Background:
Frailty is increasing in prevalence. Because patients with frailty are often perceived to have a less favorable risk/benefit profile, they may be less likely to receive new pharmacologic treatments. We investigated the efficacy and tolerability of dapagliflozin according to frailty status in patients with heart failure with mildly reduced or preserved ejection fraction randomized in DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure).
Methods:
Frailty was measured using the Rockwood cumulative deficit approach. The primary end point was time to a first worsening heart failure event or cardiovascular death.
Results:
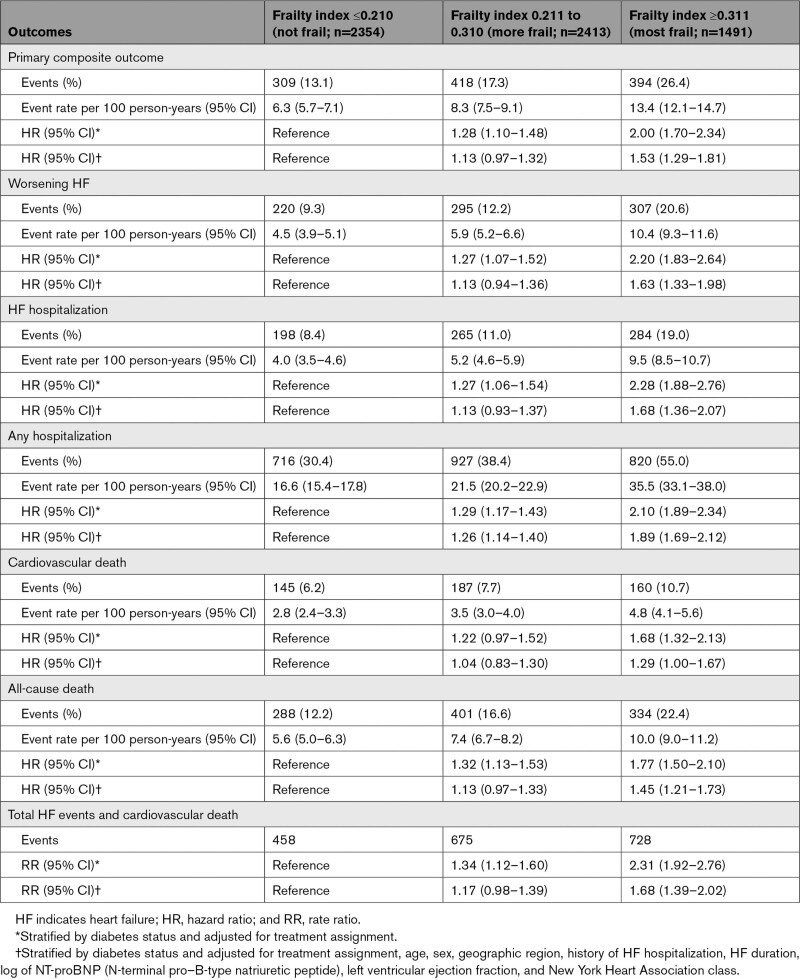
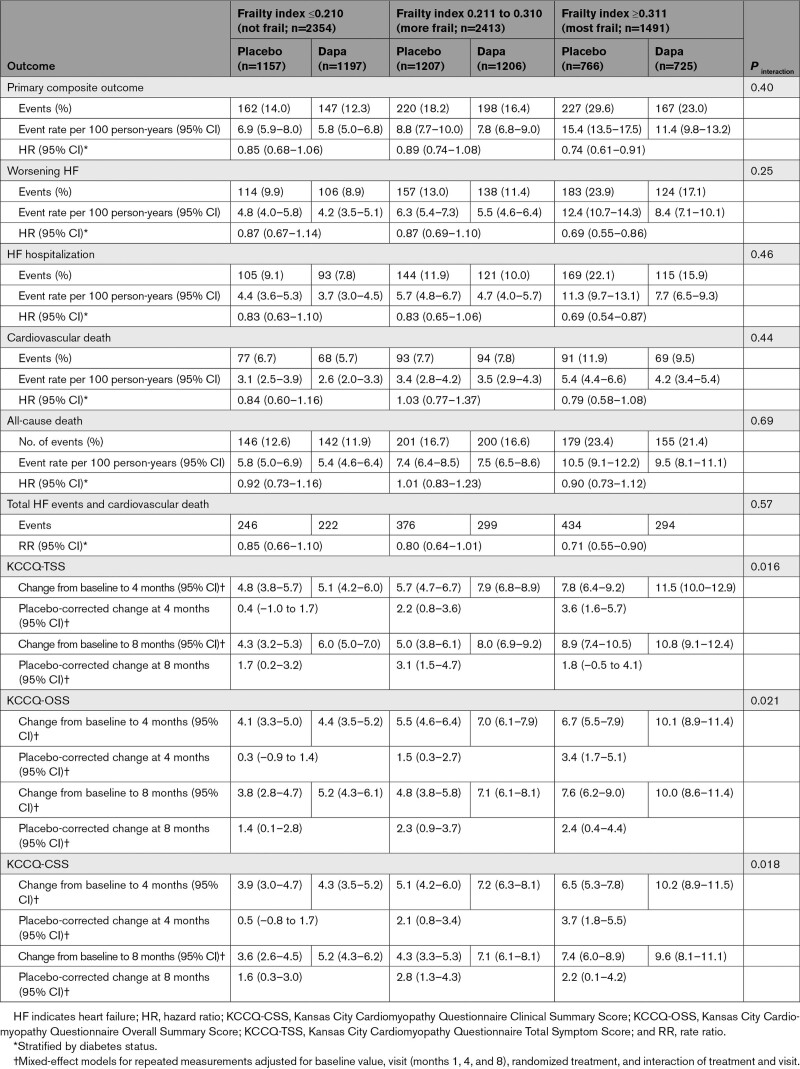
Of the 6263 patients randomized, a frailty index (FI) was calculable in 6258. In total, 2354 (37.6%) patients had class 1 frailty (FI ≤0.210; ie, not frail), 2413 (38.6%) had class 2 frailty (FI 0.211–0.310; ie, more frail), and 1491 (23.8%) had class 3 frailty (FI ≥0.311; ie, most frail). Greater frailty was associated with a higher rate of the primary end point (per 100 person-years): FI class 1, 6.3 (95% CI 5.7–7.1); class 2, 8.3 (7.5–9.1); and class 3, 13.4 (12.1–14.7; P<0.001). The effect of dapagliflozin (as a hazard ratio) on the primary end point from FI class 1 to 3 was 0.85 (95% CI, 0.68–1.06), 0.89 (0.74–1.08), and 0.74 (0.61–0.91), respectively (Pinteraction=0.40). Although patients with a greater degree of frailty had worse Kansas City Cardiomyopathy Questionnaire scores at baseline, their improvement with dapagliflozin was greater than it was in patients with less frailty: placebo-corrected improvement in Kansas City Cardiomyopathy Questionnaire Overall Summary Score at 4 months in FI class 1 was 0.3 (95% CI, −0.9 to 1.4); in class 2, 1.5 (0.3–2.7); and in class 3, 3.4 (1.7–5.1; Pinteraction=0.021). Adverse reactions and treatment discontinuation, although more frequent in patients with a greater degree of frailty, were not more common with dapagliflozin than with placebo irrespective of frailty class.
Conclusions:
In DELIVER, frailty was common and associated with worse outcomes. The benefit of dapagliflozin was consistent across the range of frailty studied. The improvement in health-related quality of life with dapagliflozin occurred early and was greater in patients with a higher level of frailty.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03619213.
Keywords: clinical trial, dapagliflozin, frailty, heart failure
Clinical Perspective.
What Is New?
In a prespecified analysis of DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure), greater frailty was associated with more impairment of health status and clinical outcomes in patients with heart failure and mildly reduced or preserved ejection fraction.
The beneficial effects of dapagliflozin, compared with placebo, on clinical outcomes were consistent regardless of frailty class, but the improvements in symptoms, physical function, and quality of life were larger in patients with the greatest level of frailty.
Adverse events were not more common in individuals randomized to receive dapagliflozin compared with placebo irrespective of frailty class.
What Are the Clinical Implications?
The risk/benefit balance related to frailty in patients with heart failure and mildly reduced or preserved ejection fraction was favorable for dapagliflozin.
These findings should challenge any clinical reluctance to introduce dapagliflozin in patients perceived to be frail.
Frailty, a syndrome characterized by a decline in homeostatic reserves across multiple physiologic systems and increased vulnerability to endogenous and exogenous stressors, is an increasing health burden globally.1–4 The implications of frailty are substantial not only for public health but also for individual patients, who are not only at greater risk of outcomes such as hospital admission and premature death, but also of falls, reduced mobility, impaired quality of life, institutional placement, social isolation, and loneliness.1–4 Because physiologic reserves decline with both age and number of comorbidities, frailty is related to but not the same as aging and multimorbidity. Frailty can also occur in younger people and those without comorbidities, and poor appetite, fatigue, reduced mobility, and declining cognition, all of which are manifestations of frailty, are not specific to a particular disease.1–4
Although heart failure (HF) and frailty are 2 distinct conditions, they often coexist, and each increases the likelihood and complicates the course of the other. Patients with HF are up to 6 times more likely to have frailty than the general population and the catabolic/anabolic imbalance in HF may accelerate the development of frailty.5–9 Patients with HF and frailty also have a substantially higher risk of functional decline, hospital admissions, and death compared with patients with HF in the absence of frailty.9–15 There has been increasing interest in evaluating the effects of new HF treatments in patients with frailty. There is a common perception that evidence-based therapies are less effective in individuals with frailty and there are concerns that these patients have more treatment intolerance and experience more adverse drug reactions and drug interactions and thus are more likely to discontinue treatment.9,10,16–18 Given the anticipation of a less favorable risk/benefit profile in patients with frailty, clinicians may be more reluctant to initiate new therapies in such individuals.9,10,16–18 However, there is little evidence to support this assumption, and patients with HF and frailty may experience greater absolute benefits in worsening HF events and health-related quality of life with certain pharmacologic therapies and aerobic exercise training.10,11,15,19–23 This is particularly important given the likely role of hospitalization and worsening of HF in concert with accelerating frailty.
In DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure), dapagliflozin, compared with placebo, reduced the risk of worsening HF events or cardiovascular death and improved symptoms in 6263 patients with HF with mildly reduced ejection fraction (HFmrEF) or HF with preserved ejection fraction (HFpEF).24 In this prespecified analysis, we examined the efficacy and safety of dapagliflozin according to frailty status using the Rockwood cumulative deficit approach.
Methods
DELIVER was a randomized, double-blind, controlled trial in patients with HF and mildly reduced or preserved left ventricular ejection fraction (LVEF) comparing the efficacy and safety of dapagliflozin 10 mg once daily compared with matching placebo in addition to standard care. The design, baseline characteristics, and primary results of DELIVER have been published.24–27 The trial protocol was approved by the ethics committees of all participating institutions and all patients provided written informed consent. The corresponding author had full access to all the trial data and takes responsibility for their integrity and the data analysis. Data underlying the findings described in this article may be obtained following AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Study Participants
Key inclusion criteria were age >40 years, HF diagnosis >6 weeks with at least intermittent use of diuretic treatment, New York Heart Association (NYHA) functional class II through IV, LVEF >40%, evidence of structural heart disease (either left atrial enlargement or left ventricular hypertrophy), and NT-proBNP (N-terminal pro–B-type natriuretic peptide) concentration ≥300 pg/mL (>600 pg/mL if atrial fibrillation or flutter was noted on the ECG at enrollment). Both ambulatory and hospitalized patients were eligible. Key exclusion criteria were type 1 diabetes, estimated glomerular filtration rate (eGFR) <25 mL/min/1.73 m2, and systolic blood pressure <95 mm Hg. A complete list of exclusion criteria is provided in the article describing the study design.25
Frailty Index
In the current analysis, frailty was assessed using the Rockwood cumulative deficit approach, which has been described in detail previously.10,11,28–30 Standard criteria for constructing a frailty index (FI) using this approach are as follows: at least 30 items are required; items must be associated with health status; items must cover a range of body systems and not be isolated to 1 system; and items must not be part of normal aging or saturate too early (eg, presbyopia), but they should generally increase with age. We created a 30-item FI; these items were derived from medical history, vital signs, laboratory data, and the EQ-5D (5-domain EuroQoL) questionnaire (health-related quality of life measures, including functional status; Table S1). A score was assigned for each nonmissing item and the FI score was calculated as the sum of these scores divided by the total number of nonmissing items, with higher scores indicating greater frailty. Binary variables (eg, a history of myocardial infarction) were scored 0 or 1 (absent or present); ordinal variables (eg, quality of life measures) were scored from 0 to 1 in increments of 0.25 (with a score of 1 indicating the greatest severity); and continuous variables (eg, creatinine) were categorized and scored as 0 or 1 (normal or abnormal). Patients were excluded if they had >20% missing items.10,11,31–33 Patients were divided into the following 3 subgroups: FI ≤0.210 (FI class 1 [not frail, as defined previously]); FI 0.211 to 0.310 (FI class 2 [moderately frail]); and FI ≥0.311 (FI class 3 [most frail]).
Trial Outcomes
The primary outcome in DELIVER was the composite of worsening HF (HF hospitalization or urgent HF visit) or cardiovascular death. Secondary outcomes were total HF events (first and repeat HF hospitalization or an urgent visit for worsening HF) and cardiovascular death, change from baseline to 8 months in the Kansas City Cardiomyopathy Questionnaire (KCCQ) Total Symptom Score (KCCQ-TSS), cardiovascular death, and all-cause mortality. In the current analysis, we also examined the change from baseline to 8 months in the KCCQ Overall Summary Score (KCCQ-OSS) and KCCQ Clinical Summary Score (KCCQ-CSS) and the change in KCCQ scores from baseline to 4 months, worsening HF, HF hospitalization, and any hospitalization.
Prespecified safety analyses included serious adverse events, adverse events leading to discontinuation of trial treatment, and selected adverse events, including volume depletion, renal adverse events, amputation, major hypoglycemia, and diabetic ketoacidosis, for consistency across reporting in trials.
Statistical Analyses
Baseline characteristics were summarized as frequencies with percentages, means with SD, or medians with interquartile ranges. Differences in baseline characteristics were tested using the Cochran-Armitage trend test for binary variables, the Cochran-Mantel-Haenszel test for categorical variables, and the Jonckheere-Terpstra test and analysis of variance test for non-normal and normally distributed continuous variables, respectively.
Regardless of treatment allocation, time-to-event data were evaluated using the Kaplan-Meier estimator (all-cause death), the Aalen-Johansen estimator (all outcomes except all-cause death), and Cox proportional hazards models, stratified according to diabetes status, and adjusted for treatment assignment, and hazard ratios (HRs) with 95% CIs were reported for FI (with FI class I as the reference). Total (first and recurrent) events were evaluated with semiparametric proportional rates models,34 stratified according to diabetes status and adjusted for treatment assignment, and rate ratios (RRs) with 95% CIs were reported. In addition, HRs and RRs, stratified according to diabetes status and adjusted for treatment assignment, age, sex, geographic region, history of HF hospitalization, HF duration, log of NT-proBNP, LVEF, and NYHA functional class, were reported; variables that were part of the FI were not adjusted for, because the categorization of FI into the 3 classes was conditioned on these variables.
To compare the effects of dapagliflozin with placebo, time-to-event data and total (first and recurrent) events were evaluated with Cox proportional hazards models and semiparametric proportional rates models, respectively, and these models were stratified according to diabetes status. HRs and RRs with 95% CI within each FI class were reported. The effect of dapagliflozin was also examined according to continuous FI as a fractional polynomial. The difference between treatment groups in the change in KCCQ-TSS, KCCQ-CSS, and KCCQ-OSS scores from baseline to 8 months was analyzed using mixed-effect models for repeated measurements and adjusted for baseline value, visit (month 1, 4, and 8), treatment assignment, and interaction of treatment and visit. The least squares mean differences with 95% CI between treatment groups within each FI class were reported. The interaction term between treatment assignment and visit was included to examine the effect of dapagliflozin, compared with placebo, on the mean change in KCCQ scores at 4 and 8 months. To test for interaction between the treatment effect of dapagliflozin and FI, the Wald test was used for the Cox proportional hazards models, the semiparametric proportional rates models, and the mixed effect models for repeated measurements.
All analyses were conducted using SAS version 9.4 (SAS Institute) and STATA version 17.0.
Results
Patient Characteristics
Of the 6263 patients randomized in DELIVER, FI was calculable for 6258 patients. The numbers of patients with missing data for individual components of the FI are shown in Tables S2 and S3. The distribution of FI is shown in Figure S1. Mean FI was 0.248 (SD 0.092) and median FI was 0.242 (interquartile range, 0.183–0.308; range, 0–0.633). In total, 2354 (37.6%) patients had class 1 frailty (FI ≤0.210; ie, not frail), 2413 (38.6%) class 2 frailty (FI 0.211–0.310; ie, more frail), and 1491 (23.8%) class 3 frailty (FI ≥0.311; ie, most frail).
Baseline characteristics according to FI class are presented in Table 1. Compared with patients with lower FI, those with higher FI (worse frailty) were older, more often White (and less often Asian), more likely to have cardiovascular and noncardiovascular comorbidities, and more often smokers. They also had higher systolic blood pressure, body mass index, NT-proBNP (irrespective of AF on ECG) level, and hemoglobin A1c level, but lower eGFR. Patients with higher FI were more likely to have a longer duration of HF, lower LVEF, and worse NYHA functional class and KCCQ scores than those with lower FI (ie, less frailty).
Table 1.
Baseline Characteristics According to the Frailty Index
Outcomes According to FI
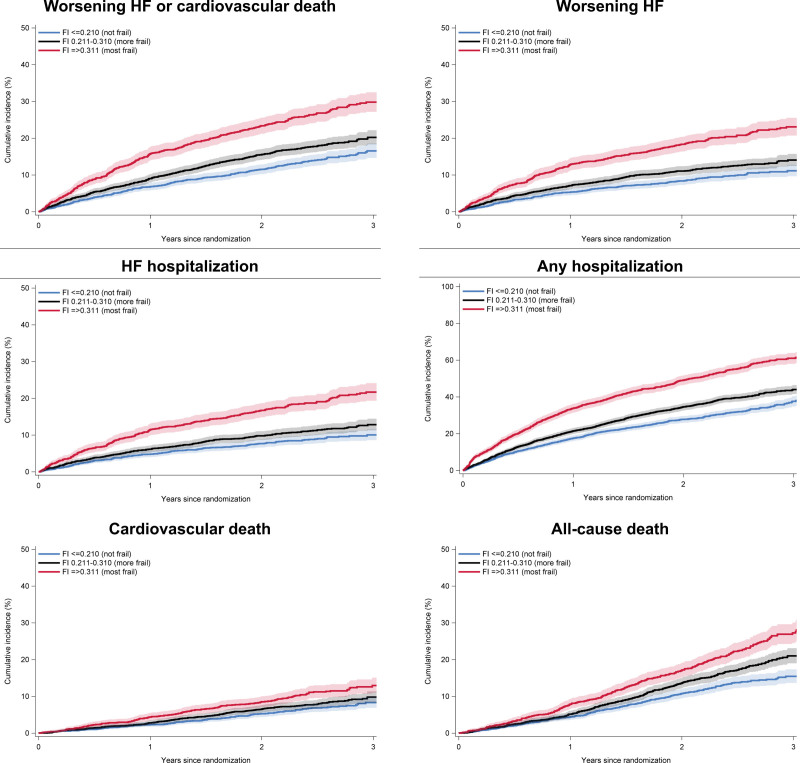
Compared with patients in FI class 1 (the least frail), those in FI class 3 (the frailest) had a higher risk of worsening HF or cardiovascular death, worsening HF, HF hospitalization, any hospitalization, cardiovascular death, all-cause death, and total HF events and cardiovascular death, even after adjustment for known prognostic variables (Figure 1 and Table 2). Compared with individuals in FI class 1, those in FI class 2 also had a higher risk of these outcomes, although the associations between FI class 2 and these outcomes were not statistically significant (except for any hospitalization) after adjustment for prognostic variables.
Figure 1.
Cumulative incidence of outcomes according to frailty index. FI indicates frailty index; and HF, heart failure.
Table 2.
Outcomes According to the Frailty Index
Effects of Dapagliflozin on Clinical Outcomes According to Frailty Index
Primary Composite Outcome
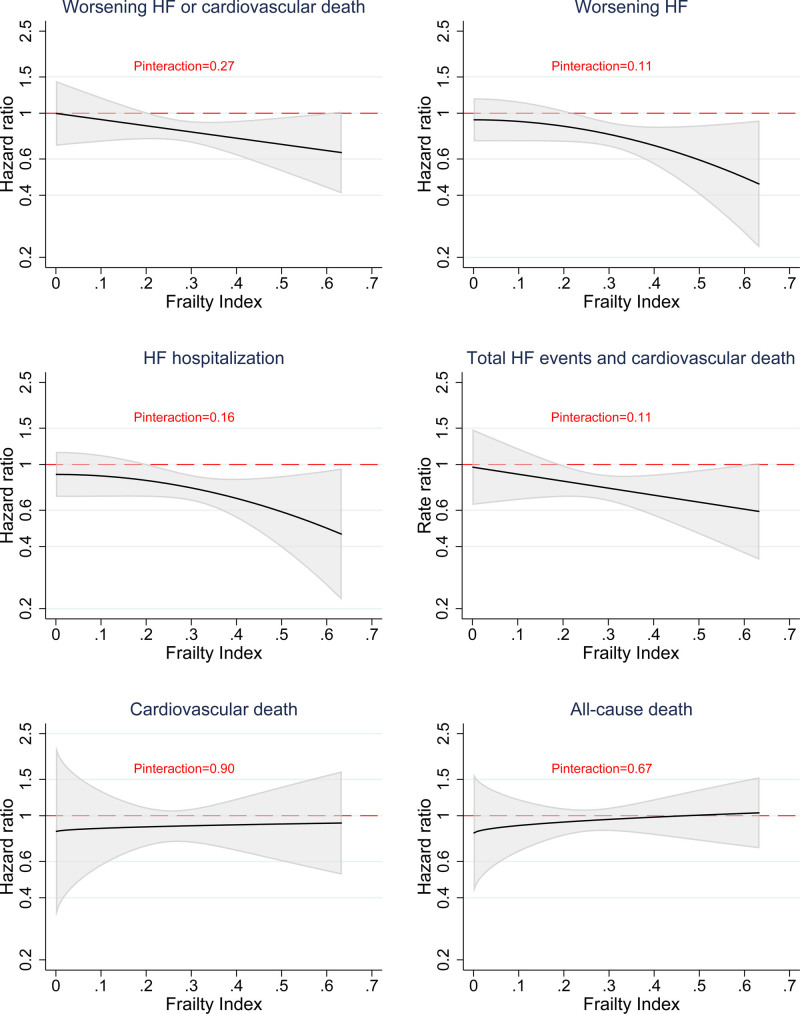
Compared with placebo, dapagliflozin reduced the risk of worsening HF or cardiovascular death across FI classes. The HRs from lowest to highest FI class were 0.85 (95% CI, 0.68–1.06), 0.89 (95% CI, 0.74–1.08), and 0.74 (95% CI, 0.61–0.91), respectively. There was no interaction between FI class (as an ordinal variable) and the effect of dapagliflozin on the primary outcome (Pinteraction=0.40; Table 2). The effect of dapagliflozin was also consistent across the spectrum of continuous FI (Pinteraction=0.27; Figure 2).
Figure 2.
Effects of dapagliflozin compared with placebo on outcomes according to frailty index. Models are stratified by diabetes status. HF indicates heart failure.
In the overall trial, the HR for the primary composite end point with dapagliflozin compared with placebo was 0.82 (95% CI, 0.73–0.92); applying a relative risk reduction of 18% to the placebo event rate to each FI class resulted in a number of patients needed to treat of 40, 31, and 19, respectively, to prevent 1 primary event over the median follow-up of 2.3 years.
Secondary Outcomes
The effect of dapagliflozin was consistent across FI classes for worsening HF, HF hospitalization, cardiovascular death, all-cause death, and the composite of total HF events and cardiovascular death (Pinteraction for all outcomes ≥0.25; Table 2 and Figure 3). The effect of dapagliflozin on these outcomes was also consistent across the spectrum of continuous FI (Pinteraction≥0.11; Figure 2).
Figure 3.
Effects of dapagliflozin compared with placebo on clinical events according to frailty index. Models are stratified by diabetes status. HF indicates heart failure.
In the overall trial, the HR for HF hospitalization with dapagliflozin compared with placebo was 0.77 (95% CI, 0.67–0.89); applying a relative risk reduction of 23% to the placebo event rate in each FI class resulted in numbers needed to treat of 48, 37, and 20 in FI classes 1 to 3, respectively, to prevent at least 1 hospital admission for worsening HF over the median follow-up of 2.3 years.
Symptoms and Health Status Measured Using the KCCQ
At baseline, 5793 patients (92.6%) had available KCCQ data. At 8 months, 4485 patients (71.7% of the study population; 74.3% of the study population alive) had available KCCQ data and 1773 did not (220 because of death, 1553 because of other reasons than death). The effect of dapagliflozin on the mean change in KCCQ scores was modified by FI class; larger increases (improvements) were seen with dapagliflozin, compared with placebo, at 4 and 8 months among patients with a higher FI (ie, greater frailty; Table 3).
Table 3.
Effects of Dapagliflozin Compared With Placebo on Outcomes According to the Frailty Index
Safety Analyses
The proportions of patients who discontinued trial treatment or experienced adverse events increased with increasing frailty. However, there were no differences between treatments (dapagliflozin versus placebo) across all FI classes (Table 4).
Table 4.
Adverse Events in Patients Assigned to Dapagliflozin or Placebo According to the Frailty Index
Discussion
In this prespecified analysis of DELIVER, ≈63% of patients were categorized as frail. Greater frailty was associated with more impairment in health status and worse clinical outcomes, including hospitalizations and death. The beneficial effects of dapagliflozin compared with placebo on clinical outcomes were consistent regardless of frailty class. The improvements in symptoms, physical function, and quality of life were larger in the patients with the greatest degree of frailty. Adverse events, although more frequent in patients with a greater degree of frailty, were not more common in those randomized to dapagliflozin compared with placebo.
Prevalence of and Outcomes According to Frailty
The mean FI in DELIVER, calculated using the Rockwood cumulative deficits approach, was 0.248 (SD 0.092). In general, FI ≤0.210 is considered not frail. In people >65 years of age participating in the UK Biobank, the mean FI was 0.129.35 Other population studies have reported a mean FI ranging from 0.14 to 0.16.36,37 In a trial comparing aspirin with placebo in 19 114 people ≥70 years of age living in the United States (≥65 years of age in US minorities) or Australia who did not have cardiovascular disease, persistent physical disability, or dementia, the mean FI was 0.11 and only 8.1% of participants were frail.38 Even in patients >80 years of age enrolled in 2 hypertension trials, the median FI was only 0.17 to 0.18.39,40
The higher FI among participants in DELIVER contrasts strikingly with these earlier reports, confirming that frailty is much more prevalent in patients with HFmrEF or HFpEF than in the people participating in the studies mentioned. The mean FI in DELIVER (0.248) was lower than in patients with HFpEF in TOPCAT–Americas (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist; mean FI, 0.37) but similar to that in the much larger and more global (and therefore more comparable) PARAGON-HF trial (Prospective Comparison of ARNI With ARB Global Outcomes in Heart Failure With Preserved Ejection Fraction; mean FI, 0.227), which also enrolled patients with HFpEF.11 Using the same approach, the FI in the generally younger patients with HFrEF in PARADIGM-HF (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure), ATMOSPHERE (Aliskiren Trial of Minimizing Outcomes in Patients With Heart Failure; mean FI, 0.250), and DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; 0.216) was similar to that in DELIVER and PARAGON-HF, confirming that frailty is common in all HF phenotypes and not confined to the very elderly.10,15
Impact of Frailty
Increasing frailty was accompanied by a large difference in KCCQ scores at baseline (which were 16 to 17 points lower in patients with the most compared with the least frailty), showing that greater frailty was associated with much more impairment of health-related quality of life and symptoms. The magnitude of the difference in KCCQ scores between patients with more and less frailty was similar to that observed in both PARAGON-HF and DAPA-HF.15
Increasing frailty was also associated with worse outcomes during follow-up. In DELIVER, as in PARAGON-HF and the 3 large HFrEF trials described, there was a graded relationship between FI and the standard outcomes reported (ie, the rates of hospitalization for HF and cardiovascular death increased with increasing FI).10,15 However, the gradients in the association between frailty and the broader outcomes of hospital admission for any cause and death from any cause were, if anything, steeper than for the more specific HF outcomes, emphasizing the more general effect of frailty on health.
Treatment Effect of Dapagliflozin According to Frailty
As mentioned previously, the risk/benefit profile of pharmacologic therapy is often considered less favorable in patients with frailty, leading to underuse and discontinuation of recommended treatments in such individuals.9,10,16–18 In our study, whereas greater frailty was associated with higher rates of adverse events and discontinuation of randomized treatment, neither was more common in the dapagliflozin group than in the placebo group. The efficacy of dapagliflozin was not diminished in patients with the greatest degree of frailty. We found no statistically significant interaction between frailty and the effects of dapagliflozin compared with placebo for any of the outcomes assessed in our categorical analysis, which was confirmed when FI was analyzed as a continuous variable. Indeed, there was a trend toward a greater effect on worsening HF events in patients with a greater degree of frailty, consistent with what was observed with sacubitril/valsartan in PARAGON-HF. Because of the considerably higher event rate in the patients with the greatest degree of frailty, the absolute benefit was twice as large in these individuals as in participants without frailty, emphasizing the importance of counteracting any tendency to therapeutic nihilism in patients deemed to be frail.
These findings in DELIVER were consistent with those using dapagliflozin in DAPA-HF and with sacubitril/valsartan in patients with both HFrEF and HFpEF.15 Although the major effect in patients with HFpEF was on HF hospitalization, this is of potentially great importance given the likely role of hospital admission in accelerating frailty, the prevention and treatment of which has become a key priority in clinical medicine.9,41
Improvement of health status is a major goal of treatment in patients with HF and especially in patients with a greater degree of frailty, who have greater symptom burden and worse health-related quality of life than patients without frailty. The improvement in symptom burden and quality of life with dapagliflozin was significantly larger in patients with greater frailty. This benefit was apparent as early as 4 months after starting treatment. Symptom control and continuation of daily activities are important for patients with HF and may help prevent the development of frailty and progression of existing frailty in these individuals.
Limitations
The inclusion and exclusion criteria in DELIVER precluded the enrollment of patients with the greatest level of frailty and it is likely that the participants in DELIVER were less frail than patients with HFmrEF and HFpEF in the general population. Although the effects of dapagliflozin on clinical outcomes were consistent across the range of FI in DELIVER (0 to 0.633), our results may not be generalizable to all patients with HF, and it is possible that the beneficial effects of this therapy may be attenuated in patients with a high degree of frailty. We were not able to test other frailty scores that include assessments of muscle strength and functional capacity as these measurements were not made in DELIVER. In addition, given the observational nature of the analyses on the association between FI and clinical outcomes, the possibility of residual confounding cannot be excluded fully despite adjustment for measured, known confounders in our analyses.
Conclusions
In DELIVER, greater frailty was associated with greater impairment of health status and worse clinical outcomes. The relative risk reduction in clinical events with dapagliflozin was consistent across frailty classes. The improvement in health-related quality of life with dapagliflozin occurred early and was greater in patients with greater frailty. Adverse events were not more common in individuals randomized to dapagliflozin compared with placebo, irrespective of frailty class. Therefore, the risk/benefit balance related to frailty was favorable for dapagliflozin. These findings should challenge any clinical reluctance to introduce this new therapy in patients perceived to be frail.
Article Information
Sources of Funding
DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure) was funded by AstraZeneca. Professors McMurray and Jhund are supported by British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217.
Disclosures
Dr Butt reports advisory board honoraria from Bayer. Dr Jhund’s employer, the University of Glasgow, has been remunerated by AstraZeneca for work on DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) and DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure). Dr Jhund has received speakers and consulting fees from Novartis and Boehringer Ingelheim, and grants from AstraZeneca and Boehringer Ingelheim. Dr De Boer’s institution, the University Medical Center Groningen, has received research grants and fees (outside the submitted work) from AstraZeneca, Abbott, Boehringer Ingelheim, Cardio Pharmaceuticals GmbH, Ionis Pharmaceuticals, Inc, Novo Nordisk, and Roche. Dr de Boer has received speaker fees from Abbott, AstraZeneca, Bayer, Novartis, and Roche outside the submitted work. Dr Chiang has received honoraria and consultation fees from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, and Sanofi. Dr Desai has received grants and personal fees from AstraZeneca during the conduct of the study; personal fees from Abbott, Biofourmis, Boston Scientific, Boehringer Ingelheim, Corvidia, DalCor Pharma, Relypsa, Regeneron, and Merck; grants and personal fees from Alnylam and Novartis; and personal fees from Amgen outside the submitted work. Dr Drożdż has received personal and institutional research support for DELIVER from AstraZeneca. Dr Hernandez has received research support from American Regent, AstraZeneca, Boehringer Ingelheim, Merck, Novartis, and Verily and has served as a consultant or on the Advisory Board for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cytokinetics, MyoKardia, Merck, Novartis, and Vifor. Dr Inzucchi has served on clinical trial committees or as a consultant to AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Lexicon, Merck, Pfizer, vTv Therapeutics, Abbott, and Esperion and has given lectures sponsored by Astra-Zeneca and Boehringer Ingelheim. Dr Katova has received fees for serving as national coordinator from Novartis and AstraZeneca. Dr Kitakaze has received grants or contracts from the Japanese government through the Japan Agency for Medical Research and Development, Japan Heart Foundation, and has received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from AstraZeneca, Ono, Novartis, Tanabe-Mitsubishi, Japan Medical Data Center, Takeda, Pfizer, Daiichi Sankyo, Otsuka, Sanofi, Boehringer Ingelheim, Amgen, Kowa, Toyama-Kagaku, Kureha, Viatris, and Mochida. Dr Kosiborod has received research grant support from AstraZeneca and Boehringer Ingelheim; has served as a consultant or on an advisory board for Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Esperion Therapeutics, Janssen, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, and Vifor Pharma; has received other research support from AstraZeneca; and has received honorarium from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. Dr Lam is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from AstraZeneca, Bayer, Boston Scientific, and Roche Diagnostics; has served as a consultant or on the advisory board/steering committee/executive committee for Actelion, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma, Us2.ai, Janssen Research & Development LLC, Medscape, Merck, Novartis, Novo Nordisk, Radcliffe Group Ltd, Roche Diagnostics, Sanofi, and WebMD Global LLC; and serves as the cofounder and non-executive director of Us2.ai. Dr Langkilde is an employee and shareholder of AstraZeneca. Dr Lindholm is an employee and shareholder of AstraZeneca. Dr Martinez has received personal fees from AstraZeneca. Dr Merkely has received personal fees from AstraZeneca and Servier. Dr Petersson is an employee and shareholder of AstraZeneca. Dr Kerr Saraiva has received research grant support from Pfizer, Daiichi Sankyo, Sanofi, Boehringer Ingelheim, Novo Nordisk, AstraZeneca, Janssen, Amgen, and Novartis and has received honorarium from Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Amgen. Dr Shah has received personal or institutional research support for DELIVER from AstraZeneca. Dr Vaduganathan has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, and Relypsa; speaker engagements with Novartis and Roche Diagnostics; and participates on clinical end point committees for studies sponsored by Galmed and Novartis. Dr Vardeny has received personal or institutional research support for DELIVER from AstraZeneca. Dr Wilderäng is an employee and shareholder of AstraZeneca. Dr Claggett has received consulting fees from Boehringer Ingelheim. Dr Solomon has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, Bristol Myers Squibb, Celladon, Cytokinetics, Eidos, Gilead, GlaxoSmithKline, Ionis, Lilly, Mesoblast, MyoKardia, National Institutes of Health/National Heart, Lung, and Blood Institute, NeuroTronik, Novartis, Novo Nordisk, Respicardia, Sanofi Pasteur, Theracos, and Us2.ai; and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi Sankyo, GlaxoSmithKline, Lilly, Merck, MyoKardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi-Pasteur, Dinaqor, Tremeau, CellPro-Thera, Moderna, American Regent, and Sarepta. Dr McMurray has received payments through Glasgow University from work on clinical trials, consulting, and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, Dal-Cor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos and personal lecture fees from the Corpus, Abbott, Hikma, Sun Pharmaceuticals, Medscape/Heart.Org, Radcliffe Cardiology, Servier Director, and Global Clinical Trial Partners.
Supplemental Material
Tables S1–S3
Figure S1
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- ATMOSPHERE
- Aliskiren Trial of Minimizing Outcomes in Patients With Heart Failure
- DAPA-HF
- Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure
- DELIVER
- Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure
- eGFR
- estimated glomerular filtration rate
- EQ-5D
- 5-domain EuroQoL
- FI
- frailty index
- HF
- heart failure
- HFmrEF
- heart failure with mildly reduced ejection fraction
- HFpEF
- heart failure with preserved ejection fraction
- HR
- hazard ratio
- KCCQ
- Kansas City Cardiomyopathy Questionnaire
- KCCQ-CSS
- Kansas City Cardiomyopathy Questionnaire Clinical Summary Score
- KCCQ-OSS
- Kansas City Cardiomyopathy Questionnaire Overall Summary Score
- KCCQ-TSS
- Kansas City Cardiomyopathy Questionnaire Total Symptom Score
- LVEF
- left ventricular ejection fraction
- NT-proBNP
- N-terminal pro–B-type natriuretic peptide
- NYHA
- New York Heart Association
- PARADIGM-HF
- Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure
- PARAGON-HF
- Prospective Comparison of ARNI With ARB Global Outcomes in Heart Failure With Preserved Ejection Fraction
- RR
- rate ratio
- TOPCAT
- Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist
Circulation is available at www.ahajournals.org/journal/circ
This manuscript was sent to Ileana Piña, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.122.061754.
For Sources of Funding and Disclosures, see page 1222.
Contributor Information
Jawad H. Butt, Email: jawad.butt@glasgow.ac.uk.
Pardeep S. Jhund, Email: pardeep.jhund@glasgow.ac.uk.
Jan Belohlávek, Email: jan.belohlavek@vfn.cz.
Rudolf A. de Boer, Email: r.a.de.boer@umcg.nl.
Chern-En Chiang, Email: cechiang@vghtpe.gov.tw.
Akshai S. Desai, Email: adesai@bwh.harvard.edu.
Adrian F. Hernandez, Email: adrian.hernandez@duke.edu.
Silvio E. Inzucchi, Email: silvio.inzucchi@yale.edu.
Tzvetana Katova, Email: katova@hearthospital.bg.
Masafumi Kitakaze, Email: kitakaze@zf6.so-net.ne.jp.
Mikhail N. Kosiborod, Email: mkosiborod@saint-lukes.org.
Carolyn S.P. Lam, Email: carolyn.lam@duke-nus.edu.sg.
Daniel Lindholm, Email: daniel.lindholm@me.com.
Erasmus Bachus, Email: erasmus.bachus@astrazeneca.com.
Felipe Martinez, Email: Dr.martinez@usa.net.
Béla Merkely, Email: merkely.study@gmail.com.
Magnus Petersson, Email: magnus.petersson@astrazeneca.com.
Sanjiv J. Shah, Email: Sanjiv.shah@northwestern.edu.
Muthiah Vaduganathan, Email: muthu@fsm.northwestern.edu.
Orly Vardeny, Email: ovardeny@umn.edu.
Ulrica Wilderäng, Email: ulrica.wilderang@astrazeneca.com.
Brian L. Claggett, Email: BCLAGGETT@BWH.HARVARD.EDU.
Scott D. Solomon, Email: ssolomon@bwh.harvard.edu.
References
- 1.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255 [DOI] [PubMed] [Google Scholar]
- 3.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6 [DOI] [PubMed] [Google Scholar]
- 4.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative observational study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x [DOI] [PubMed] [Google Scholar]
- 6.Khan H, Kalogeropoulos AP, Georgiopoulou VV, Newman AB, Harris TB, Rodondi N, Bauer DC, Kritchevsky SB, Butler J. Frailty and risk for heart failure in older adults: the Health, Aging, and Body Composition study. Am Heart J. 2013;166:887–894. doi: 10.1016/j.ahj.2013.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denfeld QE, Winters-Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: a systematic review and meta-analysis. Int J Cardiol. 2017;236:283–289. doi: 10.1016/j.ijcard.2017.01.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bielecka-Dabrowa A, Ebner N, dos Santos MR, Ishida J, Hasenfuss G, von Haehling S. Cachexia, muscle wasting, and frailty in cardiovascular disease. Eur J Heart Fail. 2020;22:2314–2326. doi: 10.1002/ejhf.2011 [DOI] [PubMed] [Google Scholar]
- 9.Vitale C, Jankowska E, Hill L, Piepoli M, Doehner W, Anker SD, Lainscak M, Jaarsma T, Ponikowski P, Rosano GMC, et al. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail. 2019;21:1299–1305. doi: 10.1002/ejhf.1611 [DOI] [PubMed] [Google Scholar]
- 10.Dewan P, Jackson A, Jhund PS, Shen L, Ferreira JP, Petrie MC, Abraham WT, Desai AS, Dickstein K, Køber L, et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction: an analysis of PARADIGM-HF and ATMOSPHERE. Eur J Heart Fail. 2020;22:2123–2133. doi: 10.1002/ejhf.1832 [DOI] [PubMed] [Google Scholar]
- 11.Sanders NA, Supiano MA, Lewis EF, Liu J, Claggett B, Pfeffer MA, Desai AS, Sweitzer NK, Solomon SD, Fang JC. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail. 2018;20:1570–1577. doi: 10.1002/ejhf.1308 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Yuan M, Gong M, Tse G, Li G, Liu T. Frailty and clinical outcomes in heart failure: a systematic review and meta-analysis. J Am Med Dir Assoc. 2018;19:1003–1008.e1. doi: 10.1016/j.jamda.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 13.Bottle A, Kim D, Hayhoe B, Majeed A, Aylin P, Clegg A, Cowie MR. Frailty and co-morbidity predict first hospitalisation after heart failure diagnosis in primary care: population-based observational study in England. Age Ageing. 2019;48:347–354. doi: 10.1093/ageing/afy194 [DOI] [PubMed] [Google Scholar]
- 14.Vidán MT, Blaya-Novakova V, Sánchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18:869–875. doi: 10.1002/ejhf.518 [DOI] [PubMed] [Google Scholar]
- 15.Butt JH, Dewan P, Merkely B, Belohlávek J, Drożdż J, Kitakaze M, Inzucchi SE, Kosiborod MN, Martinez FA, Tereshchenko S, et al. Efficacy and safety of dapagliflozin according to frailty in heart failure with reduced ejection fraction: a post hoc analysis of the DAPA-HF trial. Ann Intern Med. 2022;175:820–830. doi: 10.7326/M21-4776 [DOI] [PubMed] [Google Scholar]
- 16.Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018;72:351–366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 17.Brunner-La Rocca H-P, Linssen GC, Smeele FJ, van Drimmelen AA, Schaafsma H-J, Westendorp PH, Rademaker PC, van de Kamp HJ, Hoes AW, Brugts JJ. Contemporary drug treatment of chronic heart failure with reduced ejection fraction: the CHECK-HF registry. JACC Heart Fail. 2019;7:13–21. doi: 10.1016/j.jchf.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 18.Veenis JF, Brunner-La Rocca HP, Linssen GCM, Geerlings PR, Van Gent MWF, Aksoy I, Oosterom L, Moons AHM, Hoes AW, Brugts JJ. Age differences in contemporary treatment of patients with chronic heart failure and reduced ejection fraction. Eur J Prev Cardiol. 2019;26:1399–1407. doi: 10.1177/2047487319835042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtin D, Dukelow T, James K, O’Donnell D, O’Mahony D, Gallagher P. Deprescribing in multi-morbid older people with polypharmacy: agreement between STOPPFrail explicit criteria and gold standard deprescribing using 100 standardized clinical cases. Eur J Clin Pharmacol. 2019;75:427–432. doi: 10.1007/s00228-018-2598-y [DOI] [PubMed] [Google Scholar]
- 20.Milner A, Braunstein ED, Umadat G, Ahsan H, Lin J, Palma EC. Utility of the modified frailty index to predict cardiac resynchronization therapy outcomes and response. Am J Cardiol. 2020;125:1077–1082. doi: 10.1016/j.amjcard.2019.12.049 [DOI] [PubMed] [Google Scholar]
- 21.Kubala M, Guédon-Moreau L, Anselme F, Klug D, Bertaina G, Traullé S, Buiciuc O, Savouré A, Diouf M, Hermida J-S. Utility of frailty assessment for elderly patients undergoing cardiac resynchronization therapy. JACC Clin Electrophysiol. 2017;3:1523–1533. doi: 10.1016/j.jacep.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 22.Pulignano G, Del Sindaco D, Di Lenarda A, Tarantini L, Cioffi G, Gregori D, Tinti MD, Monzo L, Minardi G. Usefulness of frailty profile for targeting older heart failure patients in disease management programs: a cost-effectiveness, pilot study. J Cardiovasc Med. 2010;11:739–747. doi: 10.2459/JCM.0b013e328339d981 [DOI] [PubMed] [Google Scholar]
- 23.Pandey A, Segar MW, Singh S, Reeves G, O’Connor C, Pina I, Whellan D, Kraus W, Mentz R, Kitzman D. Frailty status modifies the efficacy of exercise training among patients with chronic heart failure and reduced ejection fraction: an analysis from the HF-ACTION trial. Circulation. 2022;146:80–90. doi: 10.1161/CIRCULATIONAHA.122.059983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon SD, McMurray JV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction [published online August 27, 2022]. N Engl J Med. doi: 10.1056/NEJMoa2206286. https://www.nejm.org/doi/10.1056/NEJMoa2206286 [Google Scholar]
- 25.Solomon SD, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Lindholm D, et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. Eur J Heart Fail. 2021;23:1217–1225. doi: 10.1002/ejhf.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon SD, Vaduganathan M, Claggett BL, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. Baseline characteristics of patients with HF with mildly reduced and preserved ejection fraction: DELIVER trial. JACC Heart Fail. 2022;10:184–197. doi: 10.1016/j.jchf.2021.11.006 [DOI] [PubMed] [Google Scholar]
- 27.Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, Desai AS, Vaduganathan M, Gasparyan SB, Bengtsson O, Lindholm D, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER [published online August 27, 2022]. Nat Med. doi: 10.1038/s41591-022-01971-4. https://www.nature.com/articles/s41591-022-01971-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 30.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. doi: 10.1111/jgs.12420 [DOI] [PubMed] [Google Scholar]
- 32.Blodgett JM, Theou O, Howlett SE, Rockwood K. A frailty index from common clinical and laboratory tests predicts increased risk of death across the life course. GeroScience. 2017;39:447–455. doi: 10.1007/s11357-017-9993-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60:464–470. doi: 10.1016/j.archger.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 34.Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Ser B Stat Methodol. 2000;62:711–730. doi: 10.1111/1467-9868.00259 [Google Scholar]
- 35.Williams DM, Jylhävä J, Pedersen NL, Hägg S. A frailty index for UK Biobank participants. J Gerontol A Biol Sci Med Sci. 2019;74:582–587. doi: 10.1093/gerona/gly094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoogendijk EO, Theou O, Rockwood K, Onwuteaka-Philipsen BD, Deeg DJH, Huisman M. Development and validation of a frailty index in the Longitudinal Aging Study Amsterdam. Aging Clin Exp Res. 2017;29:927–933. doi: 10.1007/s40520-016-0689-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong JJ, Mitnitski A, Launer LJ, White LR, Rockwood K. Frailty in the Honolulu-Asia Aging Study: deficit accumulation in a male cohort followed to 90% mortality. J Gerontol A Biol Sci Med Sci. 2015;70:125–131. doi: 10.1093/gerona/glu089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan J, Espinoza S, Ernst ME, Ekram ARMS, Wolfe R, Murray AM, Shah RC, Orchard SG, Fitzgerald S, Beilin LJ, et al. Validation of a deficit-accumulation frailty index in the Aspirin in Reducing Events in the Elderly Study and its predictive capacity for disability-free survival. J Gerontol A Biol Sci Med Sci. 2022;77:19–26. doi: 10.1093/gerona/glab225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warwick J, Falaschetti E, Rockwood K, Mitnitski A, Thijs L, Beckett N, Bulpitt C, Peters R. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the Hypertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with depression aged 80 and over. BMC Med. 2015;13:78. doi: 10.1186/s12916-015-0328-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pajewski NM, Williamson JD, Applegate WB, Berlowitz DR, Bolin LP, Chertow GM, Krousel-Wood MA, Lopez-Barrera N, Powell JR, Roumie CL, et al. Characterizing frailty status in the Systolic Blood Pressure Intervention Trial. J Gerontol A Biol Sci Med Sci. 2016;71:649–655. doi: 10.1093/gerona/glv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.