ABSTRACT
Purpose
This study aimed to investigate the effects of 12 wk of omega-3 fatty acid supplementation during endurance training on omega-3 index (O3I) and indicators of running performance in amateur long-distance runners.
Methods
Twenty-six amateur male long-distance runners ≥29 yr old supplemented omega-3 fatty acid capsules (OMEGA group, n = 14; 2234 mg of eicosapentaenoic acid and 916 mg of docosahexaenoic acid daily) or medium-chain triglycerides capsules as placebo (medium-chain triglyceride [MCT] group, n = 12; 4000 mg of MCT daily) during 12 wk of endurance training. Before and after intervention, blood samples were collected for O3I assessment, and an incremental test to exhaustion and a 1500-m run trial were performed.
Results
O3I was significantly increased in the OMEGA group (from 5.8% to 11.6%, P < 0.0001). A significant increase in V̇O2peak was observed in the OMEGA group (from 53.6 ± 4.4 to 56.0 ± 3.7 mL·kg−1⋅min−1, P = 0.0219) without such change in MCT group (from 54.7 ± 6.8 to 56.4 ± 5.9 mL·kg−1⋅min−1, P = 0.1308). A positive correlation between the change in O3I and the change in running economy was observed when data of participants from both groups were combined (−0.1808 ± 1.917, P = 0.0020), without such an effect in OMEGA group alone (P = 0.1741). No effect of omega-3 supplementation on 1500-m run results was observed.
Conclusions
Twelve weeks of omega-3 fatty acid supplementation at a dose of 2234 mg of eicosapentaenoic acid and 916 mg of docosahexaenoic acid daily during endurance training resulted in the improvement of O3I and running economy and increased V̇O2peak without improvement in the 1500-m run trial time in amateur runners.
Key Words: OMEGA-3 INDEX (O3I), POLYUNSATURATED FATTY ACIDS, RUNNING PERFORMANCE, ENDURANCE TRAINING, RUNNING ECONOMY
Omega-3 fatty acids include α-linolenic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), characterized by the first double bond on the third carbon atom from the methyl end of the fatty acyl chain. There is growing evidence that synthesis de novo of EPA and, in particular, DHA is limited in the human body, and sources of preformed EPA and DHA, e.g., seafood, especially fatty fish or supplements should be consumed (1,2). Despite this, athlete’s intake of sources of omega-3 fatty acids is often inadequate (3,4). Harris and von Schacky (5) proposed the so-called omega-3 index (O3I) as a valid indicator of omega-3 PUFA status, reflecting both intake of these fatty acids and their biological and health effects. O3I is the sum of EPA and DHA expressed as a percent of total fatty acids in erythrocytes. It is proposed that values >8% are associated with the greatest cardioprotection, whereas values <4% are associated with the least (5). O3I has been recognized as the best marker of omega-3 PUFA status associated with many health indicators and outcomes in the general population (6); however, its relation with physical performance indicators in athletes is poorly understood. Observations on amateur and competitive athletes confirm low O3I values. For example, in 106 German elite winter endurance athletes, only one had an O3I in the target range of >8%, and the average O3I value of the others was 4.97% ± 1.19% (7). Analysis conducted on collegiate athletes, professional basketball players, and trained but not professional endurance athletes confirm low values of the O3I and its increase after supplementation with omega-3 PUFA (4,8,9). A recent systematic review summarizing randomized placebo-controlled trials in athletes revealed that omega-3 PUFA supplementation improved cognitive function (e.g., reduction of reaction time and improvement of mood state), promoted skeletal muscle recovery, and attenuated proinflammatory cell responses (10).
The effect of omega-3 fatty acid supplementation on exercise performance is unclear, although several studies show positive effects on oxygen kinetics: cycling efficiency or maximal oxygen uptake (10). To date, the longest study where physical performance parameters were analyzed lasted 10 wk with the applied dose of 1.60 g of EPA and 1.04 g of DHA daily (11). The length and the dose of omega-3 fatty acid supplementation seem to be crucial because of the incorporation of EPA + DHA into target tissues, which would be reflected in erythrocyte membranes and O3I. Maximal incorporation of EPA and DHA into erythrocytes is related to erythrocyte turnover: in a 12-month controlled intervention trial conducted on healthy individuals, Browning and coauthors (12) revealed that it takes 55 and 136 d for EPA and DHA, respectively, to achieve peak incorporation into erythrocytes in the case of a supplementation dose of 3.27 g of EPA + DHA for 4 d·wk−1.
Given the paucity of long-term studies using omega-3 fatty acid supplements in athletes showing relation between O3I values and physical performance indicators, there is a need for further work in this area. Accordingly, we determined the effects of 12 wk of EPA + DHA supplementation (2234 mg and 916 mg·d−1, respectively) compared with medium-chain triglycerides (MCT) as placebo in dose 4000 mg·d−1 during endurance training on O3I and physical performance indicators in amateur runners. We hypothesize that this duration and dosage of omega-3 PUFA will result in significant incorporation of EPA and DHA into erythrocytes membranes and increase O3I to values considered as a target range (i.e., >8%). Moreover, using the longest duration and the highest dose of supplementation of the studies conducted so far, we hypothesize that this will increase V̇O2peak and improve running economy (RE) to a degree that will translate into better running performance.
METHODS
Ethical approval
The study was approved by the Bioethical Committee of Regional Medical Society in Gdańsk (NKBBN/628/2019) and conducted according to the Declaration of Helsinki. After comprehensive details of the study protocol were explained orally and in writing, all participants provided their written informed consent.
Participants
Forty amateur male long-distance runners were recruited through advertisements on the Internet. Inclusion criteria included age between 29 and 42 yr and completion of an official 10 km race over the 2016 and 2020 time period with a time result between 37 and 57 min. The exclusion criteria included chronic diseases, cigarette smoking, or use of prescribed medications or dietary supplements, including omega-3 fatty acids. On the day of familiarization with the laboratory conditions and the treadmill test, participants were allocated sequential numbers that were then used as the identifiable characteristic. Assignment to each group (OMEGA or MCT) using an online randomizer (http://www.randomizer.org) took place on the first day of the actual exercise tests. All participants agreed to carry out only the training courses included in the program and were instructed to continue with their habitual dietary patterns for the duration of the intervention.
Overview of study design
The trial was conducted in the Laboratory of Physical Exercise and Department of Biochemistry of the Academy of Physical Education and Sport in Gdansk. After inclusion, participants were randomly assigned to one of the two groups: OMEGA or MCT providing either omega-3 fatty acids or MCT. All participants completed a progressive endurance training supervised by a track and field coach. The parallel randomized trial consisted of three 4-wk phases, for a total of 12 wk together with simultaneous supplementation. A graded exercise test to exhaustion with assessment of V̇O2peak, RE, and a 1500-m run trial was carried out before and after completion of the exercise training program. Each test was preceded by a standardized breakfast for all participants consumed 1 h before the test began. Blood collection and weight assessment were performed when participants were in a fasting state. Figure 1 outlines the experimental protocol.
FIGURE 1.
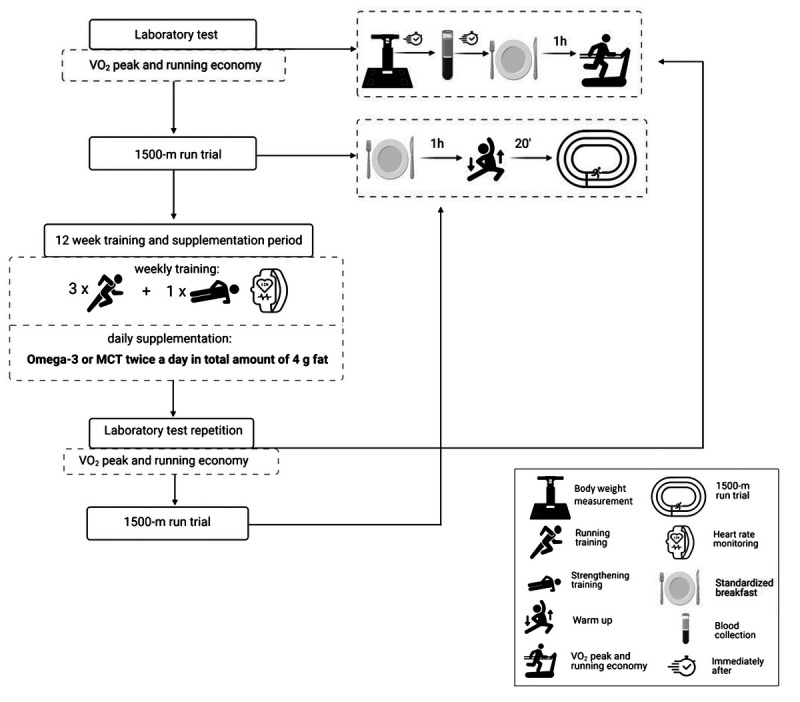
General experimental design.
Omega-3 PUFA supplementation
Throughout the study, all participants took four identical-looking capsules each day (two in the morning and two in the evening) containing either omega-3 fatty acids or MCT. The omega-3 capsules provided 2234 mg of EPA and 916 mg of DHA daily (Omega-3 double plus, NAMED SPORT, Italy), whereas the MCT capsules contained 4000 mg of MCT (MCT Oil; Now Foods, Bloomingdale, IL). The dose of omega-3 fatty acids is consistent with the dosage applied in the study of Browning and coauthors (12). To maintain certainty of the amount of each fatty acid and the general quality of the supplements containing omega-3 fatty acids, a product certified by the International Fish Oil Standard was selected. The International Fish Oil Standard program verifies the amount of each fatty acid and the content of heavy metals, dioxins, and rate of oxidation. A publicly available batch report of the supplements used in the study indicated that the amounts of individual acids were in accordance with the manufacturer’s claims, and content of heavy metals, dioxins, and rate of oxidation did not exceed accepted standards. Moreover, both supplements were certified by the informed-sport program, under which products are tested for substances banned by the World Anti-Doping Agency. To avoid a potential recognition of supplements, participants were informed that they were all taking omega-3 fatty acids in one of two chemical forms. On the day of arrival at the laboratory, 1 h before the graded exercise test and the 1500-m run trial, participants consumed the same standardized breakfast. Breakfast was a replication of a typical prestart meal and consisted of wheat roll with butter and jam and half a banana.
Total energy value and amount of carbohydrate, protein, and fat was 290 kcal, 49 g, 5 g, and 8 g, respectively. Dietary intake over 3 d (2 d from week and 1 d from weekend) was recorded in the first and the last week of the program. Participants used the MyFitnessPal mobile application to record the meals they consumed. Before using the app for the first time, the basic functions were demonstrated to all participants. Moreover, the Web site ilewazy.pl was presented to participants, so they could more easily estimate the portions they consumed when kitchen scales were not available. If recorded meals were not precise, participants were asked to clarify the information. Collected dietary records where then analyzed using nutrition analysis software (Kcalmar.pro, Poland). Every food item in meals, with the consumed amount, was entered to the nutrition analysis software, and total dietary energy, carbohydrate, protein, and fat content were calculated.
Exercise testing
Before (week 0) and after completion (week 13) of the exercise training program, participants were submitted to a graded exercise test to exhaustion on a motorized treadmill (h/p Cosmos, Saturn, Germany) to determine whether omega-3 fatty acids combined with endurance training might positively affect the endurance potential of runners. Before the intervention, the participant’s body weight and height were measured (analyzer InBody 720 and stadiometer Seca 213, respectively), then they were familiarized with the laboratory conditions and the treadmill test.
First, participants stood on the treadmill for 2 min to make sure the measuring equipment was ready and to measure the resting values. Thereafter, runners walked for 5 min at 5 km·h−1 speed and with a 1.5% inclination as a warm-up before starting the test. Every next stage lasted 3 min aimed to reach steady-state V̇O2 (13), and the treadmill belt was accelerated starting from 8 × 1 km·h−1 per stage up to 12 km·h−1. Then the inclination of the treadmill was increased to 5%, 10%, and 15% at 12 km·h−1 speed until volitional exhaustion. During both tests, heart rate (HR) was monitored (Polar RS400; Polar Electro Oy, Kempele, Finland) to define the highest value (HRmax) during each test. Minute ventilation (V̇E), oxygen uptake (V̇O2), carbon dioxide output (V̇CO2), and RER were continuously measured using a breath-by-breath analyzer (Oxycon Pro, Jaeger, Germany), which was calibrated before each test following the manufacturer’s recommendations. Measurements were averaged in 10-s intervals. V̇O2peak was obtained as the highest 30-s mean value recorded during the test. Running economy was measured as an oxygen cost from the last 50 s of each stage to 12 km·h−1 speed and was expressed as milliliters per kilogram per minute (14), and RE analysis was performed up to RER <1. All measurements were performed at similar time of day ±2 h and constant environmental conditions (18°C–20°C and humidity 40%–45%). Additionally, participants were informed to avoid strenuous exercise for 24 h before and caffeine and alcohol consumption for 12 h before laboratory tests. One week after the graded exercise test, participants took part in a 1500-m run time trial on an indoor 200-m track. The time was recorded with a handheld stopwatch to the nearest 0.1 s. During both tests, participants received strong verbal encouragement.
Training protocol
The training protocol lasted 12 wk and was built based on undulatory load manipulation 3:1, which was suggested to be effective to prevent overtraining and stress due to oscillations between volume/intensity according to Costa et al. (15) with slight modifications. Hence, participants performed endurance training 3 times per week. One additional training per week aimed to strengthen core muscles to reduce the risk of lower extremity injuries was also included in protocol (16). Training intensity was prescribed according to the first ventilatory threshold and ventilatory anaerobic threshold (VT1 and VAT), respectively, and their associated HR values were obtained during the laboratory testing. The threshold-based method was described as better than the HR reserve–based method to design more individualized exercise prescriptions that will enhance training efficacy and limit training unresponsiveness (17). Consequently, participants trained in three HR zones: [Z1: ≤HR@VT1 + 5 bpm; Z2: (>HR@VT1 + 5 bpm) to (≤HR@VAT-5 bpm); Z3: >HR@VAT-5 bpm], and their average training times spent in every mesocycle were (~80%–15%–5%) in zones (Z1–Z2–Z3), respectively, accordingly to previous authors (18) with slight modifications. On the last (12th) week, the tapering procedure was performed, whereby the training load was reduced to 70% from the volume obtained in the 11th week to reduce accumulated fatigue. Participant’s training activity (training volume, intensity, and energy expenditure) was monitored by a Polar M430 wristwatch and an H9 HR chest sensor. All running tests and training procedures were supervised by a track and field coach.
Erythrocyte fatty acid analysis
Fasting blood samples were collected from participants by a nurse into 4-mL sodium citrate vacutainer tubes (BD Vacutainer®, Franklin Lakes, NJ) and centrifuged at 4°C (4000g for 10 min). After centrifugation, erythrocytes were collected with a disposable pasteur pipette and transferred into eppendorfs, which were stored in a − 80°C freezer until further analysis. Erythrocyte EPA and DHA were assessed using gas chromatography as described elsewhere (19). Briefly, erythrocyte lipids were extracted into chloroform–methanol, and fatty acid methyl esters (representing the erythrocyte fatty acids) were formed by heating the lipid extract with methanolic sulfuric acid. The fatty acid methyl esters were separated by gas chromatography on a Hewlett Packard 6890 gas chromatograph fitted with a BPX-70 column using the settings and run conditions described elsewhere (19). Fatty acid methyl esters were identified by comparison with run times of authentic standards. Data are expressed as weight % of total fatty acids. O3I was calculated by summing the percentages of EPA and DHA according to Harris and von Schacky (5).
Statistical analysis
The sample size calculation was based on changes in oxygen consumption during graded exercise test to exhaustion assessed as V̇O2peak, as this was the primary outcome of the study. A typical value for V̇O2peak in population of recreational long-distance runners is about 54 mL·kg−1⋅min−1 with an SD of about 5 (20).
It is considered that an 8% increase in V̇O2peak is meaningful in amateur runners (21). A sample size of 18 participants per group (i.e., 36 participants in total) would give 70% power to detect this difference as significant with alpha = 0.05. In order to account for a dropout rate of 10%, 40 participants were recruited. Statistical analysis was performed using the tools of GraphPad Prism 7. Arithmetic means, SD, and significance levels of differences between means were calculated. A two-way repeated-measures ANOVA was used to investigate the significance of differences between groups and time. Significant main effects were further analyzed using the Bonferroni corrected post hoc test. Changes (Δ) in both groups were compared using an independent samples t-test. Correlations between variables were evaluated using the Pearson correlation coefficient. All analyses used a significance level of P < 0.05.
RESULTS
Participant flow through the study
Participants excluded from the final analysis completed insufficient (<80%) training sessions (n = 3) or withdrew from the study for health (n = 9) or personal reasons (n = 1). Moreover, one participant from MCT group increase intake of omega-3 fatty acids during study; therefore, he was also excluded from statistics. Participant flow through the study is presented in Figure 2. From the 40 participants enrolled, 26 completed the entire study and their characteristic is shown in Table 1.
FIGURE 2.
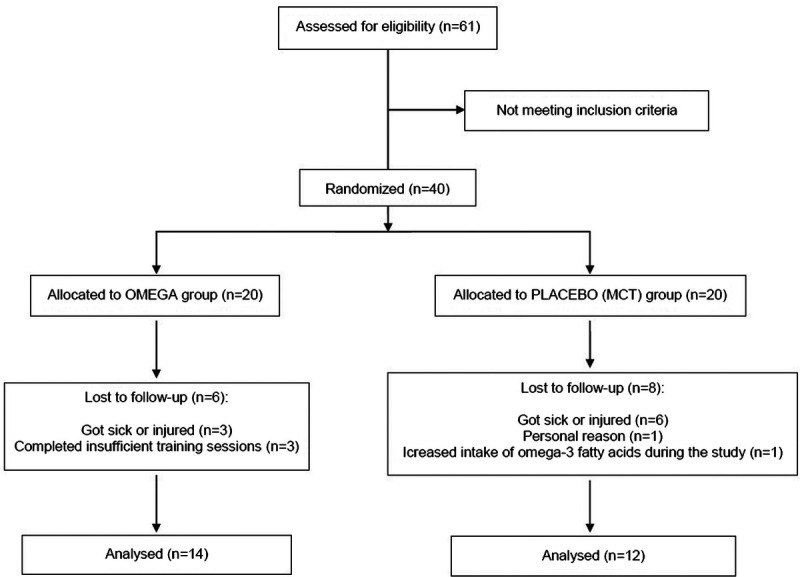
Flow of participants through the study.
TABLE 1.
Characteristics of participants who completed the study.
| Variable | Omega (n = 14) | MCT (n = 12) |
|---|---|---|
| Age (yr) | 37 ± 3 | 37 ± 4 |
| Body mass (kg) | 76.3 ± 11 | 78.0 ± 8 |
| Height (cm) | 181 ± 7 | 180 ± 4 |
| V̇O2 peak (mL·kg−1⋅min−1) | 53.6 ± 4 | 54.7 ± 7 |
| Personal best in 10-km run between 2016 and 2020 (min) | 45 ± 4 | 46 ± 5 |
Data are presented as mean ± SD.
Erythrocyte EPA, DHA, and O3I
The percentage values of erythrocyte EPA, DHA, and O3I pre- and postintervention in the OMEGA and MCT groups are presented in Figures 3 and 4. There was no difference in baseline values of either omega-3 PUFA or O3I between the groups (OMEGA group: 1.1% EPA, 4.7% DHA, 5.8% O3I; MCT group: 1.2% EPA, 4.4% DHA, 5.6% O3I; all P > 0.9999). Twelve weeks of omega-3 fatty acid supplementation during endurance training increased both omega-3 PUFA and O3I in the OMEGA group (to 4.9% ± 1.1% EPA, 6.7% ± 0.8% DHA, 11.6% ± 1.7% O3I; all P < 0.0001) without significant changes in the MCT group (to 1.1% EPA, 4.5% DHA, 5.6% O3I; all P > 0.9999). At the end of the intervention period EPA, DHA and O3I were significantly higher in OMEGA group than in MCT group (all P < 0.0001).
FIGURE 3.
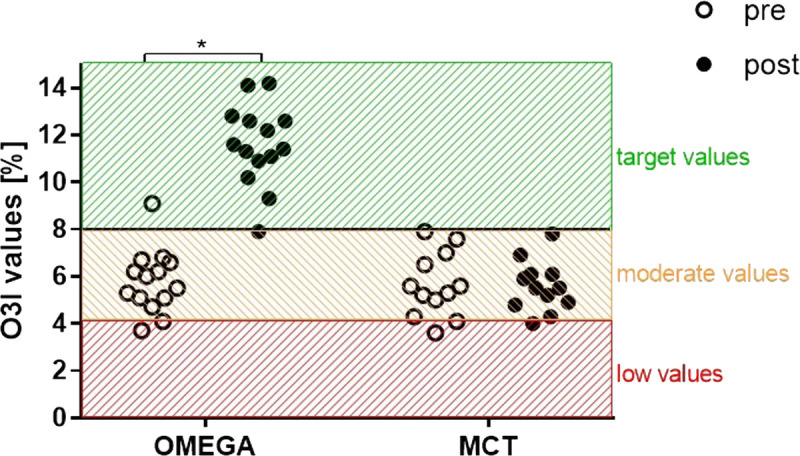
Effect of supplementation with omega-3 PUFA or MCT on individual values of O3I before and after the 12-wk intervention. *P < 0.0001.
FIGURE 4.
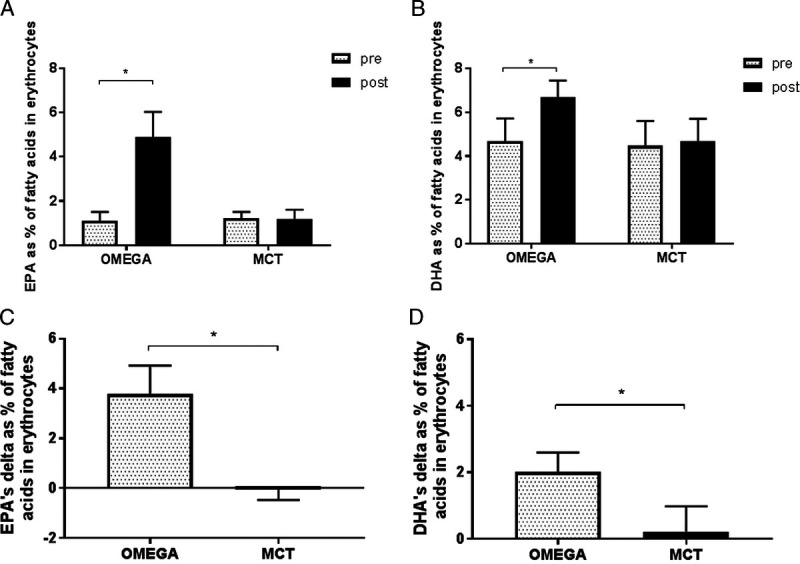
Effect of supplementation with omega-3 PUFA or MCT on erythrocyte EPA (A) and DHA (B) before and after the 12-wk intervention and change from baseline in EPA (C) and DHA (D) compared between the two groups. Data are expressed as mean. Error bars indicate ± SD, *P < 0.0001.
V̇O2peak, RE, and 1500-m run trial
There was no significant difference between groups in change in V̇O2peak over the 12-wk intervention period (P = 0.6764) (Fig. 5B). However, a significant increase in V̇O2peak from pre- to postintervention in OMEGA group was observed (from 53.6 ± 4.4 to 56.0 ± 3.7 mL·kg−1⋅min−1, P = 0.0219) with no significant change in MCT group (from 54.7 ± 6.8 to 56.4 ± 5.9 mL·kg−1⋅min−1, P = 0.1308) (Fig. 5A). Increase in V̇O2peak was seen in 13 (93%) out of 14 participants in the OMEGA group, whereas in the MCT group, improvements were visible in 9 (75%) out of 12 runners.
FIGURE 5.
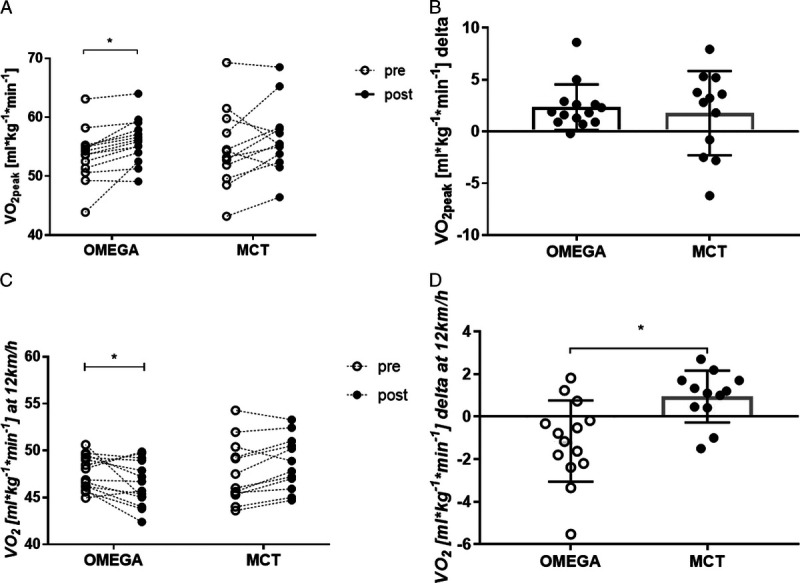
Effect of training and supplementation on peak oxygen consumption (A) (mL·kg−1⋅min−1) before and after the 12-wk intervention, change in peak oxygen consumption (B) (mL·kg−1⋅min−1) in the two groups over the 12-wk intervention, oxygen utilization (C) (mL·kg−1⋅min−1) during submaximal treadmill running at 12 km·h−1 before and after the 12-wk intervention, change in oxygen utilization (D) (mL·kg−1⋅min−1) in the two groups over the 12-wk intervention. Data are expressed as mean. Error bars indicate ± SD, *P < 0.05.
Moreover, oxygen uptake at 12 km·h−1 changed in both groups: the RE increased significantly in the OMEGA group (from 47.6 ± 1.8 to 46.5 ± 2.4 mL·kg−1⋅min−1, P = 0.0295), whereas it decreased in the MCT group (from 47.7 ± 3.3 to 48.7 ± 2.9 mL·kg−1⋅min−1, P = 0.1127) (Fig. 5C). The change in oxygen uptake over the 12-wk intervention period was significantly different between groups (P = 0.0033) (Fig. 5D). When results before and after the 12-wk intervention from all participants were combined, correlation highlighted the relationship between O3I and oxygen cost of submaximal running (Fig. 6A, P = 0.0338; Fig. 6B, P = 0.0020). There was significant improvement in completion of the 1500-m run trial in both groups from pre- to postintervention; however, results did not differ between groups over the study period (OMEGA group from 356.3 to 344.9 s, P = 0.0002; MCT group from 362.1 to 347.3 s, P < 0.0001; pre- to postintervention between groups, P > 0.9999).
FIGURE 6.
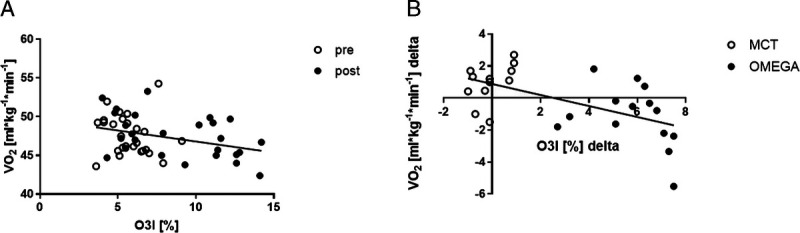
Correlation between O3I and oxygen cost of submaximal running when: OMEGA and MCT groups were combined before and after the 12-wk intervention (A). B. Results postintervention minus preintervention (Δ) in OMEGA and MCT groups were combined.
Physiological and nutritional variables
Table 2 summarizes physiological and nutritional variables obtained from the participants at the beginning and after completing the intervention program. There was no difference in weekly training volume (P = 0.7399), energy expenditure (P = 0.1828), and HRmax (P = 0.4624) between the groups. However, in both groups, there was a significant increase in HRmax at VAT (%) postintervention compared with preintervention (OMEGA group from 91.7 ± 2.6 to 93.9 ± 2.8, P = 0.0331; MCT group from 90.8 ± 3.9 to 95.2 ± 3.7, P = 0.0001). Total energy (kcal·d−1), carbohydrate, and protein (g·kg−1⋅d−1) intake did not differ pre- to postintervention within either group (OMEGA group P > 0.9999, P = 0.5442, P = 0.5777; MCT group P = 0.1973, P > 0.9999, P = 0.7721, respectively).
TABLE 2.
Physiological and nutritional variables according to treatment group.
| Variable | Omega | MCT | ||
|---|---|---|---|---|
| Weekly training volume (km) | 30.95 ± 2.47 | 31.5 ± 5.51 | ||
| Energy expenditure (kcal·d−1) | 2515 ± 445 | 2748 ± 415 | ||
| V̇O2peak (mL·kg−1·min−1) | Pre | 53.61 ± 4.36 | Pre | 54.66 ± 6.76 |
| Post | 55.96 ± 3.72* | Post | 56.44 ± 5.89 | |
| HRmax (bpm) | Pre | 190 ± 9 | Pre | 186 ± 9 |
| Post | 189 ± 9 | Post | 184 ± 7 | |
| HRmax at VAT (%) | Pre | 91.71 ± 2.65 | Pre | 90.81 ± 3.95 |
| Post | 93.89 ± 2.77* | Post | 95.20 ± 3.69** | |
| Body mass (kg) | Pre | 76.30 ± 10.96 | Pre | 78.03 ± 7.70 |
| Post | 76.55 ± 11.32 | Post | 77.0 ± 7.35* | |
| Energy and nutrient intake (per day) | ||||
| Energy (kcal) | Pre | 2393 ± 453 | Pre | 2456 ± 587 |
| Post | 2429 ± 420 | Post | 2338 ± 627 | |
| Carbohydrate (g) | Pre | 301 ± 63 | Pre | 310 ± 111 |
| Post | 289 ± 46 | Post | 302 ± 127 | |
| Protein (g) | Pre | 98 ± 20 | Pre | 99 ± 20 |
| Post | 102 ± 17 | Post | 95 ± 17 | |
| Fat (g)a | Pre | 83 ± 27 | Pre | 86 ± 18 |
| Post | 92 ± 27* | Post | 79 ± 15 | |
Data are presented as mean ± SD.
*P < 0.05 for post- vs preintervention value.
**P < 0.01 for post- vs preintervention value.
aStatistically significant difference in groups (Δ) with a trend of higher intake in the O3I group and lower intake in the MCT group.
There was a statistically significant difference in fat intake between the two groups with a significantly higher fat intake in the OMEGA group (from 83.4 ± 25.9 to 91.9 ± 25.9 g, P = 0.0321) and lower; however, not significant fat intake in the MCT group (P = 0.0943).
DISCUSSION
The main finding of the study is that 12 wk of supplementation with omega-3 fatty acids at a dose of 2234 mg of EPA and 916 mg of DHA daily shifts erythrocyte O3I to values considered as a target range for cardiovascular health. Moreover, this duration and dose of supplementation during endurance training increased V̇O2peak and improved RE at velocity 12 km·h−1 with no effect on 1500-m run trial results. Insufficient values of O3I in active individuals are well described. In a study including vegan and omnivorous endurance athletes, Cradock et al. (8) showed suboptimal O3I in both groups: 4.13% in vegans and 5.40% in omnivores, respectively. Similarly, O3I below the desirable values was demonstrated in German national elite winter endurance athletes (4.97% ± 1.19%), professional basketball players from the NBAG League (5.02% ± 1.19%), and collegiate athletes, representing diverse disciplines throughout the United States (4.33% ± 0.81%) (4,7,22). Our observations are in agreement with these reports, indicating that amateur runners had mean baseline O3I of around 5.7% (5.8% and 5.6% in OMEGA and MCT groups, respectively).
Twelve weeks with omega-3 fatty acid supplementation at a dose of 2234 mg of EPA and 916 mg of DHA daily during endurance training increased O3I in all but one participant in OMEGA group to mean of 11.4%, which is considered to be well within the O3I target range (5). Moreover, an increase in O3I correlated with an increase in RE at velocity 12 km·h−1 when results post- minus pre-12-wk intervention of participants from both groups were combined. Improvements in exercise economy as an effect of supplementation with omega-3 fatty acids have previously been shown in both amateur and competitive athletes (9,23,24). In an 8-wk double-blind, parallel design study in well-trained cyclists, Peoples et al. (23) showed that 3.2 g·d−1 of omega-3 fatty acids reduced whole-body O2 consumption throughout 60 min of sustained submaximal cycling. Contrary to our observations, peak oxygen consumption in these cyclists was not changed, which may be related to their high level of training status or quite high compared with other data (above 9%) baseline O3I values (23). Improved economy of cycling during the physiologically demanding time trial in trained cyclists and runners was also revealed by Hingley et al. (9) after 8 wk of supplementation with a dose of 560 mg of DHA + 140 mg of EPA a day. Despite an elevation in O3I (from 4.7% ± 0.2% to 6.3% ± 0.3%), the values did not achieve the recommended O3I >8%, which may be related to the low dose of EPA + DHA used. A study conducted by Kawabata et al. (24) with recreational players of American football, rugby, baseball, and basketball is consistent with other observations in trained individuals: 8 wk of daily supplementation with 914 mg of EPA and 399 mg of DHA increased exercise economy during a steady-state submaximal cycloergometer test. In one crossover study with trained cyclists, researchers observed an increase in V̇O2max after 3 wk of supplementation with a daily dose of 660 mg of EPA and 440 mg of DHA (25).
In contrast to this report, an earlier study conducted by Raastad et al. (11) showed no changes in V̇O2max and running performance in well-trained soccer players receiving 1.60 g of EPA and 1.04 g of DHA a day through 10-wk period. Exercise economy together with V̇O2max, lactate threshold, and critical power are all strongly related to endurance exercise performance (26). Therefore, studies showing increased exercise economy, V̇O2max, or V̇O2peak provide a rationale to further explore this topic together with the potential underlying mechanisms. Supplementation with omega-3 fatty acids reduces exercise-induced inflammation in athletes through decreasing in proinflammatory omega-6 fatty acids (27) and AA/EPA ratio (28). Given the large cross-sectional study indicating that inverse relationship between V̇O2max and C-reactive protein is modified by omega-3 fatty acid levels (29), this may be the case. Moreover, an increase in insulin sensitivity due to unsaturation of skeletal muscle membranes (30), improved calcium handling by skeletal muscle sarcoplasmic reticulum (23), and improved endothelial function via increase in NO release (25) should be taken into account in searching for potential mechanisms of action. Of note, in the present study, 13 out of 14 participants in the OMEGA group showed an improved V̇O2peak compared with a variable response in the MCT group, in which only 9 out of 12 runners improved their results. This may suggest better adaptation to endurance training in response to omega-3 fatty acid supplementation, as has been observed with several other dietary supplements (31). Still, neither our nor previous reports support the hypothesis that long-term supplementation with omega-3 fatty acids enhances exercise performance. Duration and dose of omega-3 supplementation are crucial factors determining the amount of fatty acids incorporated into erythrocyte membranes, and more than 4 months is needed to reach the highest concentration of DHA in case of a supplementation dose of 1.5 g of EPA and 1.77 g of DHA for 4 d·wk−1 (12).
Compared with previous studies in which performance indicators were assessed, our supplementation protocol (2234 mg of EPA and 916 mg of DHA daily for 12 wk) was a higher dose over a longer supplementation period (9,23–25). However, what values of O3I are sufficient for amateur and competitive athletes to optimize athletic performance remains a question to be answered in future studies.
Our study has some limitations that must be highlighted. Running economy is typically determined by measuring the consumption of oxygen when the steady state of V̇O2 is observed (13). We recognized steady-state conditions when runners had RER <1 during treadmill running (13,32); however, the concentration of lactic acid was not assessed. Considering that lactate threshold (LT) is one of the indicators of disturbance in V̇O2 steady state (26,33), it should be included in future research. Animal studies showed that DHA is incorporated into the membranes of fast-oxidative glycolytic fibers (type IIA) of skeletal muscle (34). These muscle fibers have both a high oxidative and glycolytic capacity, and because of their increased activation during moments of high energy demand (35), we decided to perform a 1500-m run trial. Our participants typically perform distances from 10 km to a marathon; therefore, lack of experience and unfamiliarization at such a short distance as 1500-m may influence the outcome of the run trial, and this must be taken into consideration when interpreting our findings. Future studies with omega-3 supplementation should also consider prescreening, during which individuals with similar baseline O3I should be selected (36).
CONCLUSIONS
In conclusion, 12 wk of omega-3 fatty acid supplementation at a dose of 2234 mg of EPA and 916 mg of DHA daily during an endurance running program increased O3I to values currently considered as a target range. This duration and dose of supplementation combined with endurance training increased peak oxygen consumption and improved RE in amateur runners without affecting their performance.
Acknowledgments
This study was funded by the National Science Centre (Poland), grant number 2018/31/N/NZ7/02962. Methodological and technical support from Maciej Jurczak, Sylwester Kujach, Bartosz Weron, and Wieslaw Ziolkowski was crucial for conducting the study and words of appreciation must be expressed toward them. The authors thank the participants of the study—our runners, without whose persistence and trust this project would not have come into being. No conflicts of interest, financial or otherwise, are declared by the authors. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Competing interests: None of the authors of this paper has a competing interest.
Conception and design of the experiments were undertaken by M.T., Z. J., P. C. C., and J. A. Collection, assembly, analysis, and interpretation of data were undertaken by M. T., Z. J., M. C., R. U., H. L. F., P. C. C., M. S., and J. A. Drafting the work or revising it critically for important intellectual content was undertaken by M. T., Z. J., M. C., P. C. C., and J. A. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Clinical registry: The study was registered at https://www.clinicaltrials.gov/ with identifier NCT04780451.
Contributor Information
ZBIGNIEW JOST, Email: zbigniew.jost@awf.gda.pl.
MACIEJ CHROBOCZEK, Email: maciej.chroboczek@awf.gda.pl.
ROBERT URBAŃSKI, Email: robert.urbanski@awf.gda.pl.
PHILIP C. CALDER, Email: P.C.Calder@soton.ac.uk.
HELENA L. FISK, Email: H.Fisk@soton.ac.uk.
MATEUSZ SPRENGEL, Email: msprengel451@gmail.com.
JĘDRZEJ ANTOSIEWICZ, Email: jant@gumed.edu.pl.
REFERENCES
- 1.Baker EJ, Miles EA, Burdge GC, Yaqoob P, Calder PC. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog Lipid Res. 2016;64:30–56. [DOI] [PubMed] [Google Scholar]
- 2.Calder PC. Very long chain omega-3 (n-3) fatty acids and human health. Eur J Lipid Sci Technol. 2014;116(10):1280–300. [Google Scholar]
- 3.Heileson JL Elliott A Buzzard JA, et al. A cross-sectional analysis of whole blood long-chain ω-3 polyunsaturated fatty acids and its relationship with dietary intake, body composition, and measures of strength and power in collegiate athletes. J Am Nutr Assoc. 2023;42(1):94–100. [DOI] [PubMed] [Google Scholar]
- 4.Davis J-K, Freese EC, Wolfe AS, Basham SA, Stein KMW. Evaluation of omega-3 status in professional basketball players. J Strength Cond Res. 2021;35(7):1794–9. [DOI] [PubMed] [Google Scholar]
- 5.Harris WS, Von Schacky C. The omega-3 index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39(1):212–20. [DOI] [PubMed] [Google Scholar]
- 6.Harris WS, Del Gobbo L, Tintle NL. The omega-3 index and relative risk for coronary heart disease mortality: estimation from 10 cohort studies. Atherosclerosis. 2017;262:51–4. [DOI] [PubMed] [Google Scholar]
- 7.Von Schacky C, Kemper M, Haslbauer R, Halle M. Low omega-3 index in 106 German elite winter endurance athletes: a pilot study. Int J Sport Nutr Exerc Metab. 2014;24(5):559–64. [DOI] [PubMed] [Google Scholar]
- 8.Craddock JC, Probst YC, Neale EP, Peoples GE. A cross-sectional comparison of the whole blood fatty acid profile and omega-3 index of male vegan and omnivorous endurance athletes. J Am Coll Nutr. 2022;41(3):333–41. [DOI] [PubMed] [Google Scholar]
- 9.Hingley L, Macartney MJ, Brown MA, McLennan PL, Peoples GE. DHA-rich fish oil increases the omega-3 index and lowers the oxygen cost of physiologically stressful cycling in trained individuals. Int J Sport Nutr Exerc Metab. 2017;27(4):335–43. [DOI] [PubMed] [Google Scholar]
- 10.Lewis NA, Daniels D, Calder PC, Castell LM, Pedlar CR. Are there benefits from the use of fish oil supplements in athletes? A systematic review. Adv Nutr. 2020;11(5):1300–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raastad T, Høstmark AT, Strømme SB. Omega-3 fatty acid supplementation does not improve maximal aerobic power, anaerobic threshold and running performance in well-trained soccer players. Scand J Med Sci Sports. 1997;7(1):25–31. [DOI] [PubMed] [Google Scholar]
- 12.Browning LM Walker CG Mander AP, et al. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr. 2012;96(4):748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saunders PU, Pyne DB, Telford RD, Hawley JA. Factors affecting running economy in trained distance runners. Sports Med. 2004;34(7):465–85. [DOI] [PubMed] [Google Scholar]
- 14.Jones AM Kirby BS Clark IE, et al. Physiological demands of running at 2-hour marathon race pace. J Appl Physiol (1985). 2021;130(2):369–79. [DOI] [PubMed] [Google Scholar]
- 15.Costa P Simão R Perez A, et al. A randomized controlled trial investigating the effects of undulatory, staggered, and linear load manipulations in aerobic training on oxygen supply, muscle injury, and metabolism in male recreational runners. Sports Med Open. 2019;5(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Blaiser C, Roosen P, Willems T, Danneels L, Bossche LV, De Ridder R. Is core stability a risk factor for lower extremity injuries in an athletic population? A systematic review. Phys Ther Sport. 2018;30:48–56. [DOI] [PubMed] [Google Scholar]
- 17.Wolpern AE, Burgos DJ, Janot JM, Dalleck LC. Is a threshold-based model a superior method to the relative percent concept for establishing individual exercise intensity? A randomized controlled trial. BMC Sports Sci Med Rehabil. 2015;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esteve-Lanao J, Foster C, Seiler S, Lucia A. Impact of training intensity distribution on performance in endurance athletes. J Strength Cond Res. 2007;21(3):943–9. [DOI] [PubMed] [Google Scholar]
- 19.Fisk HL, West AL, Childs CE, Burdge GC, Calder PC. The use of gas chromatography to analyze compositional changes of fatty acids in rat liver tissue during pregnancy. J Vis Exp. 2014;85:51445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertuzzi R Bueno S Pasqua LA, et al. Bioenergetics and neuromuscular determinants of the time to exhaustion at velocity corresponding to VO2max in recreational long-distance runners. J Strength Cond Res. 2012;26(8):2096–102. [DOI] [PubMed] [Google Scholar]
- 21.Zinner C, Schäfer Olstad D, Sperlich B. Mesocycles with different training intensity distribution in recreational runners. Med Sci Sports Exerc. 2018;50(8):1641–8. [DOI] [PubMed] [Google Scholar]
- 22.Wilson PB, Madrigal LA. Associations between whole blood and dietary omega-3 polyunsaturated fatty acid levels in collegiate athletes. Int J Sport Nutr Exerc Metab. 2016;26(6):497–505. [DOI] [PubMed] [Google Scholar]
- 23.Peoples GE, McLennan PL, Howe PRC, Groeller H. Fish oil reduces heart rate and oxygen consumption during exercise. J Cardiovasc Pharmacol. 2008;52(6):540–7. [DOI] [PubMed] [Google Scholar]
- 24.Kawabata F, Neya M, Hamazaki K, Watanabe Y, Kobayashi S, Tsuji T. Supplementation with eicosapentaenoic acid-rich fish oil improves exercise economy and reduces perceived exertion during submaximal steady-state exercise in normal healthy untrained men. Biosci Biotechnol Biochem. 2014;78(12):2081–8. [DOI] [PubMed] [Google Scholar]
- 25.Żebrowska A, Mizia-Stec K, Mizia M, Gąsior Z, Poprzęcki S. Omega-3 fatty acids supplementation improves endothelial function and maximal oxygen uptake in endurance-trained athletes. Eur J Sport Sci. 2015;15(4):305–14. [DOI] [PubMed] [Google Scholar]
- 26.Jones AM. The physiology of the world record holder for the women’s marathon. Int J Sports Sci Coaching. 2006;1(2):101–16. [Google Scholar]
- 27.Andrade PMM, Ribeiro BG, Bozza MT, Costa Rosa LFB, Tavares do Carmo MG. Effects of the fish-oil supplementation on the immune and inflammatory responses in elite swimmers. Prostaglandins Leukot Essent Fatty Acids. 2007;77(3–4):139–45. [DOI] [PubMed] [Google Scholar]
- 28.Davinelli S Intrieri M Ali S, et al. Omega-3 index and AA/EPA ratio as biomarkers of running-related injuries: an observational study in recreational runners. Eur J Sport Sci. 2021;1–9. doi: 10.1080/17461391.2021.1998643. [DOI] [PubMed] [Google Scholar]
- 29.Farley G, Riggs DW, Bhatnagar A, Hellmann J. Omega-3 polyunsaturated fatty acids modify the inverse association between systemic inflammation and cardiovascular fitness. Clin Nutr. 2021;40(6):4097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med. 1993;328(4):238–44. [DOI] [PubMed] [Google Scholar]
- 31.Rothschild JA, Bishop DJ. Effects of dietary supplements on adaptations to endurance training. Sports Med. 2020;50(1):25–53. [DOI] [PubMed] [Google Scholar]
- 32.Conley DL, Krahenbuhl GS. Running economy and distance running performance of highly trained athletes. Med Sci Sports Exerc. 1980;12(5):357–60. [PubMed] [Google Scholar]
- 33.Hoff J, Støren Ø, Finstad A, Wang E, Helgerud J. Increased blood lactate level deteriorates running economy in world class endurance athletes. J Strength Cond Res. 2016;30(5):1373–8. [DOI] [PubMed] [Google Scholar]
- 34.Macartney MJ, Peoples GE, Treweek TM, McLennan PL. Docosahexaenoic acid varies in rat skeletal muscle membranes according to fibre type and provision of dietary fish oil. Prostaglandins Leukot Essent Fatty Acids. 2019;151:37–44. [DOI] [PubMed] [Google Scholar]
- 35.Stellingwerff T, Maughan RJ, Burke LM. Nutrition for power sports: middle-distance running, track cycling, rowing, canoeing/kayaking, and swimming. J Sports Sci. 2011;29(Suppl 1):S79–89. [DOI] [PubMed] [Google Scholar]
- 36.von Schacky C. Omega-3 index in 2018/19. Proc Nutr Soc. 2020;79(4):381–7. doi: 10.1017/S0029665120006989. [DOI] [PubMed] [Google Scholar]


