Abstract
The recent approval of novel, oral antivirals for the treatment of COVID-19 has significantly altered the outpatient management of patients diagnosed with COVID-19. From a community pharmacy perspective, the two treatment options for mild to moderate COVID-19 are Paxlovid™ (nirmatrelvir/ritonavir) and Lagevrio™ (molnupiravir). While the availability of these antivirals has expanded community pharmacists’ capability to provide care for patients diagnosed with COVID-19, many community pharmacists may struggle to effectively operationalize these agents in practice. This commentary provides a review of Paxlovid™ and Lagevrio™ clarifying the differences between each medication and their respective places in therapy, as well as suggestions and best-practices to operationalize the provision of these services in community pharmacies. These considerations are necessary to support and inform community pharmacy practice when providing oral COVID-19 antiviral therapy.
Keywords: Community pharmacists, COVID-19, Paxlovid, Lagevrio, oral antivirals
Introduction
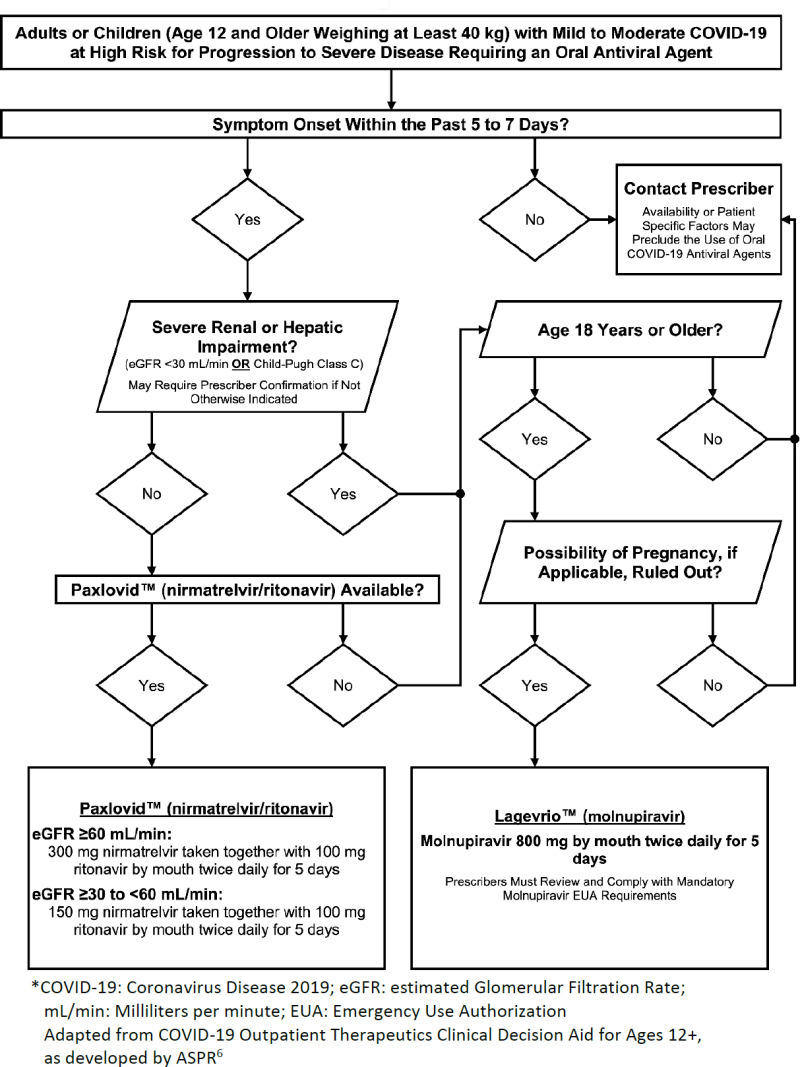
The recent approval of novel, oral antivirals for the treatment of COVID-19 has significantly altered the outpatient management of patients diagnosed with COVID-19, particularly those at high risk for progression to hospitalization or death. From a community pharmacy perspective, the two treatment options for mild to moderate COVID-19 are Paxlovid™ (nirmatrelvir/ritonavir) and Lagevrio™ (molnupiravir).1-3 Paxlovid™ is a combination peptidomimetic protease inhibitor boosted with ritonavir, whereas molnupiravir is a nucleoside analogue.2,3 As a boosted protease inhibitor, it is important to consider that Paxlovid™ has inherent drug-drug interactions, contraindications, and renal dosing considerations.2 Lagevrio™, in comparison, has fewer drug interactions, but is contraindicated in patients who are pregnant or nursing.3 In addition to drug and pregnancy contraindications, each antiviral treatment has specific dosing, administration, and counseling considerations.1 When compared to placebo, both Paxlovid™ and Lagevrio™ reduced risk of hospitalization and death in non-hospitalized, unvaccinated adults with mild-to-moderate COVID-19.4,5 While the availability of these antivirals has expanded community pharmacists’ capability to provide care for patients diagnosed with COVID-19, many community pharmacists may struggle to effectively operationalize these agents in practice. It is our goal to provide condensed guidance for community pharmacists surrounding the use of Paxlovid™ and Lagevrio™ in the outpatient setting. Figure 1 provides guidance to aid community pharmacists in identifying appropriate usage of COVID-19 antiviral therapies.
Figure 1.
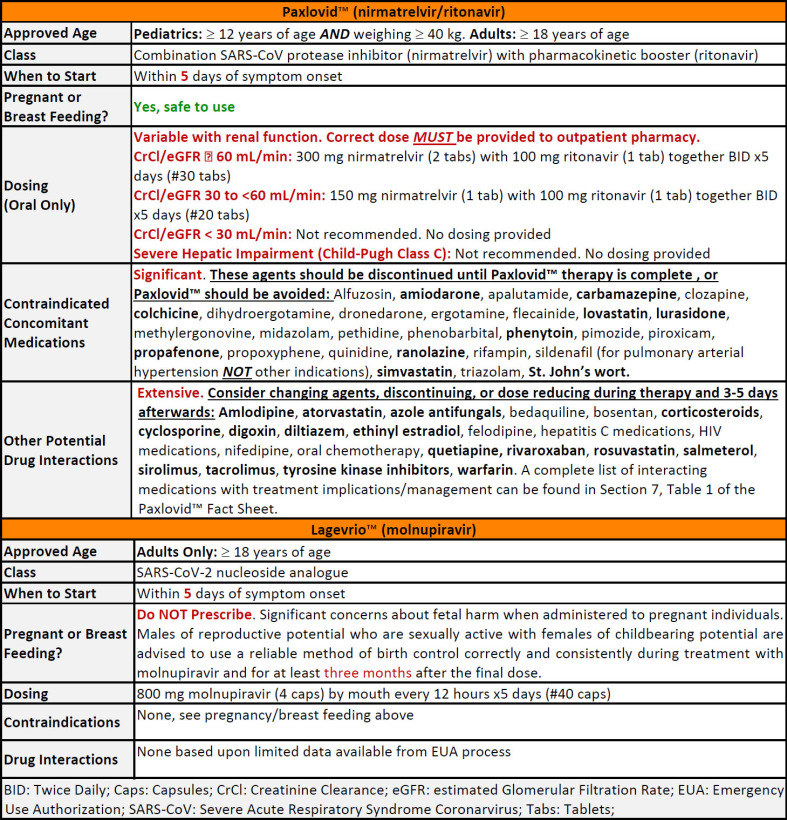
Additionally, Table 1 provides considerations for the use of these agents, and brief correspondence clarifying the differences between each medication and their respective places in therapy.
Table 11,2:
Clinical Applications and Implications
As these treatment options become widely available and employed, it is important for community pharmacists to begin preparing and planning for the integration of these antivirals into workflow and daily operations. One of the largest considerations for offering these medications in community practice is obtaining accurate and detailed medication histories. This portion of the patient care process becomes crucial as patients may opt to fill their antiviral prescriptions at pharmacies other than those at which they have previously filled prescriptions. This can leave a considerable gap in the patient’s medication history, creating the potential for unrecognized drug-drug interactions. Implementing the use of a guided, detailed patient questionnaire when dispensing prescriptions for Paxlovid™ and Lagevrio™ may streamline patient encounters, and assist in obtaining detailed medication histories, especially for non-established patients. Patient questionnaires should screen for medications contraindicated or at high risk for interaction with Paxlovid™ therapy (Table 1), date of symptom onset, as well as pregnancy and/or breast-feeding status. The patients’ primary care provider should also be obtained in the event there are considerations regarding treatment with Paxlovid™ or Lagevrio™ that require provider discussion. Obtaining this information is crucial for community pharmacists to make informed decisions on appropriate COVID-19 therapy options.
Community pharmacists must also consider which medications should be discontinued, dose-reduced, or modified for 3 to 5 days following Paxlovid™ therapy (Table 1).2,6 Medications contraindicated with Paxlovid™ therapy should be stopped, with appropriate alternatives to therapy being recommended. In patients without readily available, non-interacting alternatives, eligibility for Lagevrio™ therapy should be assessed.2,3,6 However, a judicious evaluation of a patient’s medication history should be performed before modifying medication regimens or recommending alternative therapies. Simply having a prescription for an interacting medication is not a sufficient reason for modification of COVID-19 oral antiviral therapy. Rather, medication indication (e.g. sildenafil), dose, and frequency (e.g. “PRN” or as needed) should also be considered.
Adverse reactions should also be addressed when counseling for Lagevrio™ and Paxlovid™. Hypersensitivity reactions (e.g. anaphylaxis and angioedema) can occur with both agents, while dermatologic reactions (e.g., erythema of skin, skin rash, and urticaria) have been more frequently reported with Lagevrio™.2,3 Altered sense of taste, hypertension, diarrhea, and myalgia have also been reported with Paxlovid™, though some of these generalized side-effects may be reported with COVID-19 infection as well.2 These potential side-effects should be reviewed with patients upon medication dispensing.
Depending on availability, renal dose adjustments for Paxlovid™ may require a pharmacist to physically manipulate the medication packaging prior to dispensing.1,2 With the original packaging, pharmacists must remove one nirmatrelvir tablet from each blister card and cover the dosing instructions on the front of the carton, as well as the empty areas of the blister card, with a renal dose adjustment sticker. This sticker provides updated dosing instructions for patients with renal impairment and reflects the changes made to medication packaging.1,2 However, Pfizer has also released updated “renal” packaging that obviates the need for physical manipulation in those requiring a renal dose adjustment.7 The original, 300 mg nirmatrelvir and 100 mg ritonavir, packaging may still be used in these patients, provided the packaging is modified as previously described.
Beyond assessing for dosing considerations and obtaining accurate, thorough medication histories, attention must also be paid to planning for provider correspondence. Recommendations for modifying therapeutic regimens, clarifying renal function, and notification when mutual patients obtain Paxlovid™ or Lagevrio™ are discussions community pharmacists should be prepared to have with providers.2,3 Incorporation of correspondence into workflow will require informing and training pharmacy staff, including pharmacy technicians, on screening tools utilized, processes for patients receiving anti-viral therapy, and referral to pharmacists for clinical discussion with both patients and providers.
Conclusion
The provision of Paxlovid™ and Lagevrio™ requires extensive planning and consideration for community pharmacists. As the use of these medications becomes more widespread, it is imperative for community pharmacists to serve as medication experts by ensuring appropriate antiviral use, incorporating COVID-19 specific correspondence into operations, offering provider consultation on appropriate treatment options, and providing specific counseling recommendations to patients. Community pharmacists remain integral to the use of outpatient treatments for mild to moderate COVID-19, particularly as society navigates the new, oral antiviral treatment process.
The opinions expressed in this paper are those of the author(s).
Disclosures of Conflicts of Interest: The authors declare no conflicts of interest
References
- 1.HHS Office of the Assistant Secretary for Preparedness and Response (ASPR). Federal Response to COVID-19: Therapeutics Clinical Implementation Guide. U.S. Department of Health and Human Services. Updated May 6, 2022. Accessed May 23, 2022. https://aspr.hhs.gov/COVID-19/Therapeutics/Documents/USG-COVID19-Tx-Playbook.pdf
- 2.PaxlovidTM (nirmatrelvir and ritonavir) [Emergency Use Authorization - package insert]. New York, NY: Pfizer Laboratories. April 14 2022; [Google Scholar]
- 3.LagevrioTM (molnupiravir) [Emergency Use Authorization - package insert]. Whitehouse Station, NJ: Merck. March 2022; [Google Scholar]
- 4.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med. 2022;386(15):1397-1408. doi: 10.1056/NEJMoa2118542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022;386(6):509-520. doi: 10.1056/NEJMoa2116044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.HHS Office of the Assistant Secretary for Preparedness and Response (ASPR). COVID-19 Outpatient Therapeutics Clinical Decision Aid for Ages 12+. U.S. Department of Health and Human Services. Updated April 18, 2022. Accessed April 20, 2022. https://aspr.hhs.gov/COVID-19/Therapeutics/Documents/COVID-Therapeutics-Decision-Aid.pdf
- 7.HHS Office of the Assistant Secretary for Preparedness and Response (ASPR). New Paxlovid Dose Pack Authorized by FDA. U.S. Department of Health and Human Services. Updated April 14, 2022. Accessed April 20, 2022, https://aspr.hhs.gov/COVID-19/Therapeutics/updates/Pages/important-update-14apr2022.aspx