 7.Darwin K H, Miller V L. Molecular basis of the interaction of Salmonella with the intestinal mucosa. Clin Microbiol Rev. 1999;12:405–428. doi: 10.1128/cmr.12.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekperigin H E, Nagaraja K V. Salmonella. Vet Clin North Am Food Anim Pract. 1998;14:17–29. [PubMed] [Google Scholar]
- 9.Feng J, Li Y, Hashad M, Schurr E, Gros P, Adams L G, Templeton J W. Bovine natural resistance associated macrophage protein 1 (Nramp1) gene. Genome Res. 1996;6:956–964. doi: 10.1101/gr.6.10.956. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Prada C M, Hoover D L, Tall B D, Venkatesan M M. Human monocyte-derived macrophages infected with virulent Shigella flexneri in vitro undergo a rapid cytolytic event similar to oncosis but not apoptosis. Infect Immun. 1997;65:1486–1496. doi: 10.1128/iai.65.4.1486-1496.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fields P I, Groisman E A, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243:1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- 12.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost A J, Bland A P, Wallis T S. The early dynamic response of the calf ileal epithelium to Salmonella typhimurium. Vet Pathol. 1997;34:369–386. doi: 10.1177/030098589703400501. [DOI] [PubMed] [Google Scholar]
- 14.Galán J E, Curtiss R. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hensel M, Shea J E, Baumler A J, Gleeson C, Blattner F, Holden D W. Analysis of the boundaries of Salmonella pathogenicity island 2 and the corresponding chromosomal region of Escherichia coli K-12. J Bacteriol. 1997;179:1105–1111. doi: 10.1128/jb.179.4.1105-1111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersh D, Monack D M, Smith M R, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilbi H, Moss J E, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell R A, Yuan J, Sansonetti P J, Zychlinsky A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IapB. J Biol Chem. 1988;273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 18.Jesenberger V, Procyk K J, Yuan J, Reipert S, Baccarini M. Salmonella-induced caspase-2 activation in macrophages: a novel mechanism in pathogen-mediated apoptosis. J Exp Med. 2000;192:1035–1045. doi: 10.1084/jem.192.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones B D, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee A K, Detweiler C S, Falkow S. OmpR regulates the two-component system SsrA-SsrB in Salmonella pathogenicity island 2. J Bacteriol. 2000;182:771–781. doi: 10.1128/jb.182.3.771-781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindgren S W, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundberg U, Vinatzer U, Berdnik D, Gabain A, Baccarini M. Growth phase-regulated induction of Salmonella-induced macrophage apoptosis correlates with transient expression of SPI-1 genes. J Bacteriol. 1999;181:3433–3437. doi: 10.1128/jb.181.11.3433-3437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monack D M, Hersh D, Ghori N, Bouley D, Zychlinsky A, Falkow S. Salmonella exploits caspase-1 to colonize Peyer's patches in a murine typhoid model. J Exp Med. 2000;192:249–258. doi: 10.1084/jem.192.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochman H, Soncini F C, Solomon F, Groisman E A. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price R E, Templeton J W, Smith III R, Adams L G. Ability of mononuclear phagocytes from cattle naturally resistant or susceptible to brucellosis to control in vitro intracellular survival of Brucella abortus. Infect Immun. 1990;58:879–886. doi: 10.1128/iai.58.4.879-886.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qureshi T, Templeton J W, Adams L G. Intracellular survival of Brucella abortus, Mycobacterium bovis BCG, Salmonella dublin, and Salmonella typhimurium in macrophages from cattle genetically resistant to Brucella abortus. Vet Immunol Immunopathol. 1995;50:1–10. doi: 10.1016/0165-2427(95)05492-8. [DOI] [PubMed] [Google Scholar]
- 28.Rojas M, Olivier M, Gros P, Barrera L F, Garcia L F. TNF-α and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J Immunol. 1999;162:6122–6131. [PubMed] [Google Scholar]
- 29.Ruemmele F M, Dionne S, Levy E, Seidman E G. TNFα-induced IEC-6 cell apoptosis requires activation of ice caspases whereas complete inhibition of the caspase cascade leads to necrotic cell death. Biochem Biophys Res Commun. 1999;260:159–166. doi: 10.1006/bbrc.1999.0734. [DOI] [PubMed] [Google Scholar]
- 30.Snedecor G W, Cochran W G. Statistical methods. Ames: Iowa State University Press; 1994. [Google Scholar]
- 31.Stojiljkovic I, Baumler A J, Heffron F. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J Bacteriol. 1995;177:1357–1366. doi: 10.1128/jb.177.5.1357-1366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sukupolui S, Edelstein A, Rhen M, Normark S J, Pfeifer J D. Development of a murine model of chronic Salmonella infection. Infect Immun. 1997;65:838–842. doi: 10.1128/iai.65.2.838-842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornberry N A, Rano T A, Peterson E P, Rasper D M, Timkey T, Garcia-Calvo M, Houtzager V M, Nordstrom P A, Roy S, Vaillancourt J P, Chapman K T, Nicholson D W. A combinatorial approach defines specificities of members of the caspase family and granzyme B. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 34.Tsolis R M, Adams L G, Hantman M J, Scherer C A, Kimbrough T, Kingsley R A, Ficht T A, Miller S I, Baumler A J. SspA is required for lethal Salmonella enterica serovar Typhimurium infections in calves but is not essential for diarrhea. Infect Immun. 2000;68:3158–3163. doi: 10.1128/iai.68.6.3158-3163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsolis R M, Adams L G, Ficht T A, Baumler A J. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect Immun. 1999;67:4879–4885. doi: 10.1128/iai.67.9.4879-4885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsolis R M, Townsend S M, Miao E A, Miller S I, Ficht T A, Adams L G, Baumler A J. Identification of a putative Salmonella enterica serotype typhimurium host range factor with homology to IpaH and YopM by signature-tagged mutagenesis. Infect Immun. 1999;67:6385–6393. doi: 10.1128/iai.67.12.6385-6393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Der Velden A W M, Lindgren S W, Worley M J, Heffron F. Salmonella pathogenicity island 1-independent induction of apoptosis in infected macrophages by Salmonella enterica serotype Typhimurium. Infect Immun. 2000;68:5702–5709. doi: 10.1128/iai.68.10.5702-5709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson P R, Galyov E E, Paulin S M, Jones P W, Wallis T S. Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect Immun. 1998;66:1432–1438. doi: 10.1128/iai.66.4.1432-1438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson P R, Gautier A V, Paulin S M, Bland A P, Jones P W, Wallis T S. Salmonella enterica serovars Typhimurium and Dublin can lyse macrophages by a mechanism distinct from apoptosis. Infect Immun. 2000;68:3744–3747. doi: 10.1128/iai.68.6.3744-3747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong K K, McClelland M, Stillwell L C, Sisk E C, Thruston S J, Saffer J D. Identification and sequence analysis of a 27-kilobase chromosomal fragment containing a Salmonella pathogenicity island located at 92 minutes on the chromosome map of Salmonella enterica serovar Typhimurium LT2. Infect Immun. 1998;66:3365–3371. doi: 10.1128/iai.66.7.3365-3371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood W M, Jones M A, Watson P R, Hedges S, Wallis T S, Galyov E E. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol Microbiol. 1998;29:883–891. doi: 10.1046/j.1365-2958.1998.00984.x. [DOI] [PubMed] [Google Scholar]
- 42.Wray C, Sojka W J. Experimental Salmonella typhimurium infection in calves. Res Vet Sci. 1978;25:139–143. [PubMed] [Google Scholar]