Abstract
Since disease is a natural aspect of life, human deep space missions will largely depend on preventing disease, diagnosis, and treatment. Pharmaceuticals are used to identify, treat, prevent, or cure illnesses, but they are unstable on Earth and even more so in space. What if the pharmacist could prepare small quantities of medicines in space, on site, as needed? The alteration in pharmacokinetic and pharmacodynamic (PK-PD) and pharmacogenomics with flying and medications will need to be customised for each person individually and specifically at the point of need because of drug stability issues. We can’t meet the expense of bringging everything we might need, so pharmacists must devise ways to manufacture medications in-situ and on-demand. With this skill, pharmacists would be able to fulfill the demand of any exploration mission that involved spaceflight with robust pharmaceuticals that would be stable enough to last the duration of the mission, comprehensive enough to treat all potential medical events, safe, and effective, notwithstanding the known PK-PD and pharmacogenetic alterations that take place during spaceflight. The purpose of this article was to review topics related with Astropharmacy. The topics include: the need of Astropharmacy in space, health-related problems caused by hostile space conditions, storage problems in space, methods to establish the stability and effectiveness of pharmaceutical products in space, and alteration in human physiology including PK-PD and pharmacogenomics and highlight the pharmacist’s potential roles in the pharmacies orbiting the space.
Keywords: Astropharmacy, pharmacist, space
Introduction
Human ambition in space exploration is limitless[1]. By the landing of Apollo 11 on the moon on July 20, 1969, mankind has initiated the beginning of the interstellar traveling era[2]. The next breakthrough after reaching the moon was visiting and then colonization of Mars. National Aeronautics and Space Administration (NASA) has announced its willingness to put a man on Mars by the 2030s[3]. The time that will be spent on this long trip will be about three years during which astronauts will face different hostile conditions that will impact their health[4]. In addition, health-related problems[5] and accidents[6] may occur during the mission which requires medical emergency intervention. Investigations showed that astronauts had to take at least one medication during their relatively short missions into space[7]. Thus, ensuring the availability of enough stock from a broad range of drugs and medicines is mandatory when traveling to space.
The role of medications will not be confined to treating sickness conditions[8] but also in emergency cases such as shock[9], trauma[10], and bleeding[11] as well as protecting the bodies of astronauts from adverse environmental conditions in space.
However, the required effect of the medicinal dosage form in space flight may not work as could be expected on Earth due to several reasons. Human body physiology[12] is altered in space with subsequent modification in the bioavailability and pharmacokinetics-pharmacodynamics (PK-PD) of the drug[12, 13]. The stability of the pharmaceutical dosage form is questionable in space where the storage conditions are different from the Earth especially with high levels of radiations[14] which may affect the product adversely resulting in faster degradation for sensitive materials. Also, it may be important to modify the formula and packaging of the medicinal product for the convenience of use in microgravity conditions in space. Importantly, a specific type of shield may be developed as a lining layer in the spacecraft to protect and enclose not only cosmonauts but also the sensitive materials and even electronics from the destructive effect of radiation from space[15, 16].
In addition to the quality of pharmaceuticals, spaceflight alters human physiology[12] due to fluid shifts, muscle and bone loss, immune system dysregulation, and changes in the gastrointestinal tract (GIT) and metabolic enzymes[17]. These alterations may change the PK-PD of medications[12, 13] used by people and subsequently might impact drug efficacy and safety. Most commonly, medications are administered during space missions to treat sleep disturbances, allergies, space motion sickness, pain, and sinus congestion[18]. These medications are administered under the assumption that they act in a similar way as on the Earth, an assumption that has not been investigated systematically yet. Furthermore, PK-PD studies during spaceflight are also lacking. Apart from PK-PD changes, genetic alteration[19] could be created during the trip in the space shuttle. These alterations along with physiological changes and their resulting PK-PD changes must be considered to determine their ultimate impact on medication efficacy and safety during spaceflight. The current lack of knowledge regarding altered PK-PD acts as a significant barrier to the successful treatment and prevention of medical events and robustness of any pharmacotherapy onboard.
The purpose of this article was to review topics related with Astropharmacy. The topics include: the need of Astropharmacy in space, health-related problems caused by hostile space conditions, alteration in human physiology including PK-PD and pharmacogenomics and investigate the pharmacist’s potential roles in the space.
Why Astropharmacy in Space?
Bringing pharmaceuticals in each space mission would add load to the mission[20]. There would need to be supplied of the media and chemicals required to grow the bacteria and isolate the drugs, Petri dishes and flasks to grow them in, and microfluidic equipment to separate them[21]. All of these issues highlight the need for Astropharmacy in space that will allow protein drugs to be produced “on demand,” even in the remote environment of space, using genes as templates for cellular or cell-free expression of proteins[22]. In addition, the microgravity environment of space can disrupt several systems within the human body. For example, bones and muscles deteriorate, leading to osteoporosis and other problems; the filtration rate of kidneys increases, which can create kidney stones; the redistribution of fluid in the body can lead to “chicken legs” and puffy faces, and cardiovascular systems can experience extreme stress. Luckily, protein-based drugs, many of which are approved by regulators for safety and efficacy and are routinely used clinically here on Earth (like insulin, for example), could be the key to treating many of the illnesses that people develop in space[23].
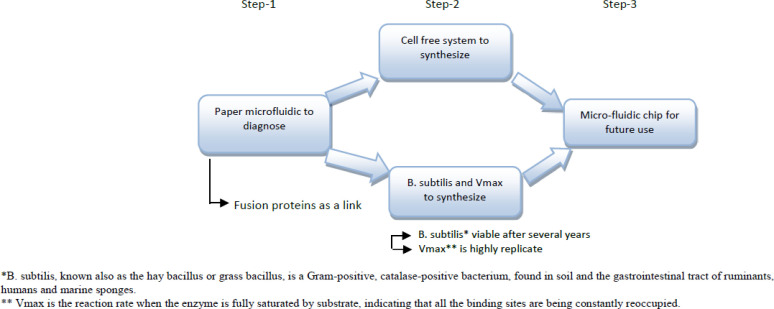
The Astro-pharmacist has to do to make instant biopharmaceuticals by using some cultural medium[24]. The cells begin to secrete whatever is programmed to make, such as teriparatide, a hormone to treat osteoporosis, or G-CSF, a bone marrow stimulator that produces white blood cells. For synthesis of on-site medicine in the space, there will be three necessary steps[25]. First, pharmacists need to design paper-based micro fluidic devices using fusion proteins as a link for the purpose of diagnosis. Second, pharmacists utilize cellular and cell-free systems for drug manufacturing. Cellular system uses bacteria, such as Vibrio natriegens (Vmax) or Bacillus subtilis. Vmax can be stabilized by lyophilization, and Bacillus subtilis can be stabilized in the spore form. Both stabilized forms can be stored for years in a dry state, without the requirement for refrigeration. Vmax is a highly replicative bacterium with a doubling time < 14 minutes, and B. subtilis spores have been shown to be viable after several years in Earth orbit. Alternatively, a cell-free system takes advantage of the transcriptional and translational machinery to generate high protein yields by operating outside the constraints of a living cell. Both systems can synthesize drugs on demand, allowing bypassing shelf-life and storage limitations, which will become necessary when long-duration space flight becomes a reality. Third, after drug synthesis, the next step will be drug purification on a microfludic chip. These chips can be automated for future access and ease of use with only minimal training[24, 26]. Figure 1 shows the drug manufacturing steps inside the Astropharmacy.
Figure 1.
Drug manufacturing in the Astropharmacy by pharmacist[22]
The Health-Related Problems in Space
Some researchers have listed the health-related problems due to harsh space conditions on the human body which may be exacerbated during long-term missions more than low-earth orbital ones[5]. Many of these problems are linked to microgravity conditions. The description of these health related problems reported in International Space Station (ISS) and Space Shuttle [35] missions 1988–1995 are as follows:
Zero-G sickness: Similar to motion sickness experienced on earth during the flight in airplane and sea travel, imbalance felt by the human brain center - that is responsible for spatial orientation - causes nausea and severe feeling of unwell-being which may last for several days. This problem occurred in 1968 during Apollo 8 mission to Moon to an astronaut [27].
Mental health: When a human subject is being left in a confined space for a long time with the only limited view through the small window into the darkness, the mental health, emotions, and thoughts may be impacted in a way that could impact the mission adversely [28].
Muscle weakness: Muscle weakness during space travel is also among the health problems which could be expected that should be minimized by regular daily exercises in an area dedicated to a gym in the spacecraft and held by a belt to the exercise machine [27].
Visual deterioration: Space fights that last for more than six months impact eye function due to changes in its structure as declared by NASA. Eye aiming and location determination of the objects could be impaired under zero-G[29].
Bone problem: The weakening of human bones may occur in a prolonged state of weightlessness [30].
Head congestion: A fluid shift that occurs in zero-G condition results in the accumulation of the body fluid in the head with consequent puffy face appearance of the astronaut [27].
Decreased efficiency of the immune system: Exposure of humans to a myriad of stressors along the whole spaceflight mission may contribute to immune system dysfunction, in addition to over-sensitization of some cosmonaut manifested as hypersensitivity signs [31, 32].
Radiation hazards: Earth’s magnetic field provides a protective shield from radiations for those on it or low orbiting space shuttles or stations. However, in deep space cosmonauts become exposed to various types of hazardous radiations: non-ionizing ultraviolet and ionizing (solar energetic particles, trapped particles, and galactic cosmic radiation) [33].
Medical emergency: Emergency cases are numerous and different scenarios could be expected in space such as trauma, fractures, injuries, and bleeding [34].
Pharmaceutical Product Stability and Effectiveness in Space
People on long-term settlement in the space may not be able to take paracetamol to treat a headache or antibiotics to fight infection. Scientists at the Johnson Space Center[36] have shown that the effectiveness of drugs declines more rapidly in space. The team said longer missions have increased the need for drugs in space. On Earth, medication is typically designed to be stored for a couple of years from the manufacture date. They normally need to be kept in precise conditions, such as away from direct sunlight or in a cool, dry space. The research team investigated whether the unique environment of space - including radiation, excessive vibrations, microgravity, a carbon dioxide rich environment and variations in humidity and temperature - affected drugs’ effectiveness.
The outer space radiation environment represents a combination of charged particles originating from galactic cosmic radiation (GCR) solar particle events, and particles—primarily electrons and protons—trapped by the magnetic field of Earth[37]. The high Z energetic particles—constituents of GCR—can induce significant damage when absorbed by spacecraft structures, depositing more energy per unit path depth and generating upon interaction with materials (through fragmentation process) secondary sources of radiation[14, 16]. Moreover, a possible contributor to tissue damage and carcinogenesis may be the high ionization power of GCR radiation[38].
As the distance from Earth and the duration of missions will increase during interplanetary travel, so does the radiation exposure in the intravehicular environment. This statement also applies to onboard medical kits that may experience drug instability during long-distance spaceflight[14]. The effect of direct ionization on new drug delivery systems has been studied, the sterilization processes leading to radiolysis products[39] and to degradation in polyester carriers in time. Even though it has been evidenced that indirect ionization induces reduced damage in drugs compared to direct ionization, liquid formulations are nonetheless prone to alterations due to less concentrated target molecules than in solid forms. In this case, drug degradation implies free radicals formation, such as oxygen, which results from the breakdown of water in aqueous formulations[14]. Still, little is known about the effect of low doses on drugs in all its complexity. Currently, radiosterilization of pharmaceuticals implies doses in the range of 25kGy–50kGy, exceeding by far the ~0.5Gy that would be accumulated during Martian missions. Providing radiosterilization doses for such short time durations as minutes or hours substantially surpasses the estimated radiation dose-rates amassed over several years of interplanetary travels. It has been put forward that drug stability should be preserved when exposed to low radiation levels, like those anticipated for distant space missions, since at high doses/dose-rates, such as those delivered during radiosterilization, they remain stable. However, many drugs exhibited better stability at increased dose-rates. This observation may be associated with the activity of radical species[14]. The stability of nalidixic acid, spectinomycin and rifampicin exposed to gamma radiation up to 205kGy has been studied with reference to their UV-Vis and FTIR spectra in Reference[40]. The overall observation was that all investigated antibiotics proved to be sensitive to doses up to 24kGy, spectinomycin being the most vulnerable to gamma rays. The higher concentration for at least one byproduct that was generated upon radiation exposure is in direct connection with the increase in radiation dose.
Regarding the type of pharmaceutical formulations, solutions are usually acknowledged to be less stable compared to solid or semisolid forms. In particular, water-based drug solutions can lead to frequent and fast degradation reactions, taking into consideration the interaction of drug molecules with reactive species generated by water. Therefore Kim et al.[41]observed that only liquid pharmaceutical formulations have been studied in the context of space radiation. It has been supposed that if the vulnerable liquid formulations are found to be stable, then the solid formulations should pose no further concern. In apparent disagreement, there have been several reports, outlined by Blue et al., [14] indicating that even drugs in solid or powder state can exhibit radiation-induced instability.
During long-duration spaceflights safe, effective and stable pharmaceuticals must be a requisite part of the missions. At the beginning of human space exploration, only three medicines were taken onto the mission[16], but during the early stages of Space Shuttles, more than 500 individual doses of 31 different drugs have been administered, with 83% of astronauts using at least one medicine[13]. Medical kits on ISS comprise a large number of pharmaceuticals in different formulations alongside diagnostic and therapeutic equipment[16]. Antimicrobial agents in ISS medical kits, aside from antibiotics, include antifungal, antiviral and antiparasitic drugs, thus making possible the prevention, treatment and control of a broad spectrum of infections via different routes of administration[16]. Many studies investigated radiation-induced physico-chemical modifications in pharmaceuticals at sterilization equivalent or higher doses (up to several hundred kGy). For example, powder formulations of methylxanthine derivatives, such as caffeine, theophylline and theobromine, have been exposed to ionizing radiation via an electron beam in the dose range of 25kGy–400kGy, resulting in no color changes and relatively high radiochemical stability up to sterilization doses[42]. On the contrary, metoclopramide aqueous formulations subjected to both gamma rays and high-energy electrons displayed color changes and generated degradation byproducts[43]. The solid-state β-blockers irradiated by high e-beam also showed color and melting point modifications[44]. As an interesting fact, two cephalosporin antibiotics with similar molecular structures—which would suggest that they might have similar responses to the same radiation sources and doses—exhibit somehow different radiosensitivity, cephradine being highly unstable following gamma radiation, while cefotaxime presents < 0.1% degradation and high stability[45].
Pharmaceuticals can become unstable through alteration of either their physical or their chemical properties. Alteration of physical properties includes changes in appearance or consistency; alteration of chemical properties includes loss of potency, alteration of excipients, excipient-active ingredient interactions, or toxic degradation[46]. In order to determine that a pharmaceutical is unchanged by exposure to the radiation environment, a drug must be demonstrated following exposure to having no significant alteration of its active pharmaceutical ingredients API(s) while at the same time have no significant development of degradation products that are either toxic themselves or in some way alter the pharmaceutical properties of the original medication[47]. The USP provides guidelines for acceptable API content in medications approved by the FDA, commonly within 10% of label-specified content. A medication would be considered radiosensitive if API concentration fails to meet USP requirements following radiation exposure. Alterations of API can affect drug potency, efficacy, and safety, rendering the drug less effective, ineffective, or potentially dangerous. There have been very few examinations of pharmaceuticals actually exposed to the space environment. Table 1 summarizes the pharmaceuticals that were exposed to the space environment, only a limited number tackled their stability.
Table 1.
Stability studies carried out on drugs subjected to the space environment.
| Drug | Formulation | Duration in Space | Stability Criteria | Remarks | Reference |
|---|---|---|---|---|---|
| Acetaminophen Aspirin Loratidine Ibuprofen Pseudoephedrine |
Solid | 550 days | Degradation products | Alteration in APIs Impurities in one or more formulation |
Wotring, V. E. (2016). The AAPS journal, 18(1), 210–216. |
| Acyclovir Amoxicilin Azithromyxcine Cobalamine Clotriamzole Cefadroxil Levofloxacine Lidocain Metronidazole Mupricoin Phenytoin Temazepam |
Solid Semi-solid |
14–880 days | Physio-chemical properties | Alteration in physio-chemical properties Impurities in one or more formulation |
Du, Brian, et al. The AAPS journal, 13(2), 299–308. |
| Multi-vitamins Once a day Women’s multi-vitamin |
Solid | 14–20 days | API content B complex |
Alteration in APIs Impurities in one or more formulation |
Chuong, Monica C., et al. Journal of pharmaceutical and biomedical analysis 55.5 (2011): 1197–1200. |
| Vitamin D | Solid | 13 days | API content | Time-associated instability | Zwart, S. R., et al. Journal of food science 74.7 (2009): H209–H217. |
Medication usage reporting during flight suggests that medications may not be as effective in managing medical concerns as expected[15]. A study published by Daniels et al., [48] revealed that crewmembers did report that some medications were less effective than expected in managing common complaints. In 1999, Putcha et al.[49] published reports of medication ineffectiveness during Space Shuttle flights, identifying 13 different medications that crewmembers reported as “not effective“ or “mildly effective“ in treating their symptoms, including oxymetazoline, zolpidem, flurazepam, aspirin, promethazine, temazepam, pseudoephedrine, acetaminophen, simethecone, bisacodyl, and the combination medications of promethazine/dextroamphetamine and phenylephrine/phenylpropanolamine. In 2014, Barger et al.[50] published a discussion of sleep-medication use during Space Shuttle and ISS missions. According to this study, in 17–19% of cases where crewmembers took a sleep medication (zolpidem or zaleplon), a second dose was taken during the same night. This suggests that initial dosing may not have been as effective as desired, highlighting a potentially diminished response to medication during flight.
Space Environment and Human Physiology
The spaceflight environment is different from what humans are accustomed to on Earth[51]. It causes alterations in most physiological processes to some degree, mainly due to the effects of space radiation and the absence of convection, hydrostatic pressure, buoyancy, sedimentation and gravitational loading[52]. There is limited knowledge regarding alterations of pharmacokinetics such as absorption, distribution metabolism, and excretion of a medication and pharmacodynamics in the space environment. As the human body undergoes significant physiological and metabolic changes during spaceflight, it stands to reason that the effects of pharmaceuticals may change during flight. Alterations of hepatic blood flow due to fluid shifts from gravitational unloading may lead to altered hepatic metabolism and variable enzyme activity. Delayed gastric emptying due to microgravity conditions, associated space motion sickness, or side effects of medications used to control spaceflight-induced nausea may alter drug absorption in the GIT. Reduced total body water due to fluid shifting and renal excretion may alter the volume of distribution for consumed drugs. Variable protein expression, altered serum albumin levels, and altered renal blood flow may further affect drug absorption, distribution, metabolism, and excretion. However, research on the impact of spaceflight induced physiological changes on pharmaceutical activity has largely been limited to observational reports and analog studies[53]. The further information about the physiological hazards associated with space travel can be found in the link https://sitn.hms.harvard.edu/flash/2013/space-human-body/ [54].
Microgravity causes fluid shift from the lower to the upper part of the body and thoracic expansion that conjointly gives rise to cardiovascular changes, like increased cardiac preload, stroke volume and cardiac output[55], alongside decreased plasma volume[56] and reduced red blood cell (RBC) mass by the destruction of newly produced RBCs. It has been indicated that blood and fluid redistribution can also cause spaceflight-associated neuro-ocular syndrome. The fluid shift and/or the sensory conflict hypothesis can be the underlying cause for space adaptation syndrome[57]. A reduced gravitational environment may deteriorate perception and impair the function of motor skills, reflexes and coordination. Microgravity is liable for musculoskeletal modifications too, such as diminished muscle mass and strength, loss in lean body mass, decreased bone density and significant loss of calcium through excretion, which leads to increased risk of kidney stone. Gastrointestinal and metabolic alterations, including changed gastric emptying, increased intestinal transit rate and variations in enzyme activity, have been likewise observed subsequent to spaceflight. In such cases, the sought pharmacotherapeutic results may not be attained in space with dosing regimens established in terrestrial gravity.
Surprisingly, very limited research has been conducted to determine whether the expected earth-based PK-PD of a drug is altered in a microgravity environment. However, we know that in such an environment, multiple physiologic processes undergo changes to compensate for the loss of a gravity vector; these adaptations follow variable time courses. Some of these physiologic changes may contribute to differences in pharmacokinetics. With regard to oral bioavailability of medications during spaceflight, several factors, including alterations in drug dissolution rate in gastric juices, gastric emptying, gastric or intestinal absorption, hepatic first-pass metabolism, and intestinal blood flow, could all be influenced by microgravity. Other conditions related to early microgravity exposure could also influence bioavailability, including space motion sickness or changes in gut microflora and gut enzymatic release and distribution [13, 58-61]. In a small study involving 5 astronauts from 3 shuttle missions, acetaminophen was administered (650 mg as two 325-mg tablets orally). Salivary samples rather than blood sample were analyzed to determine the pharmacokinetics of the drug during ground-based testing and during flight. A decrement in absorption of acetaminophen was observed in space compared with ground based testing, as noted by a consistently lower maximum salivary concentration (Cmax) and greater time to reach peak concentration (Tmax) in the test group[62]. Moreover, salivary concentrations of acetaminophen varied greatly among individual astronauts when measured over several flight days; the reasons for this are unclear, but factors may include changes in gut motility, gut absorption, and space motion sickness[63].
Tissue binding of medications can be altered because of protein loss secondary to muscle and tissue atrophy,[63, 64] redistribution of plasma proteins out of the central compartment,[65] alterations in blood lipid levels,[66] or reduced erythrocyte production[67]. Thus, volume losses coupled with reduced tissue binding could alter the distribution of a medication throughout the body, which could influence therapeutic and toxic effects. Microgravity could also affect drug elimination via the kidneys, the skin, or the pulmonary route. As suggested by antiorthostatic bed rest studies, it could also affect liver metabolism of drugs owing to changes in perfusion secondary to the redistribution of blood. No data on human hepatic drug metabolism in space are available. However, several metabolic changes have been observed during human spaceflight, and they may imply that enzymatic activity in space is altered owing to the influence of such factors as changes in plasma adrenocorticotropic hormone, thyrotropin, plasma renin activity, and antidiuretic hormone levels[68].
Pharmacogenomics and Space
Pharmacogenomics is the study of how genes influence an individual’s response to medication[69]. By extension, pharmacogenomics is the precise analysis of gene variants and influence the regulation of drug metabolism and attendant development of therapeutic strategies[70, 71]. Whereas traditional PK-PD methods are applied to populations in order to understand the range of a drug’s effects, pharmacogenomics can be used to personalize drug therapy to an individual[72]. In short, pharmacogenomics represents an emergent method available to tailor drug therapy to the individual astronaut, so that the drug countermeasure solution optimizes the chance for benefit (efficacy), while minimizing the chance for adverse events (safety)[73]. While the application of pharmacogenomics is progressing on Earth, there is presently almost no application of pharmacogenomics in space. This represents a substantial gap in our capability, but it also represents an opportunity to better enable humans to thrive in the space environment. Preemptive pharmacogenetic typing of astronauts, e.g. for selected CYP isoforms and drug transporters, has been suggested as means for supporting individualization of pharmacological treatment[74]. However, no findings from such studies are currently available. The contribution of genetics by itself to pharmacokinetic variability in space is unknown. Environmental factors might be pronounced especially during the first days after launch (as was reported for acetaminophen[75] and initially after return to Earth, which may also encompass the entire duration of touristic flights. For longer stays in space, the ISS offers more uniform environment in terms of nutrition, temperature, and fitness. Notably, the ISS has hosted astronauts of many nations[76] including the United States, Russia, China, India, Japan, Brazil, Israel, and the United Arab Emirates. Current pharmacogenetic data which guide treatment in some ethnic populations do not apply to others, and can even lead to under- or overestimation of the dose. Astronauts additionally vary in gender and age. The youngest astronaut, Gherman Titov [75] was 25 years when he was launched as the second human in orbit, and John Glen[77] was 77 years old when he flew aboard the Space Shuttle for his second mission. Current astronauts neither young nor old, but the age of space tourists and their medical background are likely to be variable. This does not rule out the importance of gaining pharmacogenetic data and connecting it to outcomes in future studies in space.
Role of Pharmacist in the Space
The role of the space medicine doctor has been discussed in the literature, there is significantly less dialogue surrounding the role of the pharmacist with regards to the health of space participants. While a small proportion of pharmacists are actively involved with space participant health in the space sector, their role has much more potential, being primarily centred around medication utilisation reviews for astronauts at the ISS[78]. Tina Bayuse[79] is the first pharmacist to work for NASA. At NASA, pharmacists mainly focus on preparing “convenience” and “contingency” medical kits for astronauts at the International Space Station. The main difference between the 2 kits is that the convenience kit contains medicines that one would usually take on a trip, while the contingency kit is stocked for emergencies and contains items like antibiotics and cardiac life support. Pharmacists decide what goes into the kit and then pack them into the flight kits[80]. Table 5 summarized the role of pharmacist in both the patient and the space mission.
In addition, pharmacist can also play a significant role in medication management and medication research[81]. Medication management encompassed safeguarding the space traveller’s health, like space tourists, by conducting medication reviews (pre-and post-flight), medication advice (digital Astro-telepharmacy information services during spaceflight) and developing personalized medication. Medication research included novel drug development, innovative manufacturing, and understanding clinical applications of the PK-PD changes of medications in space. Table 2 summarizes the potential roles of pharmacist’s in both the patient and the space mission.
Table 2.
Role of pharmacist in space
| Category | Role |
|---|---|
| Evolution of medical domain | Personalised medication by developing targeted therapies using advancements in genomics Targeted and personalised therapies optimised for specific patient groups in the spcae |
| Patient care | Drug tolerance testing Countermeasure support |
| Compounding | Combining, altering or mixing ingredients/medications suit the needs of astronaut physiology and genetics in the space Preparing “convenience” and “contingency” medical kits Development of personalised medicine include myriad inorganic as well as organic strategies |
| Dispensing | Handling medications and prescriptions during the space mission includes supply of dose administration aids (blister packs) |
| Clinicalpharmacokinetic monitoring | Designing drug dosage regimens based on PK-PD Recommending or scheduling measurements of drug concentrations in biological fluids Monitoring and adjusting dosage regimens on the basis of pharmacologic responses and biological fluid Evaluating responses to drug therapy Educating crew members about pharmacokinetic principles and appropriate indications for clinical pharmacokinetic monitoring Developing quality assurance programs for documenting improved patient outcomes |
| Pharmacy integration | Patient safety issues Drug information |
| Clinical pharmacogenomics | Advocating for the rational and ethical use of pharmacogenomics testing Ordering pharmacogenomics tests when appropriate Optimizing medication therapy based on pharmacogenomics test results Providing information and educating crew members in spaceflight Supporting and participating in research |
| Pharmacovigillance | Kit inspection Storage of pharmaceutical Drug recalls |
| Drug information | Medication monographs Drug recalls Pharmaceutical technology update Medical checklist procedures |
| Break | A break from any tasks includes food and toilet breaks, and social interactions in the space. |
| Commercial space travel | Ensure people health Ensure essential medicine availability Ensure alternative medicine availability Ensure availability of alternative treatments |
Limitations
This review had some limitations worth mentioning. First, we did not argue how the space environment will impact the antimicrobial resistance of pharmaceuticals. Second, we did not discuss the essential medicine (EM) required in Astropharmacy. Third, types of emergencies encountered during space trips and permanent settlement of humans in the space were also other limitations. Fourth, we did not discuss the formulary development and licensing roles of pharmacists about the Astromedicines.
The majority of this review has focused on the potential for pharmacokinetic changes as a consequence of microgravity exposure. Whether pharmacodynamics (the relationship of drug concentration to the intensity of its effect on the body) is affected by microgravity is even less certain. Lastly, we did not raise question what type of training and education do pharmacists need to work in zero gravity? If Astropharmacy technology is successfully developed, this knowledge will help improve the quality of healthcare for people in space by alleviating concerns. More studies are needed to evaluate these limitations and the future roles of pharmacists and modern pharmacy practices in space. Consequently, it is expected this research might stimulate debate as well as stimulate further research on these topics. Multidisciplinary studies, concerning these limitations, are required.
Concluding Comments
Overall, this review presents the pharmaceuticals’ stability issues due to microgravity conditions in direct connection with their implementation during space missions. Furthermore, it is shown that deep space exploration alters human physiology and pharmacogenetics ultimately resulting in the alteration of PK-PD. Two major challenges have to be accounted for: space radiation and microgravity whose effects on humans and pharmaceuticals are influenced by the duration and final target of the missions along with the phase of the flight. Pharmacists need to think about these issues that may arise later in life and prepare adequately for those. Additionally, pharmacists need to consider how space travel affects a person and his or her vulnerability to certain health-related problems or side effects to medications as well.
The opinions expressed in this paper are those of the authors.
Acknowledgments
Author Contributions: SA and MAR has generated the idea and discussed it with Professor Jon C. Schommer, Later on; Professor Jon C. Schommer provided the advice, conception, and design of the study. All other authors listed have made a substantial and intellectual contribution to the work and approved it for publication.
Acknowledgments: I am thankful to Dr. Muhammad Ahmer Raza for his support in writing this paper, for his motivation, and immense knowledge. His guidance helped me all the time in writing this paper.
Funding: None
Institutional Review Board Statement: Not applicable
Informed Consent Statement: All authors approved the manuscript
Data Availability Statement: Not applicable
Conflicts of Interest: All authors declare no competing interests
References
- 1.McCurdy, H.E., Space and the American imagination. 2011: JHU Press. [Google Scholar]
- 2.Long, K., Exploring the Solar System and Beyond, in Deep Space Propulsion. 2012, Springer. p. 77-98. [Google Scholar]
- 3.GETTING SICK IN SPACE ON THE WAY TO MARS. [cited 2022 6 Febraury]; Available from: https://pursuit.unimelb.edu.au/articles/getting-sick-in-space-on-the-way-to-mars.
- 4.NASA to explore how 3-year Mars mission could affect humans. [cited 2022 6 February]; Available from: https://www.bgr.in/news/nasa-to-explore-how-3-year-mars-mission-could-affect-humans-543342/.
- 5.Kandarpa, K., Schneider V., and Ganapathy K., Human health during space travel: An overview. Neurology India, 2019. 67(8): p. 176. [DOI] [PubMed] [Google Scholar]
- 6.Accidents and Disasters in Spaceflight History. [cited 2022 21 July]; Available from: https://www.britannica.com/list/7-accidents-and-disasters-in-spaceflight-history#:~.
- 7.Pharma on Mars: Getting Drugs Ready for Deep Space Travel. [cited 2022 6 February]; Available from: https://pharma.elsevier.com/pharma-rd/pharma-mars-drugs-ready-deep-space-travel/.
- 8.Strughold, H., The Role of Medicine in the Space Age. USAF JAG Bull., 1962. 4: p. 21. [Google Scholar]
- 9.Bain, B., Nervous Shock: Time and Space. International Journal of Bahamian Studies, 2015. 21(1): p. 22-26. [Google Scholar]
- 10.Miller, L.A., Chest wall, lung, and pleural space trauma. Radiologic Clinics, 2006. 44(2): p. 213-224. [DOI] [PubMed] [Google Scholar]
- 11.Sturdevant, R.W., Fallen Astronauts: Heroes Who Died Reaching for the Moon. Air Power History, 2005. 52(1): p. 66-68. [Google Scholar]
- 12.Graebe, A., et al. , Physiological, pharmacokinetic, and pharmacodynamic changes in space. The Journal of Clinical Pharmacology, 2004. 44(8): p. 837-853. [DOI] [PubMed] [Google Scholar]
- 13.Tietze, K.J. and Putcha L., Factors affecting drug bioavailability in space. The Journal of Clinical Pharmacology, 1994. 34(6): p. 671-676. [DOI] [PubMed] [Google Scholar]
- 14.Blue, R.S., et al. , Limitations in predicting radiation-induced pharmaceutical instability during long-duration spaceflight. npj Microgravity, 2019. 5(1): p. 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blue, R.S., et al. , Supplying a pharmacy for NASA exploration spaceflight: challenges and current understanding. npj Microgravity, 2019. 5(1): p. 1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta, P. and D. Bhayani, Impact of space environment on stability of medicines: challenges and prospects. Journal of pharmaceutical and biomedical analysis, 2017. 136: p. 111-119. [DOI] [PubMed] [Google Scholar]
- 17.Bayonove, J., et al. , Biological changes observed on rice and biological and genetic changes observed on tabacco after space flight in the orbital station SALYUT-7 (Biobloc III experiment). Advances in Space Research, 1984. 4(10): p. 97-101. [DOI] [PubMed] [Google Scholar]
- 18.Hodkinson, P., et al. , An overview of space medicine. BJA: British Journal of Anaesthesia, 2017. 119(suppl_1): p. i143-i153. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen, E.E., Hansen M.M., and Loeschcke V., Genetic variation in time and space: microsatellite analysis of extinct and extant populations of Atlantic salmon. Evolution, 1999. 53(1): p. 261-268. [DOI] [PubMed] [Google Scholar]
- 20.Manufacturing medicines in space.. [cited 2022 21 July]; Available from: https://www.pharmaceutical-technology.com/analysis/medicines-in-space-astronauts-make-own-drugs/.
- 21.Halldorsson, S., et al. , Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosensors and Bioelectronics, 2015. 63: p. 218-231. [DOI] [PubMed] [Google Scholar]
- 22.Astropharmacy.; Available from: https://neo.life/2021/01/to-boldly-go-where-no-pharma-has-gone-before/.
- 23.National Aeronautics and Space Administration.; Available from: https://www.nasa.gov/about/whats_next.html.
- 24.Bhatia, S., Naved T., and Sardana S., Role of pharmacists in delivering biotechnological products. [Google Scholar]
- 25.Bhatia, S., Naved T., and Sardana S., Introduction to Pharmaceutical Biotechnology, Volume 3; Animal tissue culture and biopharmaceuticals. 2019. [Google Scholar]
- 26.Astropharmacies could grow drugs on demand for future space travelers.; Available from: https://neo.life/2021/01/to-boldly-go-where-no-pharma-has-gone-before/.
- 27.Eissa, M.E.A.M., Long March to Live on Mars: Medication and Physiological Challenges. EC Pharmacology and Toxicology, 2018. 6. [Google Scholar]
- 28.Kanas, N., et al. , Psychology and culture during long-duration space missions. Acta Astronautica, 2009. 64(7-8): p. 659-677. [Google Scholar]
- 29.NASA Finds that Space Flight Impacts Astronauts’ Eyes and Vision.. [cited 2022 7 February]; Available from: https://www.aao.org/eye-health/news/nasa-space-flight-impacts-astronauts-eyes-vision.
- 30.Space Bones. [cited 2022 7 February]; Available from: https://science.nasa.gov/science-news/science-at-nasa/2001/ast01oct_1.
- 31.Frippiat, J.-P., et al. , Towards human exploration of space: The THESEUS review series on immunology research priorities. npj Microgravity, 2016. 2(1): p. 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Astronauts’ spinal muscles shrink and weaken after long stays in space. [cited 2022 10 February]; Available from: https://www.theverge.com/2016/10/25/13392824/astronauts-back-pain-health-space-spinal-muscles.
- 33.Duda, K.R., et al. , HUMAN SIDE OF SPACE EXPLORATION AND HABITATION. Handbook of Human Factors and Ergonomics, 2021: p. 1480-1511. [Google Scholar]
- 34.Kuypers, M.I., Emergency and wilderness medicine training for physician astronauts on exploration class missions. Wilderness & Environmental Medicine, 2013. 24(4): p. 445-449. [DOI] [PubMed] [Google Scholar]
- 35.Tavassoli, M., Medical problems of space flight. Am J Med, 1986. 81(5): p. 850-4. [DOI] [PubMed] [Google Scholar]
- 36.Huamn Spaceflight Capabilities. Johnson SPace Center. [cited 2022 7 Febraury]; Available from: https://www.nasa.gov/centers/johnson/capabilities/hhp/.
- 37.Cucinotta, F.A. and Durante M., Cancer risk from exposure to galactic cosmic rays: implications for space exploration by human beings. The lancet oncology, 2006. 7(5): p. 431-435. [DOI] [PubMed] [Google Scholar]
- 38.Chancellor, J.C., et al. , Limitations in predicting the space radiation health risk for exploration astronauts. npj Microgravity, 2018. 4(1): p. 1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abuhanoğlu, G. and Özer A.Y., Radiation sterilization of new drug delivery systems. Interventional Medicine and Applied Science, 2014. 6(2): p. 51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smarandache, A., Moeller R., and Pascu M., UV-Vis and FTIR Spectroscopic Investigations of Gamma-Ray Irradiated Antibiotics. Rom. Rep. Phys, 2018. 70: p. 602. [Google Scholar]
- 41.Kim, M. and Plante I., An assessment of how radiation incurred during a mars mission could affect food and pharmaceuticals. Wyle Science, Technology, and Engineering Group—NASA: Houston, TX, USA, 2015. [Google Scholar]
- 42.Marciniec, B., et al. , Analytical study on irradiated methylxanthine derivatives. Journal of thermal analysis and calorimetry, 2013. 111(3): p. 2165-2170. [Google Scholar]
- 43.Maquille, A., Jiwan J.-L.H., and Tilquin B., Radiosterilization of drugs in aqueous solutions may be achieved by the use of radioprotective excipients. International journal of pharmaceutics, 2008. 349(1-2): p. 74-82. [DOI] [PubMed] [Google Scholar]
- 44.Marciniec, B., et al. , The influence of radiation sterilisation on some β-blockers in the solid state. Thermochimica acta, 2011. 514(1-2): p. 10-15. [Google Scholar]
- 45.Hasanain, F., et al. , Gamma sterilization of pharmaceuticals—a review of the irradiation of excipients, active pharmaceutical ingredients, and final drug product formulations. PDA journal of pharmaceutical science and technology, 2014. 68(2): p. 113-137. [DOI] [PubMed] [Google Scholar]
- 46.CARSTENSEN, J.T., Drug stability: principles and practices. Drugs and the pharmaceutical sciences, 1990. 43: p. X-520. [Google Scholar]
- 47.Wotring, V.E., Space pharmacology. 2012. [Google Scholar]
- 48.Daniels, V. and Bayuse T., Risk of ineffective or toxic medications during long-duration exploration spaceflight. Oral Presentation, NASA Human Systems Risk Board, 2018. [Google Scholar]
- 49.Putcha, L., et al. , Pharmaceutical use by US astronauts on space shuttle missions. Aviation, space, and environmental medicine, 1999. 70(7): p. 705-708. [PubMed] [Google Scholar]
- 50.Barger, L.K., et al. , Prevalence of sleep deficiency and use of hypnotic drugs in astronauts before, during, and after spaceflight: an observational study. The Lancet Neurology, 2014. 13(9): p. 904-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wotring, V., Space pharmacology: how space affects pharmacology. Drug Discovery and Evaluation: Methods in Clinical Pharmacology, 2020: p. 519-531. [Google Scholar]
- 52.Thirsk, R., et al. , The space-flight environment: the International Space Station and beyond. Cmaj, 2009. 180(12): p. 1216-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandia, P., Saivin S., and Houin G., The influence of weightlessness on pharmacokinetics. Fundamental & clinical pharmacology, 2005. 19(6): p. 625-636. [DOI] [PubMed] [Google Scholar]
- 54.Springel, M., The Human Body in Space: Distinguishing Fact from Fiction. The human body in space: Distinguishing fact from fiction. Harvard University, August 12, 2013. http://sitn.hms.harvard.edu/flash/2013/space-human-body, 2013. [Google Scholar]
- 55.Norsk, P., Adaptation of the cardiovascular system to weightlessness: surprises, paradoxes and implications for deep space missions. Acta Physiologica, 2020. 228(3): p. e13434. [DOI] [PubMed] [Google Scholar]
- 56.Norsk, P., et al. , Revised hypothesis and future perspectives. American journal of kidney diseases, 2001. 38(3): p. 696-698. [DOI] [PubMed] [Google Scholar]
- 57.Heer, M. and Paloski W.H., Space motion sickness: incidence, etiology, and countermeasures. Autonomic Neuroscience, 2006. 129(1-2): p. 77-79. [DOI] [PubMed] [Google Scholar]
- 58.Smirnov, K., Role of the gravitation factor in the development of changes in the digestive system. Fiziologicheskii Zhurnal SSSR Imeni IM Sechenova, 1986. 72(4): p. 484-489. [PubMed] [Google Scholar]
- 59.Smirnov, K.V. and Ugolev A.M., Digestion and absorption. Space biology and medicine, 1993. 3: p. 211. [Google Scholar]
- 60.Groza, P., Bordeianu A., and Boca A., Modifications of the digestive tract in rats submitted to an orbital flight aboard the Soviet satellite Cosmos 1129. Physiologie (Bucarest), 1983. 20(1): p. 35-44. [PubMed] [Google Scholar]
- 61.Amidon, G.L., DeBrincat G.A., and Najib N., Effects of gravity on gastric emptying, intestinal transit, and drug absorption. The Journal of Clinical Pharmacology, 1991. 31(10): p. 968-973. [DOI] [PubMed] [Google Scholar]
- 62.Cintron, N., Putcha L., and Vanderploeg J., Inflight pharmacokinetics of acetaminophen in saliva. Results of the life sciences DSOs conducted aboard the space shuttle, 1981. 1986: p. 27-42. [Google Scholar]
- 63.LeBlanc, A., et al. , Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Neuronal Interact, 2000. 1(2): p. 157-60. [PubMed] [Google Scholar]
- 64.Fitts, R., et al. , Prolonged space flight‐induced alterations in the structure and function of human skeletal muscle fibres. The Journal of physiology, 2010. 588(18): p. 3567-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leach, C.S., et al. , Regulation of body fluid compartments during short-term spaceflight. Journal of Applied Physiology, 1996. 81(1): p. 105-116. [DOI] [PubMed] [Google Scholar]
- 66.Leach, C.S., et al. , Cholesterol in serum lipoprotein fractions after spaceflight. Aviation, space, and environmental medicine, 1988. 59(11 Pt 1): p. 1034-1037. [PubMed] [Google Scholar]
- 67.Alfrey, C.P., et al. , Control of red blood cell mass in spaceflight. Journal of applied physiology, 1996. 81(1): p. 98-104. [DOI] [PubMed] [Google Scholar]
- 68.Derendorf, H., Pharmacokinetic/pharmacodynamic consequences of space flight. The Journal of Clinical Pharmacology, 1994. 34(6): p. 684-691. [DOI] [PubMed] [Google Scholar]
- 69.What is pharmacogenomics? [cited 2022 21 July]; Available from: https://medlineplus.gov/genetics/understanding/genomicresearch/pharmacogenomics/#:~.
- 70.Relling, M.V. and Evans W.E., Pharmacogenomics in the clinic. Nature, 2015. 526(7573): p. 343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Whirl‐Carrillo, M., et al. , Pharmacogenomics knowledge for personalized medicine. Clinical Pharmacology & Therapeutics, 2012. 92(4): p. 414-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidt, M.A., et al. , Why personalized medicine is the frontier of medicine and performance for humans in space. New Space, 2020. 8(2): p. 63-76. [Google Scholar]
- 73.Schmidt, M.A., Schmidt C.M., and Goodwin T.J., Pharmacogenomics in Spaceflight. Handbook of Space Pharmaceuticals, 2022: p. 389-427. [Google Scholar]
- 74.Flockhart, D., Drug interactions: cytochrome P450 drug interaction table. Indiana University School of Medicine, 2007. 2010. [Google Scholar]
- 75.Eyal, S., How do the pharmacokinetics of drugs change in astronauts in space? 2020, Taylor & Francis. p. 353-356. [DOI] [PubMed] [Google Scholar]
- 76.NASA International Cooperation.. Available from: https://www.nasa.gov/mission_pages/station/cooperation/index.html.
- 77.John Glenn returns to space. [cited 2022 22 July]; Available from: https://www.history.com/this-day-in-history/john-glenn-returns-to-space#:.
- 78.Nguyen, V., Pharmacists out of this world. Canadian Pharmacists Journal, 2018. 151(6): p. 366-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.How Tina Bayuse became the first pharmacist at NASA. [cited 2022 8 February]; Available from: https://pharmaceutical-journal.com/article/news/how-tina-bayuse-became-the-first-pharmacist-at-nasa.
- 80.Nguyen, V., Pharmacists out of this world. Canadian pharmacists journal : CPJ = Revue des pharmaciens du Canada : RPC, 2018. 151(6): p. 366-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sawyers, L., et al. , Astropharmacy: Pushing the boundaries of the pharmacists’ role for sustainable space exploration. Research in Social and Administrative Pharmacy, 2022. [DOI] [PubMed] [Google Scholar]